Abstract
Purpose
We describe the characteristics, treatments and survival of patients with spina bifida in whom end stage renal disease developed from 2004 through 2008 in the United States Renal Data System.
Materials and Methods
We used ICD-9-CM code 741.* to identify individuals with spina bifida using hospital inpatient data from 1977 to 2010, and physician and facility claims from 2004 to 2008. We constructed a 5:1 comparison group of patients with end stage renal disease without spina bifida matched by age at first end stage renal disease service, gender and race/ethnicity. We assessed the risk of mortality and of renal transplantation while on dialysis using multivariate cause specific proportional hazards survival analysis. We also compared survival after the first renal transplant from the first end stage renal disease service to August 2011.
Results
We identified 439 patients with end stage renal disease and spina bifida in whom end stage renal disease developed at an average younger age than in patients without spina bifida (41 vs 62 years, p <0.001) and in whom urological issues were the most common primary cause of end stage renal disease. Compared to patients with end stage renal disease without spina bifida those who had spina bifida showed a similar mortality hazard on dialysis and after transplantation. However, patients with end stage renal disease without spina bifida were more likely to undergo renal transplantation than patients with spina bifida (HR 1.51, 95% CI 1.13–2.03). Hospitalizations related to urinary tract infections were positively associated with the risk of death on dialysis in patients with end stage renal disease and spina bifida (HR 1.42, 95% CI 1.33–1.53).
Conclusions
Spina bifida was not associated with increased mortality in patients with end stage renal disease on dialysis or after renal transplantation. Proper urological and bladder management is imperative in patients with spina bifida, particularly in adults.
Keywords: kidney failure, chronic, spinal dysraphism, urinary tract infections, transplantation, dialysis
Spina bifida occurs when the neural tube fails to close properly during early fetal development.1 In the United States SB affected 3.7/10,000 live births from 1999 to 2001.2 Most individuals with SB have neurogenic bladder resulting from neuronal injury to the lower urinary tract and central dysfunction due to central nervous system complications. Neurogenic bladder is a major risk factor for progressive renal damage. These patients are prone to renal deterioration secondary to recurrent UTIs or hostile bladder changes. The hostile bladder in SB cases may cause high bladder pressure, bladder-sphincter dyssynergia and vesicoureteral reflux, which may result in upper urinary tract deterioration, hydronephrosis and pyelonephritis, and renal scarring.3,4 Thus, lifelong bladder management is necessary to ensure proper drainage, capacity and compliance.
Although most individuals with SB have normal renal function at birth, about 26% of adults with SB have some degree of renal damage.5 Some SB cases progress to ESRD, requiring renal transplantation or dialysis.5 Previous studies in patients with ESRD who had SB were limited and often restricted to single center investigations, and they primarily focused on renal transplantation6–11 rather than on dialysis.12,13 To our knowledge there has been no population based examination of patients with ESRD and SB in the United States.
The goals of this study were to 1) describe the sociodemographic and clinical characteristics of patients with ESRD and SB in the American population, 2) compare the probabilities of renal transplantation and death while on dialysis and identify associated risk factors in patients with SB and in a demographically matched group without SB, and 3) compare survival in patients with vs without SB after transplantation.
MATERIALS AND METHODS
Data Source and Study Population
We performed a population based, retrospective, matched cohort study using USRDS data.14 We obtained access to the 2011 Standard Analytical File and the physician/supplier claims reported to Medicare from 2004 to 2008. The USRDS collects data on all patients who access the Medicare ESRD program in the United States. The database includes demographic and diagnosis data, biochemical values, dialysis claims, and information on treatment history, hospitalization events and physician/supplier services.15
The study cohort included patients with ESRD who had a first ESRD service date between 2004 and 2008. Individuals with SB were identified as those with ICD-9-CM code 741.* in the obtained data sources. Any patient with ESRD who had at least 1 inpatient claim or 2 outpatient claims for SB was classified as having SB. All other patients were considered not to have SB. From the latter group we matched 5 patients to each patient with ESRD and SB based on age at first ESRD service (in 5-year groups), gender and race/ethnicity (nonHispanic white, nonHispanic black, Hispanic and other).
Study Variables
We compared demographic and clinical characteristics at the first ESRD service between patients with ESRD with vs without SB in the study cohort, including incident age (age at first ESRD service), gender, race/ethnicity, ESRD causes and average yearly number of hospitalizations for UTI. In patients who had valid records in the 1995 or 2005 version of the MMS (Medicare and Medicaid Services) MER (Medical Evidence Report, form CMS-2728-U3) we compared the prevalence of comorbid conditions, physical impairment (inability to ambulate or transfer), insurance coverage (employer group, Medicaid or Medicare) and laboratory data (hemoglobin and serum albumin) at ESRD onset.
We considered renal transplantation and death on dialysis as mutually exclusive (competing) events.16–18 Time to event was calculated in months from the dialysis start date to the recorded date of transplantation or death, the end of the study period (August 2011) or loss to followup. Survival after transplantation was calculated from the date of the first transplantation until death, loss to followup or end of the study period.
Statistical Analysis
We assessed differences in characteristics among patients with ESRD with vs without SB using the t-test for continuous variables and the chi-square test for independence for categorical variables. All hypothesis tests were 2-sided with statistical significance considered at p <0.05.
We estimated the cumulative incidence function of transplantation/death on dialysis. Differences in cumulative incidence functions between the SB/nonSB groups were compared using the Pepe and Mori tests.19 We estimated Kaplan-Meier cumulative survival curves after the first transplantation. The log rank test was used to compare survival after the first transplantation between patients with ESRD and SB, and matched patients without SB.
On multivariate analysis of death on dialysis we estimated cause specific HRs using Cox proportional hazard models with patients censored at the time of the competing event. We adjusted for age, gender, race/ethnicity and other predictors that showed p <0.2 on univariate analysis. We tested for violations of proportional hazards by stratifying and inspecting the Schoenfeld residuals. Analysis was done with SAS® 9.3 and Stata™ 12.
This study was approved by the institutional review board of the National Center on Birth Defects and Developmental Disabilities, CDC (Centers for Disease Control and Prevention) under a data use agreement between the authors and the NIDDK. A NIDDK officer reviewed the manuscript for privacy content and approved it before submission.
RESULTS
ESRD Patient Characteristics
With vs without SB in entire study sample
Of the 549,710 incident patients with ESRD between 2004 and 2008 we identified 439 with SB who had demographic and clinical profiles distinct from those of other patients with ESRD (see table). Compared to incident ESRD patients without SB those with ESRD who had SB were on average younger (41 vs 62 years) and a higher percent were female (50.8% vs 44.2%), and nonHispanic white (67.5% vs 53.2%) and Hispanic (16.6% vs 13.5%).
Table I.
Demographic and clinical characteristics of patients with ESRD by SB status during 2004 through 2008 in 2011 USRDS
| Baseline | SB | No SB
|
p Value vs SB
|
||
|---|---|---|---|---|---|
| Matched Cohort | Entire Cohort | Matched Cohort | Entire Cohort | ||
| 439 | 2,195 | 549,271 | |||
| Mean ± SE age at 1st ESRD service | 40.79 ± 0.91 | 40.75 ± 0.41 | 62.37 ± 0.02 | 0.96 | <0.001 |
| No. age (%): | |||||
| 0-Less than 20 | 72 (16.4) | 360 (16.4) | 6,550 (1.2) | ||
| 20-Less than 40 | 143 (32.6) | 715 (32.6) | 43,282 (7.9) | ||
| 40-Less than 60 | 152 (34.6) | 760 (34.6) | 168,473 (30.7) | ||
| 60+ | 72 (16.4) | 360 (16.4) | 330,958 (60.3) | 1 | <0.001 |
| No. female (%) | 223 (50.8) | 1,115 (50.8) | 242,575 (44.2) | 1 | 0.0051 |
| No. race/ethnicity (%): | |||||
| NonHispanic white | 293 (67.5) | 1,465 (67.5) | 288,841 (53.2) | ||
| NonHispanic black | 54 (12.4) | 270 (12.4) | 151,189 (27.9) | ||
| Hispanic | 72 (16.6) | 360 (16.6) | 73,126 (13.5) | ||
| Other | 15 (3.5) | 75 (3.5) | 29,275 (5.4) | 1 | <0.001 |
| No. ESRD primary cause (%): | |||||
| Diabetes | 77 (17.5) | 681 (31.0) | 242,362 (44.1) | ||
| Hypertension | 59 (13.4) | 333 (15.2) | 149,962 (27.3) | ||
| Glomerulonephritis | 23 (5.2) | 399 (18.2) | 39,963 (7.3) | ||
| Cystic kidney | Not reported* | 96 (4.4) | 12,854 (2.3) | ||
| Urological | 125 (28.5) | 71 (3.2) | 9,718 (1.8) | ||
| Other | 132 (30.1) | 448 (20.4) | 65,256 (11.9) | ||
| Unknown | 19 (4.3) | 167 (7.6) | 29,156 (5.3) | <0.001 | <0.001 |
| No. insurance (%): | |||||
| Employer group | 76 (17.5) | 763 (35.2) | 138,567 (25.5) | <0.001 | <0.001 |
| Medicaid | 245 (56.5) | 687 (31.7) | 135,682 (25.0) | <0.001 | <0.001 |
| Medicare | 217 (50.0) | 508 (23.4) | 286,485 (52.8) | <0.001 | 0.25 |
| Mean ± SE No. UTI hospitalizations/yr | 1.24 ± 0.16 | 0.41 ± 0.15 | 0.82 ± 0.01 | <0.001 | <0.001 |
| Mean ± SE % families in poverty in resident county | 14.26 ± 0.27 | 13.77 ± 0.12 | 14.32 ± 0.01 | 0.056 | 0.87 |
| No. comorbid condition (%): | |||||
| Inability to ambulate or transfer | 130 (30.0) | 83 (3.8) | 35,184 (6.5) | <0.001 | <0.001 |
| Tobacco use, current smoker | 25 (5.8) | 145 (6.7) | 31,897 (5.9) | 0.52 | 1 |
| Alcohol or drug dependence | Not reported* | 68 (3.1) | 13,583 (2.5) | 0.16 | 0.54 |
| Diabetes | 109 (25.1) | 785 (36.2) | 281,690 (51.9) | <0.001 | <0.001 |
| Hypertension history | 288 (66.4) | 1,613 (74.3) | 449,693 (82.9) | 0.0009 | <0.001 |
| Congestive heart failure | 53 (12.2) | 387 (17.8) | 175,597 (32.4) | 0.0040 | <0.001 |
| Ischemic heart disease | 30 (6.9) | 235 (10.8) | 120,910 (22.3) | 0.0146 | <0.001 |
| Other cardiac disease | 23 (5.3) | 170 (7.8) | 82,737 (15.3) | 0.071 | <0.001 |
| Cerebrovascular disease | 12 (2.8) | 101 (4.7) | 50,821 (9.4) | 0.092 | <0.001 |
| Peripheral vascular disease | 30 (6.9) | 156 (7.2) | 76,371 (14.1) | 0.92 | <0.001 |
| Chronic obstructive pulmonary disease | 16 (3.7) | 98 (4.5) | 47,014 (8.7) | 0.52 | <0.001 |
| Ca | Not reported* | 80 (3.7) | 38,079 (7.0) | 0.11 | <0.001 |
| No. hemoglobin less than 10 gm/dl (%) | 206 (52.3) | 991 (49.5) | 237,567 (47.5) | 0.32 | 0.062 |
| No. serum albumin less than 3.5 gm/dl (%) | 204 (61.3) | 995 (57.6) | 270,158 (64.9) | 0.23 | 0.17 |
Fewer than 10 patients not reported according to NIDDKD data user agreement.
Causes of ESRD differed significantly between patients with vs without SB. Diabetes and hypertension were listed as the major causes of ESRD in about 30.9% of SB and about 71.4 % of nonSB cases. Urological causes, including benign neoplasms of the kidney and urinary system, chronic pyelonephritis, kidney calculus, urinary calculus and urinary obstruction, accounted for 28.5% and 1.8% of ESRD causes in SB and nonSB cases, respectively. Patients with SB averaged more annual hospitalizations related to UTI (1.24 vs 0.82). They were more likely to have Medicaid insurance (56.5% vs 25.0%) and less likely to have employer based health insurance (17.5% vs 25.5%) than patients without SB.
Patients with ESRD who had SB were significantly more likely than those without SB to have recorded physical impairments such as inability to ambulate or transfer (30.0% vs 6.5%). There was little difference in reported tobacco use (5.8% vs 5.9%), or alcohol or drug dependence (1.8% vs 2.5%) in incident ESRD cases with vs without SB. Most other comorbid conditions were recorded less frequently in ESRD cases with SB, including diabetes (25.1% vs 51.9%), history of hypertension (66.4% vs 82.9%), congestive heart failure (12.2% vs 32.4%), ischemic heart disease (6.9% vs 22.3%), other cardiac disease (5.3% vs 15.3%), cerebrovascular disease (2.8% vs 9.4%), peripheral vascular disease (6.9% vs 14.1%), chronic obstructive pulmonary disease (3.7% vs 8.7%) and cancer (2.1% vs 7.0%). Incident ESRD cases with or without SB showed similar frequencies of reported hemoglobin and serum albumin values.
Compared to 5:1 matched cohort without SB
Patients with ESRD and SB, and the matched cohort without SB had different reported primary causes of ESRD (see table). Those with SB had a higher percent of reported urological conditions (28.5% vs 3.2%). The rate of UTI hospitalization in patients with ESRD and SB was about 3 times the rate in the matched ESRD cohort without SB (1.24 vs 0.41). Patients with ESRD and SB reported significantly more physical limitations, such as inability to ambulate or transfer (30.0% vs 3.8%), than the matched cohort. Compared to patients with ESRD without SB those with SB were more likely to report having public insurance with more than half reporting Medicaid (56.5% vs 31.7%) or Medicare (50% vs 23.4%).
Death and Transplantation on Dialysis
Cumulative incidence
Of the 439 study patients with ESRD and SB 160 (36%) died at a total of 20,970 patient-months of observation. Mean followup from first ESRD service date to death/transplantation/study end was 48 months. A total of 89 patients (20.3%) with ESRD and SB underwent renal transplantation during the study period compared to 695 (31.7%) in the matched ESRD cohort without SB (p <0.001). Median time from first ESRD service to first renal transplantation was about 1 year longer in ESRD cases with SB than in the matched cohort without SB (50.2 vs 38.8 months, p = 0.03).
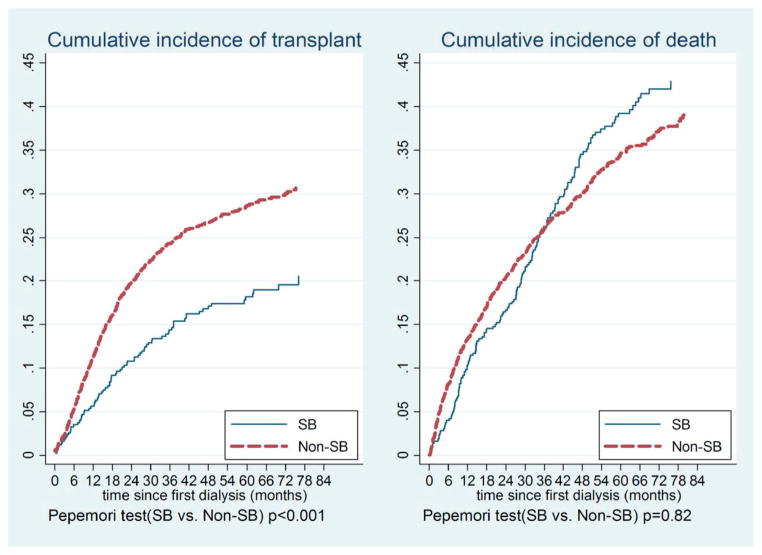
Figure 1 shows the estimated cumulative incidence of transplantation and death. Overall patients with ESRD and SB had a probability of death on dialysis that was similar to that of the matched cohort without SB (p = 0.82). For example, the probability of death by 6, 36 and 60 months after starting dialysis in ESRD cases with SB was 0.04, 0.26 and 0.39, comparable to the 0.08, 0.26 and 0.35, respectively, in the matched cohort without SB. On the other hand, patients with ESRD and SB had a lower probability of transplantation than the matched cohort without SB (p <0.001). For example, the probability of transplantation by 6, 36 and 60 months after starting dialysis in ESRD cases with SB was 0.035, 0.14 and 0.18, significantly lower than the 0.055, 0.24 and 0.29, respectively, in the nonSB matched cohort.
Figure 1.
Cumulative incidence of transplantation and death in patients with ESRD and SB, and in matched cohort without SB in 2011 USRDS. Probability of transplantation by 6, 36 and 60 months after starting dialysis was 0.035, 0.14 and 0.18, significantly less than 0.055, 0.24 and 0.29, respectively, in matched nonSB cohort.
Associated factors
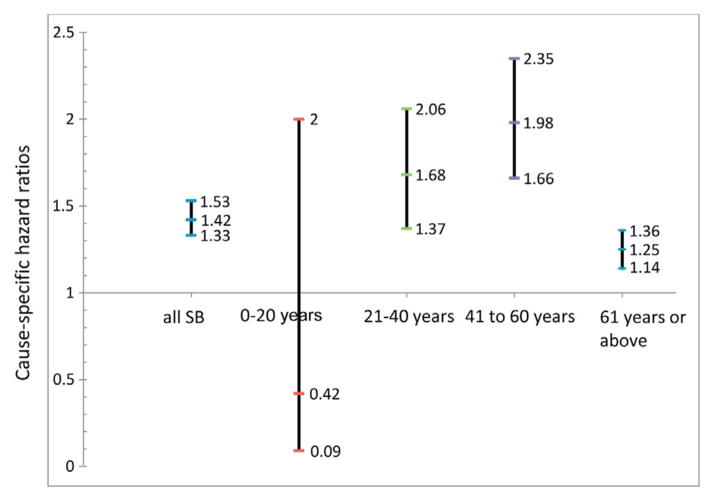
Figure 2 shows HRs for death according to the annual number of hospitalizations related to UTI in patients with SB by age. In patients with ESRD and SB for each additional annual UTI hospitalization the hazard of death on dialysis increased by 42%. The effect of each annual hospitalization related to UTI was especially salient in young adults 21 to 40 years old (HR 1.68, 95% CI 1.37–2.06) and in middle-aged adults 41 to 60 years old (HR 1.98, 95% CI 1.66–2.35).
Figure 2.
Cause specific HRs of average yearly UTI hospitalizations by death on dialysis in multivariate Cox models adjusted for age, race/ethnicity, gender, ESRD primary cause and comorbid conditions in patients with SB only and stratified by age in 2011 USRDS. In patients with ESRD and SB hazard of death on dialysis increased by 42% for each additional annual UTI hospitalization.
The results of multivariate Cox models for transplantation on dialysis revealed that patients with ESRD without SB were more likely to undergo renal transplantation than patients with ESRD and SB (HR 1.51, 95% CI 1.13–2.03).
Survival in Patients with Transplant
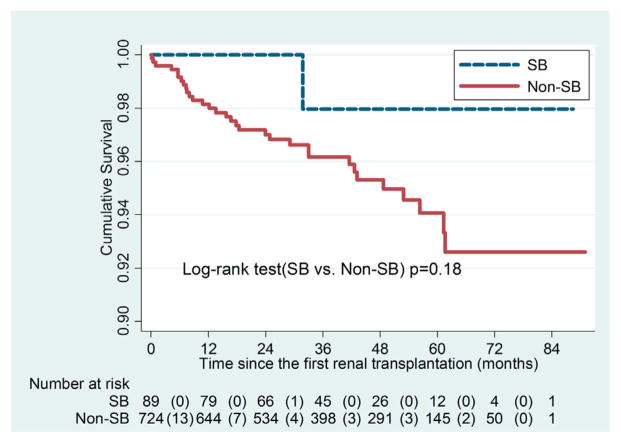
Of the 439 patients with ESRD and SB 89 (20.3%) underwent renal transplantation during the study period compared to 724 of 2,195 (33.0%) in the matched cohort with ESRD without SB (p <0.001). Average time under observation after receiving the first transplant was 39.8 months (maximum 90.9), during which 33 deaths occurred, including in 1 patient with ESRD and SB, and 32 without SB. Figure 3 shows Kaplan-Meier survival curves for patients with vs without SB who received a first renal transplant. There was no statistical difference in patient survival after the first renal transplantation (p = 0.18).
Figure 3.
Kaplan-Meier survival curves of transplanted patients with ESRD and SB, and matched comparison group without SB. There was no statistical difference in patient survival after first renal transplantation (p = 0.18). Values in parentheses indicate transplantation events.
DISCUSSION
We examined the characteristics and survival of 439 patients with ESRD and SB in the USRDS from 2004 to 2008. Several important sociodemographic and clinical characteristics of these patients differed from those of patients with ESRD without SB. Those with ESRD and SB were younger (age 40 vs 62 years) and had urological issues as the leading cause of ESRD, which accounted for 29% of the primary causes of ESRD. Diabetes was the leading cause of ESRD (31%) in patients without SB. This observation underscores the need for lifelong urological evaluation and management as well as lifelong screening for renal scarring and renal complications in patients with SB.
Previous studies suggested that an early structured bladder regimen beginning immediately after birth could preserve kidney function in children with SB.20,21 However, compliance and followup are difficult for several reasons. Many adults with SB lose coordinated urological care after leaving specialized pediatric SB clinics.22,23 Additionally, most SB programs do not have specialists in nephrology to assist with long-term followup and prevention of ESRD in these patients. Lastly, serum creatinine is a poor measure of renal function in patients with poor muscle mass such as those with SB24 and, thus, recognizing renal dysfunction in this population may be quite delayed. It is imperative to monitor for upper tract changes by ultrasound at regular intervals and perform nuclear medicine studies such as dimercapto-succinic acid to assess for renal scarring. Future studies in this population should include renal function monitoring with serum creatinine correlated with cystatin C or the nuclear medicine glomerular filtration rate.
Our study suggests that hospitalizations related to UTIs were associated with an increased risk of mortality on dialysis in patients with SB. This highlights the importance of ongoing urological management in this population. Although the current urological management approach in patients with SB can be effective,25,26 in the United States there are currently no standardized protocols to manage the urinary tract and monitor kidney function. A recent Dutch study suggested that there is a gap between practice and recommended guidelines to evaluate bladder and kidney function in adults with SB.27 Effective urological treatment in patients with SB, especially adults with SB, remains a challenge.
Our study reveals that survival up to 84 months on dialysis and after receiving a transplant in patients with ESRD and SB is comparable to that in demographically matched patients with ESRD without SB. This finding is consistent with previous findings.7,9,10,28 Thus, dialysis and renal transplantation appear to be viable treatments in patients with SB. However, patients with ESRD and SB were less likely to undergo renal transplantation than matched patients without SB even after adjusting for demographic, clinic and economic factors. This important finding warrants future studies to confirm disparities in access to transplants according to SB status and determine the reasons for such disparities.
Our study has several limitations. 1) We used administrative data sets to identify individuals with SB. Diagnosis codes for SB may not be accurate on all medical claims, although we only included cases with 1 inpatient code or 2 outpatient codes for SB to decrease this error. 2) Followup was relatively short, which prevented us from studying long-term transplantation outcomes. 3) Due to the nature of the USRDS data system, in which data are entered when patients have accessed the ESRD services, we lacked the complete natural history of urological management and renal damage in patients with SB. Also, information on intermittent catheterization while on dialysis or after transplantation is unavailable. Thus, we could not identify risk factors for renal impairment in patients with SB. This is an important topic for future research.
CONCLUSIONS
This study demonstrates that ESRD developed in patients with SB at younger ages than in patients without SB and urological issues were the most common primary cause of ESRD. Proper urological and bladder management is imperative in patients with SB, particularly in adults. The finding that patients with SB were less likely to undergo transplantation than their peers without SB despite comparable survival rates also points to potential disparities in access to renal transplantation.
Acknowledgments
USRDS provided data access.
Abbreviations and Acronyms
- ESRD
end stage renal disease
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- SB
spina bifida
- USRDS
United States Renal Data System
- UTI
urinary tract infection
Footnotes
Study received approval from the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, USRDS or National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Kaplan KM, Spivak JM, Bendo JA. Embryology of the spine and associated congenital abnormalities. Spine J. 2005;5:564. doi: 10.1016/j.spinee.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 2.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 3.Thorup J, Biering-Sorensen F, Cortes D. Urological outcome after myelomeningocele: 20 years of follow-up. BJU Int. 2011;107:994. doi: 10.1111/j.1464-410X.2010.09681.x. [DOI] [PubMed] [Google Scholar]
- 4.Torre M, Guida E, Bisio G, et al. Risk factors for renal function impairment in a series of 502 patients born with spinal dysraphisms. J Pediatr Urol. 2011;7:39. doi: 10.1016/j.jpurol.2010.02.210. [DOI] [PubMed] [Google Scholar]
- 5.Veenboer PW, Bosch JL, van Asbeck FW, et al. Upper and lower urinary tract outcomes in adult myelomeningocele patients: a systematic review. PLoS One. 2012;7:e48399. doi: 10.1371/journal.pone.0048399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malakounides G, Lee F, Murphy F, et al. Single centre experience: Long term outcomes in spina bifida patients. J Pediatr Urol. 2013;9:585. doi: 10.1016/j.jpurol.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Hamdi M, Mohan P, Little DM, et al. Successful renal transplantation in children with spina bifida: long term single center experience. Pediatr Transplant. 2014;8:167. doi: 10.1046/j.1399-3046.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 8.Blanco M, Medina J, Pamplona M, et al. Outcome of renal transplantation in adult patients with augmented bladders. Transplant Proc. 2009;41:2382. doi: 10.1016/j.transproceed.2009.06.106. [DOI] [PubMed] [Google Scholar]
- 9.Luke PP, Herz DB, Bellinger MF, et al. Long-term results of pediatric renal transplantation into a dysfunctional lower urinary tract. Transplantation. 2003;76:1578. doi: 10.1097/01.TP.0000090866.00241.0C. [DOI] [PubMed] [Google Scholar]
- 10.Mendizábal S, Estornell F, Zamora I, et al. Renal transplantation in children with severe bladder dysfunction. J Urol. 2005;173:226. doi: 10.1097/01.ju.0000148369.34519.86. [DOI] [PubMed] [Google Scholar]
- 11.Power RE, O’Malley KJ, Little DM, et al. Long-term followup of cadaveric renal transplantation in patients with spina bifida. J Urol. 2002;167:477. doi: 10.1016/S0022-5347(01)69067-0. [DOI] [PubMed] [Google Scholar]
- 12.Grunberg J, Rebori A, Verocay MC. Peritoneal dialysis in children with spina bifida and ventriculoperitoneal shunt: one center’s experience and review of the literature. Perit Dial Int. 2003;23:481. [PubMed] [Google Scholar]
- 13.Ram Prabahar M, Sivakumar M, Chandrasekaran V, et al. Peritoneal dialysis in a patient with neurogenic bladder and chronic kidney disease with ventriculoperitoneal shunt. Blood Purif. 2008;26:274. doi: 10.1159/000126923. [DOI] [PubMed] [Google Scholar]
- 14.Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126:2234. doi: 10.1097/PRS.0b013e3181f44abc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 16.Noordzij M, Leffondré K, van Stralen KJ, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira L, Rodrigues A, Carvalho MJ, et al. Modelling competing risks in nephrology research: an example in peritoneal dialysis. BMC Nephrol. 2013;24:110. doi: 10.1186/1471-2369-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobbedez T, Verger C, Ryckelynck JP, et al. Is assisted peritoneal dialysis associated with technique survival when competing events are considered? Clin J Am Soc Nephrol. 2012;7:612. doi: 10.2215/CJN.10161011. [DOI] [PubMed] [Google Scholar]
- 19.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12:737. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 20.Dik P, Klijn AJ, van Gool JD, et al. Early start to therapy preserves kidney function in spina bifida patients. Eur Urol. 2006;49:908. doi: 10.1016/j.eururo.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 21.Wide P, Glad Mattsson G, Mattsson S. Renal preservation in children with neurogenic bladder-sphincter dysfunction followed in a national program. J Pediatr Urol. 2012;8:187. doi: 10.1016/j.jpurol.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Mourtzinos A, Stoffel JT. Management goals for the spina bifida neurogenic bladder: a review from infancy to adulthood. Urol Clin North Am. 2010;37:527. doi: 10.1016/j.ucl.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad I, Granitsiotis P. Urological follow-up of adult spina bifida patients. Neurourol Urodyn. 2007;26:978. doi: 10.1002/nau.20447. [DOI] [PubMed] [Google Scholar]
- 24.Quan A, Adams R, Ekmark E, et al. Serum creatinine is a poor marker of glomerular filtration rate in patients with spina bifida. Dev Med Child Neurol. 1997;39:808. doi: 10.1111/j.1469-8749.1997.tb07547.x. [DOI] [PubMed] [Google Scholar]
- 25.Clayton DB, Brock JW, Joseph DB. Urologic management of spina bifida. Dev Disabil Res Rev. 2010;16:88. doi: 10.1002/ddrr.92. [DOI] [PubMed] [Google Scholar]
- 26.Filler G, Gharib M, Casier S, et al. Prevention of chronic kidney disease in spina bifida. Int Urol Nephrol. 2012;44:817. doi: 10.1007/s11255-010-9894-5. [DOI] [PubMed] [Google Scholar]
- 27.Veenboer PW, Ruud Bosch JL, de Kort LM. Assessment of bladder and kidney functioning in adult spina bifida patients by Dutch urologists: a survey. Neurourol Urodyn. 2014;33:289. doi: 10.1002/nau.22413. [DOI] [PubMed] [Google Scholar]
- 28.Müller T, Arbeiter K, Aufricht C. Renal function in meningomyelocele: risk factors, chronic renal failure, renal replacement therapy and transplantation. Curr Opin Urol. 2002;12:479. doi: 10.1097/00042307-200211000-00006. [DOI] [PubMed] [Google Scholar]