Abstract
Introduction
ERα function is crucial for development of normal mammary gland as well as in the process of progression of breast cancer cells.
Signals that target receptor levels contribute to regulate estrogens effects in the cells. An intricate cross-regulation has been documented between ERα and TGF-β down-stream molecules: SMAD2, SMAD3 and SMAD4, that can bind ERα and regulate their signaling. Thus, identification of natural anticancer drugs able to influence the latter effect might provide alternative choices for breast cancer treatment. Taking into account our previous published data we wanted to study the effect of 5-Methoxypsoralen (bergapten) on ERα and on TGF-β pathway.
Methods
RT-PCR and W.B. were performed to evaluate the effect of bergapten on the ERα expression protein and the TGF-β –down stream signaling molecules. siRNA for Smad4 and TGF- β RII was also done to evaluate their involvement on the bergapten-induced responses.
Results
We reported that bergapten, a coumarin containing compound, effectively depletes ERα in MCF-7 breast cancer sensitive cells and in tamoxifen-resistant clone. The decrease of ERα protein after bergapten treatment results from the ubiquitine-proteasome pathway as demonstrated by the use of MG-132. IP experiments with ER antibody, demonstrated that the protein has physical interaction with SMAD4 and poly-ubiquitine and the amount of ubiquitinated receptor, linked to SMAD4, is greater under bergapten. The crucial role played by SMAD4, in this process, emerges from the observation that in breast cancer cells, silencing of SMAD4, resulted in increased expression of endogenous ERα in both control and bergapten-treated cells, compared to wild type cells. The same results were confirmed in siRNA TGF-β RII cells.
Conclusions
The results suggest a novel negative regulation of ERα by TGF-β/SMAD4 in breast cancer cells and indicate that the SMAD4 protein is involved in the degradation of ERα induced by bergapten. We propose that bergapten may efficiently act as a natural antitumoral agent, able to deplete ERα from breast cancer tamoxifen-sensitive and resistant cells, thereby retraining the effect of membrane signals targeting ERα and in such way its mitogenic potentiality.
INTRODUCTION
Estrogens have been recognized as a key carcinogenic factor in breast cancer. Ligands of estrogen receptors (ERs) induce a conformational change that leads the dissociation of HSP90 followed by ERα dimerization, and binding to estrogen response elements in estrogens–responsive genes. Agonists and antagonists-bound Estrogen receptor recruit either coactivators or corepressors, respectively, regulating gene transcription.
Gene amplification or overexpression of ERα was found in some breast cancer. Approximately 70% of breast cancers are ERα positive and estrogen dependent. Moreover, the ER status is a basic prognostic marker for primary invasive breast cancer and an indicator for an individual hormonal therapy. The most commonly used antiestrogens: OH-tamoxifen and ICI 182,780, block estrogen–stimulated tumor growth and have demonstrated efficacy for treatment and prevention of ER-positive breast cancer [1,2]. However, long-term tamoxifen treatment is associated with estrogen-like action in endometrial tissue leading to a high risk for development of uterine adenocarcinoma. In addition, development of acquired resistance to tamoxifen represents the major clinical problem during endocrine treatment in ER-positive breast cancer. A number of studies have suggested that enhanced growth factor signalling, via various signal transduction pathways, may account for endocrine resistant breast tumour growth [3,4].
In fact, altered expression and activation of EGFR/HER2, IGF-1R and their key downstream signaling components MAPK/ERK (mitogen activated protein kinases/extracellular signaling regulated kinases) and PI3K/Akt (phosphatidylinositol-3-kinase/protein kinase B) can elicit anti-estrogen resistance through crosstalk with estrogen receptor (ER) signalling [5]. Thus, identification of novel antiestrogen agents may provide alternative choices for breast cancer treatment. Currently, there is a huge scientific and commercial interest in the discovery of potent, safe and selective anticancer drugs. Coumarins are natural compounds found in many plants that possess medical value by itself and its modified derivatives.
They belong to the flavonoid class of plant secondary metabolites, which have been found to exhibit a variety of biological activities, usually associated with low toxicity adressing considerable interest because of their diverse pharmacological properties like anti-HIV [6], anticoagulant [7], antibacterial [8], antioxidant [9], dyslipidemic and anti-tumoral effects [10]. Among these properties, cytotoxic effects were most extensively examined [11,12]. Recently it has been reported that neo-tanshinlactone, a coumarin containing compound, showed significant inhibition against two ER+ human breast cancer cell lines and was 10-fold more potent and 20-fold more selective than Tamoxifen [13].
In addition, our data have demonstrated how 5-methoxypsoralen, exerts both antiproliferative effects and induces pro-apoptotic responses in human breast cancer cells. Besides, in ER-positive MCF-7 cells 5-methoxypsoralen “per se” is also able to counteract the stimulatory action of IGF-I/E2 on breast cancer cell growth and progression [14].
Additionally, in established breast cancer cell lines, a correlation has been observed between estrogen receptor content and sensitivity to transforming growth factor beta (TGF-β) [15]. The role of TGF-β in breast cancer is ambiguous, since it was shown to display both tumor-suppressing and enhancing effects. However, the downstream signalling components of this growth factor: SMAD2, SMAD3 and SMAD4 have been previously reported to bind ER and to regulate ER signalling [16,17].
In the present study, we have demonstrated how 5-methoxypsoralen in breast cancer Tamoxifen sensitive and Tamoxifen-resistant cells is able to deplete ER protein, through a degradative process, that sees the involvement of the SMAD4 protein.
MATERIAL and METHODS
Materials
DMEM/F12, RPMI 1640, MEM, l-glutamine, penicillin, streptomycin, fetal bovine serum, BSA, and PBS were purchased from Eurobio (Les Ullis Cedex, France). Triazol reagent was obtained from Invitrogen (Carlsbad, CA), and FuGENE 6 was from Roche Applied Science (Indianapolis, IN). Taq DNA polymerase, 100-bp DNA ladder, was provided by Promega Corp. (Madison, WI). Aprotinin, leupeptin, phenylmethylsulfonyl fluoride, sodium orthovanadate, 5-methoxypsoralen, estradiol, MG-132 were purchased from SIGMA (Milan, ITALY).
Antobodies used in this study, anti-ERα, anti-cyclin D1, anti glyceraldehydephosphatedehydrogenase (GAPDH), anti-lamin B, anti-Ub, anti-TGFβ RII, anti-phospho ERK, anti-ERK, anti-phospho JNK and anti-JNK were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); anti-SMAD2, anti-SMAD3, anti-phospho SMAD3, anti-SMAD4, anti phospho p38 MAPK and anti-p38 MAPK, from EPITOMICS, Inc. (California); anti-phospho Smad2 from NOVUS BIOLOGICALS, LLC (Littleton, CO, USA). ECL System was purchased from Amersham Pharmacia (Buckinghamshire, UK).
Cell cultures and treatments
MCF-7 breast cancer cells were grown in DMEM/F12 medium, supplemented with 7.5% FCS, 1% penicillin/streptomycin and 1% glutamine, while ZR-75 cells were maintained in RPMI 1640 medium enriched with 10% of FBS, 1% penicillin/streptomycin and 1% glutamine. Tamoxifen-resistant MCF7-TR1 cells were generated in the laboratory of Dr. Fuqua as described [18]. Cells were grown in MEM supplemented with 5% fetal bovine serum (FBS) 1% penicillin/streptomycin and 10μg/ml insulin. All cell types were incubated at 37°C in 5% CO2. Sub confluent cell cultures, synchronized for 24 hours in DMEM without phenol red and serum (PRF-SFMDMEM), were used for all experiments.
As it concerns the preparation of 5-MOP solution for the cellular treatment the light source has been accurately avoided since any experiment was performed under the cabinet laminar flow with the extinguished light. Furthermore, the 5-MOP solution was always kept in a dark bottle.
Cell proliferation assays
All cell types, treated and untreated with Bergapten for 48 and 96h in a single schedule and in combination with OH-Tamoxifen were collected for cell viability.
Cell growth was examined using the method of transcriptional and translational (MTT) colorimetric assay. At the above indicated time points, 100μl of MTT (5mg/ml) were added to each well, and the plates were incubated for 4h at 37°C. Then, 1ml 0.04N HCl in isopropanol was added to solubilise the cells. The absorbance was measured with the Ultrospec 2100 Prospectrophotometer (Amersham-Biosciences, Italy) at a test wavelength of 570 nm.
Immunoprecipitation and Western blot analysis
All cell types, following Bergapten treatments, as showed in the corresponding figures, were harvested and lysed in 500μl of lysis buffer, containing 50mM HEPES pH 7.5, 150mM NaCl, 1.5mM MgCl2, 10mM EGTA pH 7.5, 10% Glycerol, 1% Triton X-100 and protease inhibitors (2μM Na3VO4, 1% PMSF, 20μg/ml aprotinin).
Cytoplasmic protein lysates were obtained with a buffer containing 50mM HEPES, pH 7.5, 150mM NaCl, 1.5mM MgCl2, 10mM EGTA, pH 7.5, 10% glycerol, 1% TritonX-100 and protease inhibitors (2μM Na3VO4, 1% PMSF, 20μg/ml aprotinin). Following the collection of cytoplasmic proteins, the nuclei were lysed with the buffer containing 20mM KOH–HEPES, pH 8, 0.1mM EDTA, 5mM MgCl2, 0.5M NaCl, 20% glycerol, 1% Np-40 and inhibitors (as above).
For coimmunoprecipitation experiments, we used 500μg of total cellular protein and 1μg of ER alpha monoclonal antisera overnight, followed by protein A/G agarose precipitation with rotation at 4°C for 2h. Immunoprecipitated proteins were washed thrice with HNTG buffer. Equal amounts of cell extract and immunoprecipitated proteins were resolved under denaturing conditions by electrophoresis in 8% polyacrylamide gels containing SDS (SDS–PAGE), and transferred to nitrocellulose membranes by electroblotting. After blocking the transferred nitrocellulose membranes were incubated with primary antibodies overnight at 4°C, with secondary antibodies goat anti-mouse or goat anti-rabbit antisera (1:3000) for 1h at room temperature and developed with enhanced chemi luminescence reagents.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Assay
Total cellular RNA was extracted using TRIZOL reagent (Invitrogen) as suggested by the manufacturer. The purity and integrity were checked spectroscopically and by gel electrophoresis before carrying out the analytical procedures. The evaluation of gene expression was performed by semiquantitative RT-PCR method. For ERα the primers were: 5′-AGATCCAAGGGAACGAGCT-3′ (forward); 5′-TTCTCCAGGTAGTAGGGCA-3′ (reverse); and internal control gene, 36B4, the primers were: 5′-CTCAACATCTCCCCCTTCTC-3′ (forward) and 5′-CAAATCCCATATCCTCGTCC-3′ (reverse).
RNA silencing
For SMAD4 and TGF-β type II receptor gene silencing experiments, custom synthesized siRNA (Ambion) annealed duplexes were used for effective depletion of SMAD4 and TGF- β type II receptor mRNA. A scrambled siRNA that does not match with any human mRNA was used as a control for non-sequence-specific effects (Ambion). Growing cells were switched to PRF for 24h. After that, cells were trypsinized and transfected in suspension with 5nM siRNA (siSMAD4 or scrambled siRNA) in 35-mm dishes, using Lipofectamine 2000 (Invitrogen), following the manufactuer’s instructions. Cells were incubated with the siRNA-Lipofectamine 2000 complex at 37°C for 4h and then switched to fresh PRF and treated or not with Bergapten (20μM and 50μM) for 4h before analysis.
Anchorage-independent soft agar growth assays
Cells (5000/well) were plated in 4ml of 0.35% agarose with 5% charcoal-stripped FBS in phenol red-free MEM, in a 0.7% agarose base in 6-well plates. Two days after plating, media containing control vehicle or hormonal treatments (E2 10nM and bergapten 50μM) was added to the top layer, and the appropriate media was replaced every 2 days. After 14 days, 150μl of MTT was added to each well and allowed to incubate at 37°C for 4h. Plates were then placed in 4°C overnight and colonies ≥ 50μm diameters from triplicate assays were counted. Data are the mean colony number of three plates and representative of two independent experiments [18].
Statistical analysis
All data were expressed as the mean ± SD of at least three independent experiments. The data were analyzed by analysis of variance using the STATPAC computer program.
RESULTS
Bergapten inhibits breast cancer cell growth and antagonizes the stimulatory action of anti-estrogen in MCF-7 Tamoxifen resistant cells
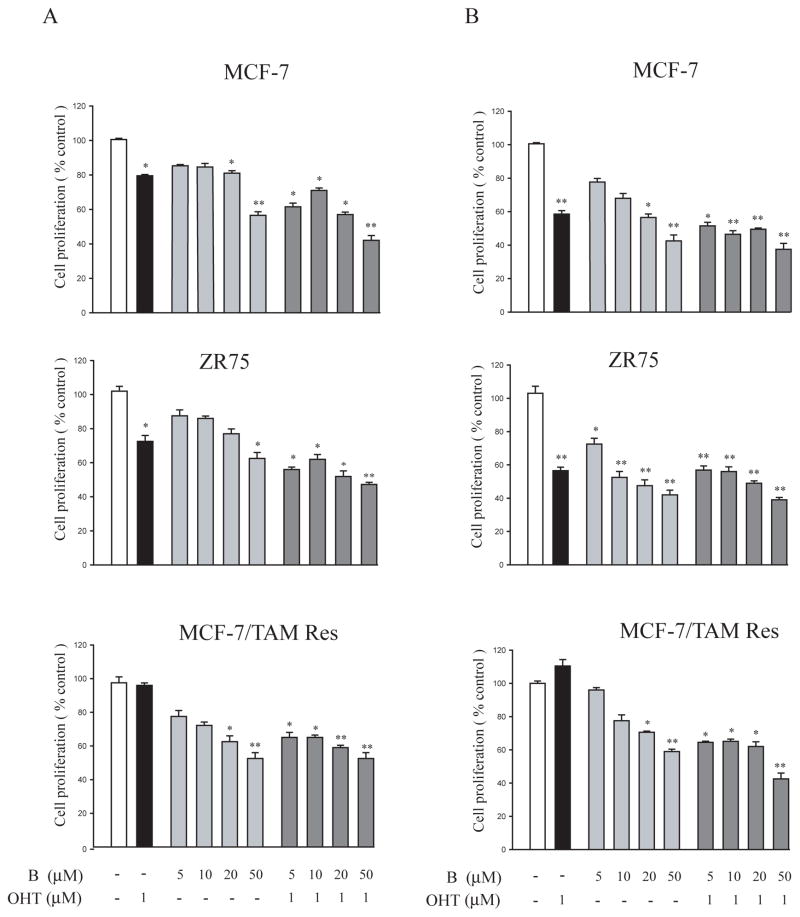
To asses the effect of bergapten on cell proliferation: MCF-7, ZR-75 breast cancer cells and MCF-7 Tamoxifen resistant (MCF-7/TAM Res) cells were treated for 48h and 96h with different doses of the drug in a single schedule and in combination with OH-Tamoxifen. As shown in Figure 1, cell growth was assessed by MTT assay. Treatment with bergapten, at both times, inhibited cell growth in a dose-dependent manner. In MCF-7 and ZR-75 cells the psoralen (20μM, 50μM) at 48h (Figure 1A) enhances the antiproliferative activity of OH-Tamoxifen. In addition, in MCF-7/TAM Res clone bergapten, after 96 h, antagonizes the stimulatory action of anti-estrogen even at low doses (5μM and 10μM) (Figure 1B).
Figure 1. Cell viability of MCF-7, ZR-75 and MCF-7/TAM Res after bergapten and OH-Tamoxifen treatments.
MCF-7, ZR-75 and MCF-7/TAM Res breast cancer cells seeded in six-well plates (100 000 cells/wells) were treated for 48h (A) and 96h (B) with 5, 10, 20, 50μM of bergapten in a single schedule and in combination with OH-Tamoxifen (1μM). The values are expressed as percentage of the control, determined by standardizing untreated cells to 100%. B: bergapten; OHT: OH-Tamoxifen. Triplicate results are expressed as mean+-S.D. (n=4). * P<0.05; ** P<0.01 as compared to untreated control cells.
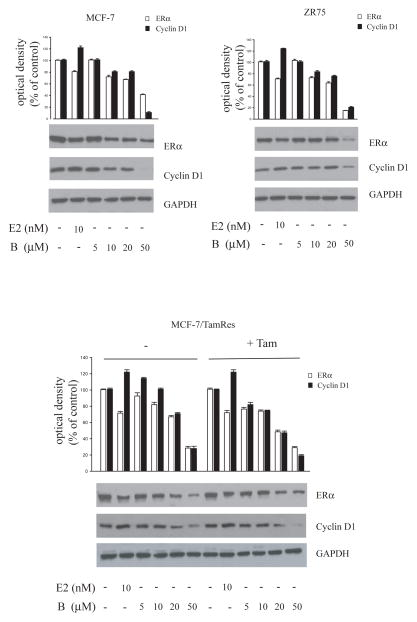
Bergapten down-regulates estrogen receptor α protein content and decreases estrogen response gene expression
To test the effect of the drug on ERα expression, we treated for 24h breast cancer cells with the same doses of bergapten used for cell growth. Western blot analysis of whole–cell lysates of MCF-7 and ZR-75 cells showed that the protein content of ERα was decreased by the highest concentrations of bergapten (Figure 2). At the same time, we examined the effect of the treatment on ERα-mediated gene expression. The incubation with bergapten reduces cyclin D1 protein in both cell types, taking as positive control the cyclin D1 expression upon E2 exposure. A total suppressive effect of ERα was obtained in MCF-7 cells under bergapten 50μM. The same down-regulatory effect was reproduced in MCF-7/TAM Res cells either in the absence or in the presence of OH-Tamoxifen maintained during the experimental time (Figure 2).
Figure 2. Bergapten treatment lowers ERα and cyclin D1 in breast cancer cells.
MCF-7, ZR-75 and MCF-7/Tam Res cells were treated with E2 10nM and increasing amounts of bergapten (B) (5, 10, 20, 50μM) for 24h. A set of MCF-7/Tam Res cells were also maintained with OH-Tamoxifen (T1μM) during the experimental procedure. GAPDH was used as loading control. Results are representative of three independent experiments.
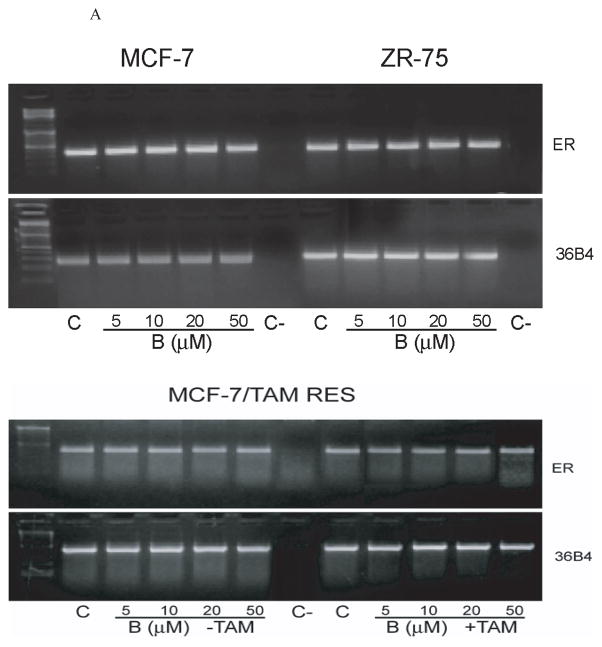
To ascertain if bergapten-mediated ER down-regulation was due to inhibitory effect induced on ER gene transcription, we performed RT-PCR to detect ERα mRNA level upon the cumarine exposure. As shown in Figure 3, bergapten does not affect ERα mRNA levels in MCF-7, ZR-75 and MCF-7/ TAM Res cells. This reasonably addressed the potential role of post-trascriptional mechanisms in determining the bergapten-induced ERα down-regulation.
Figure 3. ERα RT-PCR assay in breast cancer cells under Bergapten.
mRNA expression of ERα in MCF-7, ZR-75 and MCF-7/TAM Res cells treated for 24h with increasing concentrations of bergapten (B) as indicated. MCF-7/Tam Res were also treated with bergapten alone or in combination with OH-Tamoxifen. The housekeeping gene 36B4 was determined as control.
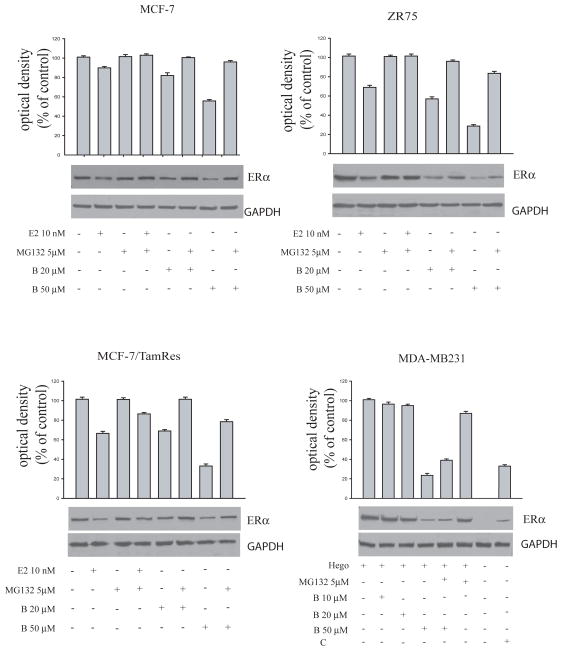
Bergapten promotes ERα degradation via the ubiquitine-proteasome pathway
To evaluate the potential molecular mechanism by which bergapten inhibits ER protein expression, we focused on ubiquitin–proteasome pathway. MCF-7, ZR-75 and MCF-7/TAM Res cells were treated with 20μM and 50μM bergapten in the presence or absence of the proteasomal inhibitor MG-132 (5μM). In all three cell lines, reduction in ERα by bergapten was prevented by the proteasomal inhibitor MG-132, suggesting that bergapten could induce ERα degradation via the proteasome degradative pathway (Figure 4).
Figure 4. Bergapten induces ERα degradation via the ubiquitin-proteasome pathway in MCF-7, ZR-75, MCF-7 Tam Res and MDA-MB 231 cells.
MCF-7, ZR-75, MCF-7/Tam Res and estrogen receptor negative MDA-MB 231 cells, transiently overexpressing ERα, were treated with E2 10nM and/or bergapten (B) at indicated concentrations in the presence or in the absence of proteasome inhibitor MG-132 (5μM). This inhibitor was added to the cells 30′ before and continued during the bergapten treatment for 4h. The level of ERα was detected by Western blot with anti-ERα antibody. GAPDH was used as loading control. C: ER+ breast cancer cell lysate. Results are representative of four independent experiments.
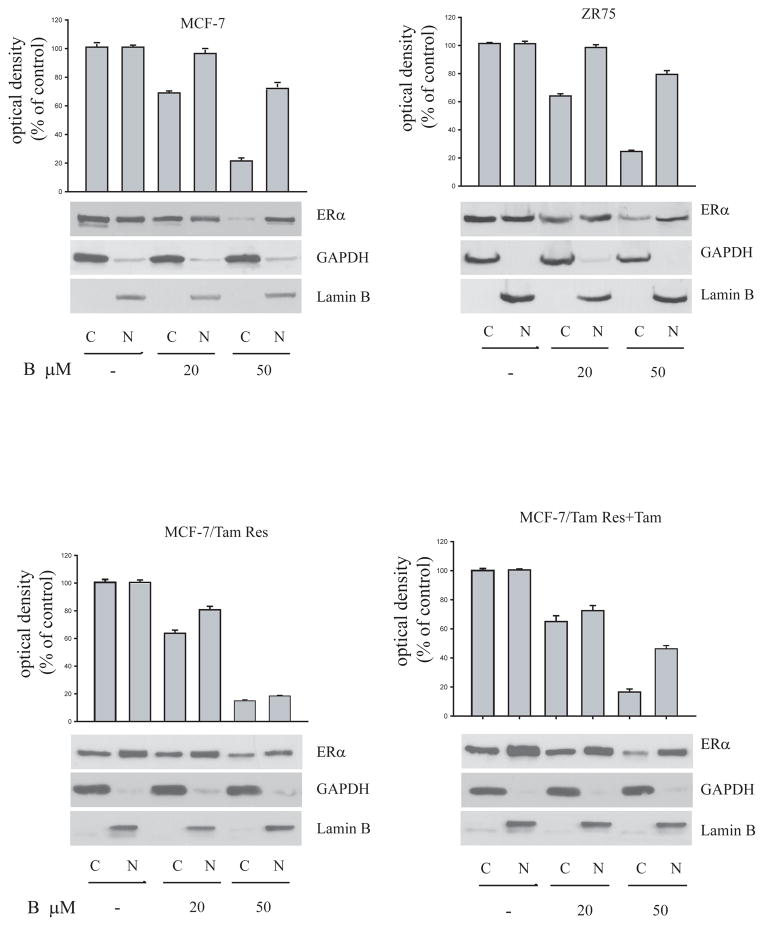
All these data were confirmed in estrogen receptor negative MDA-MB 231 cells, overexpressing ectopically the ERα through transient trasfection (Fig 4). Indeed, MG-132 incubated with bergapten increases the ERα level compared to that obtained by bergapten alone (Figure 4). Furthermore, in order to evaluate in which cellular compartment ERα degradation occurs we performed Western Blot analysis in extranuclear and nuclear fractions of MCF-7, ZR-75 and MCF-7/TAM Res cells under bergapten treatment. The lowering of ERα induced by psoralen (20μM and 50μM) occurs prevalently in the cytoplasmic fraction of breast cancer cells (Figure 5).
Figure 5. ER protein in cytosolic and nuclear extracts of breast cancer cells.
Immunoblot analysis of ERα in cytosolic (C) and nuclear (N) protein lysates of MCF-7, ZR-75 and MCF-7/Tam Res cells treated for 24h with bergapten (B) as indicated. MCF-7/Tam Res + Tam: cells maintained also with OH-Tamoxifen (T1μM) during the experimental procedure.
Lamin B and GAPDH were used, respectively, as control of nuclear and cytoplasmatic fraction. These results are representative of four independent experiments.
All results revealed that bergapten affects ERα stability in both wild-type breast cancer cells than in Tamoxifen-resistant clone.
Infuence of SMAD4 in the bergapten–induced ERα ubiquitination
ER protein is a cross-road of different intracellular signallings which can modulate ER activity during breast tumorigenesis. Recently, it has been reported how ERα may directly interact with SMADs, the effectors of the TGF-β pathway and, in this respect, to be inversely related to them [16,17].
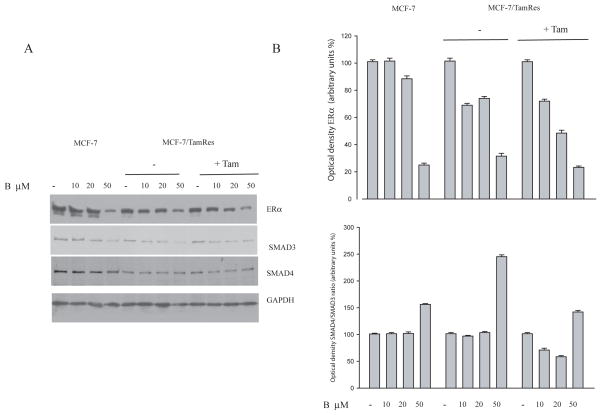
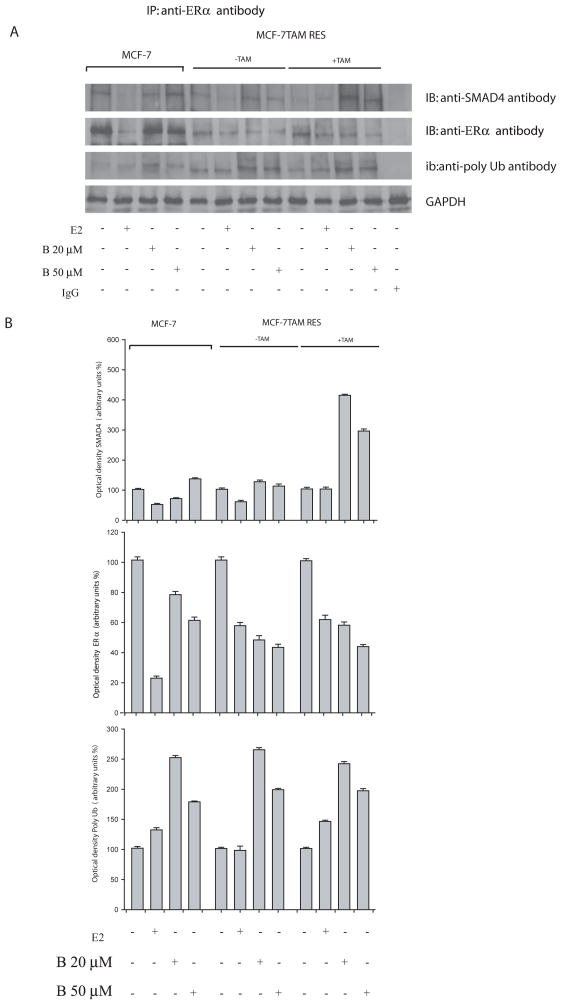
In our study, we wanted to ascertain if bergapten, by depleting ERα, might also affect the expression levels of SMAD proteins. Western Blot analysis performed in MCF-7 and MCF-7/TAM Res cells reveals that SMAD4 protein was enhanced upon bergapten treatment, resulting in an increase of the SMAD4/SMAD3 ratio as illustrated in the figure (Figure 6A, B). Furthermore, immunoprecipitation experiments with anti-ERα antibody followed by Immunoblotting with anti-SMAD4 and anti-Poly-Ub antibodies show that ER/SMAD4/ Poly-Ub are co-associated in a tripartite complex and that bergapten enhances the amount of ERα complexed to SMAD4 (Figure 7A). The same filter, stripped and reprobed for SMAD3, showed no association of ERα with the protein (data not shown).
Figure 6. Immunoblot of ERα and SMAD4/SMAD3 in breast cancer cells.
(A) The protein lysates of MCF-7 and MCF-7/Tam Res cells treated for 24h with different concentrations of bergapten (B) as indicated, were immunoblotted with anti-ERα, anti-SMAD3 and anti-SMAD4. GAPDH was used as loading control. MCF-7/Tam Res cells were maintained (+Tam) or not (−Tam) with OH-Tamoxifen (T1μM) during the experimental procedure IgG: negative control of Immunoprecipitation experiment. Results are representative of three independent experiments.
(B) Optical density of the ERα and SMAD4/SMAD3 ratio.
Figure 7. Co-association between ER Poly-Ub and SMAD4 in breast cancer cells.
(A) MCF-7 and MCF-7/Tam Res cells, treated with E2 10nM and bergapten (B 20, 50μM) for 4h, were lysated and cellular extracts were immunoprecipitated (IP) with anti-ERα antibody, resolved by SDS-PAGE, and immunoblotted (IB) with anti-SMAD4, anti-ERα and anti-ubiquitin antibodies. Prior the immunoprecipitation experiment an aliquote of the lysate corresponding to 30μg of protein was loaded to determine GAPDH, as loading control. MCF-7/Tam Res cells were maintained (+Tam) or not (−Tam) with OH-Tamoxifen (T1μM) during the experimental procedure. Results are representative of three independent experiments.
(B) Optical density of the SMAD4, ERα and Poly Ubiquitin.
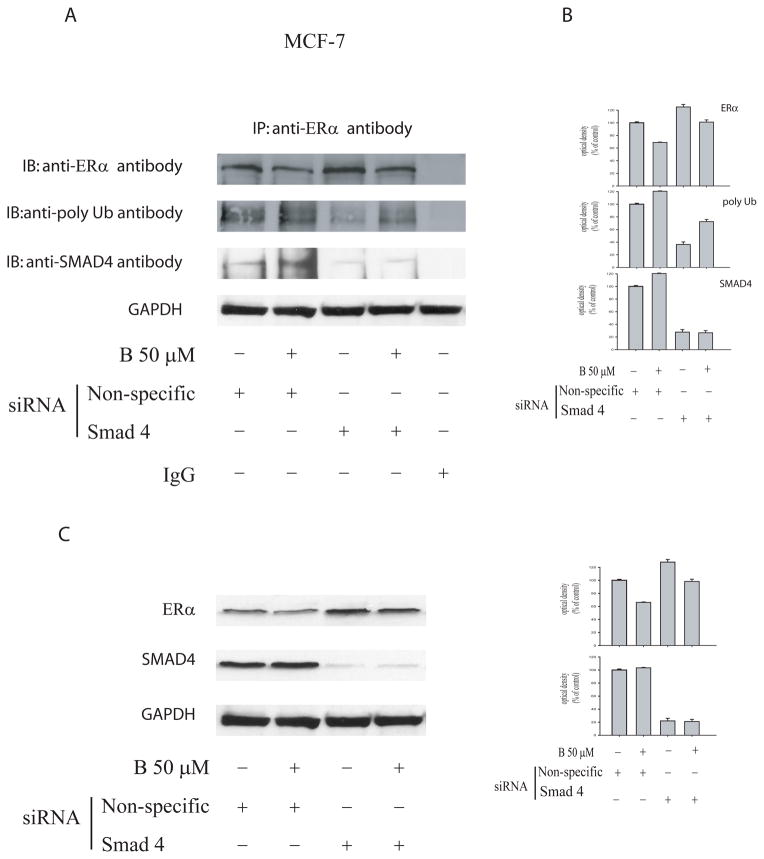
In order to further evaluate the role of SMAD4 in the ER-ubiquitination process we targeted SMAD4 with siRNA. IP experiments of MCF-7 cell lysates with anti-ERα antibody followed by Western Blotting for ERα, showed that SMAD4 knockdown induces a greater retention of ERα, so as to be longer expressed, compared to that one obtained in wild type cells. This addresses how in the latter circumstance a minor ER ubiquitination occurs (Figure 8A, B).
Figure 8. IP experiment in MCF-7 cells bearing silencing of SMAD4.
(A) MCF-7 cells were transfected with either non-targeting siRNA or SMAD4 specific siRNA. The cells were then untreated or were treated with 50μM bergapten for 4h. Cellular extracts were underwent to immunoprecipitation (IP) experiments with anti-ERα antibody, resolved by SDS-PAGE, and immunoblotted (IB) with anti-ERα, anti-poly-ubiquitin and anti-SMAD4 antibodies. IgG: negative control of Immunoprecipitation experiment.
(B) Optical density of the ERα, poly-Ubiquitin and SMAD4.
(C) Prior the immunoprecipitation experiment an aliquote of the lysates corresponding to 30μg of protein was loaded to determine the ERα and SMAD4 protein levels. GAPDH is taken as a loading control. Results are representative of three independent experiments.
A higher expression of ERα in siRNA SMAD4 MCF-7 cells, compared to wild type cells, was also confirmed by Western Blot (Figure 8C).
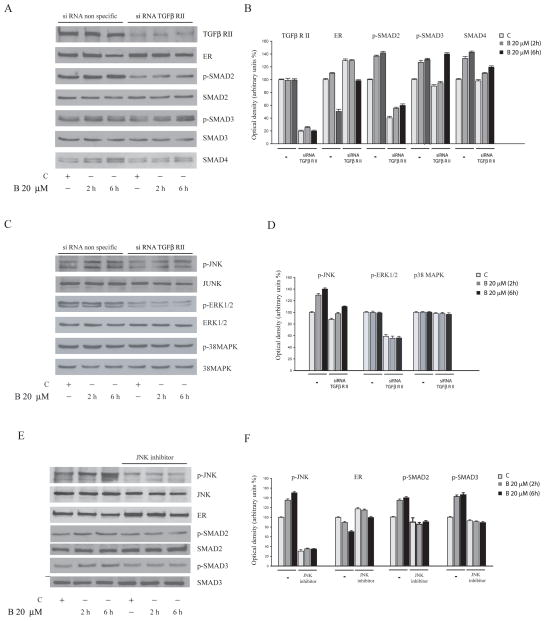
The results confirm that SMAD4 physically interact with ERα and expression of SMAD4 is necessary to mediate the bergapten-induced ER degradation. Taking into account that SMAD4 is a down-stream signalling of TGF-β, we wanted to evaluate the role of TGF-β receptor on ERα protein content both in bergapten treated and untreated MCF-7 cells. To this aim we transiently transfected MCF-7 cells with siRNA TGFbeta-RII and we stimulated the cells for short time with the drug in order to assess the phosphorylation signals down-stream the TGF-β pathway. It is worth to mention how, in this circumstance, as expected, we observed a dramatic reduction of SMAD4 and a marked up-regulation of ERα compared to the co respective experimental conditions reported in the negative siRNA transfected cells (Figure 9A).
Figure 9. TGF-β signalling proteins evaluated in MCF-7 cells underwent bergapten treatment.
MCF-7 cells were transfected with either non targeting siRNA or TGF-β RII specific siRNA. The cells were untreated (C) or treated with bergapten (B) 20μM for 2h and 6h. (A) Immunoblots of TGF-β RII, ER, phospho-SMAD2, phospho-SMAD3, total SMAD2, total SMAD3, and SMAD4 are indicated; (C) Immunoblots of phospho–JNK, JNK, phospho-ERK1/2, ERK1/2, phospho-p38MAPK, p38MAPK (E) Immunoblots of phospho-JNK, JNK, phospho-SMAD2, phospho-SMAD3, total SMAD2, total SMAD3, and ERα in MCF-7 cells untreated or treated with bergapten and in cells pre-treated for 1h with the JNK kinase inhibitor SP600125 (10 μM); (B, D, F) Densitometric analysis of the corresponding proteins.
In addition, in cells bearing siRNA TGF-β RII under bergapten treatment it has been shown a reduction of phospho-SMAD2 and phospho-SMAD3, with respect to scrambled siRNA control (Figure 9A).
To evaluate the potential involvement of MAPKs in the phosphorylation of SMADs under bergapten treatment we reprobed the filters for anti-p38 kinase, anti p-ERK1/2 and for anti p-JNK. In these experimental conditions, bergapten was able to up-regulate p-JNK, which appears clearly blunted in the presence of siRNA TGF-β RII, while no substantially modifications were reported for p-ERK1/2 and p38MAPK (Figure 9C).
Indeed, the use of the specific JNK-kinase inhibitor SP600125 reverses the p-SMAD2 and p-SMAD3 activation and leads to an increase of ERα protein (Figure 9E). Thus, we may conclude that bergapten is able to stimulate TGF-β signalling, which through JNK activation, phosphorylates SMAD2, SMAD3 and enhances the expression of SMAD4. This latter protein binds ERα and drives its ubiquitination.
Bergapten influences the anchorage-independent growth induced by Estradiol
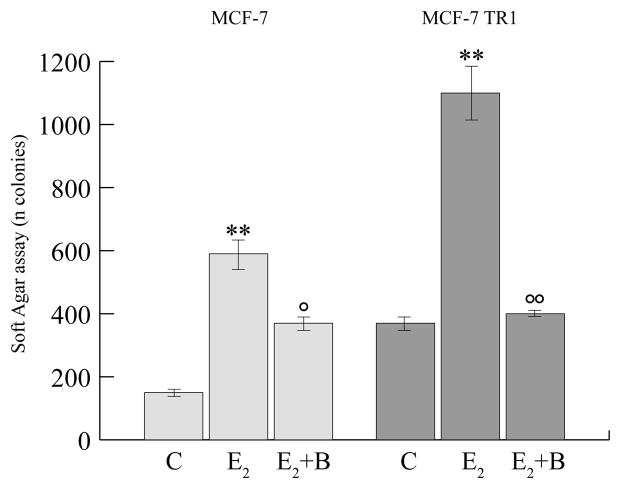
We next have evaluated the effects of bergapten on the anchorage independent growth upon E2-exposure of MCF-7 and MCF-7/TAM Res clone.
Our data have shown that psoralen is able to antagonize the stimulatory action induced by E2 on cell growth in soft agar, and, in a higher extent, in Tamoxifen resistant clone (Figure 10).
Figure 10. Soft agar growth assay of MCF- and MCF-7/TAM Res cells.
MCF-7 and MCF-7/Tamoxifen resistant cells (MCF-7TR1) were seeded (5,000/well) in 0.35% agarose and then treated with vehicle (C), E2 (10nM), bergapten (B50μM). Cells were allowed to grow for 14 days and the number of colonies ≥ 50μm were quantified and the results were graphed. ** P< 0.01 versus untreated cells; ° P< 0.05 ; °° P< 0.01 versus E2 condition.
DISCUSSION
Estrogen receptor (ER) has become an important target in the treatment of hormones-responsive breast cancer. Unfortunately, most patients initially responding to anti-estrogen therapies develop pharmacological resistance. The potential mechanisms of endocrine resistance are not fully understood, but multifactorial determinants may be involved, such as the growth factor signalling and altered ER regulation.
Therefore, depletion of ERα from breast cancer may give particularly powerful advance to block mitogenic signals, even those coming from the ER/growth factor crosstalk, preventing the development of endocrine resistance.
We showed previously that cumarine-derivate compound: 5-MOP (bergapten) inhibits human breast cancer growth, by increasing p53 and p21 expression, and induces a functional activation of pro-apoptotic response [14]. In this study we reported that 5-MOP in breast cancer cells is able to down-regulate ERα protein, without affecting mRNA-ERα level, and to decrease estrogen response gene expression such as cyclin D1. This suggests, how very likely, the down-regulation of ERα upon exposure to bergapten does not involve a transcriptional mechanism. Indeed, the use of MG-132, a proteasome inhibitor, reverses the down-regulation of ER under bergapten, addressing the effect of such molecule in enhancing the degradative pathway of the receptor protein. It is worth to mention how these effects were also reproduced in MDA-MB 231 cells overexpressing ectopically the ER alpha through transient transfection experiments. Western Blot analysis of ERα performed respectively in the nuclear and cytosolic cell lysates evidences that under bergapten treatment the decrease of ERα is detected in the cytoplasmatic fraction of the cells, suggesting that the degradative process of the receptor occurs prevalently in the cytoplasmatic fraction.
It is extremely intriguing to observe that the above reported effect of bergapten on ERα degradation is reproduced in breast cancer Tamoxifen-resistant cells, where we have reported a marked decrease of cell proliferation, evidencing an efficient response of these cells to the effect of psoralen.
It is worth to mention how the enhanced anchorage independent growth upon E2 exposure is drastically attenuated in the presence of the combined exposure of bergapten, emphasizing, furthermore, the boostering action of the psoralen on ERα-degradation in breast cancer cells.
These results reflect the anti-tumoral properties of the molecule and call other published data demonstrating how some coumarin derivatives can be potent inhibitors of proliferation of aromatase and estrogen receptor positive breast cancer cells [19,20].
The amount of ERα protein in the cell is a major determinant of the regulation of its own transcriptional activity. Steady state of ERα level is maintained by a dynaminc balance between protein synthesis and breakdown. Many factors regulate the endogenous levels of ERα in the cells and this, in turn, influences the interactions of the receptor protein with specific coactivating or corepressing transcription elements. Control of ERα expression is an important means to modulate cellular responses to growth.
Several observations have documented the crosstalk between ERα activity and TGF-β signalling. Indeed, previous reports have demonstrated how ERα is able to physically interact with SMAD2, SMAD3 and SMAD4 and to abrogate TGF-β signalling cascade [16,17,21].
While TGF- β signalling has been demonstrated to stimulate ERα transcriptional activity, the complex of SMAD3 and SMAD4 inhibits its activity [16,22].
In our study we proved that in breast cancer-treated cells the down-regulation of ERα coincides with the increase of SMAD4/SMAD3 ratio, implying a functional relationship between SMAD4 and ER.
Indeed, to gain further insight on these two proteins, we have examined the interaction between ERα/SMAD4 and poly-ubiquitine in control and in bergapten-treated cells.
As here demonstrated, the three proteins co-associated and although the level of ERα in the immunoprecipitate is lowered by the treatment, the amount of ubiquitinated receptor, linked to SMAD4, is greater compared to that obtained in control.
So, SMAD4 appears to be an important factor that participates in the ERα ubiquitination process under bergaten treatment.
The crucial role played by SMAD4, in the above mentioned process, emerges from the observation that in breast cancer cells, bearing the silencing SMAD4, the expression of endogenous ERα is better preserved in both control and treated–cells, compared to that one obtained in wild type cells. The phenomenon was also observed in MCF-7 cells silenced for TGF-β RII. In the same vein, IP experiments confirmed that SMAD4 silenced in MCF-7 cells lowers the ubiquitination of ERα, thereby highlighting a negative relationship between the two proteins. In addition, in the presence of the JNK specific inhibitor the activation of SMAD proteins was partially reversed, while concomitantly the ERα content was enhanced.
These findings suggest that SMAD4 protein, down-stream the TGF-β signalling, is crucially involved in the degradative process of the receptor.
In addition, the observation that SMAD4 is up-regulated following bergapten treatment, highlights a new molecular mechanism through which psoralen might affect ERα stability and thereby regulate breast cancer cell progression.
Results in the same direction, have been published regarding the involvement of proteasomal degradation of ERα by TGF-β in breast cancer cells, all this points to a functional crosstalk between TGF-β and estrogen receptor signaling in breast cancer [23].
Besides, apart from the apoptotic function of bergapten, according to our previously published data, the present paper has highlighted a novel role of the molecule in regulating ERα protein stability via the ubiquitin-proteasome pathway.
Conclusions
This study, once again, draws attention to the anticancer properties of psoralen, emphasizing how the molecule can unfold its functional activity even in absence of photoactivation.
Based on what we just said, we propose that bergapten may efficiently act as a natural antitumoral agent, able to deplete ERα from breast cancer tamoxifen-sensitive and resistant cells thereby preventing crosstalk between the receptor and growth factor mitogenic signalling.
Acknowledgments
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC 2011, grant IG 11595) and PRIN grant 2008; we thank Dr. P. Cicirelli for technical assistance, University of Calabria, Cosenza.
Abbrevations
- B
Bergapten
- 5-MOP
5-Methoxypsoralen
- ERα
estrogen receptor α
- TGF-β
Trasforming growth factor beta
- MAPK
mitogen-activated protein kinase
- Poly Ub
Poly-ubiquitin
Footnotes
Author’ contributions
PML designed and wrote the paper, GF designed and performed most of the experiments, RP and ZD designed siRNA and performed transfection experiments; PM and ML brought out IPs; CG and VA performed proliferation and soft agar assay; CS, AS, SD and DAF analyzed some of the data, interpreted and drafted the results; FSWA provided MCF-7/TAM Res clone and revised the manuscript; SA coordinated all the experimental designs and revised the manuscript.
All authors read and approved the final manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Furr BJ, Jordan VC. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25(2):127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, Mc-Cue BL, Wakeling AE, McClelland RA, Manning DL, Nicholson RI. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87:746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SR, Head J, Pancholi S, Detre S, Martin LA, Smith IE, Dowsett M. Integration of signal transduction inhibitors with endocrine therapy: an approach to overcoming hormone resistance in breast cancer. Clin Cancer Res. 2003 Jan;9(1 Pt 2):524S–32S. [PubMed] [Google Scholar]
- 4.Morrow M, Jordan VC. Molecular mechanisms of resistance to tamoxifen therapy in breast cancer. Arch Surg. 1993;128:1187–1191. doi: 10.1001/archsurg.1993.01420230015002. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Moerkens M, Ramaiahgari S, de Bont H, Price L, Meerman J, van de Water B. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011 May 19;13(3):R52. doi: 10.1186/bcr2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma T, Liu L, Xue H, Li L, Han C, Wang L, Chen Z, Liu G. Chemical library and structure-activity relationships of 11-demethyl-12-oxo calanolide a analogues as anti-HIV-1 agents. J Med Chem. 2008;51(5):1432–1446. doi: 10.1021/jm701405p. [DOI] [PubMed] [Google Scholar]
- 7.Kidane AG, Salacinski H, Tiwari A, Bruckdorfer KR, Seifalian AM. Aticoagulant and antiplatelet agents. Their clinical and device application (s) together with usages to engineer surface. Biomacromolecules. 2004;5:798–813. doi: 10.1021/bm0344553. [DOI] [PubMed] [Google Scholar]
- 8.Appendino G, Percalli E, Fuzzati N, Arnoldi L, Stavri M, Gibbons S, Ballero M, Maxia AJ. Antimycobacterial coumarins from the sardinian giant fennel (Ferula communis) J Nat Prod. 2004;67 (12):2108–10. doi: 10.1021/np049706n. [DOI] [PubMed] [Google Scholar]
- 9.Kontogiorgis CA, Hadjipavlou LD. Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg Med Chem Lett. 2004;14(3):611–4. doi: 10.1016/j.bmcl.2003.11.060. [DOI] [PubMed] [Google Scholar]
- 10.Sashidhara KV, Rosaiah JN, Kumar A, Bhatia G, Khanna AK. Synthesis of novel benzocoumarin derivatives as lipid lowering agents. Bioorg Med Chem Lett. 2010;20(10):3065–3069. doi: 10.1016/j.bmcl.2010.03.103. [DOI] [PubMed] [Google Scholar]
- 11.Kostova I. Synthetic and natural coumarins as cytotoxic agents. Curr Med Chem Anticancer Agents. 2005;5(1):29–46. doi: 10.2174/1568011053352550. [DOI] [PubMed] [Google Scholar]
- 12.Musa MA, Cooperwood JS, Khan MO. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem. 2008;15(26):2664–79. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sashidhara KV, kumar A, Kumar M, Sarkar J, Sinha S. Synthesis and in vitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents. Bioorg Med Chem Lett. 2010;20(24):7205–7211. doi: 10.1016/j.bmcl.2010.10.116. [DOI] [PubMed] [Google Scholar]
- 14.Panno ML, Giordano F, Palma MG, Bartella V, Rago V, Maggiolini M, Sisci D, Lanzino M, De Amicis F, Andò S. Evidence that bergapten, independently of its photoactivation, enhances p53 gene expression and induces apoptosis in human breast cancer cells. Curr Cancer Drug Targets. 2009;9(4):469–481. doi: 10.2174/156800909788486786. [DOI] [PubMed] [Google Scholar]
- 15.Arteaga CL, Tandon AK, Von Hoff DD, Osborne CK. Transforming growth factor beta: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res. 1988;48:3898–3904. [PubMed] [Google Scholar]
- 16.Matsuda T, Yamamoto T, Muraguchi A, Saatcioglu F. Cross-talk between transforming growth factor-beta and estrogen receptor signaling through Smad3. J Biol Chem. 2001;276:42908–42914. doi: 10.1074/jbc.M105316200. [DOI] [PubMed] [Google Scholar]
- 17.Ito I, Hanyu A, Wayama M, Goto N, Katsuno Y, Kawasaki S, Nakajima Y, Kajiro M, Komatsu Y, Fujimura A, Hirota R, Murayama A, Kimura K, Imamura T, Yanagisawa J. Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J Biol Chem. 2010;285(19):14747–55. doi: 10.1074/jbc.M109.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano C, Cui Y, Barone I, Andò S, Mancini MA, Berno V, Fuqua SWA. Growth factor-induced resistance to tamoxifen is associated with a mutation of estrogen receptor α and its phosphorylation at serine 305. Breast Cancer Res Treat. 2010;119:71–85. doi: 10.1007/s10549-009-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Cho M, Karlsberq K, Zhou D, Yuan YC. Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J Biol Chem. 2004;279 (46):48071–48078. doi: 10.1074/jbc.M406847200. [DOI] [PubMed] [Google Scholar]
- 20.Macaulay VM, Bates PJ, McLean MJ, Rowlands MG, Jenkins TC, Ashworth A, Neidle S. Inhibition of aromatase expression by a psoralen-linked triplex-forming oligonucleotide targeted to a coding sequence. FEBS Lett. 1995;372(2–3):222–228. doi: 10.1016/0014-5793(95)00987-k. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Wu Y, Gathings B, Wan M, Li X, Grizzle W, Liu Z, Lu C, Mao Z, Cao X. Smad4 as a transcription corepressor for estrogen receptor alpha. J Biol Chem. 2003;278(17):15192–15200. doi: 10.1074/jbc.M212332200. [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, Wu L, Frost AR, Grizzle W, Cao X, Wan M. Dual effects of TGF-beta on ER alpha-mediated estrogenic transcriptional activity in breast cancer. Mol cancer. 2009;27(8):1–11. doi: 10.1186/1476-4598-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrel TA, Brueggemeier RW. Increased proteasome-dependent degradation of estrogen receptor-alpha by TGF-beta1 in breast cancer cell lines. J Cell Biochem. 2003 Jan 1;88(1):181–90. doi: 10.1002/jcb.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]