Abstract
Background. The pathophysiology of female genital schistosomiasis (FGS) is only partially understood. This study aims to describe the histopathological findings, polymerase chain reaction (PCR) results, and gynecological manifestations of FGS in women with different intensities of Schistosoma haematobium infection.
Methods. Women aged 15–35 years living in an S. haematobium-endemic area in Madagascar underwent pelvic and colposcopic examinations. Small biopsy specimens were obtained from lesions and examined histopathologically. Schistosoma PCR was done on urine, biopsy, cervicovaginal lavage, and genital mucosal surface specimens.
Results. Sandy patches and rubbery papules were found in 41 of 118 women (35%). Rubbery papules reflected an intense cellular immune reaction dominated by eosinophils, epithelial erosion, and viable ova. There was a significant decrease in the prevalence of rubbery papules with age, even after adjustment for urinary ova excretion. The sandy patches with grains showed moderate cellular immune reaction and ova (viable and/or calcified). They were most prevalent in cases with low-intensity urinary S. haematobium infection. Forty-two percent of women with Schistosoma-negative urine specimens had at least 1 genital specimen test positive for Schistosoma by PCR.
Conclusions. The results indicate a diversity of lesions caused by S. haematobium and a dynamic evolution of the genital lesions. Schistosoma PCR may give an indication of the diagnosis.
Keywords: Schistosoma haematobium, female genital schistosomiasis (FGS), histopathology, polymerase chain reaction (PCR), Madagascar, reproductive health
Schistosoma haematobium may cause pathology in the urinary and genital tracts. In the urinary tract, morbidity is correlated with intensity of infection, as indicated by the number of eggs excreted in the urine [1]. Up to 75% of women excreting S. haematobium ova in the urine may have ova in the lower genital tract.
However, female genital schistosomiasis (FGS) may also occur in the absence of urinary egg excretion [2, 3]. FGS is rarely seen without use of a colposcope and is often overlooked even by those who have this tool. In remote areas, where most patients live, the cost of the equipment, the logistical difficulties associated with light sources, electricity, and clean instruments, as well as the invasive nature of the biopsy and gynecological examination, may result in failure to diagnose FGS. Furthermore, identification of lesions requires several weeks of training and inspection of the entire intravaginal surfaces, including the fornices and the anterior and posterior vaginal walls [3]. The clinical pathology of FGS was described in 1949, but there is still a lack of diagnostic standards, partly because of the ethical limitations in obtaining specimens appropriate for analysis [3, 4]. Therefore, the pathophysiology of FGS is only partially understood [3, 5–7]. Furthermore, neither gynecology nor infectious diseases textbooks contain information about the macroscopic manifestations of the disease.
This study aims to describe the clinical manifestations and histopathological characteristics of genital mucosal schistosomiasis lesions and to study these findings in relation to Schistosoma DNA in genital tissue. The diagnostic potential of specimens collected from the surfaces of the vagina and the cervix were evaluated in women with high- and low-intensity S. haematobium infection and in those without urinary S. haematobium egg excretion.
MATERIALS AND METHODS
Study Area
The study was performed in the district of Miandrivazo in Western Madagascar in 2010. It is an area mainly of subsistence rice farming, with scattered tobacco farming. Five villages were included that had similar socioeconomic conditions and healthcare services. At the time of the study, the national antischistosomal treatment program had not reached the district.
Study Design and Population
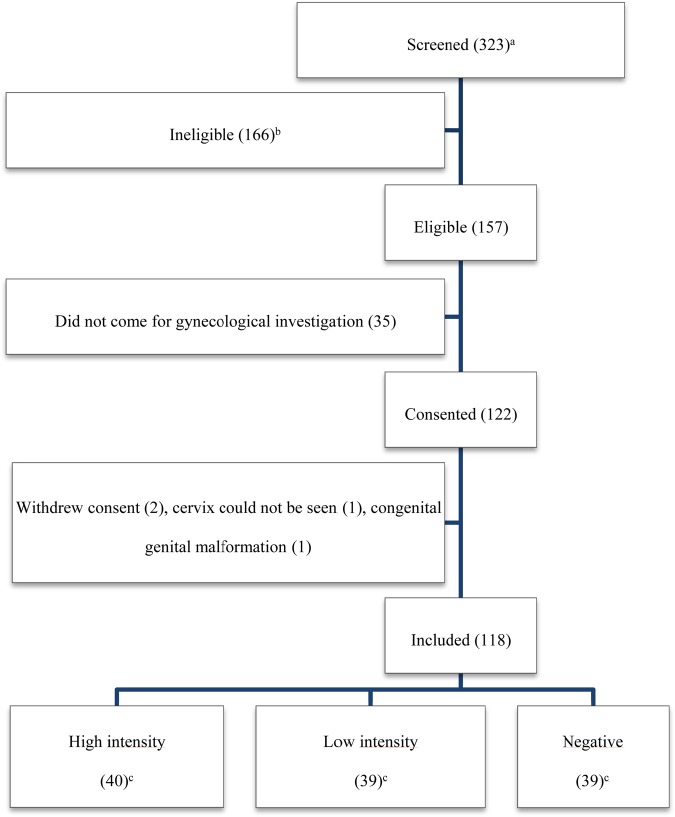
A community-based cross-sectional study was performed among women 15–35 years of age. They had lived in the villages for >5 years and had not been treated for schistosomiasis in the past 2 years. Women who were pregnant or naive to sexual intercourse were excluded. On the basis of microscopy findings in 3 urine specimens, women were divided into the following 3 groups: women who had a mean egg count of >50 ova per 10 mL urine and were from 4 villages where schistosomiasis is highly endemic (hereafter, the high-intensity group); women who had a mean egg count of <20 ova per 10 mL and were from the same villages as those in the high-intensity group (hereafter, the low-intensity group); and microscopy-negative women from a village where schistosomiasis has a low endemicity (hereafter, the microscopy-negative group). Eligible women who consented to the gynecological study were consecutively enrolled by convenience until the planned number of participants was reached (Figure 1).
Figure 1.
Inclusion into gynecological investigations for female genital schistosomiasis. Numbers of subjects are specified in parentheses. aNumber of women who came forth after the information meetings in 5 villages; the total population count is unknown; bA total of 107 women were excluded because the Schistosoma haematobium intensity did not match the study group requirements, 6 were pregnant, 13 had no history of sexual intercourse, 14 did not provide 3 urine specimens, 10 had resided in the area for <5 years, 7 had received treatment in the past 2 years, and/or 4 were outside the age range. A woman may have had several reasons for exclusion; cIntensity was determined by S. haematobium ova excretion in 3 urine specimens. High intensity was defined as >50 ova per 10 mL, and low intensity was defined as <20 ova per 10 mL. The negative women were from a village with low schistosomiasis endemicity.
Medical History, Gynecological Inspection, and Colposcopy
A female physician (F. R.) performed a reproductive health interview in the Malagasy language. Gynecological examination and colposcopy took place in a custom-built mobile clinic. Women menstruating on the day of the examination were asked to come back later. Two female physicians (E. F. K. and B. S. R.) examined the surfaces of the vulva, vagina, and cervix according to a standardized protocol [8, 9]. The findings were discussed until a consensus was reached.
Three FGS-associated lesions are described: (1) grainy sandy patches, defined as areas with distinct single or clusters of oblong grains (approximately 0.05 × 0.2 mm) in the cervicovaginal mucosa [8]; (2) homogenous sandy patches, defined as homogenous foci without detectable grains at 15 times the original magnification [6, 8]; and (3) rubbery papules, previously described in the urinary bladder mucosa only, defined as spheroid, firm, beige, smooth papules (size, 0.3–1.2 mm) in the cervicovaginal mucosa [10]. Malignant-looking lesions constituted friable, ulcerative, and papillomatous surfaces with abnormal blood vessels. Contact bleeding was defined as fresh blood originating from the mucosal surface upon insertion of the speculum.
The exact location of the biopsy was documented by a pointer and a photocolposcope (Olympus OSC 500, with a mounted E420, 10.0 megapixels; Olympus America, Center Valley, Pennsylvania). Biopsy specimens were collected with a small 2.3-mm biopsy forceps (Endo-Flex, Voerde, Germany) and preserved in 5% formaldehyde.
Histopathological Analysis
Each formaldehyde-preserved biopsy specimen was embedded in paraffin. From each biopsy specimen, 10 sections with a thickness of 5 µm were pooled for DNA isolation. The remaining parts were cut into 3.5-µm thick sections until no more tissue remained. Sections were stained with hematoxylin and eosin for histopathological examination. Only biopsy specimens that contained both epithelium and stroma were included because S. haematobium ova are rarely found in the epithelium [11].
Diagnosis of Possible Confounding Bacterial and Viral Infection
Samples were collected by a swab (Micro Rheologics, Brescia, Italy), placed in a tube (Genelock; Sierra Molecular, Sonora, California), frozen, and tested for Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, Trichomonas vaginalis (by an in-house test; Statens Serum Institut (SSI), Copenhagen, Denmark), and Schistosoma DNA by polymerase chain reaction (PCR) analysis. Ulcers were swabbed for PCR analysis of herpes simplex virus type 2 and Haemophilus ducreyi (SSI). One swab specimen (obtained and transported using the Copan Universal Transport Medium kit; Copan Diagnostics, Murrieta, California) was collected for detection of human papillomavirus [12], and 1 swab specimen was placed in 0.5 mL of sterile normal saline for microscopic examination for bacterial vaginosis by the Amsel criteria [8]. Serum specimens were tested by a rapid plasma reagin test (RPR 500; Newmarket Laboratories, Kentford, United Kingdom) and a Treponema pallidum hemagglutination assay (ELITech Group, Puteaux, France). Urine specimens were tested for N. gonorrhoeae (SSI), C. trachomatis (SSI), M. genitalium (SSI), and T. vaginalis (SSI) by PCR.
Urine Filtration, Papanicolaou (Pap) Smears, and Schistosoma PCR
Three consecutive urine samples were examined by microscopy before women were invited to participate in the gynecological investigation. For PCR analysis, a fourth urine sample was collected on the day of the gynecological investigation. Cervicovaginal lavage was performed by spraying 10 mL of 0.9% NaCl on the cervical and inner half of the vaginal surfaces, pulling it back into the syringe, and spraying again; the process was performed 4 times. A mucosal sterile swab was applied over the cervix and vaginal walls and rinsed out in 1 mL of 0.9% NaCl. After the ordinary deposition onto the Papanicolaou smear slide, the endocervical brush was rinsed out in 1.8 mL of 0.9% NaCl. A cytobrush was applied to lesions. If there was no lesion, the brush was moved over the cervix and vaginal walls. Each specimen was collected in a preallocated site and sequence.
The Schistosoma genus-specific PCR was performed with some minor modifications [13]. In brief, following a heating step and treatment with sodium dodecyl sulphate and proteinase K, DNA was extracted from 200-µL samples, using QIAmp spin columns (Qiagen Benelux; Venlo, the Netherlands). Phocin herpesvirus 1 was added to the lysis buffer as an internal control. The internal transcribed spacer 2–based real-time PCR was used [13]. DNA isolation and set up of the PCR were performed with a custom-made automated liquid handling station (Hamilton, Switzerland). Amplification, detection, and analysis were performed with the CFX real-time detection system (Bio-Rad laboratories). Negative and positive control samples were included in each amplification run. The Schistosoma PCR was classified as strongly positive (threshold cycle [Ct], < 30), moderately positive (30 ≤ Ct < 35), or weakly positive (35 ≤ Ct < 50) [14]. All laboratories were blind to subject characteristics.
Ethical Considerations
Ethical clearance was granted by the Committee of Ethics at the Ministry of Health in Madagascar (number 031-CE/MINSAN 4 June 2010). Human experimentation guidelines of the US Department of Health and Human Services and/or those of the authors’ institutions were followed in the conduct of the research. To limit the risk of iatrogenic infection, the study was conducted in an area where human immunodeficiency virus had not been found among 1341 tested individuals (Monthly Report, DHS, Miandrivazo, 2008–2009). Furthermore, for the same reason, biopsy specimens were only taken from persons with FGS-associated lesions or suspicion of malignancy. A biopsy was only to be performed if it was felt that the participating woman would be able to abstain from sex or would consistently use condoms for 2 weeks following biopsy. Only one woman was excluded at this point of the investigation. Information about the study was provided to potential participants in Malagasy, and consent was received from participants in Malagasy. A female physician (P. R.) explained the purpose of the study, the procedures, the anticipated benefits and possible negative consequences of participation, matters of privacy and confidentiality, and the possibility to withdraw at any point. Participants were allowed to ask questions at any time. Illiterate women provided written consent by placing a fingerprint on the consent form, and literate women signed the consent form. The women were asked confirmative questions at every step until completion of the examination and treatment. A copy of the consent form was handed out to each study participant. After the examination, women who had undergone biopsy were offered condoms.
The patients were informed of the findings, effects, and possible side effects of treatment with praziquantel. Women with egg excretion in urine or with FGS were offered a single dose of praziquantel (40 mg/kg body weight). Sexually transmitted infections (STIs) were treated in accordance with the Malagasy syndromic approach. Those who withdrew from the study, sex partners of participants, and other community members received the same possibility of STI treatment with praziquantel, and referrals in a neighboring location. All documentation was coded. The code list linking personal identities and results was stored securely in the Institut Pasteur de Madagascar.
Statistical Analysis
It was hypothesized that 40% of females in the low-intensity group would have FGS, while <2% of women in the microscopy-negative group would have FGS [3]. Assuming a 95% confidence interval (CI) and 80% power, a sample size of 22 women with low-intensity infection and 22 with microscopy-negative findings would be needed to demonstrate a statistical difference between the 2 groups. Likewise, it was found that 22 women with high-intensity infection were needed to compare clinical manifestations in low- and high-intensity groups. The sample size was increased to 40 women in each group to compensate for uncertainties and withdrawals.
The Statistical Package for the Social Sciences, version 16.0 (IBM, New York, New York), and EpiInfo, version 3.2.2 (Center for Disease Control and Prevention, Atlanta, Georgia), were used for statistical analysis. χ2 tests the Fisher exact tests were performed, and odds ratios (ORs), 95% CIs, and likelihood ratios for trends were calculated. Logistic regression analysis was used for variables with a P value of <.25 in the bivariate analysis.
RESULTS
Of the 323 women screened, 118 were eligible and consented to the gynecological investigations (Figure 1). The median overall age was 20.5 years (interquartile range [IQR], 18–25 years), 19 years (IQR, 17–23 years) in the high-intensity group, 22 years (IQR, 19–26 years) in the low-intensity group, and 23 years (IQR, 19–29 years) in the microscopy-negative group. The median egg counts in groups 1 and 2 were 266 ova (IQR, 110–796 ova) and 4 ova (IQR, 1–9 ova), respectively. Biopsies of 31 lesions were performed using a 2.3-mm punch. Only 19 of these biopsy specimens contained stroma and could be evaluated histopathologically.
Macroscopic and Colposcopic Findings
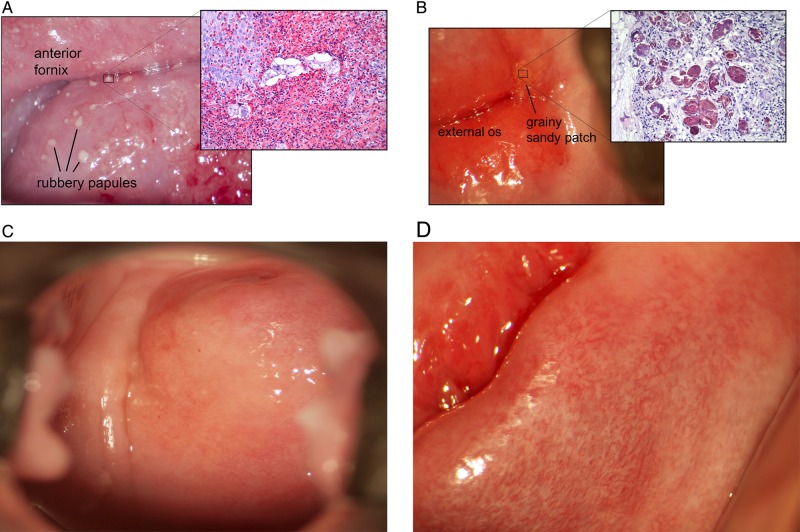
FGS was diagnosed in 41 women (35%) on the basis of finding 48 lesions (Table 1). These were rubbery papules (Figure 2A), sandy patches appearing as single or clustered grains (Figure 2B), and sandy patches appearing as homogenous yellow sandy areas (Figure 2C). Rubbery papules and contact bleeding were only found in women with eggs excreted in urine, with frequencies of 15 of 79 (19%) and 16 of 79 (20%), respectively. Rubbery papules decreased in prevalence as the intensity of schistosomiasis decreased (Table 1). Sandy patches with grains, however, were most prevalent in the low-intensity group. Twenty-nine women (25%) were found to have abnormal mucosal blood vessels (Figure 2D). One woman had a malignant-looking lesion, which was confluent with rubbery papules (not shown). There was no overall association between the intensity of urinary S. haematobium infection (as measured by egg excretion in urine) and colposcopic findings (data not shown). There was a significant decrease in the prevalence of rubbery papules with increasing age, even after adjustment for urinary ova excretion (adjusted OR, 0.85; 95% CI, .74–.99; P = .037). Similarly, the prevalence of sandy patches presenting as grains increased with increasing age, although this was not significant (adjusted OR, 1.07; 95% CI, .98–1.17; P = .13). The prevalence of homogenous sandy patches was not associated with age (P = .82).
Table 1.
Colposcopy Findings in 118 Study Participants, by Intensity of Urinary Schistosomiasis
| Colposcopya | High-Intensity Urinary S. haematobium Infection, no. (%) (n = 40)b | Low-Intensity Urinary S. haematobium Infection, no. (%) (n = 39)b | No S. haematobium Egg Excretion, no. (%) (n = 39)b | Overall Colposcopy Results, no. (%) (n = 118)b | Likelihood Ratio P value |
|---|---|---|---|---|---|
| Rubbery papulesc | 9 (23) | 6 (15) | 0 (0) | 15 (13) | .001 |
| Sandy patches constituting grainsd | 7 (18) | 11 (28) | 3 (8) | 21 (18) | .05 |
| Sandy patches appearing homogenous, yellow arease | 2 (5) | 3 (8) | 7 (18) | 12 (10) | .15 |
| Contact bleedingf | 7 (18) | 9 (23) | 0 (0) | 16 (14) | .001 |
| Abnormal mucosal blood vesselsg | 11 (28) | 9 (23) | 9 (23) | 29 (25) | .87 |
| Papillomatous tumorh | 4 (10) | 1 (3) | 2 (5) | 7 (6) | .36 |
| Leukoplakiai | 2 (5) | 0 (0) | 3 (8) | 5 (4) | .11 |
| Polypj | 2 (5) | 0 (0) | 2 (5) | 4 (3) | .19 |
| Other tumork | 1 (3) | 1 (3) | 1 (3) | 3 (3) | 1.00 |
| Ulcerl | 0 (0) | 0 (0) | 1 (3) | 1 (1) | .33 |
| Malignant-looking lesionm | 1 (3) | 0 (0) | 0 (0) | 1 (1) | .33 |
a One patient may have several types of lesions
b Intensity was determined on the basis of Schistosoma haematobium ova excretion in 3 urine specimens. High intensity was defined as >50 ova per 10 mL, and low intensity was defined as <20 ova per 10 mL. Individuals with no eggs detected were from a village of low endemicity. See “Materials and Methods” section for additional details.
c Smooth, spheroid, pustuloid, and firm beige papules of different size in the cervicovaginal epithelium.
d Distinct, oblong grains approximately 0.05 by 0.2 mm in the cervicovaginal epithelium.
e Sandy-looking areas without distinct grains in the cervicovaginal epithelium.
f Bleeding from the cervical epithelium at just slight touch of the surface during examination.
g Convoluted (cork screw), reticular, circular and/or branched, unevenly calibered blood vessels in the cervicovaginal mucosa.
h Sessile mass in the mucosa or vulva; whitish in color and often with a cauliflower-like appearance.
i Elevated white plaque on the mucosal surface, visible with or without acetic acid.
j Smooth, pedunculated mass originating from the cervicovaginal mucosal surface.
k Tumor not included in any of the other definitions.
l Demarcated area with loss of the epithelial layer; deep or superficial.
m Friable, ulcerative, papillomatous surface and abnormal blood vessels.
Figure 2.
Manifestations of female genital schistosomiasis on the cervix uteri. A, Rubbery papules are smooth, firm, like a rubber ball, they constitute viable ova and a rich eosinophilic reaction. B, Grainy sandy patches constitute calcified and occasional viable ova. C, Homogenous sandy patch. D, Abnormal blood vessels.
Histopathological Findings
Table 2 shows the results of the histopathological investigations. Histopathological examination of the rubbery papules (Figure 2A) showed S. haematobium ova surrounded in most cases by a significant eosinophil response and epithelial erosion (Splendore-Hoeppli phenomenon [15]). Eleven of the rubbery papules had stroma adequate for histological evaluation. These contained a median count of 8 Schistosoma ova per high-power field (HPF; IQR, 2–12 ova/HPF), and there were viable eggs in 9 of 11 tissue samples. Sandy patches appearing as single or clustered grains were characterized by a more moderate immune reaction (Figure 2B). Biopsy specimens revealed a median of 11 ova/HPF (IQR, 2–48 ova/HPF). The malignant-looking lesion contained viable and nonviable S. haematobium only; no neoplastic cells were detected. Neither dysplasia nor atypia were seen in the biopsy specimens. Other lesion types were not biopsied.
Table 2.
Histological Results for 118 Study Participants
| Gynecological Findinga (No. of Biopsy Specimensb) | Ova Viability, No.c | Ova Density, Median (Range)d | Tissue Eosinophiliae | Overall Tissue Immune Response | Epithelial Hyperplasiae | Patient Age, y Median (Range) |
|---|---|---|---|---|---|---|
| Rubbery papules (n = 11) | Viable, 9; calcified, 2; mixed, 0 | 8 (1–14) | Mild, 4; moderate, 3; marked, 4 | Moderate, 3; marked, 8 | Moderate, 9; marked, 2 | 19 (15–25) |
| Sandy patches constituting grains (n = 4) | Viable, 3; calcified, 1; mixed, 1 | 11 (2–80) | None, 1; mild, 2; moderate, 1 | Moderate, 3; marked, 1 | None, 1; mild, 2; moderate, 1 | 23 (15–32) |
The biopsied homogenous sandy patches, patient median age 20 years (range 15–35) did not contain stroma and were not adequate for histopathological examination with regard to schistosomiasis.
a By colposcopy.
b Biopsy specimens with sufficient amount of stroma for examination of schistosome ova.
c Ova were defined as viable if miracidial structures such as eosinophilic glands or germinal cells were identified, whereas ova containing a dark purple stain identified histologically as calcification were defined as calcified.
d Median (range) of ≥1 section per lesion per high-power field.
e Hyperplasia of squamous epithelium in ectocervical biopsy specimens.
PCR Results
Table 3 shows the PCR results related to lesion type. Only rubbery papules and sandy patches were biopsied, but other specimens were PCR positive even in the absence of FGS-associated lesions. Table 4 shows PCR findings in relation to the 3 intensity groups. A greater percentage of women with eggs detected in urine than women with microscopy-negative findings had Schistosoma-positive genital specimens. However, 42% of the microscopy-negative women had at least 1 PCR-positive genital specimen. Urine specimens tested positive by PCR for all women with high-intensity infection. Only 62% of women with low-intensity infection had PCR-positive urine specimens. Thirteen percent of women in the microscopy-negative group had PCR-positive urine specimens.
Table 3.
Polymerase Chain Reaction (PCR) Results for 118 Study Participants, by Specimen Tested
| Colposcopy Findinga | Targeted Lesion Biopsy Specimen | Cervicovaginal Lavage Fluid | Mucosal Swab Specimen | Endocervical Brush Specimen | Lesion Brush Specimen |
|---|---|---|---|---|---|
| Rubbery papulesb (4 also had GSP or HSP) | 11/13 (85) | 15/15 (100) | 15/15 (100) | 10/15 (67) | 13/15 (87) |
| Sandy patches constituting grainsc (5 also had RP and/or HSP) | 7/10 (70) | 10/21 (48) | 5/21 (24) | 5/21 (24) | 4/18 (22) |
| Sandy patches appearing homogenous, yellow areasd (3 also had GSP, and/or RP) | 3/4 (75) | 7/12 (58) | 6/12 (50) | 6/12 (50) | 5/12 (42) |
| Contact bleedinge (14 also had RP, GSP, or HSP) | NA | 11/16 (69) | 9/16 (56) | 7/16 (56) | 10/16 (63) |
| Abnormal mucosal blood vesselsf (22 also had RP, GSP, or HSP) | NA | 15/28 (54) | 11/28 (39) | 8/29 (28) | 12/28 (43) |
| Papillomatous tumorg (2 also had RP, GSP, and /or HSP) | NA | 2/3 (67) | 2/3 (67) | 0/3 (0) | 2/3 (67) |
| Leukoplakiah (2 also had RP, GSP, and/or HSP) | NA | 4/5 (80) | 3/5 (60) | 2/5 (40) | 3/5 (60) |
| Polypi (2 also had RP, GSP, or HSP) | NA | 2/4 (50) | 1/4 (25) | 1/4 (25) | 1/3 (33) |
| Other tumorj (2 also had RP, GSP, and/or HSP) | NA | 2/3 (67) | 2/3 (67) | 2/3 (67) | 3/3 (100) |
| Ulcerk (the patient also had HSP) | NA | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| Malignant-looking lesionl (the patient also had RP and HSP) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) |
Data are no. of participants with specimens testing positive/no. tested (%). PCR results are considered positive regardless of the strength (ie, strong, moderate, or weak) of the positive response (see Table 5).
Abbreviations: GSP, sandy patches constituting grains; HSP, sandy patches appearing homogenous, yellow areas; NA, not applicable; RP, rubbery papules.
a One patient may have several types of lesions.
b Spheroid, pustuloid, and firm beige papules of different size in the cervicovaginal epithelium.
c Distinct, oblong grains approximately 0.05 by 0.2 mm in the cervicovaginal epithelium.
d Sandy-looking areas without distinct grains in the cervicovaginal epithelium.
e Bleeding from the cervical epithelium at just slight touch of the surface during examination.
f Convoluted (cork screw), reticular, circular and/or branched, unevenly calibered blood vessels in the cervicovaginal mucosa.
g Sessile mass in the mucosa or vulva; whitish in color and often with a cauliflower-like appearance.
h Elevated white plaque on the mucosal surface, visible with or without acetic acid.
i Smooth, pedunculated mass originating from the cervicovaginal mucosal surface.
j Tumor not included in any of the other definitions.
k Demarcated area with loss of the epithelial layer; deep or superficial.
l Friable, ulcerative, papillomatous surface and abnormal blood vessels.
Table 4.
Schistosoma Polymerase Chain Reaction Findings in Different Specimens from 118 Study Participants, by Intensity of Urinary Schistosomiasis
| Specimen Type | High-Intensity Urinary S. haematobium Infectiona | Low-Intensity Urinary S. haematobium Infectiona | No S. haematobium Egg Excretiona | All Groups Combined | Likelihood Ratio P value |
|---|---|---|---|---|---|
| Cervicovaginal lavageb | 30/40 (75.0) | 18/39 (46.2) | 7/38 (18.4) | 55/117 (47.0) | <.001 |
| Mucosa swabc | 24/40 (60.0) | 12/39 (30.8) | 8/38 (21.1) | 44/117 (37.6) | .001 |
| Endocervical brushd | 14/39 (35.9) | 5/39 (12.8) | 4/39 (10.3) | 23/117 (19.7) | .009 |
| Lesion brushe | 29/38 (76.8) | 14/35 (40.0) | 4/31 (12.9) | 47/104 (45.2) | <.001 |
| Urine | 40/40 (100) | 24/39 (61.5) | 5/39 (12.8) | 69/118 (58.5) | <.001 |
Data are no. of participants with specimens testing positive/no. tested (%). Denominators within columns vary owing to missing data.
a Intensity was determined on the basis of Schistosoma haematobium ova excretion in 3 urine specimens. High intensity was defined as >50 ova per 10 mL, and low intensity was defined as <20 ova per 10 mL. Individuals with no eggs detected were from a village of low endemicity. See “Materials and Methods” section for additional details.
b A total of 10 mL of 0.9% NaCl was sprayed on the cervical and inner half of the vaginal surfaces, pulled it back into the syringe, and sprayed again; the process was performed 4 times.
c A sterile swab was applied over the cervix and vaginal walls and rinsed in 1 mL of 0.9% NaCl.
d The endocervical brush that had been used for Papanicolaou smear was rinsed in 1.8 mL of 0.9% NaCl following the ordinary deposition unto the Papanicolaou smear slide.
e A cytobrush was applied to lesions suspected to be caused by schistosomiasis. If there was no lesion, the brush was moved over the cervix and the vaginal walls.
All 15 women with rubbery papules had PCR-positive specimens and high levels of Schistosoma DNA. Tables 3 and 5 show that <50% of the lesions with grains tested positive by PCR. Sandy patches appearing as homogenous yellow areas yielded PCR-positive results in 42%–58%. Only 70% of women (20 of 31) who underwent biopsy under the suspicion of genital schistosomiasis had PCR-positive specimens. Only 1 of 14 women with histopathologically verified S. haematobium ova had a PCR-negative specimen. There was no significant association between any of the tested sexually transmitted diseases and the presence of rubbery papules, sandy patches, contact bleeding, or abnormal mucosal blood vessels (data not shown).
Table 5.
Graded Schistosoma Polymerase Chain Reaction (PCR) Results, by Lesion Type
| Lesion Type, Specimen Type | Strongly Positive PCR, No. (%) | Moderately Positive PCR, No. (%) | Weakly Positive PCR, No. (%) | Negative PCR, No. (%) | Likelihood Ratio P value |
|---|---|---|---|---|---|
| Rubbery nodules | |||||
| Cervicovaginal lavagea (n = 15) | 15 (100) | 0 (0) | 0 (0) | 0 (0) | <.001 |
| Mucosal swabb (n = 15) | 11 (74) | 2 (13) | 2 (13) | 0 (0) | <.001 |
| Endocervical brushc (n = 15) | 4 (27) | 4 (27) | 2 (13) | 5 (33) | <.001 |
| Lesion brushd (n = 13) | 12 (92) | 1 (8) | 0 (0) | 0 (0) | <.001 |
| Sandy patches appearing as single or clustered grains | |||||
| Cervicovaginal lavagea (n = 21) | 4 (19) | 0 (0) | 6 (29) | 11 (52) | .08 |
| Mucosal swabb (n = 21) | 4 (19) | 1 (5) | 0 (0) | 16 (76) | .13 |
| Endocervical brushc (n = 21) | 3 (14) | 2 (10) | 0 (0) | 16 (76) | .18 |
| Lesion brushd (n = 18) | 4 (22) | 0 (0) | 0 (0) | 14 (78) | .01 |
| Sandy patches appearing as homogenous yellow areas | |||||
| Cervicovaginal lavagea (n = 12) | 6 (50) | 0 (0) | 1 (8) | 5 (42) | .24 |
| Mucosal swabb (n = 12) | 5 (42) | 1 (8) | 0 (0) | 6 (50) | .13 |
| Endocervical brushc (n = 12) | 2 (17) | 3 (25) | 1 (8) | 6 (50) | .08 |
| Lesion brushd (n = 10) | 5 (50) | 0 (0) | 0 (0) | 5 (50) | .04 |
Schistosoma PCR results were classified as strongly positive (threshold cycle [Ct], < 30), moderately positive (30 ≤ Ct < 35), or weakly positive (35 ≤ Ct < 50).
a A total of 10 mL of 0.9% NaCl was sprayed on the cervical and inner half of the vaginal surfaces, pulled it back into the syringe, and sprayed again; the process was performed 4 times.
b A sterile swab was applied over the cervix and vaginal walls and rinsed in 1 mL of 0.9% NaCl.
c The endocervical brush that had been used for Papanicolaou smear was rinsed in 1.8 mL of 0.9% NaCl following the ordinary deposition unto the Papanicolaou smear slide.
d A cytobrush was applied to lesions suspected to be caused by schistosomiasis. If there was no lesion, the brush was moved over the cervix and the vaginal walls.
DISCUSSION
This population study describes a lesion in the cervicovaginal mucosa that has not been reported to date. We have named it “rubbery papule” because of its firm consistency and spherical and papulous appearance. The rubbery papule constitutes severe eosinophilic inflammation, mostly around viable-looking ova. Women with this lesion tested positive for Schistosoma by PCR, which is in agreement with the presence of viable ova. The prevalence of the rubbery papules was the highest in teenagers. This could suggest recent infection and lesions in an early stage. Sandy patches constituted more-moderate inflammation, and not all patients had PCR-positive findings, as has been reported previously [8]. Genital contact bleeding, rubbery papules, and sandy patches were found significantly more often in patients with urine specimens positive for S. haematobium eggs.
The sandy patches have been described in previous studies on the African continent. To our knowledge, our study in Madagascar is the first to document rubbery papules in the genitalia. Morphologically similar lesions were observed in the bladder by cystoscopy in Egypt in 1946 [10]. Subsequent studies have mentioned “whitish or yellowish, occasional confluent, mucosal dots” found in the genital region of humans and primates [16, p 442]. It is possible that these were rubbery papules. A review of 4000 images by some of the current authors, from previous studies in Malawi, Zimbabwe, and South Africa, showed no such lesions [17]. It is therefore possible that local Malagasy factors might have affected the manifestations of FGS, such as the schistosome strain, the genetic differences between Malagasy and the Africa mainland human populations, or local nutritional factors [18–22].
The coexistence of tissue eosinophilia and viable ova indicate ongoing ova deposition from living worms, whereas the calcified ova and moderate inflammation indicate that ova were deposited some time ago. This pattern concurs with the grading scale for bladder pathologies published by Von Lichtenberg et al in the 1970s [6, 7, 16]. Although good effect of treatment has been reported for urinary bladder pathology, it has been suggested that the sandy patches are established in childhood and are a result of a chronic infection [7, 23–25]. People living in Schistosoma-endemic areas are often exposed from infancy [26]. Women have had worms (and possibly also lesions) for many years by the time they undergo a gynecological investigation. The finding of calcified ova, therefore, could indicate that the sandy patches might be a manifestation of childhood infection and that it may not be easy to treat as has been indicated previously in one report [25].
Women in the high-intensity group had a higher probability of having genital specimens positive for Schistosoma by PCR, rubbery papules, and sandy patches. This might indicate contamination from the urinary tract. However, the specimens were taken by a syringe with an extension tip in the inner third of the vagina. Furthermore, some cases with Schistosoma-urine negative specimens (ie, women with no ova and no Schistosoma DNA in urine) were found to have Schistosoma DNA in genital specimens. This may indicate that the 2 organs need not be affected synchronously. The genital tests were better in diagnosing FGS than PCR or microscopy of urine specimens, although contamination between the organs cannot be precluded. Even so, this study confirms that the genital sampling methods and PCR analysis are not sufficient [27].
Because of ethical concerns, only women with sandy patches, rubbery papules, and/or malignant-looking lesions underwent biopsy. This posed a major limitation to the study and made it impossible to calculate sensitivity and specificity. Likewise, blood vessels were not biopsied for fear of causing severe bleeding. The sample size was limited, and the results indicate that there were no true-negative controls, as is unfortunately the case for most schistosomiasis research [4, 9, 11]. A small biopsy punch was chosen to make targeted biopsies of lesions. However, even when surfaces did not look edematous or inflamed, the genital tissue of these nonpregnant women was unusually soft, and biopsy specimens were difficult to obtain. We had never encountered such soft tissue in the thousands of women examined in our previous work in Zimbabwe, Malawi, and South Africa [8, 9], and hence this small biopsy punch was not useful as we had anticipated.
This study adds rubbery papules to the list of genital lesions associated with S. haematobium infection in females. The findings confirm that live and dead S. haematobium ova may cause pathology in genital tissue [27]. Further, patients with a series of Schistosoma-negative results of urine tests may have Schistosoma DNA in genital specimens. In this population, Schistosoma PCR contributed to the diagnosis of rubbery papules, but it was not strong in the diagnosis of sandy patches and abnormal blood vessels, which may constitute inflammation around dead ova. At the point of care, clinical inspection must be the mainstay for diagnosis, and in research it should be combined with one of the laboratory tests [28]. Further studies are needed to explore the clinical algorithms that may lead to diagnosis at the point of care and diagnostic tools that can objectively identify the lesions. Moreover, it is not known what the different lesions represent in terms of health risks and symptoms. Studies are needed to explore the health effects of the different lesions, including the abnormal mucosal blood vessels. Finally, the optimal timing of anti-schistosomal treatment [29] and the implications for infections by agents such as human immunodeficiency virus and human papillomavirus should be explored.
Notes
Acknowledgments. We thank the study participants, field assistants, and technicians in Madagascar; Drs G. Poggensee and W. E. Secor, for comments and feedback in the preparation of study design, study protocol; C. G. Aurlund, for assistance in the technical assistance with manuscript preparation; the Rigshospitalet, Copenhagen, and Dr J. Skov Jensen, Statens Serum Institut, Copenhagen, for assisting with STI analyses; Dr J. J. Verweij and Mr E. A. T. Brienen, Leiden University Medical Center, for the Schistosoma PCR analysis; Dr M. F. D. Baay, University of Antwerp, for the HPV analyses; and the Institut Pasteur de Madagascar and the Ministry of Health in Madagascar, for implementation and facilitation of the study and data collection.
Disclaimer. There was no involvement by the funders in the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the paper for publication.
Financial support. This work was supported by the University of Copenhagen with the support from the Bill and Melinda Gates Foundation (subgrant OPPGH5344); and the Center for Imported and Tropical Diseases, Oslo University Hospital.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sacko M, Magnussen P, Keita AD, et al. Impact of Schistosoma haematobium infection on urinary tract pathology, nutritional status and anaemia in school-aged children in two different endemic areas of the Niger River Basin, Mali. Acta Trop 2011; 120(suppl 1):S142–50. [DOI] [PubMed] [Google Scholar]
- 2.Renaud G, Devidas A, Develoux M, Lamothe F, Bianchi G. Prevalence of vaginal schistosomiasis caused by Schistosoma haematobium in an endemic village in Niger. Trans R Soc Trop Med Hyg 1989; 83:797. [DOI] [PubMed] [Google Scholar]
- 3.Kjetland EF, Leutscher PD, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol 2012; 28:58–65. [DOI] [PubMed] [Google Scholar]
- 4.Charlewood GP, Shippel S, Renton H. Schistosomiasis in gynaecology. J Obstet Gynaecol Br Emp 1949; 56:367–85. [DOI] [PubMed] [Google Scholar]
- 5.Badawy AH. Schistosomiasis of the cervix. Br Med J 1962; 1:369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Von Lichtenberg F, Edington GM, Nwabuebo I, Taylor JR, Smith JH. Pathologic effects of schistomiasis in Ibadan Western State of Nigeria. II. Pathogenesis of lesions of the bladder and ureters. Am J Trop Med Hyg 1971; 20:244–54. [DOI] [PubMed] [Google Scholar]
- 7.Smith JH, Elwi A, Kamel IA, von Lichtenberg F. A quantitative post mortem analysis of urinary schistosomiasis in Egypt. II. Evolution and epidemiology. Am J Trop Med Hyg 1975; 24:806–22. [DOI] [PubMed] [Google Scholar]
- 8.Kjetland EF, Ndhlovu PD, Mduluza T, et al. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg 2005; 72:311–9. [PubMed] [Google Scholar]
- 9.Kjetland EF, Poggensee G, Helling-Giese G, et al. Female genital schistosomiasis due to Schistosoma haematobium. Clinical and parasitological findings in women in rural Malawi. Acta Trop 1996; 62:239–55. [DOI] [PubMed] [Google Scholar]
- 10.Kirkaldy-Willis WH. Cystoscopy in the diagnosis and treatment of Bilharzia haematobium infection. Br J Surg 1946; 34:189–94. [DOI] [PubMed] [Google Scholar]
- 11.Helling-Giese G, Sjaastad A, Poggensee G, et al. Female genital schistosomiasis (FGS): relationship between gynecological and histopathological findings. Acta Trop 1996; 62:257–67. [DOI] [PubMed] [Google Scholar]
- 12.Baay MF, Kjetland EF, Ndhlovu PD, et al. Human papillomavirus in a rural community in Zimbabwe: the impact of HIV co-infection on HPV genotype distribution. J Med Virol 2004; 73:481–5. [DOI] [PubMed] [Google Scholar]
- 13.Obeng BB, Aryeetey YA, de Dood CJ, et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol 2008; 102:625–33. [DOI] [PubMed] [Google Scholar]
- 14.Pillay P, Taylor M, Zulu SG, et al. Real-time PCR detection of Schistosoma-DNA in small volume urine samples reflects focal distribution of urogenital schistosomiasis in primary school girls of KwaZulu Natal, South Africa. Am J Trop Med Hyg 2014; 90:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol 2008; 35:979–88. [DOI] [PubMed] [Google Scholar]
- 16.Sadun EH, Von Lichtenberg F, Cheever AW, Erickson DG, Hickman RL. Experimental infection with Schistosoma haematobium in chimpanzees. Am J Trop Med Hyg 1970; 19:427–58. [DOI] [PubMed] [Google Scholar]
- 17.Norseth HM, Ndhlovu PD, Kleppa E, et al. The colposcopic atlas of schistosomiasis in the lower female genital tract based on studies in Malawi, Zimbabwe, Madagascar and South Africa. PLoS Negl Trop Dis 2014; 8:e3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gower CM, Gabrielli AF, Sacko M, et al. Population genetics of Schistosoma haematobium: development of novel microsatellite markers and their application to schistosomiasis control in Mali. Parasitol 2011; 138:978–94. [DOI] [PubMed] [Google Scholar]
- 19.Jordan. Schistosomiaisis, 1993.
- 20.Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 1991; 349:243–5. [DOI] [PubMed] [Google Scholar]
- 21.Wilkins HA, Blumenthal UJ, Hagan P, Hayes RJ, Tulloch S. Resistance to reinfection after treatment of urinary schistosomiasis. Trans R Soc Trop Med Hyg 1987; 81:29–35. [DOI] [PubMed] [Google Scholar]
- 22.Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg 2006; 75:83–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Hegertun IEA, Sulheim Gundersen KM, Kleppa E, et al. S. haematobium as a common cause of genital morbidity in girls: a cross-sectional study of children in South Africa. PLoS Negl Trop Dis 2013; 7:e2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatz C, Mayombana C, de Savigny D, et al. Ultrasound scanning for detecting morbidity due to Schistosoma haematobium and its resolution following treatment with different doses of praziquantel. Trans R Soc Trop Med Hyg 1990; 84:84–8. [DOI] [PubMed] [Google Scholar]
- 25.Kjetland EF, Mduluza T, Ndhlovu PD, et al. Genital schistosomiasis in women - a clinical in vivo 12-months’ study following treatment with praziquantel. Trans R Soc Trop Med Hyg 2006; 100:740–52. [DOI] [PubMed] [Google Scholar]
- 26.Stothard JR, Gabrielli AF. Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends Parasitol 2007; 23:83–6. [DOI] [PubMed] [Google Scholar]
- 27.Kjetland EF, ten Hove RJ, Gomo E, et al. Schistosomiasis PCR in vaginal lavage as an indicator of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg 2009; 81:1050–5. [DOI] [PubMed] [Google Scholar]
- 28.Kjetland EF, Norseth HM, Taylor M, et al. Classification of the lesions observed in female genital schistosomiasis. Int J Gynaecol Obstet 2014; 127:227–8. [DOI] [PubMed] [Google Scholar]
- 29.Kjetland EF, Ndhlovu PD, Kurewa EN, et al. Prevention of gynecologic contact bleeding and genital sandy patches by childhood anti-schistosomal treatment. Am J Trop Med Hyg 2008; 79:79–83. [PubMed] [Google Scholar]