Abstract
Background
Juvéderm Voluma XC is a volumizing hyaluronic acid filler used for correction of age-related midface volume deficit (MVD).
Objectives
The effectiveness of Juvéderm Voluma XC was examined from the patient perspective.
Methods
Patients with moderate to severe age-related MVD (N = 235) received Juvéderm Voluma XC. At quarterly follow-up visits for 2 years, patients rated treatment outcomes on the Global Aesthetic Improvement Scale (GAIS), overall satisfaction with facial appearance, satisfaction with midfacial regions, achievement of treatment goal, Look and Feel of the Midface (LAFM), and Self-Perception of Age (SPA). Patients recorded treatment-site responses in 30-day diaries.
Results
At 6 months and 2 years after treatment, 92.8% and 79.0% of patients, respectively, rated their cheek volume as improved/much improved on the GAIS. Improvement in satisfaction with facial appearance was noted by 89.8% of patients at 6 months and 75.8% at 2 years. Increased satisfaction with outer and lower cheek areas and cheek-bone projection and clinically significant improvements in LAFM were noted through month 24. Treatment goals were achieved by 67.8% of patients at 6 months and 49.0% at 2 years. Patients reported looking, on average, 5 years younger at 6 months and 3 years younger at 2 years. The most common treatment site responses were tenderness, swelling, firmness, and lumps/bumps; most were mild to moderate in severity and lasted ≤2 weeks.
Conclusions
Juvéderm Voluma XC for age-related MVD is effective and well-tolerated from the patient perspective, with results lasting up to 2 years.
Level of Evidence
4 Therapeutic
Therapeutic
Visible signs of facial aging result, in part, from the loss of structural support over time. As the midface ages, volume loss occurs in the skin, bone, muscle, and superficial and deep fat.1-6 Volume loss of deep midfacial fat reduces support for the medial cheek compartment, diminishing projection of the midface.1,2 Gravity, coupled with volume loss of buccal fat, results in inferior migration of fat compartments, leading to deepening of the nasojugal fold and development of a crescent-shaped hollow beneath the lower edge of the orbicularis oculi muscle.4 In addition, the suspensory ligaments elongate, resulting in a lowering of the malar fat pad and cheek skin.7,8 Tonal changes to individual muscles and changes in skin quality, such as the development of wrinkles and drooping skin, may also contribute to the appearance of aging.9,10
Nonsurgical aesthetic procedures, such as those aimed at restoring volume to the midface, are voluntary; therefore, patient satisfaction is critical for evaluating treatment effectiveness.11 In studies of facial aesthetics, outcomes that measure factors such as patient satisfaction, perception of overall aesthetic change, and impact on quality of life are key components. In addition to directly assessing patient satisfaction, such outcomes measure experiences, preferences, and perceptions that affect satisfaction with treatment.12 A highly satisfied patient may reflect positive impacts of aesthetic procedures, such as improved self-image and self-esteem.13-16 It has been noted that the desire to improve self-image is a key motivator for deciding to undergo an aesthetic procedure,17 and a survey by the American Society for Dermatologic Surgery lists a desire to appear younger and more attractive and feel more confident as consumers' top reasons for seeking aesthetic procedures.18
Injectable hyaluronic acid (HA) fillers are used in aesthetic procedures to reduce the appearance of sagging skin and skin folds by replacing skin HA and increasing the skin's water-binding capacity.9 Juvéderm Voluma XC (Allergan, Inc., Irvine, CA) is an HA gel with lidocaine approved by the United States (US) Food and Drug Administration (FDA) for midface volumizing to correct age-related volume deficit. While products designed with Hylacross technology (eg, Juvéderm Ultra Plus) comprise mostly high molecular weight HA, products developed with the patented Vycross technology, including Juvéderm Voluma XC, utilize a proprietary mix of low and high molecular weight HA. Because of their unique composition and crosslinking process, Vycross-based products maintain a tightly crosslinked network of HA chains. Consequently, such products are less hygroscopic than Hylacross technology-based fillers, and exhibit minimal gel swelling.19 In addition, Vycross technology allows for products with varied HA concentrations and physicochemical properties tailored to different indications. Together, these features help to provide lasting clinical duration despite the reduced concentration of HA.19 A pivotal, single-blind, randomized, controlled study demonstrated the safety and effectiveness of Juvéderm Voluma XC for treatment of midface volume deficit (MVD).20 Primary effectiveness results were based on assessments of overall MVD on the validated 6-point Allergan Mid-Face Volume Deficit Scale (MFVDS). In this study, 85.6% of patients treated with Juvéderm Voluma XC improved by 1 point or more on the MFVDS at month 6 (primary endpoint), and the difference in responder rates between the Juvéderm Voluma XC treatment group and a no-treatment control group was statistically significant (P < .001). Correction of MVD with Juvéderm Voluma XC lasted for up to 2 years.
The objectives of the current analysis were to examine patient-reported outcomes from the pivotal clinical study designed to assess the safety and effectiveness of Juvéderm Voluma XC for the treatment of MVD.
METHODS
Study Design
A multicenter, single-blind, randomized, controlled study was conducted at 15 sites in the US and Canada from August 2009 to June 2012. Inclusion and exclusion criteria were previously reported.20 Briefly, the study enrolled patients 35 to 65 years of age who desired cheek augmentation to correct moderate, significant, or severe age-related MVD. Patients who had undergone cosmetic facial plastic surgery (other than rhinoplasty more than 2 years before enrollment), tissue grafting, or tissue augmentation with silicone, fat, or permanent or semipermanent fillers, as well as those who planned to undergo such procedures during the study timeframe, were excluded.
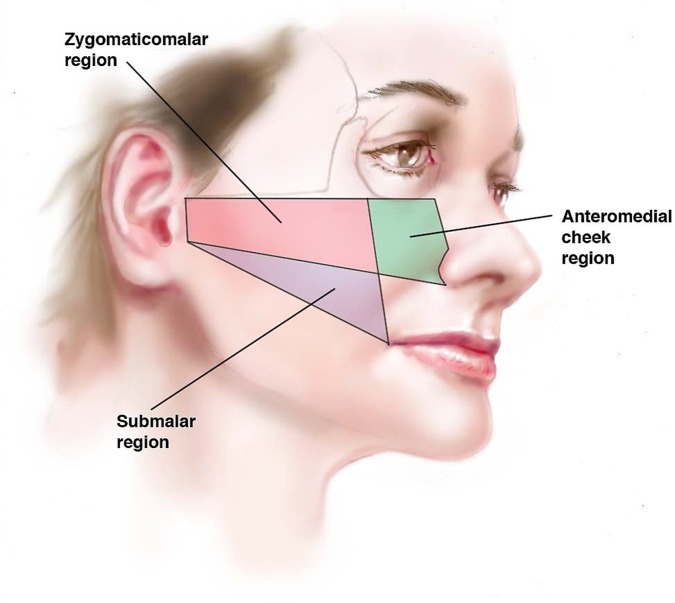
Patients were randomized to 20 mg/mL Juvéderm Voluma XC (n = 235) or to a no-treatment control (n = 47). A no-treatment control was chosen because there were no products approved by the US FDA for an age-related MVD-treatment indication that could serve as an injection control. As each subsequent patient qualified for study entry, the investigator contacted Allergan for treatment assignment based on a previously generated randomization schedule. Control patients received treatment after month 6 (ie, after the primary study endpoint evaluation). Initial treatment was followed by an optional touch-up 1 month later if optimal cheek augmentation was not achieved based on investigator and patient assessment. Areas of treatment included the zygomaticomalar region, the anteromedial cheek, and the submalar region (Figure 1).20,21 The study protocol was registered at www.clinicaltrials.gov (NCT00978042) and was approved by applicable institutional review boards (ie, Quorum Review IRB, Seattle, WA; Saint Louis University, St. Louis, MO; The University of Texas Southwestern Medical Center at Dallas IRB, Dallas, TX). All patients provided written informed consent prior to any study-related procedures.
Figure 1.
Diagram showing the midface treatment areas.
Response Measures and Statistics
Outcome Measures
The following assessments were completed by patients in the treatment group at baseline and every 3 months for 2 years after the last treatment: the Global Aesthetic Improvement Scale (GAIS), satisfaction with midfacial regions, and treatment goals assessment. Patients rated overall aesthetic improvement on the GAIS by comparing their appearance at follow-up visits against 3-dimensional digital (Vectra 3D Surface Imaging System, Canfield Scientific, Fairfield, NJ). photographs taken before treatment on a 5–point scale ranging from much improved to much worse.22 Patients also rated level of satisfaction on a scale from 0 to 4 (0, not at all satisfied; 4, completely satisfied) for each of the following midface regions: outer and lower cheek areas, prominence or projection of cheek bones, area on either side of nose, tear troughs (area under lower eyelids), and nasolabial folds (area between corners of nose and corners of mouth). Patients set treatment goals before treatment and, at all follow-up visits, assessed whether their treatment goals had been met.
Patients in the treatment group completed the following assessments at baseline and at 1, 6, 12, 18, and 24 months after the last treatment: overall satisfaction with facial appearance, Look and Feel of the Midface (LAFM), and Self-Perception of Age (SPA). Overall satisfaction with facial appearance was assessed with a questionnaire comprising 11 items rated on a 5-point scale from 0 (not at all, never, or no, depending upon the question) to 4 (completely, always, definitely). For the LAFM assessment, patients evaluated their level of concern about the look and feel of their cheeks while relaxed and in motion on an 11-point Likert scale from 0 (not at all) to 10 (very much); this 9-item questionnaire was patterned after the Facial Lines Outcomes (FLO-11)23-25 and Look and Feel questionnaires.26 The SPA, a single-item validated questionnaire, was used to assess whether patients felt they looked their chronological age, looked younger, or looked older, and, if applicable, the number of years younger or older they felt they looked.23,24,26
Safety
Using diaries, patients in the treatment and control groups reported the presence, location, severity, and duration of any common treatment-site responses (CTRs) for 30 days after each treatment.
Statistics
Compared with baseline (pretreatment) scores, a mean improvement of 3 points on the LAFM or a statistically significant improvement (demonstrated by a lower confidence limit of the change in mean score greater than 0) in mean posttreatment satisfaction with facial appearance score were considered clinically significant. Overall patient satisfaction with facial appearance scores was calculated as the mean score from the responses to the 6 questions multiplied by 25, resulting in a scale from 0 (least satisfaction) to 100 (greatest satisfaction). Summary tables and descriptive statistics were used in the presentation of results.
All effectiveness results are reported for patients in the treatment group. Patients in the control group did not perform self-assessments during the first 6-month period of the study, as they were aware that they had not received treatment and were thus not blinded to treatment condition.
RESULTS
Patients
Demographic data for the treatment group are summarized in Table 1. Patients in the treatment group had investigator-rated baseline MVD of moderate (51.5%), significant (41.7%), or severe (3.8%).20 Grades on the MFVDS and corresponding key descriptors include: severe (severe concavity, significant prominence of bony landmarks), significant (significant concavity, moderate prominence of bony landmarks), moderate (moderate concavity, mild prominence of bony landmarks), mild (mild concavity), minimal (flattening in the zygomaticomalar region, anteromedial cheek, and/or submalar region), and none (fullness in the zygomaticomalar region, anteromedial cheek, and/or submalar region).20 Demographics for the control group have been previously reported and were similar to those for the treatment group.20
Table 1.
Demographic Characteristics
| Characteristic | Treatment Group (n = 235) |
|---|---|
| Sex, n (%) | |
| Female | 189 (80.4) |
| Male | 46 (19.6) |
| Age, median (range) | 56 years (35-65 years) |
| Race, n (%) | |
| White | 137 (58.3) |
| Black | 44 (18.7) |
| Hispanic | 35 (14.9) |
| Asian | 9 (3.8) |
| Other | 10 (4.3) |
| Fitzpatrick skin type, n (%) | |
| I | 6 (2.6) |
| II | 62 (26.4) |
| III | 67 (28.5) |
| IV | 43 (18.3) |
| V | 44 (18.7) |
| VI | 13 (5.5) |
Injection Volumes
The mean injection volume (both sides of the face) for initial and touch-up treatments combined was 6.68 mL (range, 1.2-13.9 mL). The mean injection volume for initial treatment was 5.09 mL and 1.93 mL for touch-up treatment. Mean injection volumes for initial and touch-up treatments combined for the zygomaticomalar region, anteromedial cheek, and submalar region were 2.39 mL (range, 0.1-7.0 mL), 2.12 mL (range, 0.4-5.7 mL), and 2.42 mL (range, 0.2-10.0 mL), respectively. Both subcutaneous and supraperiosteal injection planes were used for 67.3%, 54.9%, and 24.5% of injections in the zygomaticomalar region, anteromedial cheek, and submalar region, respectively; the remainder of injections used only 1 of the injection planes. Additional details of treatment were previously reported.20
Effectiveness
Not all patients completed all outcome measurements at all visits; thus patient numbers vary between assessments. Six months after treatment, 92.8% of patients rated their midface volume as improved or much improved on the GAIS; a majority (79.0%) of patients still provided these ratings at 2 years (Figure 2). Overall satisfaction with facial appearance scores improved from baseline in 89.8% of patients at 6 months and in 75.8% of patients at 2 years (Figure 3). At all time points, increases from baseline in mean scores were statistically significant. The percentage of patients with improved satisfaction with facial appearance at month 6 was high across all injection volumes but increased with greater injection volumes. By injection volume quartile (initial plus touch-up volume), the percentage of patients with improved satisfaction was 78.4% for ≤4.6 mL, 89.8% for 4.6 to ≤6.5 mL, 94.3% for 6.5 to ≤8.9 mL, and 96.2% for >8.9 mL.
Figure 2.
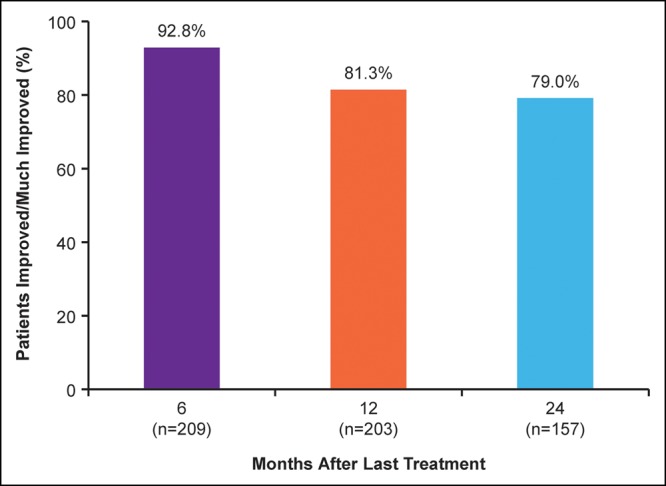
Percentage of patients who rated themselves as improved or much improved on the Global Aesthetic Improvement Scale.
Figure 3.
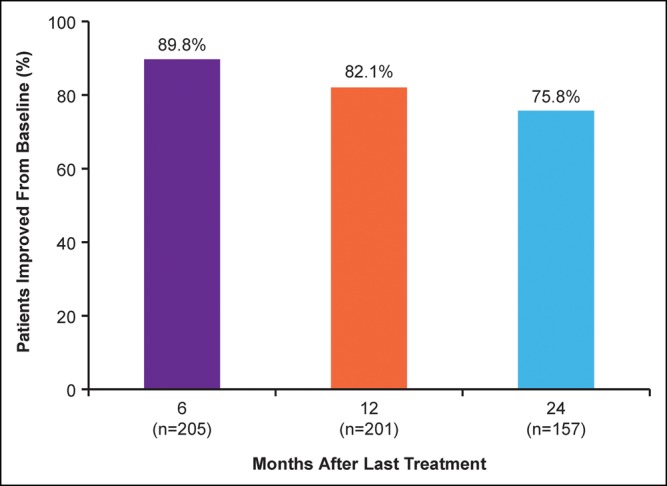
Percentage of patients with improved scores for overall satisfaction with facial appearance since baseline.
The percentage of patients who were somewhat to completely satisfied with their outer and lower cheek areas improved from 59.0% at baseline to 97.6% at 6 months and remained well above baseline at 12 months (93.1%) and 2 years (89.8%) (Table 2). Satisfaction with cheek-bone projection improved from 69.7% at baseline to 97.6% at 6 months and was maintained at 91.7% at 2 years. Increases in patient satisfaction were also noted in regard to untreated, adjacent midfacial areas (eg, tear troughs, nasolabial folds, and areas on either side of the nose) (Table 2). For example, less than half of patients (47.2%) were satisfied with their tear troughs before MVD treatment. However, at 6 months and 2 years, satisfaction increased to 84.7% and 77.7%, respectively.
Table 2.
Patient Satisfaction With Midfacial Regions
| Midfacial Region | Percentage (n/N) of Patients Satisfieda |
|||
|---|---|---|---|---|
| Baseline | Month 6 | Month 12 | Month 24 | |
| Outer and lower cheek areas | 59.0 (138/234) | 97.6 (202/207) | 93.1 (188/202) | 89.8 (141/157) |
| Prominence or projection of cheek bones | 69.7 (163/234) | 97.6 (204/209) | 95.5 (191/200) | 91.7 (143/156) |
| Area on either side of nose | 53.6 (125/233) | 95.7 (198/207) | 94.0 (187/199) | 91.0 (142/156) |
| Tear troughsb | 47.2 (110/233) | 84.7 (177/209) | 80.1 (161/201) | 77.7 (122/157) |
| Nasolabial foldsc | 31.2 (73/234) | 73.6 (153/208) | 69.0 (138/200) | 56.7 (89/157) |
aPatients who rated scores of 1 (somewhat satisfied), 2 (moderately satisfied), 3 (very satisfied), or 4 (completely satisfied) on a scale of 0 to 4. bArea under lower eyelids. cArea between corners of nose and corners of mouth.
At 6 months, more than two-thirds of patients (67.8%) agreed that their treatment goals had been met. More than half of patients in the treatment group (58.4%) agreed that their treatment goals were attained through month 12, and 49.0% of patients still agreed at 2 years.
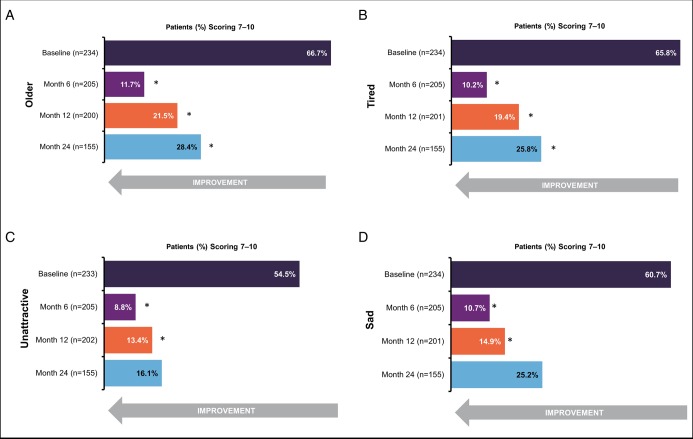
At baseline, a majority of patients rated their cheeks as making them look older, tired, unattractive, and sad. Clinically significant improvements in mean satisfaction scores (change of ≥3 points in mean satisfaction score since baseline) were noted for the categories of older and tired at 6, 12, and 24 months and for the categories of sad and unattractive at 6 and 12 months (Figure 4). In the 5 additional categories, patients noted both at baseline and subsequent time points that their cheeks did not feel hard, lumpy/grainy, or unnatural and did not look uneven or unnatural.
Figure 4.
Percentage of patients rating scores of 7 to 10 on the assessment of the look and feel of the midface. (A) Cheeks make me look older than I want to look; (B) Cheeks make me look tired, even when I am not; (C) Cheeks make me look unattractive; (D) Cheeks make me look sad, even when I am not. Scores ranged from 0 to 10, where 0 = not at all, 5 = somewhat, and 10 = very much. Significant improvements in mean score were noted in all categories at 6 and 12 months and in the categories of older and tired at 24 months. *Clinically significant change from baseline in mean satisfaction score (defined a priori as change of ≥3 points).
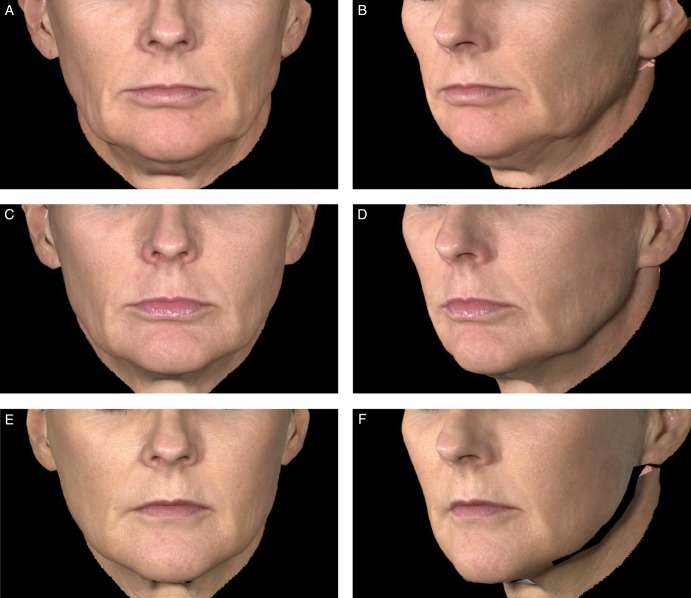
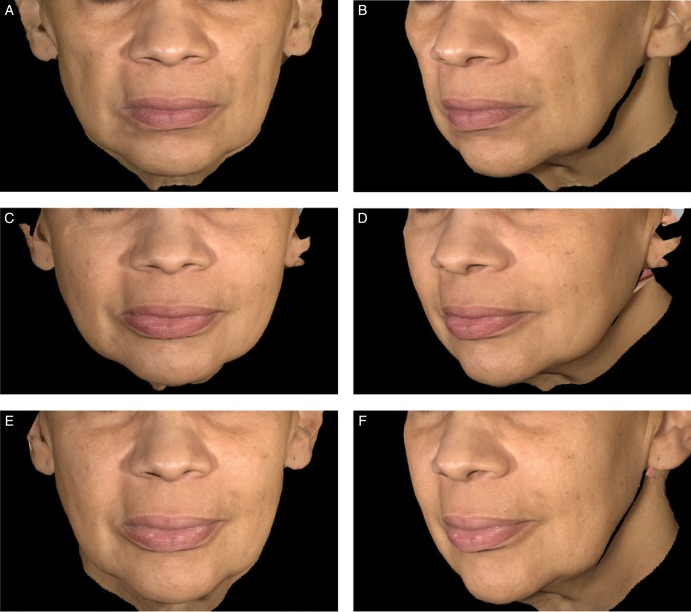
Representative examples of aesthetic outcomes following treatment with Juvéderm Voluma XC are shown in Figures 5 and 6.
Figure 5.
Aesthetic outcomes with Juvéderm Voluma XC for this 48-year-old woman before treatment (A, B), at 6 months (C, D), and at 24 months (E, F) after treatment with 6.3 mL of Juvéderm Voluma XC in the midface.
Figure 6.
Aesthetic outcomes with Juvéderm Voluma XC for this 59-year-old woman before treatment (A, B), at 6 months (C, D), and at 24 months (E, F) after treatment with 8.9 mL of Juvéderm Voluma XC in the midface.
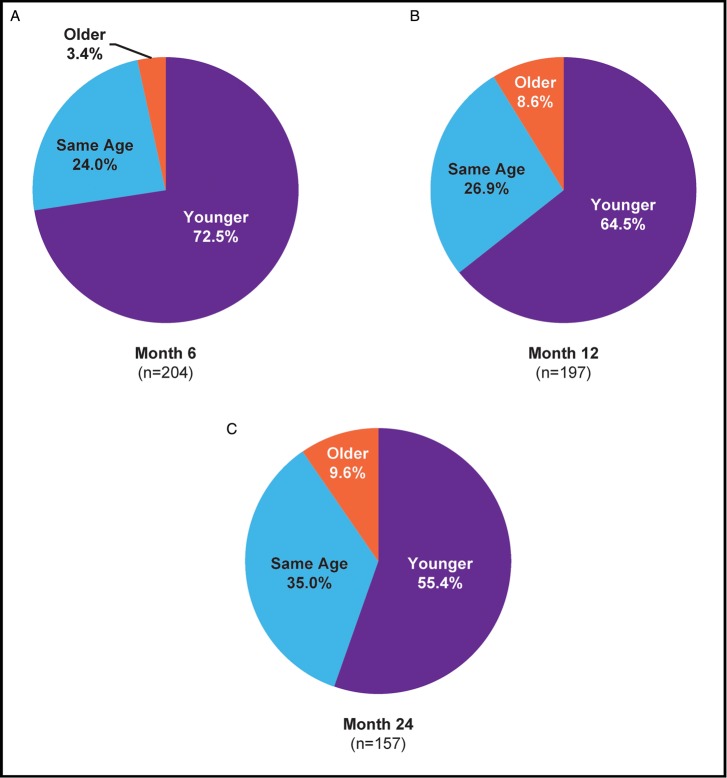
At each time point following treatment with Juvéderm Voluma XC, a majority of patients perceived themselves as looking younger versus baseline, from 72.5% at month 6 to 55.4% at month 24 (Figure 7). The mean decrease in perceived age was 4.9 years at month 6, 3.8 years at month 12, and 3.3 years at month 24.
Figure 7.
Percentage of patients reporting that their perceived age was younger than their chronological age, older, or the same compared with baseline at 6 (A), 12 (B), and 24 (C) months after treatment.
Patients in the control group who received treatment at month 6 of the study reported similar improvements in effectiveness results over time compared with the treatment group. For example, the percentage of patients in the control group who rated their midface volume as improved or much improved on the GAIS was 86.7% at 6 months, 73.1% at 12 months, and 68.4% at 18 months after they received treatment.
Safety
For the 265 patients who completed safety diaries after initial treatment (234 patients in the primary follow-up period and 31 control patients treated in the extended follow-up period), the most frequently reported CTRs in patient diaries were tenderness, swelling, firmness, and lumps/bumps (Table 3). Most CTRs (80.7%) were mild or moderate in severity. The duration of CTRs was ≤2 weeks for 55.4% of patients.
Table 3.
Patient-Reported Common Treatment Responses
| Diary-Reported Term | Patients (%) (N = 265a) | Mild/Moderate Severityb (%) | Resolved Within 2 Weeksb (%) |
|---|---|---|---|
| Tenderness | 92.1 | 96.3 | 88.5 |
| Swelling | 85.7 | 90.3 | 91.6 |
| Firmness | 82.3 | 92.2 | 76.6 |
| Lumps/bumps | 81.1 | 90.2 | 66.0 |
| Bruising | 77.7 | 88.9 | 85.0 |
| Pain | 66.4 | 97.7 | 97.2 |
| Redness | 66.0 | 96.0 | 96.0 |
| Discoloration | 41.1 | 89.9 | 89.9 |
| Itching | 38.5 | 89.2 | 100 |
aN denotes the number of patients who completed the diary after initial treatment. bThe denominator for percentages is the number of patients who reported an incidence of that treatment response in the diary.
DISCUSSION
These results demonstrate that Juvéderm Voluma XC treatment for age-related MVD has a long-term positive effect on overall aesthetic look, patient satisfaction, and perception of appearance. A majority of patients were highly satisfied across a number of different measures, with more than 75% of treated patients completely satisfied with overall facial appearance for up to 2 years. Patients also perceived themselves to appear younger than their chronological age. These results were also reflected in the attainment of treatment goals and indicate that individuals seeking treatment with Juvéderm Voluma XC are likely to achieve the younger midface appearance they desire.
There are a limited number of published studies focusing on patient-reported outcomes with volumizing HA fillers. High levels of patient-reported improvement on the GAIS regarding the use of volumizing HA fillers for MVD have been previously reported.22,27,28 Authors studying volumizing HA fillers have also reported patient satisfaction, patient likelihood of returning for additional treatment, and patient willingness to recommend treatment to friends.22,27,29,30 The current study is unique in that the authors report on multiple patient-reported outcome measures to fully assess facial aesthetic outcomes from the patient's perspective.
A clinically significant response in facial aesthetics can support treatment satisfaction, providing further evidence of successful outcomes from the patient's perspective. In this study, a clinically significant response refers to the point at which patients perceive and report meaningful improvements in their midface volume. Based on predefined criteria, the authors of the current study found that patients treated with Juvéderm Voluma XC perceived clinically significant improvement in the look and feel of their midface and also experienced a clinically significant increase in overall satisfaction with their facial appearance.
In addition to high rates of satisfaction with treated midfacial regions, increases in patient satisfaction were also observed in untreated areas of the midface, including the tear troughs, the nasolabial folds, and areas on either side of the nose. These observations are likely associated with the impact of midface refilling on the surrounding anatomy. For example, adding filler to the midface may restore structural support to the medial cheek compartment, reducing the prominence of tear troughs and the nasolabial folds. Thus, the benefits of treatment are not centered on the exact area of injection but extend in a multitiered fashion.
One limitation of this study is that not all of the patient-reported outcomes were validated. Further, clinical significance is largely a qualitative assessment, and predefined criteria for the determination of clinical significance were subjective. Because patients were not blinded with regard to treatment, the outcomet measures may have been slightly biased. However, it should be noted that patient-rated improvement on the GAIS at 6 months was consistent with blinded investigator ratings at this time point (with 82.2% of investigators and 92.8% of patients rating midface volume as improved or much improved at 6 months), as previously reported.20 Another limitation was the lack of blunt cannula use, as the researchers employed a variety of injection techniques using needles to inject into the subcutaneous and supraperiosteal planes. Additional study is required to determine the safety and effectiveness of Juvéderm Voluma XC injection administered via blunt cannulae vs needles. The authors of the current study did not compare Juvéderm Voluma XC with another filler. This limitation underscores the lack of an agreed-upon standard for midfacial filling; given that there is no US FDA-approved filler for the midface other than Juvéderm Voluma XC, the closest standard would be fat injection, which is a surgical alternative.
The safety profile observed in this study was comparable to that of other fillers.28,31 CTRs were minor and of short duration.
CONCLUSIONS
High patient satisfaction and improvements in self-image may be the most essential outcomes and motivators for facial aesthetic procedures. On multiple measures, patients treated with Juvéderm Voluma XC for age-related MVD experienced improved satisfaction with their midface and overall facial aesthetic look and appearance for up to 2 years after treatment.
Disclosures
Dr Few serves on an advisory board for Allergan, Inc., (Irvine, CA) and Mentor (Santa Barbara, CA); as a remunerated consultant for Allergan, Inc., Medicis (Scottsdale, Arizona), Palomar (Westford, MA), Ulthera (Mesa, Arizona), and Venus Concept (Toronto, Ontario Canada); as a remunerated speaker for Allergan, Inc., Ulthera, and Venus Concepts; and has received research grants as an investigator for Allergan, Inc. and Medicis. Dr Cox is a remunerated consultant and speaker and serves on an advisory board for Allergan, Inc. She also receives research grants as an investigator for Allergan, Inc. Dr Paradkar-Mitragotri is a former employee of Allergan, Inc. and Ms Murphy is an employees of Allergan, Inc. and holds stock and/or stock options in that company.
Funding
Allergan, Inc. (Irvine, CA), the manufacturer of the device discussed in this article, designed and funded the study and performed statistical analysis of the data. Writing and editorial support for this article was provided by Linda Romagnano, PhD, of Peloton Advantage (Parsippany, NJ). The opinions expressed in this article are those of the authors. The authors received no honorarium/fee or other form of financial support related to the development of this article.
REFERENCES
- 1.Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;1197:2219-2227. [DOI] [PubMed] [Google Scholar]
- 2.Rohrich RJ, Pessa JE. The retaining system of the face: histologic evaluation of the septal boundaries of the subcutaneous fat compartments. Plast Reconstr Surg. 2008;1215:1804-1809. [DOI] [PubMed] [Google Scholar]
- 3.Shaw RB, Jr., Katzel EB, Koltz PF, et al. Aging of the facial skeleton: aesthetic implications and rejuvenation strategies. Plast Reconstr Surg. 2011;1271:374-383. [DOI] [PubMed] [Google Scholar]
- 4.Gierloff M, Stohring C, Buder T, Gassling V, Acil Y, Wiltfang J. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg. 2012;1291:263-273. [DOI] [PubMed] [Google Scholar]
- 5.Pessa JE, Rohrich RJ. Discussion: Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg. 2012;1291:274-275. [DOI] [PubMed] [Google Scholar]
- 6.Pontius AT, Chaiet SR, Williams EF., III Midface injectable fillers: have they replaced midface surgery? Facial Plast Surg Clin North Am. 2013;212:229-239. [DOI] [PubMed] [Google Scholar]
- 7.Few JW, Llorente O. Transconjunctival deep plane midface rejuvenation. In: de Castro C, Boehm K, Codner M, eds. Midface Surgery, Techniques in Aesthetic Plastic Surgery. New York, NY: Sanders; 2009:161-171. [Google Scholar]
- 8.Seitz IA, Llorente O, Few JW. The transconjunctival deep-plane midface lift: a 9-year experience working under the muscle. Aesthet Surg J. 2012;326:692-699. [DOI] [PubMed] [Google Scholar]
- 9.Jones D. Volumizing the face with soft tissue fillers. Clin Plast Surg. 2011;383:379-390. [DOI] [PubMed] [Google Scholar]
- 10.Le Louarn C, Buthiau D, Buis J. Structural aging: the facial recurve concept. Aesthetic Plast Surg. 2007;313:213-218. [DOI] [PubMed] [Google Scholar]
- 11.Cliff SH. Patient satisfaction measures: clinical data. Cosmet Dermatol. 2007;20(suppl 3):27-31. [Google Scholar]
- 12.Clancy CM, Eisenberg JM. Outcomes research: measuring the end results of health care. Science. 1998;2825387:245-246. [DOI] [PubMed] [Google Scholar]
- 13.Dayan SH, Arkins JP, Patel AB, Gal TJ. A double-blind, randomized, placebo-controlled health-outcomes survey of the effect of botulinum toxin type a injections on quality of life and self-esteem. Dermatol Surg. 2010;36(Suppl 4):2088-2097. [DOI] [PubMed] [Google Scholar]
- 14.Ching S, Thoma A, McCabe RE, Antony MM. Measuring outcomes in aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg. 2003;1111:469-480. [DOI] [PubMed] [Google Scholar]
- 15.MacPherson S. Self-esteem and cosmetic enhancement. Plast Surg Nurs. 2005;251:5-20. [DOI] [PubMed] [Google Scholar]
- 16.Kosowski TR, McCarthy C, Reavey PL, et al. A systematic review of patient-reported outcome measures after facial cosmetic surgery and/or nonsurgical facial rejuvenation. Plast Reconstr Surg. 2009;1236:1819-1827. [DOI] [PubMed] [Google Scholar]
- 17.Haas CF, Champion A, Secor D. Motivating factors for seeking cosmetic surgery: a synthesis of the literature. Plast Surg Nurs. 2008;284:177-182. [DOI] [PubMed] [Google Scholar]
- 18.American Society for Dermatologic Surgery. ASDS Survey: 3 in 10 Consumers Considering Cosmetic Procedures. https://www.asds.net/_Media.aspx?id=7976 Accessed August 11, 2014. [Google Scholar]
- 19.Bernardin A, Pierre S, Pather S, Roca Martinez A, Fugazza C, Lebreton P. Vycross™: an innovative dermal filler technology [poster]. In: Presented at: the Anti-Aging Medicine European Congress, October 11-12, 2013; Paris, France. [Google Scholar]
- 20.Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;3911:1602-1611. [DOI] [PubMed] [Google Scholar]
- 21.Hinderer UT. Malar implants for improvement of the facial appearance. Plast Reconstr Surg. 1975;562:157-165. [DOI] [PubMed] [Google Scholar]
- 22.Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carruthers J, Carruthers A. Botulinum toxin type A treatment of multiple upper facial sites: patient-reported outcomes. Dermatol Surg. 2007;33(1 Spec No.):S10-S17. [DOI] [PubMed] [Google Scholar]
- 24.Fagien S, Cox SE, Finn JC, Werschler WP, Kowalski JW. Patient-reported outcomes with botulinum toxin type A treatment of glabellar rhytids: a double-blind, randomized, placebo-controlled study. Dermatol Surg. 2007;33(1 Spec No.):S2-S9. [DOI] [PubMed] [Google Scholar]
- 25.Carruthers A, Carruthers J. A single-center dose-comparison study of botulinum neurotoxin type A in females with upper facial rhytids: assessing patients’ perception of treatment outcomes. J Drugs Dermatol. 2009;810:924-929. [PubMed] [Google Scholar]
- 26.Carruthers J, Carruthers A, Monheit GD, Davis PG. Multicenter, randomized, parallel-group study of onabotulinumtoxinA and hyaluronic acid dermal fillers (24-mg/ml smooth, cohesive gel) alone and in combination for lower facial rejuvenation: satisfaction and patient-reported outcomes. Dermatol Surg. 2010;36(Suppl 4):2135-2145. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann K. Volumizing effects of a smooth, highly cohesive, viscous 20-mg/mL hyaluronic acid volumizing filler: prospective European study. BMC Dermatol. 2009;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeLorenzi C, Weinberg M, Solish N, Swift A. The long-term efficacy and safety of a subcutaneously injected large-particle stabilized hyaluronic acid-based gel of nonanimal origin in esthetic facial contouring. Dermatol Surg. 2009;35(Suppl 1):313-321. [DOI] [PubMed] [Google Scholar]
- 29.Carruthers J, Carruthers A, Tezel A, Kraemer J, Craik L. Volumizing with a 20-mg/mL smooth, highly cohesive, viscous hyaluronic acid filler and its role in facial rejuvenation therapy. Dermatol Surg. 2010;36(Suppl 3):1886-1892. [DOI] [PubMed] [Google Scholar]
- 30.Kestemont P, Cartier H, Trevidic P, et al. Sustained efficacy and high patient satisfaction after cheek enhancement with a new hyaluronic acid dermal filler. J Drugs Dermatol. 2012;11(1 Suppl):s9-16. [PubMed] [Google Scholar]
- 31.Baumann LS, Shamban AT, Lupo MP, et al. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: a multicenter, double-masked, randomized, within-subject study. Dermatol Surg. 2007;33(Suppl 2):S128-S135. [DOI] [PubMed] [Google Scholar]