Abstract
Volumetric muscle loss (VML) is a disabling condition in which current clinical procedures are suboptimal. The field of tissue engineering has many promising strategies for the creation of functional skeletal muscle in vitro. However, there are still two key limitations that prevent it from becoming a solution for treating VML. First, engineered muscle tissue must be biocompatible to facilitate muscle tissue regrowth without generating an immune response. Second, engineered muscle constructs must be scaled up to facilitate replacement of clinically relevant volumes of tissue (centimeters in diameter). There are currently no tissue engineering strategies to produce tissue constructs that are both biocompatible and large enough to facilitate clinical repair. However, recent advances in tissue engineering using synthetic scaffolds, native scaffolds, or scaffold-free approaches may lead to a solution for repair of VML injuries.
Keywords: acellular, electrospinning, micropatterning, myoblast, myotube, satellite cell, scaffold-free
The field of skeletal muscle tissue engineering has taken great strides since Vandenburgh's first work in 1988 using cultured avian myotubes in collagen-coated tissue culture plates [1]. Since that time, the ability to engineer skeletal muscle and other tissues in vitro has ushered in a new era of tissue engineering and regenerative medicine. Promising research is currently being performed in bioengineering, materials science and physiology programs around the world [1–5], reflecting the necessary cross-disciplinary collaboration to formulate innovative solutions for these often complex and debilitating musculoskeletal injuries. Specifically, volumetric muscle loss (VML), characterized by extensive structural and functional damage to skeletal muscle from either blunt or sharp trauma, chronic denervation or, in many cases, the direct consequence of tumor extirpation, overwhelms the body's own repair systems. Repair technologies are currently limited and could benefit tremendously from advances in tissue engineering. If successful, skeletal muscle tissue engineering will be uniquely equipped for the treatment of VML with the ultimate goal being the accurate repair or replacement of skeletal muscle defects to recapitulate the premorbid state in both form and function.
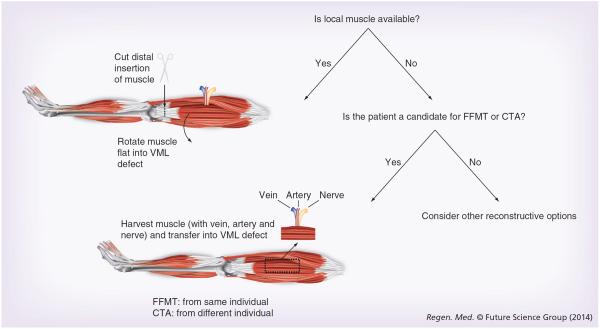
VML is a disabling condition for which surgical repair is limited by a unique set of challenges [2,6]. Standard operative treatment consists of replacing the damaged or lost muscle with healthy, well-vascularized, innervated autogenous skeletal muscle tissue from outside of the zone of injury (Figure 1). If muscle tissue is moved with its neurovascular supply intact, then the procedure is referred to as a muscle flap. Muscle flaps are often the procedure of choice to treat VML of upper and lower extremity musculoskeletal injuries, of which free functional muscle transfer (FFMT) is the most common [4,7]. This procedure involves the transplantation of a donor muscle along with its accompanying nerve, artery and vein to a new location on the body where it is revascularized and reinnervated. It differs from a traditional muscle flap in that the nerve, artery and vein are cut and sewn back together in FFMT. Thus, FFMT can move skeletal muscle anywhere on the body while traditional muscle flaps are restricted to an area defined by the length of their artery and nerve. For example, in the case of chronic facial paralysis in which irreversible atrophy of the facial musculature occurs, transfer of the gracilis muscle as a FFMT is used for facial reanimation [1,4–6]. The advantage of FFMT is the ability of the reconstructive surgeon to select a donor muscle that meets certain requirements of the defect by adjusting for size, bulk and orientation to optimize functional outcomes. Furthermore, volitional movement of the donor muscle is possible following reinnervation. Limitations of FFMT include donor site morbidity and operative times are approximately 4–6 h compared with 1–2 h of traditional muscle flap procedures. Further drawbacks include extended rehabilitation limited by reinnervation to the motor end plates in the donor muscle and the technical demands of microsurgery. Traditional muscle flaps utilize donor muscle adjacent to the defect, which can be rotated or advanced without severing the native blood and nerve supply to the muscle. This approach provides an expedited return to function because no nerve regeneration is required. However, viable donor muscle may not be available, or may not adequately fill the defect, and patients usually require physiotherapy to relearn how to control the donor muscle function. Limited successes have been reported with both FFMT and other traditional muscle flap procedures because many skeletal muscle defects cannot be repaired using autogenous tissue and return to premorbid function is rare. Therefore, there is a need for an easy-touse, biocompatible, adaptable and effective tissue replacement therapy for damaged or diseased skeletal muscle tissue [1–6,8–10].
Figure 1. Reconstructive algorithm for clinical volumetric muscle loss defects.
If local uninjured muscle is available, it can be used to reconstruct the VML defect. The distal tendon is cut, allowing the muscle to be rotated into the defect based on its blood supply (the artery, vein and nerve are not severed). If local muscle is unavailable, the VML defect can be reconstructed using muscle from another part of the body within the same individual (FFMT) or from another individual (CTA). The muscle to be transferred is harvested along with its artery, vein and nerve, and these are reconnected at the recipient site to re-establish an immediate blood supply. If none of these options are possible, then alternative reconstructive approaches should be considered. CTA: Composite tissue allotransplantation; FFMT: Free functional muscle transfer; VML: Volumetric muscle loss.
Recently, composite tissue allotransplantation (CTA), or the use of vascularized, innervated allogeneic composite tissue, has emerged as a powerful technique for reconstructing some of the most functionally complex soft tissue defects, which include variable degrees of skeletal muscle loss. Most notably among them are the face, hands, abdominal wall and larynx [1,2,6,10–16]. Specifically, CTA involves the transfer of simultaneous, multitissue reconstructive units (i.e., skin, muscle, tendon and bone), such as an entire hand or face, from one individual to another in a single setting. It employs the same principles as free tissue transfer, except that CTA uses allogeneic rather than autogenous tissue. The early results are very encouraging, but require chronic immunosuppression to combat tissue rejection [2–7,13,17–21]. Thus, future investigations will need to address the side effects of chronic immunosuppression, namely visceral organ toxicity, the development of skin-based malignancies and lymphoproliferative disorders, and tissue regeneration itself [2,4–6,22]. Again, tissue engineering approaches may be a better solution for tissue replacement therapy, by simply avoiding the pitfalls of CTA including finding a suitable donor, optimization of the patient's immunosuppressive drug regimen, or eliminating the need for long-term surveillance to monitor for tissue rejection, if autogenous tissue is engineered.
Skeletal muscle tissue engineering may be able to overcome many of the complications and obstacles encountered in the treatment of VML. Today, tissue engineered skeletal muscle implants have not been developed for clinical use, but many developing technologies exist which share the same fundamental approach [4,5,7–10,23–25]; recapitulation of myogenesis by induction of muscle satellite cells toward development of whole-organ implants. The satellite cell is a skeletal muscle-specific stem cell that normally remains quiescent between the sarcolemma and basal lamina of the mature muscle fiber [1,4–6,12–16,26–28], and becomes activated in response to traumatic injury or microinjury induced by mechanical loading. Once activated, satellite cells migrate to the site of muscle injury and undergo asymmetric division to a `determined' state and migrate to the site of muscle injury [1–6,8–10,26–28]. At the injury site, determined satellite cells are induced to myoblasts, proliferate and begin to fuse with other myoblasts or existing injured myofibers to form multinucleated myofibers that produce the characteristic protein machinery responsible for muscular contraction. Satellite cells are strongly imprinted to a myogenic lineage [4,5,11,18,23,29], making them a useful cell source for skeletal muscle tissue engineering applications. To date, no clinically viable muscle constructs have been developed for skeletal muscle repair or replacement, but recent advancements in the field demonstrate that a safe and effective tissue engineered muscle construct is a realistic goal for the near future.
While a variety of cell sources have been utilized in attempts to aid in skeletal muscle tissue regeneration following VML, Pax7+ muscle-derived satellite cells are the primary cell considered for muscle regeneration strategies. This positive Pax7 expression is considered the requisite marker for muscle cells' `stemness' and thus has been used to validate the muscle lineage of cells used for skeletal tissue regeneration experiments [30,31]. In addition to the satellite cell, the microenvironment of the stem cell, the stem cell niche, is highly specific and is composed of the stem cells themselves, extracellular matrix (ECM), vascular and neural networks, different types of surrounding cells, and various diffusible molecules (reviewed by [32,33]. Several important factors (e.g., IGF-1, FGF, HGF and matrix metalloproteinases), have all demonstrated an ability to alter the niche and thus myogenic function. Even brief periods of satellite cell growth in vitro leads to a marked reduction in proliferative and renewal capacity in vivo [30], suggesting the signals that maintain satellite cell and satellite cell niche communication are also temporally sensitive. It is thus challenging as many of the complex and dynamic external and internal niche factors are still being identified; as such, engineered muscle tissue and other muscle-derived interventions must be cognizant of their interactions within the niche itself to optimize biocompatibility and functional success.
While the satellite cell is the primary stem cell population in muscle, other progenitors reside within the muscle tissue (as reviewed by [34–36]). For example, mesoangioblasts, pericytes, fibroadipogenic progenitors, Pax3+, Sk-34, CD45+/Sca1+ and PW1+/Pax7− interstitial cells have all been implicated in contributing to regeneration and repopulation of the satellite cell pool. These heterogeneous cell populations all possess the capacity to influence the myogenic lineage and/or the local stem cell environment. For example, unlike satellite cells, mesenchymal progenitors when transplanted demonstrate little or no contribution to myogenic or regenerative capacity, yet have been shown to promote myotube formation and differentiation in co-culture [37].
Although satellite cells are the most well characterized for tissue engineering applications, maintaining their stem cell-like properties in vitro is challenging; in addition a significant number of satellite cells is required. As such, other cell sources have been investigated for muscle engineering purposes. For example, Rossi et al. actually compared the use of satellite cells and the more abundant muscle progenitor cells (MPC) on hydrogel scaffolds for tissue engineering [38]. Unfortunately the results demonstrated that MPCs yielded inadequate regeneration and limited contribution to muscle regeneration. Vessel-associated progenitor cells, like meso angio blasts and pericytes, have also been utilized as muscle engineering cell sources. Fuoco et al. delivered mesoangioblasts with an injectable polyethylene glycol–fibrinogen hydrogel adjuvant and demonstrated enhanced cell engraftment in acute and chronic muscle degeneration repair [39]. Adipose tissue-derived stromal cells have advantages in tissue engineering as adipose tissue is not typically limiting and have the potential to differentiate into a skeletal muscle lineage. Kim et al. utilized injectable poly(lactic-co-glycolic acid) sphere scaffolds to transplant adipose tissue-derived stromal cells to demonstrate their differentiation and in vivo muscle generation [40]. Other mesenchymal stem cells and nonmuscle-derived progenitors also have the potential for skeletal muscle tissue engineering, however, each has their own advantages and drawbacks (see [34,41,42]). The complexity of these varied cell populations as well as the influential local muscle environment highlights the necessity for a proficient and complete understanding of satellite cells and the other multiple cell types to develop efficacious therapies involving engineered tissue.
A plethora of approaches have been employed to culture muscle precursor cells into muscle tissue with the capacity to functionally restore muscle deficits [5,8–11,21,23]. Three primary tissue engineering technologies exist for the fabrication of such engineered muscle tissue: assembled scaffolds of synthetic polymers, naturally occurring ECM proteins of collagen or fibrin, or micropatterned surfaces; acellularized tissue ECMs; and scaffold-free (or self-assembled) tissue engineering. Scaffold-free technologies utilize fibroblasts obtained from skeletal muscle co-cultured with muscle satellite cells to create cell monolayers that self-assemble into 3D constructs [17,19,21,26,43,44]. Our scaffoldless tissue engineered muscle constructs are unique in that they are co-cultured with engineered tendon constructs and thus are fabricated with functional tendon ends [11,19,23,45,46].
In keeping with the limited therapeutic modalities currently available for VML, functional outcomes of VML treatment could be improved through the development of successful tissue engineered skeletal muscle technologies. Fabrication of small functional muscle tissue constructs has been accomplished via scaffold and scaffold-free approaches [5,9,10,13,21,45]. The success of a tissue engineered skeletal muscle technology as a clinically relevant treatment for VML will require constructs which are mechanically robust enough for implantation, are fabricated from cells that do not generate an immunogenic response, and are competent to direct muscle regeneration and in vitro culture conditions that replicate the skeletal muscle microenvironment to direct myogenesis within the construct. These challenges are shared among all skeletal muscle tissue engineering approaches, therefore this review will focus on the two most significant challenges in engineering clinically relevant constructs for VML repair: the ability of the technology to provide a microenvironment on which satellite cells are able to thrive, grow and differentiate during the regeneration of muscle tissue; and the ability of the technology to be scaled up to produce muscle tissue large enough to replace clinically relevant volumes of tissue. The purpose of this review is to highlight the many approaches to skeletal muscle tissue engineering with emphasis on their utility as effective tools for the treatment of VML.
Synthetic scaffolds
Bioabsorbable synthetic scaffolds have been designed to provide temporary support for muscle regrowth, allowing myofibroblasts time to rebuild native ECM [4,5,8,9,11,22,47]. These synthetic scaffolds are fabricated with three promising technologies: electrospinning, micropatterning and gel-based scaffolds. Electrospinning uses synthetic polymers to create uniformly aligned and densely packed fibrous matrices resembling skeletal muscle ECM by accelerating charged polymer strands to a collector drum. These fibrous matrices have been fabricated of one or more biocompatible polymers and promote cell adhesion, nutrient diffusion, cell proliferation and have favorable mechanical properties [8,47,48]. Furthermore, the structural protein collagen can be electrospun to create fibrous matrices independently or in conjunction with precision molded or machined surfaces [49–52]. The second technology, micro-patterned substrates, is fabricated by molding or printing liquid polymer matrix material into nanoscale channels designed to align myotubes and promote cell fusion. These microfabricated hydrogel substrates allow for finely tuned special features, such as grooves and wells with specific placement of proteins to direct cell attachment, proliferation and fate [53,54]. Finally, gel-based scaffolds of fibrin, collagen, and other ECM proteins are seeded with MPCs in their commercially available gel form [3,17,19,46], or can be suspended into matrices with a determined pore size by cryogelation [18,55].
Synthetic scaffolds have several distinct advantages that may be useful for engineering a clinically viable tissue engineered construct. Synthetic scaffolds fabricated by electrospinning, micropatterning or in gel form can be used to fabricate large, shape-specific scaffolds for repair of large muscle defects (centimeters in diameter), are relatively cheap to produce, and are not limited by donor tissue availability. Synthetic scaffolds can be fabricated with ideal pore sizes, mechanical stiffness and nanoscale contours to promote satellite cell adhesion and migration within the scaffold. In addition, parallel alignment of regenerating muscle is essential for optimal force production. Thus, the ability to fabricate scaffolds that promote the parallel alignment of the proliferating muscle precursor cells is very advantageous as muscle cells must be aligned in the direction of eventual force production for optimal muscle functionality. While this technology holds a lot of promise for the future of tissue engineering, synthetic scaffolds have several disadvantages currently limiting their use in VML repair. First, utilization of a preformed rigid scaffold has the potential to interfere with and reduce muscle force production by inhibiting myofiber force transmission to tendon ends [11]. A second issue that arises with synthetic scaffolds is low cellular adhesion and proliferation during construct formation. Despite these limitations, synthetic scaffolds remain the most studied technology in skeletal muscle tissue engineering [3,8,11,47].
Electrospun scaffolds
Electrospinning organizes biocompatible polymers into thin sheets of fibrous meshes from a wide variety of biocompatible polymers and is a cost-effective, rapid technique which allows for manipulations of scaffold pore size, matrix density, and fiber diameter and composition [8–10,47,48]. These are all important for muscle tissue engineering because they all impact satellite cell adhesion, migration, differentiation and myotube alignment [26,47,56,57]. Electrospun scaffolds are fabricated using biodegradable polymers such as polylactic acid or polycaprolactone, or ECM proteins, such as collagen [8,9,46,47,51,56]. A successful scaffold will not only degrade as the native tissue regrows, but will also facilitate myogenesis in the scaffold. Because of their low cost, quick time of fabrication, the size of resulting scaffolds and the ability to manipulate parameters important for satellite cell adhesion and myotube formation, electrospun scaffolds are an attractive tissue engineering approach to VML restoration.
Collagen electrospun scaffolds seeded with C2C12 cells were implanted in the gastrocnemius of mice, resulting in slow degradation of the scaffold, which became vascularized, and facilitated the regeneration of muscle fibers [46]. These constructs, however, had low biocompatibility, necessitating the use of immune-deficient mice to complete the study. In our review of the literature, this is the only study reporting the utilization of an electrospun tissue construct to repair skeletal muscle. Several studies have been published which test biocompatibility in vitro, but cellular proliferation is low in comparison with native protein-based scaffolds, acellularized organs or scaffold-free approaches [9,21,47]. While some research has focused on electrospinning meshes of collagen and elastin for use in tissue engineering, further research into biocompatible materials for successful electrospun scaffold fabrication must be done to improve cellular proliferation, migration and myotube formation, and reduce immune rejection.
Micropatterned scaffolds
A second approach to synthetic scaffold engineering is micropatterning synthetic polymer scaffolds onto which cells are seeded. By providing a directed, contoured structure, micropatterned surfaces are able to provide a greater surface area for cell attachment, allowing for more focal adhesions, which enhance cellular migration, proliferation and differentiation compared with a standard tissue culture plate [5,17,57–59]. Substrates are molded into channels or grooves and subsequently seeded with satellite or C2C12 cells, which are then allowed to differentiate and form networked monolayer of aligned myotubes [11,57,59,60]. Micropatterned substrates themselves cannot be implanted, as this would require removal of the monolayers from the substrate, which is difficult and damaging to the cells [2,5,6]. Currently, monolayers can be removed from micropatterned substrates through the use of a biodegradable mandrel [21,57], but this difficult process still results in very small constructs and remains a significant limitation [26,57]. Some studies have focused on transferring monolayers from micropatterned substrates to biodegradable mandrels or hydrogels for use in tissue repair [57,60,61]. While the use of micropatterned scaffolds for tissue repair is limited, these scaffolds are being used successfully to assess the effects of various nanoscale contours on myotube fusion and alignment [5,11,27,57,59,60,62–64]. The alignment of myotubes is essential for muscle tissue engineering, as parallel alignment of myotubes will allow the construct to generate the greatest force when implanted. Constructs generating larger forces will facilitate a better repair outcome when implanted for VML injuries. However, a significant limitation to this technology is scaling the micropatterned scaffold up to a clinically relevant size for utilization for VML repair. Future ideas will need to be developed to determine ways of fabricating these micro-patterned layers into larger 3D structures that will allow for successful VML repair.
Protein-based scaffolds
Several ECM proteins have been adopted for scaffold fabrication for skeletal muscle tissue engineering. Fibrin, collagen and other ECM proteins are used to form scaffolds in their commercially available gel forms or can be suspended into matrices with a determined pore size by cryogelation [3,55,65]. Fibrin gels have been studied extensively as a native scaffold in gels and preformed microfibers for musculoskeletal tissue engineering [18,45,65]. Recently, the cryogelation technique has been developed to fabricate specific scaffold pore sizes while using native proteins [19,55] by slowly freezing an aqueous protein solution. One study used fibrin-based scaffolds seeded with adult human myoblasts in vitro and then implanted the construct in a mouse partial-thickness tibialis anterior muscle injury [3]. A total of 10 weeks after implantation, skeletal muscle fibers were observed histologically, and mean tetanic force of the tibialis anterior had recovered to approximately 90% of uninjured values. The skeletal muscle constructs formed using fibrin-gel scaffolds showed rapid proliferation and differentiation of satellite cells, and allow for an influx of host satellite cells when implanted in mouse tibialis anterior [3,65].
Immune rejection remains the biggest limitation of protein-based scaffolds, requiring many implantation studies to use a severe combined immunodeficiency mouse [46,66]. In addition, scale-up of protein-based scaffolds is a major limitation. Constructs produced using protein-based scaffolds are small due to the limited diffusion of oxygen and other nutrients, with diameters less than 200 μm, requiring up to ten constructs to replace 30 mg of resected tissue [3,65]. Constructs of this size are suitable to replace small volumetric deficits in rodent models, but integration of a vascular network in vitro will be required to fabricate larger implants. Finally, achieving parallel alignment of myotubes is essential for muscle functionality, so constructs formed on scaffolds of this type must use other means to align myotubes. Thus, ECM protein-based scaffolds provide an excellent microenvironment for satellite cell proliferation and myotube formation, but are currently limited by small size and complications with immune rejection.
In conclusion, research on synthetic scaffolds demonstrates that modification of the physical microenvironment of muscle satellite cells can direct them into aligned structures, which have a muscle-like morphology when assessed by myosin immunohistochemistry [9,56]. Electrospinning, micropatterning and protein-based synthetic scaffolds have all been shown to promote cell adhesion and migration, which are essential for muscle growth and differentiation [58,67,68]. These studies collectively demonstrate that electrospinning, micropatterning or protein-based assembled scaffolds are able to direct muscle satellite cells and C2C12 cells into forming monolayers of networked and fused myotubes [8,47,57]. Because of the adaptability of these various technologies, some laboratories are beginning to combine micropatterning and electrospinning [50] to produce the precise topographical features to promote cell orientation, proliferation, and differentiation. Hybrid hydrogel scaffolds, which are fabricated by chemically crosslinking hydrogel substrates with structural proteins such as fibrinogen or chitosan, have also been fabricated to better reproduce the topographical and biochemical niche to promote cellular viability, differentiation, and alignment [69–71]. Due to the ability to carefully control the structure and composition of synthetic scaffolds, electrospun, micropatterned and protein-based scaffolds are a promising approach to skeletal muscle tissue engineering for VML functional repair.
Acellularized tissue scaffolds
Acellularized tissue scaffolds are a promising approach for engineering tissue replacements for repair of VML injuries. Acellularized tissue scaffolds are attractive because they offer a preformed, native ECM, which can be either preseeded with satellite cells, or treated to attract endogenous satellite cells. The native microenvironment and mechanical properties are similar to the tissue being repaired [23,72–75], allowing for optimal satellite cell adhesion and migration [58,73,74], which are essential in myogenesis. Additionally, acellularization of tissue conserves the architectural features of the tissue, such as the vascular bed [72], which are features other implanted tissue constructs would have to develop after implantation. When implanted, acellularized scaffold constructs integrate readily with endogenous tissue, and demonstrate early signs of neoangiogenesis [23].
Recent work has demonstrated that there is no significant benefit to using skeletal muscle tissue for creating the acellular matrix for use in muscle repair [73]. Acellularized quadriceps, hamstring and intestine-derived scaffolds were seeded with C2C12 cells and implanted in the abdominal wall of rat (external and internal oblique) in a VML injury model. Thirty-five days after implantation, intestine-derived scaffolds had more completely disintegrated than the muscle-based scaffolds and myosin heavy chain immunohistochemistry revealed that muscle fiber structure had been restored using either scaffold, but functional outcomes were not assessed. Another study using acellularized bladder as scaffolds implanted into a nude mouse latissimus dorsi VML injury model demonstrated the ability to restore tetanic isometric force generation to 70% of the amount generated when uninjured [23,76].
Acellularized tissue scaffolds have some disadvantages in their clinical utility as a method for VML repair. Scale-up to a size useful for VML in larger muscles remains a difficulty, as myoblasts cannot proliferate and differentiate more than 150 μm from a nutrient source [43]. Utilization of larger acellularized tissue often results in a necrotic core and restricts the overall size of the acellularized tissue that can be implanted [43,77]. New in vitro studies suggest that vascular networks can be formed in vitro in scaffold-free constructs, potentially alleviating the in vitro and eventual in vivo issues that arise with diffusion [78]. One way to facilitate satellite cell migration through a matrix would be to increase the matrix pore size, but it is more difficult to fine-tune matrix pore size, ECM stiffness and composition in an acellularized tissue scaffold compared with a synthetic scaffold because the structure of the scaffold depends on the starting tissue. Another limitation of acellularized tissue scaffolds is the limited donor tissue available for acellularization, and the potential for immune rejection from an allogenic tissue source. Acellularization of adult tissue is a promising approach for fabrication of constructs appropriate to restore function in VML injuries because of the mature ECM structure acellularized tissue scaffolds offer for skeletal muscle tissue engineering. Future studies into vascularization of these larger acellularized tissues must be accomplished to make this technology useful for large VML repairs in humans.
Scaffold-free technologies
Scaffold-free tissue engineering technologies do not use a preformed scaffold, and instead rely on satellite cells and fibroblasts in co-culture to self-assemble into cylindrical muscle constructs [17–21,26,44,78]. Fibroblasts obtained from muscle that were placed in the co-culture system assemble an ECM in vitro, and provide support to satellite cells as they proliferate and form myotubes. Constructs assembled in this way have parallel myotubes aligned axially between two constraint anchors placed during monolayer roll-up. The muscle monolayer will interface with engineered tendon to form functional myotendinous junctions and tendon ends in vitro, resulting in the fabrication of a skeletal muscle tissue construct that replicates the structure of skeletal muscle in vivo.
These scaffold-free muscle constructs form functional interfaces with nerve tissue as well, and quickly reorganizes when implanted in the biceps femoris in F344 rats [17,21]. The scaffold-free constructs were implanted for 1 week at an ectopic site to assess the effect of an in vivo environment on the tissue constructs. These 3D muscle constructs were implanted ectopically along the biceps femoris muscle, were anchored at the biceps femoris tendon ends and a section of the sciatic nerve routed to the construct for innervation. Thus, the implanted constructs moved dynamically with the endogenous muscle. After 1 week in vivo, the construct showed advances in the phenotype indicated by the development of an epimysium-like outer layer of connective tissue and an increase in myosin heavy chain content. Analysis of cross-sections of the constructs showed 20.2% more myofiber staining in constructs than in in vitro controls. Implanted muscle constructs showed a developing capillary system and α-bungarotoxin clustering suggestive of neuromuscular junctions. Longitudinal sections of the explanted muscle stained with α-actinin showed the presence of the developed sarcomeres. In addition, the engineered muscle constructs increased in contractile strength with an average maximum isometric force increase from 127 to 445 μN, and an average maximum specific force increase from 1075 to 1630 Pa. The explanted constructs had maximum isometric force increases of 245% compared with in vitro controls that only increased by 25% [1]. Additionally, no signs of immune rejection were observed in or around the tissue construct [21].
In a separate study, scaffold-free constructs implanted in mice demonstrated histological continuity 2 weeks after implantation, and hindlimb grip strength was significantly improved by the implantation of scaffold-free constructs in place of the extensor digitorum longus muscle [78]. Scaffold-free constructs are biocompatible, as all components of the finished construct are naturally occurring in vivo, and constructs may be fabricated from an autogenic cell source, reducing the risk of immune rejection. Scaffold-free constructs quickly reorganize when implanted to form advanced muscle characteristics including a capillary system, an epimysium-like layer of connective tissue, and an increase in myofiber content. The constructs can be readily innervated with a branch of a neighboring nerve.
While the scaffold-free technology provides an excellent microenvironment for satellite cell growth and myotube formation, scale-up of scaffold-free tissue constructs remains a limitation. Dennis and Kosnik showed that myoblasts cannot proliferate and differentiate more than 150 μm away from a source of oxygen and other nutrients, which limits the cross-sectional area that scaffold-free constructs can develop in vitro without a functional vascular network [43]. Development of a vascular network in vitro may resolve this limitation. In fact, a new study by Carosio et al. demonstrated that the earliest forms of a vascular network can be formed in vitro in scaffold-free constructs, potentially alleviating the in vitro and eventual in vivo issues that arise with diffusion [78]. An additional limitation towards the success of this technology lies in the time required for myoblasts and fibroblasts to assemble an ECM robust enough for construct formation. Currently, it takes 3–4 weeks to grow an implantable muscle-tendon construct in vitro. If autogenic constructs are to be used for VML repair, nearly a month would pass between obtaining a tissue biopsy and implantation of finished constructs to restore VML. Therefore, scaffold-free constructs may be more appropriate for chronic reconstruction rather than acute repair of traumatic injury. Despite these current limitations, scaffold-free technologies have been used to fabricate multiphasic constructs of muscle–tendon–bone and bone–ligament used successfully as skeletal tissue replacements tissues for VML in small rodent models and ligament in large animal models [18,21,26,79].
Future perspective
The field of tissue engineering has many promising strategies for the creation of functional skeletal muscle in vitro. However, there are several limitations that prevent it from becoming a solution for treating VML. First, engineered muscle tissue must be biocompatible to facilitate muscle tissue regrowth without generating an immune response. Second, engineered muscle constructs must be scaled up to facilitate replacement of clinically relevant volumes of tissue (centimeters in diameter). There are currently no tissue engineering strategies to produce tissue constructs that are both biocompatible and large enough to facilitate clinical repair. In addition, current technologies clearly demonstrate that only small gains in construct contractility can be achieved through manipulation of the tissue culture process [26], and that the largest gains are demonstrated when constructs are implanted [21]. Significant work has been done to replicate the in vivo environment during the fabrication of tissue constructs using electrical stimulation [9,11,80,81], growth factors [26], substrate stiffness [58,82] and oxygen levels [27] to improve construct structure and function in vitro; however, the body remains the most complete bioreactor available with significant gains seen after skeletal muscle constructs are implanted for as little as 1 week [21]. The future direction for skeletal muscle tissue engineering may be to focus more on effective ways of implanting these tissues in a VML repair model rather than trying to enhance the phenotype of constructs in vitro.
Many exciting and promising technologies are being developed to enhance progenitor cell adhesion, proliferation, differentiation and alignment through careful engineering of scaffolds designed to replicate the endogenous microenvironment. Future development of synthetic scaffolds should focus on continued development of protein-based electrospun constructs, and implantation of these constructs in VML injury models [46,47,49]. Hybrid micropatterned and electrospun substrates are being adapted to promote the growth and differentiation of MPCs, while studies using modified hydrogels are incorporating structural proteins such as fibrin and chitosan to better replicate the progenitor cell niche in vitro [50,69,70]. Future studies using native protein-based scaffolds should focus on shaping these technologies for use in skeletal muscle VML injury models. Acellularized organ-based scaffolds should continue to focus on developing methods to produce larger constructs by introducing technologies such as a functional in vitro vascular networks as carried out by Carosio et al. [23,76,78,83].
Thus, fabrication of engineered skeletal muscle tissue initiated in vitro must first overcome the two limitations described above: constructs must be biocompatible and scaled up to clinically relevant sizes. To address the biocompatibility obstacle, scaffold-free tissue engineered skeletal muscle constructs are fabricated from an autogenic cell source, eliminating the potential for immune rejection. The biocompatibility of our scaffold-free engineered muscle tissue was demonstrated when our constructs were implanted for 1 week into an ectopic site and showed no evidence of immune rejection, as indicated by a lack of macrophage and neutrophil infiltration [21]. Implantation of scaffold-free constructs near the tibialis anterior muscle demonstrated histological continuity when implanted in mice [78]. While these acute studies are promising, longer-term experiments that investigate biocompatibility and functionality of the constructs must be done.
The most significant limitation remaining for a scaffold-free approach is the ability to scale constructs up to a size appropriate for large tissue replacement. While synthetic and acellular scaffolds may be large in size, many are still limited by a lack of adequate nutrients in the core of the construct. In static in vitro culture, scaffold-free constructs larger than 800 μm in diameter cannot be fabricated, due to limited nutrient availability at the core of the construct. To address this issue, several laboratories are focusing on the development of a functional vascular system in vitro, which would allow fabrication of larger constructs [84–87]. Other laboratories have focused on selecting more appropriate biomaterials to promote cellular proliferation and differentiation [53,58,88]. One approach our laboratory has taken to address the problem of construct scale-up is multiple construct fusion (Figure 2). In the fabrication of tissue for ligament repair models, we observed that constructs over 1 mm in diameter can be fabricated using multiple constructs 1 mm in diameter and aligning them in parallel just prior to implantation [18]. Using this technique, we find that the constructs fuse together longitudinally creating a construct many millimeters wide but only 1 mm thick. Just prior to implantation, these flat constructs are rolled into a cylinder with a diameter of approximately 4–5 mm (Figure 3). Future studies will validate the application of this approach to scaffold-free skeletal muscle to produce large constructs appropriate for repair of VML injuries.
Figure 2. Model for parallel fusion of constructs to fabricate large-diameter muscle tissue.
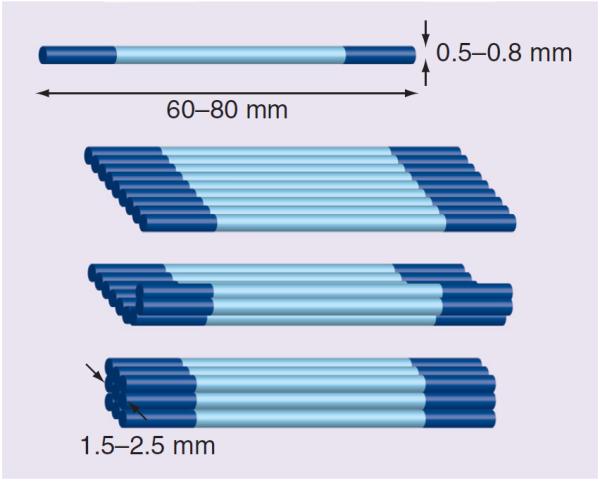
Small in vitro constructs (0.5–0.8 mm in diameter) are lined up in parallel and allowed to fuse to form a muscle construct width of 2.5 mm and a thickness of 1.5 mm.
Figure 3. Utilization of large tissue culture plates to increase construct size.

Construct length is determined by the size of the tissue culture plate the monolayer is fabricated in. Use of larger culture plates will allow for fabrication of muscle constructs up to 18 cm in length. The construct shown was cultured in a square culture plate, allowing the construct to form along the diagonal to form constructs in excess of 15 cm.
A second approach our laboratory has taken to address the issue of scale-up is to increase construct size through the use of larger culture plates. In our hands, construct size is limited radially by diffusion of oxygen and other nutrients, but length is only limited by the size of the sterile culture plate available for monolayer formation. Published work from our laboratory has produced larger constructs through the use of 60 mm tissue culture plates [21]. Furthermore, unpublished pilot studies from our laboratory have demonstrated that larger constructs can be fabricated by using 100 and 150 mm tissue culture plates to fabricate constructs 1 mm in diameter, and 15 cm in length. These constructs can be aligned in parallel to fuse into a sheet of constructs 5 mm in width and 1 mm in thickness before being rolled into a cylindrical construct just prior to implantation (Figure 3). Cylindrical constructs formed using this technique are up to 1 cm in diameter and over 15 cm in length. In the future, our laboratory plans to overcome the limitation of length of construct scale-up by having even larger customized sterile tissue culture plates fabricated for our technologies.
In conclusion, scaffold-free engineered skeletal muscle is biocompatible, autogenic and rapidly remodels when implanted. Currently, the major limitation for scaffold-free engineered muscle is the scale-up of constructs to clinically relevant volumes of tissue. Leveraging current technologies in our laboratory for the fabrication of engineered ligament, we will produce muscle constructs large enough to use our scaffold-free approach for reconstruction of VML in a large animal facial muscle model.
Executive summary.
Synthetic scaffolds provide a defined structure for satellite cell growth & differentiation
-
■
Electrospun scaffolds of biocompatible polymers may be rapidly and reproducibly fabricated, but are limited by poor biocompatibility.
-
■
Micropatterning provides an optimum 2D structure for satellite cell motility and myoblast fusion, but is limited in its ability to form 3D constructs.
-
■
Protein-based scaffolds have been developed that are able to restore some volumetric muscle loss (VML) model injuries, but are complicated by poor biocompatibility and limited by size.
Acellularized scaffolds retain structural features of adult extracellular matrix
-
■
Acellular tissue scaffolds have been fabricated from skeletal muscle, bladder, and intestinal extracellular matrix, but cannot be easily customized for the specific needs of a VML injury.
Scaffold-free technologies enable fabrication of tissues tailor-made for a specific application
-
■
Scaffold-free engineered tissues form functional interfaces with tendons and nerves, and remodel quickly when implanted ectopically. These self-assembled tissues remain limited by their small size.
Conclusion
-
■
Tissue engineered muscle must be biocompatible to facilitate muscle regrowth, and must be scaled up to a clinically relevant size.
-
■
Synthetic scaffold, acellularized scaffold and scaffold-free approaches have been developed to fabricate tissues in vitro with the goal of repairing VML injuries.
-
■
Significant work has been done to enhance construct structure and function in vitro, but the largest advances have been observed when constructs are implanted.
Acknowledgments
This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Financial & competing interests disclosure The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell. Dev. Biol. 1988;24(3):166–174. doi: 10.1007/BF02623542. [DOI] [PubMed] [Google Scholar]
- 2.Ninagawa NT, Isobe E, Hirayama Y, et al. Transplantated mesenchymal stem cells derived from embryonic stem cells promote muscle regeneration and accelerate functional recovery of injured skeletal muscle. Biores. Open Access. 2013;2(4):295–306. doi: 10.1089/biores.2013.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page RL, Malcuit C, Vilner L, et al. Restoration of skeletal muscle defects with adult human cells delivered on fibrin microthreads. Tissue Eng. Part A. 2011;17(21–22):2629–2640. doi: 10.1089/ten.TEA.2011.0024. [DOI] [PubMed] [Google Scholar]
- 4.Chuang D. Free tissue transfer for the treatment of facial paralysis. Facial Plast. Surg. 2008;24(2):194–203. doi: 10.1055/s-2008-1075834. [DOI] [PubMed] [Google Scholar]
- 5.Koning M, Harmsen MC, van Luyn MJA, Werker PMN. Current opportunities and challenges in skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2009;3(6):407–415. doi: 10.1002/term.190. [DOI] [PubMed] [Google Scholar]; ■■ Discusses the current challenges faced by skeletal muscle tissue engineering technologies and gives a concise review of the current state of the art in tissue engineering of skeletal muscle and the opportunities and challenges for future clinical applicability.
- 6.Grogan BF, Hsu JR. Skeletal Trauma Research Consortium. Volumetric muscle loss. J. Am. Acad. Orthop. Surg. 2011;19(Suppl. 1):S35–S37. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]; ■ Review on the current challenges and treatments for volumetric muscle loss.
- 7.Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2011;347(3):759–774. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]; ■■ Excellent article on the regeneration of skeletal muscle. The three stages of skeletal muscle regeneration and the potential pitfalls in the development of regenerative medicine strategies for the restoration of functional skeletal muscle in situ are discussed.
- 8.Ladd MR, Lee SJ, Stitzel JD, Atala A, Yoo JJ. Co-electrospun dual scaffolding system with potential for muscle–tendon junction tissue engineering. Biomaterials. 2011;32(6):1549–1559. doi: 10.1016/j.biomaterials.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 9.McKeon-Fischer KD, Freeman JW. Characterization of electrospun poly (l-lactide) and gold nanoparticle composite scaffolds for skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2010;5(7):560–568. doi: 10.1002/term.348. [DOI] [PubMed] [Google Scholar]
- 10.Klumpp D, Horch RE, Kneser U, Beier JP. Engineering skeletal muscle tissue – new perspectives in vitro and in vivo. J. Cell. Mol. Med. 2010;14(11):2622–2629. doi: 10.1111/j.1582-4934.2010.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed LE, Guilak F, Guo XE, et al. Advanced tools for tissue engineering: scaffolds, bioreactors, and signaling. Tissue Eng. 2006;12(12):3285–3305. doi: 10.1089/ten.2006.12.3285. [DOI] [PubMed] [Google Scholar]; ■■ Excellent review from the top researchers in the area of tissue engineering. Contains the collective views expressed at the second session of the workshop `Tissue Engineering: The Next Generation', which was devoted to the tools of tissue engineering: scaffolds, bioreactors, and molecular and physical signaling.
- 12.Siemionow M, Papay F, Alam D, et al. Near-total human face transplantation for a severely disfigured patient in the USA. Lancet. 2009;374(9685):203–209. doi: 10.1016/S0140-6736(09)61155-7. [DOI] [PubMed] [Google Scholar]
- 13.Dubernard JM, Lengelé B, Morelon E, et al. Outcomes 18 months after the first human partial face transplantation. N. Engl. J. Med. 2007;357(24):2451–2460. doi: 10.1056/NEJMoa072828. [DOI] [PubMed] [Google Scholar]
- 14.Levi DM, Tzakis AG, Kato T, et al. Transplantation of the abdominal wall. Lancet. 2003;361(9376):2173–2176. doi: 10.1016/S0140-6736(03)13769-5. [DOI] [PubMed] [Google Scholar]
- 15.Strome M, Stein J, Esclamado R, et al. Laryngeal transplantation and 40-month follow-up. N. Engl. J. Med. 2001;344(22):1676–1679. doi: 10.1056/NEJM200105313442204. [DOI] [PubMed] [Google Scholar]
- 16.Jones JW, Gruber SA, Barker JH, Breidenbach WC. Successful hand transplantation. One-year follow-up. Louisville Hand Transplant Team. N. Engl. J. Med. 2000;343(7):468–473. doi: 10.1056/NEJM200008173430704. [DOI] [PubMed] [Google Scholar]
- 17.Larkin LM, Van der Meulen JH, Dennis RG, Kennedy JB. Functional evaluation of nerve–skeletal muscle constructs engineered in vitro. In Vitro Cell. Dev. Biol. Anim. 2006;42(3):75–82. doi: 10.1290/0509064.1. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Smietana MJ, Kostrominova TY, Wojtys EM, Larkin LM, Arruda EM. Engineered bone–ligament–bone constructs for anterior cruciate ligament replacement. Tissue Eng. Part A. 2012;18(1–2):103–116. doi: 10.1089/ten.tea.2011.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin LM, Calve S, Kostrominova TY, Arruda EM. Structure and functional evaluation of tendon–skeletal muscle constructs engineered in vitro. Tissue Eng. 2006;12(11):3149–3158. doi: 10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baltich J, Hatch-Vallier L, Adams AM, Arruda EM, Larkin LM. Development of a scaffoldless engineered nerve using a nerve–fibroblast co-culture. In Vitro Cell. Dev. Biol. Anim. 2010;46(5):438–444. doi: 10.1007/s11626-009-9260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams ML, Kostrominova TY, Arruda EM, Larkin LM. Effect of implantation on engineered skeletal muscle constructs. J. Tissue Eng. Regen. Med. 2013;7(6):434–442. doi: 10.1002/term.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation – how much of the promise has been realized? Nat. Med. 2005;11(6):605–613. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- 23.Machingal MA, Corona BT, Walters TJ, et al. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng. Part A. 2011;17(17–18):2291–2303. doi: 10.1089/ten.tea.2010.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sah RL, Ratcliffe A. Translational models for musculoskeletal tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 2010;16(1):1–3. doi: 10.1089/ten.teb.2009.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Describes the utility of various translational models for engineered tissues and regenerative medicine therapies. Provides an effective and succinct summary of the status of various animal models in musculoskeletal regenerative medicine.
- 25.Monge C, Ren K, Berton K, Guillot R, Peyrade D, Picart C. Engineering muscle tissues on microstructured polyelectrolyte multilayer films. Tissue Eng. Part A. 2012;18(15–16):1664–1676. doi: 10.1089/ten.tea.2012.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weist MR, Wellington MS, Bermudez JE, et al. TGF-β1 enhances contractility in engineered skeletal muscle. J. Tissue Eng. Regen. Med. 2013;7(7):562–571. doi: 10.1002/term.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koning M, Werker PM, van Luyn MJ, Harmsen MC. Hypoxia promotes proliferation of human myogenic satellite cells: a potential benefactor in tissue engineering of skeletal muscle. Tissue Eng. Part A. 2011;17(13–14):1747–1758. doi: 10.1089/ten.tea.2010.0624. [DOI] [PubMed] [Google Scholar]
- 28.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 2001;91(2):534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]; ■ Highlights the origin and markers of the satellite cell population, regulation by growth factors, and response to physiological and pathological stimuli. Includes potential therapeutic uses of satellite cells and identifies future research areas for satellite cell biology.
- 29.Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J. Histochem. Cytochem. 2011;59(1):33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montarras D. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309(5743):2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 31.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456(7221):502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Discusses some of the recent advances on the functions of satellite cells and their niche during the process of skeletal muscle regeneration.
- 33.Pannérec A, Marazzi G, Sassoon D. Stem cells in the hood: the skeletal muscle niche. Trends Mol. Med. 2012;18(10):599–606. doi: 10.1016/j.molmed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Judson RN, Zhang RH, Rossi FMA. Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle: collaborators or saboteurs? FEBS J. 2013;280(17):4100–4108. doi: 10.1111/febs.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J. Clin. Invest. 2010;120(1):11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 2006;54(11):1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 37.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12(2):153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi CA, Flaibani M, Blaauw B, et al. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011;25(7):2296–2304. doi: 10.1096/fj.10-174755. [DOI] [PubMed] [Google Scholar]
- 39.Fuoco C, Salvatori ML, Biondo A, et al. Injectable polyethylene glycol–fibrinogen hydrogel adjuvant improves survival and differentiation of transplanted mesoangioblasts in acute and chronic skeletal-muscle degeneration. Skeletal Muscle. 2012;2(1):24. doi: 10.1186/2044-5040-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim M, Choi YS, Yang SH, et al. Muscle regeneration by adipose tissue-derived adult stem cells attached to injectable PLGA spheres. Biochem. Biophys. Res. Commun. 2006;348(2):386–392. doi: 10.1016/j.bbrc.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 41.Fishman JM, Tyraskis A, Maghsoudlou P, et al. Skeletal muscle tissue engineering: which cell to use? Tissue Eng. Part B Rev. 2013;19(6):503–515. doi: 10.1089/ten.TEB.2013.0120. [DOI] [PubMed] [Google Scholar]; ■ Provides an extensive review of various cell types considered for skeletal muscle tissue engineering and the factors that affect their usefulness in tissue regeneration strategies.
- 42.Rossi CA, Pozzobon M, De Coppi P. Advances in musculoskeletal tissue engineering: moving towards therapy. Organogenesis. 2010;6(3):167–172. doi: 10.4161/org.6.3.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dennis RG, Kosnik PE. Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell. Dev. Biol. Anim. 2000;36(5):1–10. doi: 10.1290/1071-2690(2000)036<0327:EAICPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Kostrominova TY, Calve S, Arruda EM, Larkin LM. Ultrastructure of myotendinous junctions in tendon–skeletal muscle constructs engineered in vitro. Histol. Histopathol. 2009;24(5):541–550. doi: 10.14670/hh-24.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao H, Zhou GQ. Development and progress of engineering of skeletal muscle tissue. Tissue Eng. Part B Rev. 2009;15(3):319–331. doi: 10.1089/ten.teb.2009.0092. [DOI] [PubMed] [Google Scholar]
- 46.Ma J, Holden K, Zhu J, Pan H, Li Y. The application of collagen-scaffolds seeded with myoblasts to repair skeletal muscle defects. J. Biomed. Biotechnol. 2011;2011:1–9. doi: 10.1155/2011/812135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aviss KJ, Gough JE, Downes S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur. Cell. Mater. 2010;19:193–204. doi: 10.22203/ecm.v019a19. [DOI] [PubMed] [Google Scholar]
- 48.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Walters BD, Stegemann JP. Strategies for directing the structure and function of collagen biomaterials across length scales. Acta Biomater. 2013:1–14. doi: 10.1016/j.actbio.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Wong YS, Wen F, et al. Human mesenchymal stem-cell behaviour on direct laser micropatterned electrospun scaffolds with hierarchical structures. Macromol. Biosci. 2012;13(3):299–310. doi: 10.1002/mabi.201200318. [DOI] [PubMed] [Google Scholar]
- 51.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27(5):724–734. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D. Fiber-based tissue engineering: progress, challenges, and opportunities. Biotechnol. Adv. 2013;31(5):669–687. doi: 10.1016/j.biotechadv.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lutolf MP, Doyonnas R, Havenstrite K, Koleckar K, Blau HM. Perturbation of single hematopoietic stem cell fates in artificial niches. Integr. Biol. 2009;1(1):59. doi: 10.1039/b815718a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elowsson L, Kirsebom H, Carmignac V, Durbeej M, Mattiasson B. Porous protein-based scaffolds prepared through freezing as potential scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 2012;23(10):2489–2498. doi: 10.1007/s10856-012-4713-4. [DOI] [PubMed] [Google Scholar]
- 56.Ku SH, Lee SH, Park CB. Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation. Biomaterials. 2012;33(26):6098–6104. doi: 10.1016/j.biomaterials.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 57.Huang NF, Lee RJ, Li S. Engineering of aligned skeletal muscle by micropatterning. Am. J. Transl. Res. 2010;2(1):43–55. [PMC free article] [PubMed] [Google Scholar]
- 58.Engler AJ. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang NF, Thakar RG, Wong M, Kim D, Lee RJ, Li S. Tissue engineering of muscle on micropatterned polymer films. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004;7:4966–4969. doi: 10.1109/IEMBS.2004.1404373. [DOI] [PubMed] [Google Scholar]
- 60.Huang NF, Patel S, Thakar RG, et al. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006;6(3):537–542. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- 61.Huang G, Li L, Wen Y. Functional reconstruction with tissue engineered myoblast in facial muscle of rat. Hua Xi Kou Qiang Yi Xue Za Zhi. 2003;21(6):432–434. [PubMed] [Google Scholar]
- 62.Millar NL, Reilly JH, Kerr SC, et al. Hypoxia: a critical regulator of early human tendinopathy. Ann. Rheum. Dis. 2012;71(2):302–310. doi: 10.1136/ard.2011.154229. [DOI] [PubMed] [Google Scholar]
- 63.Zhao J, Zhang P, Qin L, Pan XH. Hypoxia is essential for bone–tendon junction healing: the molecular biological evidence. Int. Orthop. 2011;35(6):925–928. doi: 10.1007/s00264-010-1157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Zhu L, Chen X, Fan M. Effects of hypoxia on proliferation and differentiation of myoblasts. Med. Hypotheses. 2007;69(3):629–636. doi: 10.1016/j.mehy.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 65.Huang YC, Dennis RG, Larkin L, Baar K. Rapid formation of functional muscle in vitro using fibrin gels. J. Appl. Physiol. 2005;98(2):706–713. doi: 10.1152/japplphysiol.00273.2004. [DOI] [PubMed] [Google Scholar]
- 66.Thorrez L, Vandenburgh H, Callewaert N, et al. Angiogenesis enhances factor IX delivery and persistence from retrievable human bioengineered muscle implants. Mol. Ther. 2006;14(3):442–451. doi: 10.1016/j.ymthe.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 67.Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS ONE. 2011;6(1):e15978. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hinds S, Bian W, Dennis RG, Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials. 2011;32(14):3575–3583. doi: 10.1016/j.biomaterials.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26(15):2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 70.Garnica-Palafox IM, Sánchez-Arévalo FM, Velasquillo C, et al. Mechanical and structural response of a hybrid hydrogel based on chitosan and poly (vinyl alcohol) cross-linked with epichlorohydrin for potential use in tissue engineering. J. Biomater. Sci. Polym. Ed. 2013:1–19. doi: 10.1080/09205063.2013.833441. [DOI] [PubMed] [Google Scholar]
- 71.Ding K, Yang Z, Zhang YL, Xu JZ. Injectable thermosensitive chitosan/β-glycerophosphate/collagen hydrogel maintains the plasticity of skeletal muscle satellite cells and supports their in vivo viability. Cell Biol. Int. 2013;37(9):977–987. doi: 10.1002/cbin.10123. [DOI] [PubMed] [Google Scholar]
- 72.Perniconi B, Costa A, Aulino P, Teodori L, Adamo S, Coletti D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials. 2011;32(31):7870–7882. doi: 10.1016/j.biomaterials.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 73.Wolf MT, Daly KA, Reing JE, Badylak SF. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials. 2012;33(10):2916–2925. doi: 10.1016/j.biomaterials.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mase VJ, Hsu JR, Wolf SE, et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33(7):511. doi: 10.3928/01477447-20100526-24. [DOI] [PubMed] [Google Scholar]
- 75.Cozad MJ, Bachman SL, Grant SA. Assessment of decellularized porcine diaphragm conjugated with gold nanomaterials as a tissue scaffold for wound healing. J. Biomed. Mater. Res. A. 2011;99(3):426–434. doi: 10.1002/jbm.a.33182. [DOI] [PubMed] [Google Scholar]
- 76.Corona BT, Machingal MA, Criswell T, et al. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng. Part A. 2012;18(11–12):1213–1228. doi: 10.1089/ten.tea.2011.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borschel GH, Dennis RG, Kuzon WM. Contractile skeletal muscle tissue-engineered on an acellular scaffold. Plast. Reconstr. Surg. 2004;113(2):595–602. doi: 10.1097/01.PRS.0000101064.62289.2F. [DOI] [PubMed] [Google Scholar]
- 78.Carosio S, Barberi L, Rizzuto E, Nicoletti C, Del Prete Z, Musaro A. Generation of ex vivo-vascularized muscle engineered tissue (X-MET) Sci. Rep. 2013;3:1420. doi: 10.1038/srep01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smietana MJ, Syed-Picard FN, Ma J, Kostrominova T, Arruda EM, Larkin LM. The effect of implantation on scaffoldless engineered bone constructs. In Vitro Cell. Dev. Biol. Anim. 2009;45(9):512–522. doi: 10.1007/s11626-009-9216-3. [DOI] [PubMed] [Google Scholar]
- 80.Kaji H, Ishibashi T, Nagamine K, Kanzaki M, Nishizawa M. Electrically induced contraction of C2C12 myotubes cultured on a porous membrane-based substrate with muscle tissue-like stiffness. Biomaterials. 2010;31(27):6981–6986. doi: 10.1016/j.biomaterials.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 81.Ahadian S, Ramón-Azcón J, Ostrovidov S, et al. A contactless electrical stimulator: application to fabricate functional skeletal muscle tissue. Biomed. Microdevices. 2013;15(1):109–115. doi: 10.1007/s10544-012-9692-1. [DOI] [PubMed] [Google Scholar]
- 82.Sakar MS, Neal D, Boudou T, et al. Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab. Chip. 2012;12(23):4976–4985. doi: 10.1039/c2lc40338b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ. Implantation of in vitro tissue engineered muscle repair (TEMR) constructs and bladder acellular matrices (BAM) partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss (VML) injury. Tissue Eng. Part A. 2013 doi: 10.1089/ten.tea.2012.0761. doi:10.1089/ten.TEA.2012.0761. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tosun Z, McFetridge PS. Improved recellularization of ex vivo vascular scaffolds using directed transport gradients to modulate ECM remodeling. Biotechnol. Bioeng. 2013;110(7):2035–2045. doi: 10.1002/bit.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee IH, Yu HS, Lakhkar NJ, et al. Development, characterisation and biocompatibility testing of a cobaltcontaining titanium phosphate-based glass for engineering of vascularized hard tissues. Mater. Sci. Eng. C. 2013;33(4):2104–2112. doi: 10.1016/j.msec.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 86.Li H, Chang J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater. 2013;9(6):6981–6991. doi: 10.1016/j.actbio.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 87.Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ. Decellularization for whole organ bioengineering. Biomed. Mater. 2013;8(1):014106. doi: 10.1088/1748-6041/8/1/014106. [DOI] [PubMed] [Google Scholar]
- 88.Karande TS, Ong JL, Agrawal CM. Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Ann. Biomed. Eng. 2004;32(12):1728–1743. doi: 10.1007/s10439-004-7825-2. [DOI] [PubMed] [Google Scholar]