Abstract
The opioid system consists of three receptors, mu, delta, and kappa, which are activated by endogenous opioid peptides processed from three protein precursors, proopiomelanocortin, proenkephalin, and prodynorphin. Opioid receptors are recruited in response to natural rewarding stimuli and drugs of abuse, and both endogenous opioids and their receptors are modified as addiction develops. Mechanisms whereby aberrant activation and modifications of the opioid system contribute to drug craving and relapse remain to be clarified. This review summarizes our present knowledge on brain sites where the endogenous opioid system controls hedonic responses and is modified in response to drugs of abuse in the rodent brain. We review 1) the latest data on the anatomy of the opioid system, 2) the consequences of local intracerebral pharmacological manipulation of the opioid system on reinforced behaviors, 3) the consequences of gene knockout on reinforced behaviors and drug dependence, and 4) the consequences of chronic exposure to drugs of abuse on expression levels of opioid system genes. Future studies will establish key molecular actors of the system and neural sites where opioid peptides and receptors contribute to the onset of addictive disorders. Combined with data from human and nonhuman primate (not reviewed here), research in this extremely active field has implications both for our understanding of the biology of addiction and for therapeutic interventions to treat the disorder.
I. INTRODUCTION
The discovery of the opioid system stems from the use of opium in ancient history. Opium, extracted from poppy seeds (Papaver somniferum), has powerful pain-relieving properties and produces euphoria. Morphine, named after the god Morpheus, is the most active ingredient of opium. Today morphine remains the most widely used pain killer in contemporary medicine, despite an array of adverse side effects (respiratory depression, constipation, drowsiness, tolerance, and dependence). Heroin was synthesized chemically by morphine diacetylation in the late 1800s and was commercialized as the first nonaddictive opiate to treat cough and asthma. The strong addictive properties of heroin were soon acknowledged, and both heroin and opium were prohibited in 1910. Today heroin is a main illicit drug of abuse, and opiate addiction represents a major public health issue (for review, see Ref. 394).
The existence of opioid binding sites in the brain was established in 1973, and these were later referred as to mu, delta, and kappa opioid receptors. In 1975, two pentapeptides, Met- and Leu-enkephalin, were characterized as the first endogenous ligands for these receptors. Many peptides followed, forming the opioid peptide family (5). Enkephalins, dynorphins, and β-endorphin are produced by proteolytic cleavage of large protein precursors known as preproenkephalin (Penk), preprodynorphin (Pdyn), and proopiomelanocortin (POMC), respectively. All opioid peptides share a common NH2-terminal Tyr-Gly-Gly-Phe signature sequence, which interacts with opioid receptors. Genes encoding opioid peptide precursors were isolated in the late 70s-early 80s, and receptor cloning was achieved later. The first opioid receptor gene was isolated by expression cloning in 1992 and soon followed by the identification of several homologous genes (reviewed in Ref. 190). The opioid receptor gene family includes four members encoding mu (Oprm1), delta (Oprd1), kappa (Oprk1), and the nonopioid orphaninFQ/nociceptin (Oprl1) receptors. Opioid receptors are membrane receptors with a seven-transmembrane topology. These receptors belong to the large G protein-coupled receptor superfamily, which comprises several hundred members within the mammalian genome.
Opioid peptides and receptors are broadly expressed throughout peripheral and central nervous systems and have been the subject of intense investigations for several decades. The opioid system plays a central role in nociception and analgesia, and the main aspects of opioid-regulated pain mechanisms have been reviewed recently (102, 430). The opioid system also regulates numerous physiological functions, including responses to stress, respiration, gastrointestinal transit, as well as endocrine and immune functions. Most aspects of endogenous opioid activity are reviewed comprehensively each year (for example, see Ref. 41).
Importantly, this system also plays a key role in modulating mood and well-being, as well as addictive behaviors. How does this neuromodulatory system mediate or regulate the rewarding properties of drugs of abuse and contribute to the development of addiction? These questions are being investigated actively; relevant data were reviewed and discussed several years ago (131, 362, 389, 409). Here we review current knowledge of the role of the opioid system in hedonic control and of genetic regulations of the system following drug exposure, with a specific emphasis on recent data from rodent models and a focus on the neurocircuitry and behavioral aspects of opioid function.
II. ANATOMY OF THE BRAIN OPIOID SYSTEM
Opioid receptors and peptides are both broadly expressed throughout the brain. Figure 1 presents an updated overview of the anatomy of the opioid system in the rodent brain (rat and mouse). Figure 1A summarizes the distribution pattern of mu, delta, and kappa opioid binding sites (receptor protein), as determined by ligand autoradiography (144, 176, 193, 212, 226, 229, 230, 298, 343, 346) and the distribution of cell bodies expressing opioid receptors, based on the detection of mRNAs by in situ hybridization (ISH) (99, 129, 154, 227, 398). Figure 1B illustrates the distribution of opioid peptide containing neuronal fibers and cell bodies as assessed by immunohistochemistry (28, 107, 114, 187-189, 208, 226, 230), with ISH studies completing the mapping of opioid cell bodies (150, 251, 258, 296, 297).
Fig. 1.
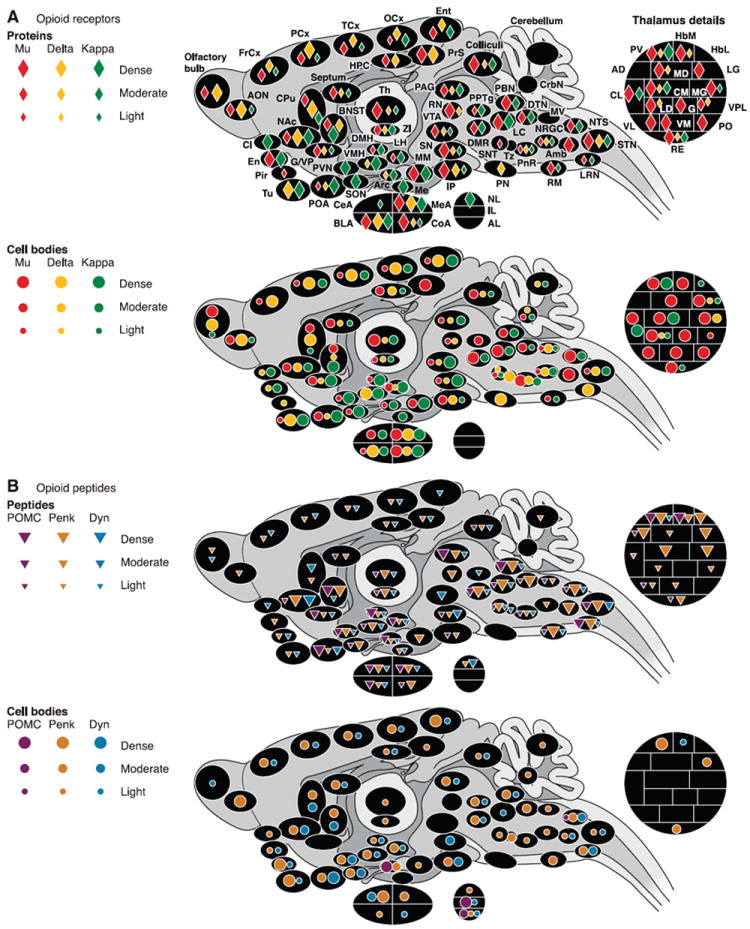
Anatomical distribution of opioid receptors (A) and peptides (B) in the rodent brain (rat and mouse). Only brain regions for which data are available in the literature are represented. Colors correspond to each of the three opioid receptor or peptide precursor. Densities are represented by symbols of different sizes, from low to high. A: receptors. Top panel represents the distribution of opioid receptor proteins as determined by ligand autoradiography. Maximal Bmax (receptor densities, radiolabeled ligands) values reported in the literature for mu and delta receptors were ~170–200 fmol/mg tissue equivalent (IP and olfactory bulbs, respectively). Maximal Bmax values recorded for kappa receptors were 80–100 fmol/mg (Cl; Refs. 144, 193, 346). Bottom panel summarizes the localization of cell bodies expressing opioid receptors based on the detection of mRNAs by in situ hybridization. B: peptides. Top panel depicts the pattern of distribution of opioid peptides by immunohistochemistry. Bottom panel maps cell bodies expressing opioid peptides, as evaluated both by immunohistochemical and in situ hybridization studies. Note: for immunohistochemical mapping, data based on antibodies for peptide precursors were used in priority. When not available, data based on antibodies for final peptides were used, with priority given to peptides issued from a single precursor (β-endorphin and dynorphin). Refer to text for further comments. Amb, nucleus ambiguus; AD, anterodorsal thalamus; AL, anterior lobe, pituitary; AON, anterior olfactory nucleus; Arc, arcuate nucleus, hypothalamus; BLA, basolateral nucleus, amygdala; BNST, bed nucleus of the stria terminalis; CeA, central nucleus, amygdala; Cl, claustrum; CL, centrolateral thalamus; CM, centromedial thalamus; CoA, cortical nucleus, amygdala; CPu, caudate putamen; CrbN, cerebellar nuclei; DMH, dorsomedial hypothalamus; DMR, dorsal and medial raphé; DTN, dorsal tegmental nucleus; En, endopiriform cortex; Ent, entorhinal cortex; FrCx, frontal cortex; G, nucleus gelatinosus, thalamus; G/VP, globus pallidus/ventral pallidum; HbL, lateral habenula; HbM, medial habenula; HPC, hippocampus; IL, intermediate lobe, pituitary; IP, interpeduncular nucleus; LC, locus coeruleus; LD, laterodorsal thalamus; LG, lateral geniculate, thalamus; LH, lateral hypothalamus; LRN, lateral reticular nucleus; MD, mediodorsal thalamus; Me, median eminence; MEA, median nucleus, amygdala; MG, medial geniculate; MM, medial mammillary nucleus; MV, medial vestibular nucleus; NAc, nucleus accumbens; NL, neuronal lobe, pituitary; NRGC, nucleus reticularis gigantocellularis; NTS, nucleus tractus solitarius; OCx, occipital cortex; PAG, periaqueductal gray; PCx, parietal cortex; Pir, piriform cortex; PN, pontine nucleus; PnR, pontine reticular; PO, posterior thalamus; POA, preoptic area; PPTg, pedunculopontine nucleus; PrS, presubiculum; PV, paraventricular thalamus; PVN, paraventricular hypothalamus; RE, reuniens thalamus; RN, red nucleus; RM, raphé magnus; SON, supraoptic nucleus; SN, substancia nigra; SNT, sensory trigeminal nucleus; STN, spinal trigeminal nucleus; TCx, temporal cortex; Th, thalamus; Tu, olfactory tubercle; Tz, trapezoid nucleus; VL, ventrolateral thalamus; VM, ventromedial thalamus; VMH, ventromedial hypothalamus; VPL, ventroposterolateral thalamus; VTA, ventral tegmental area; ZI, zona incerta.
Opioid receptors are expressed primarily in the cortex, limbic system, and brain stem. Binding sites for the three opioid receptors overlap in most structures, but some structures exhibit higher expression of one receptor over the others. Mu is the most expressed opioid receptor in the amygdala [but not the central nucleus, amygdala (CeA)], thalamus (TH), mesencephalon, and some brain stem nuclei. Kappa is the most represented receptor in the basal anterior forebrain, including the claustrum (Cl) and endopiriform cortex (En), olfactory tubercle (Tu), striatum (caudate putamen and nucleus accumbens), preoptic area (POA), hypothalamus, and pituitary. The delta receptor is the most abundant receptor in the olfactory tract (olfactory bulbs, anterior olfactory nucleus, Tu, medial amygdala) and in the cortices, including whole neocortex and regions of the amygdala (AMG) that derive ontogenically from the cortex (basolateral, cortical, and median nuclei of the AMG; Ref. 364), and is also highly expressed in the striatum. Mu and kappa coexist in most structures, whereas the distribution of delta is more restricted (low expression in the hypothalamus, thalamus, mesencephalon, and brain stem). In a few structures, only one receptor type is detected: mu binding sites only are detected in four thalamic nuclei (lateral geniculate thalamus, ventrolateral thalamus, ventromedial thalamus, and posterior thalamus), the sensory trigeminal nucleus (SNT) and nucleus ambiguus (Amb), and delta binding sites are singly represented in the pontine nucleus (PN). Kappa binding sites only are found in seven brain regions that are part of the stress axis (Cl, paraventricular hypothalamus, arcuate nucleus, supraoptic nucleus, Me, CeA, and pituitary). Notably, ligand autoradiography studies (144, 193, 226, 227, 229, 230, 343, 346) failed to detect significant kappa binding in the hippocampus, although consistent pharmacological and electrophysiological data indicate the presence of kappa receptors in this region (for example, Refs. 89, 106, 207, 340, 416).
The sites of opioid receptor expression (mRNA) generally match the distribution of binding sites (protein), suggesting that many neurons synthesizing opioid receptors are local neurons. Some structures show both mRNA and binding sites for only one receptor type: delta in the PN, mu in the Amb and some thalamic nuclei (ventrolateral, ventromedial, and posterior), and kappa in the Me. Noticeable exceptions exist to the general concomitant presence of mRNA and binding sites, with most frequent cases being detectable transcripts in the absence of binding sites (obvious for delta receptor). Such mismatches could reveal that receptors synthesized in some brain regions (mRNA only) are mostly transported to projection areas where they are localized presynaptically (binding sites only). Alternatively, these mismatches may reflect a lower sensitivity of ligand autoradiography in detecting binding sites compared with high detection efficiency in ISH studies.
Opioid peptide immunoreactivity (IR) in projection fibers overlaps largely with the localization of opioid receptors. Penk is the most abundant and widely distributed opioid precursor and is best detected in the thalamus, where it overlaps with mu receptors. Pdyn is present in most brain structures, with the highest concentration in the nucleus accumbens (NAc) and near absence in the thalamus. POMC shows a more restricted distribution and is absent from cortical structures except for the AMG.
Penk-expressing cell bodies (mRNA and immunoreactivity) are the most abundant in the brain. Pdyn cell bodies are also widespread, with a hot spot in the hypothalamus matching high kappa receptor density. In contrast, POMC cell bodies are highly restricted and only detected in three regions of the brain: the arcuate nucleus of the hypothalamus (Arc), nucleus tractus solitarius (NTS, brain stem), and pituitary [anterior lobe (AL) and intermediate lobe (IL)]. However, some mRNA can be detected in other regions by PCR (211). Neurons from the Arc and NTS project mostly to limbic, mesencephalic, and brain stem subcortical regions, where high POMC IR is detected. Mismatches exist between the distribution of opioid peptide IR and the localization of cell bodies, with the most frequent cases being IR detected in fibers with no visible cell bodies (obvious for POMC). These discrepancies between peptide and cell body maps, assessed by the same IR technique, suggest that an important proportion of opioid peptides is released by projecting neurons.
Altogether, the anatomical studies show widespread expression of most components of the opioid system. Pharmacological studies have shown best affinities of β-endorphin and enkephalin for mu and delta receptors, and preferred binding of dynorphins at the kappa receptors, although selectivity factors do not exceed one-order of magnitude (228). The overall anatomical distribution of peptides and receptors is consistent with the notion of distinct Penk-POMC/mu-delta and Pdyn/kappa systems at some brains sites.
III. LOCAL PHARMACOLOGICAL MANIPULATION OF THE OPIOID SYSTEM
In the wild, animals spend most of their time engaged in behaviors necessary for their survival and that of their genes, such as foraging for food and water, avoiding predators, looking for sexual partners, or caring for pups. These goal-directed behaviors must be flexible and plastic so that animals can constantly adapt to their environment (185). In this context, emotions have evolved that encourage animals to engage in behaviors with a beneficial outcome and avoid behaviors that could reduce their chance of survival (58). Positive emotions, such as pleasure, hedonism, or reward, when associated with the ability to learn from experience, can act to increase the probability of the occurrence of a particular behavior, a phenomenon called positive reinforcement (see Refs. 112, 326).
The view that distributed interconnected neural systems could support positive reinforcement developed from the discovery of specific brain sites supporting self-stimulation (271) and the concurrent identification of ascending monoamine pathways (87). The mesolimbic dopaminergic projections, that originate from the ventral tegmental area (VTA) and project to various regions of the forebrain with a major input to the NAc, occupy a central position within the reinforcement circuit (20, 58, 112, 168, 183, 185, 270). Nonetheless, accumulating experimental evidence argues against dopamine (DA) mediation of reward processes per se (37, 58, 112, 326, 333, 412). As a result of this debate, the concept of the reinforcement circuit has expanded beyond the VTA-NAc to include other structures (202, 265; see Fig. 2).
Fig. 2.
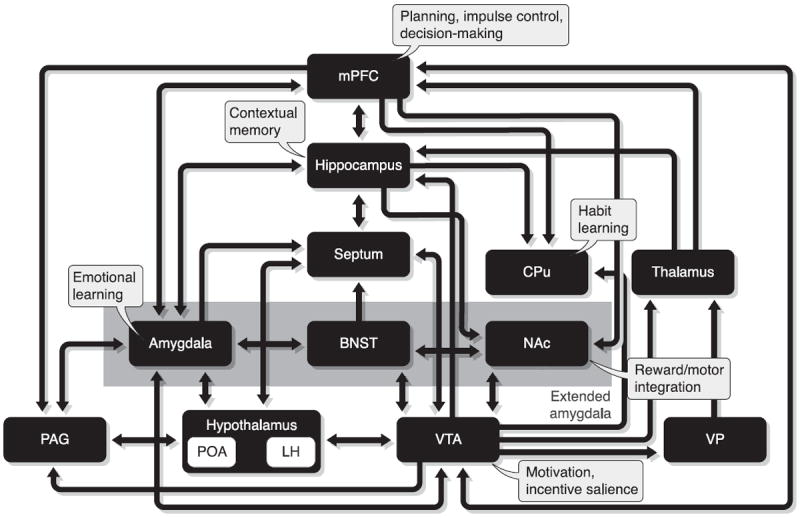
Schematic representation of the brain reinforcement circuit. Brain sites of reinforcement are integrated in a circuit based on their connectivity and putative functional roles. BNST, bed nucleus of the stria terminalis; CPu, caudate putamen; LH, lateral hypothalamus; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; PAG, periaqueductal gray; POA, preoptic area; VP, ventral pallidum; VTA, ventral tegmental area. [Adapted from Kelley (183) and Koob and Le Moal (202).]
Opioid peptides and receptors are expressed throughout the reinforcement network, placing the opioid system in a key position to modulate this circuit. Experimental data have accumulated over nearly 50 years showing that the opioid system is involved with reinforcement processes. Globally, systemic mu and, to a lesser extent, delta agonists produce positive reinforcement, whereas kappa agonists induce aversion, hallucinations, and malaise. Conversely, mu and delta antagonists suppress the positive reinforcing properties of natural rewards and opiate or nonopioid drugs, whereas kappa antagonists facilitate these effects (reviewed in Refs. 339, 389, 390). Systemic drug effects, however, reflect multiple actions of opioid compounds at several brain sites, not all of which are involved in reinforcement. In this section, we focus on studies using intracranial injection techniques that allow a precise localization of brain sites where opioids modulate reinforcement. Methodological issues related to pharmacological, anatomical, and behavioral specificities following intracranial infusions have been reviewed previously (44, 136, 171).
A. The Endogenous Opioid System and Natural Reinforcement
Among numerous natural appetitive stimuli, food, drinks, and potential mates are the most potent to trigger positive reinforcement in animals and, as such, are widely used in animal studies. Studies using food reinforcement have raised considerable interest in neuroscience and produced extensive experimental evidence that the opioid system plays a role in the reinforcing properties of diets (solid or liquid). Less is known about the neurobiological processes supporting sexual reinforcement, although some studies indicate that here also the opioid system is recruited.
1. Food reinforcement
A complex, widespread neuronal circuitry mediates sensory, metabolic, and integrative processes controlling food intake (39) and opioid peptides and receptors are present in most regions of this network (38, 138, 185). Feeding can be qualified as either homeostatic (providing the necessary intake of calories to sustain life) or nonhomeostatic (driven by other processes, such as reward/hedonics, and often resulting in a higher intake than needed). Opioids are involved in both types of food intake, depending on the brain region, suggesting that this system plays a role in energy-driven feeding as well as food hedonics. Glass et al. (138) and later Bodnar (42) reviewed the vast amount of work produced over the last three decades on the pharmacology of feeding behavior. These authors dedicated specific sections to studies using intracerebral microinjection techniques, which we update here with a particular focus on food reinforcement. Brain sites where agonists and antagonists modulate feeding behavior are summarized in Figure 3 (data from literature reviewed below, see also Refs. 175, 383).
Fig. 3.
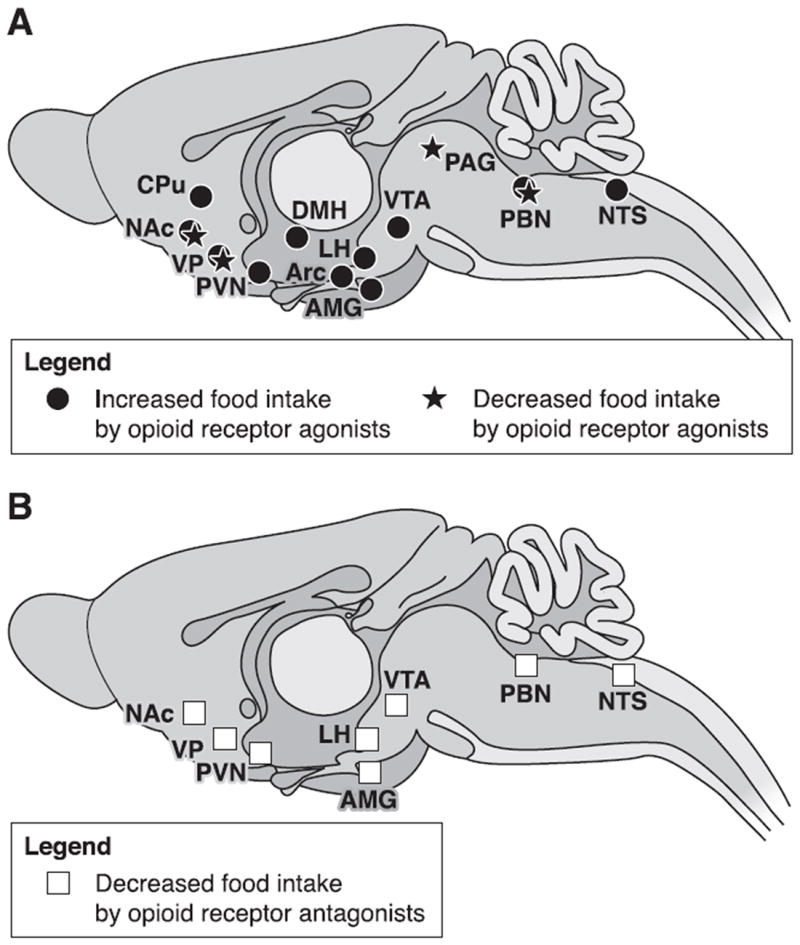
Brain sites where opioid agonists or antagonists modulate food intake. A: brain sites where opioid receptor agonists injected locally either increase or decrease food intake. B: brain sites where microinjections of opioid receptor antagonists decrease feeding behavior. Food was either standard chow or palatable (energy-dense) diet. Food intake was either basal or induced by food deprivation, intracerebral electrical stimulation, or local pharmacological injection. Opioid agonists and antagonists modulate food intake mainly through regulation of hedonic evaluation, but also contribute to these behaviors by modulating integration of sensory processes or the regulation of energy needs. AMG, amygdala; Arc, arcuate nucleus, hypothalamus; CPu, caudate putamen; DMH, dorsomedial hypothalamus; LH, lateral hypothalamus; NAc, nucleus accumbens; NTS, nucleus tractus solitarius; PAG, periaqueductal gray; PBN, parabrachial nucleus; PVN, paraventricular hypothalamus; VP, ventral pallidum; VTA, ventral tegmental area.
A) NAC AND VTA
In accordance with the view of a reinforcement circuit centered on dopaminergic structures and their anatomical targets, research on the food reinforcement has focused on the NAc and VTA. These two structures play a pivotal role in the control of food intake (19, 185). Injections of mu or delta but not kappa receptor agonists into the NAc or VTA stimulate feeding (reviewed in Refs. 19, 42). The hyperphagia induced by intra-NAc infusion of mu receptor agonists depends on macronutrient content and the taste of the food. Kelley and collaborators (19, 184) proposed that activating the ventral striatal opioid system encodes the positive affect induced by tasty and/or high-energy foods and triggers behavioral responses associated with food-seeking. Consistent with this idea, Pecina and Berridge (287, 288) demonstrated that the shell of the NAc contains a hedonic hotspot in which the stimulation of mu receptors increases the “liking” for food reward, as measured by the amplification of positive affective orofacial reactions to sucrose in rats, and the “wanting” for food as reflected by increased eating behavior. Opioid stimulation in a larger region surrounding this hotspot also triggers food intake but has no effect on “liking” reactions to taste (287, 288). The inactivation of mu receptors in the shell of the NAc by infusion of the irreversible mu receptor antagonist β-funaltrexamine (β-FNA) induced a persistent decrease in the consumption of a palatable glucose solution, with no effect on the intake of standard chow. In comparison, the peptidic mu receptor antagonist CTAP produced only a transient decrease in glucose consumption. These results provide further evidence that the NAc supports responding for orosensory reward (400). Consistent with this view, Woolley et al. (413) explored the role of accumbal opioid receptors in flavor preference. In a first study, the authors investigated the role of mu opioid receptors in the NAc in a flavor choice paradigm where two nutritionally identical, but differentially flavored, palatable food pellets were available. Intra-NAc DAMGO selectively increased, whereas naltrexone decreased, consumption of the preferred food (413). Using a sensory specific satiety paradigm, these authors demonstrated that the mu receptor agonist DAMGO injected into the NAc selectively increases consumption of a prefed flavor, reversing the sensory specific satiety effect, while the antagonist naltrexone potentiated this effect. Conversely, injecting the kappa receptor agonist U-50,488H into the NAc of rats decreased the consumption of the prefed flavor, but increased the intake of the non-prefed flavor. Together, these data suggest that opioid peptides released in the NAc during consumption of palatable foods can produce opposite effects on flavor preference depending on the opioid receptors they activate (414, 415).
The mechanism by which the striatal opioid system interacts locally with other neurotransmitters to regulate feeding behavior remains to be clarified. High-fat feeding induced by intra-NAc DAMGO was unaffected by prior infusion of D1 and D2, AMPA, or nicotinic receptor antagonists into this structure. In contrast, the opioid receptor antagonist naltrexone blocked DAMGO-induced feeding, and the muscarinic receptor antagonist scopolamine injected into the NAc reduced feeding in both DAMGO-treated and control rats. These data suggest that the effects of ventral striatal opioid receptor stimulation on palatable food intake are independent of the activation of DA and glutamate receptors but may recruit cholinergic signaling (408).
Opioid regulation of food consumption in the NAc depends not only on local mechanisms, but also on connections with distant brain sites (185); in that respect, the cross-talk between the NAc and VTA has been particularly well studied. Food intake elicited by infusion of the mu receptor agonist DAMGO into the VTA was dose-dependently decreased by injections of the opioid receptor antagonist naltrexone or the D1 DA receptor antagonist SCH-233390 into the NAc (220). Together with previous data from the same group (221), these results suggest that opioid peptides and DA interact within the VTA-NAc pathway (and reciprocal) to regulate feeding. Moreover, the general opioid receptor antagonist naltrexone, and mu (β-FNA) or delta (naltrindole), but not kappa (nor-binal-torphimine) receptor antagonists preinfused into the VTA dose-dependently reduced DAMGO-induced feeding elicited from the NAc. Reciprocally, nonselective antagonists infused into the NAc decreased mu-induced feeding triggered from the VTA. Thus the bidirectional opioid-opioid feeding interaction between the VTA and NAc involves all opioid receptor subtypes (43). The accumbal opioid system has been shown to interact with orexinergic signaling to modulate food intake. The opioid receptor antagonist naltrexone injected into the NAc shell suppressed the increase in food-intake produced by an infusion of orexin-A into the lateral hypothalamus (LH, 365). Moreover, infusing the orexin-1 receptor antagonist SB-334867 into the VTA blocked the increase in high-fat appetite induced by DAMGO injected into the NAc. This result suggests that activation of accumbal mu receptors recruits orexin neurons in the perifornical region. In turn, orexin released from perifornical neurons would stimulate orexin-1 receptors in the VTA to facilitate the intake of palatable food (425).
Together with previous results published in the field (reviewed in Refs. 42, 138, 185), the above experimental data clearly indicate that mu opioid receptors potently induce feeding behavior from the NAc or VTA, an effect exerted through regulation of the affective response to food, i.e., palatability.
B) VENTRAL PALLIDUM
As a major output structure of the NAc (421), the ventral pallidum (VP) plays a role in food reinforcement (39). Interestingly, the VP contains a similar hedonic hotspot as the NAc in its posterior section (287, 347, 371), where stimulation of mu opioid receptors generates an increase in both “liking” and “wanting” for food reward. The mu receptor agonist DAMGO, when injected into the posterior VP, increased hedonic “liking” reactions to oral sucrose but conversely suppressed these reactions when infused into the anterior and central VP. Concerning the “wanting” aspect, DAMGO stimulated food intake when injected into the posterior and central VP and suppressed eating when delivered into the anterior VP. These results show that opioid-mediated increases in food reward and eating behavior in the VP are related but dissociable (347). Injecting the mu receptor agonist DAMGO into the VP produced a biphasic effect on saccharin intake and further demonstrated the modulatory role of VP mu receptors on the consumption of palatable food (338).
Smith and Berridge (347) looked for neurobiological and functional interactions between the two opioid hedonic hotspots of the NAc and VP. Naloxone injected into the NAc blocked the increase in hedonic “liking” reactions triggered by intra-VP injection of DAMGO, and reciprocally blocked “liking” reactions triggered by intra-NAc injection of DAMGO when injected into the VP. When injected into the NAc, naloxone attenuated the stimulation of food intake (“wanting”) produced by intra-VP DAMGO. In contrast, when injected into the VP, naloxone failed to affect the increased food consumption induced by intra-NAc DAMGO. Thus the NAc opioid hotspot is sufficient to stimulate eating behavior and is independent of VP opioid activation (347). In conclusion, compared with NAc and VTA receptors, pallidal mu opioid receptors are the most efficient in modulating food intake.
C) HYPOTHALAMUS
The hypothalamus plays a central role in the control of food intake and the regulation of energy balance, through its multiple connections with brain stem, limbic, and cortical structures (39, 185, 259). Among the numerous hypothalamic nuclei, the paraventricular hypothalamus (PVN), lateral hypothalamus (LH), Arc, and dorsomedial hypothalamus (DMH) have been identified as sites where opioid peptides modulate eating (42, 185, 355). Activating mu receptors in the PVN by local agonist injection produces an increase in food intake. Conversely, blocking mu or kappa receptors, but not delta receptors, reduces deprivation-induced feeding (reviewed in Ref. 42). Naleid and collaborators tested the hypothesis, based on their previous results (137), that opioids in the PVN would modulate food intake based on energy needs more than palatability. Intra-PVN DAMGO stimulated sucrose and fat feeding in rats that preferred fat diets, but had no effect on either diet intake in sucrose-preferring animals. Naltrexone decreased fat intake in both groups but had no effect on sucrose intake. Thus activation of opioid receptors in the PVN modulates feeding depending on diet preference and nutrient type, as well as on energy needs (263). Finally, morphine injected into the LH stimulated feeding behavior (215). Whether this effect involves a role of LH opioids on the hedonic aspect of food, however, remains to be examined.
D) AMYGDALA
AMG lesions implicate the AMG in food intake (39, 159). Within the AMG, the CeA is traditionally considered as part of the taste pathway (269) and has been proposed to regulate the emotional aspects of food intake (138). The mu receptor agonist DAMGO injected into the CeA increased food intake (134, 213). Conversely, infusion of the opioid receptor antagonist naltrexone decreased food intake, but only for preferred diets, suggesting that endogenous opioids in this structure are involved in modulating food reward rather than energy needs (137). Intra-NAc DAMGO-induced feeding depends on the integrity of AMG function, as it was completely suppressed by pharmacological inactivation of either the basolateral or central nuclei of the AMG (BLA and CeA; Ref. 407). Furthermore, Kim et al. (192) presented evidence for the existence of a bidirectional opioid connection between the shell of the NAc and the CeA. Indeed, naltrexone injected into the CeA reduces food intake triggered by DAMGO infused into the NAc, and vice versa (192). Thus the AMG, and more specifically the CeA, are part of an opioid limbic network modulating food reinforcement.
E) PARABRACHIAL NUCLEUS
The parabrachial nucleus (PBN) is part of the taste pathway and is involved in sensory mechanisms modulating food intake (39, 269). Mu receptor activation in this region triggers food intake, whereas kappa receptor stimulation or mu receptor blockade decreases feeding behavior (60, 260, 261, 267, 410). A long-lasting decrease in the consumption of standard, but not palatable, chow was observed after an infusion of the irreversible mu receptor antagonist β-FNA into the PBN, as well as a blockade of the increase in palatable food intake induced by intra-PBN DAMGO (401). These results suggest that opioids in the PBN, unlike the structures reviewed above, play a role in the control of homeostatic rather than reward-driven feeding.
F) NTS
The NTS contains the first central synapse of the gustatory system (374) and as such is an essential link in the chain of food reward. Activation of mu receptors in the NTS increased feeding, an effect suppressed by mu receptor blockade in the CeA (139). Moreover, opioid receptor antagonists injected into the NTS reduced feeding responses elicited by intra-PVN injections of neuropeptide Y (NPY; reviewed in Ref. 42). Further study will be needed to determine whether opioids in the NTS modulate homeostatic or reward-driven feeding.
2. Sexual reinforcement
As with food intake, the control of the multiple aspects of sexual behavior relies on a complex neurocircuitry, where the components of the opioid system are well represented (73, 135, 166, 248, 295, 387). Opioids are commonly described as exerting an inhibitory influence on male and female sexual activity. Indeed, systemic opioid receptor agonists impair, and antagonists facilitate, sexual behavior (9). Local injections of opioid receptor agonists and antagonists have provided a partial mapping of the brain sites where endogenous opioids influence sexual behavior.
A) NAC AND VTA
Local injections of opioid receptor antagonists into the mesoaccumbens DA pathway have failed to reveal a major contribution of accumbal opioid system to sexual behavior. The kappa receptor antagonist nor-BNI injected into the NAc increased female directed behavior in male rats (214). However, when using a conditioned place preference (CPP) protocol to specifically assess sexual reinforcement (see Refs. 286, 294), Agmo and Gomez (2) could not detect an effect of intra-NAc injections of the opioid receptor antagonist methylnal-oxonium on an ejaculation-induced CPP. Similarly, the opioid receptor antagonist naloxone infused into the NAc did not affect paced mating-induced CPP in female rats (126). In contrast, opioid agonists, when injected at low doses into the VTA, facilitated sexual behavior in male rats, whereas naloxone decreased the percentage of sexually active rats (reviewed in Ref. 385) and prevented the anticipatory behavioral activation prior to the introduction of a female rat (386). Injections of the kappa receptor agonist nor-BNI into the VTA increased female directed behavior (214). It is not clear, however, whether intra-VTA opioid effects on sexual behavior are mediated through reinforcement mechanisms or an effect on somatomotor control of sexual performance. Taken together, experimental data indicate that the opioid system in the VTA, and to a lesser extent in the NAc, modulates sexual behavior.
B) HYPOTHALAMUS
The hypothalamus is critically involved in coordinating the behavioral sequences as well as autonomic and endocrine responses associated with reproductive behavior (248, 314, 342). In the anterior region of the hypothalamus, the sexual dimorphic medial preoptic area (MPOA) has received much attention as a critical brain substrate for the control of sexual behavior (23, 104, 166, 295, 387), where opioids can exert their modulatory effects (9, 166, 387). Briefly, opioid receptor agonists injected directly into the MPOA inhibited or delayed masculine copulatory activity in rats, except for low doses of morphine or dynorphin-A-(1—13) (reviewed in Ref. 387; see also Refs. 241, 385) and inhibited female sexual behavior under certain conditions (reviewed in Refs. 292, 293). Conversely, opioid receptor blockade in the MPOA can facilitate male sexual behavior depending on the experimental conditions (387). These data indicate that opioid receptors in the MPOA participate to the control of sexual behavior. Interestingly, some authors have used CPP to measure sexual reinforcement and its modulation by opioidergic manipulations in the MPOA. The opioid receptor antagonist methylnaloxonium infused into the MPOA blocked a CPP produced by ejaculation (2). Consistent with this, naloxone microinjected into the MPOA, before each conditioning session, suppressed paced mating-induced CPP in female rats (126). These latter data suggest that opioids in the MPOA are involved, more specifically, with sexual reinforcement.
At the interface between the central and peripheral autonomic nervous systems, the PVN plays an important role in the control of genital responses (248). In males, the PVN participates in the control of penile erection (10) under the inhibitory influence of endogenous opioids. Indeed, when injected into this structure, opioid agonists markedly impaired penile erection (reviewed in Refs. 9 and 10).
The ventromedial nucleus of the hypothalamus (VMH) is involved in female sexual behavior, under the critical influence of estrogens (57, 117). Opioid peptides and opiates injected into the VMH can inhibit female sexual behavior (reviewed in Ref. 292). Such inhibition could reflect an influence of opioids in the VMH on sexual reinforcement, as suggested by the suppression of paced mating-induced CPP observed following intra-VMH naloxone infusion in female rats (126).
C) AMYGDALA
The cortical and medial divisions of the AMG, which send a major projection to the MPOA, have been implicated in the neural control of sexual performance, more specifically in the processing of sex-related olfactory information (387). The activation of opioid receptors in the corticomedial AMG may exert an inhibitory influence on this processing (246, 247). However, a role for this region in sexual reinforcement cannot be excluded, as naloxone infused into the medial nucleus of the AMG (MeA) suppressed paced mating-induced CPP in female rats (126). The basolateral division of the AMG (BLA) plays a role in conditional aspects of sexual behavior (315) and expresses high densities of opioid receptors. Unfortunately, the role of these receptors in sexual reinforcement has not yet been studied.
3. Conclusion
The data reviewed above clearly demonstrate that brain opioids play a critical role in modulating food and sexual reinforcement.
With regard to food, intracerebral pharmacological studies, in accordance with systemic data, support the now widely accepted view that the central opioid system mediates the hedonic evaluation (palatability) of energy-dense foods (19, 24, 42, 282, 289). Thus opioids in reward sites (VTA, NAc, VP, AMG) participate preferentially in the modulation of nonhomeostatic feeding. New animal models of food reinforcement have been developed that tease out rewarding from motivational/learning aspects of reinforcement and show how opioids in these regions are mainly involved in the former and DA in the latter (19, 24, 289). Opioids in the brain stem (NTS, PBN) also participate in the integration of sensory and metabolic aspects of food intake. In addition, opioids in the hypothalamus (PVN, Arc) are involved in the regulation of energy needs, in tight connect with reward structures where they also control the emotional processing of food intake (138). The role of opioid peptides and receptors in other brain structures involved in the control of feeding behavior, especially limbic structures such as the hippocampus, septum, or bed nucleus of the stria terminalis (BNST), has not been examined.
Studies in the field of sexual reinforcement clearly implicate central opioids, especially in the MPOA. Other naturally reinforced behaviors, most notably social behaviors such as pair bonding, mother-infant attachment, and social play, also recruit the brain opioid system (reviewed in Ref. 390; see also Refs. 252, 256, 264, 284, 418). To date, however, data from intracerebral pharmacological studies are too scarce to draw a clear picture of the relevant neuronal network.
B. The Endogenous Opioid System and Drugs of Abuse
Drug addiction is a chronic disorder that builds up from initial recreational drug use and progresses towards compulsive drug seeking and intake. The reinforcing properties of abused drugs are thought to be responsible, in interaction with various environmental factors, for the initiation of drug taking. Once repeated drug use is established, complex neuroadaptative mechanisms develop that lead to dependence, craving, and relapse and contribute to the maintenance of repeated drug intoxication (201). A current hypothesis in the field of drug addiction is that drugs of abuse abnormally recruit neuronal pathways and transmitter systems responding to natural reinforcement and progressively alter their function (112, 168, 186, 266, 356). The mesolimbic DA system has received most attention in this regard. The opioid system, which mediates hedonic evaluation of natural rewards, represents another key substrate for the deleterious effects of drugs of abuse. Indeed, the reinforcing properties of many abused drugs depend on the activation of mu opioid receptors (for review, see Refs. 72, 191), which thus may be a potential molecular gateway to drug addiction (72).
Research with animal models has led to significant progress in understanding the neurobiological basis of drug reinforcement and addiction. Most of these models assess the direct and acute reinforcing properties (positive or negative) of drugs. As such, these models explore the initial stage of drug addiction, characterized by repeated intoxication in nondependent individuals (201). Two models are most widely used: CPP and operant self-administration (see Refs. 244, 329, 378 for methodology). In these two models, drugs can be administered intracerebrally, either to map brain sites of reinforcement or to study how activation in these sites interferes with reinforcement induced by systemic drug administration.
Figure 4 illustrates the location of brain sites where opioid receptors contribute to drug reinforcement, both opiate and nonopiate. In this section we update reviews presented a decade ago by Shippenberg and Elmer (339), McBride et al. (244), and Van Ree et al. (389).
Fig. 4.
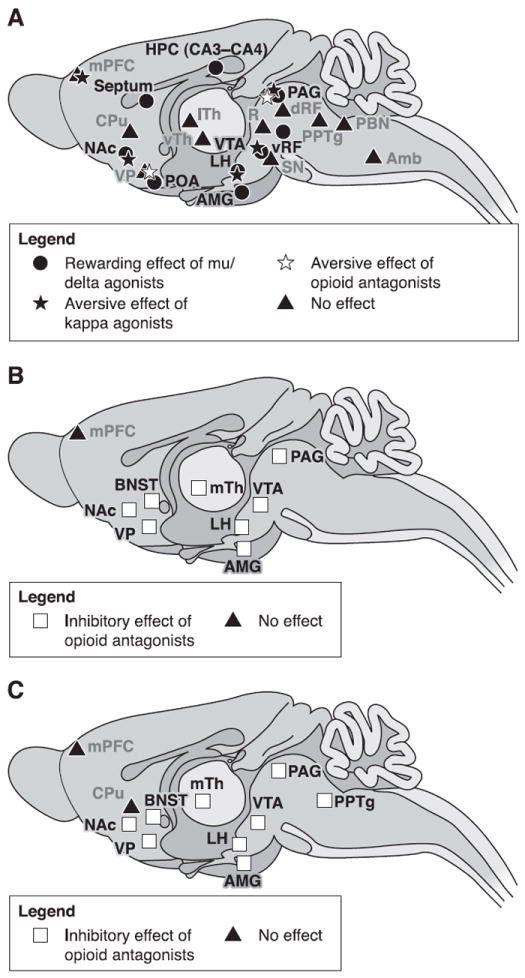
Brain sites where opioid agonists or antagonists modulate drug reinforcement. A: brain regions where injections of opioid agonists have direct positive or negative reinforcing properties. B: brain sites where microinjections of opioid antagonists inhibit the reinforcing effects of opioid drugs given systematically. C: brain areas where local injections of opioid antagonists decrease reinforcing properties of systemic nonopioid drugs (ethanol, cocaine, or nicotine). Reinforcement was assessed using animal models of drug-induced conditioned place preference or self-administration. Brain regions represented on this figure were reported to be sensitive to local opioid injections at least once in the literature. Conflicting results might have been reported in other studies, most often using a different animal model. Noteworthy, most brain areas where opioid manipulations impact on drug reinforcement express all three types of opioid receptors. Amb, nucleus ambiguus; AMG, amygdala; BNST, bed nucleus of the stria terminalis; CPu, caudate putamen; dRF, dorsal reticular formation; HPC, hippocampus; LH, lateral hypothalamus; lTh, lateral thalamus; mPFC, medial prefrontal cortex; mTh, medial thalamus; NAc, nucleus accumbens; PBN, parabrachial nucleus; PAG, periaqueductal gray; POA, preoptic area; PPTg, pedunculopontine nucleus; R, red nucleus; SN, substancia nigra; VP, ventral pallidum; vRF, ventral reticular formation; VTA, ventral tegmental area; vTh, ventral thalamus.
1. Drug reinforcement
A) VTA AND NAC
The mesolimbic dopaminergic system has long been considered the major neurobiological substrate mediating opiate reinforcement (25, 101, 197, 339, 389). Consequently, the characterization of opioid-sensitive brain sites has focused mainly on the VTA and NAc.
Van Ree and de Wied (388) were the first to demonstrate that rats readily self-administer an opioid receptor agonist into the VTA; a follow-up study confirmed this finding (47). Since then, an overwhelming number of studies using either intracerebral self-administration or CPP in rats and mice have confirmed the involvement of the VTA in opiate reinforcement (reviewed in Refs. 244, 339, 389). Further work has attempted to identify, more precisely, the opioid receptors and neurotransmitter systems recruited by intra-VTA opiate reinforcement. Intra-VTA infusions of a subthreshold dose of heroin, combined with a nonrewarding systemic dose of the benzodiazepine alprazolam (which facilitates GABA transmission), produced a significant CPP (395). This finding indicates that systemic facilitation of GABA tone potentiates the reinforcing properties of centrally applied opiate drugs. The mu receptor agonist endomorphin induced a CPP when injected into the VTA or NAc, an effect suppressed by a prior infusion of the mu receptor antagonist CTOP and the opioid receptor antagonist 3-methoxynaltrexone in the VTA or NAc, respectively (369). In another study, the mu receptor agonist endomorphin induced a CPP when infused into the posterior VTA, but not the anterior VTA or the NAc. Moreover, rats self-administered endomorphin into the VTA. These results indicate that mu receptors in the VTA are critically involved in reinforcement and that the VTA is not functionally homogeneous (422). Wild-type and delta opioid receptor knockout mice, but not mu receptor knockout mice, self-administered morphine into the VTA, and systemic naloxone suppressed self-administration. Mu, but not delta, receptors in the VTA thus appear to be critical for the rewarding properties of intra-VTA morphine infusions (96).
The mechanism by which accumbal opioid receptors contribute to opiate reinforcement remains debatable. Early evidence indicated that rats self-administer opioid receptor agonists into the NAc (140, 272), but intra-NAc infusions of mu or delta receptor agonists do not support a CPP (reviewed in Ref. 244). In contrast, intra-NAc microinjections of the kappa receptor agonist U-50,488H produce a conditioned place aversion (CPA; Ref. 22). Preinfusing (+)-morphine (inactive isomer) into the posterior shell of the NAc attenuated a CPP induced by (−)-morphine (active isomer) injected into this structure (370). A CPP to systemic morphine in rats was suppressed by pretest intra-NAc injection of the mu receptor agonist DAMGO, the delta receptor agonist DPDPE, or the kappa receptor agonist U-50,488H. The authors proposed that stimulation of mu and delta opioid receptors in the NAc compete with systemic opiate seeking by producing direct reinforcing effects, while stimulation of kappa receptors produces aversive effects (217). Mice self-administer morphine into the NAc, but not into the caudate putamen (CPu), an effect that is reduced by systemic naloxone. These results suggest that the NAc, but not the CPu, is involved in the reinforcing properties of opiates (93). In a subsequent study, the authors observed that the D2/D3 DA receptor antagonist sulpiride administered before testing reduced (although transiently) intra-VTA but not intra-NAc, morphine self-administration. This work shows that maintenance of intra-VTA, but not intra-NAc, self-administration involves activation of D2/D3 DA receptors (95).
Intra-accumbal injections of opioid receptor antagonists suppress systemic opiate reinforcement (reviewed in Refs. 244, 339, 389). Recent studies have further characterized this phenomenon. The irreversible mu receptor antagonist β-FNA injected into the caudal, but not the rostral part, of the NAc decreased the reinforcing properties of intravenous heroin. Thus the caudal portion of the nucleus accumbens might be more critically involved in mediating the reinforcing properties of opiates (236). The opioid receptor antagonist methylnaloxonium reduced heroin self-administration when injected into the shell of the NAc of morphine-dependent rats, whereas it had no effect in nondependent rats (but see Ref. 382). The authors propose that opioid receptors in the NAc contribute to opioid reinforcement only in dependent animals (396). When injected into the NAc, the mu receptor antagonist CTOP, the D1 DA receptor antagonist SCH23390, the D2 DA receptor antagonist raclopride or the combination of SCH23390 and CTOP, but not of the delta receptor antagonist naltrindole, dose-dependently decreased breakpoints to self-administer intravenous speedball (combination of cocaine and heroin). Therefore, the activation of DA and mu receptors, but not delta receptors, in the NAc is critical for the reinforcing effects of speedball (79).
Opioid receptors in the VTA and NAc not only contribute to opiate reinforcement, but also contribute to the reinforcing effects of nonopioid, systemic drug injections. A number of studies have explored this phenomenon over the last decade, using local injections of opioid receptor agonists or antagonists and a variety of drugs including cocaine, speedball, nicotine, and alcohol.
The participation of opioid receptors in the VTA and/or the NAc in ethanol reinforcement has generated considerable interest over the last decade, with consistent data from operant models of ethanol oral self-administration. Intra-VTA microinjection of the opioid receptor antagonist methylnaloxonium immediately before a test session decreased an ethanol-induced CPP in mice, whereas intra-NAc methylnaloxonium had no effect. This study suggests that a CPP to ethanol depends primarily on the activation of opioid receptors in the VTA (29). Infusion of the opioid receptor antagonist methylnaloxonium into the AMG or the NAc decreased operant responding for ethanol solution in rats, at lower doses in the AMG than in the NAc, indicating that opioid receptors in the AMG and NAc may be involved in the regulation of ethanol self-administration (155). Microinjecting the delta receptor antagonist naltrindole into the NAc or BLA reduced operant responding for ethanol solution in rats, whereas the mu antagonist CTOP was effective only in the AMG. Thus mu and delta opioid receptors in the NAc and BLA are involved in the regulation of ethanol self-administration (169). A selective decrease in operant behavior for ethanol, but not saccharin, solution was observed in alcohol-preferring rats after an injection of the mu receptor antagonist nalmefene into the VTA or NAc. When injected into the hippocampus, nalmefene reduced both ethanol and saccharin consumption. This work provides evidence that nalmefene suppresses ethanol-motivated behavior through the blockade of opioid receptors in the NAc or VTA (178). In a two bottle choice paradigm, the delta receptor agonist DPDPE injected into the VTA decreased, while the delta receptor antagonist TIPP-Ψ increased, ethanol intake in rats. These findings suggest that activation of delta receptors in the VTA acts to inhibit ethanol consumption (232). Altogether, these results demonstrate that operant responding for ethanol solution depends on the activation of mu and delta opioid receptors in the NAc and AMG, and to a lesser extent on the recruitment of VTA opioid receptors.
Opioid receptors in the VTA and NAc contribute to cocaine reinforcement. The mu receptor antagonist CTAP injected into the core of the NAc or rostral VTA, but not the caudal VTA, CPu or medial shell of the NAc attenuated the development of a cocaine CPP. In contrast, CTAP infused into the medial shell, but not the core, of the NAc blocked the expression of a cocaine-induced CPP (349). In rats trained to self-administer intravenous cocaine, infusing the mu receptor agonist DAMGO into the VTA decreased operant behavior. In contrast, the mu receptor antagonist CTOP reduced self-administration only slightly, and the kappa receptor agonist U-50,488H and antagonist norbinaltorphimine (norBNI) had no consistent effect. These data show that the activation of ventral tegmental mu, but not kappa, receptors interfere with cocaine reinforcement (81). Similarly, infusing the irreversible mu receptor antagonist β-FNA into either the NAc or the VTA attenuated systemic cocaine self-administration under a progressive ratio schedule (402). In a subsequent study by the same group, the delta receptor antagonist naltrindole 5′-isothiocyanate (5′-NTII) decreased systemic cocaine self-administration when microinjected into the NAc, but increased operant responding for the drug when injected into the VTA, and had no effect when injected into the AMG, indicating that delta receptors can modulate cocaine reinforcement in a different direction depending on the brain site (403). Finally, infusing the irreversible mu receptor antagonist β-FNA into the VTA (or the VP, see below), but not into the NAc, of trained rats, reduced rates of responding for a speedball to levels of responding for cocaine infusions, suggesting that heroin facilitates cocaine self-administration by attenuating feedback inhibition from the NAc to the VTA (235).
As for cocaine, pharmacological manipulation of opioid receptors in the VTA or NAc alters intravenous nicotine self-administration. In rats trained to self-administer nicotine, a high dose of the mu receptor agonist DAMGO into the VTA reduced operant responding for nicotine, with no effect on cocaine self-administration. The authors concluded that nicotine exerts its reinforcing properties by recruiting mu receptors in the VTA (82).
In conclusion, the data reviewed above demonstrate the contribution of mu and possibly delta receptors in the VTA and NAc to mediate the positive reinforcing properties of opiates. Moreover, receptor blockade in these two regions decreases conditioned responses for nonopioid drugs given peripherally, strongly suggesting that the reinforcing effects of such drugs involve local release of endogenous opioids.
B) VENTRAL PALLIDUM
In addition to its participation in food reinforcement, the VP contributes to drug reinforcement (88, 143, 157, 165). However, only a few studies have assessed the contribution of VP opioids to drug intake over the past decade. In one example, morphine injections into this region produced a CPP (278). Interestingly, naloxone injected into the VP produced CPA, an effect that was reproduced using the mu antagonist CTOP. These results suggest that mu receptors in the VP, activated by endogenous opioid peptides, modulate basal affective states (345).
Experimental evidence suggests that activation of opioid receptors in the VP contributes to systemic opiate reinforcement. Repeated intrapallidal morphine injections facilitated a CPP induced by a low dose of systemic morphine. Peripheral pretreatment with the opioid receptor antagonist naloxone or dopaminergic antagonists suppressed the CPP, indicating that the facilitatory effects of intra-VP morphine involve activation of mu and dopaminergic receptors (424).
Few studies have addressed a possible contribution of the pallidal opioid system to cocaine reinforcement. A CPP to cocaine was attenuated when intrapallidal naloxone was administered before the test session, at a dose that had no aversive properties, indicating that mu receptors in the VP are involved in mediating cocaine reinforcement (345). The mu receptor antagonist CTAP injected into the VP reduced reinstatement of drug seeking induced by a cocaine-priming injection (367). Conversely, intra-VP morphine reinstated operant behavior, an effect blocked by coinjection of CTAP, and facilitated by systemic cocaine administration. The authors conclude that cocaine seeking is modulated, in part, through corelease of enkephalins and GABA from NAc projections to the VP (367). Finally, β-FNA into the VP shifted the dose-response curve for a cocaine/heroin combination towards that maintained by cocaine alone. Thus pallidal mu-opioid receptors contribute to the facilitating effect of heroin on cocaine reinforcement (235). Taken together, these data provide strong evidence that the activation of mu receptors in the VP participates in mediating cocaine reinforcement. The possibility that the region also participates in the reinforcing properties of other drugs of abuse remains unexplored.
C) AMG AND EXTENDED AMG
Beyond a critical involvement in emotional learning, the AMG plays a key role in reward evaluation and appetitive conditioning (21, 158, 262, 327). Moreover, the CeA shares with the shell of the NAc and the BNST similar morphology, immunoreactivity, and connectivity. Together, these structures form the extended amygdala (6, 61, 97), which plays a key role in reinforcement (196, 198, 201, 334, 399).
The experimental evidence that the AMG is involved in opiate reinforcement is inconsistent (122, 312, 423). Mice self-administered morphine into the AMG (94). However, intra-amygdala (lateral nucleus) injections of morphine failed to produce CPP in rats (278). The direct rewarding or aversive effects of opioid receptor agonists and antagonists injected into the AMG have not been studied further; thus the role of the dense amygdalar opioid system in opiate reinforcement remains to be clarified. One study addressed the role of opioid receptors in the two other major components of the extended AMG, the shell of the NAc and BNST. The opioid receptor antagonist methylnaloxonium dose-dependently suppressed heroin self-administration when injected into the BNST, whereas it had no effect in nondependent rats. The authors suggest that opioid receptors in the BNST participate to the reinforcing effects of opiates in dependent animals (396).
With regard to nonopioid reinforcement, some studies have explored the contribution of opioid peptide release in the AMG to the reinforcing properties of ethanol. Infusion of the opioid receptor antagonist methylnaloxonium into the AMG decreased operant responding for ethanol solution in rats, at lower doses than did injections into the NAc (155). Moreover, microinjecting the delta receptor antagonist naltrindole into the NAc or the BLA reduced operant responding for an ethanol solution, whereas the mu receptor antagonist CTOP was effective only in the AMG (169). In line with these results, the opioid receptor antagonist naltrexone infused into the CeA, but not more dorsally into the CPu, reduced operant responding for ethanol in alcohol-preferring rats. Naltrexone also reduced sucrose-maintained responding when sucrose was presented alone, but not when sucrose was concurrently given with ethanol. These data indicate that opioid receptors within the CeA selectively modulate ethanol-maintained responding (118). Altogether, these studies suggest that mu and delta receptors in the AMG, and more precisely in the BLA and CeA, play an important role in modulating ethanol reinforcement.
D) OTHER, LESS STUDIED, STRUCTURES
The septum was the first brain region to be identified as a brain stimulation site in rats (271). However, the role of its two main divisions, the lateral septum (LS) and medial septum (MS), in reinforcement has not been much explored further (336). Rats self-administer morphine (357) and Met-enkephalin (358) into the septum. More recently, mice were reported to self-administer morphine into the LS and the MS, suggesting that both main septal divisions are involved in morphine reinforcement (66). Systemic pre-treatment with the opioid receptor antagonist naloxone, the D1 DA receptor antagonist SCH-23390 or the D2 antagonist sulpiride prevented intra-LS morphine self-administration. This work indicates that intra-LS morphine self-administration depends on opioid and dopaminergic mechanisms (209). We are not aware of studies using local injections of opioid antagonists to evaluate the role of septal opioid receptors and peptides in mediating systemic opiate or nonopioid drug reinforcement.
The hippocampus has a critical role in learning and memory processes but is also implicated in motivation and drug reinforcement, through a direct interaction with the NAc (105, 154, 174, 335, 373). A few studies have addressed the role of the dorsal hippocampus in opiate reinforcement using local injections of morphine (reviewed in Refs. 244, 339). Altogether the data suggest that morphine injected into the CA3 and CA4/dentate gyrus, but not the CA1, of the dorsal hippocampus, produces direct reinforcing effects. Such effects were not further characterized during the last decade. Moreover, to our knowledge, the role of hippocampal opioid peptide release in systemic opiate or nonopioid drug reinforcement has not been investigated.
In addition to an essential involvement in food reinforcement, the hypothalamus has been proposed to modulate drug intake (103, 151, 281, 325). Rats self-administer morphine into the LH (273), which is a major brain site for electrical self-stimulation. Intra-LH morphine supports self-administration in mice (64, 65, 93). However, results from CPP studies are less consistent (reviewed in Ref. 244). Injection of the opioid receptor antagonist naltrexone into the LH interfered with intravenous heroin self-administration in rats, suggesting that opioid receptors in this structure contribute to opiate reinforcement (80). Unfortunately, no pharmacological manipulations of the LH opioid system have been performed since 1997, either to investigate the direct reinforcing effects of opioid receptor agonists or antagonists or to identify a role for LH opioid system in mediating nonopioid drug reinforcement. Opioids in the MPOA are of interest for their contribution to sexual reinforcement (see sect. IIIA2). The peptide agonist d-Ala2-Met5-enkephalinamide (DALA) microinjected into the MPOA produced CPP in rats, suggesting that the release of opioid peptides in this region could represent one of the anatomical substrates for sexual reinforcement (1).
Electrophysiological, neuroimaging, and lesion studies have suggested that the medial region of the thalamus contributes to reinforcement (18, 124, 182, 243, 405). However, very little is known about the involvement of the abundant thalamic population of mu-opioid receptors in mediating drug reinforcement. Microinjections of the irreversible mu receptor antagonist β-FNA into the medial thalamus, prior to each conditioning session, blocked the acquisition of a CPP for morphine. In contrast, β-FNA infused after the last morphine conditioning session, and 23 h before the test, had no effect on a CPP. Thus mu-opioid receptors in the medial thalamus may play a role in the acquisition, but not the expression, of a morphine CPP (147).
In addition to well-characterized roles in defensive behavior and pain control, the periaqueductal gray (PAG) participates in drug reinforcement (48). Opiates injected into the PAG exert direct rewarding effects, inducing self-administration as well as a CPP (reviewed in Refs. 64, 244). Moreover, the nonselective opioid receptor antagonist naloxone injected into the PAG can suppress CPP to systemic morphine, indicating that opioid peptide release in this region is necessary for the expression of a morphine CPP (278). In contrast, high doses of morphine injected directly into the dorsal part of the PAG produced a CPA, an effect that was not blocked by previous microinjection of the mu receptor antagonist CTOP, but was significantly reduced by prior systemic treatment with the kappa receptor antagonist nor-BNI. Microinjecting CTOP alone, or the kappa receptor agonist U,50488H, produced an aversion, whereas injections of Nor-BNI did not. These results suggest that blocking mu or activating kappa receptors in the PAG both produce a CPA (330).
Some experimental evidence suggests that the pedunculopontine tegmental nucleus (PPTg) is involved in drug reinforcement (277) and more particularly in systemic opiate reinforcement (279), although local injection of morphine failed to produce a CPP in rats (278). Over the last decade, two studies have implicated the PPTg opioid system in cocaine and nicotine reinforcement. In trained rats, microinjection of the mu receptor agonist DAMGO reduced intravenous cocaine or nicotine self-administration under a fixed ratio schedule of reinforcement. The mu receptor antagonist CTOP did not affect self-administration, but reversed the effects of DAMGO when coadministered with it. These data demonstrate that mu receptors in the PPTg influence cocaine and nicotine reinforcement (81, 83).
2. Dependence
Dependence reflects the development of complex neuronal adaptations in response to repeated and/or prolonged drug administration. Dependence is revealed, when drug use ceases, by a complex withdrawal syndrome associating physical (or somatic) signs with an intensely aversive emotional state also called motivational withdrawal (199). In animal models of physical dependence, the experimenter scores various somatic signs after spontaneous (discontinuation of treatment) or precipitated (injection of a pharmacological antagonist) withdrawal of the drug (see, for example, Refs. 51, 225). Animal models of motivational withdrawal include withdrawal-induced disruption of a previously acquired operant task for food or a CPA after pairing of an environment with precipitated withdrawal (15, 361). Very few studies have combined these withdrawal models with an intracerebral pharmacological approach to explore the role of opioids in dependence, and they all dealt with opiate drugs.
A) VTA AND NAC
Only weak somatic signs were scored after injecting opioid receptor antagonists into the VTA or NAc of morphine-dependent rats (69, 177), indicating that these regions play only a minor role in physical dependence to opiates. In contrast, intra-NAc and, to a lesser extent, intra-VTA injections of the opioid receptor antagonist methynaloxonium disrupted operant responding for food and induced a CPA in morphine-dependent rats (203, 361). These results indicate that opioid receptor inactivation in the NAc can reproduce the aversive stimulus effects of opiate withdrawal and designate opioids in the NAc as an important brain substrate for motivational aspects of dependence and aversive states (see also Refs. 59, 199).
B) EXTENDED AMG
The AMG is not a critical brain substrate for physical opiate dependence. Indeed, injecting opioid receptor antagonists into this structure produced only weak somatic signs of withdrawal in morphine-dependent rats (69, 177). Injections of methynal-oxonium into the amygdaloid complex produced a CPA in morphine-dependent rats at moderate doses, suggesting that opioids in this region can modulate motivational aspects of dependence to opiates (361). Despite a recent evidence for a role of the BNST in opiate dependence (14, 348), the contribution of local opioids in this structure to this phenomenon remains unexplored.
C) PAG
The PAG contributes to physical drug dependence (48, 54, 69). Bozarth and Wise (46) showed that repeated morphine injections into this structure produce somatic signs of withdrawal after challenge with a systemic opiate antagonist. This result was replicated in further studies using morphine (45) or the enkephalin analog [d-Ala2,Met5]-enkephalinamide (120). Moreover, opioid receptor antagonists trigger a severe physical withdrawal syndrome when injected into the PAG of adult or infant rats made dependent on morphine (reviewed in Ref. 69; see also Ref. 177). Altogether, these results indicate that opioids in the PAG participate in physical opiate dependence. In contrast, blocking opioid receptors in this structure by injecting locally methynaloxonium had no effect on a previously acquired operant responding for food (203) or only produced a mild CPA (361) in morphine-dependent rats, suggesting that periaqueductal opioids play a limited role in the motivational effects of opiate withdrawal.
D) LOCUS COERULEUS
The role of the locus coeruleus (LC) in physical opiate dependence is now questioned (55, 69, 98, 197, 224, 309). Some experimental evidence supports the idea that opioids in this region are recruited during physical withdrawal. Indeed, intra-LC injections of opioid receptor antagonists in rats made dependent on systemic morphine produced severe somatic signs of withdrawal, whereas chronic infusion of morphine into the LC induced physical dependence in rats (reviewed in Ref. 69).
E) OTHER STRUCTURES
A weak withdrawal syndrome was observed after injecting opioid receptor antagonists into various hypothalamic nuclei, the raphé magnus (RM) and spinal cord of morphine-dependent rats (69, 177), suggesting that opioid receptors in these regions may contribute to physical dependence to opiates. Injections of the opioid receptor antagonist methylnaloxonium into the mediodorsal thalamic nucleus failed to induce a CPA in morphine-dependent rats except at high doses, suggesting that opioids in this structure do not play a major role in the motivational effects of opiate dependence (361).
3. Conclusion
Data reviewed in the present section, together with previously published results, demonstrate that the endogenous opioid system plays a key role in drug reinforcement. Numerous brain regions, which express opioid receptors, have been identified as directly supporting the reinforcing effects of opioids. Most of these regions also contribute to systemic opioid or nonopioid drug reinforcement (see Fig. 4). The opioid system, however, is expressed more broadly, and mapping of opioid-sensitive brain sites involved in drug reinforcement remains incomplete.
As the likely consequence of a half century focus on DA-dependent mechanisms, most studies have narrowed their interest to the VTA and its main anatomical targets. These revealed the crucial contribution of opioids in these structures, but did not clarify opioid-DA interactions in the control of drug reinforcement. As mentioned in the introduction of this section, experimental evidence refutes a role for DA in hedonic processes per se (37, 58, 112, 291, 310, 326, 333, 391, 412). Taken together with data reviewed above and results from food reinforcement studies, these arguments lead us to propose that opioids mediate the hedonic response to drugs of abuse, whereas DA is preferentially involved in motivational and/or learning aspects of drug reinforcement. Hence, investigations of opioid-sensitive sites of reinforcement that are not direct targets of VTA DA neurons deserve further extension.
C. Perspectives: Extending Studies to Animal Models of Drug Addiction
Reinforcing properties of drugs are considered critical for the initiation of drug use but are insufficient to explain the maintenance of compulsive drug intake (201). At present, the exploration of opioid-sensitive brain sites has identified anatomical substrates for reinforced behaviors, as well as sites involved in acute physical and motivational withdrawal, but has not addressed endogenous opioids and receptors contributing to long-term neuroadaptations underlying drug abuse. New animal models of drug intake have recently emerged in an attempt to reproduce the behavioral criteria for addiction as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM IV; Ref. 13) and thus to show better face validity with human addiction. Among such criteria, the progressive increase in frequency and duration of drug use has successfully been mimicked in animals using the escalation model of drug intake (3, 4, 199). The difficulty in stopping or limiting drug use (compulsive drug intake) has been studied in animals using progressive-ratio or second-order schedules of reinforcement (12, 113, 280, 332). Craving for drug(s) persists over years after the cessation of drug taking and often leads to relapse, another feature of human addiction that was transposed to animals using protocols of drug reinstatement (111, 179, 181, 334). Models of resistance to punishment have provided a means to explore the persistence of drug seeking despite harmful consequences (109, 290, 392). Finally, multidimensional models take into account several hallmarks of addiction to predict the development of addictive-like behavior in rats (33, 34, 100). Combined with local pharmacological manipulations, or refined gene targeting approaches (see below), all of these animal models can be used to assess the role of opioids in the entire addiction cycle, from occasional use to dependence and relapse.
IV. GENETIC MANIPULATION OF THE OPIOID SYSTEM, REINFORCEMENT, AND DRUG DEPENDENCE
More recently, genetic approaches using knockout animals have confirmed a role for the opioid system in drug reinforcement and dependence. At present, available knockout lines produce a complete gene deletion throughout the body, and these genetic models provide no anatomical information on sites where receptors or peptides operate. Nonetheless, examination of these mutant mice has allowed unequivocal identification of the receptors or peptides involved in a number of behavioral responses. Opioid system knockout studies have previously been reviewed (128, 191), and major findings in the context of drug abuse are updated and summarized here.
A. Opioid Receptor Knockout Mice
1. Mu receptors
Recent studies have demonstrated an essential role of mu receptors in mediating natural rewards. Mu receptor knockout mice showed decreased motivation to eat (285) and diminished food-anticipatory behavior (180). In the context of social interactions, mu receptor knockout pups showed reduced maternal attachment in several behavioral paradigms (256).
Many more studies have addressed the role of mu receptors in drug reinforcement and dependence in these mutant mice. Mu opioid receptor knockout mice are insensitive to morphine, demonstrating that mu receptors are the primary molecular target for the prototypical opiate in vivo. Opiate reward was tested in several studies. Morphine (240, 253, 350) and heroin (71, 234) CPP as well as morphine self-administration (30) were abolished in the mu mutant. Furthermore, the reinforcing properties of nonopioid drugs of abuse are generally diminished in mu receptor knockout mice. In these animals, nicotine (35) and delta9-tetrahydrocannabinol (THC; Ref. 133) induced CPP were undetectable, alcohol self-administration was abolished (31, 317), and ethanol consumption was decreased (31, 317). At present, the role of mu receptors in psychostimulant reinforcement is unclear, in that a cocaine CPP was unchanged (71), increased (31), or decreased (148). However, cocaine self-administration was reduced (239), suggesting that mu receptors also contribute to cocaine reward. Finally, a CPP to 3,4-methyl-enedioxymethamphetamine (MDMA) was unchanged (318). In sum, the data reveal that mu receptors mediate the rewarding properties of most drugs of abuse and therefore represent a key molecular switch for the initiation of addictive behaviors (see Ref. 72). Interestingly, and relevant to drug abuse, mu receptor knockout mice showed decreased motor impulsivity, suggesting for the first time a facilitatory role of mu receptors in disinhibition (276).
Mu receptor knockout mice also show reduced adaptive responses following chronic exposure to drugs of abuse. Nicotine withdrawal was reduced (35) and handle-induced convulsions induced by chronic exposure to ethanol vapors were decreased (132) in the mu receptor knockout mice. Furthermore, THC withdrawal was reduced in single mu (218) and double mu-delta knockout mice (62). Mu receptors, therefore, also contribute to long-term neuroadaptations to nonopioid drugs of abuse.
2. Delta receptors
The analysis of delta receptor knockout animals appeared highly interesting, in that behavioral phenotypes often differ or even oppose phenotypes of mu receptor knockout animals. Delta receptor mutants showed increased anxiety levels and a depressive-like behavior (116). Directly relevant to drug abuse, delta receptor knockout mice showed increased ethanol self-administration, and ethanol intake reduced the innate high-anxiety levels in these animals (316). There was no detectable change in a THC CPP (133). Morphine CPP was reduced (68). Finally, delta receptor knockout mice showed increased motor impulsivity, suggesting a facilitatory role of delta receptor activity on inhibitory controls (276). Altogether, the data suggest that delta receptors regulate emotional behaviors, drug reinforcement, and impulsivity in a unique way that influences the development of addictive behaviors differently from mu receptors. At present, and in contrast to mu receptors, the direct implication of delta receptors in hedonic control has not been demonstrated.
3. Kappa receptors
Pharmacological studies have long shown that kappa receptor activation is aversive in animal models. Kappa receptors have been proposed to oppose mu receptors in the regulation of hedonic homeostasis (351) and potentially show hallucinogenic activity, as revealed by pharmacological effects of Salvinorin A (see Ref. 417). The notion that kappa receptor activity is aversive, and negatively modulates reward, was strengthened by a number of studies using kappa receptor knockout mice. Deletion of the kappa receptor gene did not modify a morphine CPP (343) but abolished aversive effects of the kappa receptor agonist U,50488H in a CPA paradigm (343) and enhanced a THC CPP (133). In contrast, kappa receptor knockout mutants showed reduced ethanol CPP (204). Finally, kappa receptor knockout mice showed potentiated cocaine CPP induced by stress (249), consistent with the notion that kappa receptors also counteract reward processes under stressful conditions.
B. Opioid Peptide Knockout Mice
1. Penk and POMC
Penk and β-endorphin knockout mice have often been examined in parallel studies. Endogenous enkephalin activity has long been proposed to positively regulate hedonic homeostasis (341). In accordance with this notion, a naloxone-induced reduction in sucrose consumption was intact in β-endorphin knockout mice but absent in Penk knockout mice and double Penk β-endorphin knockout mice (153). These data suggest that naloxone reduces sucrose intake in wild-type animals by inhibiting enkephalin (and/or dynorphin, see below), but not β-endorphin, signaling. Moreover, Penk knockout mice, and not β-endorphin knockout mice, failed to show an aversion to naloxone (344). A CPA to a kappa receptor agonist (344) and a CPP to morphine (233, 268, 344) were preserved, indicating that associative learning is not altered in Penk knockout mice (344). These results suggest that the aversive properties of naloxone result from the blockade of an endogenous enkephalinergic tone. A CPP to morphine was normal in β-endorphin and double Penk β-endorphin knockout mice, showing that opiate reinforcement is not modified when these peptides are absent (344).
Recent alcohol studies failed to show any phenotype in Penk and β-endorphin mutants. The animals showed no change in baseline ethanol preference or CPP (194, 305). Both Penk and β-endorphin mutants learned to self-administer ethanol (152), and basal sucrose consumption was unaltered in the two mutants (152, 153). In contrast, stress experiments distinguished the two lines. Stress-induced ethanol consumption was reduced in β-endorphin but not Penk mutant mice, confirming the reported role of β-endorphin in stress responses (305).
Nicotine effects were investigated in Penk knockout mice only. A nicotine CPP was absent in the knockout mice, and nicotine withdrawal was attenuated, demonstrating altogether that Penk-derived peptides contribute to nicotine responses (36).
2. Prodynorphin
Pdyn knockout mice showed unaltered sucrose consumption under basal conditions but were insensitive to the suppressive effect of naloxone on this consumption. This last result suggests that naloxone decreases sucrose intake in wild-type mice by blocking dynorphin (and/or enkephalin, see above) signaling (153). Pdyn knockout mice displayed lower sensitivity to nicotine reinforcement, in accordance with the proposed role of kappa systems in negative reinforcement. Nicotine produced a normal CPP in the Pdyn mutant, but the percent acquisition of intravenous nicotine self-administration was shifted to the left, indicating enhanced nicotine reward in these animals (123). In addition, Pdyn mutant and kappa receptor knockout mice were impaired in the potentiation of a cocaine CPP triggered by stress, suggesting a connection between stress and drug abuse via the endogenous kappa opioid system (250).
Finally, as for Penk and β-endorphin knockout mutants, several responses to alcohol were unchanged, including acute ethanol withdrawal, ethanol-induced CPP, and conditioned taste aversion to ethanol (40).
C. Conclusion
Altogether, the analysis of knockout mice clarified the role of each opioid receptor in a large number of behavioral responses and has identified highly distinct activity patterns for each receptor. Relevant to drug intake, genetic data demonstrate that mu receptors contribute to the reinforcing properties of most drugs of abuse, whereas kappa receptors induce dysphoria and counteract mu receptors in regulating hedonic homeostasis. With regard to other aspects of addictive behaviors, the data show a role for mu receptors in drug dependence, for kappa receptors in stress-induced drug intake, and for delta receptors in emotional control. Data from opioid peptide knockout mice show milder phenotypes. As an example, no phenotype in alcohol response was detected for any peptide mutant line, while all three receptor mutants show significant and distinct responses to alcohol. Data from peptide knockout mice implicate Penk in hedonic control and reveal β-endorphin and Pdyn activities in experimental situations involving stress. At present, none of the phenotypes from peptide knockout animals overlaps with those of receptor mutant mice. This may be due to technical difficulties in comparing different knockout lines under identical experimental conditions. Also, it is likely that the potential Penk/mu-delta, POMC/mu, and Pdyn/kappa partnerships suggested by pharmacological in vitro analysis and distribution studies (see sect. I) operate in some parts of the neural circuitry but are undetectable in integrated behavioral responses. Alternatively, redundancy in opioid peptide activities may minimize the effects of genetic inactivation of a single gene.
Finally, we would like to emphasize that the deletion of opioid peptide genes in mice is the only available approach to identifying the opioid peptide function, which is difficult to address by biochemical techniques and cannot be examined by pharmacological methods that manipulate receptors only.
D. Perspectives: Towards Genetic Manipulation of the Opioid System in Targeted Neuronal Populations
In the future, conditional gene knockout will be achieved for genes of the opioid system. These novel tools are being developed to limit the genetic inactivation in space and/or time. Regionally controlled knockout lines will allow us to identify neural sites where defined receptor protein or peptide populations operate within addiction circuits. Importantly, the use of transgenic Cre lines will permit targeting of opioid receptor or peptide genes within selected neuronal populations, such as GABAergic, or aminergic neurons, a goal which cannot be achieved by local pharmacological or viral approaches. The latter methodology will provide invaluable insights into opioid-mediated mechanisms of drug abuse at molecular and cellular levels. Finally, inducible gene knockout will allow the temporal control of gene deletions and reveal critical time periods for opioid system intervention as addiction develops.
V. MODIFICATIONS OF OPIOID SYSTEM GENE EXPRESSION UNDER CHRONIC DRUG EXPOSURE
At present, anatomical studies combined with local pharmacological manipulations have mainly addressed sites of opioid-mediated reinforcement (see sects. II and III). Drugs of abuse produce reinforcement after a single or limited drug exposure. This process does not necessarily lead to the development of addictive behaviors and is therefore considered a necessary, although not sufficient, step towards drug abuse. In contrast, chronic exposure to drugs of abuse, particularly repeated exposure-withdrawal cycles, produces broad long-term and likely irreversible modifications within reinforcement networks, as well as in associated circuits controlling cognitive and emotional behaviors. These modifications, in turn, contribute to the transition from recreational drug use to compulsive drug taking and abuse (199, 360). A vast amount of literature now indicates that the opioid system is altered following chronic drug exposure, and at many brain sites, and data are reviewed in this section with a focus on adaptations at the transcriptional level.
Recent research has developed large-scale gene profiling approaches to investigate genetic regulations involved in the development of addiction that could lead to loss of control and relapse. Transcriptome studies represent a rapidly moving field of research and have recently been reviewed elsewhere (8, 216, 245, 304, 313, 419). In the present review, we have focused on genes encoding opioid receptors and peptides. Two experimental situations have been investigated, a drug-dependent state where animals are repeatedly exposed to drugs of abuse and a drug-withdrawn state where drug levels decrease in dependent animals, either due to termination of drug administration or precipitated by antagonist treatment. Data from these experiments are reviewed below. Regulations of opioid peptide genes, which appear to be better detected than those of receptor genes, are summarized in Figure 5.
Fig. 5.
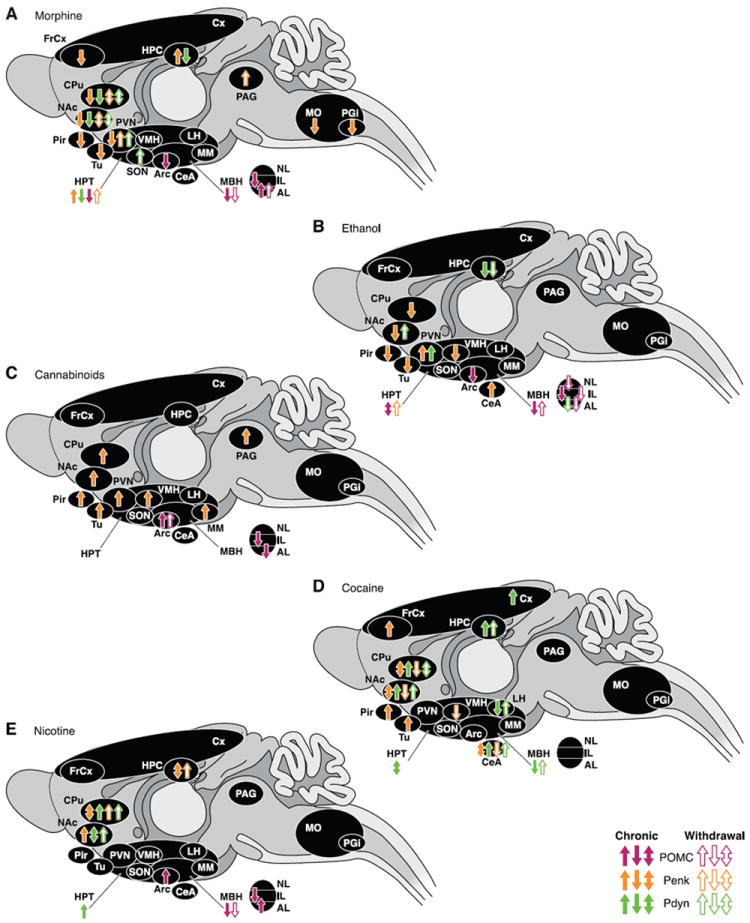
Regulation of opioid peptide genes after chronic exposure to, or withdrawal from, drugs of abuse. Each panel represents brain areas where modifications of endogenous opioid peptide transcript levels were described after chronic exposure to, or withdrawal from, morphine (A), ethanol (B), cannabinoid agonist (C), cocaine (D), and nicotine (E). Chronic drug administration (full arrows) corresponds to repeated injections (constant or escalating doses), pellets implantation, minipump infusions, or self-administration of the drug of abuse. Withdrawal (hatched arrows) was either spontaneous (cessation of injections or removal of pellets) or induced by antagonist injection. Many studies have also reported negative results (no detectable regulation) that are not represented on the figure but discussed in the text. AL, anterior lobe, pituitary; Arc, arcuate nucleus; CeA, central nucleus, amygdala; CPu, caudate putamen; Cx, cortex; FrCx, frontal cortex; HPC, hippocampus; HPT, hypothalamus; IL, intermediate lobe, pituitary; LH, lateral hypothalamus; MBH, medial basal hypothalamus; MM, medial mammillary nucleus; MO, medulla oblongata; NAc, nucleus accumbens; NL, neuronal lobe, pituitary; PAG, periaqueductal gray; PGi, nucleus paragigantocellularis; Pir, piriform cortex; PVN, paraventricular hypothalamus; SON, supraoptic nucleus; Tu, olfactory tubercle; VMH, ventromedial hypothalamus.
A. Opioid Receptor mRNA Levels
1. Mu receptors
A) MORPHINE
Mu receptors have been the focus of most studies. Surprisingly, only few studies have reported changes in mu receptor gene expression after chronic morphine treatment. An upregulation of mu receptor transcripts was observed in the mediobasal hypothalamus (MBH; Ref. 53), and a downregulation was detected in the NAc, CPu, and PAG (368). Otherwise, most studies failed to detect changes in mu receptor gene expression in a variety of brain regions, including the NAc, CPu, PAG, LH, AMG, POA, RM, hypothalamus (HPT), sensorimotor cortex, and septum (49, 52, 63, 322, 426). The many negative results suggest that chronic stimulation of mu receptors by morphine does not necessarily modify receptor mRNA levels.
Only two studies investigated the regulations of mu receptor mRNA in morphine withdrawal. An increase in mu receptor gene transcription in the NAc, CPu, and LH was reported within 12 h following cessation of morphine injections (426). No modification of mu receptor mRNA levels was detected either in spontaneous (48 h abstinence) or naltrexone-induced withdrawal (63).
B) OTHER DRUGS
Several studies have used animal models in the search for genetic regulations triggered by excessive ethanol consumption (for recent reviews, see Refs. 85, 108, 156, 195). In some studies, levels of mu receptor mRNA were assessed before or after ethanol consumption in mice and rats selected on the basis of ethanol preference. Basal levels of mu receptor transcripts were similar between preferring and nonpreferring strains in the HPT and striatum (52, 141, 411) as well as in the NAc, hippocampus (HPC), frontal cortex (FrCx), but not inferior colliculus, where higher levels were observed in alcohol-preferring animals (141). Following chronic ethanol, mu receptor transcripts were unchanged in the striatum of both strains (337, 411), but decreased in the HPT, an effect still measurable up to 3 wk after cessation of ethanol consumption, specifically in the alcohol-preferring animals (411).
Many different modalities of chronic cocaine or psychostimulant treatment have been used to investigate the regulation of opioid receptor expression. Generally, literature in the field suggests an increase in mu receptor gene expression in several brain structures. Levels of mu receptor mRNA were increased in the NAc and rostral cingulate cortex after chronic exposure to cocaine or after cocaine CPP, whereas no change was detected in the CPu (16, 210, 381, 397). One report showed no change in mu receptor transcripts in both dorsal and ventral striatal areas following a cocaine binge (18). Chronic exposure to amphetamine increased mu receptor gene expression in the rostral CPu and decreased the expression in the shell of the NAc (393). In the FrCx, mRNA levels for mu receptors were increased or not modified depending on the protocol of drug administration, using a cocaine CPP (210) or chronic binge (18) paradigm, respectively. Mu receptor transcripts were not modified in the AMG following a cocaine CPP (210) or chronic cocaine treatment (427), or in the VTA and olfactory bulbs after chronic cocaine treatment (17, 397). Several groups investigated the effects of withdrawal from cocaine or amphetamine on mu receptor transcription and observed either an increase or no change in mRNA levels. Mu receptor mRNA levels were increased in the FrCx after cocaine withdrawal (18) and in the VTA following withdrawal from amphetamine (222). No modifications were detected in the CPu (18) and AMG (427) after cocaine withdrawal, in the shell of the NAc after amphetamine withdrawal (222), or in the HPT following withdrawal from chronic cocaine treatment (427) or a cocaine CPP (210). These results suggest that the upregulation of mu receptor gene expression observed after chronic psychostimulant treatment in the VTA and FrCx persists under a withdrawal state.
One study has assessed the effects of nicotine exposure on mu receptor gene transcription. An upregulation of mu receptor mRNA was observed in the VTA following chronic nicotine treatment, while the expression was not modified in the NAc (397). We are not aware of studies reporting regulations of the mu receptor gene expression after chronic cannabinoid treatment.
2. Delta receptors
A) MORPHINE
The delta receptor has been the least studied receptor with regard to transcriptional regulation after chronic drug exposure. Delta receptor transcription was investigated after chronic morphine treatment in several brain areas, including the striatum, PAG (52, 368), and Th (52), and no modification was detected.
B) OTHER DRUGS
As for mu receptors, delta receptor mRNA levels under basal conditions were similar in the striatum of both alcohol-preferring and alcohol-avoiding strains of mice (411). Chronic exposure to ethanol did not change delta transcripts in the striatum (337, 411) or HPT (411) of these animals. Interestingly, 3 wk after the cessation of ethanol consumption, delta receptor transcripts were increased in the striatum of alcohol-avoiding but not -preferring mice (411). No modification of the expression level of delta receptors was observed in the NAc and olfactory bulbs after chronic cocaine (17). As for mu receptors, delta receptor transcripts were increased in the VTA but not the shell of the NAc following withdrawal from chronic amphetamine (222). To our knowledge, regulations of delta receptor gene expression following either chronic cannabinoids or nicotine treatment have not been investigated.
3. Kappa receptors
A) OPIATES
Kappa receptor gene expression was downregulated in the NAc, striatum, and PAG (368) and upregulated in the MBH (53) following chronic morphine. Chronic intracerebroventricular infusion of the kappa receptor agonist butorphanol increased levels of kappa receptor transcripts in various brain areas including the cerebral cortex, striatum, Th, HPC, and pons (366).
B) OTHER DRUGS
Kappa receptor expression was most often downregulated after chronic treatment with ethanol or cocaine, while no data are available with respect to nicotine and cannabinoid exposure. A downregulation of kappa receptor transcription was observed in the VTA and NAc following chronic ethanol (323). Regarding cocaine administration, results appear more contradictory, with no change of kappa receptor transcripts in the NAc and CPu following repeated cocaine (352, 381) and decreased kappa receptor expression in the NAc and VTA when cocaine was administered alone or in combination with ethanol (323). Levels of kappa receptor transcripts were decreased in the substantia nigra (SN) under chronic binge cocaine (352, 354), but not after withdrawal (352).
B. Opioid Peptide mRNA Levels
The effect of chronic exposure to drugs of abuse on opioid peptide gene expression has been broadly investigated. Regulations of Penk, Pdyn, and POMC transcripts under chronic drug exposure or in withdrawal states are illustrated in Figure 5 for morphine, ethanol, cannabinoids, cocaine, and nicotine. Levels of Penk and Pdyn transcripts were investigated in many areas of the brain and under several drugs of abuse. In contrast, POMC expression has been less well studied, probably due to the restricted distribution of this opioid peptide in the brain.
1. Proenkephalin
A) MORPHINE
Changes in Penk mRNA following chronic morphine have been widely examined in the NAc and CPu where the transcript is highly expressed (see Fig. 1B), and most studies consistently report a downregulation (26, 130, 372, 375, 380). Decreased expression following chronic morphine was also described in other brain areas, like the HPT (PVN, 149), FrCx, (26), medulla oblongata (MO), and nucleus paragigantocellularis (PGi; Ref. 384). However, increased levels of Penk mRNA were detected in the HPC (26), whole cortex, and spinal cord (145). Regulations of Penk expression were compared in Lewis and Fisher rat strains, the former displaying higher rates of morphine self-administration than the latter. Lewis rats displayed lower basal levels of Penk mRNA than Fisher rats in the CPu and NAc. After morphine self-administration, Penk expression was reduced in the CPu, Tu, and piriform cortex (Pir) of Lewis rats and increased in the core of the NAc of Fisher rats (328). Penk transcription was decreased under morphine withdrawal in the CPu (130, 380), NAc (375), pons, and spinal cord (145). In contrast, increased levels of Penk mRNA were observed in the striatum (145) and HPT (145, 219). Interestingly, depending on how withdrawal was induced, spontaneously or by injecting an opioid antagonist, a decrease or no change in Penk expression was measured in the rostral PAG. Both withdrawal paradigms, however, induced Penk expression in the caudal PAG (120, 121).
B) OTHER DRUGS
As for chronic morphine treatment, chronic ethanol reduced Penk messenger levels in the whole striatum (84, 274), Pir (274), and Tu (84, 274). Within the HPT, Penk mRNA expression was decreased in the VMH (274) and increased in the PVN (67, 274). Chronic free-choice ethanol consumption in rats increased Penk transcripts within the CeA and intercalated nuclei of the AMG (84). Noteworthy, two studies reported no change in Penk expression in the CPu after chronic ethanol exposure (238, 303).
As opposed to morphine and ethanol exposure, chronic administration of cannabinoid agonists (THC, CP-55,940, or R-methanandamide) increased levels of Penk mRNA in the NAc and CPu (78, 110, 223, 231, 275), Tu and Pir (275), HPT (both PVN and VMH; Refs. 76, 77, 231), as well as in the mammillary area and PAG (231). However, no change in Penk mRNA levels was detected in the CPu, NAc (231), and spinal cord (74). Following cannabinoid withdrawal, Penk expression was upregulated in the CPu, NAc, Tu, and Pir and returned to normal 14 days following cessation of drug (275).
Many different paradigms of drug administration were used to study the regulation of Penk expression after chronic cocaine exposure, with variable results. Several studies reported increased Penk mRNA levels in the CPu (299, 354, 359, 363) and NAc (167, 237, 299). Other investigations showed no change in Penk expression in the CPu (18, 91, 237, 352, 429), NAc (7, 429), cortex (90), FrCx (18), and CeA of AMG (376, 429). Spontaneous withdrawal from chronic cocaine was associated with decreased Penk transcription in the CPu and NAc (299), VMN (86), and CeA (86), although no effect on Penk gene regulation in the CPu (11, 18, 352, 363), NAc (11), or FrCx (18) was reported. Increased levels of Penk mRNA in the CPu, NAc, Tu, and Pir were observed in animals abstinent from cocaine self-administration (86). As for cocaine, chronic amphetamine exposure produced variable results on Penk expression depending on the brain area examined, with increased Penk mRNA levels in the FrCx (257) and decreased Penk expression in the CeA (376) as well as anterior medial CPu (257).
The effect of chronic nicotine exposure on Penk expression has been poorly investigated, and results appear controversial. Penk mRNA level was increased (172, 238), not changed (161), or decreased (162) in the CPu and NAc following chronic nicotine administration. In other brain areas, Penk transcription was either upregulated (HPC, 162) or unchanged (HPC and HPT; Ref. 161). Withdrawal from chronic nicotine was associated with increased Penk transcription in the CPu (162, 172) and HPC (162). When 2 wk of nicotine treatment was followed by 16 h of abstinence, a challenge dose of nicotine did not modify levels of Penk transcripts in the CPu and NAc (238).
2. POMC
Studies examining the regulation of POMC expression after chronic drug exposure are restricted to the hypothalamic region and the pituitary. To our knowledge, modulations of POMC transcription in the NTS have not been assessed.
A) MORPHINE
Repeated morphine administration decreased POMC expression in the MBH (53, 404) and Arc (50, 115, 125, 254, 255). Levels of POMC mRNA were not modified after morphine withdrawal in the MBH (404) and Arc (125, 163). Decreased levels of POMC transcripts were observed in the HPT when withdrawal was precipitated by naltrexone, whereas no regulation was detected following spontaneous withdrawal (255).
Chronic morphine administration increased (160) or did not modify (219) levels of POMC transcripts in the AL of the pituitary gland and decreased POMC transcription in the IL (160). Increased POMC expression was reported in the pituitary gland after morphine withdrawal (219).
B) OTHER DRUGS
Levels of POMC transcripts in the MBH decreased following chronic ethanol administration and increased 3 wk after gradual removal of ethanol (308). Chronic cannabinoid agonists (THC or CP-55,940) increased levels of POMC transcripts in the Arc (75, 77, 275), and this increase lasted up to 14 days following cessation of drug (275). POMC gene expression was not changed in the HPT following either a cocaine CPP protocol (210) or a 2-wk chronic binge cocaine treatment (427). No further change was observed after a short period of withdrawal (427). Following chronic nicotine, POMC mRNA levels were either increased (164) or unchanged (161) in the Arc, as well as decreased in the MBH (306). The downregulation of POMC gene transcription was still observed after 21 days of spontaneous withdrawal from nicotine (306).
In the AL of the pituitary, POMC mRNA levels were not changed after chronic cannabinoid (75) and alcohol (301, 307) exposure and increased after chronic nicotine (161). Increased levels of POMC transcripts were detected in the anterior pituitary following cocaine CPP (210), but no change after a 2-wk binge treatment (427). Alcohol withdrawal decreased (307) or did not change (301) POMC mRNA in the anterior pituitary. In the IL, POMC expression was decreased after chronic alcohol (301) and chronic nicotine (161).
3. Prodynorphin
A) OPIATES
Repeated morphine exposure decreased Pdyn or Dyn expression in the CPu and NAc in several studies (56, 130, 302, 372). An upregulation in these two regions (375) or no change (406) was also reported. Decreased Pdyn mRNA levels were observed in the HPC (56) and HPT (26, 56). Chronic treatment with kappa receptor agonists (U-69593 or U-50,488H) consistently decreased levels of Pdyn mRNA in the CPu, HPC, and FrCx (70, 321). In the HPT, Pdyn expression was either downregulated (321) or upregulated (70).
Decreased Dyn gene expression was observed in the CPu and NAc after repeated morphine administration. Dyn mRNA levels were still down 24 h after cessation of drug treatment and went back to control levels after 3 days (130). Other studies have shown opposite effects, with increased Pdyn transcription in these areas following spontaneous morphine withdrawal (302, 375). Different regulations of Pdyn expression in the CPu and rostral NAc were reported after morphine withdrawal depending on how the drug was administered. Indeed, Pdyn transcription was augmented following withdrawal from chronic intermittent injections, and unchanged following cessation of an escalating treatment (372). An increase in Pdyn expression was detected within the CPu, HPT, and FrCx after withdrawal from chronic treatment with the kappa receptor agonist U-69593 (70).
B) OTHER DRUGS
Several studies have assessed the effects of chronic exposure to nonopioid drugs, with the exception of cannabinoids, on Pdyn transcription.
Pdyn transcription after chronic exposure to ethanol was increased in the HPT (67, 146), decreased in the HPC (300), and unchanged in the CPu and NAc (84, 237), Tu (84), and anterior pituitary (301). Withdrawal from ethanol induced an increase in Pdyn messenger in the CPu (27), Tu (27), and NAc (27, 303), and mRNA levels went back to control levels after 96 h (303). The decrease in hippocampal Pdyn transcription measured after chronic ethanol was also following 2 days of spontaneous withdrawal (300). Levels of Pdyn mRNA were also downregulated in the anterior pituitary (301). No change in Pdyn gene expression was detected in the Pir after ethanol withdrawal (27).
Pdyn transcription was increased in the CPu after repeated cocaine exposure (18, 91, 92, 167, 320, 352-354, 359, 363, 406, 420) and cocaine self-administration (429). In the NAc, changes in Pdyn expression are less consistent. Pdyn expression increased (237) or did not change (7) after repeated cocaine injections. An increase in Pdyn expression was observed following a cocaine binge as well as repeated amphetamine injections (376). Hence, chronic exposure to psychostimulants, but not morphine, induced Pdyn transcription in the striatal area. In a study comparing the effects of extensive running (as a natural reinforcer) with those of cocaine or morphine exposure, Pdyn mRNA increased within the CPu after both cocaine and running and did not change after morphine (406). Levels of Pdyn transcripts after chronic cocaine or amphetamine were increased in the dentate gyrus of the HPC (376). In contrast, no change in hippocampal Pdyn transcription was detected following chronic intracerebroventricular infusion of cocaine (320) or repeated exposure to methamphetamine (319). Chronic methamphetamine treatment increased the levels of Pdyn transcripts in the HPT (319), while intracerebroventricular cocaine (320) or cocaine CPP (428) decreased hypothalamic Pdyn expression. Cocaine binge did not modify Pdyn messengers in the HPT (420, 428). Chronic amphetamine (376) but not cocaine (376, 420) induced Pdyn expression in the AMG. In a protocol of cocaine self-administration, no modification of Pdyn mRNA was observed in the CeA (429). Cocaine exposure did not change Pdyn transcription in the FrCx (420) or whole cortex, except at a high dose (90). Withdrawal from cocaine either decreased (363), increased (18), or did not change (352) levels of Pdyn mRNA in the CPu. Withdrawal from amphetamine induced an increase in Pdyn in both CPu and NAc (377). Short-term withdrawal from chronic escalating doses of cocaine increased Pdyn expression in the LH (428). Finally, increased levels of Pdyn transcripts in the dentate gyrus of the HPC were still detected after early withdrawal from amphetamine (376).
As for Penk, results obtained for Pdyn mRNA levels following chronic nicotine appear variable, with either no change (161, 238) or increased levels (173) in the CPu. High levels of Pdyn transcripts in the CPu could be detected after nicotine withdrawal (173). A decrease in Pdyn expression was observed in the ventral shell of the NAc after repeated nicotine injections (237). Pdyn transcripts were also increased in the HPT and unchanged in the HPC following chronic nicotine treatment (161).
C. Conclusion
Gene expression studies have revealed adaptive modifications of the opioid system in response to repeated drug treatments and following cessation of drug treatment. Data from withdrawn animals are variable, likely due to the diversity of withdrawal conditions and the dynamic nature of the process. In contrast, a picture emerges from studies of animals under chronic drug treatment. Both repeated morphine and alcohol administration produce consistent downregulation of opioid peptides in many brain areas, whereas chronic psychostimulants upregulate those genes. This observation reflects the possibility that distinct classes of drugs of abuse produce opposing neuroadaptations of endogenous opioids. Chronic narcotic (opioids) or sedative (ethanol) drugs may repress the endogenous opioid tone as a result of repeated activation of this system, whereas psychostimulants may activate the opioid system as a feedback mechanism counteracting the stimulant properties of the drugs. This is, however, highly speculative, and more data are needed to substantiate this hypothesis. Data from cannabinoid and nicotine studies are presently too scarce to be discussed in this context.
D. Perspectives: Adaptations of Opioidergic Systems, Significance, and Implications
The significance of opioid system regulations to drug addiction remains an open question. Natural genetic variations have been proposed to modulate susceptibility to addiction (379). Variation in expression levels of opioid receptors and peptides across individuals may also play a role. Some genetic studies support this view (note that this entire research field was not reviewed here). Similarly, it has been proposed that repeated exposure to drugs of abuse dysregulates the endogenous opioid system and that these modifications in turn contribute to drug craving, seeking, and relapse (186, 200, 324). The data show changes in the expression of both receptors and peptides in drug-dependent and withdrawn animals. However, the causal relationship between those changes and the development of addictive behaviors is presently unclear. Although genetic manipulations of the opioid system have established that each component of the opioid system regulates some aspects of drug abuse (see sect. IV), local or neuron-targeted gene knockout and knock-down approaches will be necessary to understand how specific regulations trigger adaptations leading to compulsive drug use.
Finally, transcriptome studies allow exploration of genetic regulation on a genome-wide basis. Regulations of opioid peptide and receptor genes are likely accompanied by other molecular adaptations that occur at pre- and postsynaptic levels, respectively, and reflect coordinated adaptive changes at both circuit and integrated levels. Today the availability of reporter transgenic mice expressing fluorescently labeled neuronal populations (142), combined with advanced cell sorting and gene hybridization techniques, permit extensive gene profiling in sets of defined neurons. Future experiments will undoubtedly describe regulatory signaling networks at the level of opioidergic neurons and their targets (32) and provide a comprehensive view of opioid-associated adaptations at the systems level.
VI. GENERAL CONCLUSION
Opioid receptors and peptides are strongly expressed in reinforcement circuits in the mammalian brain. In this review we have focused specifically on rodent models that allow extensive experimental manipulations, hence the exploration of molecular and cellular mechanisms of addiction. Pharmacological activation or blockade of opioid receptor activity at several sites of the brain reinforcement network has demonstrated a role for these receptors in hedonic evaluation of natural rewards, as well as in reinforcing properties of drugs of abuse. Genetic inactivation of the six genes encoding opioid receptors and peptides has produced a large variety of reward-relevant behavioral phenotypes. Together, pharmacological and genetic studies have definitely established a role for the opioid system in behavioral responses and adaptations to most drugs of abuse and highlighted the distinct implication of each component of the system in drug reward and dependence. However, pharmacological studies have not established the molecular nature of the receptors and peptides involved, and genetic studies have provided no anatomical information. Expression studies have revealed modifications in levels of opioid receptor and peptide transcripts following chronic exposure to drugs of abuse, at several brain sites and mainly in the reinforcement circuit. Although these data are suggestive of a role for gene regulation in the development of addiction, the correlative nature of this approach does not examine the causal link between changes in gene expression and behavioral adaptations to drugs of abuse.
Ultimately, and although opioid system function is considered an old research field, many aspects of opioidcontrolled behaviors deserve further clarification. Advanced cellular and molecular approaches to studying neurotransmitter systems in vivo, as well as imaging techniques in rodents, have emerged and provide valuable information on this topic. Future studies will focus on specific neuronal populations within brain circuits. Refined genetic targeting approaches (127) combined with receptor imaging (331) and pharmacology should conclusively identify neuroanatomical localization of genetically defined key opioid peptides and receptor populations that contribute to the development of behavioral adaptations. Furthermore, the use of innovative animal models of addiction will clarify the implications of opioid receptors and peptides in human addictive disorders (201, 329). Finally, large-scale transcriptome approaches in selected neurons, combined with epigenetic studies (311), should broaden the analysis of adaptations of opioidergic circuits to drugs of abuse.
Opiates have been used for thousands of years and abused for almost two centuries. Recently, opiate therapies have been developed to treat heroin and alcohol addiction (see Refs. 206, 283). Data from rodent models have provided invaluable insights into cellular and molecular mechanisms of opioid-mediated mechanisms of hedonic homeostasis and drug abuse. Rodent-based approaches will further the development of testable hypotheses driven by noninvasive imaging (119) and gene association studies (170, 205, 242) in humans and likely lead to the use of opioid receptors as targets in novel therapeutic strategies for treating addiction, or developing opiate drugs to treat other psychiatric disorders.
Acknowledgments
We apologize to authors whose original work was not cited directly. We thank Cella Olmstead and Claire Gavériaux-Ruff for critical reading of the manuscript. We also thank C. Evans, R. Maldonado, and G. Koob for fruitful collaborations over the past years.
GRANTS
This work was supported by the Centre National de la Recherche Scientifique, Institut de la Santé et de la Recherche Médicale, and Université de Strasbourg. We thank the Agence Nationale pour la Recherche, the European Union (GENADDICT/FP6 005166), and the National Institutes of Health (NIAAA AA-16658, NIDA DA-16768, and NIDA DA-05010) for financial support.
References
- 1.Agmo A, Gomez M. Conditioned place preference produced by infusion of Met-enkephalin into the medial preoptic area. Brain Res. 1991;550:343–346. doi: 10.1016/0006-8993(91)91339-3. [DOI] [PubMed] [Google Scholar]
- 2.Agmo A, Gomez M. Sexual reinforcement is blocked by infusion of naloxone into the medial preoptic area. Behav Neurosci. 1993;107:812–818. doi: 10.1037//0735-7044.107.5.812. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 5.Akil H, Owens C, Gutstein H, Taylor L, Curran E, Watson S. Endogenous opioids: overview and current issues. Drug Alcohol Depend. 1998;51:127–140. doi: 10.1016/s0376-8716(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 6.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez Fischer D, Schafer MK, Ferger B, Gross S, Westermann R, Weihe E, Kuschinsky K. Sensitization to the behavioural effects of cocaine: alterations in tyrosine hydroxylase or endogenous opioid mRNAs are not necessarily involved. Naunyn-Schmiedebergs Arch Pharmacol. 2001;363:288–294. doi: 10.1007/s002100000332. [DOI] [PubMed] [Google Scholar]
- 8.Ammon-Treiber S, Hollt V. Morphine-induced changes of gene expression in the brain. Addict Biol. 2005;10:81–89. doi: 10.1080/13556210412331308994. [DOI] [PubMed] [Google Scholar]
- 9.Argiolas A. Neuropeptides and sexual behaviour. Neurosci Biobehav Rev. 1999;23:1127–1142. doi: 10.1016/s0149-7634(99)00068-8. [DOI] [PubMed] [Google Scholar]
- 10.Argiolas A, Melis MR. Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Prog Neurobiol. 2005;76:1–21. doi: 10.1016/j.pneurobio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Arroyo M, Baker WA, Everitt BJ. Cocaine self-administration in rats differentially alters mRNA levels of the monoamine transporters and striatal neuropeptides. Brain Res. 2000;83:107–120. doi: 10.1016/s0169-328x(00)00205-9. [DOI] [PubMed] [Google Scholar]
- 12.Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology. 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- 13.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 14.Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann NY Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- 15.Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology. 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- 16.Azaryan AV, Clock BJ, Rosenberger JG, Cox BM. Transient upregulation of mu opioid receptor mRNA levels in nucleus accumbens during chronic cocaine administration. Can J Physiol Pharmacol. 1998;76:278–283. [PubMed] [Google Scholar]
- 17.Azaryan AV, Coughlin LJ, Buzas B, Clock BJ, Cox BM. Effect of chronic cocaine treatment on mu- and delta-opioid receptor mRNA levels in dopaminergically innervated brain regions. J Neurochem. 1996;66:443–448. doi: 10.1046/j.1471-4159.1996.66020443.x. [DOI] [PubMed] [Google Scholar]
- 18.Bailey A, Yuferov V, Bendor J, Schlussman SD, Zhou Y, Ho A, Kreek MJ. Immediate withdrawal from chronic “binge” cocaine administration increases mu-opioid receptor mRNA levels in rat frontal cortex. Brain Res. 2005;137:258–262. doi: 10.1016/j.molbrainres.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 20.Balleine BW. Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 21.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuro-anatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- 23.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- 25.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 26.Basheer R, Tempel A. Morphine-induced reciprocal alterations in G alpha s and opioid peptide mRNA levels in discrete brain regions. J Neurosci Res. 1993;36:551–557. doi: 10.1002/jnr.490360507. [DOI] [PubMed] [Google Scholar]
- 27.Beadles-Bohling AS, Wiren KM. Alteration of kappa-opioid receptor system expression in distinct brain regions of a genetic model of enhanced ethanol withdrawal severity. Brain Res. 2005;1046:77–89. doi: 10.1016/j.brainres.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 28.Beaulieu J, Champagne D, Drolet G. Enkephalin innervation of the paraventricular nucleus of the hypothalamus: distribution of fibers and origins of input. J Chem Neuroanat. 1996;10:79–92. doi: 10.1016/0891-0618(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 29.Bechtholt AJ, Cunningham CL. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav Neurosci. 2005;119:213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- 30.Becker A, Grecksch G, Brodemann R, Kraus J, Peters B, Schroeder H, Thiemann W, Loh HH, Hollt V. Morphine self-administration in mu-opioid receptor-deficient mice. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;361:584–589. doi: 10.1007/s002100000244. [DOI] [PubMed] [Google Scholar]
- 31.Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Hollt V. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn-Schmiedeberg’s Arch Pharmacol. 2002;365:296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- 32.Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J, Lardenois A, Thibault C, Dembele D, Le Merrer J, Becker JA, Poch O, Kieffer BL. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci. 2008;27:2973–2984. doi: 10.1111/j.1460-9568.2008.06273.x. [DOI] [PubMed] [Google Scholar]
- 33.Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65:863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci. 2002;22:10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, Zimmer A, Maldonado R. Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J Neurosci. 2005;25:1103–1112. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 38.Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 40.Blednov YA, Walker D, Martinez M, Harris RA. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006;40:73–86. doi: 10.1016/j.alcohol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodnar RJ. Endogenous opiates and behavior: 2006. Peptides. 2007;28:2435–2513. doi: 10.1016/j.peptides.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Bodnar RJ, Lamonte N, Israel Y, Kandov Y, Ackerman TF, Khaimova E. Reciprocal opioid-opioid interactions between the ventral tegmental area and nucleus accumbens regions in mediating mu agonist-induced feeding in rats. Peptides. 2005;26:621–629. doi: 10.1016/j.peptides.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Bozarth MA. Neuroanatomical boundaries of the reward-relevant opiate-receptor field in the ventral tegmental area as mapped by the conditioned place preference method in rats. Brain Res. 1987;414:77–84. doi: 10.1016/0006-8993(87)91327-8. [DOI] [PubMed] [Google Scholar]
- 45.Bozarth MA. Physical dependence produced by central morphine infusions: an anatomical mapping study. Neurosci Biobehav Rev. 1994;18:373–383. doi: 10.1016/0149-7634(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 46.Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- 47.Bozarth MA, Wise RA. Heroin reward is dependent on a dopaminergic substrate. Life Sci. 1981;29:1881–1886. doi: 10.1016/0024-3205(81)90519-1. [DOI] [PubMed] [Google Scholar]
- 48.Brandao ML. Involvement of opioid mechanisms in the dorsal periaqueductal gray in drug abuse. Rev Neurosci. 1993;4:397–405. doi: 10.1515/revneuro.1993.4.4.397. [DOI] [PubMed] [Google Scholar]
- 49.Brodsky M, Elliott K, Hynansky A, Inturrisi CE. CNS levels of mu opioid receptor (MOR-1) mRNA during chronic treatment with morphine or naltrexone. Brain Res Bull. 1995;38:135–141. doi: 10.1016/0361-9230(95)00079-t. [DOI] [PubMed] [Google Scholar]
- 50.Bronstein DM, Przewlocki R, Akil H. Effects of morphine treatment on pro-opiomelanocortin systems in rat brain. Brain Res. 1990;519:102–111. doi: 10.1016/0006-8993(90)90066-k. [DOI] [PubMed] [Google Scholar]
- 51.Broseta I, Rodriguez-Arias M, Stinus L, Minarro J. Ethological analysis of morphine withdrawal with different dependence programs in male mice. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:335–347. doi: 10.1016/s0278-5846(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 52.Buzas B, Rosenberger J, Cox BM. Mu and delta opioid receptor gene expression after chronic treatment with opioid agonist. Neuroreport. 1996;7:1505–1508. doi: 10.1097/00001756-199606170-00013. [DOI] [PubMed] [Google Scholar]
- 53.Byrnes EM. Chronic morphine exposure during puberty induces long-lasting changes in opioid-related mRNA expression in the mediobasal hypothalamus. Brain Res. 2008;1190:186–192. doi: 10.1016/j.brainres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Cabral A, Isoardi N, Salum C, Macedo CE, Nobre MJ, Molina VA, Brandao ML. Fear state induced by ethanol withdrawal may be due to the sensitization of the neural substrates of aversion in the dPAG. Exp Neurol. 2006;200:200–208. doi: 10.1016/j.expneurol.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Caille S, Espejo EF, Reneric JP, Cador M, Koob GF, Stinus L. Total neurochemical lesion of noradrenergic neurons of the locus ceruleus does not alter either naloxone-precipitated or spontaneous opiate withdrawal nor does it influence ability of clonidine to reverse opiate withdrawal. J Pharmacol Exp Ther. 1999;290:881–892. [PubMed] [Google Scholar]
- 56.Candeletti S, Lopetuso G, Cannarsa R, Cavina C, Romualdi P. Effects of prolonged treatment with the opiate tramadol on prodynorphin gene expression in rat CNS. J Mol Neurosci. 2006;30:341–347. doi: 10.1385/JMN:30:3:341. [DOI] [PubMed] [Google Scholar]
- 57.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 58.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 59.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carr KD, Aleman DO, Bak TH, Simon EJ. Effects of parabrachial opioid antagonism on stimulation-induced feeding. Brain Res. 1991;545:283–286. doi: 10.1016/0006-8993(91)91298-f. [DOI] [PubMed] [Google Scholar]
- 61.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann NY Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- 62.Castane A, Robledo P, Matifas A, Kieffer BL, Maldonado R. Cannabinoid withdrawal syndrome is reduced in double mu and delta opioid receptor knockout mice. Eur J Neurosci. 2003;17:155–159. doi: 10.1046/j.1460-9568.2003.02409.x. [DOI] [PubMed] [Google Scholar]
- 63.Castelli MP, Melis M, Mameli M, Fadda P, Diaz G, Gessa GL. Chronic morphine and naltrexone fail to modify mu-opioid receptor mRNA levels in the rat brain. Brain Res. 1997;45:149–153. doi: 10.1016/s0169-328x(96)00305-1. [DOI] [PubMed] [Google Scholar]
- 64.Cazala P. Dose-dependent effects of morphine differentiate self-administration elicited from lateral hypothalamus and mesencephalic central gray area in mice. Brain Res. 1990;527:280–285. doi: 10.1016/0006-8993(90)91147-9. [DOI] [PubMed] [Google Scholar]
- 5.Cazala P, Darracq C, Saint-Marc M. Self-administration of morphine into the lateral hypothalamus in the mouse. Brain Res. 1987;416:283–288. doi: 10.1016/0006-8993(87)90908-5. [DOI] [PubMed] [Google Scholar]
- 66.Cazala P, Norena A, Le Merrer J, Galey D. Differential involvement of the lateral and medial divisions of the septal area on spatial learning processes as revealed by intracranial self-administration of morphine in mice. Behav Brain Res. 1998;97:179–188. doi: 10.1016/s0166-4328(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 67.Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31:249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 68.Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christie MJ, Williams JT, Osborne PB, Bellchambers CE. Where is the locus in opioid withdrawal? Trends Pharmacol Sci. 1997;18:134–140. doi: 10.1016/s0165-6147(97)01045-6. [DOI] [PubMed] [Google Scholar]
- 70.Collins S, D’Addario C, Romualdi P, Candeletti S, Izenwasser S. Regulation of dynorphin gene expression by kappa-opioid agonist treatment. Neuroreport. 2002;13:107–109. doi: 10.1097/00001756-200201210-00025. [DOI] [PubMed] [Google Scholar]
- 71.Contarino A, Picetti R, Matthes HW, Koob GF, Kieffer BL, Gold LH. Lack of reward and locomotor stimulation induced by heroin in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2002;446:103–109. doi: 10.1016/s0014-2999(02)01812-5. [DOI] [PubMed] [Google Scholar]
- 72.Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Coolen LM, Allard J, Truitt WA, McKenna KE. Central regulation of ejaculation. Physiol Behav. 2004;83:203–215. doi: 10.1016/j.physbeh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 74.Corchero J, Avila MA, Fuentes JA, Manzanares J. delta-9-Tetrahydrocannabinol increases prodynorphin and proenkephalin gene expression in the spinal cord of the rat. Life Sci. 1997;61:PL 39–43. doi: 10.1016/s0024-3205(97)00405-0. [DOI] [PubMed] [Google Scholar]
- 75.Corchero J, Fuentes JA, Manzanares J. delta 9-Tetrahydrocannabinol increases proopiomelanocortin gene expression in the arcuate nucleus of the rat hypothalamus. Eur J Pharmacol. 1997;323:193–195. doi: 10.1016/s0014-2999(97)00144-1. [DOI] [PubMed] [Google Scholar]
- 76.Corchero J, Fuentes JA, Manzanares J. Gender differences in proenkephalin gene expression response to delta9-tetrahydrocan-nabinol in the hypothalamus of the rat. J Psychopharmacol. 2002;16:283–289. doi: 10.1177/026988110201600401. [DOI] [PubMed] [Google Scholar]
- 77.Corchero J, Manzanares J, Fuentes JA. Repeated administration of delta9-tetrahydrocannabinol produces a differential time related responsiveness on proenkephalin, proopiomelanocortin and corticotropin releasing factor gene expression in the hypothalamus and pituitary gland of the rat. Neuropharmacology. 1999;38:433–439. doi: 10.1016/s0028-3908(98)00195-6. [DOI] [PubMed] [Google Scholar]
- 78.Corchero J, Romero J, Berrendero F, Fernandez-Ruiz J, Ramos JA, Fuentes JA, Manzanares J. Time-dependent differences of repeated administration with delta9-tetrahydrocannabinol in proenkephalin and cannabinoid receptor gene expression and G-protein activation by mu-opioid and CB1-cannabinoid receptors in the caudate-putamen. Brain Res. 1999;67:148–157. doi: 10.1016/s0169-328x(99)00053-4. [DOI] [PubMed] [Google Scholar]
- 79.Cornish JL, Lontos JM, Clemens KJ, McGregor IS. Cocaine and heroin (“speedball”) self-administration: the involvement of nucleus accumbens dopamine and mu-opiate, but not delta-opiate receptors. Psychopharmacology. 2005;180:21–32. doi: 10.1007/s00213-004-2135-9. [DOI] [PubMed] [Google Scholar]
- 80.Corrigall WA. Heroin self-administration: effects of antagonist treatment in lateral hypothalamus. Pharmacol Biochem Behav. 1987;27:693–700. doi: 10.1016/0091-3057(87)90196-1. [DOI] [PubMed] [Google Scholar]
- 81.Corrigall WA, Coen KM, Adamson KL, Chow BL. Manipulations of mu-opioid and nicotinic cholinergic receptors in the pontine tegmental region alter cocaine self-administration in rats. Psychopharmacology. 1999;145:412–417. doi: 10.1007/s002130051075. [DOI] [PubMed] [Google Scholar]
- 82.Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and gamma-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology. 2000;149:107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- 83.Corrigall WA, Coen KM, Zhang J, Adamson L. Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology. 2002;160:198–205. doi: 10.1007/s00213-001-0965-2. [DOI] [PubMed] [Google Scholar]
- 84.Cowen MS, Lawrence AJ. Alterations in central preproenkephalin mRNA expression after chronic free-choice ethanol consumption by fawn-hooded rats. Alcohol Clin Exp Res. 2001;25:1126–1133. [PubMed] [Google Scholar]
- 85.Crabbe JC. Review: neurogenetic studies of alcohol addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3201–3211. doi: 10.1098/rstb.2008.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crespo JA, Manzanares J, Oliva JM, Corchero J, Palomo T, Ambrosio E. Extinction of cocaine self-administration produces a differential time-related regulation of proenkephalin gene expression in rat brain. Neuropsychopharmacology. 2001;25:185–194. doi: 10.1016/S0893-133X(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 87.Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- 88.Dallimore JE, Mickiewicz AL, Napier TC. Intra-ventral pallidal glutamate antagonists block expression of morphine-induced place preference. Behav Neurosci. 2006;120:1103–1114. doi: 10.1037/0735-7044.120.5.1103. [DOI] [PubMed] [Google Scholar]
- 89.Daumas S, Betourne A, Halley H, Wolfer DP, Lipp HP, Lassalle JM, Frances B. Transient activation of the CA3 Kappa opioid system in the dorsal hippocampus modulates complex memory processing in mice. Neurobiol Learn Mem. 2007;88:94–103. doi: 10.1016/j.nlm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Daunais JB, McGinty JF. Acute and chronic cocaine administration differentially alters striatal opioid and nuclear transcription factor mRNAs. Synapse. 1994;18:35–45. doi: 10.1002/syn.890180106. [DOI] [PubMed] [Google Scholar]
- 91.Daunais JB, McGinty JF. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Brain Res. 1995;29:201–210. doi: 10.1016/0169-328x(94)00246-b. [DOI] [PubMed] [Google Scholar]
- 92.Daunais JB, McGinty JF. The effects of D1 or D2 dopamine receptor blockade on zif/268 and preprodynorphin gene expression in rat forebrain following a short-term cocaine binge. Brain Res. 1996;35:237–248. doi: 10.1016/0169-328x(95)00226-i. [DOI] [PubMed] [Google Scholar]
- 93.David V, Cazala P. Anatomical and pharmacological specificity of the rewarding effect elicited by microinjections of morphine into the nucleus accumbens of mice. Psychopharmacology. 2000;150:24–34. doi: 10.1007/s002130000425. [DOI] [PubMed] [Google Scholar]
- 94.David V, Cazala P. A comparative study of self-administration of morphine into the amygdala and the ventral tegmental area in mice. Behav Brain Res. 1994;65:205–211. doi: 10.1016/0166-4328(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 95.David V, Durkin TP, Cazala P. Differential effects of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology. 2002;160:307–317. doi: 10.1007/s00213-001-0981-2. [DOI] [PubMed] [Google Scholar]
- 96.David V, Matifas A, Gavello-Baudy S, Decorte L, Kieffer BL, Cazala P. Brain regional Fos expression elicited by the activation of mu- but not delta-opioid receptors of the ventral tegmental area: evidence for an implication of the ventral thalamus in opiate reward. Neuropsychopharmacology. 2008;33:1746–1759. doi: 10.1038/sj.npp.1301529. [DOI] [PubMed] [Google Scholar]
- 97.De Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann NY Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- 98.Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 99.DePaoli AM, Hurley KM, Yasada K, Reisine T, Bell G. Distribution of kappa opioid receptor mRNA in adult mouse brain: an in situ hybridization histochemistry study. Mol Cell Neurosci. 1994;5:327–335. doi: 10.1006/mcne.1994.1039. [DOI] [PubMed] [Google Scholar]
- 100.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 101.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dickenson AH, Kieffer BL. Opiates: basic mechanisms. In: McMahon SB, Koltzenburg M, editors. Textbook of Pain. New York: Elsevier; 2005. pp. 427–442. [Google Scholar]
- 103.DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- 104.Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 105.Dong Z, Han H, Wang M, Xu L, Hao W, Cao J. Morphine conditioned place preference depends on glucocorticoid receptors in both hippocampus and nucleus accumbens. Hippocampus. 2006;16:809–813. doi: 10.1002/hipo.20216. [DOI] [PubMed] [Google Scholar]
- 106.Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res. 2007;163:245–263. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- 107.Drolet G, Van Bockstaele EJ, Aston-Jones G. Robust enkephalin innervation of the locus coeruleus from the rostral medulla. J Neurosci. 1992;12:3162–3174. doi: 10.1523/JNEUROSCI.12-08-03162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 110.Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- 111.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 113.Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- 114.Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- 115.Fang Y, Kelly MJ, Ronnekleiv OK. Proopiomelanocortin (POMC) mRNA expression: distribution and region-specific downregulation by chronic morphine in female guinea pig hypothalamus. Brain Res. 1998;55:1–8. doi: 10.1016/s0169-328x(97)00348-3. [DOI] [PubMed] [Google Scholar]
- 116.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 117.Flanagan-Cato LM. Estrogen-induced remodeling of hypothalamic neural circuitry. Front Neuroendocrinol. 2000;21:309–329. doi: 10.1006/frne.2000.0204. [DOI] [PubMed] [Google Scholar]
- 118.Foster KL, McKay PF, Seyoum R, Milbourne D, Yin W, Sarma PV, Cook JM, June HL. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29:269–284. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- 119.Fowler JS, Volkow ND, Kassed CA, Chang L. Imaging the addicted human brain. Sci Pract Perspect. 2007;3:4–16. doi: 10.1151/spp07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fukunaga Y, Inoue N, Miyamoto M, Kishioka S, Yamamoto H. Effects of peptidase inhibitors, [d-Ala2,Met5]-enkephalinamide and antiserum to methionine-enkephalin microinjected into the caudal periaqueductal gray on morphine withdrawal in rats. Jpn J Pharmacol. 1998;78:455–461. doi: 10.1254/jjp.78.455. [DOI] [PubMed] [Google Scholar]
- 121.Fukunaga Y, Nishida S, Inoue N, Kishioka S, Yamamoto H. Increase of preproenkephalin mRNA in the caudal part of peri-aqueductal gray by morphine withdrawal in rats: a quantitative in situ hybridization study. Brain Res. 1996;42:128–130. doi: 10.1016/s0169-328x(96)00158-1. [DOI] [PubMed] [Google Scholar]
- 122.Gadd CA, Murtra P, De Felipe C, Hunt SP. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J Neurosci. 2003;23:8271–8280. doi: 10.1523/JNEUROSCI.23-23-08271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galeote L, Berrendero F, Andreea Bura S, Zimmer A, Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol. 2008:1–11. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- 124.Garcia MM, Brown HE, Harlan RE. Alterations in immediate-early gene proteins in the rat forebrain induced by acute morphine injection. Brain Res. 1995;692:23–40. doi: 10.1016/0006-8993(95)00625-z. [DOI] [PubMed] [Google Scholar]
- 125.Garcia de Yebenes E, Pelletier G. Opioid regulation of proopiomelanocortin (POMC) gene expression in the rat brain as studied by in situ hybridization. Neuropeptides. 1993;25:91–94. doi: 10.1016/0143-4179(93)90087-q. [DOI] [PubMed] [Google Scholar]
- 126.Garcia-Horsman SP, Agmo A, Paredes RG. Infusions of naloxone into the medial preoptic area, ventromedial nucleus of the hypothalamus, and amygdala block conditioned place preference induced by paced mating behavior. Horm Behav. 2008;54:709–716. doi: 10.1016/j.yhbeh.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 127.Gaveriaux-Ruff C, Kieffer BL. Conditional gene targeting in the mouse nervous system: insights into brain function and diseases. Pharmacol Ther. 2007;113:619–634. doi: 10.1016/j.pharmthera.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 128.Gaveriaux-Ruff C, Kieffer BL. Opioid receptor genes inactivated in mice: the highlights. Neuropeptides. 2002;36:62–71. doi: 10.1054/npep.2002.0900. [DOI] [PubMed] [Google Scholar]
- 129.George SR, Zastawny RL, Briones-Urbina R, Cheng R, Nguyen T, Heiber M, Kouvelas A, Chan AS, O’Dowd BF. Distinct distributions of mu, delta and kappa opioid receptor mRNA in rat brain. Biochem Biophys Res Commun. 1994;205:1438–1444. doi: 10.1006/bbrc.1994.2826. [DOI] [PubMed] [Google Scholar]
- 130.Georges F, Stinus L, Bloch B, Le Moine C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur J Neurosci. 1999;11:481–490. doi: 10.1046/j.1460-9568.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- 131.Gerrits MA, Lesscher HB, van Ree JM. Drug dependence and the endogenous opioid system. Eur Neuropsychopharmacol. 2003;13:424–434. doi: 10.1016/j.euroneuro.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 132.Ghozland S, Chu K, Kieffer BL, Roberts AJ. Lack of stimulant and anxiolytic-like effects of ethanol and accelerated development of ethanol dependence in mu-opioid receptor knockout mice. Neuropharmacology. 2005;49:493–501. doi: 10.1016/j.neuropharm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 133.Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Giraudo SQ, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the central nucleus of the amygdala and in the paraventricular nucleus in the rat. Brain Res. 1998;782:18–23. doi: 10.1016/s0006-8993(97)01140-2. [DOI] [PubMed] [Google Scholar]
- 135.Giuliano F, Rampin O. Central neural regulation of penile erection. Neurosci Biobehav Rev. 2000;24:517–533. doi: 10.1016/s0149-7634(00)00020-8. [DOI] [PubMed] [Google Scholar]
- 136.Givens B, Sarter M. Modulation of cognitive processes by transsynaptic activation of the basal forebrain. Behav Brain Res. 1997;84:1–22. doi: 10.1016/s0166-4328(96)00146-5. [DOI] [PubMed] [Google Scholar]
- 137.Glass MJ, Billington CJ, Levine AS. Naltrexone administered to central nucleus of amygdala or PVN: neural dissociation of diet and energy. Am J Physiol Regul Integr Comp Physiol. 2000;279:R86–R92. doi: 10.1152/ajpregu.2000.279.1.R86. [DOI] [PubMed] [Google Scholar]
- 138.Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- 139.Glass MJ, Briggs JE, Billington CJ, Kotz CM, Levine AS. Opioid receptor blockade in rat nucleus tractus solitarius alters amygdala dynorphin gene expression. Am J Physiol Regul Integr Comp Physiol. 2002;283:R161–R167. doi: 10.1152/ajpregu.00480.2001. [DOI] [PubMed] [Google Scholar]
- 140.Goeders NE, Lane JD, Smith JE. Self-administration of methionine enkephalin into the nucleus accumbens. Pharmacol Biochem Behav. 1984;20:451–455. doi: 10.1016/0091-3057(84)90284-3. [DOI] [PubMed] [Google Scholar]
- 141.Gong J, Li XW, Lai Z, Froehlich JC, Yu L. Quantitative comparison of mu opioid receptor mRNA in selected CNS regions of alcohol naive rats selectively bred for high and low alcohol drinking. Neurosci Lett. 1997;227:9–12. doi: 10.1016/s0304-3940(97)00289-9. [DOI] [PubMed] [Google Scholar]
- 142.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 143.Gong W, Neill D, Justice JB., Jr Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 1996;707:64–74. doi: 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- 144.Goody RJ, Oakley SM, Filliol D, Kieffer BL, Kitchen I. Quantitative autoradiographic mapping of opioid receptors in the brain of delta-opioid receptor gene knockout mice. Brain Res. 2002;945:9–19. doi: 10.1016/s0006-8993(02)02452-6. [DOI] [PubMed] [Google Scholar]
- 145.Gudehithlu KP, Bhargava HN. Modulation of preproenkephalin mRNA levels in brain regions and spinal cord of rats treated chronically with morphine. Peptides. 1995;16:415–419. doi: 10.1016/0196-9781(94)00199-g. [DOI] [PubMed] [Google Scholar]
- 146.Gulya K, Orpana AK, Sikela JM, Hoffman PL. Prodynorphin and vasopressin mRNA levels are differentially affected by chronic ethanol ingestion in the mouse. Brain Res. 1993;20:1–8. doi: 10.1016/0169-328x(93)90105-x. [DOI] [PubMed] [Google Scholar]
- 147.Guo N, Garcia MM, Taylor BK, Zadina JE, Harlan RE. Blockade of micro-opioid receptors in the medial thalamus inhibits acquisition, but not expression, of morphine-induced conditioned place preference. Neuroscience. 2008;151:948–954. doi: 10.1016/j.neuroscience.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 148.Hall FS, Goeb M, Li XF, Sora I, Uhl GR. mu-Opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Brain Res. 2004;121:123–130. doi: 10.1016/j.molbrainres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 149.Harbuz M, Russell JA, Sumner BE, Kawata M, Lightman SL. Rapid changes in the content of proenkephalin A and corticotrophin releasing hormone mRNAs in the paraventricular nucleus during morphine withdrawal in urethane-anaesthetized rats. Brain Res. 1991;9:285–291. doi: 10.1016/0169-328x(91)90074-8. [DOI] [PubMed] [Google Scholar]
- 150.Harlan RE, Shivers BD, Romano GJ, Howells RD, Pfaff DW. Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J Comp Neurol. 1987;258:159–184. doi: 10.1002/cne.902580202. [DOI] [PubMed] [Google Scholar]
- 151.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 152.Hayward MD, Hansen ST, Pintar JE, Low MJ. Operant self-administration of ethanol in C57BL/6 mice lacking beta-endorphin and enkephalin. Pharmacol Biochem Behav. 2004;79:171–181. doi: 10.1016/j.pbb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 153.Hayward MD, Schaich-Borg A, Pintar JE, Low MJ. Differential involvement of endogenous opioids in sucrose consumption and food reinforcement. Pharmacol Biochem Behav. 2006;85:601–611. doi: 10.1016/j.pbb.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Herraez-Baranda LA, Carretero J, Gonzalez-Sarmiento R, Rodriguez RE. Kappa opioid receptor is expressed in the rat cerebellar cortex. Cell Tissue Res. 2005;320:223–228. doi: 10.1007/s00441-004-1048-6. [DOI] [PubMed] [Google Scholar]
- 155.Heyser CJ, Roberts AJ, Schulteis G, Koob GF. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin Exp Res. 1999;23:1468–1476. [PubMed] [Google Scholar]
- 156.Higuchi S, Matsushita S, Kashima H. New findings on the genetic influences on alcohol use and dependence. Curr Opin Psychiatry. 2006;19:253–265. doi: 10.1097/01.yco.0000218595.54054.7a. [DOI] [PubMed] [Google Scholar]
- 157.Hiroi N, White NM. The ventral pallidum area is involved in the acquisition but not expression of the amphetamine conditioned place preference. Neurosci Lett. 1993;156:9–12. doi: 10.1016/0304-3940(93)90426-l. [DOI] [PubMed] [Google Scholar]
- 158.Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 159.Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- 160.Hollt V, Haarmann I. Differential alterations by chronic treatment with morphine of pro-opiomelanocortin mRNA levels in anterior as compared to intermediate pituitary lobes of rats. Neuropeptides. 1985;5:481–484. doi: 10.1016/0143-4179(85)90059-9. [DOI] [PubMed] [Google Scholar]
- 161.Hollt V, Horn G. Effect of nicotine on mRNA levels encoding opioid peptides, vasopressin and alpha 3 nicotinic receptor subunit in the rat. Clin Invest. 1992;70:224–231. doi: 10.1007/BF00184655. [DOI] [PubMed] [Google Scholar]
- 162.Houdi AA, Dasgupta R, Kindy MS. Effect of nicotine use and withdrawal on brain preproenkephalin A mRNA. Brain Res. 1998;799:257–263. doi: 10.1016/s0006-8993(98)00454-5. [DOI] [PubMed] [Google Scholar]
- 163.Houshyar H, Manalo S, Dallman MF. Time-dependent alterations in mRNA expression of brain neuropeptides regulating energy balance and hypothalamo-pituitary-adrenal activity after withdrawal from intermittent morphine treatment. J Neurosci. 2004;24:9414–9424. doi: 10.1523/JNEUROSCI.1641-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang LZ, Winzer-Serhan UH. Nicotine regulates mRNA expression of feeding peptides in the arcuate nucleus in neonatal rat pups. Dev Neurobiol. 2007;67:363–377. doi: 10.1002/dneu.20348. [DOI] [PubMed] [Google Scholar]
- 165.Hubner CB, Koob GF. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res. 1990;508:20–29. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- 166.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- 168.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 169.Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 170.Ikeda K, Ide S, Han W, Hayashida M, Uhl GR, Sora I. How individual sensitivity to opiates can be predicted by gene analyses. Trends Pharmacol Sci. 2005;26:311–317. doi: 10.1016/j.tips.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 171.Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 172.Isola R, Zhang H, Duchemin AM, Tejwani GA, Neff NH, Hadjiconstantinou M. Met-enkephalin and preproenkephalin mRNA changes in the striatum of the nicotine abstinence mouse. Neurosci Lett. 2002;325:67–71. doi: 10.1016/s0304-3940(02)00240-9. [DOI] [PubMed] [Google Scholar]
- 173.Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Dynorphin and prodynorphin mRNA changes in the striatum during nicotine withdrawal. Synapse. 2008;62:448–455. doi: 10.1002/syn.20515. [DOI] [PubMed] [Google Scholar]
- 174.Ito R, Robbins TW, Pennartz CM, Everitt BJ. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci. 2008;28:6950–6959. doi: 10.1523/JNEUROSCI.1615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jenck F, Gratton A, Wise RA. Opioid receptor subtypes associated with ventral tegmental facilitation of lateral hypothalamic brain stimulation reward. Brain Res. 1987;423:34–38. doi: 10.1016/0006-8993(87)90821-3. [DOI] [PubMed] [Google Scholar]
- 176.Jomary C, Gairin JE, Cros J, Meunier JC. Autoradiographic localization of supraspinal kappa-opioid receptors with [125I-Tyr1, d-Pro10]dynorphin A-(1–11) Proc Natl Acad Sci USA. 1988;85:627–631. doi: 10.1073/pnas.85.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jones KL, Barr GA. Injections of an opioid antagonist into the locus coeruleus and periaqueductal gray but not the amygdala precipitates morphine withdrawal in the 7-day-old rat. Synapse. 2001;39:139–151. doi: 10.1002/1098-2396(200102)39:2<139::AID-SYN5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 178.June HL, Cummings R, Eiler WJ, 2nd, Foster KL, McKay PF, Seyoum R, Garcia M, McCane S, Grey C, Hawkins SE, Mason D. Central opioid receptors differentially regulate the nalmefene-induced suppression of ethanol- and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharmacology. 2004;29:285–299. doi: 10.1038/sj.npp.1300338. [DOI] [PubMed] [Google Scholar]
- 179.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 180.Kas MJ, van den Bos R, Baars AM, Lubbers M, Lesscher HM, Hillebrand JJ, Schuller AG, Pintar JE, Spruijt BM. Mu-opioid receptor knockout mice show diminished food-anticipatory activity. Eur J Neurosci. 2004;20:1624–1632. doi: 10.1111/j.1460-9568.2004.03581.x. [DOI] [PubMed] [Google Scholar]
- 181.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 182.Kawagoe T, Tamura R, Uwano T, Asahi T, Nishijo H, Eifuku S, Ono T. Neural correlates of stimulus-reward association in the rat mediodorsal thalamus. Neuroreport. 2007;18:683–688. doi: 10.1097/WNR.0b013e3280bef9a6. [DOI] [PubMed] [Google Scholar]
- 183.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 184.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 185.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- 186.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Khachaturian H, Lewis ME, Haber SN, Houghten RA, Akil H, Watson SJ. Prodynorphin peptide immunocytochemistry in rhesus monkey brain. Peptides. 1985;6(Suppl 2):155–166. doi: 10.1016/0196-9781(85)90149-4. [DOI] [PubMed] [Google Scholar]
- 188.Khachaturian H, Lewis ME, Watson SJ. Enkephalin systems in diencephalon and brainstem of the rat. J Comp Neurol. 1983;220:310–320. doi: 10.1002/cne.902200305. [DOI] [PubMed] [Google Scholar]
- 189.Khachaturian H, Watson SJ, Lewis ME, Coy D, Goldstein A, Akil H. Dynorphin immunocytochemistry in the rat central nervous system. Peptides. 1982;3:941–954. doi: 10.1016/0196-9781(82)90063-8. [DOI] [PubMed] [Google Scholar]
- 190.Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cell Mol Neurobiol. 1995;15:615–635. doi: 10.1007/BF02071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 192.Kim EM, Quinn JG, Levine AS, O’Hare E. A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Res. 2004;1029:135–139. doi: 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 193.Kitchen I, Slowe SJ, Matthes HW, Kieffer B. Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res. 1997;778:73–88. doi: 10.1016/s0006-8993(97)00988-8. [DOI] [PubMed] [Google Scholar]
- 194.Koenig HN, Olive MF. Ethanol consumption patterns and conditioned place preference in mice lacking preproenkephalin. Neurosci Lett. 2002;325:75–78. doi: 10.1016/s0304-3940(02)00242-2. [DOI] [PubMed] [Google Scholar]
- 195.Kohnke MD. Approach to the genetics of alcoholism: a review based on pathophysiology. Biochem Pharmacol. 2008;75:160–177. doi: 10.1016/j.bcp.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 196.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 198.Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 199.Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 202.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 203.Koob GF, Wall TL, Bloom FE. Nucleus accumbens as a substrate for the aversive stimulus effects of opiate withdrawal. Psychopharmacology. 1989;98:530–534. doi: 10.1007/BF00441954. [DOI] [PubMed] [Google Scholar]
- 204.Kovacs KM, Szakall I, O’Brien D, Wang R, Vinod KY, Saito M, Simonin F, Kieffer BL, Vadasz C. Decreased oral self-administration of alcohol in kappa-opioid receptor knock-out mice. Alcohol Clin Exp Res. 2005;29:730–738. doi: 10.1097/01.alc.0000164361.62346.d6. [DOI] [PubMed] [Google Scholar]
- 205.Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev. 2005;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- 206.Kreek MJ, Zhou Y, Butelman ER, Levran O. Opiate and cocaine addiction: from bench to clinic and back to the bench. Curr Opin Pharmacol. 2009;9:74–80. doi: 10.1016/j.coph.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lantos TA, Gorcs TJ, Palkovits M. Immunohistochemical mapping of neuropeptides in the premamillary region of the hypothalamus in rats. Brain Res. 1995;20:209–249. doi: 10.1016/0165-0173(94)00013-f. [DOI] [PubMed] [Google Scholar]
- 209.Le Merrer J, Gavello-Baudy S, Galey D, Cazala P. Morphine self-administration into the lateral septum depends on dopaminergic mechanisms: evidence from pharmacology and Fos neuroimaging. Behav Brain Res. 2007;180:203–217. doi: 10.1016/j.bbr.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 210.Leri F, Zhou Y, Goddard B, Cummins E, Kreek MJ. Effects of high-dose methadone maintenance on cocaine place conditioning, cocaine self-administration, and mu-opioid receptor mRNA expression in the rat brain. Neuropsychopharmacology. 2006;31:1462–1474. doi: 10.1038/sj.npp.1300927. [DOI] [PubMed] [Google Scholar]
- 211.Leriche M, Cote-Velez A, Mendez M. Presence of pro-opiomelanocortin mRNA in the rat medial prefrontal cortex, nucleus accumbens and ventral tegmental area: studies by RT-PCR and in situ hybridization techniques. Neuropeptides. 2007;41:421–431. doi: 10.1016/j.npep.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 212.Lesscher HM, Bailey A, Burbach JP, Van Ree JM, Kitchen I, Gerrits MA. Receptor-selective changes in mu-, delta- and kappa-opioid receptors after chronic naltrexone treatment in mice. Eur J Neurosci. 2003;17:1006–1012. doi: 10.1046/j.1460-9568.2003.02502.x. [DOI] [PubMed] [Google Scholar]
- 213.Levine AS, Olszewski PK, Mullett MA, Pomonis JD, Grace MK, Kotz CM, Billington CJ. Intra-amygdalar injection of DAMGO: effects on c-Fos levels in brain sites associated with feeding behavior. Brain Res. 2004;1015:9–14. doi: 10.1016/j.brainres.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 214.Leyton M, Stewart J. The stimulation of central kappa opioid receptors decreases male sexual behavior and locomotor activity. Brain Res. 1992;594:56–74. doi: 10.1016/0006-8993(92)91029-e. [DOI] [PubMed] [Google Scholar]
- 215.Li D, Olszewski PK, Shi Q, Grace MK, Billington CJ, Kotz CM, Levine AS. Effect of opioid receptor ligands injected into the rostral lateral hypothalamus on c-fos and feeding behavior. Brain Res. 2006;1096:120–124. doi: 10.1016/j.brainres.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 216.Li MD, Konu O, Kane JK, Becker KG. Microarray technology and its application on nicotine research. Mol Neurobiol. 2002;25:265–285. doi: 10.1385/MN:25:3:265. [DOI] [PubMed] [Google Scholar]
- 217.Liang J, Li Y, Ping X, Yu P, Zuo Y, Wu L, Han JS, Cui C. The possible involvement of endogenous ligands for mu-, delta- and kappa-opioid receptors in modulating morphine-induced CPP expression in rats. Peptides. 2006;27:3307–3314. doi: 10.1016/j.peptides.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 218.Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther. 2001;298:1007–1014. [PubMed] [Google Scholar]
- 219.Lightman SL, Young WS., 3rd Corticotrophin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. J Physiol. 1988;403:511–523. doi: 10.1113/jphysiol.1988.sp017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.MacDonald AF, Billington CJ, Levine AS. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res. 2004;1018:78–85. doi: 10.1016/j.brainres.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 221.MacDonald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R999–R1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- 222.Magendzo K, Bustos G. Expression of amphetamine-induced behavioral sensitization after short- and long-term withdrawal periods: participation of mu- and delta-opioid receptors. Neuropsychopharmacology. 2003;28:468–477. doi: 10.1038/sj.npp.1300063. [DOI] [PubMed] [Google Scholar]
- 223.Mailleux P, Vanderhaeghen JJ. δ-9-Tetrahydrocannabinol regulates substance P and enkephalin mRNAs levels in the caudateputamen. Eur J Pharmacol. 1994;267:R1–3. doi: 10.1016/0922-4106(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 224.Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 225.Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- 226.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 227.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 228.Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98. doi: 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- 229.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 230.Mansour A, Watson SJ. Anatomical distribution of opioid receptors in mammalian: an overview. In: Hertz A, editor. Opioids I. Berlin: Springer-Verlag; 1993. pp. 79–105. [Google Scholar]
- 231.Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Brain Res. 1998;55:126–132. doi: 10.1016/s0169-328x(97)00371-9. [DOI] [PubMed] [Google Scholar]
- 232.Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Marquez P, Baliram R, Gajawada N, Friedman TC, Lutfy K. Differential involvement of enkephalins in analgesic tolerance, locomotor sensitization, and conditioned place preference induced by morphine. Behav Neurosci. 2006;120:10–15. doi: 10.1037/0735-7044.120.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Marquez P, Baliram R, Kieffer BL, Lutfy K. The mu opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology. 2007;52:1336–1341. doi: 10.1016/j.neuropharm.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Martin TJ, Coller M, Co C, Smith JE. Micro-opioid receptor alkylation in the ventral pallidum and ventral tegmental area, but not in the nucleus accumbens, attenuates the effects of heroin on cocaine self-administration in rats. Neuropsychopharmacology. 2008;33:1171–1178. doi: 10.1038/sj.npp.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Martin TJ, Kim SA, Lyupina Y, Smith JE. Differential involvement of mu-opioid receptors in the rostral versus caudal nucleus accumbens in the reinforcing effects of heroin in rats: evidence from focal injections of beta-funaltrexamine. Psychopharmacology. 2002;161:152–159. doi: 10.1007/s00213-002-1008-3. [DOI] [PubMed] [Google Scholar]
- 237.Mathieu-Kia AM, Besson MJ. Repeated administration of cocaine, nicotine and ethanol: effects on preprodynorphin, preprotachykinin A and preproenkephalin mRNA expression in the dorsal and the ventral striatum of the rat. Brain Res. 1998;54:141–151. doi: 10.1016/s0169-328x(97)00338-0. [DOI] [PubMed] [Google Scholar]
- 238.Mathieu AM, Caboche J, Besson MJ. Distribution of preproenkephalin, preprotachykinin A, and preprodynorphin mRNAs in the rat nucleus accumbens: effect of repeated administration of nicotine. Synapse. 1996;23:94–106. doi: 10.1002/(SICI)1098-2396(199606)23:2<94::AID-SYN5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 239.Mathon DS, Lesscher HM, Gerrits MA, Kamal A, Pintar JE, Schuller AG, Spruijt BM, Burbach JP, Smidt MP, van Ree JM, Ramakers GM. Increased GABAergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice. Neuroscience. 2005;130:359–367. doi: 10.1016/j.neuroscience.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 240.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 241.Matuszewich L, Ormsby JL, Moses J, Lorrain DS, Hull EM. Effects of morphiceptin in the medial preoptic area on male sexual behavior. Psychopharmacology. 1995;122:330–335. doi: 10.1007/BF02246262. [DOI] [PubMed] [Google Scholar]
- 242.Mayer P, Hollt V. Pharmacogenetics of opioid receptors and addiction. Pharmacogenet Genomics. 2006;16:1–7. doi: 10.1097/01.fpc.0000182781.87932.0d. [DOI] [PubMed] [Google Scholar]
- 243.McAlonan GM, Robbins TW, Everitt BJ. Effects of medial dorsal thalamic and ventral pallidal lesions on the acquisition of a conditioned place preference: further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience. 1993;52:605–620. doi: 10.1016/0306-4522(93)90410-h. [DOI] [PubMed] [Google Scholar]
- 244.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 245.McClung CA. The molecular mechanisms of morphine addiction. Rev Neurosci. 2006;17:393–402. doi: 10.1515/revneuro.2006.17.4.393. [DOI] [PubMed] [Google Scholar]
- 246.McGregor A, Herbert J. The effects of beta-endorphin infusions into the amygdala on visual and olfactory sensory processing during sexual behaviour in the male rat. Neuroscience. 1992;46:173–179. doi: 10.1016/0306-4522(92)90016-u. [DOI] [PubMed] [Google Scholar]
- 247.McGregor A, Herbert J. Specific effects of beta-endorphin infused into the amygdala on sexual behaviour in the male rat. Neuroscience. 1992;46:165–172. doi: 10.1016/0306-4522(92)90015-t. [DOI] [PubMed] [Google Scholar]
- 248.McKenna KE. The neurophysiology of female sexual function. World J Urol. 2002;20:93–100. doi: 10.1007/s00345-002-0270-7. [DOI] [PubMed] [Google Scholar]
- 249.McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Merchenthaler I, Maderdrut JL, Cianchetta P, Shughrue P, Bronstein D. In situ hybridization histochemical localization of prodynorphin messenger RNA in the central nervous system of the rat. J Comp Neurol. 1997;384:211–232. [PubMed] [Google Scholar]
- 252.Miranda-Paiva CM, Ribeiro-Barbosa ER, Canteras NS, Felicio LF. A role for the periaqueductal grey in opioidergic inhibition of maternal behaviour. Eur J Neurosci. 2003;18:667–674. doi: 10.1046/j.1460-9568.2003.02794.x. [DOI] [PubMed] [Google Scholar]
- 253.Mizoguchi H, Wu HE, Narita M, Sora I, Hall SF, Uhl GR, Loh HH, Nagase H, Tseng LF. Lack of mu-opioid receptor-mediated G-protein activation in the spinal cord of mice lacking Exon 1 or Exons 2 and 3 of the MOR-1 gene. J Pharmacol Sci. 2003;93:423–429. doi: 10.1254/jphs.93.423. [DOI] [PubMed] [Google Scholar]
- 254.Mocchetti I, Costa E. In vivo studies of the regulation of neuropeptide stores in structures of the rat brain. Neuropharmacology. 1987;26:855–862. doi: 10.1016/0028-3908(87)90062-1. [DOI] [PubMed] [Google Scholar]
- 255.Mocchetti I, Ritter A, Costa E. Down-regulation of proopiomelanocortin synthesis and beta-endorphin utilization in hypothalamus of morphine-tolerant rats. J Mol Neurosci. 1989;1:33–38. doi: 10.1007/BF02896854. [DOI] [PubMed] [Google Scholar]
- 256.Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 257.Morales-Mulia M, Panayi F, Lambas-Senas L, Scarna H, Mendez M. Changes in proenkephalin mRNA expression in forebrain areas after amphetamine-induced behavioural sensitization. Pharmacol Biochem Behav. 2007;87:232–240. doi: 10.1016/j.pbb.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 258.Morita Y, Zhang JH, Hironaka T, Tateno E, Noguchi K, Sato M, Kiyama H, Tohyama M. Postnatal development of preproenkephalin mRNA containing neurons in the rat lower brainstem. J Comp Neurol. 1990;292:193–213. doi: 10.1002/cne.902920204. [DOI] [PubMed] [Google Scholar]
- 259.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 260.Moufid-Bellancourt S, Razafimanalina R, Velley L. Interaction between mu and kappa receptors located in the parabrachial area in the opioid control of preference threshold for saccharin: modulatory role of lateral hypothalamic neurones. Behav Pharmacol. 1996;7:798–809. [PubMed] [Google Scholar]
- 261.Moufid-Bellancourt S, Velley L. Effects of morphine injection into the parabrachial area on saccharin preference: modulation by lateral hypothalamic neurons. Pharmacol Biochem Behav. 1994;48:127–133. doi: 10.1016/0091-3057(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 262.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 263.Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol. 2007;293:R99–R105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- 264.Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 265.Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 266.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 267.Nicklous DM, Simansky KJ. Neuropeptide FF exerts pro- and anti-opioid actions in the parabrachial nucleus to modulate food intake. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1046–R1054. doi: 10.1152/ajpregu.00107.2003. [DOI] [PubMed] [Google Scholar]
- 268.Niikura K, Narita M, Okutsu D, Tsurukawa Y, Nanjo K, Kurahashi K, Kobayashi Y, Suzuki T. Implication of endogenous beta-endorphin in the inhibition of the morphine-induced rewarding effect by the direct activation of spinal protein kinase C in mice. Neurosci Lett. 2008;433:54–58. doi: 10.1016/j.neulet.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 269.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- 270.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 271.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 272.Olds ME. Reinforcing effects of morphine in the nucleus accumbens. Brain Res. 1982;237:429–440. doi: 10.1016/0006-8993(82)90454-1. [DOI] [PubMed] [Google Scholar]
- 273.Olds ME, Williams KN. Self-administration of d-Ala2-Met-enkephalinamide at hypothalamic self-stimulation sites. Brain Res. 1980;194:155–170. doi: 10.1016/0006-8993(80)91325-6. [DOI] [PubMed] [Google Scholar]
- 274.Oliva JM, Manzanares J. Gene transcription alterations associated with decrease of ethanol intake induced by naltrexone in the brain of Wistar rats. Neuropsychopharmacology. 2007;32:1358–1369. doi: 10.1038/sj.npp.1301237. [DOI] [PubMed] [Google Scholar]
- 275.Oliva JM, Ortiz S, Palomo T, Manzanares J. Behavioural and gene transcription alterations induced by spontaneous cannabinoid withdrawal in mice. J Neurochem. 2003;85:94–104. doi: 10.1046/j.1471-4159.2003.01627.x. [DOI] [PubMed] [Google Scholar]
- 276.Olmstead C, Ouaggazzal AM, Kieffer BL. Deletion of mu opioid receptors in mice decreases impulsivity. PLosONE. In press. [Google Scholar]
- 277.Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of lesions of various CNS sites. Behav Neurosci. 1997;111:1313–1323. doi: 10.1037//0735-7044.111.6.1313. [DOI] [PubMed] [Google Scholar]
- 278.Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci. 1997;111:1324–1334. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- 279.Olmstead MC, Munn EM, Franklin KB, Wise RA. Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci. 1998;18:5035–5044. doi: 10.1523/JNEUROSCI.18-13-05035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Olmstead MC, Parkinson JA, Miles FJ, Everitt BJ, Dickinson A. Cocaine-seeking by rats: regulation, reinforcement and activation. Psychopharmacology. 2000;152:123–131. doi: 10.1007/s002130000498. [DOI] [PubMed] [Google Scholar]
- 281.Olson VG, Green TA, Neve RL, Nestler EJ. Regulation of morphine reward and feeding by CREB in the lateral hypothalamus. Synapse. 2007;61:110–113. doi: 10.1002/syn.20344. [DOI] [PubMed] [Google Scholar]
- 282.Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav. 2007;91:506–512. doi: 10.1016/j.physbeh.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 283.Oslin DW, Berrettini WH, O’Brien CP. Targeting treatments for alcohol dependence: the pharmacogenetics of naltrexone. Addict Biol. 2006;11:397–403. doi: 10.1111/j.1369-1600.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 284.Panksepp J, Nelson E, Bekkedal M. Brain systems for the mediation of social separation-distress and social-reward. Evolutionary antecedents and neuropeptide intermediaries. Ann NY Acad Sci. 1997;807:78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x. [DOI] [PubMed] [Google Scholar]
- 285.Papaleo F, Kieffer BL, Tabarin A, Contarino A. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur J Neurosci. 2007;25:3398–3405. doi: 10.1111/j.1460-9568.2007.05595.x. [DOI] [PubMed] [Google Scholar]
- 286.Paredes RG. Evaluating the neurobiology of sexual reward. Ilar J. 2008;50:15–27. doi: 10.1093/ilar.50.1.15. [DOI] [PubMed] [Google Scholar]
- 287.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic “liking” for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- 289.Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 290.Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- 291.Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 292.Pfaus JG, Everitt BJ. The psychopharmacology of sexual behavior. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Forth Generation of Progress. New York: Raven; 1995. pp. 742–758. [Google Scholar]
- 293.Pfaus JG, Gorzalka BB. Opioids and sexual behavior. Neurosci Biobehav Rev. 1987;11:1–34. doi: 10.1016/s0149-7634(87)80002-7. [DOI] [PubMed] [Google Scholar]
- 294.Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: a review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- 295.Phillips-Farfan BV, Fernandez-Guasti A. Endocrine, neural and pharmacological aspects of sexual satiety in male rats. Neurosci Biobehav Rev. 2009;33:442–455. doi: 10.1016/j.neubiorev.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 296.Poulin JF, Castonguay-Lebel Z, Laforest S, Drolet G. Enkephalin co-expression with classic neurotransmitters in the amygdaloid complex of the rat. J Comp Neurol. 2008;506:943–959. doi: 10.1002/cne.21587. [DOI] [PubMed] [Google Scholar]
- 297.Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol. 2006;496:859–876. doi: 10.1002/cne.20956. [DOI] [PubMed] [Google Scholar]
- 298.Pradhan AA, Clarke PB. Comparison between delta-opioid receptor functional response and autoradiographic labeling in rat brain and spinal cord. J Comp Neurol. 2005;481:416–426. doi: 10.1002/cne.20378. [DOI] [PubMed] [Google Scholar]
- 299.Przewlocka B, Lason W. Adaptive changes in the proenkephalin and D2 dopamine receptor mRNA expression after chronic cocaine in the nucleus accumbens and striatum of the rat. Eur Neuropsychopharmacol. 1995;5:465–469. doi: 10.1016/0924-977x(95)80005-m. [DOI] [PubMed] [Google Scholar]
- 300.Przewlocka B, Lason W, Przewlocki R. Repeated ethanol administration decreases prodynorphin biosynthesis in the rat hippocampus. Neurosci Lett. 1992;134:195–198. doi: 10.1016/0304-3940(92)90515-9. [DOI] [PubMed] [Google Scholar]
- 301.Przewlocka B, Lason W, Przewlocki R. Repeated ethanol differently affects opioid peptide biosynthesis in the rat pituitary. Neuroendocrinology. 1994;60:331–336. doi: 10.1159/000126766. [DOI] [PubMed] [Google Scholar]
- 302.Przewlocka B, Turchan J, Lason W, Przewlocki R. The effect of single and repeated morphine administration on the prodynorphin system activity in the nucleus accumbens and striatum of the rat. Neuroscience. 1996;70:749–754. doi: 10.1016/s0306-4522(96)83012-0. [DOI] [PubMed] [Google Scholar]
- 303.Przewlocka B, Turchan J, Lason W, Przewlocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett. 1997;238:13–16. doi: 10.1016/s0304-3940(97)00829-x. [DOI] [PubMed] [Google Scholar]
- 304.Przewlocki R. Opioid abuse and brain gene expression. Eur J Pharmacol. 2004;500:331–349. doi: 10.1016/j.ejphar.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 305.Racz I, Schurmann B, Karpushova A, Reuter M, Cichon S, Montag C, Furst R, Schutz C, Franke PE, Strohmaier J, Wienker TF, Terenius L, Osby U, Gunnar A, Maier W, Bilkei-Gorzo A, Nothen M, Zimmer A. The opioid peptides enkephalin and beta-endorphin in alcohol dependence. Biol Psychiatry. 2008;64:989–997. doi: 10.1016/j.biopsych.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Rasmussen DD. Effects of chronic nicotine treatment and withdrawal on hypothalamic proopiomelanocortin gene expression and neuroendocrine regulation. Psychoneuroendocrinology. 1998;23:245–259. doi: 10.1016/s0306-4530(98)00003-1. [DOI] [PubMed] [Google Scholar]
- 307.Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal. 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- 308.Rasmussen DD, Boldt BM, Wilkinson CW, Mitton DR. Chronic daily ethanol and withdrawal. 3. Forebrain pro-opiomelanocortin gene expression and implications for dependence, relapse, and deprivation effect. Alcohol Clin Exp Res. 2002;26:535–546. [PubMed] [Google Scholar]
- 309.Rasmussen K. The role of the locus coeruleus and N-methyl-d-aspartic acid (NMDA) and AMPA receptors in opiate withdrawal. Neuropsychopharmacology. 1995;13:295–300. doi: 10.1016/0893-133X(95)00082-O. [DOI] [PubMed] [Google Scholar]
- 310.Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- 311.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rezayof A, Zarrindast MR, Sahraei H, Haeri-Rohani AH. Involvement of dopamine D2 receptors of the central amygdala on the acquisition and expression of morphine-induced place preference in rat. Pharmacol Biochem Behav. 2002;74:187–197. doi: 10.1016/s0091-3057(02)00989-9. [DOI] [PubMed] [Google Scholar]
- 313.Rhodes JS, Crabbe JC. Gene expression induced by drugs of abuse. Curr Opin Pharmacol. 2005;5:26–33. doi: 10.1016/j.coph.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 314.Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 315.Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- 316.Roberts AJ, Gold LH, Polis I, McDonald JS, Filliol D, Kieffer BL, Koob GF. Increased ethanol self-administration in delta-opioid receptor knockout mice. Alcohol Clin Exp Res. 2001;25:1249–1256. [PubMed] [Google Scholar]
- 317.Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- 318.Robledo P, Mendizabal V, Ortuno J, de la Torre R, Kieffer BL, Maldonado R. The rewarding properties of MDMA are preserved in mice lacking mu-opioid receptors. Eur J Neurosci. 2004;20:853–858. doi: 10.1111/j.1460-9568.2004.03532.x. [DOI] [PubMed] [Google Scholar]
- 319.Romualdi P, Donatini A, Capobianco A, Ferri S. Methamphetamine alters prodynorphin gene expression and dynorphin A levels in rat hypothalamus. Eur J Pharmacol. 1999;365:183–186. doi: 10.1016/s0014-2999(98)00905-4. [DOI] [PubMed] [Google Scholar]
- 320.Romualdi P, Donatini A, Izenwasser S, Cox BM, Ferri S. Chronic intracerebroventricular cocaine differentially affects prodynorphin gene expression in rat hypothalamus and caudate-putamen. Brain Res. 1996;40:153–156. doi: 10.1016/0169-328x(96)00091-5. [DOI] [PubMed] [Google Scholar]
- 321.Romualdi P, Lesa G, Ferri S. Chronic opiate agonists downregulate prodynorphin gene expression in rat brain. Brain Res. 1991;563:132–136. doi: 10.1016/0006-8993(91)91525-6. [DOI] [PubMed] [Google Scholar]
- 322.Ronnekleiv OK, Bosch MA, Cunningham MJ, Wagner EJ, Grandy DK, Kelly MJ. Downregulation of mu-opioid receptor mRNA in the mediobasal hypothalamus of the female guinea pig following morphine treatment. Neurosci Lett. 1996;216:129–132. [PubMed] [Google Scholar]
- 323.Rosin A, Lindholm S, Franck J, Georgieva J. Downregulation of kappa opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neurosci Lett. 1999;275:1–4. doi: 10.1016/s0304-3940(99)00675-8. [DOI] [PubMed] [Google Scholar]
- 324.Roth-Deri I, Green-Sadan T, Yadid G. Beta-endorphin and drug-induced reward and reinforcement. Prog Neurobiol. 2008;86:1–21. doi: 10.1016/j.pneurobio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 325.Roth-Deri I, Mayan R, Yadid G. A hypothalamic endorphinic lesion attenuates acquisition of cocaine self-administration in the rat. Eur Neuropsychopharmacol. 2006;16:25–32. doi: 10.1016/j.euroneuro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 326.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated fore-brain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 327.Salinas JA, McGaugh JL. The amygdala modulates memory for changes in reward magnitude: involvement of the amygdaloid GABAergic system. Behav Brain Res. 1996;80:87–98. doi: 10.1016/0166-4328(96)00023-x. [DOI] [PubMed] [Google Scholar]
- 328.Sanchez-Cardoso P, Higuera-Matas A, Martin S, del Olmo N, Miguens M, Garcia-Lecumberri C, Ambrosio E. Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats. Neuropharmacology. 2007;52:931–948. doi: 10.1016/j.neuropharm.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 329.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 330.Sante AB, Nobre MJ, Brandao ML. Place aversion induced by blockade of mu or activation of kappa opioid receptors in the dorsal periaqueductal gray matter. Behav Pharmacol. 2000;11:583–589. doi: 10.1097/00008877-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 331.Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci USA. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology. 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- 333.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 334.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 335.Sharifzadeh M, Haghighat A, Tahsili-Fahadan P, Khalaj S, Zarrindast MR, Zamanian AR. Intra-hippocampal inhibition of protein kinase AII attenuates morphine-induced conditioned place preference. Pharmacol Biochem Behav. 2006;85:705–712. doi: 10.1016/j.pbb.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 336.Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 337.Shen J, Chan KW, Chen BT, Philippe J, Sehba F, Duttaroy A, Carroll J, Yoburn BC. The effect of in vivo ethanol consumption on cyclic AMP and delta-opioid receptors in mouse striatum. Brain Res. 1997;770:65–71. doi: 10.1016/s0006-8993(97)00747-6. [DOI] [PubMed] [Google Scholar]
- 338.Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23:1596–1604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- 339.Shippenberg TS, Elmer GI. The neurobiology of opiate reinforcement. Crit Rev Neurobiol. 1998;12:267–303. doi: 10.1615/critrevneurobiol.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- 340.Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 341.Shoblock JR, Maidment NT. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience. 2007;149:642–649. doi: 10.1016/j.neuroscience.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 342.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 343.Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci. 2005;21:1379–1384. doi: 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- 345.Skoubis PD, Maidment NT. Blockade of ventral pallidal opioid receptors induces a conditioned place aversion and attenuates acquisition of cocaine place preference in the rat. Neuroscience. 2003;119:241–249. doi: 10.1016/s0306-4522(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 346.Slowe SJ, Simonin F, Kieffer B, Kitchen I. Quantitative autoradiography of mu-, delta- and kappa1 opioid receptors in kappa-opioid receptor knockout mice. Brain Res. 1999;818:335–345. doi: 10.1016/s0006-8993(98)01201-3. [DOI] [PubMed] [Google Scholar]
- 347.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Soderman AR, Unterwald EM. Cocaine reward and hyperactivity in the rat: sites of mu opioid receptor modulation. Neuroscience. 2008;154:1506–1516. doi: 10.1016/j.neuroscience.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS, Uhl GR. Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology. 2001;25:41–54. doi: 10.1016/S0893-133X(00)00252-9. [DOI] [PubMed] [Google Scholar]
- 351.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Spangler R, Ho A, Zhou Y, Maggos CE, Yuferov V, Kreek MJ. Regulation of kappa opioid receptor mRNA in the rat brain by “binge” pattern cocaine administration and correlation with pre-prodynorphin mRNA. Brain Res. 1996;38:71–76. doi: 10.1016/0169-328x(95)00319-n. [DOI] [PubMed] [Google Scholar]
- 353.Spangler R, Unterwald EM, Kreek MJ. “Binge” cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- 354.Spangler R, Zhou Y, Maggos CE, Schlussman SD, Ho A, Kreek MJ. Prodynorphin, proenkephalin and kappa opioid receptor mRNA responses to acute “binge” cocaine. Brain Res. 1997;44:139–142. doi: 10.1016/s0169-328x(96)00249-5. [DOI] [PubMed] [Google Scholar]
- 355.Stanley BG, Lanthier D, Leibowitz SF. Multiple brain sites sensitive to feeding stimulation by opioid agonists: a cannula-mapping study. Pharmacol Biochem Behav. 1988;31:825–832. doi: 10.1016/0091-3057(88)90391-7. [DOI] [PubMed] [Google Scholar]
- 356.Stefano GB, Bianchi E, Guarna M, Fricchione GL, Zhu W, Cadet P, Mantione KJ, Casares FM, Kream RM, Esch T. Nicotine, alcohol and cocaine coupling to reward processes via endogenous morphine signaling: the dopamine-morphine hypothesis. Med Sci Monit. 2007;13:RA91–102. [PubMed] [Google Scholar]
- 357.Stein EA, Olds J. Direct intracerebral self-administration of opiates in the rat. Soc Neurosci Abstr. 1977;302 [Google Scholar]
- 358.Stein EA, Zerneskie J. Is reward behavior mediated by an endogenous opiate system? Soc Neurosci Asbstr. 1979;573 [Google Scholar]
- 359.Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Stephens DN, Duka T. Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 2008;363:3169–3179. doi: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- 362.Stolerman IP. Components of drug dependence: reinforcement, discrimination and adaptation. Biochem Soc Symp. 1993;59:1–12. [PubMed] [Google Scholar]
- 363.Svensson P, Hurd YL. Specific reductions of striatal prodynorphin and D1 dopamine receptor messenger RNAs during cocaine abstinence. Brain Res. 1998;56:162–168. doi: 10.1016/s0169-328x(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 364.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 365.Sweet DC, Levine AS, Kotz CM. Functional opioid pathways are necessary for hypocretin-1 (orexin-A)-induced feeding. Peptides. 2004;25:307–314. doi: 10.1016/j.peptides.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 366.Tanaka S, Fan LW, Tien LT, Park Y, Liu-Chen LY, Rockhold RW, Ho IK. Butorphanol dependence increases hippocampal kappa-opioid receptor gene expression. J Neurosci Res. 2005;82:255–263. doi: 10.1002/jnr.20620. [DOI] [PubMed] [Google Scholar]
- 367.Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci. 2005;25:4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Teodorov E, Modena CC, Sukikara MH, Felicio LF. Preliminary study of the effects of morphine treatment on opioid receptor gene expression in brain structures of the female rat. Neuroscience. 2006;141:1225–1231. doi: 10.1016/j.neuroscience.2006.04.071. [DOI] [PubMed] [Google Scholar]
- 369.Terashvili M, Wu HE, Leitermann RJ, Hung KC, Clithero AD, Schwasinger ET, Tseng LF. Differential conditioned place preference responses to endomorphin-1 and endomorphin-2 microinjected into the posterior nucleus accumbens shell and ventral tegmental area in the rat. J Pharmacol Exp Ther. 2004;309:816–824. doi: 10.1124/jpet.103.059287. [DOI] [PubMed] [Google Scholar]
- 370.Terashvili M, Wu HE, Schwasinger ET, Hung KC, Hong JS, Tseng LF. (+)-Morphine attenuates the (−)-morphine-produced conditioned place preference and the mu-opioid receptor-mediated dopamine increase in the posterior nucleus accumbens of the rat. Eur J Pharmacol. 2008;587:147–154. doi: 10.1016/j.ejphar.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- 372.Tjon GH, Voorn P, Vanderschuren LJ, de Vries TJ, Michiels NH, Jonker AJ, Klop H, Nestby P, Mulder AH, Schoffelmeer AN. Delayed occurrence of enhanced striatal preprodynorphin gene expression in behaviorally sensitized rats: differential long-term effects of intermittent and chronic morphine administration. Neuroscience. 1997;76:167–176. doi: 10.1016/s0306-4522(96)00363-6. [DOI] [PubMed] [Google Scholar]
- 373.Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity. Behav Brain Res. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- 374.Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of rat. J Neurophysiol. 1995;73:2144–2162. doi: 10.1152/jn.1995.73.6.2144. [DOI] [PubMed] [Google Scholar]
- 375.Turchan J, Lason W, Budziszewska B, Przewlocka B. Effects of single and repeated morphine administration on the prodynorphin, proenkephalin and dopamine D2 receptor gene expression in the mouse brain. Neuropeptides. 1997;31:24–28. doi: 10.1016/s0143-4179(97)90015-9. [DOI] [PubMed] [Google Scholar]
- 376.Turchan J, Maj M, Przewlocka B, Przewlocki R. Effect of cocaine and amphetamine on biosynthesis of proenkephalin and prodynorphin in some regions of the rat limbic system. Pol J Pharmacol. 2002;54:367–372. [PubMed] [Google Scholar]
- 377.Turchan J, Przewlocka B, Lason W, Przewlocki R. Effects of repeated psychostimulant administration on the prodynorphin system activity and kappa opioid receptor density in the rat brain. Neuroscience. 1998;85:1051–1059. doi: 10.1016/s0306-4522(97)00639-8. [DOI] [PubMed] [Google Scholar]
- 378.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 379.Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, Naiman D, Liu QR. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify “connectivity constellation” and drug target genes with pleiotropic effects. Ann NY Acad Sci. 2008;1141:318–381. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Uhl GR, Ryan JP, Schwartz JP. Morphine alters preproenkephalin gene expression. Brain Res. 1988;459:391–397. doi: 10.1016/0006-8993(88)90658-0. [DOI] [PubMed] [Google Scholar]
- 381.Unterwald EM, Kreek MJ, Cuntapay M. The frequency of cocaine administration impacts cocaine-induced receptor alterations. Brain Res. 2001;900:103–109. doi: 10.1016/s0006-8993(01)02269-7. [DOI] [PubMed] [Google Scholar]
- 382.Vaccarino FJ, Pettit HO, Bloom FE, Koob GF. Effects of intra-cerebroventricular administration of methyl naloxonium chloride on heroin self-administration in the rat. Pharmacol Biochem Behav. 1985;23:495–498. doi: 10.1016/0091-3057(85)90027-9. [DOI] [PubMed] [Google Scholar]
- 383.Vaccarino FJ, Taube MR. Intra-arcuate opiate actions stimulate GRF-dependent and protein-selective feeding. Peptides. 1997;18:197–205. doi: 10.1016/s0196-9781(96)00283-5. [DOI] [PubMed] [Google Scholar]
- 384.Van Bockstaele EJ, Peoples J, Menko AS, McHugh K, Drolet G. Decreases in endogenous opioid peptides in the rat medullocoerulear pathway after chronic morphine treatment. J Neurosci. 2000;20:8659–8666. doi: 10.1523/JNEUROSCI.20-23-08659.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Van Furth WR, van Emst MG, van Ree JM. Opioids and sexual behavior of male rats: involvement of the medial preoptic area. Behav Neurosci. 1995;109:123–134. doi: 10.1037//0735-7044.109.1.123. [DOI] [PubMed] [Google Scholar]
- 386.Van Furth WR, van Ree JM. Sexual motivation: involvement of endogenous opioids in the ventral tegmental area. Brain Res. 1996;729:20–28. doi: 10.1016/s0006-8993(96)00225-9. [DOI] [PubMed] [Google Scholar]
- 387.Van Furth WR, Wolterink G, van Ree JM. Regulation of masculine sexual behavior: involvement of brain opioids and dopamine. Brain Res. 1995;21:162–184. doi: 10.1016/0165-0173(96)82985-7. [DOI] [PubMed] [Google Scholar]
- 388.Van Ree JM, de Wied D. Involvement of neurohypophyseal peptides in drug-mediated adaptive responses. Pharmacol Biochem Behav. 1980;13(Suppl 1):257–263. doi: 10.1016/s0091-3057(80)80039-6. [DOI] [PubMed] [Google Scholar]
- 389.Van Ree JM, Gerrits MA, Vanderschuren LJ. Opioids, reward and addiction: an encounter of biology, psychology, and medicine. Pharmacol Rev. 1999;51:341–396. [PubMed] [Google Scholar]
- 390.Van Ree JM, Niesink RJ, Van Wolfswinkel L, Ramsey NF, Kornet MM, Van Furth WR, Vanderschuren LJ, Gerrits MA, Van den Berg CL. Endogenous opioids and reward. Eur J Pharmacol. 2000;405:89–101. doi: 10.1016/s0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- 391.Van Ree JM, Ramsey N. The dopamine hypothesis of opiate reward challenged. Eur J Pharmacol. 1987;134:239–243. doi: 10.1016/0014-2999(87)90172-5. [DOI] [PubMed] [Google Scholar]
- 392.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 393.Vecchiola A, Collyer P, Figueroa R, Labarca R, Bustos G, Magendzo K. Differential regulation of mu-opioid receptor mRNA in the nucleus accumbens shell and core accompanying amphetamine behavioral sensitization. Brain Res. 1999;69:1–9. doi: 10.1016/s0169-328x(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 394.Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 395.Walker BM, Ettenberg A. Intra-ventral tegmental area heroin-induced place preferences in rats are potentiated by peripherally administered alprazolam. Pharmacol Biochem Behav. 2005;82:470–477. doi: 10.1016/j.pbb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 396.Walker JR, Ahmed SH, Gracy KN, Koob GF. Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]
- 397.Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 398.Wang H, Wessendorf MW. Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience. 2002;109:619–634. doi: 10.1016/s0306-4522(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 399.Waraczynski MA. The central extended amygdala network as a proposed circuit underlying reward valuation. Neurosci Biobehav Rev. 2006;30:472–496. doi: 10.1016/j.neubiorev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 400.Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ. Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. Eur J Neurosci. 2006;23:1605–1613. doi: 10.1111/j.1460-9568.2006.04674.x. [DOI] [PubMed] [Google Scholar]
- 401.Ward HG, Simansky KJ. Chronic prevention of mu-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology. 2006;187:435–446. doi: 10.1007/s00213-006-0463-7. [DOI] [PubMed] [Google Scholar]
- 402.Ward SJ, Martin TJ, Roberts DC. Beta-funaltrexamine affects cocaine self-administration in rats responding on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2003;75:301–307. doi: 10.1016/s0091-3057(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 403.Ward SJ, Roberts DC. Microinjection of the delta-opioid receptor selective antagonist naltrindole 5′-isothiocyanate site specifically affects cocaine self-administration in rats responding under a progressive ratio schedule of reinforcement. Behav Brain Res. 2007;182:140–144. doi: 10.1016/j.bbr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wardlaw SL, Kim J, Sobieszczyk S. Effect of morphine on proopiomelanocortin gene expression and peptide levels in the hypothalamus. Brain Res. 1996;41:140–147. doi: 10.1016/0169-328x(96)00084-8. [DOI] [PubMed] [Google Scholar]
- 405.Weissenborn R, Whitelaw RB, Robbins TW, Everitt BJ. Excitotoxic lesions of the mediodorsal thalamic nucleus attenuate intravenous cocaine self-administration. Psychopharmacology. 1998;140:225–232. doi: 10.1007/s002130050761. [DOI] [PubMed] [Google Scholar]
- 406.Werme M, Thoren P, Olson L, Brene S. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur J Neurosci. 2000;12:2967–2974. doi: 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- 407.Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15:1857–1860. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- 408.Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav. 2006;89:226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 409.Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 410.Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1055–R1065. doi: 10.1152/ajpregu.00108.2003. [DOI] [PubMed] [Google Scholar]
- 411.Winkler A, Buzas B, Siems WE, Heder G, Cox BM. Effect of ethanol drinking on the gene expression of opioid receptors, enkephalinase, and angiotensin-converting enzyme in two inbred mice strains. Alcohol Clin Exp Res. 1998;22:1262–1271. [PubMed] [Google Scholar]
- 412.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 413.Woolley JD, Lee BS, Fields HL. Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience. 2006;143:309–317. doi: 10.1016/j.neuroscience.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 414.Woolley JD, Lee BS, Kim B, Fields HL. Opposing effects of intra-nucleus accumbens mu and kappa opioid agonists on sensory specific satiety. Neuroscience. 2007;146:1445–1452. doi: 10.1016/j.neuroscience.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 415.Woolley JD, Lee BS, Taha SA, Fields HL. Nucleus accumbens opioid signaling conditions short-term flavor preferences. Neuroscience. 2007;146:19–30. doi: 10.1016/j.neuroscience.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 416.Wright RC, Ingenito AJ. Blockade of dorsal hippocampal kappa-opioid receptors increases blood pressure in normotensive and isolation-induced hypertensive rats. Neuropeptides. 2003;37:127–132. doi: 10.1016/s0143-4179(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 417.Yan F, Roth BL. Salvinorin A: a novel and highly selective kappa-opioid receptor agonist. Life Sci. 2004;75:2615–2619. doi: 10.1016/j.lfs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 418.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 419.Yuferov V, Nielsen D, Butelman E, Kreek MJ. Microarray studies of psychostimulant-induced changes in gene expression. Addict Biol. 2005;10:101–118. doi: 10.1080/13556210412331308976. [DOI] [PubMed] [Google Scholar]
- 420.Yuferov V, Zhou Y, LaForge KS, Spangler R, Ho A, Kreek MJ. Elevation of guinea pig brain preprodynorphin mRNA expression and hypothalamic-pituitary-adrenal axis activity by “binge” pattern cocaine administration. Brain Res Bull. 2001;55:65–70. doi: 10.1016/s0361-9230(01)00496-8. [DOI] [PubMed] [Google Scholar]
- 421.Zahm DS, Zaborszky L, Alones VE, Heimer L. Evidence for the coexistence of glutamate decarboxylase and Met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res. 1985;325:317–321. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]
- 422.Zangen A, Ikemoto S, Zadina JE, Wise RA. Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci. 2002;22:7225–7233. doi: 10.1523/JNEUROSCI.22-16-07225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zarrindast MR, Ahmadi S, Haeri-Rohani A, Rezayof A, Jafari MR, Jafari-Sabet M. GABA(A) receptors in the basolateral amygdala are involved in mediating morphine reward. Brain Res. 2004;1006:49–58. doi: 10.1016/j.brainres.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 424.Zarrindast MR, Ebrahimi-Ghiri M, Rostami P, Rezayof A. Repeated pre-exposure to morphine into the ventral pallidum enhances morphine-induced place preference: involvement of dopaminergic and opioidergic mechanisms. Behav Brain Res. 2007;181:35–41. doi: 10.1016/j.bbr.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 425.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- 427.Zhou Y, Bendor JT, Yuferov V, Schlussman SD, Ho A, Kreek MJ. Amygdalar vasopressin mRNA increases in acute cocaine withdrawal: evidence for opioid receptor modulation. Neuroscience. 2005;134:1391–1397. doi: 10.1016/j.neuroscience.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 428.Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Ziolkowska B, Stefanski R, Mierzejewski P, Zapart G, Kostowski W, Przewlocki R. Contingency does not contribute to the effects of cocaine self-administration on prodynorphin and proenkephalin gene expression in the rat forebrain. Brain Res. 2006;1069:1–9. doi: 10.1016/j.brainres.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 430.Zollner C, Stein C. Opioids. Handb Exp Pharmacol. 2007:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]


