Abstract
Rationale
Embryonic stem cells (ESCs) hold great promise for cardiac regeneration but are susceptible to various concerns. Recently, salutary effects of stem cells have been connected to exosome secretion. ESCs have the ability to produce exosomes however their effect in the context of the heart is unknown.
Objective
Determine the effect of ESC-derived exosome for the repair of ischemic myocardium and whether c-kit+ CPCs function can be enhanced with ESC exosomes
Methods and Results
This study demonstrates that mouse ESC derived exosomes (mES Ex) possess ability to augment function in infarcted hearts. mES Ex enhanced neovascularization, cardiomyocyte survival and reduced fibrosis post infarction consistent with resurgence of cardiac proliferative response. Importantly, mES Ex augmented cardiac progenitor cell (CPC) survival, proliferation and cardiac commitment concurrent with increased c-kit+ CPCs in vivo 8 weeks after in vivo transfer along with formation of bonafide new cardiomyocytes in the ischemic heart. miRNA array revealed significant enrichment of miR290–295 cluster and particularly miR-294 in ESC exosomes. The underlying basis for the beneficial effect of mES Ex was tied to delivery of ESC specific miR-294 to CPCs promoting increased survival, cell cycle progression and proliferation.
Conclusions
mES Ex provide a novel cell free system that utilizes the immense regenerative power of ES cells while avoiding the risks associated with direct ES or ES derived cell transplantation and risk of teratomas. ESC exosomes possess cardiac regeneration ability and modulate both cardiomyocyte and CPC based repair programs in the heart.
Keywords: Embryonic stem cells, exosomes, miRNA, cardiac repair, cardiac progenitor cells, microvesicles
INTRODUCTION
Endogenous myocardial repair in response to injury has been reported to involve limited self-division of pre-existing cardiomyocytes and the activation and differentiation of resident cardiac stem cells (CSC)1–4. However, the insufficiency of these responses to meaningful repair paved the way for administration of exogenous stem cell based therapies. Adoptive transfer of different cell types has been associated with enhanced cardiac function in patients with cardiovascular diseases5, 6 and animal models of heart failure7, 8. Despite these promising results, poor survival and low retention of the donated stem cell population9, 10 remains a significant limitation prompting research into new alternative remedies.
Pluripotent stem cells including both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) hold immense promise for cardiac regeneration since they possess unparalleled differentiation ability11. Although, cardiomyocytes derived from ESCs have been shown to improve cardiac regeneration and function in animal models of heart failure, however, this also has been reported to enhance arrhythmogenic response 12–13. In spite of their impressive cardiac repair ability, teratoma formation has been observed14, 15 after transplantation of an unpurified ESC derived cardiomyocyte population. Derivation of induced pluripotent cells has solved the issues with the availability of autologous ES cells, however, ES or iPS derived cells may still suffer the same difficulties in cell retention, coupling and survival in ischemic myocardium as is noted for adult stem cells. Thus, there is a critical need for exploiting the powerful regenerative capacity of pluripotent cells while avoiding the problems associated with cell transplantation.
Discovery of cell-free components such as exosomes 16 capable of instigating cell analogous response in target cells may provide a promising alternative for cardiac protection and regeneration17–19. Novel, non-traditional use of cell-free components of ESC/iPS, such as exosomes, which carry ES-specific miRs and proteins may still allow for harnessing the regenerative power of these cells to augment and modulate endogenous repair mechanisms.
In this manuscript, we report that mES- exosome delivery in the heart after myocardial infarction stimulates and augments CPC and cardiomyocyte proliferation based endogenous myocardial repair, which in part involves transfer of ES-specific microRNA-294. Our data suggests that ESC/iPS derived exosomes represent a novel cell-free system for enhancing endogenous cardiac repair after pathological injury and bypass limitations of adoptive cell transplantation.
METHODS
Cell culture and differentiation
Mouse embryonic stem cells (mES) isolated from C57Bl/6 were obtained from ATCC and cultured in DMEM (high glucose) with 15% FBS and supplemented with β-mercaptoethanol (100μM), Non-essential amino acids (100 μM), Leukemia inhibitory factor (LIF; 1000U/ml) and penicillin/streptomycin (50ug/ml each). Mouse embryonic fibroblasts (MEF) were cultured in DMEM with 10% FBS, Non-essential amino acids, (100 μM) and penicillin/streptomycin (50ug/ml each). H9c2 myoblasts and Human umbilical vein endothelial cells (HUVECs) were maintained in their respective culture mediums. CPCs from syngeneic male FVB mice were cultured in cardiac stem cell media and were differentiated as previously described 20 with 10 8 mol/L dexamethasone treatment for 7days. Additional detail in online supplement.
Exosome isolation and labeling
mES and MEF cells were cultured for 40 hours followed by collection and purification by ultracentrifugation of exosomes as described previously 21. The purified exosome fraction was re-suspended in saline for use. Purified exosomes were labeled with PKH26 Red Fluorescent Cell Linker Kit for in vitro studies according to the manufacturer’s protocol. Additional detail in online supplement.
Dynamic light scattering
Exosome size analysis was carried out by dynamic light scattering measurement as described previously22. Briefly, exosomes were suspended in phosphate-buffered saline (PBS) containing 2 mM ethylenediaminetetraacetic acid (EDTA); then, dynamic light-scattering measurements were performed with a Zetasizer Nano ZS (Malvern Instruments Ltd, Worcestershire, UK). Additional detail in online supplement.
Electron microscopy
Cells were fixed with 4% paraformaldehyde and processed, contrasted and embedded as described previously21. Transmission electron microscopy images were obtained with an FEI (Hillsboro, OR, USA) Tecnai Spirit G2 transmission electron microscope operating at 120 kV. Additional detail in online supplement.
Immunoblot
Immunoblot analysis was performed as described previously 23 with additional detail in online supplement.
Immunohistochemistry
Immunocytochemistry, TUNEL assays, and immunohistochemistry were performed as previously described 22–24 with additional information in online supplement and a list of antibodies in Supplementary Table II.
TaqMan® Array MicroRNA
Single-stranded cDNA is synthesized from all samples using the TaqMan® MicroRNA Reverse Transcription Kit (Part Number 4366593) and the Megaplex™ RT Primers, Rodent Pool Set v3.0 (Part Number 4444746) as described in the Applied Biosystems protocol “Megaplex™ Pools for microRNA Expression Analysis (Part Number 4399721 Rev. C). The reverse transcription product is pre-amplified using Megaplex™ PreAmp Primers, Rodent Pool B v3.0 (4444308). The pre-amplified product is used to run real time PCR reactions using TaqMan® Universal PCR Master Mix, No AmpErase® UNG (Part Number 4324018) on a TaqMan® Array Rodent MicroRNA A+B Cards set v3.0 (Part Number 4444909). The array cards are run on a 7900HT system.
microRNA treatment and quantification
Cells are transfected with mouse miR-291a-5p, miR-294-3p, miR-295-3p (mimics) or negative control mimics. CPCs are grown in DMEM/F12 media without antibiotics and transfected with either miRNA mimics or controls (25nM, Invitrogen, CA, USA) using Lipofectamine RNAiMAX (Invitrogen, CA, USA) for 24 hrs as per manufacturer instructions25. Total RNA from CPCs and the heart is extracted using the miRNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. Real time reactions were performed in triplicate on a 7500FAST Real-Time PCR system (Applied Biosystems, CA USA). Ct values were averaged and normalized to snoRNA236. Relative expression was determined by the ΔΔCt comparative threshold method. Detailed methods are provided in the online supplemental information.
Oxygen consumption rates
A Seahorse Bioscience XF96 extracellular flux analyzer was utilized to measure oxygen consumption rates (OCR) in cardiac progenitor cells +/− exosomes with modification of a previously reported protocol (detailed in online supplement).
Animal studies
All mice (male C57BL/6, 8–12 weeks old) used in this study were obtained from The Jackson Laboratories (Bar Harbor, ME). All surgical procedures and animal care protocols were approved by the Temple University Animal Care and Use Committee.
Induction of acute myocardial infarction
Mice underwent surgery to ligate the left anterior descending coronary artery as reported previously 24 followed by administration of exosomes from mES cells (n=6) and MEF (n=6) cells suspended in saline intramyocardially into the left ventricular wall (border zone) at two different locations immediately after left anterior descending ligation. The saline group underwent the same surgery but received saline without exosomes (n=6). Tissue was harvested at 5 or 14 days and 8 weeks after AMI for histological analysis.
Echocardiography
Transthoracic two-dimensional M-mode echocardiography was performed using the Vevo770 (VisualSonics, Toronto, ON, Canada) equipped with a 30-MHz transducer as described previously23, 24. Additional details in online supplement.
Statistics
Statistical analysis is performed using Student t test. Comparison of 2 or more groups is performed by 1-way ANOVA or 2-way ANOVA with Bonferroni post hoc test. P < 0.05 is considered statistically significant. Error bars represent ±SEM. Statistical analysis is performed using Graph Pad prism v 5.0 software.
RESULTS
Embryonic stem cells secrete physiologically functional exosomes
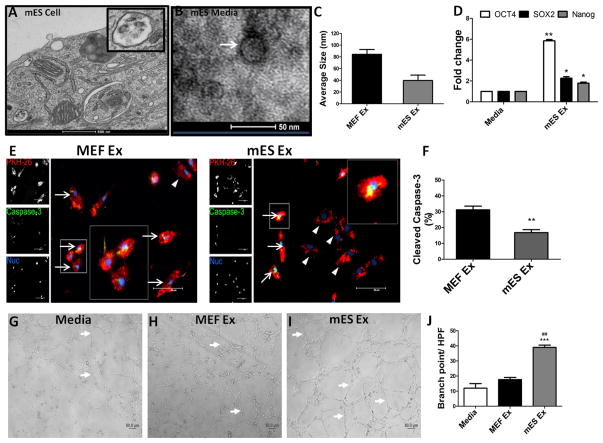
Electron microscopy and dynamic light scattering analysis of murine embryonic stem cells (mES) and embryonic fibroblasts (MEF) showed that both cell types secrete exosomes of typical size range16 (Figure 1A–C and Online Figure IA–F). Additionally, exosomes from both cells expressed exosomal marker protein, flotillin-1 indicating their cytoplasmic origin and were negative for Lamin B, a nuclear protein (Online Figure IG). Expression of ES-specific transcripts was exclusively detected in mES Ex (Online Figure IH) confirming the embryonic stem cell origin of the mES Ex.
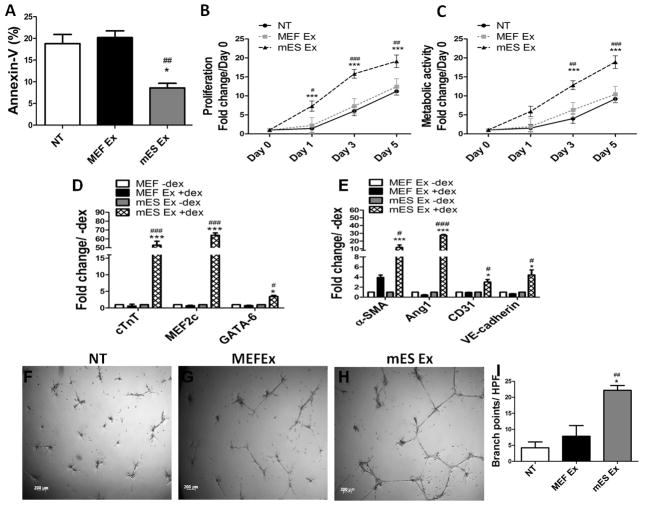
Figure 1. Characterization and functional validation of exosomes derived from embryonic stem cells (ESCs).
A) Exosome secretion from a mouse embryonic stem cell (ESC) as evidenced by electron microscopy Scale bar = 500nm, inset shows higher magnification of an ESC exosome. B) ESC culture medium shows exosome by electron microscopy, Scale bar = 50nm. C) Measurement of exosome size in mES and MEF cells by dynamic light scattering (DLS) analysis shows that mES exosome are 39.7nm in size compared to MEF ex (84.3nm) (n=4). D) Increased mRNA expression of pluripotent markers OCT4, SOX2 and Nanog in MEF cells treated with mES exosomes after 24 hrs in comparison to control cells. (n=3) media vs. mES Ex *p < 0.05, **p < 0.01, ***p < 0.001. E) Reduction in caspase3+ H9c2 cells treated with PKH-26 labeled mES ex compared to MEF ex treated cells along with corresponding quantification in F) (n=3). Arrows indicate caspase3 expressing cells while arrowhead shows H9c2 cells negative for capsase3 expression while inset show higher magnification. PKH-26 (red), Caspase3 (green) and nuclei (blue). MEF Ex vs mES Ex *p < 0.05, **p < 0.01, ***p < 0.001. G–I) Enhanced tube formation in HUVECs treated with mES Ex in comparison to MEF Ex and media treated control HUVECs. J) Quantification of branch points in HUVECs given different treatments. Media vs. mES Ex *p < 0.05, **p < 0.01, ***p < 0.001 and MEF Ex vs mES Ex #p < 0.05, ##p < 0.01, ###p < 0.001.
Ability of mES Ex to modulate cellular function was assessed in vitro using different cell types. mES Ex enhanced expression of pluripotent markers OCT-4, SOX-2 and Nanog in MEF cells 24 hrs after treatment indicating efficient delivery of exosomal cargo to target cells (Figure 1D). Cell survival after exosomal uptake was determined by labeling mES Ex and MEF Ex with PKH26 followed by administration to H9c2 myoblasts under challenge from H2O2 induced stress. A significant reduction in cleaved caspase-3 expression was observed in H9c2 myoblasts treated with mES Ex (16.8%) compared to MEF Ex treated cells (31.8%) in response to 16hrs of H2O2 challenge (Figure 1E–F). Finally, human umbilical vein endothelial cells (HUVECs) were treated with mES Ex and MEF Ex and cultured on matrigel to assess whether mES Ex can enhance in vitro tube formation. HUVEC tube formation was significantly increased exclusively after mES Ex treatment (Figure 1G–J). Collectively, results showed that mES Ex are readily up-taken by target cells and modulate target cell function including cell survival.
Intramyocardial delivery of mES Ex improved post-MI cardiac function
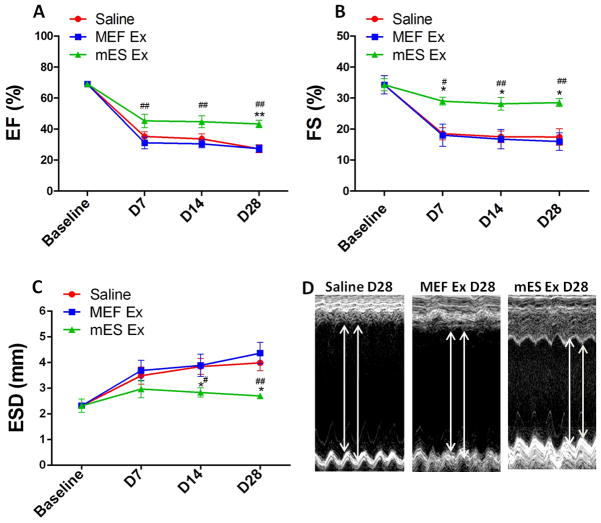
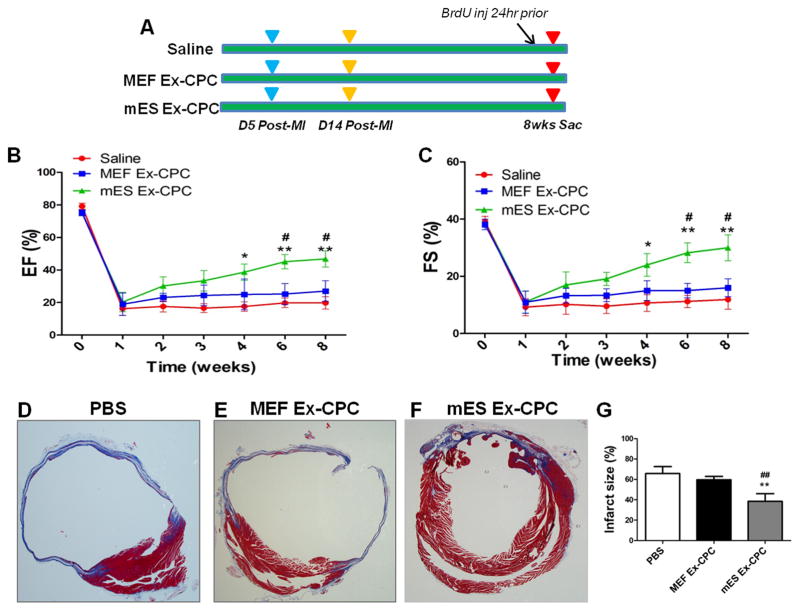
In order to assess their therapeutic efficacy in post-infarct myocardium, mES Ex were intramyocardially administered in mice at the time of myocardial infarction while MEF Ex and saline served as controls. Left ventricular contractility and function were consistently increased with mES Ex treatment as evidenced by significantly improved ejection fraction (EF; Figure 2A) and fractional shortening (FS; Figure 2B) measurements 4 weeks after infarction. Similarly, significant reduction in left ventricular end-systolic diameter (ESD, Figure 2C) was observed in mES Ex treated animals compared to control groups in conjunction with significantly improved wall motion in the mES Ex treated animals (Figure 2D) at 4 weeks. Histological analysis of the heart 4 weeks post infarction indicated decreased infarct size in mES Ex transplanted mice (20.8%) compared to MEF Ex (33.1%) and saline (32.1%) administered animals (Online Figure IIA–D). Interestingly, no tumor formation was observed in the hearts of mice transplanted with mES Ex 4 weeks after administration (Online Figure IIIA–C). Together these results provide evidence for a therapeutic role of mES Ex in augmenting cardiac function after myocardial infarction.
Figure 2. Enhanced cardiac function after myocardial infarction in mice transplanted with mES exosome.
A) Increased ejection fraction (EF) and B) fractional shortening (FS) in mice transplanted with mES exosomes (n=6) compared to mice with MEF exosomes (n=6) and saline (n=6) treated animals after 4 weeks after infarction. mES Ex, MEF Ex and saline was administered to animals at the time of infarction. C) Reduced left ventricular end-systolic diameter (LVSED) after mES Ex treatment compared to saline treatment. D) Increased wall motion in mice treated with mES exosomes after 4 weeks as evidenced by M-mode recordings by echocardiography. Saline vs. mES Ex *p < 0.05, **p < 0.01, ***p < 0.001 and MEF Ex vs mES Ex #p < 0.05, ##p < 0.01, ###p < 0.001.
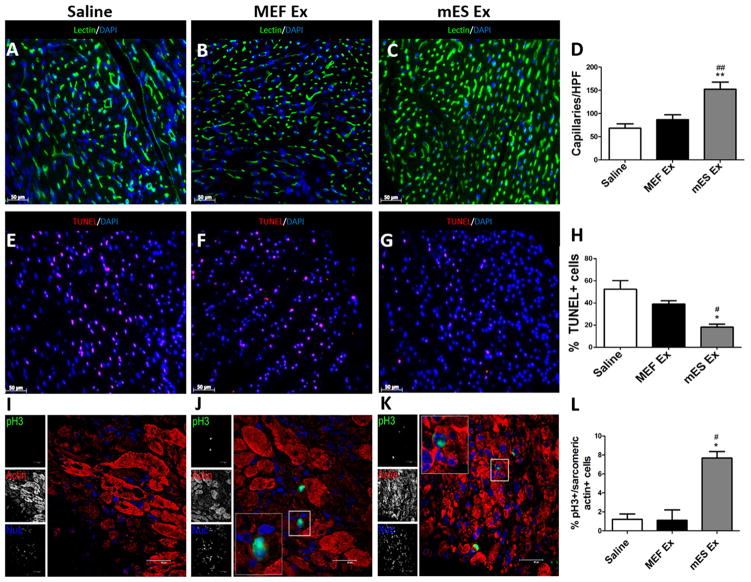
mES Exosomes augment neo-vascularization, myocyte proliferation and survival after MI
Immunohistochemical analysis of the hearts isolated from various treatment groups was carried out to determine whether mES Ex induce morphometric changes in the heart. Capillary density was significantly increased in mES Ex transplanted hearts (border zone) as evidenced by lectin staining (Figure 3A–D) together with decreased apoptosis (Figure 3E–H) compared to MEF Ex and saline groups 4 weeks after infarction. Next, analysis of heart sections in animals that were administered with BrdU 24hr prior to terminal experiments, revealed that BrdU+ cardiomyocytes were significantly increased in mES Ex hearts (5.8 fold) compared to saline 28 days after infarction (Online Figure IVA–D) coupled with increased mRNA levels of cyclins (A2, D1, D2, E1; Online Figure IV E) and decreased expression of cyclin inhibitors (p16, p19, p21, p53; Online Figure IV F) when analyzed at day 5 following myocardial infarction. Moreover, a significant increase in pH3+ cardiomyocytes in hearts treated with mES Ex compared to control hearts further supporting evidence towards myocyte cycling (Figure 3 I–L). Collectively, these results indicate that mES Ex lead to induction of cardiac protective response and promote myocyte proliferative and survival response that in turn contribute to the endogenous repair process.
Figure 3. Increased capillary density and reduced apoptosis in the hearts after myocardial infarction.
A–C) Increased capillary density after mES Ex treatment in mice 4 weeks after myocardial infarction along with corresponding quantification D). Lectin (green) and nuclei (blue) (n=6). E–G) Reduced TUNEL+ nuclei in hearts transplanted with mES Ex compared to MEF Ex and saline treated hearts. Quantification of TUNEL+ cells in H). TUNEL (magenta) and nuclei (blue) (n=6). Scale bar=50um. I–K) Enhanced cardiomyocyte cycling as evidenced by pH3+/sacromeric actin+ cells in hearts treated with mES Ex compared to MEF Ex and saline treated animals 28 days after infarction. Corresponding quantification is shown in (L). Scale bar=40um. Saline vs. mES Ex *p < 0.05, **p < 0.01, ***p < 0.001 and MEF Ex vs mES Ex #p < 0.05, ##p < 0.01, ###p < 0.001.
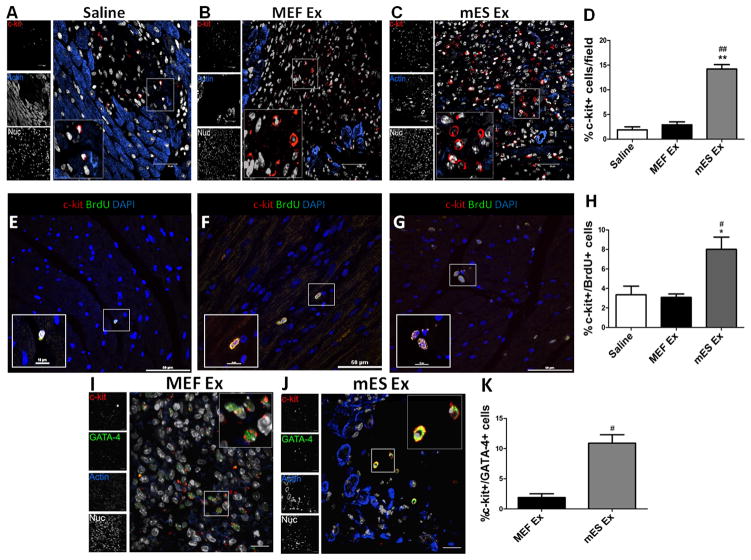
mES Ex augment resident c-Kit+ CPCs in infarcted myocardium
Resident cardiac progenitor cells (CPCs) within the heart capable of regulating cardiac homeostasis 3, 4 form an integral part of the endogenous cardiac repair response to injury 26. Since, mES Ex enable functional augmentation following myocardial damage, effect of mES Ex on CPC number, survival and proliferation was assessed in vivo. Compared to controls, the number of resident c-kit+ CPCs in the myocardium after mES Ex treatment significantly increased (Figure 4A–D). Additional characterization of c-kit+ CPCs was done by colabeling with GATA-4 that revealed a corroborating increase in c-kit+/GATA-4 CPCs in the heart treated mES Ex compared to MEF Ex heart (Figure 4I–K). Similarly, CPC proliferation, measured by c-kit+/pH3+ cells, increased by 4.1 fold (Online Figure VA–D) in conjunction with a 3.8 fold decrease in c-kit+/TUNEL+ apoptotic CPCs (Online Figure VE–H). An important aspect of mES Ex administration is whether early CPC proliferation and survival translates into long term enhancement of CPC numbers in the heart. In this respect, mES Ex administration led to approximately 2.5 fold increase in c-kit+/BrdU+ CPCs compared to control hearts 4 weeks after infarction (Figure 4E–H) indicating that mES Ex have the ability to sustain long term CPC proliferation in the heart. Therefore, these results support the postulate that mES Ex promotes CPC survival and proliferation in hearts after infarction that may be in part responsible for augmented cardiac function.
Figure 4. mES Ex promote CPC numbers and proliferation in hearts after infarction.
A–C) Increased number of c-kit+ CPCs in hearts 5 days after mES Ex transplantation compared to MEF Ex and saline treated animals (n=4). Quantification of c-kit+ cells is shown in D). c-kit (red), sarcomeric actin (blue) and nuclei (white). Scale bar=40μm. E–G) Enhanced c-kit+ /BrdU+ cells in the heart 28 days after mES Ex transplantation along with corresponding quantification (n=6). c-kit (red), BrdU (green) and nuclei (blue). Scale bar=50μm. H) Quantification of c-kit+/BrdU+ CPCs in all animal groups. I–J) Identification of CPCs by colocalization of c-kit with GATA-4 in hearts treated with mES Ex and MEF Ex along with corresponding quantification in K). c-kit (red), GATA-4 (green), sarcomeric actin (blue) and nuclei (white). Saline vs. mES Ex *p < 0.05, **p < 0.01, ***p < 0.001 and MEF Ex vs mES Ex #p < 0.05, ##p < 0.01, ###p < 0.001.
mES Ex enhance CPC survival and function both in vitro and in vivo
CPC survival, proliferation and ability for cardiac commitment in response to mES Ex treatment was assessed in vitro to corroborate findings in injured hearts receiving mES Ex. CPCs treated with mES Ex showed enhanced survival as evidenced by decreased annexin V+ cells (8.6%) compared to MEF Ex (20.2%) and non-treated CPCs (18.8%) (Figure 5A) in response to H2O2 challenge. Importantly, no significant change in CPC survival was observed after treatment with equal amount of mES media, MEF media, mES exosome free media (mES Ex free) and MEF exosome free media (MEF Ex free) (Online Figure VI A) suggesting that mES Ex were predominantly responsible for the observed survival response in CPCs with minimal or no contribution from serum exosomes. Additionally, mES Ex treatment of CPCs also resulted in significantly enhanced CPC proliferation (Figure 5B) and metabolic activity as measured by MTT assay (Figure 5C) and confirmed by Seahorse assay for oxygen consumption rates (OCR; Online Figure IX A–E). The ability of CPCs to commit to cardiac lineages is an important aspect of cardiac regenerative response and it was hypothesized that mES Ex may enhance CPC commitment towards cardiac lineages. mRNA expression of cardiomyocyte and endothelial cell markers (Figure 5D–E) was increased in CPCs treated with mES Ex compared to MEF Ex under stimulation with dexamethasone for 7days. Independent experiment on CPC tube formation ability on matrigel corroborated increased endothelial differentiation in response to mES Ex (Figure 5F–I).
Figure 5. Modulation of CPC function by mES Exosome administration.
A) Increased survival of CPCs after treatment with mES exosomes in comparison to MEF exosomes under H2O2 challenge (n=3). B) Increased CPC proliferation at day 1, 3 and 5 after mES exosome treatment compared to MEF exosomes and non-treated CPCs as evidenced by CyQuant assay (n=3). NT vs. mES Ex *p < 0.05, **p < 0.01, ***p < 0.001 and MEF Ex vs mES Ex #p < 0.05, ##p < 0.01, ###p < 0.001. C) Increased metabolic activity in CPCs at day 3 and day5 after mES Ex treatment compared to MEF Ex and non-treated CPCs as measured by MTT assay (n=3). D–E) Enhanced mRNA levels of cardiac markers such (cTnT, MEF2c and GATA-6) and endothelial markers (α-SMA, Ang1, CD31, VE-cadherin) in CPCs treated with mES Ex in the presence of dexamethasone compared to MEF Ex dex treated CPCs and non-treated controls as evidenced by qRT-PCR (n=3). NT vs. mES Ex +dex *p < 0.05, **p < 0.01, ***p < 0.001 and MEF Ex +dex vs mES Ex + dex #p < 0.05, ##p < 0.01, ###p < 0.001. F–H) Tube formation is increased in CPCs treated with mES Ex cultured on matrigel compared to MEF Ex and non-treated CPCs after 24hrs (n=3) along with corresponding quantification I).
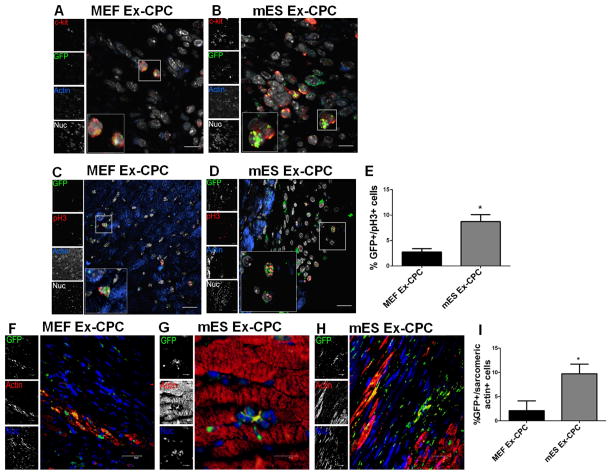
In order to elucidate whether mES Ex enhance CPC survival and function in vivo, GFP-CPCs pretreated with mES Ex and MEF Ex were transplanted after induction of myocardial infarction (Figure 6A). Long-term follow-up studies (8 weeks after MI) showed consistently improved LV function in mice receiving mES Ex treated CPCs compared to MEF Ex treated CPCs (Figure 6B–C). Moreover, significant reduction in fibrosis was observed in mES Ex-CPC hearts compared to controls (Figure 6D–G). The enhanced function was attributed to increased ability of GFP+ mES Ex-CPC to survive in the injured hearts observed mainly in the border zone, infarcted region and in close proximity to blood vessels 14 days after infarction parallel with their de novo differentiation to small myocytes (Online Figure VIB–D). Furthermore, GFP colocalized with c-kit+ CPCs confirming the identity of the adoptively transferred CPCs 5 days after infarction (Figure 7A–B). Pre-treatment with mES Ex also enhanced the proliferation of the transplanted CPCs (Figure 7C–E) along with reduction in TUNEL+ GFP cells (Online Figure VIIA–C). Interestingly, persistence of GFP+ CPCs and new GFP+ myocytes in mES Ex-CPC transplanted hearts was still evident even after 8 weeks of transplantation (Figure 7F–I) concurrent with increased contribution of GFP+ CPC to new blood vessel formation (Online Figure VII–F). Therefore, salutary effects of mES Ex on CPC survival and proliferation in infarcted hearts effectively translates into significant modulation of CPC function in vivo suggesting mES Ex as a novel regimen for enhancing CPC function and survival.
Figure 6. mES derived exosome pretreatment of CPCs enhance their potential to augment function and reduce fibrosis.
A) Representation of experimental design showing mES Ex-CPCs, MEF Ex-CPCs and PBS administration in mice after infarction. Animals were followed till 8 weeks and received injections of BrdU prior to sac. Increased cardiac function EF B) and FS C) in mES Ex-CPC hearts compared to MEF Ex-CPC and PBS transplanted hearts 8 weeks after infarction. PBS vs. mES Ex-CPC *p < 0.05, **p < 0.01, ***p < 0.001 and MEF Ex-CPC vs mES Ex-CPC #p < 0.05, ##p < 0.01, ###p < 0.001. D–G) Decreased infarct size in mES Ex-CPC hearts compared to MEF Ex-CPC and PBS transplanted hearts 8 weeks after injury. PBS vs. mES Ex-CPC *p < 0.05, **p < 0.01, ***p < 0.001 and MEF Ex-CPC vs mES Ex-CPC #p < 0.05, ##p < 0.01, ###p < 0.001.
Figure 7. Enhanced persistence and ability to form myocytes of mES derived exosomes pretreated CPCs.
A–B) Colocalization of GFP with CPC marker c-kit in the heart transplanted with mES Ex and MEF Ex pretreated cells. c-kit (red), GFP (green), sarcomeric actin (blue) and nuclei (white). Scale bar=20μm. C–D) Increased GFP+/pH3+ cells in mES Ex-CPC hearts 5 days after infarction along with corresponding quantification E). GFP (green), pH3 (red), sarcomeric actin (blue) and nuclei (white). Scale bar=20μm. mES Ex-CPC vs. MEF Ex-CPC *p < 0.05, **p < 0.01, ***p < 0.001. F) Small GFP+ myocytes in MEF Ex-CPC hearts after 8 weeks of cell delivery. Persistence of mES Ex pretreated GFP+ myocytes G) along with GFP+ myocyte formation H) in hearts 8 weeks after injury corroborating with the augmented cardiac function. I) Quantification of GFP+ sarcomeric actin+ cells in the hearts transplanted with mES Ex-CPC and MEF Ex-CPC 8 weeks after infarction. GFP (green), sarcomeric actin (red) and nuclei (blue). Scale bar=40μm. Panel E Scale bar=20μm.
mES exosomes are highly enriched for ES cell-specific miRNAs
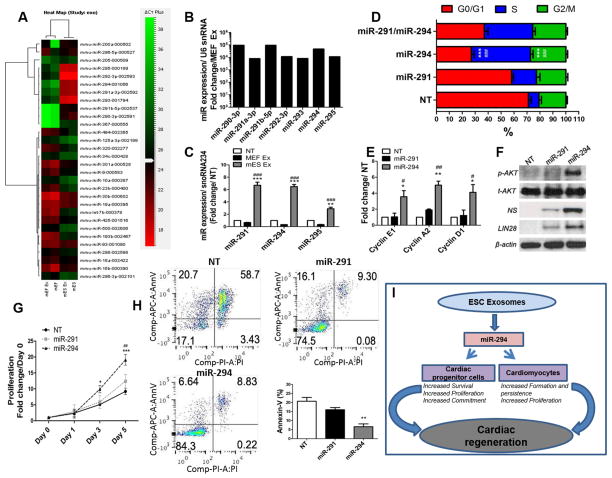
Exosomes carry cell specific proteins or mRNA/miRNA that mediate the functional effect of exosomes 22. Global miRNA profiling of mES Ex and MEF Ex demonstrated 59 miRs upregulated (>2fold) in mES Ex compared to MEF Ex while 169 showed no change (Figure 8A and Online Figure VIIIA). However, members of the ES-specific miR-290 family including miR-291, miR-294 and miR-295 demonstrated >104 fold expression in mES Ex compared to MEF exosomes (Figure 8B) confirming ESC specific origin of mES exosomes and the ability to carry ESC miRs. Previously it has been shown that miR-290 family is exclusively expressed in ESCs and forms 70% of the known miRNAs produced by ESCs 27, 28. Furthermore, members of the miR-290 family are involved in the maintenance of the unique ESC cell cycle regulating G1/S transition 29. Therefore it was hypothesized that mES Ex enriched with members of the miR-290 cluster deliver these miRs to target cells. Indeed, de novo expression of miR-291 and miR-294 was detected in mES Ex hearts while no expression of these miRs was detected in saline treated animals 5 days after infarction (Online Figure VIIIB). Concurrently, elevated levels of miR-291 (6.7 fold), miR-294 (6.4 fold) and miR-295 (2.8 fold) were detected in CPCs treated with mES Ex compared to MEF Ex treated CPCs (Figure 8C). This data demonstrates that mES exosomes are highly enriched for miR-290 family including miR-291, miR-294 and miR-295 and efficiently deliver these miRs to target cells.
Figure 8. miRNA profiling of mES derived exosomes.
A) Comparative analysis of miRs in mES Ex, MEF Ex along with mES cells and MEF cells identified significantly high expression of miRNA-290 family in mES cells and mES Ex (>104 fold) B) compared to MEF cells and MEF Ex groups. C) CPCs treated with mES Ex have enhanced expression of miR-291, miR-294 and miR-295 as confirmed by miRNA qRT-PCR (n=3). Expression of miRs was normalized to snoRNA234. NT vs. mES Ex *p < 0.05, **p < 0.01, ***p < 0.001 and MEF Ex vs mES Ex #p < 0.05, ##p < 0.01, ###p < 0.001. D) Increased number of CPCs in G2-phase of the cells cycle after treatment with miR-294 (25nM) compared to miR-291 (25nM) and miR291 (25nM)/miR294 (25nM) and non-treated control cells as analyzed by FACS based cell cycle assay (n=4). E) Enhanced mRNA expression of cyclins (E1, A2 and D1) in CPCs treated with miR-294 (25nM) compared to miR-291 (25nM) and non-treated controls (n=3). F) Increase phosphorylation of AKT in association with elevated levels of nucleostemin and LIN28 in miR-294 (25nM) treated CPCs compared to miR-291 and non-treated controls (n=3). G) Increased CPC proliferation at day 3 and 5 after miR-294 treatment compared to miR-291 and non-treated CPCs as evidenced by CyQuant assay (n=3). NT vs. miR-294 *p < 0.05, **p < 0.01, ***p < 0.001 and miR-291 vs miR-294 #p < 0.05, ##p < 0.01, ###p < 0.001. H) miR-294 treated CPCs showed reduction in Annexin-V+ cells compared to miR-291 and non-treated CPC in response to H2O2 challenge as evidenced by FACS based cell cycle assay (n=3). I) Schematic representation of therapeutic effect of ESC derived exosomes for cardiac repair after myocardial infarction. ESC exosome deliver miR-294 to the heart resulting in significant modulation of survival, proliferation and cardiac commitment of cardiac progenitor cells. At the same time, enhanced cardiomyocyte survival and proliferation take place as a consequence of ESC exosome delivery that ultimately leads significant augmentation of cardiac regeneration in the heart after myocardial infarction.
iR-294 mimics mES exosome effects on CPCs
In order to provide evidence towards a central role played by miR-290 cluster in mediating the effects of mES Ex on CPC function, miR-294 gain of function studies were carried out in CPCs. Recent evidence shows that miR-291-3p, miR-294-3p and miR-295 form the predominantly active core group of the miR-290 cluster 30. CPCs were treated with miRNA mimics for miR-291-5p, miR-294-3p and miR-295-3p in order to characterize the effect on cell cycle progression. A significant shift in the number of CPCs in S-phase of the cell cycle was observed after treatment with miR-290 mimics, however, miR-294-3p treatment enhanced accumulation of CPCs in S-phase (45.6%) together with significant reduction of the G1-phase (27.4%) compared to non-treated CPCs (G1-phase 71.0%, S-phase 8.2%) (Figure 8D). miR-291 treatment also enhanced increased S-phase transition albeit at lower magnitude (S-phase 19.2%; G1 58.0%) in CPCs. Interestingly, treatment with miR-291 and miR-294 mimics together did not lead to an additive effect on S-phase cell number compared to miR-294 alone suggesting a critical role for miR-294 in cell cycle modulation of CPCs. Similarly, mRNA expression of cyclins (E1, A2 and D1) was increased in CPCs treated with miR-294-3p mimic compared to miR-291-5p mimic and non-treated control CPCs (Figure 8E). In parallel, neonatal rat cardiomyocyte (NRCM) treated with miR-mimic for miR-294-3p showed a similar increase in mRNA levels of proliferative markers (Cyclin E1, Cyclin A2 and Cdk2, Online Figure VIII C) compared to miR-291-5p and non-treated NRCMs.
Next, underlying molecular signaling was assessed after miR- mimic treatment in CPCs. AKT phosphorylation was increased in CPCs treated with miR-294-3p mimic concomitant with elevated expression of nucleostemin, a marker for multi-potency for CPCs 31 and LIN28, a miR-binding protein that has been shown to be involved in regulating pluripotency by miR-294 32 compared to miR-291-5p mimic and non-treated controls (Figure 8F). Additionally, mRNA expression of c-myc and Klf4 were increased in miR-294-3p treated CPCs compared to non-treated cells (Online Figure VIII D). A significant increase in proliferation and survival was also evident in miR-294-3p mimic treated CPCs after H2O2 stress (Figure 8G and H respectively). Therefore, miR-294 plays a central role in regulating CPC cell cycle in association with promoting proliferation, survival and largely mimics the effect of mES Ex.
DISCUSSION
Discovery of cell-free components such as exosomes 16 capable of instigating cell analogous response in target cells may provide a promising alternative for cardiac regeneration and allow utilization of benefits associated with adoptive stem cell therapies. Recent reports suggest that exosomes derived from various stem cells enhance myocardial viability and prevent adverse remodeling of the pathological heart due to reduction in oxidative stress and AKT activation in a myocardial infarction model 17. Similarly, exosomes secreted by cardiac progenitor cells were reported to stimulate migration of endothelial cells 18 and protect ischemic myocardium from ischemia/reperfusion injury 19 validating that exosome derived from stem cells recapitulate cardiac regeneration representative of adoptively transferred stem cells. However, mechanism of exosome mediated cardiac protection remains unclear as either exosomes utilized in these studies were characteristic of stem cells with paracrine abilities or unable to activate endogenous repair processes in the heart after injury.
However, all stem cell derived exosomes are not created equal. Since exosomes largely pack small RNAs and protein representative of parent stem cell phenotype, the choice of stem cells becomes critical. Embryonic stem cells with their unique microRNA and protein content as well as signature cell cycle activity represent an attractive source of exosomes for augmentation of endogenous cardiomyocyte/CPC proliferative and survival/differentiation responses after myocardial injury. The present study demonstrates mouse embryonic stem cell (ESC) derived exosomes (mES) augment post-MI physiological and anatomical myocardial repair in cell-autonomous manner that strongly suggests cardiac therapeutic potential of mES-Ex in augmenting endogenous repair mechanisms. Importantly, data presented in this manuscript suggests that our findings can be easily translated to autologous induced pluripotent stem cells (iPS) cells thereby paving way for iPS-exosomes for potential clinical trials. Thus, proposed studies represent a novel cell free system that recapitulates ESC regenerative power for cardiac repair and circumvents concerns and limitations associated with direct cell administration.
Evidence from literature suggests that cardiomyocytes are capable of limited cell division while cardiac progenitor cells (CPCs) regulate cardiac homeostasis forming a critical axis for endogenous myocardial repair. Recently, however, the relative contribution of the endogenous c-kit+ CPCs to cardiomyogenesis has come into question 33. Despite the low occurrence of cardiomyocytes originating from endogenous CPCs observed in the above report using lineage tracing technology, existence of c-kit+ CPCs in the heart together with their ability to form cardiomyocytes, albeit few, is remarkably clear. Ideal strategies for cardiac repair would bank on not only increasing CPC function but promote cardiomyocyte replenishment in failing hearts. Indeed, our results point towards significant activation of cardiomyocyte and CPC based repair and regenerative programs in heart receiving mES-Ex. Importantly, our data provides evidence that CPCs when pre-treated with mES Ex before transplantation to ischemic myocardium survive for long-term (up to 8 weeks of experimental window) and supports the possibility for high engraftment and de novo cardiomyocyte differentiation. Thus, our findings may represent a novel strategy to enhance CPC contribution to cardiomyogenesis.
The inherent plasticity of embryonic stem cells (ESC) is argued to be an advantage for their potential application in regenerative medicine. ESCs have been used in animal studies of cardiac repair 12, 15 and transplantation of human ES-derived cardiomyocyte in primate models has recently been associated with arrythmogenic response despite myocardial regeneration13. Moreover, ethical, technical and regulatory issues as well as unavailability of autologous human ESC for cell therapy applications limit the potential therapeutic utility of ESC in humans. The remarkable discovery by Yamanaka and colleagues 34 towards the derivation of induced pluripotent cells (iPS) has solved the issue of availability of autologous pluripotent cells and despite rapid research on iPS-derived cardiac lineage cell, these cells also present some of the same burden that is associated with ES cells. Although iPS-derived cardiac cells provide a fantastic tool for disease modeling and drug screening, further work needs to be done towards generating and extensively characterizing “clinical grade” iPS cells before human cell replacement therapies can be attempted 35. Beyond these concerns, ES/iPS derivative cells, when used as cell replacement therapy, may still suffer the same difficulties in cell retention and survival in ischemic myocardium as is noted for adult stem cells. Thus, there is a critical need for exploiting the powerful regenerative capacity of pluripotent cells while avoiding the problems associated with cell transplantation and exosomes derived from pluripotent cells may provide such therapeutic tool.
The underlying molecular basis for cardioprotection observed by exosome in published studies remains unclear although it appears that exosomes directly communicate with the target cells and deliver the specific microRNAs, proteins and other small RNAs representative of their parental cell of origin 22, 36. Therefore, we postulated that ESC specific miRs involved in regulation of pluripotency, proliferation and the distinctive ESC cell cycle are consigned within exosomes derived from ESCs and are delivered to target cells. Indeed, analysis of miR expression in ES exosome revealed very high expression of ES-specific miRs especially that of miR-290 family. Elevated levels of miR-291, miR-294 and miR-295 were observed in the heart and CPCs after treatment with mES Ex suggested not only mES exosome as their source (these miRs are not expressed in adult cells or organs) but also a possible role for members of the miR-290 family in mediating the effect of mES Ex. This miRNA family comprises 14 miRNA (290–295) 30, bear a common seed sequence (AAAGUGC), are functionally dominant miRNAs in ES cells and comprise of approximately 70% of all ES miR contents. In particular, miR-291, miR-294 and miR-295 encoded in the 290 cluster are expressed exclusively during early development and ES cells and regulate ES cell cycle and self-renewal 37 with corresponding effect on proliferation and differentiation 29, 38. Indeed, overexpression of miR-294 mimics both in CPCs in vitro, recapitulated some of the similar effects as were observed by exosome treatment suggesting a direct role of ES-specific miRs in the augmentation of post-MI cardiac repair. These results are in concordance with studies that document the multifaceted role played by miR-294 in modulating cellular reprogramming 39, proliferation 37 and survival 40. In contrast, inhibition of miR-294 is an important aspect of the study and would have enabled us to compare the effect of miR-294 enriched exosomes to miR-294 alone. However, since miR-294 critically regulates various embryonic stem cell (ESCs) characteristics including pluripotency, cell cycle and proliferation as described above, altering miR294 levels leads to a complete loss in cellular properties including their survival and proliferation and in turn changing both the yield and characteristics of exosomes.
In sum, the beneficial effect of mES Ex in the heart after injury in our study suggests that cardiomyocyte survival and cell cycle entry, enhanced neovascularization and potentiation of CPC expansion, differentiation and survival is mediated by miR-294 delivered via ESC exosomes to the heart (Figure 8I). Recent studies conform to these findings and demonstrate efficiency of cardiac repair after restoration of endogenous repair processes by ex vivo delivery of therapeutic agents 7, 8. Furthermore, enhanced neovascularization by mES Ex maybe caused by increased activation and cycling of endothelial cells in the heart. Synergistic CPC adoptive transfer combined with exosome delivery or engineering of CPC with ES specific microRNAs may provide for a potential powerful therapeutic regimen preserving adoptively transferred cells and at the same time revitalizing endogenous myocardial repair processes.
Supplementary Material
Novelty and Significance.
What Is Known?
Embryonic stem cells are a promising source of cardiac myocytes, yet their use remain controversial due to ethical concerns and methodological limitations.
Stem cell derived exosomes are able to recapitulate regenerative potential of parent cells of their origin.
What New Information Does This Article Contribute?
Mouse embryonic stem cells exosomes (mES Ex) modulate cellular processes in target cells
mES Ex augment cardiac function after myocardial infarction
Hearts receiving mES Ex show enhanced activation and contribution of cardiac progenitor cells towards cardiac repair.
Salutary effects of mES Ex are mediated by the transfer of miR-294 to the heart and CPC promoting their survival and proliferation
ESCs possess the ability to form functional cardiomyocytes, but their use remains controversial. Recent identification of small vesicles called exosomes in the stem cell secretome carries significant implications for cardiac regeneration. ESC have the ability to secrete exosomes yet their role in cardiac repair is not well defined. Here, we report that mouse ESC derived exosomes (mES Ex) have the ability to modulate molecular signaling, survival and tube formation in target cells. Delivery of mES Ex in the heart after myocardial infarction leads to significant augmentation of cardiac function in conjunction with enhanced cardiac proliferative response. Moreover, mES Ex promote CPC survival, proliferation, persistence and contribution towards repair processes in the heart after myocardial infarction. The beneficial effects of mES Ex are mediated by miR-294 transfer to the heart and CPCs promoting survival and proliferation. Cardiac repair potential of mES Ex represents a novel cell free strategy that harnesses the regenerative power of ESC. Understanding the mechanisms underlying the stem cell exosome mediated cardiac repair and regeneration may be beneficial in developing an alternate cell free strategy for the treatment of cardiac diseases.
Acknowledgments
We thank all members of the Kishore lab for their valuable discussions and Dr Muniswamy Madesh for their help with confocal microscopy.
SOURCES OF FUNDING
This work was supported in part by funding from the National Institute of Health grants HL091983, HL105597, HL095874, HL053354, HL108795 and HL108806. Mohsin Khan is supported by American Heart Association Scientific development grant 15SDG22680018.
Nonstandard Abbreviations and Acronyms
- mES Ex
Mouse embryonic stem cell derived exosomes
- MEF Ex
Mouse embryonic fibroblast derived exosomes
- CPCs
Cardiac progenitor cells
- GFP
Green fluorescent protein
- TUNEL
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
- mES Ex-CPC
Mouse embryonic stem cell derived exosome pretreated cardiac progenitor cells
- MEF Ex-CPC
Mouse embryonic fibroblast derived exosome pretreated cardiac progenitor cells
- HUVEC
Human umbilical vein endothelial cells
- NRCM
Neonatal rat cardiomyocytes
- EF
Ejection fraction
- FS
Fractional shortening
- ESD
End-systolic diameter
Footnotes
DISCLOSURES
None.
References
- 1.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 2.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 4.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohsin S, Khan M, Toko H, et al. Human cardiac progenitor cells engineered with pim-i kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 10.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 11.Mayhall EA, Paffett-Lugassy N, Zon LI. The clinical potential of stem cells. Curr Opin Cell Biol. 2004;16:713–720. doi: 10.1016/j.ceb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 13.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blin G, Nury D, Stefanovic S, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 16.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 17.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase atp levels, decrease oxidative stress and activate pi3k/akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Vrijsen KR, Sluijter JP, Schuchardt MW, van Balkom BW, Noort WA, Chamuleau SA, Doevendans PA. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14:1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431:566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human cd34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S, Misener S, Kishore R, Losordo DW. Sonic hedgehog-modified human cd34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111:312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma SK, Krishnamurthy P, Barefield D, Singh N, Gupta R, Lambers E, Thal M, Mackie A, Hoxha E, Ramirez V, Qin G, Sadayappan S, Ghosh AK, Kishore R. Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-kappab. Circulation. 2012;126:418–429. doi: 10.1161/CIRCULATIONAHA.112.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thal MA, Krishnamurthy P, Mackie AR, Hoxha E, Lambers E, Verma S, Ramirez V, Qin G, Losordo DW, Kishore R. Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair. Circ Res. 2012;111:180–190. doi: 10.1161/CIRCRESAHA.112.270462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishore R, Verma SK, Mackie AR, Vaughan EE, Abramova TV, Aiko I, Krishnamurthy P. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac mirna-155 modulates fibrotic response in diabetic hearts. PLoS One. 2013;8:e60161. doi: 10.1371/journal.pone.0060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoenfeld M, Frishman WH, Leri A, Kajstura J, Anversa P. The existence of myocardial repair: Mechanistic insights and enhancements. Cardiol Rev. 2013;21:111–120. doi: 10.1097/CRD.0b013e318289d7a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marson A, Levine SS, Cole MF, et al. Connecting microrna genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific micrornas. Dev Cell. 2003;5:351–358. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific micrornas regulate the g1-s transition and promote rapid proliferation. Nature genetics. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landgraf P, Rusu M, Sheridan R, et al. A mammalian microrna expression atlas based on small rna library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiqi S, Gude N, Hosoda T, et al. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ Res. 2008;103:89–97. doi: 10.1161/CIRCRESAHA.107.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanina SA, Mifsud W, Down TA, Hayashi K, O’Carroll D, Lao K, Miska EA, Surani MA. Genome-wide identification of targets and function of individual micrornas in mouse embryonic stem cells. PLoS Genet. 2010;6:e1001163. doi: 10.1371/journal.pgen.1001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Hoxha E, Kishore R. Induced pluripotent cells in cardiovascular biology: Epigenetics, promises, and challenges. Prog Mol Biol Transl Sci. 2012;111:27–49. doi: 10.1016/B978-0-12-398459-3.00002-2. [DOI] [PubMed] [Google Scholar]
- 36.Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, Farber DB. Transfer of micrornas by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichner Z, Pall E, Kerekes A, Pallinger E, Maraghechi P, Bosze Z, Gocza E. The mir-290–295 cluster promotes pluripotency maintenance by regulating cell cycle phase distribution in mouse embryonic stem cells. Differentiation. 2011;81:11–24. doi: 10.1016/j.diff.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Zovoilis A, Smorag L, Pantazi A, Engel W. Members of the mir-290 cluster modulate in vitro differentiation of mouse embryonic stem cells. Differentiation. 2009;78:69–78. doi: 10.1016/j.diff.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific micrornas promote induced pluripotency. Nat Biotechnol. 2009;27:459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng GX, Ravi A, Calabrese JM, Medeiros LA, Kirak O, Dennis LM, Jaenisch R, Burge CB, Sharp PA. A latent pro-survival function for the mir-290–295 cluster in mouse embryonic stem cells. PLoS Genet. 2011;7:e1002054. doi: 10.1371/journal.pgen.1002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.