Abstract
The present study investigated whether the selective nociceptin opioid peptide (NOP) receptor agonist, Ro64-6198, impairs acquisition of fear conditioning through glutamatergic mechanisms. Systemic administration of Ro64-6198 (0.3 and 1 mg/kg) or the non-competitive NMDA receptor antagonist, MK-801 (0.03 and 0.1 mg/kg) prior to conditioning severely impaired contextual but not cued fear learning in C57BL/6N mice. When administered together at sub-effective doses, Ro64-6198 (0.5 mg/kg) and MK-801 (0.05 mg/kg), synergistically impaired contextual fear learning, but left cued fear learning intact. We next used the immediate shock deficit paradigm (ISD) to examine the effects of Ro64-6198 and MK-801 on contextual memory formation in the absence of the foot-shock. As expected, naive mice that were shocked briefly after being placed in the training chamber displayed no contextual fear conditioning. This learning deficit was elevated by prior exposure of mice to the training context. Furthermore, administration of Ro64-6198 and MK-801, either separately at amnesic doses (1 mg/kg and 0.1 mg/kg, respectively) or concomitantly at sub-effective doses (0.5 mg/kg and 0.05 mg/kg, respectively) significantly reduced the facilitating effects of context preexposure. These findings demonstrate the existence of functional antagonism between NOP and NMDA receptors that predominantly contributes to modulation of conditioned fear learning which involves spatial-processing demands.
Keywords: NOP receptor, Glutamate, Learning, Memory, Mice
1. Introduction
Nociceptin/orphanin FQ (N/OFQ) is a 17-amino acid neuropeptide identified as the endogenous agonist ligand of the nociceptin opioid peptide (NOP) receptor, previously referred to as opioid-receptor-like 1 (ORL1). Although N/OFQ peptide and its receptor show some structural homology with the opioid peptides and receptors, they constitute a novel neuromodulatory system that is pharmacologically distinct from the opioid family (Calo’ et al., 2000; Meis, 2003). There is growing evidence that N/OFQ–NOP system modulates learning and memory processes. N/OFQ and NOP receptors are highly expressed in cortical and limbic structures underlying learning and memory processes (septum, amygdaloid complex and the hippocampal formation) (Darland & Grandy, 1998; Neal et al., 1999a,b). Furthermore, systemic administration of the synthetic NOP receptor agonist, Ro64-6198, or intracerebral infusions of N/OFQ peptide causes memory deficits in various cognitive tasks, including step-down passive avoidance, fear conditioning, object recognition and Morris water-maze procedures (Fornari, Soares, Ferreira, Moreira, & Oliveira, 2008; Goeldner et al., 2008; Higgins et al., 2002; Nabeshima, Noda, & Mamiya, 1999; Redrobe, Calo’, Guerrini, Regoli, & Quirion, 2000; Sandin, Georgieva, Schott, Ogren, & Terenius, 1997). Conversely, mice deficient in the N/OFQ precursor peptide or in the NOP receptor displayed improved learning abilities (Higgins et al., 2002; Mamiya et al., 2003; Manabe et al., 1998). Similarly, pharmacological blockade of NOP receptors in the amygdala enhanced long-term consolidation of fear memory in rat (Roozendaal, Lengvilas, McGaugh, Civelli, & Reinscheid, 2007). Together, these findings indicate that endogenous N/OFQ peptide negatively modulates learning and memory abilities.
Although N/OFQ–NOP-receptor system role in cognition is now well accepted, the precise mechanisms of action remain poorly understood. Previous electrophysiologic and neurochemical studies have demonstrated that N/OFQ peptide inhibits neurotransmitter release and synaptic plasticity in the hippocampus and amygdala (Bongsebandhu-phubhakdi & Manabe, 2007; Kawahara, Hesselink, van Scharrenburg, & Westerink, 2004; Manabe et al., 1998; Meis, 2003; Wei & Xie, 1999; Yu, Fein, Phan, Evans, & Xie, 1997). In line with these findings, N/OFQ peptide was shown to impair passive avoidance learning in rats through noradrenergic mechanisms in the amygdala (Roozendaal et al., 2007). On the other hand, the deletion of NOP receptor gene in mice results in enhanced intrinsic activity of hippocampal NMDA receptor, which is reflected at the behavioral level by improved learning performances (Mamiya et al., 2003). Interestingly, NOP receptor knockout mice displayed improved passive avoidance learning and acquisition of contextual but not cued fear conditioning, suggesting that the functional interaction between N/OFQ and glutamatergic systems may predominantly contribute to fear leaning behavior which encompasses spatial processing. However, enhanced basal hippocampal acetylcholine levels and theta rhythm were also reported in NOP receptor knockout mice, which could account for the improved learning abilities observed in these mice (Uezu et al., 2005). Hence, to date no study has examined the direct contribution of glutamatergic component to fear learning deficits induced by NOP receptor agonists.
In the past decade, many non-peptide compounds that act on NOP receptor have been synthesized (Chiou et al., 2007; Lambert, 2008). Ro64-6198 represents one of the most selective brain-penetrant agonist identified to date (Chiou et al., 2007; Wichmann et al., 2000). When tested in vivo following systemic administration, this agonist mimics N/OFQ disruptive actions in a range of learning tasks (Higgins et al., 2002; Sandin et al., 1997; Sandin, Ogren, & Terenius, 2004; Goeldner et al., 2008). Ro64-6198 was also found to act synergistically with the non-competitive NMDA receptor antagonist, MK-801, to disrupt long-term recognition memory formation in mice, thus revealing the existence of functional antagonism between NOP and NMDA receptors activities (Goeldner et al., 2008). In light of these findings, the present study was designed to investigate whether the disruptive effects of Ro64-6198 on Pavlovian conditioned fear learning involves glutamatergic mechanisms. In the first series of experiments we evaluated the effects of Ro64-6198 on acquisition of contextual and cued fear conditioning in C57BL/6 mice using a standard fear conditioning paradigm. This consisted of one-phase training session, in which an auditory cue (conditioned stimuli, CS) was paired with foot-shocks (unconditioned stimuli, US) after familiarization of mice to the training context. Contextual and cued fear memories were assessed the following day in separate testing sessions. Because Ro64-6198 was reported to affect acute pain processing (Reiss, Wichmann, Tekeshima, Kieffer, & Ouagazzal, 2008), a comparable study with morphine was included to verify whether potent analgesic produces similar learning deficits as the NOP receptor agonist. We next examined the effects of concurrent administration of sub-effective doses of Ro64-6198 and MK-801 on acquisition of contextual and cued fear conditioning to reveal the existence of possible functional interactions between NOP and NMDA receptors. Finally, to delineate the contribution of NOP and NMDA receptors in the modulation of mnemonic processes associated to formation of a context representation we used an immediate shock deficit paradigm. This consisted of a two-phase training session in which context learning and context-shock association were temporally separated. During conditioning, a single, unsignaled foot-shock was delivered shortly after mice were placed in the context to prevent the formation of an adequate contextual representation. As expected, mice did not develop conditioned fear responses under these training conditions, a phenomenon known as the immediate shock deficit (ISD). These learning deficits could be reversed by prior exposure of mice to the training context, thus corroborating the notion that context representation can be acquired without explicit reinforcement and stored in dormancy (a form of latent learning) until being activated for further association (Fanselow, 2000; Rudy, Huff, & Matus-Amat, 2004). To evaluate the effects of Ro64-6198 and MK-801 on context representation formation, mice received drug injections before preexposure session, and contextual fear learning abilities were then assessed the following day.
2. Materials and methods
2.1. Animals
Eight week old C57BL/6N (BL6) male mice were purchased from Charles River Laboratory (France) and housed four per cage with free access to food and water. Mice were maintained on a 12:12 h light/dark cycle (lights on from 7 am to 7 pm) at 22 °C, and allowed to acclimate to housing conditions until testing age, between 12 and 15 weeks.
All experimental procedures were carried out according to European Union and French Home Office guidelines.
2.2. Drugs
Ro64-6198 (a generous gift from Hoffman-La Roche, Basel, Switzerland) and morphine (purchased from RBI, Natick, MA) were dissolved in physiologic saline (0.9% NaCl) and administered intraperitoneally (i.p.). MK-801 (purchased from Sigma–Aldrich, St. Quentin Fallavier, France) was dissolved in saline (0.9% NaCl) and injected sub-cutaneously (s.c.). All drugs were injected at a volume of 10 ml/kg with 30 min pre-treatment time. The doses of the compounds used (Ro64-6198, MK-801 and morphine) were selected based on our previous studies (Goeldner et al., 2008; Reiss et al., 2008).
2.3. Fear conditioning
Experiments were conducted in four operant chambers (28 × 21 × 22 cm, Coulbourn Instruments, Allentown US), with a plexiglass door and a metal bar floor linked to a shocker (Coulbourn Instruments). Chambers were dimly lit with a permanent house-light and equipped with a speaker for tone delivery. Chambers were enclosed in sound attenuating cabinets equiped with fans producing a 55-dB background noise. An infra-red activity monitor, placed on the ceiling of each chamber, was used to asses the animal motion. The monitor (Coulbourn Instrument; Model H24-61MC; set to mouse sensitivity) detects the change in position of the animal's infrared body-heat signature (13 nM infrared radiation) in the x, y and z axes. The activity/inactivity behavior was monitored continuously during 100 ms period. Data were expressed in duration of inactivity per 1 s (Graphic state Notation software 3.02, Coulbourn Instruments) and the total time of inactivity displayed by each subject during training and testing was counted. The data were then transformed in percentage of immobility. A highly significant correlation was obtained between this automated analysis of fear conditioning and visual monitoring of freezing behavior (for context and cue testing r consistently >0.85 and p < 0.005).
2.4. Behavioral procedure and experimental design
2.4.1. Standard fear conditioning paradigm
Mice were transferred in their home cage from the vivarium to a holding room adjacent to the room housing the conditioning chambers. After familiarization to the holding room, mice were then transported in their home cage and placed in the conditioning chambers. The conditioning session was initiated with a 4-min habituation period followed by a 20 s long tone of 20 Khz/75 dB (conditional stimulus, CS) that was coupled with a 0.4-mA foot-shock (unconditional stimulus, US) during the last second. Two minutes later, a similar CS–US pairing was presented and the mice were removed from the boxes 2 min after the foot-shock. The level of immobility monitored during the 2-min preceding the first foot-shock was used as an index of basal fear level and the 2-min after the last foot-shock was used as an index of short-term contextual fear. The following day, mice were preexposed to the conditioning chamber and immobility was monitored during 2 min to assess long-term contextual fear memory. Cued fear conditioning was assessed 5 h after context testing in modified conditioning chambers with walls and floor of a different color and texture, and no background noise. After a 2-min habituation period to this new context the tone was delivered for 2 min and conditioned fear was monitored during the whole testing session.
2.4.1.1. Experiment I. Effect of Ro64-6198 or morphine on acquisition of contextual and auditory fear conditioning
A group of naïve mice received an injection of Ro64-6198 (0, 0.3 and 1 mg/kg, n = 11–12 per dose) before conditioning and tested the following day drug free. Because Ro64-6198 was previously found to alter pain processing (Reiss et al., 2008), the effects of the prototypical analgesic morphine were studied for comparison. A group of naïve mice received an injection of analgesic doses of morphine (0, 2 and 4 mg/ kg, n = 8–9 per dose) before conditioning and tested the following day drug free.
2.4.1.2. Experiment II. Effect of MK-801 on acquisition of contextual and auditory fear conditioning
One group of naïve mice received injections of MK-801 (0, 0.03 and 0.1 mg/kg, n = 7–8 per dose) before conditioning and tested the following day drug free.
2.4.1.3. Experiment III: Effect of combined injections of Ro64-6198 and MK-801 on acquisition of contextual and auditory fear conditioning
One group of naïve mice received injections of Ro64-6198 (0.05 mg/kg, n = 7) and MK-801 (0.05 mg/kg, n = 7) either separately or concomitantly (n = 7) before conditioning and tested drug free the following day. The control group (n = 8) received conjoint injections of the corresponding vehicles.
2.4.2. Immediate shock deficit procedure
Mice were transferred in their home cage from the vivarium to a holding room adjacent to the room housing the conditioning chambers. After familiarization to the holding room (30 min), mice were preexposed to the training context on the first day without any foot-shocks. On the following day, they were placed in the same context and 10 s later, a 1 s long, 0.25 mA foot-shock was delivered. Mice were then left undisrupted for 20 s after the foot-shock. The level of immobility displayed during the last 10 s was used as an index of short-term contextual fear memory (Chang, Chen, & Liang, 2008; Fanselow, 2000; Matus-Amat, Higgins, Sprunger, Wright-Hardesty, & Rudy, 2007).
2.4.2.1. Experiment I. Effect of preexposure duration
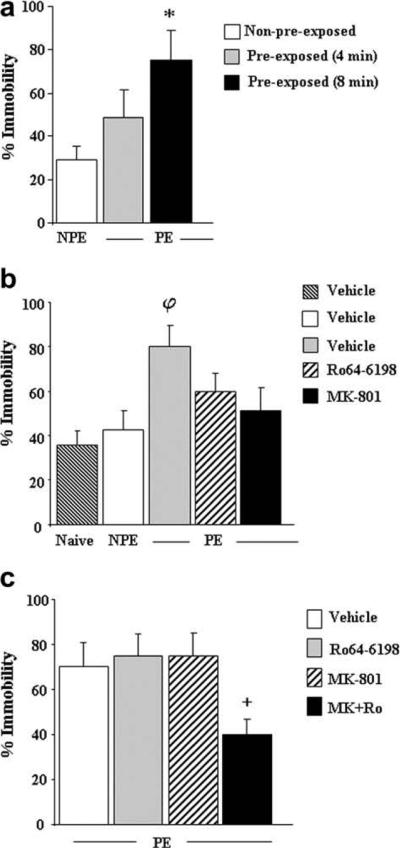
One batch of naïve mice was assigned to the following groups: preexposed group (PE, n = 8) for 4 or 8 min to the training context and control groups that were not preexposed to the context (NPE). Mice from the non-preexposed groups were left undisturbed in the holding room until the end of the experiment. On the following day, all groups were submitted to the conditioning session. The results of NPE groups from 4 to 8 min conditions were pooled together (n = 17) as no differences in immobility scores were detected between them (Fig. 3a).
Fig. 3.
Effects of Ro64-6198 and MK-801 on contextual memory formation. (a) Effects of preexposure to training context on post-shock freezing behavior during the immediate shock deficit conditioning session. Preexposed groups (PE): mice were preexposed to the training context for 4 or 8 min and submitted the following day to the immediate shock deficit (ISD) conditioning session (n = 8 per duration). NPE: Non-preexposed control group (n = 17). (b) Effects of Ro64-6198 (1 mg/kg) and MK-801 (0.1 mg/kg) on the facilitation effects of context preexposure. Preexposed groups (PE): mice received an injection of vehicle (n = 9), Ro64-6198 (n = 8) or MK-801 (n = 7) before preexposure to training context (8 min duration) and submitted the following day to ISD conditioning session. NPE: non-preexposed mice received vehicle injection in their home cage in the first day and submitted the following day to the ISD conditioning session (n = 8). Naïve: non-preexposed mice that did not experience the foot-shock during the ISD conditioning session. (c) Effects of Ro64-6198 (0.5 mg/kg) and MK-801 (0.05 mg/kg) administered alone (n = 6–8 per treatment) or conjointly (n = 7) on the facilitation effects of context preexposure. Preexposed groups (PE): mice received drug treatments before preexposure to the training chamber and submitted to the ISD conditioning session the following day. Data are expressed as mean ± SEM percentage of immobility. *p < 0.05, significantly different from NPE control group (Tukey–Kramer post-hoc test). φp < 0.05, significantly different from naïve and NPE control groups (Tukey–Kramer post-hoc test). +p < 0.05, significantly different from vehicle-treated counterparts (Tukey–Kramer post-hoc test).
2.4.2.2. Experiment II. Effect of Ro64-6198 and MK-801 on the facilitatory effects of preexposure
Mice were assigned to preexposed (PE) and non-preexposed (NPE) groups. Mice from the preexposed groups received an injection of vehicle (n = 9), Ro64-6198 (1 mg/kg, n = 8) or MK-801 (0.1 mg/kg, n = 7) prior to preexposure to the training context for 8 min. Mice from non-preexposed groups (n = 15) received an injection of vehicle and were then left undisturbed in their home cages until the end of the experiment. The following day, mice were submitted to the conditioning session. A non-preexposed control group (naïve, n = 7) which did not experience the foot-shock was used to evaluate baseline immobility level induced by exposure to novel environment.
2.4.2.3. Experiment III. Effect of combined injections of Ro64-6198 and MK-801 on the facilitatory effects of preexposure
Mice received an injection of Ro64-6198 (0.5 mg/kg, n = 6) and MK-801 (0.05 mg/ kg, n = 8) either alone or concomitantly (n = 7) before preexposure (8 min duration) to the training context. A control group (n = 7) received injections of corresponding vehicles. On the following day, all groups of mice were submitted to the conditioning session.
2.5. Statistical analysis
All data are expressed as mean group value ± standard error of the mean (SEM) and analyzed using Student's test, one-way, two-way or three-way ANOVA (StatView version 5) whenever it was appropriate. Komolgorov-Smirnov test was performed prior to ANOVA analysis to ensure that the assumption of normality was not violated. When relevant, data were submitted to Tukey–Kramer post-hoc analysis. The criterion for statistical significance was p < 0.05.
3. Results
3.1. Effects of Ro64-6198 or morprhine on contextual and cued fear learning
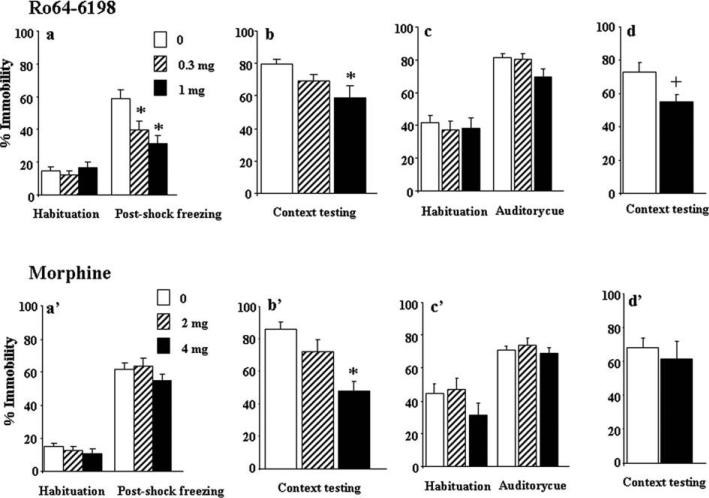
As can be seen from Fig. 1a, the control group injected with saline solution displayed high level of immobility (around 60%) following the presentation of the second foot-shock, thus indicating that mice rapidly develop fear responses during the training session. The NOP receptor agonist, Ro64-6198 (0.3 and 1 mg/kg), had no effect on basal immobility levels (or spontaneous locomotor activity, vehicle: 625 ± 36, 0.3 mg/kg: 689 ± 58 and 1 mg/kg: 721 ± 64 activity counts) during the habituation period, but it markedly reduced post-shock immobility scores (Fig. 1a). Accordingly, three-way ANOVA revealed a significant main effect of test condition [F(1,32) = 107.36, p < 0.05], treatment [F(2,32) = 3.99, p < 0.05], and a significant test condition × treatment interaction [F(2,32) = 9.51, p < 0.05]. Post-hoc analysis indicated that both 0.3 and 1 mg/kg doses significantly reduced post-shock immobility levels compared to vehicle treatment (p < 0.05, Tukey–Kramer post-hoc test). When animals were preexposed 24 h later to the training context, saline-treated mice displayed clear fear responses as reflected by the immobility scores reaching 80% (Fig. 1b). In contrast, mice that received Ro64-6198 injections displayed reduced contextual fear responses. One-way ANOVA revealed a significant main effect of treatment [F(2,32) = 3.52, p < 0.05, Fig. 1b], and post-hoc analysis indicated that 1 mg/kg dose impaired long-term retention of contextual fear compared to saline treatment (p < 0.05, Tukey–Kramer post-hoc test). Fig. 1c illustrates the effects of Ro64-6198 on cued fear learning. Two-way ANOVA revealed a significant main effect of test condition [F(1,32) = 158.43, p < 0.05], indicating that mice acquired fear responses to the auditory cue (CS). In contrast, no main effect of treatment [F(2,32) = 0.87, p > 0.05] or significant test condition × treatment interaction [F(2,32) = 1.47, p > 0.05] were detected, which indicates that Ro64-6198 failed to affect acquisition of cued fear conditioning at the doses tested.
Fig. 1.
Effects of Ro64-6198 or morphine on acquisition of contextual and cued fear conditioning. Top panels, (a), (b) and (c): effects of Ro64-6198 (0, 0.3 and 1 mg/kg, n = 11–12 per dose) on immobility scores during conditioning, context and auditory cue testing, respectively. Mice were treated with Ro64-6198 injections before conditioning, and contextual and cued fear memories were assessed the following day in separate testing sessions. (d) Lack of state-dependant effects of Ro64-6198 on contextual fear conditioning. Mice received an injection of Ro64-6198 (0 or 1 mg/kg, n = 8–10 per dose) before both training and context testing sessions. Lower panels, (a′), (b′) and (c′): Effects of morphine (0, 2 and 4 mg/kg, n = 8–9 per dose) on immobility scores during conditioning, context and auditory cue testing, respectively. Mice were treated with morphine injections before conditioning and tested the following day drug free. (d′): State-dependant effects of morphine on contextual fear conditioning. Mice received an injection of morphine (0 or 4 mg/kg, n = 9 per dose) before both training and context testing sessions. Data are expressed as mean ± SEM percentage immobility. *p < 0.05, significantly different from corresponding control group (Tukey–Kramer post-hoc test). +p < 0.05, significantly different from corresponding control group (Student's t tests).
Morphine (2 and 4 mg/kg), had no effect on basal immobility levels (or spontaneous locomotor activity, vehicle: 623 ± 27, 2 mg/kg: 685 ± 22 and 4 mg/kg: 662 ± 44 activity counts) during conditioning [p > 0.05, Fig. 1a′], but reduced long-term retention of contextual fear. One-way ANOVA revealed a significant main effect of treatment [F(2,23) = 9.79, p < 0.05, Fig. 1b′] and post-hoc analysis indicated an amnesic effects of the 4 mg/kg dose (p < 0.05, Tukey–Kramer post-hoc test). In contrast, no effect of morphine were detected on auditory fear conditioning (Fig. 1c′). Consequently, two-way ANOVA failed to reveal main effect of treatment [F(2,23) = 0.50, p > 0.05] or significant test condition × treatment interaction [F(2,32) = 1.47, p > 0.05].
Because context testing was carried out in a drug-free state, we examined whether the memory deficits could be due to state-dependent learning. To this end, a group of naïve mice received an injection of active doses of Ro64-6198 (1 mg/kg, n = 8) or morphine (4 mg/kg, n = 9) before both conditioning and context testing. The control group received saline injections (n = 10 and 9, respectively). As can be seen from Fig. 1d, mice that received Ro64-6198 treatment displayed reduced contextual fear learning compared to their counterpart control mice (p < 0.05, unpaired Student's t test). In contrast, morphine-treated mice recovered the immobility levels of the control group (p > 0.05, unpaired Student's t test, Fig. 1d′), thus indicating that the retrieval deficit observed with the 4 mg/kg dose was due to drug-state induced changes.
3.2. Effects of Ro64-6198 and 3 on contextual and cued fear learning
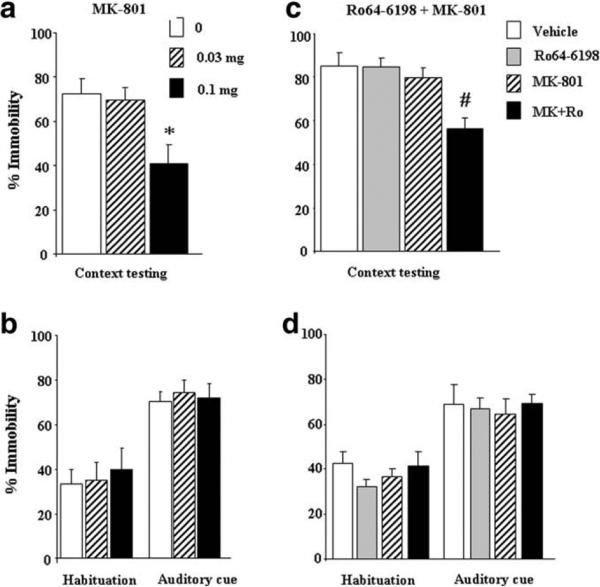
To investigate whether Ro64-6198 induced memory impairments through glutamatergic-dependent mechanisms, we first evaluated the effects of the non-competitive NMDA receptor antagonist, MK-801 (0.03 and 0.1 mg/kg), on acquisition of contextual and cued fear conditioning. During context testing, mice that received MK-801 injection displayed reduced immobility scores compared to saline-treated group (Fig. 2a). One-way ANOVA revealed a significant main effect of treatment [F(2,19) = 6.43, p < 0.05], and post-hoc analysis indicated an amnesic effect of 0.1 mg/kg dose (p < 0.05, Tukey–Kramer post-hoc test). In contrast, no effects of MK-801 were observed on auditory fear conditioning (Fig. 2b). Consequently, two-way ANOVA failed to reveal a signifi-cant main effet of treatment [F(2,19) = 0.12, p > 0.05] or a signifi-cant test condition × treatment interaction [F(2,19) = 0.69, p > 0.05].
Fig. 2.
Effects of Ro64-6198 and MK-801 on acquisition of contextual and cued fear conditioning. Left panels, (a) and (b): effects of MK-801 (0, 0.03 and 0.1 mg/kg, n = 7–8 per dose) on immobility scores during context and auditory cue testing, respectively. Mice received injections of MK-801 before conditioning and submitted the following day to context and auditory cue testing drug free. Right panels, (c) and (d): effects of Ro64-6198 and MK-801 on immobility scores during context and auditory cue testing, respectively. Mice (n = 7–8 per treatment) received separate or conjoint injections of Ro64-6198 (0.5 mg/kg) and MK-801 (0.05 mg/kg) before conditioning, contextual and cued fear memories were then assessed the following day in separate testing sessions. Data are expressed as mean ± SEM percentage immobility. *p < 0.05, significantly different from vehicle-treated group (Tukey–Kramer post-hoc test). #p < 0.05, significantly different from all other groups (Tukey–Kramer post-hoc test).
We next studied the effect of co-administration of sub-effective doses of Ro64-6198 and MK-801. During context testing, mice treated with Ro64-6198 (0.5 mg/kg) or MK-801 (0.05 mg/kg) alone displayed immobility scores comparable to their vehicle-treated counterparts, whereas the co-treated group had lower scores (Fig. 2c). Two-way ANOVA revealed a significant Ro64-6198 × MK-801 interaction [F(1,25) = 4.44, p < 0.05], and post-hoc analysis indicated that the combined treatment significantly decreased immobility level compared to all other groups (p < 0.05, Tukey–Kramer post-hoc test). As can be seen from Fig. 2d, administration of Ro64-6198 and MK-801, either alone or combined, failed to affect acquisition of cued fear conditioning. Consequently, three-way ANOVA revealed a significant main effect of test condition [F(1,25) = 80.28, p < 0.05], but failed to detect a significant main effect of treatment [F(1,25) = 0.03, and F(1,25) = 0.01, p > 0.05 for Ro64-6198 and MK-801, respectively] or a significant Ro64-6198 × MK-801 interaction [F(1,25) = 1.15, p > 0.05].
3.3. Effects of Ro64-6198 and MK-801 on contextual learning
In this series of experiments we used the immediate shock deficit paradigm to examine the effects of Ro64-6198 and MK-801 on formation of contextual memory independently of context-US association processes. To determine the amount of time required to form a robust contextual memory, mice were first preexposed to the training context for various durations (0, 4 and 8 min) in the absence of foot-shock. Conditioning session took place the following day and consisted of a single, unsignaled foot-shock, delivered 10 s after mice were placed in the context to preclude the formation of an adequate contextual representation and obtain a weak level of conditioning (immediate shock deficit phenomenon). As can be seen from Fig. 3a, the levels of post-shock immobility displayed by mice increased with increasing length of context preexposure. Accordingly, one-way ANOVA revealed a significant main effect of preexposure [F(2,28) = 5.49, p < 0.01], and post-hoc analysis indicated a strong facilitation for the 8 min duration (p < 0.05, Tukey–Kramer post-hoc test). Hence, the former preexposure duration was used in all subsequent experiments.
Fig. 3b illustrates the effects of Ro64-6198 (1 mg/kg) and MK-801 (0.1 mg/kg) on the facilitation effects of context preexposure. Overall, there were no differences in immobility scores between the non-preexposed shocked mice (NPE) and their non-shocked counterparts (naïve), thus confirming that animals are unable to develop conditioned fear responses under our training condition. Again, preexposure to the training context facilitates conditioning as illustrated by the high percentage of immobility reaching 80% for PE control mice. In contrast, PE mice that received Ro64-6198 or MK-801 injections showed reduced contextual fear conditioning. Overall ANOVA yielded a significant main effect of treatment [F(4,34) = 4.64, p < 0.01] and post-hoc analysis indicated that only the PE control group had a significantly higher immobility scores compared to naïve and NPE control groups (p < 0.05, Tukey–Kramer post-hoc test). No significant differences were detected between the other groups.
We next examined the effects of concurrent injections of sub-effective doses of Ro64-6198 (0.5 mg/kg) and MK-801 (0.05 mg/ kg). As can be seen from Fig. 3c, PE mice treated with Ro64-6198 or MK-801 alone displayed immobility scores comparable to their vehicle-treated counterparts, whereas co-treated mice had lower scores. Two-way ANOVA revealed a significant Ro64-6198 × MK-801 interaction [F(1,24) = 4.33, p < 0.05], and post-hoc analysis indicated that combined treatment significantly decreased the percentage of immobility compared to the control groups (p < 0.05, Tukey–Kramer post-hoc test). No significant differences were detected between the other groups.
4. Discussion
In line with previous studies (Stiedl, Birkenfeld, Palve, & Spiess, 2000; Bolivar, Pooler, & Flaherty, 2001), C57BL/6N mice subjected to a standard fear conditioning paradigm acquired conditioned fear responses to both training context and explicit cue. During the acquisition phase, control mice also displayed enhanced immobility levels after exposure to the final foot-shock. The increase in immobility observed at the end of the conditioning session was suggested to reflect a rapid development of conditioned fear response to the context and thus a short-term contextual fear memory (Bast, Zhang, & Feldon, 2003; Chang et al., 2008; Fanselow, 2000; Matus-Amat et al., 2007). The findings from the immediate shock deficit paradigm are also consistent with this idea. Naïve mice, shocked briefly after being placed in the context showed no sign of enhanced fear compared to their non-shocked counterparts. In contrast, those preexposed to the training context displayed enhanced immobility with increasing preexposure length, indicating that the rise in fear level observed following the foot-shock relies on context-shock association. Together, our results led further support to the notion that establishment of contextual fear involves sequential learning processes: the formation of a context representation and its association with the aversive unconditional stimulus (Fanselow, 2000; Rudy et al., 2004).
Convergent evidence suggests that contextual fear conditioning, which involves configural or spatial learning, and cued fear conditioning, which involves discrete unisensory information processing, are subserved by different but overlapping memory systems (Fanselow & Poulos, 2005; LeDoux, 2000; Rudy et al., 2004). For instance, the amygdala complex plays a key role in acquisition and expression of both contextual and cue fear conditioning, and is thus considered as a locus for the encoding and storage of CS-shock associations. On the other hand, the hippocampus is mainly involved in contextual fear learning and does not seem to be critical for cued fear conditioning. The hippocampal formation contributes to the processing of contextual information and it is required for the assembly of discrete contextual elements into a conjunctive representation. Hence, an advantage of the fear conditioning paradigm is that drug effects on amygdala- and hippocampal-dependent functions can be studied using different variant of training procedures. Our results show that pre-training administration of the non-competitive NMDA receptor antagonist, MK-801, severely impair acquisition of contextual fear conditioning, but spared cued fear learning. This is in broad agreement with previous work demonstrating that systemic and intra-hippocampal injections of NMDA receptor blockers differentially affect contextual and cued fear learning (Bardgett et al., 2003; Figueredo, Moreira, Ferreira, Fornari, & de Oliveira, in press; Gould, McCarthy, & Keith, 2002; Parada-Turska & Turski, 1990). When tested in the immediate shock deficit paradigm, MK-801 also prevented the beneficial effect of context preexposure confirming that NMDA receptor blockade impairs formation of hippocampal-dependent contextual memory. These findings extend those reported with the competitive NMDA receptor antagonist, AP-5, showing that hippocampal NMDA receptors contribute to elaboration of conjunctive contextual memory that supports fear conditioning (Chang et al., 2008; Matus-Amat et al., 2007; Sanders & Fanselow, 2003; Stote & Fanselow, 2004). Overall, the pharmacological blockade of NMDA receptors following systemic administration of MK-801 was more potent in disrupting hippocampal-dependent than amygdala-dependent fear learning although NMDA receptors are located in both structures. However, it cannot be excluded that higher doses of MK-801 would also impair cued fear learning in our paradigm.
Previous studies showed that intracerebroventricular infusions of the exogenous N/OFQ peptide are more potent in impairing contextual than auditory fear conditioning memory in rats and mice (Fornari et al., 2008; Mamiya et al., 2003). We extend these findings to show that systemic administration of the synthetic NOP receptor agonist, Ro64-6198, also differentially disrupts both types of fear learning in mice. Furthermore, the deficits in contextual fear learning persisted when Ro64-6198 was administrated before both conditioning and testing sessions indicating that it was not related to drug-state changes. This is in line with the lack of state-depend effects of N/OFQ reported in the rat fear conditioning paradigm (Fornari et al., 2008). During conditioning, Ro64-6198 also altered post-shock immobility suggesting that NOP receptor suppresses development of short-term conditioned fear response to the training context. However, these results contrast with those reported with N/OFQ peptide in rats (Fornari et al., 2008). Several factors might contribute to this discrepancy including differences in species (mice vs rats), the compounds (synthetic agonist vs peptide) and the training procedures (e.g., two tone-shock pairings vs five tone-shock pairings) used. It is worth noting that Ro64-6198 was more potent in reducing immobility behavior during conditioning than context testing thus revealing some dissociation in the mechanisms underlying development of short- and long-term conditioned fear. We previously showed that amnesic effect of 1 mg/kg Ro64-6198 in the novel object exploration task was related to suppression of hippocampal mitogen-activated-protein-kinases/extracellular-regulated-kinase (MAPK/ERK) pathway activation (Goeldner et al., 2008), which is required for long-term information storage (Ahi, Radulovic, & Spiess, 2004; Giovannini, 2006; Sweatt, 2004). Given that Ro64-6198 may still be active beyond the conditioning session, it is therefore possible that the disruptive effects of this agonist on long-term contextual fear memory formation may be due to interference with acquisition processes as well as consolidation processes which occur immediately after conditioning. The impairments in passive avoidance and contextual fear memory reported following post-training infusions of N/OFQ (Mamiya et al., 2003; Roozendaal et al., 2007) further support this idea.
We recently reported the efficacy of Ro64-6198 in acute pain assays (tail flick, hot-plate and shock-threshold tests) and show that this compound changes nociceptive threshold in C57BL/6N mouse strain, but these effects were overall weaker than those obtained with a prototypical analgesic (morphine) (Reiss et al., 2008). Hence, in the current study we tested analgesic doses (2 and 4 mg/kg) of morphine in similar conditions to verify whether the learning deficits induced by Ro64-6198 may be connected to changes in nociceptive threshold. However, marked differences in the profile of action of morphine and Ro64-6198 were noted which argue against this possibility. Unlike Ro64-6198, morphine was without effect on post-shock immobility during conditioning. Furthermore, the deficit in contextual fear learning we obtained with 4 mg/kg morphine was restored by administration of the same dose prior to testing, indicating that it was caused by drug state change. These results are in good agreement with those reported in the literature showing state-dependent learning effects of analgesic doses of morphine in aversive cognitive tasks (Homayoun, Khavandgar, & Zarrindast, 2003; Patti et al., 2006; Zarrindast et al., 2006). The differential effect of morphine on retention of contextual and auditory fear conditioning is of great interest and it shows that state-dependence is critically related to retrieval of conjunctive spatial than elemental (e.g., simple sensory cue) representation memory. In sum, the above findings strongly suggest that amnesic effects of Ro64-6198 may not be attributed to changes in pain threshold. They also demonstrate dissociation of the brain mechanisms underlying NOP and mu opioid receptors modulation of conditioned fear learning.
Several studies reported anxiolytic-like properties of NOP receptor agonists (Hirao et al., 2008; Jenck et al., 1997, 2000; Varty et al., 2008), which raises the possibility that amnesic effects of Ro64-6198 may be secondary to reduced fear perception. Because the drug was administered prior to the conditioning session we cannot completely discard this possibility, although the lack of changes in cued fear learning is not compatible with this interpretation. Furthermore, morphine, which was also reported to possess anxiolytic-like properties (Asakawa et al., 1998; Glover & Davis, 2008; Kahveci, Gulec, & Ozluk, 2006; Koks, Soosaar, Voikar, Bourin, & EVasar, 1999; Winslow, Noble, & Davis, 2007), failed to produce similar learning deficits. To confirm the effects of Ro64-6198 on mnemonic processes we therefore used the immediate shock deficit procedure, which offers the means to evaluate drug effects on contextual learning in the absence of the foot-shock. Our results show that mice treated with 1 mg/kg Ro64-6198 are unable to form a robust hippocampal-dependent contextual memory, a process that is fundamental for establishment of short- and long-term conditioned fear. The lack of effects of Ro64-6198 on basal level of immobility and spontaneous locomotor exploration argues for a specific disruption of mnemonic processes involved in contextual learning. In line with these conclusion, Ro64-6198 (1 mg/kg) was reported to produce spatial and recognition memory deficits similar to those caused by intra-hippocampal infusions of N/OFQ peptide (Higgins et al., 2002; Sandin et al., 1997; Sandin et al., 2004; Goeldner et al., 2008). Furthermore, the amnesic effects of this agonist could be reversed by the blockade of NOP receptors in dorsal hippocampus (Goeldner et al., 2008). Beyond the hippocampus, NOP receptors are located in many areas of the temporal lobe, including the perirhinal and the entorhinal cortex that also contribute to spatial learning. Indeed, encoding of discrete spatial cues into unitary conjunctive representation would require integration of multimodal sensory information, a process that involves entorhinal cortex and relies on substantial entorhinal–hippocampal cooperation (Parron, Poucet, & Save, 2006; Rudy & O'Reilly, 2001). Hence, the amnesic effects we saw following systemic administration of Ro64-6198 may likely be linked to suppression of information processing at a number of sites within the medial temporal lobe system. Further studies using intracerebral injections should help clarify the anatomic substrates mediating Ro64-6198 effects on contextual learning.
Overall, the similarity of the profile of actions of Ro64-6198 and N/OFQ peptide (Fornari et al., 2008; Mamiya et al., 2003) in fear conditioning paradigms point to a prominent modulatory influence of NOP receptors on aspects of aversive learning supported by hippocampal formation. The data obtained following drug co-administration also argue in this direction. Concomitant injection of Ro64-6198 and MK-801 at sub-effective doses severely impaired contextual but not auditory fear conditioning. These results are complementary to those reported in NOP receptor knockout mice showing that antagonism of N/OFQ transmission preferentially improved contextual fear conditioning through NMDA receptor-dependent mechanisms (Mamiya et al., 2003). The synergistic interaction detected between Ro64-6198 and MK-801 in the immediate shock deficit paradigm provide new evidence that common neural targets relay the opposite influence of NOP and NMDA receptors on formation of hippocampal-dependent contextual memory. In support of this view, we previously showed that NOP and NMDA receptors converge on ERK pathway in the hippocampus to modulate long-term recognition memory formation (Goeldner et al., 2008). Furthermore, Mamiya et al. (2003) showed that NOP and NMDA receptors modulate in an opposing manner the activity of Ca2+/calmodulin-dependent protein kinase II (CaMKII) in hippocampal slices. Interestingly, CaMKII was suggested to act as trigger of MAPK/ERK signaling pathway (Micheau & Riedel, 1999) and disruption of the function of this kinase in mice severely impairs contextual memory formation in the immediate shock deficit paradigm (Frankland et al., 2004). Collectively, these findings suggest that N/OFQ–NOP-receptor system negatively modulates hippocampal-dependent cognitive processing through suppression of glutamatergic function. This modulatory action may be mediated via post-synaptic mechanisms involving inhibition of NMDA receptor-dependent signaling transduction pathways (Goeldner et al., 2008; Mamiya et al., 2003), and/or pre-synaptic mechanisms involving inhibition of glutamate release (Meis, 2003; Schlicker & Morari, 2000; Tallent, 2008).
In conclusion, activation of NOP receptor following parenteral administration of Ro64-6198, impaired acquisition of contextual, but not auditory fear conditioning in mice. The prototypical analgesic morphine also differentially affected both types of learning, but unlike Ro64-6198 the contextual retention deficits were secondary to state-dependency effects. Most importantly, combined treatments with sub-effective doses of Ro64-6198 and MK-801, severely impaired contextual fear learning, but left cued fear learning intact. Similarly, co-administration of the two compounds either separately at amnesic doses or concomitantly at sub-effective doses reversed the preexposure facilitative effect on immediate shock deficit. Together, these findings provide new evidence that NOP receptors inhibit acquisition of contextual fear conditioning predominantly by interfering with formation of hippocampal-dependant conjunctive representation. This modulatory action is in part mediated through interaction with glutamatergic function at NMDA receptors. However, the contribution of other neurotransmitters cannot be discarded. In this regard, cholinergic system seems a strong candidate since it plays key role in contextual fear learning (Gold, 2003; Tinsley, Quinn, & Fanselow, 2004), and N/OFQ system was shown to inhibit cholinergic transmission in the hippocampus (Cavallini, Marino, Beani, Bianchi, & Siniscalchi, 2003; Uezu et al., 2005).
Acknowledgments
This work was supported by gants from the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the Université Louis Pasteur de Strasbourg (ULP), the Agence Nationale de la Recherche (Grant SynapticZinc) and the National Institute of Drug Abuse Grant (DA05010). C. Goeldner was supported by the French Gouvernement (Ministère de la Recherche et de l'Enseignement Supérieur) and the Fondation pour la Recherche Médicale (FRM). We thank Dr. Mary C. Olmstead for helpful comments on the manuscript.
References
- Ahi J, Radulovic J, Spiess J. The role of hippocampal signaling cascades in consolidation of fear memory. Behavioural Brain Research. 2004;149:17–31. doi: 10.1016/s0166-4328(03)00207-9. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Momose K, Ueno N, Fujino MA, Kasuga M. Endomorphins have orexigenic and anxiolytic activities in mice. Neuroreport. 1998;9:2265–2267. doi: 10.1097/00001756-199807130-00022. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Boeckman R, Krochmal D, Fernando H, Ahrens R, Csernansky JG. NMDA receptor blockade and hippocampal neuronal loss impair fear conditioning and position habit reversal in C57Bl/6 mice. Brain Research Bulletin. 2003;60:131–142. doi: 10.1016/s0361-9230(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: Effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Pooler O, Flaherty L. Inbred strain variation in contextual and cued fear conditioning behavior. Mammalian Genome. 2001;12:651–656. doi: 10.1007/s003350020039. [DOI] [PubMed] [Google Scholar]
- Bongsebandhu-phubhakdi S, Manabe T. The neuropeptide nociceptin is a synaptically released endogenous inhibitor of hippocampal long-term potentiation. Journal of Neuroscience. 2007;27:4850–4858. doi: 10.1523/JNEUROSCI.0876-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo’ G, Guerrini R, Rizzi A, Salvadori S, Regoli D. Pharmacology of nociceptin and its receptor: A novel therapeutic target. British Journal of Pharmacolgy. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini S, Marino S, Beani L, Bianchi C, Siniscalchi A. Nociceptin inhibition of acetylcholine efflux from different brain areas. Neuroreport. 2003;14:2167–2170. doi: 10.1097/00001756-200312020-00007. [DOI] [PubMed] [Google Scholar]
- Chang SD, Chen DY, Liang KC. Infusion of lidocaine into the dorsal hippocampus before or after the shock training phase impaired conditioned freezing in a two-phase training task of contextual fear conditioning. Neurobiology of Learning and Memory. 2008;89:95–105. doi: 10.1016/j.nlm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Chiou LC, Liao YY, Fan PC, Kuo PH, Wang CH, Riemer C, et al. Nociceptin/orphanin FQ peptide receptors: Pharmacology and clinical implications. Current Drug Targets. 2007;8:117–135. doi: 10.2174/138945007779315605. [DOI] [PubMed] [Google Scholar]
- Darland T, Grandy DK. The orphanin FQ system: An emerging target for the management of pain? British Journal of Anaesthesia. 1998;81:29–37. doi: 10.1093/bja/81.1.29. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Figueredo LZ, Moreira KD, Ferreira TL, Fornari RV, de Oliveira MG. Interaction between glutamatergic–NMDA and cholinergic–muscarinic systems in classical fear conditioning. Brain Research Bulletin. 2008;77:71–76. doi: 10.1016/j.brainresbull.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Fornari RV, Soares JC, Ferreira TL, Moreira KM, Oliveira MG. Effects of nociceptin/orphanin FQ in the acquisition of contextual and tone fear conditioning in rats. Behavioural Neuroscience. 2008;122:98–106. doi: 10.1037/0735-7044.122.1.98. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, Silva AJ. Consolidation of CS and US representations in associative fear conditioning. Hippocampus. 2004;14:557–569. doi: 10.1002/hipo.10208. [DOI] [PubMed] [Google Scholar]
- Giovannini MG. The role of the extracellular signal-regulated kinase pathway in memory encoding. Reviews in the Neurosciences. 2006;17:619–634. doi: 10.1515/revneuro.2006.17.6.619. [DOI] [PubMed] [Google Scholar]
- Glover EM, Davis M. Anxiolytic-like effects of morphine and buprenorphine in the rat model of fear-potentiated startle: Tolerance, cross-tolerance, and blockade by naloxone. Psychopharmacology. 2008;198:167–180. doi: 10.1007/s00213-008-1112-0. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Reiss D, Wichmann J, Meziane H, Kieffer BL, Ouagazzal AM. Nociceptin receptor impairs recognition memory via interaction with NMDA receptor-dependent mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in the hippocampus. Journal of Neuroscience. 2008;28:2190–2198. doi: 10.1523/JNEUROSCI.3711-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiology of Learning and Memory. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Gould TJ, McCarthy MM, Keith RA. MK-801 disrupts acquisition of contextual fear conditioning but enhances memory consolidation of cue fear conditioning. Behavioural Pharmacology. 2002;13:287–294. doi: 10.1097/00008877-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Kew JN, Richards JG, Takeshima H, Jenck F, Adam G, et al. A combined pharmacological and genetic approach to investigate the role of orphanin FQ in learning and memory. European Journal Neuroscience. 2002;15:911–922. doi: 10.1046/j.1460-9568.2002.01926.x. [DOI] [PubMed] [Google Scholar]
- Hirao A, Imai A, Sugie Y, Yamada Y, Hayashi S, Toide K. Pharmacological characterization of the newly synthesized nociceptin/orphanin FQ-receptor agonist 1-[1-(1-methylcyclooctyl)-4-piperidinyl]-2-[(3R)-3-piperidinyl]-1H-benzimidazole as an anxiolytic agent. Pharmacological Science. 2008;106:361–368. doi: 10.1254/jphs.fp0071742. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Khavandgar S, Zarrindast MR. Morphine state-dependent learning: Interactions with alpha2-adrenoceptors and acute stress. Behavioural Pharmacology. 2003;14:41–48. doi: 10.1097/00008877-200302000-00004. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau J-L, Martin JR, Kilpatrick G, Reinscheid R, Monsma FJ, et al. Orphanin FQ acts as anxiolytic to attenuate behavioral responses to stress. Proceedings of the National Academy of Sciences USA. 1997;94:4854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, et al. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: Anxiolytic profile in the rat. Proceedings of the National Academy of Sciences USA. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahveci N, Gulec G, Ozluk K. Effects of intracerebroventricularly-injected morphine on anxiety, memory retrieval and locomotor activity in rats: Involvement of vasopressinergic system and nitric oxide pathway. Pharmacology Biochemistry and Behavior. 2006;85:859–867. doi: 10.1016/j.pbb.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Hesselink MB, van Scharrenburg G, Westerink BH. Tonic inhibition by orphanin FQ/nociceptin of noradrenaline neurotransmission in the amygdala. European Journal of Pharmacolgy. 2004;485:197–200. doi: 10.1016/j.ejphar.2003.11.061. [DOI] [PubMed] [Google Scholar]
- Koks S, Soosaar A, Voikar V, Bourin M, EVasar E. BOC-CCK-4, CCK(B)receptor agonist, antagonizes anxiolytic-like action of morphine in elevated plus-maze. Neuropeptides. 1999;33:63–69. doi: 10.1054/npep.1999.0015. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. Nature Reviews. Drug Discovery. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Yamada K, Miyamoto Y, Konig N, Watanabe Y, Noda Y, et al. Neuronal mechanism of nociceptin-induced modulation of learning and memory: Involvement of N-methyl-D-aspartate receptors. Molecular Psychiatry. 2003;8:752–765. doi: 10.1038/sj.mp.4001313. [DOI] [PubMed] [Google Scholar]
- Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, et al. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behavioral Neuroscience. 2007;121:721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Meis S. Nociceptin/orphanin FQ: Actions within the brain. Neuroscientist. 2003;9:158–168. doi: 10.1177/1073858403252231. [DOI] [PubMed] [Google Scholar]
- Micheau J, Riedel G. Protein kinases: Which one is the memory molecule? Cellular and Molecular Life Science. 1999;55:534–548. doi: 10.1007/s000180050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima T, Noda Y, Mamiya T. The role of nociceptin in cognition. Brain Research. 1999;848:167–173. doi: 10.1016/s0006-8993(99)01906-x. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr., Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: Comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. Journal of Comparative Neurology. 1999b;412:563–605. [PubMed] [Google Scholar]
- Neal CR, Jr., Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. Journal of Comparative Neurology. 1999a;406:503–547. [PubMed] [Google Scholar]
- Parada-Turska J, Turski WA. Excitatory amino acid antagonists and memory: Effect of drugs acting at N-methyl-D-aspartate receptors in learning and memory tasks. Neuropharmacology. 1990;29:1111–1116. doi: 10.1016/0028-3908(90)90034-o. [DOI] [PubMed] [Google Scholar]
- Parron C, Poucet B, Save E. Cooperation between the hippocampus and the entorhinal cortex in spatial memory: A disconnection study. Behavioural Brain Research. 2006;170:99–109. doi: 10.1016/j.bbr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Patti CL, Kameda SR, Carvalho RC, Takatsu-Coleman AL, Lopez GB, Niigaki ST, et al. Effects of morphine on the plus-maze discriminative avoidance task: Role of state-dependent learning. Psychopharmacology. 2006;184:1–12. doi: 10.1007/s00213-005-0238-6. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Calo’ G, Guerrini R, Regoli D, Quirion R. [Nphe(1)]-Nociceptin (1–13)-NH(2), a nociceptin receptor antagonist, reverses nociceptin-induced spatial memory impairments in the Morris water maze task in rats. British Journal of Pharmacology. 2000;131:1379–1384. doi: 10.1038/sj.bjp.0703724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Wichmann J, Tekeshima H, Kieffer BL, Ouagazzal AM. Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64-6198, on reactivity to acute pain in mice. comparison to morphine. European Journal of Pharmacology. 2008;579:141–148. doi: 10.1016/j.ejphar.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Lengvilas R, McGaugh JL, Civelli O, Reinscheid RK. Orphanin FQ/nociceptin interacts with the basolateral amygdala noradrenergic system in memory consolidation. Learning and Memory. 2007;14:29–35. doi: 10.1101/lm.403607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: Insights from a two-process model. Neuroscience and Biobehavioral Reviews. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive Affective & Behavioral Neuroscience. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Fanselow MS. Pre-training prevents context fear conditioning deficits produced by hippocampal NMDA receptor blockade. Neurobiology of Learning and Memory. 2003;80:123–129. doi: 10.1016/s1074-7427(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Sandin J, Georgieva J, Schott PA, Ogren SO, Terenius L. Nociceptin/ orphanin FQ microinjected into hippocampus impairs spatial learning in rats. European Journal of Neuroscience. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Sandin J, Ogren SO, Terenius L. Nociceptin/orphanin FQ modulates spatial learning via ORL-1 receptors in the dorsal hippocampus of the rat. Brain Research. 2004;997:222–233. doi: 10.1016/j.brainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Morari M. Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides. 2000;21:1023–1029. doi: 10.1016/s0196-9781(00)00233-3. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Birkenfeld K, Palve M, Spiess J. Impairment of conditioned contextual fear of C57BL/6J mice by intracerebral injections of the NMDA receptor antagonist APV. Behavioural Brain Research. 2000;116:157–168. doi: 10.1016/s0166-4328(00)00269-2. [DOI] [PubMed] [Google Scholar]
- Stote DL, Fanselow MS. NMDA receptor modulation of incidental learning in Pavlovian context conditioning. Behavioral Neuroscience. 2004;118:253–257. doi: 10.1037/0735-7044.118.1.253. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Current Opinion in Neurobiology. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tallent MK. Presynaptic inhibition of glutamate release by neuropeptides: Use-dependent synaptic modification. Results and Problems in Cell Differentiation. 2008;44:177–200. doi: 10.1007/400_2007_037. [DOI] [PubMed] [Google Scholar]
- Tinsley MR, Quinn JJ, Fanselow MS. The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: Comparing pavlovian fear conditioning and inhibitory avoidance. Learning & Memory. 2004;11:35–42. doi: 10.1101/lm.70204. [DOI] [PubMed] [Google Scholar]
- Uezu K, Sano A, Sei H, Toida K, Houtani T, Sugimoto T, et al. Enhanced hippocampal acetylcholine release in nociceptin-receptor knockout mice. Brain Research. 2005;1050:118–123. doi: 10.1016/j.brainres.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Varty GB, Lu SX, Morgan CA, Cohen-Williams ME, Hodgson RA, Smith-Torhan A, et al. The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510). Journal of Pharmacology and Experimental Therapeutics. 2008;326:672–682. doi: 10.1124/jpet.108.136937. [DOI] [PubMed] [Google Scholar]
- Wei WZ, Xie CW. Orphanin FQ suppresses NMDA receptor-dependent long-term depression and depotentiation in hippocampal dentate gyrus. Learning & Memory. 1999;6:467–477. doi: 10.1101/lm.6.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann J, Adam G, Rover S, Hennig M, Scalone M, Cesura AM, et al. Synthesis of (1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one, a potent and selective orphanin FQ (OFQ) receptor agonist with anxiolytic-like properties. European Journal Medicinal Chemistry. 2000;35:839–851. doi: 10.1016/s0223-5234(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Davis M. Modulation of fear-potentiated startle and vocalizations in juvenile rhesus monkeys by morphine, diazepam, and buspirone. Biological Psychiatry. 2007;61:389–395. doi: 10.1016/j.biopsych.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Yu TP, Fein J, Phan T, Evans CJ, Xie CW. Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus. 1997;7:88–94. doi: 10.1002/(SICI)1098-1063(1997)7:1<88::AID-HIPO9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Bananej M, Khalilzadeh A, Fazli-Tabaei S, Haeri-Rohani A, Rezayof A. Influence of intracerebroventricular administration of dopaminergic drugs on morphine state-dependent memory in the step-down passive avoidance test. Neurobiology of Learning and Memory. 2006;86:286–292. doi: 10.1016/j.nlm.2006.04.002. [DOI] [PubMed] [Google Scholar]