Abstract
A commercially available ruthenium (II) PNP type pincer catalyst (Ru-Macho) promotes formation of amides and imines from alcohols and amines via an acceptorless dehydrogenation pathway. The formation of secondary amides, tertiary amides, and secondary ketimines occurs in yields ranging from 35%–95%.
Keywords: Acceptorless Dehydrogenation, Amide, Imine, Ruthenium Catalysis
1. Introduction
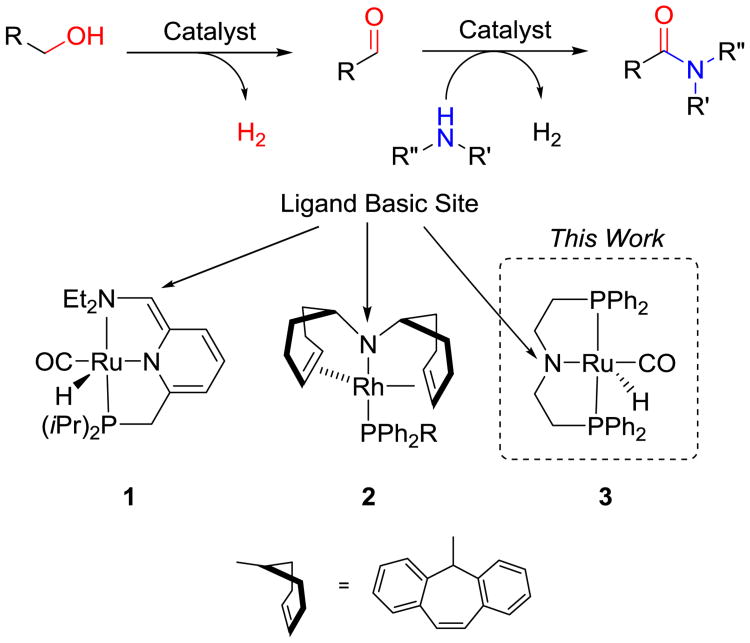
Amide bonds are prevalent in natural products, proteins, and synthetic materials. The formation of amide linkages is one of the most executed transformations in organic chemistry. Traditional methods for amide bond formation often involve harsh conditions and/or generate a stoichiometric amount of waste.1 With growing environmental concerns, there is a need for more efficient, atom economical, and environmentally friendly methods for amide synthesis. As an indication, the formation of amide bonds via green methods was named the number one challenge for organic chemists by the ACS Green Chemistry Institute in 2007.2 To address this challenge, a variety of new methodologies have emerged.1c Among them, acceptorless catalytic dehydrogenation has received particular attention for its ability to form amide bonds directly from alcohols and amines3 (Scheme 1). Catalytic acceptorless dehydrogenative amide synthesis circumvents the need for a stoichiometric oxidizing agent or sacrificial hydrogen acceptor by evolving hydrogen gas directly from the reaction.4 A number of laboratories3a-d, 5 have demonstrated promising catalysts for amide bond formation via dehydrogenation using Ruthenium catalysis. Our group previously employed the Milstein catalyst (1) for the synthesis of polyamides.6 Others have also applied acceptorless dehydrogenation to the direct synthesis of esters,7 lactones,3b, 7a imines, 5e, 8 and pyrroles3b, 9. While searching for improved polyamidation catalysts, we discovered in this work that a commercially available, relatively inexpensive catalyst (complex 3, Scheme 1) catalyzed the synthesis of a variety of secondary and tertiary amides, as well as secondary ketimines from alcohols and amines.
Scheme 1.
Acceptorless dehydrogenation of an amine and alcohol to form an amide. All catalysts shown contain a cooperative basic site on the ligand.
Given the potential of acceptorless dehydrogenation for amide bond formation, 10 we sought to identify inexpensive and robust catalysts that could produce amide bonds. Previous studies demonstrated that the catalytic cycle did not rely on redox chemistry at the metal, but rather on metal/ligand cooperation.5c, 11 In both the Milstein catalyst (1) and the Grützmacher catalyst (2), the catalytic process was proposed to proceed through cooperative interactions of substrates with the basic site of the ligand and with the electrophilic metal centerutilizing catalysts based off the works of Shvo, Murahashi and others5f, 12 (Scheme 1). A hydrogen acceptor is not necessary because the ligands play an active role in the hydrogen abstraction and liberation process. The bulky ligands in these catalysts, however, may hinder the ability of the substrate to interact with both sites of the complex, which may explain why tertiary amides are difficult to synthesize via reported acceptorless dehydrogenation systems3a, 3b, 3d, 3e, 5d. Of note, Ru-NHC complexes have also emerged as promising catalysts.3d-f
The commercially available PNP type ruthenium (II) catalyst, (RuHCl(CO)(HN(CH2CH2PPh2)2)) pioneered by the Saito group (Ru-Macho, Scheme 1, complex 3) has recently been reported to efficiently hydrogenate esters to form the corresponding alcohols.13 In this context, hydrogenation and dehydrogenation are reversible reactions, we hypothesized that this industrially relevant catalyst could be used for dehydrogenative amide formation. Complex 3 has a number of desirable attributes for our purpose. Firstly, similar to catalysts 1 and 2, complex 3 contains a basic site on the ligand to provide the desired cooperative interactions between the substrate and the metal/ligand framework (Scheme 1, complex 3). Secondly, the ligand in complex 3 is less bulky than those in 1 and 2, which may broaden the substrate scope relative to pincer type ligands. Importantly, the precursor of complex 3 is commercially available and relatively inexpensive. Lastly, it has previously demonstrated robust catalytic activity in ethyl acetate formation from ethanol7b as well as methanol water reformation,14 and is used in large scale industrial applications.13a
2. Results and Discussion
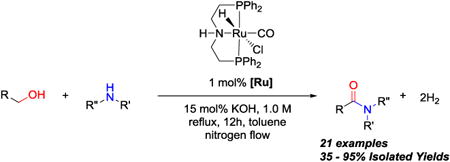
First, we investigated the feasibility of direct amidation by complex 3, by using 2-methoxyethanol and benzyl amine as model substrates (see SI, Figure S1). A number of experimental parameters, including the base, solvent, and H2 removal were varied. To form the catalytically active complex 3, the pre-catalyst 4 must be activated with a base (Scheme 2). Initial attempts using NaOEt or NaOtBu as the base resulted in no amide formation. Upon changing to sodium hydroxide as the base, however, amide bond formation was observed. Because the base appeared to have an effect on direct amidation, the base and counter-ion was further investigated. Among all bases evaluated (LiOH, NaOH, KOH, Na2CO3, K2CO3, NaOEt, and NaOtBu), potassium hydroxide promoted the highest yield of the desired amide (see SI, Figure S1). Using KOH as the base, we investigated the solvent effect for this transformation. Among the list of both polar and nonpolar solvents examined, toluene and dioxane gave the highest yield (∼66%). Because catalyst 3 can catalyze hydrogenolysis of esters and amides with the H2 generated during dehydrogenation, removal of H2 was necessary prevent the reverse transformation.13a Indeed, introducing continuous nitrogen flow through the reaction flask afforded almost quantitative amide formation.
Scheme 2.
Activation of Ru-Macho precatalyst 4 with base to form the active catalyst 3.
With this protocol in hand (1 mol% complex 4, 15 mol% KOH, reflux, toluene, nitrogen flow), several amines and alcohols were examined as coupling partners for amide bond formation. A variety of amides (Table 1, entries 1–15) were obtained in good to excellent yields, demonstrating the efficiency and versatility of complex 3 in amide bond formation. Simple linear aliphatic alcohols coupled efficiently (entries 1–2). Similarly, aliphatic alcohols with β-branching also produced amides in high yields (entries 3–4). Ether and tertiary amine groups were well-tolerated in the amidation process (entries 5 & 7). An aniline substrate, which is less nucleophilic than aliphatic amines, afforded amide in 79% yield (entry 6). To test whether optically active amines were racemized or not during the amidation, an optically pure amine was subjected to the catalytic protocol. The optically pure amide was obtained in high yield (entry 8), suggesting no significant racemization occurs during the dehydrogenative coupling (see experimental). After successful amidation with a variety of mono-amines and mono-alcohols, diamines (entry 9) and diols (entry 10) were coupled to form diamides in high yields, suggesting the applicability of making polyamides using complex 3.6
Table 1. Amide bond formation by acceptorless dehydrogenations of amines and alcohols with Ru-Macho catalyst.

| ||||
|---|---|---|---|---|
| Entry | Amine | Alcohol | Amide | Yield [%]a |
| 1 |
|
|
|
91% |
| 2 |
|
|

|
95% |
| 3 |
|

|

|
90% |
| 4 |
|
|

|
92% |
| 5 |
|
|

|
95% |
| 6 |

|
|

|
79% |
| 7 |
|
|

|
88% |
| 8 |

|
|

|
88% |
| 9 |
|
|
|
95% |
| 10 |
|
|
|
89% |
| 11 |

|
|

|
89% |
| 12 |

|
|

|
86% |
| 13 |

|
|

|
85% |
| 14 |

|
|

|
55%b |
| 15 |
|
|

|
35%c |
Reaction Conditions: 1 mmol amine, 1 mmol alcohol, 0.01 mmol 3, 0.15 mmol KOH, 1.0 mL toluene, 110 °C, 12h.
Isolated yields
24 h
24 h in 1 mL Xylene
The ability of the secondary amines to undergo coupling stands out (Table 1, entries 11–15) because previously reported acceptorless dehydrogenation catalysts have limited conversion for forming tertiary amides directly from secondary amines and alcohols3a, 3b, 3d, 5d or require high catalyst loadings3e. Morpholine and piperidine were both coupled in 89% and 86% yield, respectively (entries 11, 12). A linear secondary amine with moderate steric bulk was coupled in very good yield (entry 13). Systematically increasing steric bulk of the secondary amine resulted in decreasing yield (entries 13–15). Nevertheless, a more sterically encumbered secondary amine, dibenzylamine, underwent coupling with moderate yield (entry 15). This result suggests that further ligand tuning may open the door to the direct synthesis of more sterically hindered tertiary amides, which are very difficult to access.1c
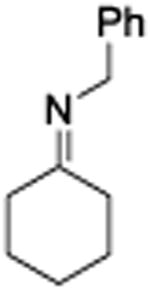
Acceptorless dehydrogenative catalysts have also been shown to afford secondary aldimines from primary alcohols and amines.5e, 8 However, synthesis of secondary ketimines via the acceptorless hydrogenation pathway remains problematic.8a, 8c Considering that complex 3 could couple secondary amines efficiently, we reasoned that ketimine formation would be feasible. Using complex 3, cyclohexanol and benzylamine were shown to undergo coupling to generate secondary ketimines (Table 2, entry 1) in nearly quantitative yields on the basis of GC-MS and 1HNMR analysis.15 Borohydride reduction of the resulting imines leads to isolated yields for the reduced products between 40-91%. Our results represent the highest yielding examples of secondary alcohols and amines undergoing dehydrogenative coupling to generate secondary ketimines. More sterically hindered and acyclic alcohols transformed to the corresponding ketimines in moderate yields (entries 2–5).
Table 2. Imine bond formation via acceptorless dehydrogenations of amines and alcohols by Ru-Macho catalyst.

| ||||
|---|---|---|---|---|
| Entry | Amine | Alcohol | Imine | Yield [%]a |
| 1 |
|

|
|
85% |
| 2 |
|

|

|
51% |
| 3 |
|

|

|
40% |
| 4 |
|

|

|
80% |
| 5 |
|

|

|
40% |
Reaction Conditions: 1 mmol amine, 1 mmol alcohol, 0.01 mmol 3, 0.15 mmol KOH, 1.0 mL toluene, 110 °C, 8 - 12h.
Isolated yields of reduced amines obtained by reduction of imine to amine with sodium borohydride.
The ability of complex 3 to form both amides and imines results from the common hemiaminal intermediate generated after the dehydrogenative coupling of the alcohol and the amine (Scheme 3). Typically, the hemiaminal (5a) undergoes another dehydrogenation via complex 3 to create the amide product (5b). However, if the R′ substituent on the hemiaminal is a moiety other than a hydrogen atom, complex 3 is unable to undergo elimination of an equivalence of dihydrogen. Thus, imine formation is favored through dehydration in the case of secondary alcohols due to no other competing pathway.
Scheme 3.

Hemiaminal 5a can either form an amide (5b) or imine (5c) depending on the identity of R′.
3. Conclusions
The catalyst 3 (Ru-Macho) investigated here has shown utility for both amide and imine bond formation through the acceptorless dehydrogenation pathway. As an advance, we demonstrate good reactivity with secondary amines for the synthesis of tertiary amides and an improved synthesis of ketimines from secondary alcohols. The combination of commercial availability, relatively low cost, and general substrate scope makes Ru-Macho an attractive catalyst for amide and imine bond formation. Future studies will focus on stereoselective variants and using these insights towards the construction of polyamides.
4. Experimental Section
4.1. General considerations
All reactions were set up under a nitrogen atmosphere in a Vacuum Atmospheres Company Glove box. Reactions carried out outside of the glove box were performed under a nitrogen atmosphere using standard Schlenk techniques. Toluene was purchased from Fischer Chemical and purged with argon for 2 hours, then dried by passing it through two columns of neutral alumina under argon pressure. Dioxane, DMF, DMSO, chlorobenzene, xylene, and DMF were purchased from Aldrich Chemical Co or Fischer Chemical, and purged with nitrogen for 30 min before use. Amines and alcohols were purchased at reagent grade or higher purity from Aldrich Chemical Co, or Fischer Chemical and purged with nitrogen for 30 minutes before use. [RuHCl(CO)(HN(CH2CH2PPh2)2)] (4) was purchased from Strem Chemicals Inc. and used as received. KOH was purchased from Fisher Chemical, ground with a mortar and pestle in the glove box to a fine powder, and stored in the glove box. Column chromatography was performed using silica gel (Dynamic Adsorbents Inc. Silica, C-18 32-63μ, 60A) and eluted using applied air pressure with the indicated solvent system. All compounds were characterized by 1HNMR, 13CNMR, ESI-MS, and IR. NMR spectra were obtained using a Bruker Cryo 500 instrument. All 1HNMR and 13CNMR are reported in ppm relative to TMS (0.00 ppm) unless otherwise noted. IR spectra were obtained using a Thermo Scientific Nicolet iS5 iD5 ATR IR spectrometer. GC-MS (CI) was performed using a GCT Premier Micromass MS Technologies mass spectrometer, coupled with a Waters 7890A gas chromatograph. ESI MS was performed using a LCT Premier Micromass MS Technologies mass spectrometer.
4.2. General Procedure for Examples in Table 1
An oven dried 10 mL round bottom flask equipped with a stir bar was brought into the glove box with a reflux condenser. The flask was charged with [RuHCl(CO)(HN(CH2CH2PPh2)2)] (4) (6.0 mg, 0.01 mmol, 1 mol %), KOH (8.2 mg, 0.15 mmol, 15 mol %), toluene (1.0 mL), an amine (1 mmol, 1.0 eq.) and an alcohol (1 mmol, 1.0 eq.) in that order. After all reagents have been added to the flask, the reflux condenser was attached and secured with a keck clamp. The top of the condenser was sealed with a septum and the whole apparatus was then removed from the glove box. Once outside the glove box, the apparatus was equipped to nitrogen flow by inserting an inlet needle supplying a positive pressure of nitrogen into the septum, and an outlet needle connected to an oil bubbler. The reaction mixture was heated at reflux in a silicone oil bath overnight (12 h), then allowed to cool to RT, and the conversion determined by GC-MS (CI). The resulting residue was subjected to flash chromatography with the indicated solvent system to obtain the purified amide in the reported isolated yield.
4.2.1. N-benzyloctanamide
(Table 1, Entry 1) Clear oil (194 mg, 91% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.44-7.36 (m, 2H) 7.34 (m, 3H) 5.72 (br, s, 1H) 4.51 (d, J = 5.5 Hz, 2H) 2.27 (t, J = 7.5 Hz, 2H) 1.72 (m, 2H) 1.36 (m, 8H) 0.94 (t, J = 7.5 hz, 3H) ppm. 13CNMR (125 MHz, CDCl3) δ 173.0, 138.5, 128.7, 127.8, 127.5, 43.5, 36.8, 31.7, 29.3, 29.1, 25.8, 22.6, 14.1 ppm. The physical data were identical in all respects to those previously reported.16
4.2.2. N-benzylbutyramide
(Table 1, Entry 2) White solid (168 mg, 95% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.35 – 7.26 (m, 5H), 6.60 (br, s, 1H), 4.45 (d, J = 7.5 Hz, 2H), 2.19 (t, J = 7 Hz, 2H), 1.69 (m, 2H), 0.96 (t, J = 7 Hz, 3H) ppm. 13CNMR (125 MHz, CDCl3) δ 173.1, 138.6, 128.7, 127.8, 127.4, 43.5, 38.6, 19.3, 13.9 ppm. The physical data were identical in all respects to those previously reported.17
4.2.3. N-benzyl-2-methylbutanamide
(Table 1, Entry 3) White solid (171 mg, 90%). 1HNMR (500 MHz, CDCl3) δ 7.34 - 7.26 (m, 5H), 5.83 (br, s, 1H), 4.44 (m, 2H), 2.14 (m, 1H), 1.70 (m, 1H), 1.43 (m, 1H), 1.16 (d, J = 5 Hz, 3H), 0.91 (t, J = 5 Hz 3H) ppm. 13CNMR (125 MHz, CDCl3) δ 176.4, 138.6, 128.7, 127.8, 127.4, 43.5, 43.3, 27.4, 17.6, 12.0 ppm. The physical data were identical in all respects to those previously reported.18
4.2.4. N-benzylbenzamide
(Table 1, Entry 4) White solid (194 mg, 92% isolated yield). (500 MHz, CDCl3) δ 7.80 (d, J = 7, 2H), 7.48 (m, 1H) 7.42 (t, J = 7 Hz, 2H), 7.36–7.28 (m, 5H), 6.48 (br, s, 1H), 4.65 (d, J = 5.5 Hz, 2H) ppm. 13CNMR (125 MHz, CDCl3) δ 167.4, 138.2, 134.4, 131.6, 128.9, 128.7, 128., 127.7, 127.0, 44.2 ppm. The physical data were identical in all respects to those previously reported.19
4.2.5. N-benzyl-2-methoxyacetamide
(Table 1, Entry 5). Clear oil (170 mg, 95% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.36–7.26 (m, 5H), 6.82 (br, s, 1H), 4.49 (d, J = 10 Hz, 2H), 3.95 (s, 2H), 3.40 (s, 3H) ppm. 13CNMR (125 MHz, CDCl3) δ 169.5, 138.0, 128.8, 127.9, 127.6, 72.0, 59.2, 42.9 ppm. The physical data were identical in all respects to those previously reported.18
4.2.6. 2-methoxy-N-(4-methoxyphenyl)acetamide
(Table 1, Entry 6) Clear oil (154 mg, 79%). HRMS (ESI/CH2Cl2) m/z calcd for C10H13NO3Na (M + Na)+: 218.0793, Found: 218.0797. 1HNMR (500 MHz, CDCl3) δ 8.14 (br, s, 1H), 7.47 (d, J = 9.0 Hz, 2H) 6.87 (d, J = 9.0 Hz, 2H) 4.01 (s, 2H) 3.80 (s, 3H), 3.50 (s, 3H) ppm. 13CNMR (125 MHz, CDCl3) δ 167.3, 156.6, 130.3, 121.6, 114.2, 72.2, 59.3, 55.5 ppm. IR (film) 3003.1, 2917.6, 2849.0, 1679.8, 1511.8, 1246.1, 1110.9, 1033.5 cm-1.
4.2.7. N-benzyl-2-(diethylamino)acetamide
(Table 1, Entry 7) Clear oil (193 mg, 88% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.71 (br, s, 1H), 7.35-7.62 (m, 5H) 4.75 (d, J = 6 Hz, 2H) 3.08 (s, 2H) 2.54 (q, J = 7.0 Hz, 4H) 0.99 (t, 6H, 7.0 Hz) ppm. 13CNMR (125 MHz, CDCl3) δ 172.1, 138.6, 128.7, 127.6, 127.4, 57.5, 48.8, 43.0, 12.4 ppm. The physical data were identical in all respects to those previously reported.20
4.2.8. (R)-2-methoxy-N-(1-phenylethyl)acetamide
(Table 1, Entry 8) Colorless solid (169 mg, 88%). [a]D25 +109.9 (c = 2.0, MeOH) 1HNMR (500 MHz, CDCl3) δ 7.52-7.24 (m, 5H), 6.85 (br, s, 1H), 5.18 (m, 1H), 3.91-3.83 (m, 2H), 3.41 (s, 3H), 1.52 (d, J = 7.5, 3H) ppm. 13CNMR (125 MHz, CDCl3) δ 168.4, 143.0, 128.8, 127.4, 126.1, 72.1, 59.1, 48.0, 21.9 ppm. The physical data were identical in all respects to those previously reported.21
4.2.9. N,N′-(1,4-phenylenebis(methylene))bis(2-methoxyacetamide)
(Table 1, Entry 9) White solid (265 mg, 95% isolated yield). HRMS (ESI/CH2Cl2) m / z calcd for C14H20N2O4Na (M + Na)+: 303.1321, Found: 303.1331. 1HNMR (500 MHz, DMSO-d6) δ 8.32 (t, J = 6.0 Hz, 1H) 7.19 (s, 4H) 4.25 (d, J = 6.0 Hz, 4H) 3.84 (s, 4H) 3.31 (s, J = 7.0 Hz, 6H) ppm. 13CNMR (125 MHz, DMSO-d6) δ 169.4, 138.5, 127.7, 72.0, 59.1, 41.9. IR (solid) 3029.1, 2939.7, 2831.2, 1650.8, 1532.0, 1197.4, 1109.3, 733.1 cm-1.
4.2.10 2,2′-((oxybis(ethane-2,1-diyl))bis(oxy))bis(N-benzylacetamide)
(Table 1, Entry 10) Clear oil (360 mg, 90% isolated yield). HRMS (ESI/CH2Cl2) m / z calcd for C22H28N2O5Na (M + Na)+: 423.1896, found 423.1888. 1HNMR (500 MHz, CDCl3) δ 7.31–7.23 (m, 10H), 7.26 (br, s, 2H) 4.43 (d, J = 5.0 Hz, 4H) 3.92 (s, 4H) 3.50 (s, 8H). 13CNMR (125 MHz, CDCl3) δ 169.8, 138.2, 128.7, 127.8, 127.5, 70.8, 70.4, 70.0, 42.8. IR (film) 3030.1, 2913.0, 1656.8, 1529.8, 1496.5, 1454.0, 1102.6, 1028.5 cm-1.
4.2.11 2-methoxy-1-(piperidin-1-yl)ethan-1-one
(Table 1, Entry 11) Clear oil (135 mg, 86% isolated yield). HRMS (ESI/CH2Cl2) m / z calcd for C8H15NO2Na (M + Na)+:180.1001, Found: 180.0997. HNMR (500 MHz, CDCl3) δ 4.10 (m, 2H), 3.55 (m, 2H) 3.42 (s, 3H), 3.39 (m, 2H), 1.65 (m, 2H), 1.57 (m, 4H). 13CNMR (125 MHz, CDCl3) δ 167.3, 59.0, 46.0, 42.9, 26.5, 25.6, 24.5. IR (film) 2926.3, 2854.6, 1644.6, 1466.0, 1117.7 cm-1.
4.2.12 Morpholino(phenyl)methanone
(Table 1, Entry 12) Clear oil (164 mg, 86% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.42 - 7.39 (m, 5H), 3.75–3.45 (m, 8H) ppm. 13CNMR (125 MHz, CDCl3) δ 170.5, 135.3, 129.9, 128.6, 127.1, 66.9 (2 carbons) ppm. The physical data were identical in all respects to those previously reported.22
4.2.13 N-benzyl-2-methoxy-N-methylacetamide
(Table 1, Entry 13) Clear oil (164 mg, 88% isolated yield). Due to constrained rotational nature of the tertiary amide, product is a mixture of two rotamers (A: major, B: minor) in a 60:40 A:B ratio. NMR experiments at 350K showed coalescence of the two rotamer peaks. 1HNMR (500 MHz, CDCl3) δ 7.42-7.24 (m, 5H)[A][B], 4.65 (s, 2H)[A], 4.58 (s, 2H)[B], 4.21 (s, 2H)[A], 4.20 (s, 2H)[B], 3.52 (s, 3H)[A], 3.48 (s, 3H)[B], 2.99 (s, 3H)[B], 2.94 (s, 3H)[A] ppm. 13CNMR (125 MHz, CDCl3) δ 169.4 [B], 169.1 [A], 137.0 [A], 136.3 [B], 129.0 [A], 128.7 [A], 128.2 [A], 127.8 [B], 127.5 [B], 126.6 [B], 71.6 [B], 71.5 [A], 59.3 [B], 59.2 [A], 52.5 [B], 51.0 [A], 33.7 [B], 33.64 [A] ppm. The physical data were identical in all respects to those previously reported.23
4.2.14 N-benzyl-N-ethyl-2-methoxyacetamide
(Table 1, Entry 14) White solid (113 mg, 55% isolated yield). Due to constrained rotational nature of the tertiary amide, product is a mixture of two rotamers (A: major, B: minor) in a 60:40 A:B ratio. NMR experiments at 350K in DMSO-d6 showed coalescence of the two rotamer peaks. HRMS (ESI/CH2Cl2) m / z calcd for C12H17NO2Na (M + Na)+:230.1157. Found [M + Na]+: 230.1148. 1HNMR (500 MHz, CDCl3) δ 7.36-7.22 (m, 5H)[A][B], 4.61 (s, 2H)[A], 4.52 (s, 2H)[B], 4.18 (s, 2H)[A], 4.11 (s, 2H)[B], 3.48 (s, 3H)[A], 3.42 (s, 2H)[B], 3.41 (q, J = 6.5 Hz, 2H)[B], 3.25 (q, J = 6.5 Hz, 2H)[A], 1.14 (t, J = 6.5 Hz, 2H)[A], 1.11 (t, J = 6.5 Hz, 2H)[B]. 13CNMR (125 MHz, CDCl3) δ 169.0 [B], 169.0 [A], 137.5 [A], 136.8 [B], 129.0 [A], 128.6 [A], 128.2 [A], 127.7 [B], 127.4 [B], 126.6 [B], 71.7 [B], 71.4 [A], 59.3 [A], 59.3 [B], 49.7 [B], 47.7 [A], 40.9 [B], 40.6 [A], 13.7 [A], 12.6 [B] ppm. IR (film) 3033.1, 2924.2, 1644.0, 1452.0, 1431.9, 1134.8, 1109.4, 1080.5 cm-1.
4.2.15 N,N-dibenzyl-2-methoxyacetamide
(Table 1, Entry 15) Clear oil (94 mg, 35% isolated yield). HRMS (ESI/CH2Cl2) m / z calcd for C17H19NO2Na (M + Na)+: 292.1313, Found: 292.1323. 1HNMR (500 MHz, CDCl3) δ 7.37–7.15 (m, 10H), 4.59 (s, 2H) 4.43 (s, 2H) 4.20 (s, 2H) 3.46 (s, 3H) ppm. 13CNMR (125 MHz, CDCl3) δ 169.5, 136.9, 136.1, 129.0, 128.6, 128.4, 127.7, 127.5, 126.6, 71.5, 59.3, 48.9, 47.9. IR (film) 3028.8, 2919.4, 1649.9, 1450.9, 1429.3, 1195.8, 1128.3, 1106.4, 1080.2 cm-1.
4.3. General Procedure for Examples in Table 1
An oven dried 10 mL round bottom flask equipped with a stir bar was brought into the glove box with a reflux condenser. The flask was charged with [RuHCl(CO)(HN(CH2CH2PPh2)2)] (4) (6.0 mg, 0.01 mmol, 1 mol %), KOH (8.2 mg, 0.15 mmol, 15 mol %), and toluene (1.0 mL), an amine (1 mmol, 1.0 eq.) and an alcohol (1 mmol, 1.0 eq.) in that order. After all reagents had been added to the flask, the reflux condenser was attached and secured with a keck clamp. The top of the condenser was sealed with a septum and the whole apparatus was removed from the glove box. Once outside the glove box, the apparatus was equipped to nitrogen flow by inserting an inlet needle supplying a positive pressure of nitrogen into the septum, and an outlet needle connected to an oil bubbler. When the transformation was deemed complete on the basis of analysis by GC-MS (CI), the reaction mixture was allowed to cool to rt. 5 mL MeOH was added, and the resulting mixture was stirred until the solution was homogeneous. NaBH4 (95 mg, 2.5 mmol, 2.5 eq.) was added through the top of the flask, exposing the reaction to the atmosphere. The solution was stirred for 1 h at room temperature. After 1 h, 3.5 mL 1M HCl was added drop-wise. The solution was then diluted with 50 mL of EtOAc, washed with 1M KOH (3 × 50 mL), and finally brine (3 × 50 mL). The organic layer was then dried with MgSO4 and the excess solvent was removed in vacuo. The resulting residue was subjected to flash chromatography.
4.3.1. N-benzylcyclohexanamine
(Table 2, Entry 1) Tan oil (158 mg, 85% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.32-7.19 (m, 5H) 3.81 (s, 2H), 1.93-1.89 (m, 2H), 1.75-1.70 (m, 2H), 1.65-1.59 (m, 1H), 1.44 (s, 1H), 1.33-1.00 (m, 6H) ppm. 13CNMR (125 MHz, CDCl3) δ 140.9, 128.3, 127.9, 126.7, 56.1, 51.0, 33.5, 26.1, 24.9. The physical data were identical in all respects to those previously reported.24
4.3.2. N-benzyl-1,1-diphenylmethanamine
(Table 2, Entry 2) White solid (139 mg, 85% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.49-7.22 (m, 15H) 4.88 (s, 1H), 3.78 (s, 2H), 1.89 (s, 1H) ppm. 13CNMR (125 MHz, CDCl3) δ 144.0, 140.5, 128.5, 128.5, 128.2, 127.4, 127.1, 127.0, 66.51, 51.9 ppm. The physical data were identical in all respects to those previously reported.25
4.3.3. N-benzylpentan-3-amine
(Table 2, Entry 3) Tan oil (71 mg, 40% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.37–7.20 (m, 5H), 3.74 (s, 2H), 2.41 (q, J = 6.0 Hz, 1H) 1.59 (s, 1H), 1.52–1.40 (m, 4H), 0.87 (t, J = 6.0 Hz, 6H) ppm. 13CNMR (125 MHz, CDCl3) δ 141.2, 128.7, 128.5, 127.1, 59.7, 51.5, 26.0, 10.2 ppm. The physical data were identical in all respects to those previously reported.26
4.3.4 N-benzyl-1-phenylethan-1-amine
(Table 2, entry 4) Tan oil (168 mg, 80% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.48 – 7.22 (m, 10H), 3.85 (q, J = 6.5 Hz, 1H), 3.70 (d, J = 13.0 Hz, 1H) 3.61 (d, J = 13.0 Hz, 1H), 1.70 (s, 1H), 1.39 (d, J = 6.5 Hz, 3H) ppm. (125 MHz, CDCl3) δ 145.6, 140.7, 128.6, 128.4, 128.2, 127.0, 126.9, 126.7, 57.5, 51.7, 24.5 ppm. The physical data were identical in all respects to those previously reported.25
4.3.5 N-benzyl-1,2,3,4-tetrahydronaphthalen-1-amine
(Table 2, Entry 5) Tan oil (94 mg, 40% isolated yield). 1HNMR (500 MHz, CDCl3) δ 7.60-7.24 (m, 9H), 4.10 (d, J = 13.0 Hz, 1H), 4.00 (d, J = 13.0 Hz, 1H), 3.95 (t, J = 5.0 Hz, 1H), 3.04-2.84 (m, 2H), 2.24-2.04 (m, 3H) 1.94-1.85 (m, 1H). 1.54 (s, 1H) ppm. 13CNMR (125 MHz, CDCl3) 142.2, 139.5, 137.7, 129.3, 129.0, 128.5, 128.3, 127.0, 126.8, 125.9, 54.9, 51.4, 29.6, 28.4, 19.3 ppm. The physical data were identical in all respects to those previously reported.25
Supplementary Material
Acknowledgments
Z.G. would like to thank the financial support received from the US National Institute of Health (DK098446) and the National Science Foundation (CHE-1012422). V.M.D would like to thank the financial support received from the US National Institute of Health (GM105938).
Footnotes
Supplementary data: These data include the substrate preparation, 1HNMR and 13CNMR spectra for all new compounds. Supplementary data associated with this article can be found in the online version, at [insert link here]
References and notes
- 1.(a) Montalbetti CAGN, Falque V. Tetrahedron. 2005;61:10827. [Google Scholar]; (b) Valeur E, Bradley M. Chem Soc Rev. 2009;38:606. doi: 10.1039/b701677h. [DOI] [PubMed] [Google Scholar]; (c) Pattabiraman VR, Bode JW. Nature. 2011;480:471. doi: 10.1038/nature10702. [DOI] [PubMed] [Google Scholar]
- 2.Constable DJC, Dunn PJ, Hayler JD, Humphrey GR, Leazer JL, Linderman RJ, Lorenz K, Manley J, Pearlman BA, Wells A, Zaks A, Zhang TY. Green Chem. 2007;9:411. [Google Scholar]
- 3.(a) Gunanathan C, Ben-David Y, Milstein D. Science. 2007;317:790. doi: 10.1126/science.1145295. [DOI] [PubMed] [Google Scholar]; (b) Schley ND, Dobereiner GE, Crabtree RH. Organometallics. 2011;30:4174. [Google Scholar]; (c) Gnanaprakasam B, Milstein D. J Am Chem Soc. 2011;133:1682. doi: 10.1021/ja109944n. [DOI] [PubMed] [Google Scholar]; (d) Nordstrom LU, Vogt H, Madsen R. J Am Chem Soc. 2008;130:17672. doi: 10.1021/ja808129p. [DOI] [PubMed] [Google Scholar]; (e) Chen C, Zhang Y, Hong SH. J Org Chem. 2011;76:10005. doi: 10.1021/jo201756z. [DOI] [PubMed] [Google Scholar]; (f) Muthaiah S, Ghosh SC, Jee JE, Chen C, Zhang J, Hong SH. J Org Chem. 2010;75:3002. doi: 10.1021/jo100254g. [DOI] [PubMed] [Google Scholar]
- 4.Gunanathan C, Milstein D. Science. 2013;341:249. doi: 10.1126/science.1229712. [DOI] [PubMed] [Google Scholar]
- 5.(a) Dam JH, Osztrovszky G, Nordstrom LU, Madsen R. Chem Eur J. 2010;16:6820. doi: 10.1002/chem.201000569. [DOI] [PubMed] [Google Scholar]; (b) Shimizu K, Ohshima K, Satsuma A. Chem Eur J. 2009;15:9977. doi: 10.1002/chem.200901896. [DOI] [PubMed] [Google Scholar]; (c) Zweifel T, Naubron JV, Grutzmacher H. Angew Chem Int Ed. 2009;48:559. doi: 10.1002/anie.200804757. [DOI] [PubMed] [Google Scholar]; (d) Srimani D, Balaraman E, Hu P, Ben-David Y, Milstein D. Adv Synth Catal. 2013;355:2525. [Google Scholar]; (e) Rigoli JW, Moyer SA, Pearce SD, Schomaker JM. Org Biomol Chem. 2012;10:1746. doi: 10.1039/c2ob06921k. [DOI] [PubMed] [Google Scholar]; (f) Naota T, Murahashi SI. Synlett. 1991;693 [Google Scholar]
- 6.Zeng HX, Guan ZB. J Am Chem Soc. 2011;133:1159. doi: 10.1021/ja106958s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Zhang J, Leitus G, Ben-David Y, Milstein D. J Am Chem Soc. 2005;127:10840. doi: 10.1021/ja052862b. [DOI] [PubMed] [Google Scholar]; (b) Nielsen M, Junge H, Kammer A, Beller M. Angew Chem Int Ed. 2012;51:5711. doi: 10.1002/anie.201200625. [DOI] [PubMed] [Google Scholar]; (c) Spasyuk D, Smith S, Gusev DG. Angew Chem Int Ed. 2012;51:2772. doi: 10.1002/anie.201108956. [DOI] [PubMed] [Google Scholar]
- 8.(a) Zhang GQ, Hanson SK. Org Lett. 2013;15:650. doi: 10.1021/ol303479f. [DOI] [PubMed] [Google Scholar]; (b) Ho HA, Manna K, Sadow AD. Angew Chem Int Ed. 2012;51:8607. doi: 10.1002/anie.201203556. [DOI] [PubMed] [Google Scholar]; (c) Gnanaprakasam B, Zhang J, Milstein D. Angew Chem Int Ed. 2010;49:1468. doi: 10.1002/anie.200907018. [DOI] [PubMed] [Google Scholar]; (d) Maggi A, Madsen R. Organometallics. 2012;31:451. [Google Scholar]; (e) Esteruelas MA, Honczek N, Olivan M, Onate E, Valencia M. Organometallics. 2011;30:2468. [Google Scholar]
- 9.(a) Zhang M, Neumann H, Beller M. Angew Chem Int Ed. 2013;52:597. doi: 10.1002/anie.201206082. [DOI] [PubMed] [Google Scholar]; (b) Michlik S, Kempe R. Nature Chem. 2013;5:140. doi: 10.1038/nchem.1547. [DOI] [PubMed] [Google Scholar]
- 10.Montag M, Zhang J, Milstein D. J Am Chem Soc. 2012;134:10325. doi: 10.1021/ja303121v. [DOI] [PubMed] [Google Scholar]
- 11.Yang XZ. Acs Catal. 2013;3:2684. [Google Scholar]
- 12.(a) Shvo Y, Blum Y, Reshef D, Menzin M. J Organomet Chem. 1982;226:C21. [Google Scholar]; (b) Murahashi SI, Naota T, Ito K, Maeda Y, Taki H. J Org Chem. 1987;52:4319. [Google Scholar]; (c) Dobson A, Robinson SD. Inorg Chem. 1977;16:137. [Google Scholar]
- 13.(a) Kuriyama W, Matsumoto T, Ogata O, Ino Y, Aoki K, Tanaka S, Ishida K, Kobayashi T, Sayo N, Saito T. Org Process Res Dev. 2011;16:166. [Google Scholar]; (b) Otsuka T, Ishii A, Dub PA, Ikariya T. J Am Chem Soc. 2013;135:9600. doi: 10.1021/ja403852e. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen M, Alberico E, Baumann W, Drexler HJ, Junge H, Gladiali S, Beller M. Nature. 2013;495:85. doi: 10.1038/nature11891. [DOI] [PubMed] [Google Scholar]
- 15.Imine formation was monitored by GC-MS (CI) and 1H NMR analysis of the reaction mixture. For ease of purification the imine was reduced with sodium borohydride to the corresponding amine to prevent hydrolysis of the imine on silica gel.
- 16.Smith SM, Thacker NC, Takacs JM. J Am Chem Soc. 2008;130:3734. doi: 10.1021/ja710492q. [DOI] [PubMed] [Google Scholar]
- 17.Chaysripongkul S, Pluempanupat W, Jang DO, Chavasiri W. Bull Korean Chem Soc. 2009;30:2066. [Google Scholar]
- 18.Watson AJA, Maxwell AC, Williams JMJ. Org Lett. 2009;11:2667. doi: 10.1021/ol900723v. [DOI] [PubMed] [Google Scholar]
- 19.Moore JD, Byrne RJ, Vedantham P, Flynn DL, Hanson PR. Org Lett. 2003;5:4241. doi: 10.1021/ol0352759. [DOI] [PubMed] [Google Scholar]
- 20.Shannon SK, Peacock MJ, Kates SA, Barany G. J Comb Chem. 2003;5:860. doi: 10.1021/cc034014n. [DOI] [PubMed] [Google Scholar]
- 21.Ditrich K. [10.1055/S-2008-1078451];Synthesis-Stuttgart. 2008 :2283. [Google Scholar]
- 22.Ueda T, Konishi H, Manabe K. Org Lett. 2013;15:5370. doi: 10.1021/ol4026815. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson NA, Zhu J, Gellman SH, Stahl SS. J Am Chem Soc. 2009;131:10003. doi: 10.1021/ja8094262. [DOI] [PubMed] [Google Scholar]
- 24.Lee OY, Law KL, Yang D. Org Lett. 2009;11:3302. doi: 10.1021/ol901111g. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberger P, Bailey AM, Crudden CM. J Am Chem Soc. 2012;134:17384. doi: 10.1021/ja307374j. [DOI] [PubMed] [Google Scholar]
- 26.Carter DS, Cai HY, Lee EK, Iyer PS, Lucas MC, Roetz R, Schoenfeld RC, Weikert RJ. Bioorg Med Chem Lett. 2010;20:3941. doi: 10.1016/j.bmcl.2010.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.