Abstract
Objective
To describe the cutaneous trunci muscle (CTM) reflex in dogs.
Study Design
Prospective descriptive study.
Animals
Normal dogs (n = 155) and 10 dogs with thoracolumbar myelopathies.
Methods
The CTM reflex caudal border was assessed from the ilial crests moving cranially until a CTM contraction was elicited. The lateral borders were evaluated at 4 levels and the distance from the midline to the lateral border was expressed as a percentage of the trunk hemicircumference. The caudal border was assessed in 10 dogs with myelopathies by 4 different observers and by 1 observer on 3 occasions; the inter- and intraobserver kappa coefficient was calculated.
Results
The CTM reflex was elicited in all dogs. Its caudal border was at L5 or L6 in 153 dogs and at L1 and L3 in 2 dogs. The lateral field of the reflex occupied >50% of the hemicircumference of the trunk at each level tested. The mean difference in measurement of the reflex caudal border was 0.55 cm between observers and 0.28 cm for the same observer. The inter- and intraobserver kappa coefficient was 0.67 and 0.87, respectively.
Conclusions
The CTM reflex is elicited caudal to L5 in most normal dogs, and the lateral sensory field extends to 50% or more of the circumference of the trunk. Inter- and intraobserver differences in assessment of the caudal border are low.
The cutaneous trunci muscle (CTM) is a thin but extensive sheet of skeletal muscle that covers most of the dorsal and lateral walls of the abdomen and thorax. It originates in the caudal gluteal region and runs cranially and ventrally to insert in the axillary region, where it is associated with the latissimus dorsi muscle and the caudal border of the deep pectoral muscle.1 It is mainly composed of type II (fast, glycolytic) fibers, and does not contain muscle spindles. Contraction of the CTM occurs as a reflex and the skin twitch is a defensive mechanism to remove foreign bodies or insects, or to increase heat production.2, 3 It may also be involved in other functions such as forced expiration, vomiting, coughing, and defecation4 and was originally described in the cat as the viscero-pannicular reflex in response to gall bladder insufflations.5 The CTM reflex, also sometimes called panniculus reflex because the CTM was formerly called the Panniculus Carnosus muscle,6 is classically elicited by applying mechanical or thermal sensory stimuli to the skin.2 The afferent input to the CTM reflex is provided by the cutaneous branches of spinal nerves that supply the skin of the thorax and abdomen.7 They enter the dorsolateral fasciculus and synapse with interneurons in the gray matter that project bilaterally and ascend in the fasciculus proprius to the motor nucleus in the cervical enlargement.2, 4, 8 In the dog, the cutaneous trunci motor nucleus is located in the ventral and ventrolateral nuclei of the ventral horn of the spinal cord primarily in the eighth cervical (C8) and first thoracic (T1) spinal cord segments, and occasionally in the C7 and T2 segments of the spinal cord.9 Motor innervation is provided by the lateral thoracic nerve that comprises a part of the brachial plexus.6, 10 The CTM reflex is commonly used to localize thoracolumbar spinal lesions in dogs but there is limited data available describing this reflex in normal dogs.10–12
Our aims for this prospective descriptive study are to describe the sensory field, and contraction strength and latency of the CTM reflex in normal dogs, and to evaluate its repeatability in myelopathic dogs.
MATERIALS AND METHODS
Inclusion Criteria
All dogs enrolled were patients of North Carolina State University Veterinary Teaching Hospital. Inclusion criteria for establishment of the normal CTM reflex sensory field included an age ≥2 years, a normal neurologic examination and hydration status, and no prior history of myelopathy. To assess the repeatability of the CTM reflex, dogs were recruited with an abnormal CTM reflex caudal border because of a transverse thoracolumbar myelopathy. Age, breed, body condition score (BCS; 9-point Nestle Purina Body Condition System), and reason for hospitalization were recorded for all dogs.
Caudal Border of the CTM Reflex
The caudal border of the CTM reflex was assessed bilaterally by pinching the skin ~2 cm lateral to the dorsal mid-line using hemostatic forceps, starting at the level of the ilial crests (considered as the 6th lumbar vertebra [L6] as defined below) and moving cranially 5 mm at a time until the CTM reflex was elicited. The border of the reflex was defined from the corresponding vertebra identified by palpating the dorsal spinous processes. If the border lay between 2 dorsal spinous processes, it was defined according to the cranial of the 2 processes. In case of asymmetry between left and right sides, the most cranial border was noted.
The effect of BCS on the vertebral level of the caudal border was examined by constructing contingency tables and performing χ2 or Fisher’s exact tests as appropriate based on number of data points. The effect of age on the vertebral level of the caudal border was examined using logistic regression. All statistical evaluations were performed using software (JMP 8.0, SAS, Cary, NC). P < .05 was considered statistically significant.
Lateral Border of the CTM Reflex
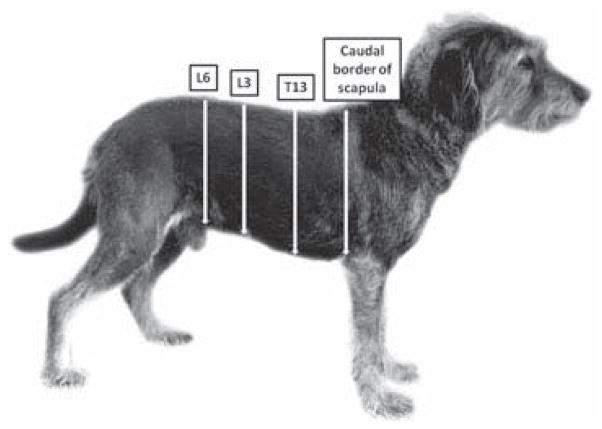
The lateral border of the CTM reflex was evaluated bilaterally at 4 vertebral levels by pinching the skin with hemostatic forceps, starting at the dorsal midline and moving ventrally 1 cm at a time until the CTM reflex could not be elicited. The levels evaluated included the caudal border of the scapulae, and the T13, L3, and L6 dorsal spinous processes (Fig 1). The distance from the midline to the lateral border was expressed as a percentage of the hemicircumference of the trunk at that level.
Figure 1.
The 4 levels tested to evaluate the lateral borders and contraction characteristics of the CTM reflex.
Strength and Latency of CTM Contraction
The strength and latency of ipsilateral and contralateral CTM contraction were described in 10 normal dogs after stimulation 2 cm from the midline bilaterally at the level of the caudal border of the scapulae, T13, L3, and L6. The strength of contraction was described as strong or weak based on visual inspection taking the amplitude and area of the contraction into account, and the contraction latency was timed. To assess fatigue, the stimulation was repeated 5 times at 5-second intervals at the same 4 levels and the number of times the muscle contracted was recorded.
Intra and Interobserver Repeatability
Ten dogs with an abnormal CTM reflex because of transverse thoracolumbar myelopathy were used to assess intra-and interobserver repeatability at identifying the caudal border of the CTM reflex. Four different observers evaluated the CTM reflex in the same dogs using hemostatic forceps and the border of the reflex was reported as the corresponding vertebra as well as a measured distance from a line drawn to link the caudal aspect of the left and right last rib. One observer also evaluated the reflex 3 times at 15–30 minute intervals in 10 dogs with both measured distances and vertebral level recorded. The mean difference in measurements of the caudal border was calculated both between observers and for the same observer. The inter- and intraobserver agreement at defining the level of the caudal border of the reflex by vertebral level was assessed by calculation of the kappa (κ) coefficient.
RESULTS
Normal dogs (n = 155) aged 2–16 years (mean ± SD, 8.25 ± 3.73 years) were studied. Thirty-four breeds were represented, 20 large breeds (>25 kg), and 14 small to medium size breeds. BCS ranged from 2–9 (mean, 6).
The CTM reflex was present in all dogs tested. The caudal border of the reflex was bilaterally symmetrical in 153 dogs. In 2 dogs, it was 1 vertebral level apart, and was elicited at L5 on 1 side and L6 on the other side. The caudal border of the reflex was located at L6 in 150 dogs (97%), at L5 in 3 dogs (2%), at L3 in 1 dog (0.65%), and at L1 in 1 dog (0.65%). The 2 dogs with the cranial lumbar borders were an 11-year-old Boxer (L1) and a 14-year-old American Cocker spaniel (L3). The Boxer presented for evaluation of degenerative joint disease, and the Cocker spaniel had a cholecystectomy as treatment for a gallbladder mucocele and had renal disease. They both had a BCS of 6. There was no significant relationship between age or BCS and level of caudal border of the CTM reflex.
The lateral borders of the CTM reflex were mapped in 50 dogs aged 2–12 years (mean, 6.11 years). The borders were bilaterally symmetrical and decreased slightly in relative distance from the midline from cranially to caudally (Table 1).
Table 1.
Lateral Border of the CTM Reflex in 50 Dogs Expressed as a Percentage of the Hemicircumference of the Trunk at Each of 4 Levels of the Trunk
| Caudal Edge of Scapula | T13 | L3 | L6 | |
|---|---|---|---|---|
| Mean (%) | 69 | 65 | 63 | 54 |
| SD | 12.72 | 11.84 | 13.69 | 14.93 |
| Range | 39–100 | 26–97 | 36–100 | 13–82 |
The intensity of the CTM contraction varied depending on the level at which the reflex was tested. The skin twitch was strong at T13 and L3, weak at L6, and inconsistent in strength at the caudal edge of the scapulae. The contralateral response was less intense than the ipsilateral response. The ipsilateral contraction of the CTM appeared to coincide to the skin stimulation in all 10 dogs and at all levels tested. The contralateral response occurred ~1 second after the stimulus was applied in 3 dogs, and was simultaneous to the ipsilateral response in the remaining dogs. When the stimulus was repeated 5 times at 5-second intervals, the CTM reflex contracted repeatedly in all but 1 dog at the level of the scapulae. This dog was extremely nervous and after 2 stimuli its muscle remained contracted for several seconds, making further responses impossible.
The 10 dogs with thoracolumbar myelopathy tested for assessment of repeatability were aged 2–14 years (mean, 6, SD: 3.06 years). Seven small to medium size breeds were represented. BCS ranged from 5 to 8 (mean, 6). All dogs were admitted paraplegic with no nociception and the level of the CTM reflex caudal border ranged from T9 to L5. The results for inter- and intraobserver assessment of the caudal border of the CTM reflex are provided in Tables 2 and 3. Mean difference in measured observation was 0.55 cm for different observers (range, 0–3 cm; SD, 0.72) and 0.28 cm for the same observer (range, 0–2 cm; SD, 0.47). When reported as the vertebral level, different observers agreed in 4 of 10 dogs, differed by 1 vertebral level in 5 dogs and 2 vertebral levels in 1 dog. The same observer found the same vertebral level in 8 dogs, and differed by 1 vertebral level in 2 dogs. The κ coefficient for agreement on the vertebral level of the CTM reflex was 0.87 for intraobserver observations, and 0.67 for interobserver observations.
Table 2.
Caudal Border of the CTM Reflex from a Line Joining the Last Ribs (Distances Measured Cranial to This Line are Reported as Negative Numbers) and the Vertebral Level in 10 Myelopathic Dogs, Recorded by 4 Observers
| Dog | Observer 1 cm/Vertebral Level
|
Observer 2 cm/Vertebral Level
|
Observer 3 cm/Vertebral Level
|
Observer 4 cm/Vertebral Level
|
||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| 1 | 2/L2 | 2.5/L2 | 2/L2 | 2.5/L2 | 2/L2 | 3/L2 | 2/L2 | 3/L2 |
| 2 | 9/L4 | 10/L5 | 7/L4 | 9/L4 | 7/L4 | 9/L4 | 7/L4 | 8/L4 |
| 3 | 6/L4 | 6/L4 | 5.5/L4 | 6/L4 | 6/L4 | 6/L4 | 6/L4 | 5.5/L4 |
| 4 | 5/L3 | 5/L3 | 6/L4 | 5/L3 | 4/L3 | 4/L3 | 5/L3 | 6/L4 |
| 5 | 6/L4 | 6/L4 | 4/L3 | 4/L3 | 3.5/L3 | 5/L3 | 4/L3 | 5/L3 |
| 6 | 3/L3 | 2/L2 | 3/L3 | 3/L3 | 3/L3 | 2/L2 | 3/L3 | 2/L2 |
| 7 | 2/L1 | −1/T12 | 1/L1 | −2/T12 | 0/T13 | −4/T11 | 0/T13 | −3/T11 |
| 8 | 8/L5 | 8/L5 | 7/L5 | 8/L5 | 7.5/L5 | 8/L5 | 7/L5 | 8/L5 |
| 9 | 5/L3 | 3/L3 | 5/L3 | 3.5/L3 | 6/L4 | 4/L3 | 5/L3 | 4.5/L3 |
| 10 | −2/T12 | −3/T11 | −2/T12 | −3/T11 | −2/T12 | −3/T11 | −2/T12 | −4/T11 |
Table 3.
Measurements (cm) of the Caudal Border of the CTM Reflex from a Line Joining the Last Ribs (Distances Measured Cranial to This Line are Reported as Negative Numbers) and as the Vertebral Level in 10 Myelopathic Dogs by the Same Observer on 3 Occasions
| Dog | First Measurement cm/Vertebral Level
|
Second Measurement cm/Vertebral Level
|
Third Measurement cm/Vertebral Level
|
|||
|---|---|---|---|---|---|---|
| L | R | L | R | L | R | |
| 1 | 3/L2 | 4/L3 | 4/L3 | 4/L3 | 4/L3 | 4/L3 |
| 2 | 3/L3 | 2/L2 | 3/L3 | 2/L2 | 3/L3 | 2/L2 |
| 3 | 7/L1 | 9/L3 | 7/L1 | 8/L3 | 7/L1 | 9/L3 |
| 4 | 0/T12 | −4/T9 | 0/T12 | −5/T9 | 0/T12 | −4/T9 |
| 5 | 4/L3 | 5/L3 | 3/L2 | 3/L2 | 3/L2 | 3/L2 |
| 6 | 8/L5 | 8/L5 | 7/L5 | 9/L5 | 8/L5 | 8.5/L5 |
| 7 | 7/L4 | 8/L5 | 7/L4 | 8/L5 | 7/L4 | 8/L5 |
| 8 | 8/L4 | 8/L4 | 8/L4 | 9/L5 | 8/L4 | 8/L4 |
| 9 | 4/L4 | 4/L4 | 4/L4 | 4/L4 | 4/L4 | 4/L4 |
| 10 | 6/L3 | 5.5/L3 | 6/L3 | 5/L3 | 5.5/L3 | 6/L3 |
DISCUSSION
The CTM reflex was present in all 155 neurologically normal dogs tested and the caudal border lay at L5 or L6 in 153 (99%) of these dogs. The caudal border of the reflex was more cranial in only 2 dogs, lying at L1 and L3, respectively. Both of these dogs were older, although there was no significant relationship between age and location of the border. The lateral borders were symmetrical with the entire lateral sensory field occupying approximately two-thirds of the hemicircumference of the trunk. The field narrowed slightly in the caudal lumbar region to occupy just over 50% of the hemicircumference. The CTM contraction was strongest with stimulation at T13 and L3, and in most dogs the contralateral muscle contraction occurred simultaneously but was weaker than the ipsilateral muscle contraction. Precise measurement of the level of the caudal border of the reflex was possible; while observers frequently did not have exact agreement on the measurements, the mean difference in measurements was only 0.55 cm for different observers and 0.28 cm for the same observer. When the level of the border was expressed as the appropriate vertebral level, intraobserver agreement, expressed as the κ coefficient, was high and interobserver agreement was moderate. Serial assessments of the CTM reflex caudal border can therefore be interpreted with confidence if there are changes greater than 1 vertebral level or 0.6 cm.
The afferent and efferent pathways of the CTM reflex have been described in early studies, using a combination of electromyography (EMG) to document muscle contraction, nerve transection, and retrograde labeling techniques.9, 10, 12, 13 These studies have demonstrated that there is somatotopic arrangement of this intersegmental reflex in dogs and guinea pigs10, 13; the lateral thoracic nerve has 4 branches that innervate different regions of the muscle, and a sensory stimulus elicits a stronger contraction of the portion of the CTM that lies at the level of the stimulus, thus producing a skin twitch in the area that is being stimulated.10, 13 This can be overridden by increasing the intensity of the stimulus, causing the entire muscle to contract.10 It has been reported that the threshold for stimulation is lowest, and the strength of muscle twitch based on EMG evaluation is greatest around T1112 similar to our findings. The same study reported a decrease in strength of muscle contraction with advancing age.12 In studies performed on guinea pigs13 and rats,2 the contralateral response is generally weaker than the ipsilateral response. This was also true in our dogs. The delay between stimulus and contraction was too short to record for ipsilateral stimulation; given the distance between the stimulus and the C8-T1 spinal cord segments and the velocity of conduction and speed of synaptic transmission, this is not surprising, but in 30% of the dogs, the contralateral response was delayed by ~1 second. Early studies also evaluated the optimal stimulus needed to elicit the CTM reflex. In guinea pigs and cats, gentle displacement of the fur is enough to elicit the reflex reliably.4, 13 Whereas displacement of the fur can elicit a CTM reflex in some dogs, the maximum response is elicited by grasping and gently compressing 2–3 mm of skin in dogs, so this technique was chosen for our study.10, 12, 14 Intense stimulation of the skin was avoided because it may involve the deeper musculature of the trunk and lead to pain and withdrawal of the animal.12, 13
In spite of the early work performed to determine the neuroanatomy of the CTM reflex, there is little objective information available on the extent and variability of the CTM reflex sensory field in normal dogs. The caudal border of the CTM reflex is commonly used to aid in localization of spinal lesions, making it important to understand the normal variability. In 153 of the 155 dogs we evaluated, the caudal border of the CTM reflex lay in the caudal lumbar spine, with only 2 older dogs having a more cranially located border. It is unclear whether the cranial location of the border in these 2 dogs is related to age, because there was no significant relationship between age and level of the border. An age-related decrease in contractile strength has been reported previously in dogs,12 and this could be related to either a change in sensory perception of the reflex, or a decrease in muscle strength. A study on human skin has demonstrated that there is a significant reduction in intra-dermal nerve fiber density with age,15 potentially changing sensory thresholds. Muscle atrophy also commonly occurs with age16 and could affect the CTM. These 2 age-related factors could conspire to reduce the CTM reflex in the more caudal lumbar spine, where the sensory threshold is higher and strength of contraction is lower than the thoracolumbar junction.12 Other metabolic disorders, such as type 2 diabetes can cause loss of skin innervation in people17 but these dogs did not suffer from metabolic conditions known to cause neuropathies. It is also possible that these 2 dogs suffered from an otherwise subclinical myelopathy, and the lack of a necropsy in these dogs makes it difficult to definitively identify the cause of the unusually cranial position of the caudal border.
The caudal border of the CTM reflex was symmetrical in most dogs tested, although 2 dogs did have an asymmetrical reflex. Asymmetry of the CTM reflex may result from a lateralizing myelopathy or brachial plexus disease, but in these instances, the dog will also show other evidence of spinal cord or brachial plexus dysfunction. We conclude that the presence of slight asymmetry at the level of the caudal border in a dog that lacks any other neurologic deficits is an uncommon but normal finding.
The lateral CTM reflex borders were bilaterally symmetrical for each level tested. The lateral field extended further ventrally than anticipated with a mean field of 69% of the hemicircumference at the level of the thorax and 54% at the level of L6. In 2 dogs, reflex CTM contractions could be elicited from the entire circumference of the thorax and trunk at the level of the caudal border of the scapula (1 dog) and at L3 (1 dog). The sensory arm of the reflex varies with species. In rats and guinea pigs, the dorsal cutaneous branches of the spinal nerves provide the afferent input2, 13 whereas in dogs and cats, it is unclear whether the lateral and ventral cutaneous branches of the thoracic and lumbar spinal nerves also participate.3, 4 The dorsal cutaneous branches are reported to supply the dorsal third of the thorax and abdomen.7 Our observations of the lateral extent of the CTM reflex are consistent with a previous report demonstrating that, in dogs, the CTM reflex afferent arm includes the dorsal and lateral cutaneous branches.10 It is also likely that the ventral cutaneous branches that are present in the T2–T12 spinal nerves7 participate in the CTM reflex as the lateral field extended to 100% bilaterally at the level of the caudal border of the scapula in one of the dogs we tested.
Summarily, the caudal border of the CTM reflex is at the level of the 6th lumbar vertebrae in most dogs, and extends laterally for more than 50% of the hemicircumference of the trunk. Although rare, in older dogs the sensory field may not extend beyond the mid or cranial lumbar level. The reflex is bilateral, with a weaker contralateral muscle contraction; the CTM contraction is strongest when the stimulus is applied in the region of the thoracolumbar junction. Asymmetry of the caudal border of reflex is rare but can be seen in clinically normal dogs. Measurement of the caudal border of the reflex from a line joining the last ribs is a precise method of monitoring this aspect of the reflex, with relatively low differences between observers and for the same observer. Agreement of the vertebral level of the caudal border of the reflex is moderate between observers and high for the same observer.
Acknowledgments
We thank Mrs. Nathalie Ricaux, MA for the artwork in Fig 1.
Footnotes
Presented in part at the American College of Veterinary Internal Medicine Annual Forum at Anaheim, CA, June 2010.
References
- 1.Pavletic MM. Pedicle grafts. In: Slatter D, editor. Textbook of small animal surgery. 3. Vol. 1. Philadelphia, PA: Saunders; 2002. pp. 292–321. [Google Scholar]
- 2.Theriault E, Diamond J. Nociceptive cutaneous stimuli evoke localized contractions in a skeletal muscle. J Neurophysiol. 1988;60:446–462. doi: 10.1152/jn.1988.60.2.446. [DOI] [PubMed] [Google Scholar]
- 3.Al-Bagdadi F. The integument. In: Evans HE, editor. Miller’s anatomy of the dog. 3. Philadelphia, PA: Saunders; 1993. pp. 98–121. [Google Scholar]
- 4.Holstege G, Blok BF. Descending pathways to the cutaneous trunci muscle motoneuronal cell group in the cat. J Neurophysiol. 1989;62:1260–1269. doi: 10.1152/jn.1989.62.6.1260. [DOI] [PubMed] [Google Scholar]
- 5.Askenaz DM, Spiegel EA. The viscero-pannicular reflex. Am J Physiol. 1935;112:573–576. [Google Scholar]
- 6.Langworthy OR. The panniculus carnosus in cat and dog and its genetical relation to the pectoral musculature. J Mammal. 1924;5:49–63. [Google Scholar]
- 7.Kitchell RL, Evans HE. The spinal nerves. In: Evans HE, editor. Miller’s anatomy of the dog. 3. Philadelphia, PA: Saunders; 1993. pp. 829–893. [Google Scholar]
- 8.Fletcher TF. Spinal cord and meninges. In: Evans HE, editor. Miller’s anatomy of the dog. 3. Philadelphia, PA: Saunders; 1993. pp. 800–828. [Google Scholar]
- 9.Krogh JE, Towns LC. Location of the cutaneous trunci motor nucleus in the dog. Brain Res. 1984;295:217–225. doi: 10.1016/0006-8993(84)90970-3. [DOI] [PubMed] [Google Scholar]
- 10.Krogh JE, Denslow JS. The cutaneus trunci muscle in spinal reflex. Electromyogr Clin Neurophysiol. 1979;19:157–164. [PubMed] [Google Scholar]
- 11.Fox MW. Clinical observations on the panniculus reflex in the dog. J Am Vet Med Assoc. 1963;142:1296–1299. [PubMed] [Google Scholar]
- 12.Krogh JE, Denslow JS, Shaddy JH. Spinal reflex thresholds as related to mechanical stresses and aging. Electromyogr Clin Neurophysiol. 1983;23:371–383. [PubMed] [Google Scholar]
- 13.Blight AR, McGinnis ME, Borgens RB. Cutaneous trunci muscle reflex of the guinea pig. J Comp Neurol. 1990;296:614–633. doi: 10.1002/cne.902960408. [DOI] [PubMed] [Google Scholar]
- 14.Doucette R, Theriault E, Diamond J. Regionally selective elimination of cutaneous thermal nociception in rats by neonatal capsaicin. J Comp Neurol. 1987;261:583–591. doi: 10.1002/cne.902610409. [DOI] [PubMed] [Google Scholar]
- 15.Chang YC, Lin WM, Hsieh ST. Effects of aging on human skin innervations. Neuroreport. 2004;15:149–153. doi: 10.1097/00001756-200401190-00029. [DOI] [PubMed] [Google Scholar]
- 16.Suetta C, Kjaer M. What are the mechanisms behind disuse and age-related skeletal muscle atrophy? Scand J Med Sci Sports. 2010;20:167–168. doi: 10.1111/j.1600-0838.2010.01121.x. [DOI] [PubMed] [Google Scholar]
- 17.Shun CT, Chang YC, Wu HP, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairment. Brain. 2004;127:1593–1605. doi: 10.1093/brain/awh180. [DOI] [PubMed] [Google Scholar]