Abstract
Background
In this retrospective study, we investigated the efficacy and safety of radioimmunotherapy with 90Yttrium- ibritumomab tiuxetan (90Y-RIT) in 9 patients with recurrent follicular lymphoma (FL) who were treated in a consolidation setting after having achieved complete (CR) or partial remission (PR) with Fludarabine, Cyclophosphamide and Rituximab (FCR).
Methods
The median age was 63 years (range 46–77). All patients were relapsed with histologically confirmed CD20-positive (grade 1 or 2) FL, at relapse they received FCR every 28 days: F (25 mg/m2x 3 days), C (1 gr/m2 day 1) and R (375 mg/m2 day 4) for 4 cycles. Those who achieved at least a PR with <25 % bone marrow involvement were treated with 90Y-RIT 11.1 or 14.8 MBq/Kg, at 3 months after completing FCR. Patients underwent a further restaging at 12 weeks after 90Y-RIT with a total body CT scan, FDG-PET/CT and bilateral bone marrow biopsy.
Results
Nine patients completed the treatment: FCR followed by 90Y-RIT (6 patients at 14.8 MBq/Kg, 3 patients at 11.1 MBq/Kg). After FCR, 7 patients obtained CR and 2 PR; after 90Y-RIT 2 patients in PR converted to CR 12 weeks later. With a median follow up of 95 months (range 20–114) since FCR and 88 months (range 13–104) since 90Y-RIT 3 deaths were not related to lymphoma; all 3 deceased patients obtained CR before 90Y-RIT and died still in CR. The median overall (OS) and progression free survival (PFS) have not been reached, in this analysis both OS or PFS are 67 % at 7.5 year. The most common grade 3 or 4 adverse events were hematologic.
Conclusions
These results confirm the long term efficacy and safety of 4 cycles of FCR followed by 90Y-RIT in relapsed grades 1 and 2 FL and suggest that this regimen could be a therapeutic option for this setting of patients, specially at age of 60–75 with no unexpected toxicities.
Keywords: Follicular lymphoma, 90Y-ibritumomab tiuxetan, Radioimmunotherapy
Introduction
Most cases of follicular lymphoma are characterized by recurrence of disease. There is usually a pattern of repeated remissions and relapses until patients become refractory to treatment. The duration of remissions becomes shorter with repeated induction attempts. Transformation to more aggressive non-Hodgkin lymphoma (NHL) occurs in 15 % to 50 % of the patients at 5 years. Therefore, it is important to have many treatment options: combination chemotherapy, radiation, immunotherapy, radioimmunotherapy and myeloablative therapy with stem-cell rescue for some patients with good performance status and responsive disease to overcome the development of resistance. A number of cytotoxic agents in combination are active in this patient population. The fludarabine, cyclophosphamide and rituximab (FCR) regimen provided encouraging results as initial or salvage therapy in patients with CLL or indolent NHL [1, 2]. Radioimmunotherapy is also an excellent modality in the treatment of NHL; the target antigen, radionuclide emission properties and chemical stability of radioimmunoconjugates are important factors that contribute to the effectiveness of RIT. 90Y can deliver a high beta energy to tumor (2–3 MeV) and 90Yttrium- ibritumomab tiuxetan (90Y-RIT ) consists of the anti-CD20 monoclonal antibody ibritumomab (an IgG1k antibody which is the murine parent immunoglobulin to rituximab) covalently bound to the chelating agent tiuxetan and radiolabeled with 90Y.
The phase III FIT trial (First-line Indolent Trial), enrolled 414 patients with stage III or IV who had attained a CR or PR after induction chemotherapy. It showed that consolidation of first remission with 90Y-RIT was highly effective with no unexpected toxicities, producing a statistically significant longer time to progression in both PR and CR patients groups. In the last update of the trial the median PFS has not yet been reached (>7.9 years) for patients in the 90Y-RIT arm and 4.9 years in control arm [3–5]. Furthermore, several phase II trials show high rates of conversion from PR to CR and significant improvements in PFS [6–14] using consolidation therapy with 90Y-RIT obtained, after initial treatment. 90Y-RIT also has been reported to be effective in patients with relapsed or refractory FL [15–17]. Here, we report updated long–term efficacy and toxicity results of 90Y-RIT consolidation in 9 patients relapsed with grade 1 and 2 FL patients responding to FCR that were treated at our Institute [18].
Results
Patients characteristics
In this retrospective analysis 9 patients had received 4 cycles of FCR followed by 90Y-RIT (6 patients at 14.8 MBq/Kg, 3 patients at 11.1 MBq/Kg). Baseline characteristics are presented in Table 1. The median age was 63 years (range 46–77), all patients were relapsed patients: 2 patients received a prior therapy, 5 patients received 2 prior treatments and 2 patients received 3 regimens. Seven patients were previously treated with rituximab plus chemotherapy, 2 patients had no previous rituximab treatment history, 1 patient received also high-dose therapy followed by autologous stem cell transplantation (Table 2).
Table 1.
Patient characteristics
| Patients (n = 9) | ||
|---|---|---|
| Male/Female | 3/6 | |
| Median Age (Range) | 63 (46–77) years | |
| Disease stage | at diagnosis | at start of FCR |
| I | 1 | 0 |
| II | 1 | 5 |
| III | 1 | 3 |
| IV | 6 | 1 |
| Bone marrow involvement | ||
| 0 % | 7 | |
| 10 % to ≤25 % | 2 | |
| Extranodal involvement | 1 (liver) | |
| FLIPI | ||
| Low | 1 | |
| Low-intermediate | 6 | |
| Intermediate-high | 2 | |
| Bulky disease | 1 | |
| B-symptoms | 0 | |
| Previous therapy including rituximab | ||
| No | 2 | |
| Yes | 7 | |
| Number of previous regimens | ||
| 1 | 2 | |
| 2 | 5 | |
| > 2 | 2 | |
Table 2.
Clinical characteristics
| Patients n | Sex/Age (y) | Previous treatment | Response to FCR | Response to RIT | Follow up (mo) since RIT |
|---|---|---|---|---|---|
| 1 | F/68 | CHOP/R,radiotherapy | CR | CR | 104 alive in CR |
| 2 | F/66 | Radiotherapy, CHOP/R | CR | CR | 88 alive in CR |
| 3 | F/57 | CHOP/R | PR | CR | 99 alive in CR |
| 4 | F/67 | CHOP/R, radiotherapy | CR | CR | 13 dead in CR |
| 5 | M/46 | CHOP/like, ASCT, IFN maintenance for 24 months | PR | CR | 83 alive in CR |
| 6 | F/61 | MACOPB/R | CR | CR | 92 alive in CR |
| 7 | M/69 | CHOP, FM/R, CyDex/R | CR | CR | 30 dead in CR (t-MDS) |
| 8 | M/57 | Chlorambucil, MACOPB/R | CR | CR | 32 dead in CR |
| 9 | F/77 | Chlorambucil, radiotherapy | CR | CR | 99 alive in CR |
CHOP cyclophosfamide, doxorubicin, vincristine, prednisone; R Rituximab; MACOPB Methotrexate, Doxorubicin, cyclophoshamide, vincristine, prednisone, bleomycin; ASCT autologous stem cell transplantation; IFN alpha interferon, FM fludarabine, mitoxantrone; Cy Dex cyclophosphamide, dexamethasone; t-MDS treatment-related myelodysplastic syndrome
Efficacy and safety
After 4 cycles of FCR 7 patients obtained CR and 2 PR, 2 patients in PR converted to CR after 90Y-RIT. In February 2015, with a median observation period of 95 months (range 20–114) since FCR and 88 months (range 13–104) since RIT, the median OS and the PFS have not been reached, 6/9 patients were alive in CR and current analysis has shown that either OS or PFS are 67 % at 7.5 year (Fig. 1). Grade 3 or 4 neutropenia occurred in 8/9 patients treated with FCR and in 9/9 patients assessable after 90Y-RIT. Subsequently to radioimmunotherapy the median neutrophil nadir was 0.8 × 109/ L (range 0.1-0.9 × 109/ L) at week 5, the median platelet count nadir was 49 × 109/ L (range 17–80 × 109/ L) at week 5. The median duration nadir for both neutrophils or platelets was 14 days. One patient developed herpes zoster infection after 8 months following valacyclovir discontinuation; another patient developed fungal infection. Both infections disappeared after specific treatment. One patient developed t-MDS (treatment-related myelodysplastic syndrome) at 26 months after 90Y-RIT. This patient before FCR and consolidation with 90Y-RIT had received 3 previous regimens: at diagnosis 6 courses of CHOP, at first relapse, 3 years later, 4 courses of FM/R (fludarabine, mitoxantrone plus rituximab) and after 1 year at the second relapse the patient received cyclophosphamide plus dexamethasone and rituximab, remaining in CR for 48 months. The patient died at 73 years of age of sepsis during support therapy for t-MDS. Other 2 patients died: 1 for acute renal failure and 1 for ictus cerebri.
Fig. 1.
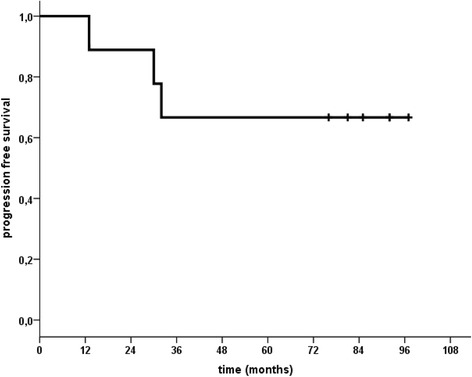
Progression Free Survival from RIT
Discussion
Cytotoxic chemotherapies lose efficacy with subsequent rounds of therapy in the retreatment of follicular lymphoma, eventually leading to refractory disease, however the question remains whether the survival of patients with FL is improving with new treatment regimens.
In the current retrospective analysis, nine patients with relapsed grade 1 and 2 FL, responding to FCR regimen and consolidated with 90Y–RIT obtained a significant high rate of response with 100 % of CR and acceptable toxicity. The conversion from PR to CR was already shown in the published phase III study (FIT-study) in first-line FL [3, 4] and also in phase II studies [5–12] of consolidation with the radioimmunotherapy agent 131 I-tositumomab after first-line induction [19, 20] thus, confirming the ability of 90Y-RIT to improve responses also in patients who are pretreated with rituximab based combination therapy [3]; even if in our two patients there is no proof that this conversion was due to RIT and not to a late response to FCR. In the FIT study, close to 17 % of the patients in the control arm, converted from PR to CR during watchful waiting [3], but our 2 patients who had higher risk of resistance already being pretreated must be considered.
In our analysis, the OS at 2 years was 89 %, at 3 years 76 % and at 4 years 61 % and OS and PFS are 67 % at 7.5 years. In another study conducted on patients with recurrent FL, treated with FCR, 75 % OS rate at 4 years and 61 % PFS rate at 4 years were registered, but in that study only 7 % of patients had been treated previously with rituximab and furthermore no patients had received combination treatment with chemotherapy plus rituximab [21]. Furthermore our results are in line with those recently described in a Japanese study [22] on 94 patients with relapsed or refractory low grade B cell non-Hodgkin lymphoma, among them 61 patients with grade 1 and 2 FL, treated with 90Y-RIT alone as salvage therapy and showing a CR rate of 69 %. In the Japanese cohort, during a median follow up of 46.5 months, the PFS rates of the first 50 patients who had undergone ≤ 2 and ≥ 3 previous regimens, and for those who achieved CR compared with those who did not were 38 and 11 months, respectively; the number of previous regimens and CR were statistically significant (p = 0.0011 and p < 0.0001, respectively). In our study 7/9 patients underwent ≤ 2 previous regimens before FCR and all of patients reached CR after 90Y-RIT with a PFS of 67 % at 7.5 years. Regarding AEs no grade 3 or 4 anemia was noted and no erythropoietic growth factors were used; there was high incidence of grade 3 or 4 neutropenia and thrombocytopenia but no platelet transfusions were necessary and granulocyte colony-stimulating factors were utilized in the majority of patients during FCR treatment and in all of them after 90Y–RIT. Despite the high incidence of grade 3 or 4 neutropenia, there were no patients requiring hospitalization for infection. We registered a case of herpes zoster infection after 8 months following valacyclovir discontinuation that disappeared after retreatment, and a case of fungal infection by conidiobolus, developed 10 months after 90Y-RIT and disappeared with itraconazole treatment. Other previous studies have already shown the low percentage of patients requiring hospitalization for infections [3, 15] and a favorable safety profile [23, 24]. A case of t-MDS with complex karyotype was diagnosed 26 months after 90Y-RIT consolidation: this patient received 3 previous regimens before FCR plus 90Y-RIT and as already mentioned the patient died of sepsis. This patient had been previously treated with topoisomerase II inhibitors, alkylating agents and purine nucleoside analogs. Czuczman et al. reported incidence of t-MDS and t-AML (treatment-related acute myeloid leukemia) after 90Y–RIT of 0.3 % per year after the diagnosis of NHL and 0.7 % per year after treatment. Most patients with t-MDS or t-AML had multiple cytogenetic aberrations, commonly on chromosomes 5 and 7, suggesting an association with previous exposure to chemotherapy. In Czuczman study, these malignancies were diagnosed at a median of 5.6 years (range 1.4 to 13.9) after the diagnosis of NHL and 1.9 years (range 0.4 to 6.3) after radioimmunotherapy [25]. The conclusion of this study was that the annualized incidences of t-MDS and t-AML were consistent with that expected in patients with NHL who had extensive previous chemotherapy and did not seem to increase after 90Y-RIT. However, in the FIT study 8 patients who developed MDS/AML were treated with 90Y-RIT, suggesting a role played by 90Y-RIT in the risk of secondary MDS/AML, thus it is reasonable to consider monitoring these patients closely. Cytogenetic testing before treatment with RIT may identify existing chromosomal abnormalities in previously treated patients, particularly those who have been treated with alkylating agents and purine analogs and would be at higher risk of developing t-MDS or t-AML.
In our series, the other two deaths were not related to progressive disease and all three deceased patients obtained CR before 90Y-RIT and died still in CR; so far the six survivors have maintained a high quality of life without having to make many visits to the hospital due to toxicity. Additional follow up is required to determine potential long-term AEs with 90Y-RIT consolidation. In our patients, the response to 90Y-RIT was assessed by CT, bone marrow biopsies and also with FDG-PET. This imaging procedure is useful to evaluate disease extension before treatment and response to RIT in FL. A study has shown that the post-90Y–RIT PET result is an independent predictive factor of PFS [26].
Conclusions
This retrospective analysis of 9 relapsed grades 1 or 2 FL heavily pretreated patients with median age 63 years demonstrates that sequential treatment with FCR and 90Y-RIT did not give rise to cumulative toxicity; it was feasible, safe and yielded high OS and PFS in patients with recurrent FL. Hematologic toxicity occurring with FCR or with 90Y-RIT was clinically controllable and acceptable in a population composed mainly of patients with a history of prior treatment using rituximab plus chemotherapy. With caution due to the low number of patients, these results suggest that this regimen could be an option used for the treatment in this setting of patients, specially at age of 60–75. 90Y-RIT as consolidation appears to be best suited to patients with low burden of disease and may be more acceptable in those who are not candidates for high dose therapy/transplant approaches.
Design and methods
The patients who were included in the current retrospective analysis had CD20+ histologically confirmed relapsed grade 1 or 2 follicular lymphoma and had received at least 1 prior treatment. In this single institution study, between August 2005 and July 2010, 9 patients at relapse had received 4 cycles of FCR: fludarabine at a dose of 25 mg/m2 i.v. on days 1 to 3; cyclophosphamide at a dose of 1 gr/ m2 i.v. on day 1 and rituximab at a dose of 375 mg/ m2 was given on day 4 of each cycle every 28 days. Patients were restaged with CT scan, FDG PET/CT and bone marrow biopsies after the last course of FCR, who had achieved at least a partial remission with < 25 % bone marrow involvement received, 12 weeks since the last course of FCR, 2 infusions of rituximab 250 mg/ m2 one week apart, with the first infusion administered alone and the second infusion followed immediately by 90 Y–RIT (14.8 MBq/Kg – 11 MBq/Kg), if the platelet number was between 100 x 109/ L and 149 x 109/ L, not exceeding a total of 1.184 MBq it was administered as a slow i.v. push over 10 min (Fig. 2). The patients were age ≥ 18 years, with WHO performance status of 0 to 2 and the last chemotherapy with or without rituximab was administered at least 3 months before start of FCR; no patient under maintenance therapy with rituximab was considered. Before starting 90Y-RIT an absolute neutrophil count ≥ 1.5 × 109 L, hemoglobin levels ≥ 9 gr/dl and a platelet count ≥ 100 × 109 L were required. None of the patients had central nervous system (CNS) involvement and positive HIV. All patients provided an informed consent according to institutional guidelines.
Fig. 2.
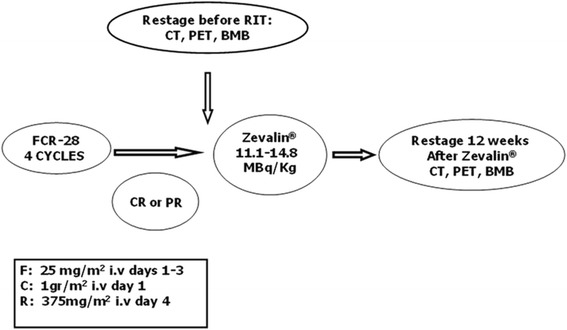
Treatment schema
No real-time quantitative PCR (RQ-PCR) evaluation of peripheral or marrow blood samples for bcl-2 t(14;18) translocation was performed at baseline nor thereafter. Safety was assessed by adverse events (AEs), with toxicity grading based on the National Cancer Institute Common Toxicity Criteria (version 4.0), clinical laboratory evaluations, and physical examinations. Filgrastim was administered when the neutrophil count was less than 1×109/L and platelet support was planned for eventual episodes of bleeding and platelet count less than 15×109/L. In patients developing grade 4 neutropenia or thrombocytopenia, the duration of cytopenia was measured from the first day of laboratory evidence of grade 4 toxicity until the last day of grade 4 toxicity without further support. OS was calculated from the date of FCR treatment to the date of death from any cause; OS was analyzed by using the Kaplan-Meier method.
Acknowledgments
The technical assistance of Mrs. Tania Merlino is greatly appreciated.
Abbreviations
- FCR
Fludarabine cyclophosphamide rituximab
- FL
Follicular lymphoma
- NHL
Non-Hodgkin lymphoma
- RIT
Radioimmunotherapy
- MeV
Megaelectronvolt
- MBq
Megabecquerel
- OS
Overall survival
- PFS
Progression free survival
- t-MDS
Treatment related myelodysplastic syndrome
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FP was the principal investigator and contributed to the study design, the writing of the report. Provision of study materials or patients: RS, MLD, DG, RK, SR, FM, MM. All the authors cited have been involved in drafting the manuscript and revising it critically for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Francesco Pisani, Phone: +39 0652665360, Email: fr.pisani@tiscali.it.
Rosa Sciuto, Email: sciuto@ifo.it.
Maria Laura Dessanti, Email: ldessanti@hotmail.com.
Diana Giannarelli, Email: giannarelli@ifo.it.
Ramy Kayal, Email: rkayal@libero.it.
Sandra Rea, Email: rea@ifo.it.
Francesco Marchesi, Email: fmarchesi@ifo.it.
Mirella Marino, Email: mirellamarino@inwind.it.
References
- 1.Tam CS, Wolf M, Prince HM, Januszewicz EH, Westerman D, Lin IK, Carney D, Seymour JF. Fludarabine, Cyclophosphamide, and Rituximab for the treatment of patients with chronic lymphocytic leukemia or indolent non-Hodgkin’s lymphoma. Cancer. 2006;106:2412–20. doi: 10.1002/cncr.21882. [DOI] [PubMed] [Google Scholar]
- 2.Czuczman MS, Koryzna A, Mohr A, Stewart C, Danohue K, Blumenson L, Bemstein ZP, McCarthy P, Alam A, Hernandez-Ilizaliturri F, Skipper M, Brown K, Chanan-Khan A, Klippestein D, Loud P, Rock MK, Benyunes M, Grillo-Lopez A, Bemstein SH. Rituximab in combination with fludarabine chemotherapy in low-grade or follicular lymphoma. J Clin Oncol. 2005;23:694–704. doi: 10.1200/JCO.2005.02.172. [DOI] [PubMed] [Google Scholar]
- 3.Morschhauser F, Radford J, Van Hoof A, Vitolo U, Soubeyran P, Tilly H, Huijgens PL, Kolstad A, d’Amore F, Diaz MG, Petrini M, Sebban C, Zinzani PL, van Oers MHJ, van Putten W, Bischof-Delaloye A, Rohatiner A, Salles G, Kuhlmann J, Hagenbeek A. Phase III trial of consolidation therapy with Yttrium-90-Ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26:5156–64. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 4.Morschhauser F, Dreyling M, Rohatiner A, Hagemeister F, Bischof-Delaloye A. Rationale for consolidation to improve progression-free survival in patients with non-Hodgkin’s lymphoma: a review of the evidence. The Oncologist. 2009;14:17–29. doi: 10.1634/theoncologist.2009-S2-17. [DOI] [PubMed] [Google Scholar]
- 5.Morschhauser F, Radford J, Van Hoof A, Botto B, Rohatiner AZ, Salles G, et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the international, randomized, phase III first-line Indolent trial. J Clin Oncol. 2013;31:1977–83. doi: 10.1200/JCO.2012.45.6400. [DOI] [PubMed] [Google Scholar]
- 6.Hainsworth J, Spigel D, Markus T, Shipley D, Thompson D, Rotman R, et al. Rituximab plus short duration chemotherapy followed by yttrium-90-ibritumomab tiuxetan as first-line treatment for patients with follicular non-hodgkin lymphoma: a phase II trial of the Sarah Cannon Oncology Research Consortium. Clin Lymphoma Myeloma. 2009;9:223–8. doi: 10.3816/CLM.2009.n.044. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs S, Swerdlow S, Kant J, Foon K, Jankowitz R, Land S, et al. Phase II trial of short course CHOP-R followed by 90 Y-ibritumomab tiuxetan and extended rituximab in previously untreated follicular lymphoma. Clin Cancer Res. 2008;21:7088–94. doi: 10.1158/1078-0432.CCR-08-0529. [DOI] [PubMed] [Google Scholar]
- 8.Zinzani PL, Tani M, Pulsoni A, Gobbi M, Perotti A, De Luca S, et al. Fludarabine and mitoxantrone followed by yttrium-90-ibritumomab tiuxetan in previously untreated patients with follicular non-Hodgkin lymphoma trial. A phase II non-randomized trial (FLUMIZ) Lancet Oncol. 2008;9:352–8. doi: 10.1016/S1470-2045(08)70039-1. [DOI] [PubMed] [Google Scholar]
- 9.Zinzani PL, Derenzini E, Pellegrini C, Rigacci L, Fabbri A, Gandolfi L, et al. Long-term efficacy and toxicity results of the FLUMIZ trial (fludarabine and mitoxantrone followed by yttrium-90 ibritumomab tiuxetan in untreated follicular lymphoma. Ann Oncol. 2012;23:805–7. doi: 10.1093/annonc/mdr633. [DOI] [PubMed] [Google Scholar]
- 10.Karmali R, Kassar M, Venugopal P, Shammo J, Fung H, Bayer R, et al. Safety and efficacy of combination therapy with fludarabine, mithoxantrone, and rituximab followed by yttrium-90 ibritumomab tiuxetan and maintenance rituximab as front-line therapy for patients with follicular or marginal zone lymphoma. Clin Lymphoma Myeloma Leuk. 2011;11:467–74. doi: 10.1016/j.clml.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin P, Neelapu S, Fanale M, Rodriguez M, Ayala A, Pro B, et al. R-FND followed by radioimmunotherapy for high-risk follicular lymphoma. Blood (ASH Annual Meeting Abstracts) 2013;112:3056. [Google Scholar]
- 12.Provencio M, Cruz Mora M, Gómez-Codina J, Quero Blanco C, Llanos M, García-Arroyo FR, de la Cruz L, Gumá Padró J, Delgado Pérez JR, Sánchez A, Alvarez Cabellos R, Rueda A. Consolidation treatment with Yttrium-90 ibritumomab tiuxetan after new induction regimen in patients with intermediate-and high-risk follicular lymphoma according to the follicular lymphoma international prognostic index: a multicentre, prospective phase II trial of the Spanish Lymphoma Oncology group. Leuk Lymphoma. 2014;55:51–5. doi: 10.3109/10428194.2013.790544. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez Ruiz AC, de la Cruz-Merino L, Provencio Pulla M. Role of consolidation with yttrium-90 ibritumomab tiuxetan in patients with advance-stage follicular lymphoma. Ther Adv Hematol. 2014;5:78–90. doi: 10.1177/2040620714532282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibatici A, Pica GM, Nati S, Vitolo U, Botto B, Ciocchetto C, et al. Safety and efficacy of 90yttrium-ibritumomab-tiuxetan for untreated follicular lymphoma patients. An Italian cooperative study. Br J Haematol. 2014;164:710–6. doi: 10.1111/bjh.12695. [DOI] [PubMed] [Google Scholar]
- 15.Witzing TE, White CA, Gordon LI, Wiseman GA, Emmanouilides C, Murray JL, Lister J, Multani PS. Safety of Yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21:1263–70. doi: 10.1200/JCO.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 16.Emmanouilides C, Witzing TE, Gordon LI, Vo K, Wiseman GA, Flinn IW, Darif M, Schilder RJ, Molina A. Treatment with Yttrium-90 ibritumomab tiuxetan at early relapse is safe and effective in patients with previously treated B-cell non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006;47:629–36. doi: 10.1080/10428190500376076. [DOI] [PubMed] [Google Scholar]
- 17.Witzing TE, Molina A, Gordon LI, Emmanouilides C, Schilder RJ, Flinn IW, Darif M, Macklis R, Vo K, Wiseman GA. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin’s lymphoma treated with Yttrium-90 ibritumomab tiuxetan. Cancer. 2007;109:1804–10. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 18.Pisani F, Maini CL, Sciuto R, Dessanti L, D’Andrea M, Assisi D, Petti MC. FCR (Fludarabine, Cyclophosphamide, Rituximab) regimen followed by 90Yttrium-ibritumomab tiuxetan consolidation for the treatment of relapsed grades 1 and 2 follicular lymphoma: a report of 9 cases. J Exp Clin Cancer Res. 2011;30:16. doi: 10.1186/1756-9966-30-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard JP, Coleman M, Kostakoglu L, Chadbum A, Cesarman E, Furman RR, Schuster MW, Niesvizky R, Muss D, Fiore J, Kroll S, Tidmarsh G, Vallabhajosula S, Goldsmith SJ. Abbreviated chemotherapy with fludarabine followed by tositumomab and iodine I-131-tositumomab for untreated follicular lymphoma. J Clin Oncol. 2005;23:5696–704. doi: 10.1200/JCO.2005.14.803. [DOI] [PubMed] [Google Scholar]
- 20.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, Fisher RI. Phase II trial of CHOP chemotherapy followed by I-131-tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: Five years follow up of Southwest Oncology Group Protocol 59911. J Clin Oncol. 2006;24:4143–4129. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 21.Sacchi S, Pozzi S, Marcheselli R, Federico M, Tucci A, Merli F, Orsucci L, Liberati M, Vallisa D, Brugiatelli M. Rituximab in combination with fludarabine and cyclophosphamide in the treatment of patients with recurrent follicular lymphoma. Cancer. 2007;110:121–8. doi: 10.1002/cncr.22740. [DOI] [PubMed] [Google Scholar]
- 22.Uike N, Choi I, Tsuda M, Haji S, Toyoda K, Suehiro Y, et al. Factors associated with effects of 90Y-ibritumomab tiuxetan in patients with relapsed or refractory low-grade B cell non-Hodgkin lymphoma: single-institution experience with 94 Japanese patients in rituximab era. Int J Hematol. 2014;100:386–92. doi: 10.1007/s12185-014-1636-5. [DOI] [PubMed] [Google Scholar]
- 23.Dreyling M, Trumper L, von Schilling C, Rummel M, Holtkamp U, Waldmann A, Wehmeyer J, Freund M. Results of a national consensus workshop: therapeutic algorithm in patients with follicular lymphoma – Role of radioimmunotherapy. Ann Hematol. 2007;86:81–7. doi: 10.1007/s00277-006-0207-0. [DOI] [PubMed] [Google Scholar]
- 24.Zinzani PL, d’Amore F, Bombardieri E, Brammer E, Codina JG, Ilidge T, Jurczak W, Linkesch W, Morschhauser F, Vandenberghe E, Van Hoof A. Consensus conference: Implementing treatment recommendations on Yttrium-90 immunotherapy in clinical practice – Report of a European workshop. Eur J Cancer. 2008;44:366–73. doi: 10.1016/j.ejca.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Czuczman MS, Emmanoulides C, Darif M, Witzig TE, Gordon LI, Revell S, Vo K, Molina A. Treatment-related myelodysplastic syndrome and acute myelogenous leukaemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol. 2007;25:4285–92. doi: 10.1200/JCO.2006.09.2882. [DOI] [PubMed] [Google Scholar]
- 26.Lopci E, Santi I, Derenzini E, Fonti C, Savelli G, Bertagna F, Bellò M, Botto M, Huglo D, Morschhauser F, Zinzani PL, Fanti S. FDG-PET in the assessment of patients with follicular lymphoma treated by ibritumomab tiuxetan Y-90: multicentric study. Ann Oncol. 2010;21:1877–83. doi: 10.1093/annonc/mdq024. [DOI] [PubMed] [Google Scholar]


