Abstract
Human artificial chromosome (HAC)-based vectors represent an alternative technology for gene delivery and expression with a potential to overcome the problems caused by virus-based vectors. The recently developed alphoidtetO-HAC has an advantage over other HAC vectors because it can be easily eliminated from cells by inactivation of the HAC kinetochore via binding of chromatin modifiers, tTA or tTS, to its centromeric tetO sequences. This provides a unique control for phenotypes induced by genes loaded into the HAC. The alphoidtetO-HAC elimination is highly efficient when a high level of chromatin modifiers as tetR fusion proteins is achieved following transfection of cells by a retrovirus vector. However, such vectors are potentially mutagenic and might want to be avoided under some circumstances. Here, we describe a novel system that allows verification of phenotypic changes attributed to expression of genes from the HAC without a transfection step. We demonstrated that a single copy of tTAVP64 carrying four tandem repeats of the VP16 domain constitutively expressed from the HAC is capable to generate chromatin changes in the HAC kinetochore that are not compatible with its function. To adopt the alphoidtetO-HAC for routine gene function studies, we constructed a new TAR-BRV- tTAVP64 cloning vector that allows a selective isolation of a gene of interest from genomic DNA in yeast followed by its direct transfer to bacterial cells and subsequent loading into the loxP site of the alphoidtetO-HAC in hamster CHO cells from where the HAC may be MMCT-transferred to the recipient human cells.
INTRODUCTION
Human artificial chromosomes or HAC-based vectors represent a novel system for gene delivery and expression that has several advantages over previous gene and cell therapy strategies (1–4). All HACs by definition contain a functional centromere and, therefore, replicate and segregate like normal chromosomes in human cells without integration into the host genome. HAC vectors are essentially limited only by our ability to generate and handle large DNA fragments, and therefore, may provide long-term expression of complete genetic loci. Several labs succeeded in complementation of gene deficiencies in human recipient cell lines and in creation of the transgenic mice using HAC vectors containing genomic copies of genes with all their regulatory elements, demonstrating their potential as therapeutic gene expression vectors (5–26).
HACs can be engineered by ‘top-down’ or ‘bottom-up’ (de novo formation) approaches (1–4,27,28). The top-down approach is based on telomere-associated chromosome fragmentation in the homologous recombination-proficient chicken DT40 cell line. The bottom-up approach includes transfection of human cells with either natural or synthetic alpha-satellite (alphoid) DNA arrays with a size bigger than 30 kb. After transfection of such arrays into human cells, de novo HACs are generated that range in size from 1 Mb to 10 Mb due to concatemerization of input constructs and amplification of alphoid DNA.
One of the most advanced HACs is the alphoidtetO-HAC, engineered using a 40 kb synthetic alphoid DNA array (29). This array contains 42-bp tetracycline operator (tetO) sequences incorporated into every second alphoid DNA monomer. After multimerization of the input DNA in human cells, the resulting alphoidtetO-HAC contains ∼6000 copies of the tetO sequence within the 1.1 mega-base size block of synthetic alphoid DNA (30). Because tetO sequences are bound with high affinity and specificity by the tet repressor (tetR), they can be targeted efficiently in vivo with tetR fusion proteins. The power of this system is that it allows for specific manipulation of the chromatin composition of a HAC kinetochore in vivo, while leaving all kinetochores of the natural chromosomes unperturbed. Targeting of chromatin-modifying proteins into the HAC kinetochore demonstrated that a balance between open and condensed chromatin is critical for kinetochore function (31–34). The frequency of HAC loss was very high when either transcriptionally competent or repressive chromatin was induced in the HAC via the tTA (tet-repressor transcriptional activator containing VP16 domain) (35) or tTS (tet-repressor transcriptional silencer - this contains the KRAB-AB domain of the SDkid-1 protein) (36). Tethering these chromatin modifiers caused a block of CENP-A loading and stripping of other proteins of the CCAN complex (the constitutive centromere-associated network) from the HAC kinetochore. As a consequence, the functional HAC kinetochore is inactivated and the HAC is lost during cell division (29,31–34,37).
The alphoidtetO-HAC with a regulated kinetochore represents a powerful system for gene function analysis because it provides a unique possibility to compare the phenotypes of target human cells with and without a functional copy of a gene inserted into the HAC. Such a rigorous control is required for proper interpretation of gene complementation studies. The power of this system was recently demonstrated in experiments for expression of three human full-length genes, VHL (∼25 kb), NBS1 (∼60 kb), HPRT (∼60 kb) and BRCA1 (90 kb) from the alphoidtetO-HAC vector transferred in patient-derived cell lines (23,38,39).
The alphoidtetO-HAC elimination by modification of centromeric chromatin is highly efficient using either retrovirus or plasmid-induced expression to provide a high level of chromatin modifiers as tetR fusion proteins. Thus, inactivation of the HAC kinetochore includes a transfection step that is potentially mutagenic. Recently, we described an approach to re-engineering the alphoidtetO-HAC that allows verification of phenotypic changes attributed to expression of genes from the HAC without a transfection step (40). In this alphoidtetO-HAC vector, a tTS-containing cassette was inserted into a gene-loading site along with a gene of interest. A relatively low level of expression of the tTS does not inactivate the HAC kinetochore but generates a self-regulating fluctuating heterochromatin on the alphoidtetO-HAC that induces fast silencing of the genes on the HAC without significant effects on HAC segregation. This silencing of the HAC-encoded genes can be recovered by simply adding a ligand (doxycycline). While the alphoidtetO-HAC vector carrying a single copy of tTS provides a simple way to verify phenotypic changes attributed to expression of genes from the HAC, persistence of the HAC with a silenced gene in cells might want to be avoided under some circumstances. For example, this is specifically required when the HAC vector is used for generation of iPS cells (3,4,41).
In this paper, we examined whether a single copy of a chromatin modifier, either the transcriptional transactivator tTA carrying the VP16 domain or the advanced transcriptional transactivator tTAVP64 carrying four tandem repeats of the VP16 domain (36), inserted into the alphoidtetO-HAC is enough to inactivate the HAC kinetochore. We demonstrated that tTAVP64 constitutively expressed from the HAC induces a fast inactivation of the HAC kinetochore leading to HAC loss. This feature of the alphoidtetO-HAC carrying tTAVP64 is consistent with its potential broad use in gene function studies and cell reprogramming.
One advantage of HAC-based technologies is the opportunity to work with full-length genes. Transformation-associated recombination (TAR) cloning allows selective isolation of any desired allele of a gene of interest as a predetermined large chromosomal fragment in yeast (42–44). Based on our observation that a single copy transgene encoding a potent chromatin modifier may inactivate a kinetochore, we constructed a new vector, TAR-BRV- tTAVP64 that links the gene isolation strategy with the regulated gene expression in the alphoidtetO-HAC.
MATERIALS AND METHODS
Construction of the tTA-containing plasmids, tetR-VP64-IRES-DsRed2 and tetR*-VP16-IRES-DsRed2
The transgene cassette tetR-VP64-IRES-DsRed2 was assembled as follows. The plasmid 264-CAG-IRES-DsRed (40) was digested with PacI/NheI, then was gel purified to remove a short (∼200 bp) fragment. Next, the tetR-NLS sequence (with complete NLS) was polymerase chain reaction (PCR) amplified from the plasmid 264-CAG-OKS-EF1-tTKRAB-DsRed-pA (kindly provided by Dr. Tomilin, Institute of Cytology, RAS, Russia) with a pair of specific primers PacI-tetR-EYFP-VP16/NheI-NLSrev (Supplementary Table S1). Then the tetR-NLS fragment was gel purified, digested by PacI/NheI and ligated with the 264-CAG-IRES-DsRed plasmid digested by PacI/NheI, producing the intermediate plasmid 264-CAG-tetR-newNLS- completeIRES-DsRed. Next, this plasmid was digested by NheI/AsiSI to insert the VP64 sequence. The VP64 PCR sequence (carrying four copies of a subsection of the VP16 domain) was PCR amplified from the Cas9D10AH840A TagBFP VP64 plasmid (kindly provided by Dr. Kagansky, Edinburgh University, Scotland) with a pair of specific primers NheI-VP64for/ AsiSI-VP64rev (Supplementary Table S1). The VP64 PCR fragment was gel purified, digested with NheI/AsiSI and then ligated with the 264-CAG-tetR-newNLS-completeIRES-DsRed plasmid digested by NheI/AsiSI, producing the final plasmid tetR-VP64-IRES-DsRed2. The transgene cassette tetR*-VP16-IRES-DsRed2 was assembled similarly except it contains only one copy of the VP16 domain. Both plasmids contain the 3'HPRT-loxP cassette that allows their insertion into the single loxP loading site of the alphoidtetO-HAC propagated in HPRT-minus hamster CHO or human HT1080 cells followed by reconstitution of the HPRT gene (45,46). Detailed physical maps of the tetR-VP64-IRES-DsRed2 and tetR*-VP16-IRES-DsRed2 plasmids are shown in Supplementary Figures S1 and S2a.
Construction of the TAR-BRV-tTAVP64 vector
The construction of the TAR-BRV- tTAVP64 vector was shown in detail in Supplementary Figure S3. Steps 1–4 removed restriction sites XmaI, EcoRI, BglII and XbaI from the pBeloBAC11 plasmid backbone (New England Biolabs). This was carried out as follows. Step 1: The plasmid pBeloBAC11 was PCR amplified with a pair of primers B161/B196 (Supplementary Table S1). PCR product was then blunt end ligated to form plasmid A096. Step 2: Plasmid A096 was PCR amplified with a pair of primers B244/B245 (Supplementary Table S1). The PCR product was then blunt end ligated to produce the intermediate plasmid A098. Step 3: Plasmid A098 was PCR amplified with a pair of primers B246/B247 (Supplementary Table S1). The PCR product was then blunt end ligated to produce the intermediate plasmid A099. Step 4: Plasmid A099 was PCR amplified with a pair of primers B248/B249 (Supplementary Table S1). The PCR product was then blunt end ligated to produce the intermediate plasmid A102. Step 5: The TAR plasmid pVC604 (22) was PCR amplified with a pair of primers B288/B287 (Supplementary Table S1). The PCR product was then blunt end ligated to produce the intermediate plasmid A106. This step removed restriction sites BglII, NheI, BclI and HindIII sites in the HIS3 gene of pVC604. Step 6: Then three fragments were ligated as follows. The first fragment, CEN6-HIS3, was PCR amplified from A106 with a pair of primers B286/B295 (Supplementary Table S1). This fragment was then digested with XhoI/BamHI. The second fragment, ARSH4, was PCR amplified from the plasmid pRS313 (47) using a pair of primers B294/B293 (Supplementary Table S1). The ARSH4 fragment was then digested with SalI/XbaI. The third fragment was amplified from the plasmid A102 using a pair of primers B022/B280 (Supplementary Table S1) and then digested with NheI/BamHI. All three fragments were then ligated to produce the intermediate plasmid A116. Step 7: The plasmid A116 was PCR amplified using a pair of primers B433/B432 (Supplementary Table S1). The PCR product was then digested with MluI and ligated to produce the intermediate plasmid A152. This step removed the ARSH4 segment and added MluI and PacI sites to the plasmid. Step 8: This step included ligation of two fragments. The first fragment, 3'HPRT (2256 bp in length) was obtained by digestion of the plasmid p264 (37) with XbaI/AscI. The second fragment was obtained by PCR amplification of pBluescript II KS (Stratagene) with a pair of primers B630/B631 (Supplementary Table S1). The B630 primer carries with it the loxP site. The PCR product was subsequently digested with NheI/MluI. Both fragments were then ligated to produce the intermediate plasmid A151. Step 9: The plasmid A153 was then digested with NheI/BamHI, while the plasmid A151 was digested with BglII/SpeI. Both fragments were then ligated to produce the intermediate plasmid A153. Step 10: This step was a single five-way ligation. The first fragment was obtained by PCR amplification of the plasmid pBlueScript II SK (Stratagene) with a pair of primers B489/B688 (Supplementary Table S1). This 2868 bp fragment was then digested with MluI/SacI. The second fragment, tDNA-CAG (2097 bp in length), was obtained by digesting the plasmid CAG-EGFP-tDNA (25,48) with SacI/XbaI digest. The third fragment, CAG intron (129 bp in length), was obtained by PCR amplification of the p264 plasmid (37) with a pair of primers B510/B509 (Supplementary Table S1). This fragment was then digested with SpeI/EcoRI. The forth fragment, tetR-VP64 (842 bp in length), was obtained by PCR amplification of the plasmid tetR-VP64-IRES-DsRed2 (see above) with a pair of primers B487/B486 (Supplementary Table S1). This fragment was digested with EcoRI/BglII. The fifth fragment, SV40pA (240 bp in length), was PCR amplified from the plasmid BRV1 (49) with a pair of primers B619/B618 (Supplementary Table S1). This fragment was then digested with BamHI/MluI. All five fragments were ligated together in a single five-way ligation to produce the intermediate plasmid A185. Step 11: This step was a single two-way ligation. The first fragment, tDNA-CAG-tetR-VP64 (3314 bp in length), was obtained by digesting the plasmid A185 with AsiSI/MluI. The second fragment was obtained by digesting the plasmid A153 with MluI/PacI. Ligation of both fragments produced the final TAR-BRV-tTAVP64 vector. A detailed physical map of the TAR-BRV-tTAVP64 plasmid is shown in Supplementary Figure S5.
Cell lines and media
The hypoxanthine phosphoribosyltransferase (HPRT)-deficient fibrosarcoma HT1080 cell line was obtained from Dr. Zoia L. Monaco (Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, UK). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% (v/v) tet system-approved fetal bovine serum (Clontech Laboratories, Inc.), 1% (v/v) Penicillin-Streptomycin (10 000 U/mL) (Life Technologies) at 37°C in 5% CO2. Hypoxanthine phosphoribosyltransferase (HPRT)-deficient Chinese hamster ovary (CHO) cells (JCRB0218) were maintained in Ham's F-12 nutrient mixture (Invitrogen) plus 10% FBS, 1% P/S and 8 μg/ml of BS. HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Atlanta Bio- logicals, Lawrenceville, GA, USA), 1% penicillin–strepto- mycin (P/S; Invitrogen, USA) with 2 μg/ml of Blasticidin S (BS; Invitrogen) at 37°C in 5% CO2. After loading of tTAVP64 -containing plasmids into the loxP site of the alphoidtetO-HAC, the HT1080 and CHO cells were cultured in 1× HAT medium, 2 μg/ml Blasticidin S and 1 μg/ml doxycycline (Life Technologies).
Loading of the tTA -containing plasmids into the loxP site of the alphoidtetO-HAC
A total of 1–3 μg of the tTAVP64 - or TAVP16 -containing construct DNA and 0.8–1 μg of the Cre expression pCpG-iCre vector DNA were co-transformed with 6 μl of X-tremeGENE 9 DNA transfection reagent (Roche) into HPRT-deficient human HT1080 and hamster CHO cells containing the alphoidtetO-HAC that were grown on a 6 well plate with DMEM, 10% FBS, 1% PenStrep, 2 μg/ml Blasticidin S (Life Technology) and 1 μg/ml doxycycline (Life Technology). HPRT-positive colonies were selected after 2–3 weeks growth in HAT medium. 5–7 clones were then selected in each experiment. Correct loading of the transgene cassette into the HAC was confirmed by genomic PCR with a specific pair of primers that diagnose reconstitution of the HPRT gene (Supplementary Table S1).
FISH analysis with a BAC probe
FISH analysis was performed as previously described (29,40). HAC-containing cells were cultured in medium with 10 μg/ml of colcemid (Invitrogen) for 3 h at 37°C. Metaphase cells were trypsinized and collected by centrifugation for 5 min at 483 x g, treated in hypotonic solution 50 mM KCl for 20 min at 37°C and washed three times in 10 ml of methanol:acetic acid (3:1) fixative solution. Cells were centrifuged at 174 x g for 5 min between washes. Cells were then diluted to the appropriate density with fixative solution, spread onto precleaned slides (Fisher Scientific) above steam (boiling water), and allowed to age 2 days at room temperature. Metaphase chromosomes on the slide were denatured by 70% formamide/2 x SSC for 2 min at 72°C. Samples were dehydrated through a 70%, 90% and 100% ethanol series for 4 min each and left to air-dry. Orange 552 dUTP (5-TAMRA-dUTP) (Abbott Molecular) labeled probes were denatured in hybridization solution at 78°C for 10 min and left at 37°C for 30 min. The hybridization mix probe was applied to the sample and incubated at 37°C overnight. Slides were washed with 0.4 x SSC, 0.3% Tween 20 for 2 min at 72°C, briefly rinsed with 2 x SSC, 0.1% Tween 20 (5s-1min) and air dried in darkness. The samples were counterstained with VectaShield mounting medium with DAPI (Vector Labs). Slides were analyzed by fluorescence microscopy. Images were captured using a DeltaVision microscopy imaging system in the CRC, LRBGE Fluorescence Imaging Facility (NIH) and analyzed using image J software (NIH). The probe used for FISH was BAC32–2-mer(tetO) DNA containing a 40 kb of alphoid-tetO array cloned into a BAC vector as described previously (29). BAC DNA was labeled using a nick-translation kit with Orange 552 dUTP (5-TAMRA-dUTP) (Abbott Molecular).
Analysis of HAC mitotic stability by FISH
Fluorescence images of human cells carrying the HAC with the tTAVP64 -containing cassette were analyzed. Cells were processed for fluorescence in situ hybridization (FISH) using the BAC32-2-mer(tetO) DNA probe after 3, 4, 7 or 14 days of culturing without HAT selection in the presence or absence of doxycycline. Metaphase spreads were screened for the presence or absence of the HAC. At least 60–140 metaphases were screened for each clone. The stability of the HAC was calculated as the percentage of alphoidtetO-HAC-containing metaphase spreads in the absence or presence of doxycycline.
Reverse-transcriptase polymerase chain reaction
The alphoidtetO non-coding transcripts were detected in HPRT-deficient HT1080 cells containing alphoidtetO-HAC by RT-PCR using specific primers listed in Supplementary Table S1. Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. A measure of 2 μg RNA was subsequently used for RT with random hexamer primers, using the Roche Transcriptor High Fidelity cDNA Synthesis Kit. Real-time PCR analysis of cDNA equivalent to ∼70 ng (alphoidtetO) input RNA was then performed using a SYBR Green Mastermix (JumpStart, Sigma) on a LightCycler480 system (Roche). Oligonucleotide primer pairs are tetO-F/tetO-R2 (Supplementary Table S1) for the alphoidtetO non-coding transcripts and actin-F/actin-R (Supplementary Table S1) for control β-actin transcripts. For each oligonucleotide primer pair and every plate, a standard curve was created using genomic DNA derived from the corresponding cell line, thereby calculating the transcript copy numbers relative to the transcript copy number for the genomic locus. Background values (no reverse transcriptase) were subtracted, and all values were normalized to β-actin expression. For time course experiments, the transcript levels were expressed relative to the dox+ values of the alphoidtetO, which was arbitrarily set to 1. Similar conditions were used to quantify transcription of the tTAVP64 from the HAC and from transiently transfected tTAVP64 -containing plasmid in human HT1080 cells. As a control, the level of transcripts for the human GAPDH gene was measured. A list of primers is presented in Supplementary Table S1.
Microcell-mediated chromosome transfer (MMCT)
The alphoidtetO HAC with the tTAVP64 -containing cassette was transferred from hamster CHO to human epithelium HeLa using a standard MMCT protocol (37,39). Microcells were collected from about 1×108 CHO cells containing the alphoidtetO-HAC carrying the tTAVP64 gene. HAC was transferred into human HeLa cell line (3×106) via fusion of microcells with target cells. For fusion we have used Neo EX HVJ Envelope Transfection Kit (Cosmo Bio, Japan). Blasticidin S (BS) was used to select resistant colonies. Blasticidin S selection (4 μg/ml) was applied 48 h later and HeLa Blasticidin S resistant clones were picked after 2–3 weeks of growth under selective conditions. Typically 1–3 BSR colonies were obtained in one MMCT experiment involving HAC transfer from hamster to human cells. Based on FISH analysis, more than 95% of cells in the colony contained a HAC.
ChIP assay and real-time PCR
ChIP with antibodies against trimethyl H3K4me3 (Upstate) and CENP-A was carried out according to a previously described method (40). Briefly, cultured cells were cross-linked in 1% formaldehyde for 10 min at 37°C. After addition of 1/10 volume of 1.25 M glycine and incubation for 5 min, fixed cells were washed twice with cold PBS buffer. Soluble chromatin was prepared by sonication (Bioruptor sonicator; Cosmo Bio) to an average DNA size of 500 bp in sonication buffer and immunoprecipitated in IP buffer (20 mM Tris-HCl, pH 8.0, 600 mM NaCl, 1 mM EDTA, 0.05% SDS, 1.0% Triton X-100, 20% glycerol, 1.5 μM aprotinin, 10 μM leupeptin, 1 mM DTT and 40 μM MG132). Protein G sepharose (Amersham, USA) blocked with BSA was added, and the antibody–chromatin complex was recovered by centrifugation. The recovery ratio of the immunoprecipitated DNA relative to input DNA was measured by real-time PCR using a CFX96 real time PCR detection system (Bio-Rad) and iQ SYBR Green Supermix (Bio-Rad). Primers for alphoidtetO repeat (tetO) as well as for control loci, 5S ribosomal DNA, are listed in Supplementary Table S1. At least three independent ChIP experiments were performed to estimate the level of enrichment.
Statistical analysis
Statistical analysis was made using Prism (GraphPad Software Inc., La Jolla, CA, USA). An unpaired Student t-test was conducted to examine HAC stability upon binding of tetR-VP64 to HAC alphoidtetO array induced by doxycycline withdrawal. P < 0.05 was considered significant.
RESULTS
Construction of the tTAVP64- and tTAVP16- containing HACs in HPRT-minus fibrosarcoma human HT1080 cells
The mega-size alphoid DNA array of the alphoidtetO-HAC contains a unique loxP site combined with the 5'-end of the HPRT sequence fragment such that gene loading events can be selected by reconstitution of a functional HPRT gene. The loxP-targeted region in the HAC also contains the drug-resistance marker hygromycin (Hygro) and the thymidine kinase gene of herpes simplex virus 1 (HS-tk) as well as the Bsr gene (repeated ∼30–40 times within the mega-size alphoid DNA array) (29,30,37).
In this study, two transcriptional transactivator tTA -containing vectors, tetR-VP64-IRES-DsRed2 (Figure 1a; Supplementary Figure S1) and tetR*-VP16-IRES-DsRed2 (Supplementary Figure S2a) were constructed (see Materials and Methods for details) and then inserted into the loxP site of the alphoidtetO-HAC in HPRT-deficient human HT1080 cells (Figure 1b) in order to determine the consequences of a single copy tTA expression on HAC stability. The tetR-VP64-IRES-DsRed2 vector contains the tTAVP64 sequence (four tandem repeats of the minimal VP16 domain DALDDFDLDML fused with TetR), the color marker DsRed2 and the 3'-end HPRT sequence fragment. The tTAVP64 and DsRed2 sequences are transcribed from the same CAG promoter but are separated by an internal ribosome entry site (IRES) sequence (Figure 1a). Therefore, while the tetR-VP64-IRES-DsRed2 vector encodes a single tTAVP64/DsRed2 transcript it is translated into two proteins: tTAVP64 (which binds to the multiple tetO-sequences in the alphoid array of the HAC) and DsRed2 (which is translated from IRES at a lower efficiency). The cHS4 insulator flanks the whole cassette. The tetR*-VP16-IRES-DsRed2 vector is identical to tetR-VP64-IRES-DsRed2 but contains only one copy of the VP16 domain. The tTAVP64 or tTAVP16 binding to tetO sequences is negatively regulated by doxycycline.
Figure 1.
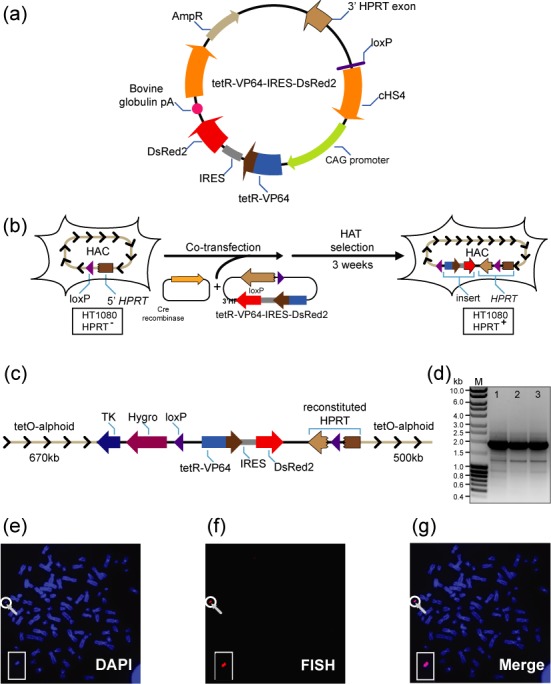
Loading of the tTAVP64-containing construct into the alphoidtetO-HAC propagated in human HPRT-minus HT1080 cells. (a) Diagram of the construct tetR-VP64-IRES-DsRed2 used in this study. The construct contains a 3′ HPRT sequence and loxP site, the tTAVP64 (tetR with the VP64 carrying four copies of VP16 activation domain) that is co-transcribed with the DsRed2 transgene under the same CAG promoter. The cHS4 insulator (54) flanks the expressing cassette from both sides. (A similar construct, tetR*-VP16-IRES-DsRed2, carrying one copy of the VP16 domain is described in Supplementary Figure S2). Both constructs were inserted into the loxP site of the alphoidtetO-HAC propagated in HPRT-deficient HT1080 cells. (b) Loading of the vector into the loxP site of the HAC is accompanied by reconstitution of the HPRT gene allowing cell selection on HAT medium. (c) A map of the resulting transgene cluster in the HAC containing TK and Hygro genes, the DsRed2 color marker and the reconstructed HPRT gene. Arrows indicate direction of transcription of the transgenes and 30–40 copies of BS incorporated in the alphoidtetO-HAC. (d) Lanes 1, 2 and 3 correspond to PCR products obtained with the genomic DNA isolated from HAC-containing clones of HT1080 cells using specific primers for the HPRT gene. The PCR products were sequenced and confirmed reconstitution of the HPRT gene. M-ladder marker. (e–g) FISH analysis of the HAC-containing HT1080 clone. Chromosomal DNA was counterstained with DAPI (blue) (e). The HAC was visualized using a BAC32–2-mer(tetO) probe (red) (f and g).
For insertion into the HAC, each vector was co-transformed along with the Cre-recombinase expression vector into HPRT-minus HT1080 cells carrying the alphoidtetO-HAC (Figure 1b). After insertion of the vector into the HAC the HPRT gene was reconstituted (Figure 1d) and the order and orientation of the transgenes is as shown in Figure 1c. The efficiency of gene loading into the HAC in HPRT-minus HT1080 cells was 1–2 × 10−4. From four to six recombinant clones were selected by growth on HAT medium after 12–15 days for each vector. All clones were selected in the presence of doxycycline to prevent tTA binding to the alphoidtetO array. Fluorescence microscopy revealed expression of the DsRed2 transgene in all analyzed clones. The presence of the autonomous form of the HACs carrying the tTAVP64 or tTAVP16 cassette was examined by FISH analysis. A fluorescence in situ hybridization image of one representative clone with the tTAVP64 cassette is shown in Figure 1e–g and with the tTAVP16 cassette in Supplementary Figure S2b. As seen, the HACs are maintained autonomously. These clones were used for further analysis.
One copy of the transcriptional transactivator tTAVP64 within alphoidtetO-HAC is enough to inactivate HAC kinetochore
To evaluate the effect of a single copy of the tTA on HAC stability, cells bearing HACs carrying the tetR*-VP16-IRES-DsRed2 or tetR-VP64-IRES-DsRed2 constructs were transferred into non-selective medium (HAT− and BS−) either with doxycycline (dox+) to inhibit binding of the tTA to the alphoidtetO array sequence or without doxycycline (dox−) to permit tTA binding. Cells were cultured in these media for different time intervals. Then a fluorescence image and FISH analysis with the probe specific for the alphoidtetO-HAC were carried out (see Materials and Methods). For each HAC-containing clone at least 70–80 metaphases were examined. No significant difference in the mitotic stability of the HAC carrying the tTAVP16 cassette was observed when cells were grown in dox+ or dox− medium (Supplementary Figure S2c), indicating that the cassette with one copy of the VP16 domain cannot inactivate the HAC kinetochore. Note that it has been previously shown that the same tTAVP16 cassette expressed from the virus or plasmid-based multiple copy vector caused loss of the alphoidtetO-HAC (29,37,39).
In contrast, a dramatic alphoidtetO HAC loss was observed in dox− medium when the tTAVP64 cassette was inserted into the HAC. Data on FISH analysis presented as a percentage of HAC-containing metaphases in different media (dox+ versus dox−) are summarized in Table 1. As seen, the absence or presence of doxycycline had essentially little, if any, effect on stability of the HAC after 3 days of culture. However, after 14 days, expression of the tTAVP64 fusion protein caused a dramatic increase in the population of cells lacking the HAC when the cells were grown in the absence of doxycycline (dox−). As a consequence, a disappearance of the DsRed2 fluorescence signal expressed from the HAC was observed when the cells were grown in the absence of doxycycline (Figure 2a and b). Note that expression of a single copy tTAVP64 from the HAC is 57-times lower than expression of the same transgene from the multiple copy vector transiently transfected into HT1080 cells (Supplementary Figure S4). Differences between two analyzed tTA cassettes is likely explained by a stronger effect of four tandem repeats of the VP16 domain in tTAVP64 on chromatin status in the HAC kinetochore compared to tTAVP16. Indeed, tTAVP64 was developed to increase the potency of transcriptional activation of tTAVP16 (36).
Table 1. Stability of the alphoidtetO-HAC/tTAVP64 in dox+ and dox− medium in human fibrosarcoma HT1080 cells.
| # clonea | Medium | 3 days | 14 days |
|---|---|---|---|
| 1 | dox+ | 99% | 93% |
| dox− | 90% | 0% | |
| 2 | dox+ | 99% | 92% |
| dox− | 99% | 0% | |
| 3 | dox+ | 80% | 85% |
| dox− | 75% | 5% | |
| 4 | dox+ | 80% | 72% |
| dox− | 70% | 1% |
aFor each HAC-containing clone at least 70–80 metaphases were examined.
Figure 2.
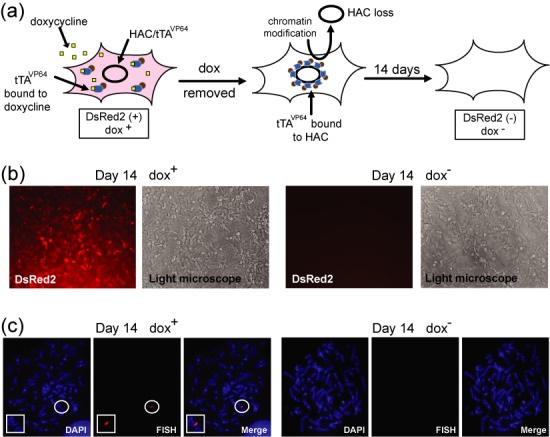
(a) A scheme representing binding of tTAVP64 protein with the HAC followed by HAC loss from the cells as result of doxycycline removal. (b) Bright and fluorescent images of one representative clone with the HAC carrying the tTAVP64-DsRed2 cassette cultured in dox+ medium and after removal doxycycline followed by 14 days of culture. Cells were cultured without HAC selection (in HAT− and BS− medium). (c) Representative images of the metaphase spread after 14 days of culture in the presence and absence of doxycycline. Chromosomal DNA was counterstained with DAPI (blue). The HAC was visualized using a BAC32–2-mer(tetO) probe (red).
Next, we examined the kinetics of HAC loss in one of HT1080 clones after 3, 4, 7 and 14 days of culture in the medium with and without doxycycline. As seen, the fraction of cells carrying the HAC decreased dramatically in dox− medium after 4 days of culture while remaining relatively stable in dox+ medium (Figure 3).
Figure 3.
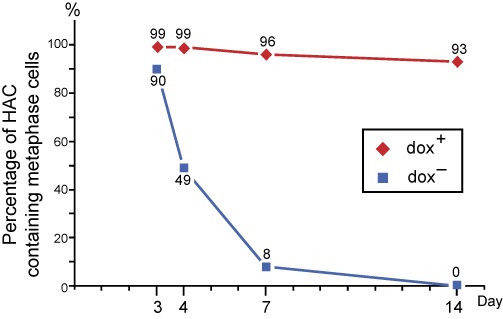
Kinetics of mitotic stability of the HAC carrying the construct tetR-VP64-IRES-DsRed2. The cells were cultured under following conditions: dox+ 3 days and dox− 3 days, dox+ 4 days and dox− 4 days, dox+ 7 days and dox− 7 days, dox+ 14 days and dox− 14 days. To quantify the HAC retention, the cells were analyzed by FISH. FISH analysis included detection of the HAC on metaphase spreads. At least 70–80 metaphase spreads were analyzed for each point. Addition of doxycycline, which prevents fusion of the tetracycline repressor from binding to tetO sequences of the HAC, blocks the inactivation of HAC kinetochore. Targeting the transcriptional transactivator tTAVP64 into the HAC kinetochore in the absence of doxycycline induces HAC loss.
Altogether, these data indicate that the expression of the tTAVP64 consisting of four copies of VP16 loaded into the alphoidtetO-HAC induces HAC loss, presumably as a result of changes in the HAC kinetochore chromatin.
Self-elimination of the alphoidtetO-HAC carrying the tTAVP64 cassette is not cell line specific
Similar results on HAC self-elimination in dox− medium were obtained in hamster ovarian CHO cells and in human epithelial HeLa cells. For these experiments, the tetR-VP64-IRES-DsRed2 construct was inserted into a single loxP site of the alphoidtetO-HAC propagated in HPRT-minus hamster CHO cells by Cre/loxP-mediated recombination (see Materials and Methods) (Figure 4a). Several clones were selected on HAT medium. Insertion into the HAC was confirmed by PCR using specific primers (Supplementary Table S1). These primers were diagnostic for reconstitution of the HPRT gene, which accompanies Cre/loxP targeting. A FISH image of one representative clone of the HAC in CHO cells in dox+ medium after 14 days of culture is shown in Figure 4b. This FISH confirmed that the HAC is maintained autonomously. One CHO clone containing alphoidtetO-HAC/tTAVP64 was chosen for transfer to human HeLa cells via MMCT (Figure 4c). FISH analysis confirmed that the HAC propagates autonomously without detectable integration into chromosomes (Figure 4d). The results on the HAC stability in two HAC-containing clones in CHO and HeLa cell lines in dox+ versus dox− medium are summarized in Table 2. As seen, expression of the tTAVP64 cassette from the HAC induces a dramatic HAC loss in the absence of doxycycline in the analyzed cell lines after 14 days of culture (Table 2; Figure 4b and d).
Figure 4.
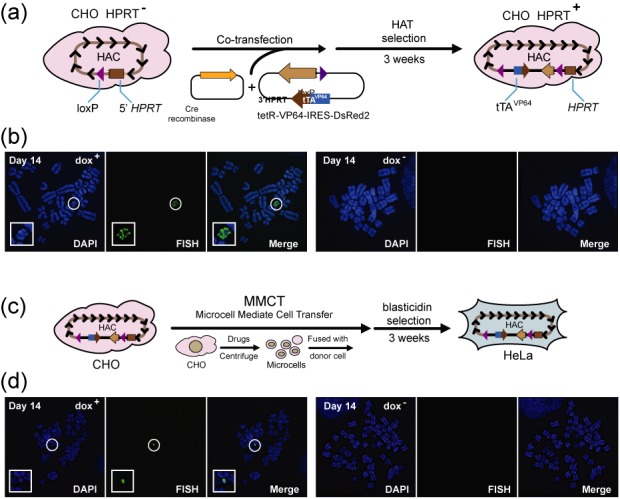
The alphoidtetO-HAC/tTAVP64 in different cell lines. (a) Insertion of the construct tetR-VP64-IRES-DsRed2 into the loxP site of the alphoidtetO-HAC propagated in HPRT-deficient hamster CHO cells by Cre/loxP mediated recombination. (b) FISH analysis of the hamster clone carrying the autonomously propagated alphoidtetO-HAC/tTAVP64 in dox+ medium and after removal doxycycline followed by 14 days of culture. (c) From CHO cells the alphoidtetO-HAC/tTAVP64 was MMCT-transferred to human HeLa cells. (d) FISH analysis of the HeLa human clone carrying the autonomously propagated alphoidtetO-HAC/tTAVP64 in dox+ medium and after removal doxycycline followed by 14 days of culture. Chromosomal DNA was counterstained with DAPI (blue). The HAC was visualized using a BAC32–2-mer(tetO) probe (green).
Table 2. Stability of the alphoidtetO-HAC/tTAVP64 in dox+ and dox− medium in hamster CHO and human HeLa cells.
| Hamster ovary CHO cells | |||
|---|---|---|---|
| # clonea | Medium | 3 days | 14 days |
| 1 | dox+ | 99% | 77% |
| dox− | 99% | 4% | |
| 2 | dox+ | 99% | 85% |
| dox− | 99% | 5% | |
| Human epithelium HeLa cells | |||
| 1 | dox+ | 99% | 93% |
| dox− | 99% | 2% | |
| 2 | dox+ | 99% | 92% |
| dox− | 99% | 3% | |
aFor each HAC-containing clone at least 130–140 metaphases were examined.
Chromatin changes in the alphoidtetO-HAC kinetochore induced by tTAVP64 tethering
Previously we have demonstrated that expression of the transcriptional activator (tTA) containing only one copy of the minimal VP16 domain DALDDFDLDML from the viral vectors produced ‘open’ chromatin in the alphoidtetO-HAC allied with a substantial elevation in transcripts specific for synthetic alphoid DNA array (29,31,34). Such a high level of transcription of the alphoidtetO array resulted in a rapid inactivation of the HAC kinetochore due not only to a complete block of CENP-A loading, but also due to stripping of pre-assembled CENP-A and other CCAN (constitutive centromere-associated network) proteins from the HAC kinetochore (32).
In this work, we examined whether a single copy of the tTAVP64 incorporated into the alphoidtetO-HAC had a similar effect on the HAC centrochromatin as multiple copies of the tTA expressed from a viral vector when a large amount of the transcriptional activator is produced.
In our first experiments, the transcription of satellite DNA in alphoidtetO array was found to be significantly increased (3-fold) after 3 days of growth in the medium lacking doxycycline in clone #1 [t-test, t(4) = 12.3554, P = 0.0002]. In clone #2, there was a significant increase (6-fold) after 2 days [t-test, t(4) = 53.9038, P < 0.0001] that further increased by 9-fold after 3 days [t-test, t(4) = 146.6633, P < 0.0001] (Figure 5a and b). These experiments demonstrate that targeting of HAC kinetochore chromatin by a single expressed copy of the tTAVP64 leads to a significant increase of transcription of non-coding alphoidtetO array after 3 days of cell growth (Figure 5a and b). These results correlate with ChIP data on enrichment of H3K4me3, a marker for the 5' end of actively transcribed genes (28,29,50,51), on alphoidtetO array in the HAC. In two independent clones, the HAC chromatin following tTAVP64 expression failed to reveal significant structural differences after one day of culture in dox− medium but was significantly (3–4 fold) increased on the alphoidtetO array of the HAC after 3 days of culture in the medium lacking doxycycline [t(4) = 31.2421, P < 0.0001 for clone #1 and t(4) = 49.8532, P < 0.0001 for clone #2] (Figure 5c and d). Thus, elevation of satellite transcription of the alphoidtetO array after 3 days was accompanied by enrichment in H3K4me3 chromatin. No significant difference in the level of CENP-A on the alphoidtetO array was observed when cells were grown in the presence or absence of doxycycline after 3 days of culture [t(4) = 2.1625, P = 0.0966] (Figure 5e). However, a longer culturing of the cells in dox− medium (4, 8 and 12 days) resulted in a dramatic decrease of the level of CENP-A (Figure 5e) followed by kinetochore inactivation and HAC loss (Figure 3) that is in agreement with our previous results on inactivation of the HAC kinetochore by tTA using viral vectors (29,31,32).
Figure 5.
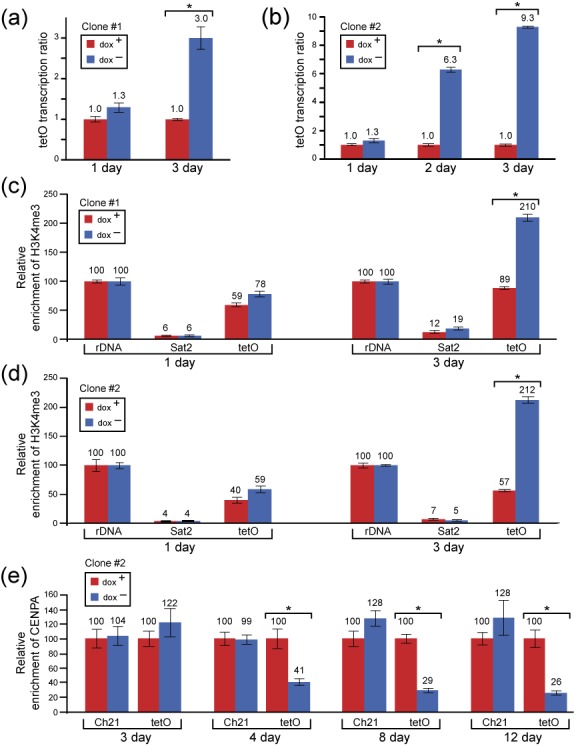
Chromatin/transcription changes in the alphoidtetO-HAC kinetochore induced by tTAVP64 tethering. (aand b) De-repression of tetO-alpha-satellite DNA transcription in the HAC when cells are grown in the medium lacking doxycycline. Quantitative RT-PCR experiments for two clones, clone #1 and clone #2, showing that the transcripts of non-coding tetO-alpha-satellite DNA repeats in the alphoidtetO-HAC are significantly repressed in the cells growing in doxycycline-containing medium compared to that of the cells that have been grown in the medium lacking doxycycline. (c and d) Chromatin immunoprecipitation (ChIP) analysis of non-coding tetO-alpha-satellite DNA repeats in the alphoidtetO-HAC in two clones using antibodies against H3K4me3 in the cells growing in doxycycline-containing medium compared to that of the cells that have been grown in the medium lacking doxycycline after 1 and 3 days of culture. Data were normalized to the internal 5S rDNA controls. (e) ChIP analysis of CENP-A chromatin in the alphoidtetO-HAC in the clone #2 cultured in the presence and absence of doxycycline for different time intervals (3, 4, 8 and 12 days of culture). Enrichment is shown relative to the chromosome 21 centromere. Error bars indicate s.d. The significant differences were calculated using Student's two-tailed t-test.
Development of a novel alphoidtetO-HAC/ tTAVP64-based system to verify phenotypic changes attributed to expression of HAC-encoded genes
The phenomenon of HAC loss caused by expression of the tTAVP64-containing chromatin modifier from the HAC can be used to develop a powerful system to verify phenotypes induced by a gene(s) loaded into the single loxP site of the alphoidtetO-HAC. This could be achieved by combining the TAR-cloning vector (43), used to selectively isolate any full-length gene from multiple human genomes, with the tTAVP64- containing modifier cassette followed by loading of this final vector along with a gene of interest into the HAC. Figure 6a shows a diagram of a newly constructed TAR-BRV- tTAVP64 cloning vector (see details in Supplementary Figure S5). This vector contains restriction sites for insertion of two unique targeting sequences (hooks) (red dots) homologous to the 5' and 3' ends of a gene of interest and restriction sites for insertion of chromatin insulators (green dots) (48), such as tRNA (52,53) or cHS4 (54) or γ -satellite DNA (55) to protect the captured gene from silencing. The vector also contains a YAC cassette that includes the HIS3 marker for selection in yeast and the CEN6 centromere for proper segregation in yeast cells as well as a BAC cassette that includes the F’ factor origin of replication and the chloramphenicol acetyltransferase (CmR) gene for selection in bacterial cells. This allows the TAR-isolated gene to be isolated either directly from yeast (56) or moved to bacterial cells without a step of YAC retrofitting with BAC sequence (39) for further gene DNA isolation. The TAR-BRV- tTAVP64 vector also contains a 3' HPRT-loxP cassette allowing further loading of the TAR-gene isolate into the unique loxP site of the alphoidtetO-HAC gene delivery vector in hamster HPRT-deficient CHO cells by Cre/loxP mediated recombination. From there the HAC may be MMCT-transferred (37,57) to the recipient human cells for further gene function analysis (23,38,39). Importantly, because the TAR-BRV- tTAVP64 vector contains the chromatin modifier that is constitutively expressed from the HAC, integrity of the HAC kinetochore and, as a consequence, expression of a gene of interest in the cells can be regulated. In the presence of doxycycline when the tTAVP64 does not bind to the tetO-sequences in the alphoidtetO-HAC, the HAC is mitotically stable and each cell contains a product of a gene loaded into the HAC. After removal of the ligand doxycycline, the tTAVP64 binds to tetO-sequences of the HAC resulting in HAC loss along with a gene of interest.
Figure 6.
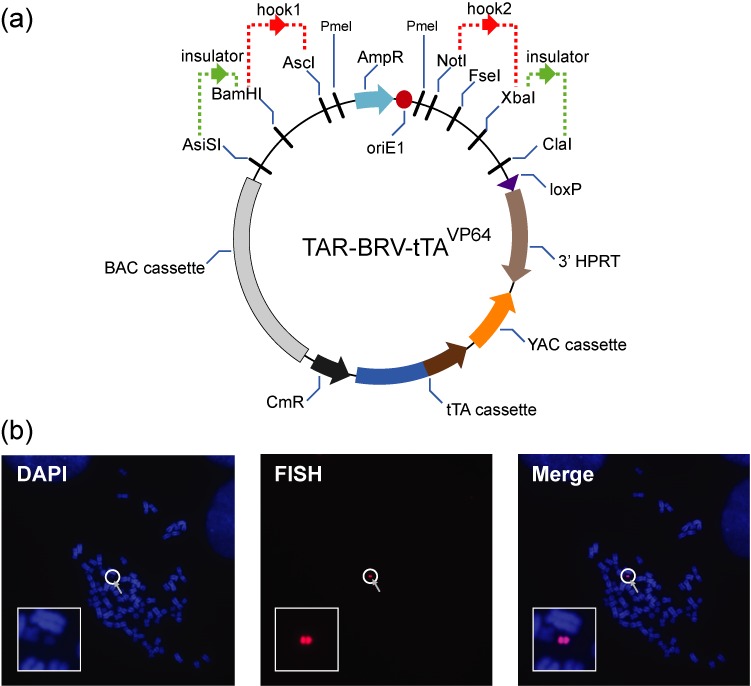
(a) A scheme of the TAR-BRV- tTAVP64 vector. This vector contains restriction sites for insertion of two unique targeting sequences (hooks) (dot red) homologous to 5' and 3' ends of a gene of interest and restriction sites for insertion of chromatin insulators to protect a gene from silencing (dot green). The vector also contains a YAC cassette that includes a yeast selectable marker HIS3 for selection in yeast and a yeast centromere CEN6 for proper segregation in yeast cells. The TAR-BRV- tTAVP64 vector also contains a BAC cassette that includes a F’ factor origin of replication and the chloramphenicol acetyltransferase (CmR) gene for selection in bacterial cells. For gene TAR cloning experiments, the TAR-BRV- tTAVP64 vector DNA should be linearized by a unique endonuclease located between the hooks to expose targeting sequences (hooks). The TAR-BRV- tTAVP64 vector also contains a 3' HPRT-loxP cassette allowing a further loading of a TAR-gene isolate into the unique loxP site of the alphoidtetO-HAC in hamster CHO cells by Cre/loxP mediated recombination, from where the HAC may be MMCT-transferred to the recipient human cells for further gene function analysis. The TAR-BRV- tTAVP64 vector also contains the tTAVP64 chromatin modifier that is constitutively expressed from the HAC. In the presence of doxycycline the tTAVP64 cannot bind to the tetO-sequences in the alphoidtetO-HAC. (b) FISH analysis of the HAC containing the TAR-BRV- tTAVP64 vector in HT1080 cells. Chromosomal DNA was counterstained with DAPI (blue). The HAC was visualized using a vector probe (red).
As a proof of principle, the TAR-BRV- tTAVP64 vector was inserted into the loxP site of the alphoidtetO-HAC in human HPRT-deficient HT1080 cells. FISH analysis proved that the HAC is maintained in the autonomous form (Figure 6b). Three randomly chosen clones were analyzed in dox+ and dox− media with the following FISH analysis to confirm HAC loss in the medium lacking doxycycline. To protect the tTAVP64 expression from silencing, the tRNA insulator (52) was inserted upstream of the CAG promoter. Significant HAC loss was observed in three out of three selected clones cultured in the absence of doxycycline after 16 days of culture (Table 3). The greater HAC loss observed for the original tetR-VP64-IRES-DsRed2 vector may be due to the difference either in the structure of the cassettes or the insulator type used (cHS4 vs tRNA) (52–54). It is known that different insulator types have a different level of gene protection (48).
Table 3. Stability of the alphoidtetO-HAC/TAR-BRV- tTAVP64 in dox+ and dox− medium in HT1080 cells.
| # clonea | Medium | 2 days | 16 days |
|---|---|---|---|
| 1 | dox+ | 96% | 73% |
| dox− | 80% | 8% | |
| 2 | dox+ | 98% | 75% |
| dox− | 84% | 8% | |
| 3 | dox+ | 98% | 72% |
| dox− | 79% | 7% |
aFor each HAC-containing clone at least 70–90 metaphases were examined.
To summarize, the TAR-BRV-tTAVP64 gene cloning vector allows to control expression of genes loaded into the alphoidtetO-HAC by simple removal of a ligand (doxycycline) that results in the kinetochore disassembly followed by the HAC loss.
DISCUSSION
The alphoidtetO-HAC is an advanced HAC-based vector for gene delivery and regulated gene expression (23,29,37,39). This HAC consists of a megabase-size synthetic, higher order alpha-satellite repeat array with a tet operator sequence (tetO) in every other alphoid monomer. This provides a significant advantage for this HAC compared to other HAC-based vectors because its kinetochore can be inactivated by tethering the tTS silencer or the tTA transcriptional transactivator or other chromatin modifiers to the tetO sequences leading to HAC loss (29,31,32,34,37). This HAC feature provides a control for phenotypes induced by test genes loaded into the alphoidtetO-HAC.
In our previous studies, induction of alphoidtetO-HAC loss was initiated by transfection of cells by viral or plasmid vectors expressing multiple copies of a chromatin modifier, a step that is potentially mutagenic (23,29,37,39). Recently we described a re-engineered alphoidtetO-HAC that allows verification of phenotypic changes attributed to expression of genes from the HAC without the potentially mutagenic transfection step (40). In that HAC, a single copy of the tTS was inserted into a unique gene-loading site along with a gene of interest. Expression of the tTS generated a self-regulating fluctuating heterochromatin on the alphoidtetO-HAC that induced a fast silencing of the gene on the HAC without significant effects on HAC segregation (40). Importantly, inactivation of a gene on the alphoidtetO-HAC/tTS was reversible by addition of doxycycline, presumably because the tTS was silencing its own expression. Thus, expression of a single auto-regulated copy of the tTS from the alphoidtetO-HAC provides an efficient method to switch off or switch on expression of the gene(s) loaded onto the HAC.
In this work, we engineered the novel alphoidtetO-HAC/ tTAVP64 and demonstrated that a single copy of the transcriptional transactivator tTAVP64 constitutively expressed from the HAC also allows a stable expression of the HAC gene(s) in the presence of doxycycline. In contrast to the alphoidtetO-HAC/tTS, culturing of the alphoidtetO-HAC/ tTAVP64 in medium without doxycycline induces a fast inactivation of the HAC kinetochore leading to HAC loss along with a loaded gene. The different effect of tTS and tTAVP64 on the HAC kinetochore function is presumably due to that the tTAVP64 does not self-silence. This provides a pure control for phenotypic changes attributed to expression of a HAC-encoded gene by returning the mutant cell line to its original state following removal of the HAC from the cell. The alphoidtetO-HAC/ tTAVP64 with a regulated kinetochore may have a specific application for generation of induced pluripotent stem (iPS) cells for regenerative medicine. It is worth noting that recent publications demonstrate the use of other HAC vectors for reprogramming mouse embryonic fibroblasts (MEFs) into induced iPS cells (5,6).
In addition, in this work we combined the alphoidtetO-HAC-based gene delivery vector with TAR gene-cloning technology and demonstrated a potential to carry out a complete cycle starting with selective gene isolation using a newly constructed TAR cloning vector, TAR-BRV- tTAVP64, followed by tTAVP64 and a gene of interest expression from the HAC vector and ended by the HAC loss along with a gene. This new vector contains a BAC cassette and, therefore, allows the direct transfer of TAR-isolated genes from yeast to bacterial cells for further BAC DNA isolation. This vector also contains a 3'HPRT-loxP cassette allowing direct gene loading into the alphoidtetO-HAC for the following complementation of gene deficiencies in human cells. The gene expression from the alphoidtetO-HAC can be eliminated by a simple removal of doxycycline from the medium because tTAVP64 is inserted along with a gene of interest into the HAC. A hypothetical scheme illustrating the entire process, starting with TAR isolation of a gene from genomic DNA in yeast using a TAR-BRV- tTAVP64 vector then proceeding to its direct transfer to bacterial cells and subsequent insertion into the alphoidtetO-HAC and ending with the verification of gene-induced phenotypes by inducing HAC loss, is diagrammed in Supplementary Figure S6. It is worth noting that a YAC/BAC/TAR gene-containing construct could also possibly be introduced into HAC-containing human cells through yeast spheroplast fusion to human cells (58).
Thus, we have designed two approaches to verify gene-induced phenotypes using HAC vectors. In the first case, when a single copy of tTS is expressed, a self-regulating fluctuating heterochromatin is generated on the alphoidtetO-HAC. This can induce a fast silencing of the gene(s) on the HAC without significant effects on HAC stability and such gene silencing is reversible by addition of doxycycline (40). In the second case, expression of a single copy of the tTAVP64 from the HAC induces a fast inactivation of the HAC kinetochore leading to irreversible HAC loss along with the loaded gene. Both approaches are useful for studies of gene function and have a potential for cell and gene therapy.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Acknowledgments
The authors thank the CRC, LRBGE Fluorescence Imaging Facility (NIH) and also Dr Karpova and Dr McNally for instructions, consultations and help with the usage of a DeltaVision microscopy imaging system.
FUNDING
Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, USA (V.L., N.K.); Kazusa DNA Research Institute Foundation (H.M.); Wellcome Trust of which W.C.E. is a Principal Research Fellow [073915]. Funding for open access charge: NIH.
Conflict of interest statement. None declared.
REFERENCES
- 1.Kazuki Y., Oshimura M. Human artificial chromosomes for gene delivery and the development of animal models. Mol. Ther. 2011;19:1591–1601. doi: 10.1038/mt.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kouprina N., Earnshaw W.C., Masumoto H., Larionov V. A new generation of human artificial chromosomes for functional genomics and gene therapy. Cell. Mol. Life Sci. 2013;70:1135–1148. doi: 10.1007/s00018-012-1113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kouprina N., Tomilin A.N., Masumoto H., Earnshaw W.C., Larionov V. Human artificial chromosome-based gene delivery vectors for biomedicine and biotechnology. Expert Opin. Drug Deliv. 2014;11:517–535. doi: 10.1517/17425247.2014.882314. [DOI] [PubMed] [Google Scholar]
- 4.Oshimura M, Kazuki Y, Iida Y, N U. eLS. Chichester: John Wiley & Sons Ltd; 2013. New Vectors for Gene Delivery: Human and Mouse Artificial Chromosomes. [Google Scholar]
- 5.Hiratsuka M., Uno N., Ueda K., Kurosaki H., Imaoka N., Kazuki K., Ueno E., Akakura Y., Katoh M., Osaki M., et al. Integration-free iPS cells engineered using human artificial chromosome vectors. Plos One. 2011;6:e25961. doi: 10.1371/journal.pone.0025961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakura Y., Ishihara C., Kurosaki H., Kazuki Y., Komatsu N., Okada Y., Doi T., Takeya H., Oshimura M. An induced pluripotent stem cell-mediated and integration-free factor VIII expression system. Biochem. Biophys. Res. Commun. 2013;431:336–341. doi: 10.1016/j.bbrc.2012.12.096. [DOI] [PubMed] [Google Scholar]
- 7.Auriche C., Carpani D., Conese M., Caci E., Zegarra-Moran O., Donini P., Ascenzioni F. Functional human CFTR produced by a stable minichromosome. EMBO Rep. 2002;3:862–868. doi: 10.1093/embo-reports/kvf174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu J., Compitello G., Stromberg G., Willard H.F., Van Bokkelen G. Efficient assembly of de novo human artificial chromosomes from large genomic loci. BMC Biotechnol. 2005;5:21. doi: 10.1186/1472-6750-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breman A.M., Steiner C.M., Slee R.B., Grimes B.R. Input DNA ratio determines copy number of the 33 kb Factor IX gene on de novo human artificial chromosomes. Mol. Ther. 2008;16:315–323. doi: 10.1038/sj.mt.6300361. [DOI] [PubMed] [Google Scholar]
- 10.Kazuki Y., Hoshiya H., Kai Y., Abe S., Takiguchi M., Osaki M., Kawazoe S., Katoh M., Kanatsu-Shinohara M., Inoue K., et al. Correction of a genetic defect in multipotent germline stem cells using a human artificial chromosome. Gene Ther. 2008;15:617–624. doi: 10.1038/sj.gt.3303091. [DOI] [PubMed] [Google Scholar]
- 11.Kuroiwa Y., Tomizuka K., Shinohara T., Kazuki Y., Yoshida H., Ohguma A., Yamamoto T., Tanaka S., Oshimura M., Ishida I. Manipulation of human minichromosomes to carry greater than megabase-sized chromosome inserts. Nat. Biotechnol. 2000;18:1086–1090. doi: 10.1038/80287. [DOI] [PubMed] [Google Scholar]
- 12.Rocchi L., Braz C., Cattani S., Ramalho A., Christan S., Edlinger M., Ascenzioni F., Laner A., Kraner S., Amaral M., et al. Escherichia coli-cloned CFTR loci relevant for human artificial chromosome therapy. Hum. Gene Ther. 2010;21:1077–1092. doi: 10.1089/hum.2009.225. [DOI] [PubMed] [Google Scholar]
- 13.Yamada H., Li Y.C., Nishikawa M., Oshimura M., Inoue T. Introduction of a CD40L genomic fragment via a human artificial chromosome vector permits cell-type-specific gene expression and induces immunoglobulin secretion. J. Hum. Genet. 2008;53:447–453. doi: 10.1007/s10038-008-0268-0. [DOI] [PubMed] [Google Scholar]
- 14.Hoshiya H., Kazuki Y., Abe S., Takiguchi M., Kajitani N., Watanabe Y., Yoshino T., Shirayoshi Y., Higaki K., Messina G., et al. A highly stable and nonintegrated human artificial chromosome (HAC) containing the 2.4 Mb entire human dystrophin gene. Mol. Ther. 2009;17:309–317. doi: 10.1038/mt.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeno M., Inagaki H., Nagata K., Morita M., Ichinose H., Okazaki T. Generation of human artificial chromosomes expressing naturally controlled guanosine triphosphate cyclohydrolase I gene. Genes Cells. 2002;7:1021–1032. doi: 10.1046/j.1365-2443.2002.00580.x. [DOI] [PubMed] [Google Scholar]
- 16.Ito M., Ikeno M., Nagata H., Yamamoto T., Hiroguchi A., Fox I.J., Miyakawa S. Treatment of nonalbumin rats by transplantation of immortalized hepatocytes using artificial human chromosome. Transplant. Proc. 2009;41:422–424. doi: 10.1016/j.transproceed.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Kazuki Y., Hiratsuka M., Takiguchi M., Osaki M., Kajitani N., Hoshiya H., Hiramatsu K., Yoshino T., Kazuki K., Ishihara C., et al. Complete genetic correction of ips cells from Duchenne muscular dystrophy. Mol. Ther. 2009;18:386–393. doi: 10.1038/mt.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroiwa Y., Kasinathan P., Sathiyaseelan T., Jiao J.A., Matsushita H., Sathiyaseelan J., Wu H., Mellquist J., Hammitt M., Koster J., et al. Antigen-specific human polyclonal antibodies from hyperimmunized cattle. Nat. Biotechnol. 2009;27:173–181. doi: 10.1038/nbt.1521. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki N., Nishii K., Okazaki T., Ikeno M. Human artificial chromosomes constructed using the bottom-up strategy are stably maintained in mitosis and efficiently transmissible to progeny mice. J. Biol. Chem. 2006;281:26615–26623. doi: 10.1074/jbc.M603053200. [DOI] [PubMed] [Google Scholar]
- 20.Voet T., Schoenmakers E., Carpentier S., Labaere C., Marynen P. Controlled transgene dosage and PAC-mediated transgenesis in mice using a chromosomal vector. Genomics. 2003;82:596–605. doi: 10.1016/s0888-7543(03)00112-5. [DOI] [PubMed] [Google Scholar]
- 21.Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa Y., Ishikura T., Hasegawa T., Watanabe T., Suzuki J., Nakayama M., Okamura Y., Okazaki T., Koseki H., Ohara O. Generating a transgenic mouse line stably expressing human MHC surface antigen from a HAC carrying multiple genomic BACs. Chromosoma. 2014;2014:1–12. doi: 10.1007/s00412-014-0488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kononenko A.V., Bansal R., Lee N.C.O., Grimes B.R., Masumoto H., Earnshaw W.C., Larionov V., Kouprina N. A portable BRCA1-HAC (human artificial chromosome) module for analysis of BRCA1 tumor suppressor function. Nucleic Acids Res. 2014;42:e164. doi: 10.1093/nar/gku870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto K., Suzuki N., Sakai K., Asakawa S., Okazaki T., Kudoh J., Ikeno M., Shimizu N. A novel mouse model for Down syndrome that harbor a single copy of human artificial chromosome (HAC) carrying a limited number of genes from human chromosome 21. Transgenic Res. 2013;23:317–329. doi: 10.1007/s11248-013-9772-x. [DOI] [PubMed] [Google Scholar]
- 25.Tedesco F.S., Gerli M.F., Perani L., Benedetti S., Ungaro F., Cassano M., Antonini S., Tagliafico E., Artusi V., Longa E., et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci. Transl. Med. 2012;4:140ra89. doi: 10.1126/scitranslmed.3003541. [DOI] [PubMed] [Google Scholar]
- 26.Tedesco F.S., Hoshiya H., D'Antona G., Gerli M.F., Messina G., Antonini S., Tonlorenzi R., Benedetti S., Berghella L., Torrente Y., et al. Stem cell-mediated transfer of a human artificial chromosome ameliorates muscular dystrophy. Sci. Transl. Med. 2011;3:96ra78. doi: 10.1126/scitranslmed.3002342. [DOI] [PubMed] [Google Scholar]
- 27.Basu J., Willard H.F. Human artificial chromosomes: potential applications and clinical considerations. Pediatr. Clin. North Am. 2006;53:843–853. doi: 10.1016/j.pcl.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Monaco Z.L., Moralli D. Progress in artificial chromosome technology. Biochem. Soc. Trans. 2006;34:324–327. doi: 10.1042/BST20060324. [DOI] [PubMed] [Google Scholar]
- 29.Nakano M., Cardinale S., Noskov V.N., Gassmann R., Vagnarelli P., Kandels-Lewis S., Larionov V., Earnshaw W.C., Masumoto H. Inactivation of a human kinetochore by specific targeting of chromatin modifiers. Dev. Cell. 2008;14:507–522. doi: 10.1016/j.devcel.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouprina N., Samoshkin A., Erliandri I., Nakano M., Lee H.S., Fu H.G., Iida Y., Aladjem M., Oshimura M., Masumoto H., et al. Organization of synthetic alphoid DNA array in human artificial chromosome (HAC) with a conditional centromere. ACS Synth. Biol. 2012;1:590–601. doi: 10.1021/sb3000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergmann J.H., Jakubsche J.N., Martins N.M., Kagansky A., Nakano M., Kimura H., Kelly D.A., Turner B.M., Masumoto H., Larionov V., et al. Epigenetic engineering: histone H3K9 acetylation is compatible with kinetochore structure and function. J. Cell Sci. 2012;125:411–421. doi: 10.1242/jcs.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergmann J.H., Martins N.M.C., Larionov V., Masumoto H., Earnshaw W.C. HACking the centromere chromatin code: insights from human artificial chromosomes. Chromosome Res. 2012;20:505–519. doi: 10.1007/s10577-012-9293-0. [DOI] [PubMed] [Google Scholar]
- 33.Bergmann J.H., Rodriguez M.G., Martins N.M.C., Kimura H., Kelly D.A., Masumoto H., Larionov V., Jansen L.E.T., Earnshaw W.C. Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J. 2011;30:328–340. doi: 10.1038/emboj.2010.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardinale S., Bergmann J.H., Kelly D., Nakano M., Valdivia M.M., Kimura H., Masumoto H., Larionov V., Earnshaw W.C. Hierarchical inactivation of a synthetic human kinetochore by a chromatin modifier. Mol. Biol. Cell. 2009;20:4194–4204. doi: 10.1091/mbc.E09-06-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sander A., Guth A., Brenner H.R., Witzemann V. Gene transfer into individual muscle fibers and conditional gene expression in living animals. Cell Tissue Res. 2000;301:397–403. doi: 10.1007/s004410000247. [DOI] [PubMed] [Google Scholar]
- 36.Beerli R.R., Segal D.J., Dreier B., Barbas C.F. Toward controlling gene expression at will: Specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14628–14633. doi: 10.1073/pnas.95.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iida Y., Kim J.H., Kazuki Y., Hoshiya H., Takiguchi M., Hayashi M., Erliandri I., Lee H.S., Samoshkin A., Masumoto H., et al. Human artificial chromosome with a conditional centromere for gene delivery and gene expression. DNA Res. 2010;17:293–301. doi: 10.1093/dnares/dsq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayabe F., Katoh M., Inoue T., Kouprina N., Larionov V., Oshimura M. A novel expression system for genomic DNA loci using a human artificial chromosome vector with transformation-associated recombination cloning. J. Hum. Genet. 2005;50:592–599. doi: 10.1007/s10038-005-0300-6. [DOI] [PubMed] [Google Scholar]
- 39.Kim J.H., Kononenko A., Erliandri I., Kim T.A., Nakano M., Iida Y., Barrett J.C., Oshimura M., Masumoto H., Earnshaw W.C., et al. Human artificial chromosome (HAC) vector with a conditional centromere for correction of genetic deficiencies in human cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:20048–20053. doi: 10.1073/pnas.1114483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kononenko A.V., Lee N.C.O., Earnshaw W.C., Kouprina N., Larionov V. Re-engineering an alphoid(tetO)-HAC-based vector to enable high-throughput analyses of gene function. Nucleic Acids Res. 2013;41:e107. doi: 10.1093/nar/gkt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iida Y., Kazuki Y., Hayashi M., Ueda Y., Hasegawa M., Kouprina N., Larionov V., Oshimura M. Bi-HAC vector system toward gene and cell therapy. ACS Synth. Biol. 2014;3:83–90. doi: 10.1021/sb400166j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kouprina N., Larionov V. Innovation - TAR cloning: insights into gene function, long-range haplotypes and genome structure and evolution. Nat. Rev. Genet. 2006;7:805–812. doi: 10.1038/nrg1943. [DOI] [PubMed] [Google Scholar]
- 43.Kouprina N., Larionov V. Selective isolation of genomic loci from complex genomes by transformation-associated recombination cloning in the yeast Saccharomyces cerevisiae. Nat. Protoc. 2008;3:371–377. doi: 10.1038/nprot.2008.5. [DOI] [PubMed] [Google Scholar]
- 44.Larionov V., Kouprina N., Graves J., Chen X.N., Korenberg J.R., Resnick M.A. Specific cloning of human DNA as yeast artificial chromosomes by transformation-associated recombination. Proc. Natl. Acad. Sci. U.S.A. 1996;93:491–496. doi: 10.1073/pnas.93.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez-Solis R., Liu P.T., Bradley A. Chromosome Engineering in Mice. Nature. 1995;378:720–724. doi: 10.1038/378720a0. [DOI] [PubMed] [Google Scholar]
- 46.Smith A.J., De Sousa M.A., Kwabi-Addo B., Heppell-Parton A., Impey H., Rabbitts P. A site-directed chromosomal translocation induced in embryonic stem cells by Cre-loxP recombination. Nat. Genet. 1995;9:376–385. doi: 10.1038/ng0495-376. [DOI] [PubMed] [Google Scholar]
- 47.Sikorski R.S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces-cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee N.C., Kononenko A.V., Lee H.S., Tolkunova E.N., Liskovykh M.A., Masumoto H., Earnshaw W.C., Tomilin A.N., Larionov V., Kouprina N. Protecting a transgene expression from the HAC-based vector by different chromatin insulators. Cell. Mol. Life Sci. 2013;70:3723–3737. doi: 10.1007/s00018-013-1362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larionov V., Kouprina N., Solomon G., Barrett J.C., Resnick M.A. Direct isolation of human BRCA2 gene by transformation-associated recombination in yeast. Proc. Natl. Acad. Sci. U.S.A. 1997;94:7384–7387. doi: 10.1073/pnas.94.14.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 51.Santos-Rosa H., Schneider R., Bannister A.J., Sherriff J., Bernstein B.E., Emre N.C.T., Schreiber S.L., Mellor J., Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 52.Ebersole T., Kim J.H., Samoshkin A., Kouprina N., Pavlicek A., White R.J., Larionov V. tRNA genes protect a reporter gene from epigenetic silencing in mouse cells. Cell Cycle. 2011;10:2779–2791. doi: 10.4161/cc.10.16.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raab J.R., Chiu J., Zhu J.C., Katzman S., Kurukuti S., Wade P.A., Haussler D., Kamakaka R.T. Human tRNA genes function as chromatin insulators. EMBO J. 2012;31:330–350. doi: 10.1038/emboj.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pikaart M.I., Recillas-Targa F., Felsenfeld G. Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev. 1998;12:2852–2862. doi: 10.1101/gad.12.18.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J.H., Ebersole T., Kouprina N., Noskov V.N., Ohzeki J.I., Masumoto H., Mravinac B., Sullivan B.A., Pavlicek A., Dovat S., et al. Human gamma-satellite DNA maintains open chromatin structure and protects a transgene from epigenetic silencing. Genome Res. 2009;19:533–544. doi: 10.1101/gr.086496.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohzeki J., Bergmann J.H., Kouprina N., Noskov V.N., Nakano M., Kimura H., Earnshaw W.C., Larionov V., Masumoto H. Breaking the HAC Barrier: histone H3K9 acetyl/methyl balance regulates CENP-A assembly. EMBO J. 2012;31:2391–2402. doi: 10.1038/emboj.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koi M., Shimizu M., Morita H., Yamada H., Oshimura M. Construction of mouse A9 clones containing a single human-chromosome tagged with neomycin-resistance gene via microcell fusion. Jpn. J. Cancer Res. 1989;80:413–418. doi: 10.1111/j.1349-7006.1989.tb02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L.P., Blankenstein T. Generation of transgenic mice with megabase-sized human yeast artificial chromosomes by yeast spheroplast-embryonic stem cell fusion. Nat. Protoc. 2013;8:1567–1582. doi: 10.1038/nprot.2013.093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


