Abstract
The changes that occur in mammalian systems following trauma and sepsis, termed systemic inflammatory response syndrome (SIRS), elicit major changes in carbohydrate, protein, and energy metabolism. If these events persist for too long they result in a severe depletion of lean body mass, multiple organ dysfunction, and eventually death. Nutritional supplementation has been investigated to offset the severe loss of protein, and recent evidence suggests that diets enriched in branched chain amino acids (BCAAs) may be especially beneficial. BCAAs are metabolized in two major steps that are differentially expressed in muscle and liver. In muscle, BCAAs are reversibly transaminated to the corresponding alpha-keto acids. To complete the degradation of the BCAAs, the alpha-keto acids must travel to the liver to undergo oxidation. The liver, in contrast to muscle, does not significantly express the branched chain amino transferase. Thus, BCAA degradation is under the joint control of both liver and muscle. Recent evidence suggests that in liver, BCAAs may perform signaling functions, more specifically via activation of the mTOR signaling pathway, resulting in positive influences on a wide variety of metabolic and synthetic functions, including increased protein translation, improved insulin resistance, increased insulin-independent glucose transport, and reduced oxidative stress following severe injury and infection. However, understanding of the system-wide effects of BCAAs that integrates both metabolic and signaling aspects is currently lacking. Further investigation in this respect will help rationalize the design and optimization of nutritional supplements containing BCAAs for critically ill patients.
Severe insults, such as burns, trauma, and infection, elicit systemic inflammatory and metabolic responses in humans as well as other mammalian organisms. These responses have presumably evolved to isolate and destroy potentially invading pathogens, as well as to promote rapid wound closure and subsequent repair of the injured tissues. Although variations depending on the type of injury and preexisting pathologies do exist, their pattern is strikingly similar for many large-scale injuries. The early phase post injury involves activation of the coagulation and complement cascades to stop bleeding and provide a first line of defense against invading microorganisms. Immune cells, such as neutrophils and T-helper cells are also recruited to the injured areas, and the liver releases acute phase proteins into the circulation, all of which contribute to killing and clearing bacteria from the host. This massive immune response requires redirecting of significant energy and metabolic resources away from other tissues, especially skeletal muscle.
Critically ill patients are at risk of complications, often stemming from infections contracted after admission to the hospital, which lead to a prolongation and potential intensification of the systemic inflammatory response, instead of a dampening of the response that would indicate recovery. The ensuing condition is called Systemic Inflammatory Response Syndrome (SIRS) and puts further strain on the host's resources, and unless controlled, leads to a significant loss of lean body mass, disseminated microvascular dysfunction, and eventually Multiple Organ Dysfunction Syndrome (MODS) and death (1). Nutritional supplementation in critically ill patients is essential for replenishing endogenous nutrients and for alleviating the loss of lean body mass due to increased proteolysis in peripheral tissues. Recent developments suggest that nutrition should not be viewed as adjunctive supportive care only but also as an active therapeutic strategy, hence the term pharmaconutrition, with the potential to modulate various detrimental effects of severe trauma at the cellular level, such as oxidative stress, excessive proteolysis, and exacerbated pro-inflammatory signaling (2).
Most studies on nutritional supplementation have focused on the amino acid composition because the hallmark of SIRS and associated metabolic derangements is the accelerated proteolysis and increased utilization of endogenous amino acids for hepatic production of acute phase proteins and gluconeogenesis, the maintenance of gut mucosal integrity, and mounting of the immune response (3). SIRS is associated with a hypermetabolic state characterized by increased energy expenditure, a negative nitrogen balance, and a net efflux of amino acids from muscle (4). Reversing these trends through the supplementation of amino acids could prevent excessive loss of lean body mass, and allow for an eventual recovery through the resolution of the inflammatory response.
Glutamine and arginine are two amino acids that received a great deal of attention in the past decades. Glutamine, the most abundant free amino acid in the body (~60% of intracellular amino acid pool in skeletal muscle), serves as an important carrier of nitrogen to viscera and the immune system. Depletion of glutamine stores within the muscle is thought to be indicative of severe muscle wasting (5), and by increasing glutamine levels in the circulation, one may reverse of slow down this depletion, thus preserving skeletal muscle mass. Glutamine is also important as a precursor in the synthesis of glutathione, a major anti-oxidant molecule in the body, and as a signaling molecule involved in the regulation of immune functions, including neutrophil phagocytosis and the expression of heat shock proteins (6).
Arginine has been labeled a conditionally essential amino acid due to the fact that, during times of stress, endogenous sources do not meet the demand for the amino acid, and therefore external intake is required. Like glutamine, arginine is an important regulator of the immune system and also precursor for the synthesis of nitric oxide (NO), which has immunoregulatory functions, including killing of pathogens and modulating cytokine production, as well as acting as scavenger of superoxide anion (7). NO is also well known as a vasodilator which allows increased blood flow to sites of injury. Various isoforms of NO synthase are responsible for NO production. The constitutively expressed form endothelial NO synthase is activated by various agonists through the release of intracellular calcium, which then triggers synthesis of relatively small amounts of NO whose effects are primarily local relaxation of the smooth muscle and vasodilatation. The inducible NO synthase is transcriptionally regulated by inflammatory mediators, and NO synthesis is then only limited by the available concentration of arginine. Disproportionate NO production caused by supraphysiological arginine supplementation may lead to excessive vasodilatation and hemodynamic instability, which are often seen in SIRS patients (8).
Branched chain amino acids (BCAAs, namely leucine, isoleucine and valine) have been shown to promote muscle protein synthesis and reduce protein catabolism; therefore, BCAA-enriched nutrition has been suggested as potentially therapeutic for critically ill patients (9). Clinical studies using BCAA supplementation, which are summarized in Table 1, are however extremely diverse with respect to study design, pathologic states, patient enrollment criteria, and supplementation regimens. As a result, to date there is no consensus whether or not BCAA-enriched nutritional supplementation is really effective and should be used. BCAAs also tend to be seen as a group of amino acids with similar properties, but in fact evidence suggests that leucine is the amino acid primarily responsible for the beneficial effects of BCAAs on protein synthesis and energy regulation, while isoleucine and valine may be dispensable (9).
Table 1.
Clinical studies investigating the effects of amino acid enriched nutritional supplementation on survival and recovery.
| Year | Author | # of patients | Condition | Duration | Administered Supplements | Outcomes | Notes |
|---|---|---|---|---|---|---|---|
| 1984 | Manelli et al. (61) | 22 | Severe burn | 5 days | BCAA-enriched (41%) vs. conventional (22% BCAA) parenteral nutrition | BCAA supplementation demonstrated improvement in protein catabolism | No beneficial effect upon nitrogen loss or nitrogen balance was shown |
| 1988 | Yu et al. (62) | 12 | Severe burn | 48-96 h | Enteral feeding with BCAA-enriched (44%) vs. conventional egg protein formulation | BCAA enriched feeding failed to demonstrate significant benefits in terms of protein synthesis or degradation | Cross-over design study, no parallel controls |
| 1990 | King et al. (63) | 14 | Severe burn | 15 days | Standard regimen (16% BCAA) vs. BCAA-enriched (31%) or a similar regimen where 65% of the leucine is replaced by KIC | Leucine enrichment reduced muscle protein breakdown compared to a standard feed, whereas KIC enrichment did not | Nitrogen balance or serum albumin levels were not significantly affected |
| 1997 | Garcia-de-Lorenzo et al. (64) | 69 | Sepsis | 11 day | Total parenteral nutrition, either 1.5 g amino acids/kg/day with 23% or 45% BCAA or 1.1 g/kg/day with 45% BCAA content | Lower mortality rate observed in patients groups with high BCAA loads. Higher plasma conc.s of BCAAs and rapid protein turnover is shown | |
| 1997 | Griffiths et al. (65) | 84 | Critically ill | 6 months | Glutamine containing parenteral nutrition vs. isonitrogenous isoenergetic control | Survival at 6 months was significantly improved in patient group receiving glutamine. Total ICU and hospital cost per survivor was reduced by half | |
| 2003 | Marchesini et al. (66) | 174 | Advanced cirrhosis | 1 year | BCAAs vs. lactoalbumin or maltodextrin | BCAA significantly reduced combined event rates compared with lactoalbumin and nonsignificantly compared with maltodextrin | Liver function tests of the patients were stable or improved with BCAA treatment |
| 2005 | Muto et al. (67) | 646 | Decompensated cirrhosis | 2 years | Orally administered BCAAs vs. diet therapy with defined daily food intake | The incidence of events decreased in the BCAA group. Serum albumin concentration increased significantly | |
| 2007 | Fukushima et al. (11) | 7 | Cirrhosis | 8 week | All subjects were received oral granular preparations consisting of BCAAs alone | Basal levels observed initially were low total albumin and high oxidized/reduced albumin ratio. BCAA supplementation improved oxidized/reduced albumin ratio | No controls BCAA supplementation increases the turnover leading to reduction in its circulation half-life and therefore reduced level of oxidation |
| 2008 | Ohno et al. (12) | 27 | HCV positive cirrhosis | 6 months | Oral granular preparations of BCAAs vs. no supplementation | BCAA supplementation reduced production of oxidative stress and microinflammation which lead to a decreased occurance of HCC | |
| 2011 | Ken et al. (68) | 236 | Living donor liver trans-plantation | 1 month pre-op | Oral supplementation with BCAAs one month before surgery or no supplementation | Incidence of bacteremia after transplantation was lower in BCAA group |
There is evidence that BCAAs may impart their beneficial effects not only by replenishing nutrients, but also by controlling the redox state. A recent study showed that BCAA-enriched amino acid diets increased longevity of mice by inducing mitochondrial biogenesis and reducing oxidative stress through signaling mechanisms that increase defenses against oxidative stress (10). Moreover, in patients with liver disease, BCAA-enriched supplements have been shown to induce a marked reduction of oxidative stress accompanied by a positive effect on protein synthesis and glucose metabolism (11, 12).
In sum, amino acid supplements can significantly impact on intracellular signaling networks, redox state, and protein synthesis rate, all of which can have far reaching therapeutic consequences. A better understanding of the comprehensive metabolic and signaling effects of amino acid supplementation in normal and “critical” states will be important to rationally design nutritional regimens containing optimal combinations of amino acids. In this review, we summarize current knowledge of the cellular mechanisms whereby BCAAs impact on metabolism and cell signaling under stress.
Metabolism of Branched-chain Amino Acids
BCAAs go across the cell membrane via one transport mechanism, and as a result compete with each other for transport into the cell (9, 13). Transport into the mitochondria is governed by yet another transport mechanism (14), which maintains different BCAA levels in the cytosol, where they putatively function as signaling molecules, and in the mitochondria, where they are ultimately degraded to produce energy. These transport mechanisms are also differentially regulated among tissues. For example, inflammatory signals tend to inhibit BCAA transport into muscle, while promoting the same into liver (15). Studies also indicate a decrease in BCAA absorption through the gut in systemic inflammatory states (16), which increases the importance of inter-tissue transport of BCAAs in these pathological states.
Metabolic degradation of all three naturally found BCAAs proceeds first by deamination followed by breakdown of branched carbon structures into simpler ones that are further oxidized via major lipid oxidation pathways in mitochondria. BCAA deamination is catalyzed by mitochondrial branched chain amino transferase (BCATm), which removes the amino group from the BCAA carbon backbone and transfers it to α-ketoglutarate to form glutamate and the corresponding branched chain α-keto acid (BCKA). This reaction is reversible and therefore provides a mechanism to redistribute nitrogen among the various BCAAs with glutamate as the intermediate (17). BCATm is either not expressed or barely expressed in liver, therefore the initial step in BCAA metabolism primarily occurs in other tissues, with skeletal muscle having the major contribution. Furthermore, glutamate produced as a byproduct of BCKA generation may be further transaminated to glutamine (18), which can be exported. Thus, muscle-derived products of BCAA metabolism include BCKAs and indirectly glutamine, both of which can taken up by the liver.
Unlike branched chain amino acids, branched chain α-keto acid transport is not governed by a single transporter, but is instead mediated via monocarboxylate transporters (MCTs), which also control the transport of lactate, pyruvate and ketone bodies through the plasma membrane (19). There are many different MCTs that are active throughout multiple tissues, indicative of organ specific metabolic regulation through transport activity. Notably, muscle has been shown to co-express multiple MCTs, including MCTs 1, 2, 4, 5, 6, and 7 (though MCT7 is found only in human muscle, not rat), indicating a need in muscle cells for high throughput transport mechanisms capable of moving multiple metabolites, including BCKAs. Studies have been conducted to assess the Km values for some of these transporters, and although many remain unknown, it is worth noting that BCKA transport is significantly higher in MCT4 than in MCTs 1 or 2 (20). Utilizing these transport mechanisms, newly metabolized BCKAs can travel from the muscle to other peripheral tissues, notably the liver, for further metabolism.
BCKAs undergo oxidative decarboxylation, which is catalyzed by the multienzyme complex BCKA dehydrogenase (BCKDH) within the mitochondria, with the end products being branched chain acyl-coenzyme A esters of the BCKA precursors. An important fact to note is that the enzyme activity ratio of BCAT:BCKDH is high in muscle, which tends to favor the release of BCKAs into the circulation instead of their oxidation via MCT transport. In liver, the ratio is reversed (because BCATm is virtually absent and BCKDH activity is high), and oxidation of BCKAs is favored (21). As a result, there is a continuous exchange of BCKAs from skeletal muscle to liver.
Unlike BCATm, BCKDH catalyzes an irreversible reaction and therefore controls the flux of BCKAs towards complete oxidation. BCKDH is regulated by a post-translational mechanism involving BCKDH kinase, which inactivates the BCKHD enzyme complex by phosphorylation. BCKAs, which are the substrates of BCKDH, also serve as inhibitors of BCKDH kinase, therefore accumulation of BCKAs causes dephosphorylation and activation of BCKHD. The leucine-derived BCKA, alpha-ketoisocaproate (KIC), is a potent inhibitor of the regulatory kinase. BCKAs derived from isoleucine and valine, alpha-keto-beta-methylbutyrate (KMV) and alpha-ketoisovalerate (KIV), respectively, have similar effects, albeit with lower inhibitory potency on the kinase (22). Therefore, it is thought that leucine-mediated regulation of this pathway is dominant. This regulatory scheme conserves BCAAs, putatively for protein synthesis, when their concentration is low, while favoring BKCA oxidation when BCAA concentration is high (23).
The foregoing has important implications for liver-muscle communication during systemic inflammation, as the major source of BCKAs reaching the liver are muscle-derived. Furthermore, BCAAs stored in the liver must either be exported to the muscle for oxidation, or locally used for protein synthesis. As detailed further below, BCAAs also have important signaling effects in addition to their metabolic fates, which creates a dual role for these molecules, namely signals in the liver, and metabolic substrates in the muscle. The distributed nature of the BCAA degradation pathways between muscle and liver may suggest competing functions (in this case, the signaling properties of BCAAs potentially compete with their use as energy sources through BCKA dehydrogenase activity) which can be resolved through the division of BCAAs and BCKAs that are achieved through inter organ compartmentalization. Figure 1 summarizes the pathways involved in BCAA metabolism in liver and muscle.
Figure 1.
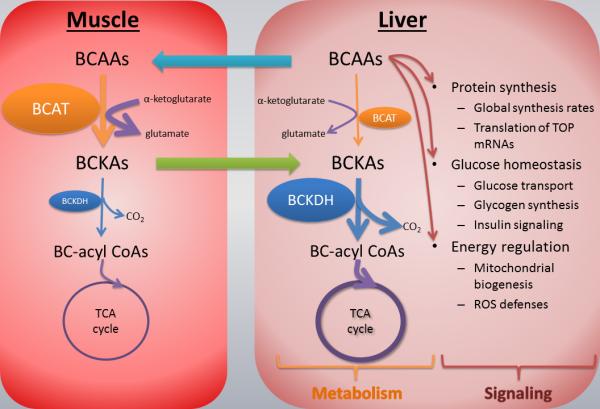
BCAA metabolism in liver and muscle. BCAAs absorbed from the gut reach the liver where they are taken up and play various signaling and protein synthetic roles. The BCAA catabolic pathway takes place within mitochondria, and consists of two major steps: reversible transamination with alpha-ketoglutarate to form BCKAs, followed by irreversible decarboxylation to form CoA compounds which enter the TCA cycle. The transamination step catalyzed by branched chain aminotransferase (BCAT) mostly occurs in muscle because this is where BCAT expression is highest. On the other hand, BCKA decarboxylation, catalyzed by branched chain ketoacid dehydrogenase (BCKDH), as well as later steps leading to complete oxidation, occur mainly in liver (19).
Signaling Functions of Branched-chain Amino Acids
Levels of amino acids, and in particular BCAAs, can affect relevant metabolic fluxes through mass action effects (i.e. via a direct effect of concentration on reaction rate), but in fact these levels are monitored through specific sensing mechanisms that initiate or terminate processes that utilize amino acids, including protein synthesis (24). Thus, BCAAs, besides their ability to serve as substrates for protein synthesis or energy production, also act as signaling molecules. For example, provision of BCAAs mimics the effect of a more complete mixture of amino acids and stimulates the initiation of mRNA translation in skeletal muscle. Among BCAAs, leucine appears to be the most potent effector of protein synthesis (25), which is not surprising given its role as a primary regulator of BCAA degradation.
Leucine Based Regulation of Translational Initiation in Eukaryotes
Leucine promotes translation via a stimulatory effect on eukaryotic initiation factor (eIF) 4F complex (eIF4F). Assembly of eIF4F complex is regulated by the repressor protein eIF binding protein-1 (4E-BP1)(25). 4E-BP1 prevents formation of active eIF4F complex by sequestering mRNA cap binding protein eIF4E and rendering it inactive. Leucine induces hyper-phosphorylation of 4E-BP1 leading to its dissociation from eIF4E, allowing eIF4F active complex formation by association of eIF4E with a large scaffold protein eIF4G (26, 27). Leucine administration also enhances the phosphorylation of ribosomal p70S6 kinase (25).
Recent studies suggest that all of these interactions are in fact downstream events controlled by the mammalian target of rapamycin (mTOR), which is a highly conserved serine/threonine kinase that regulates cell growth in response to nutrient status. mTOR appears to act as a critical signaling element sensing the intracellular availability of amino acids and whose activation triggers a downstream cascade leading to activation of eukaryotic initiation factors (28, 29). The exact mechanism by which leucine activates mTOR remains elusive, however.
Data obtained from manipulating the activity of another eukaryotic initiation factor, EIF2, suggests differences in leucine-mediated stimulation of protein synthesis between liver and muscle. GCN2 is a kinase tasked with the regulation of EIF2 through inhibition, and furthermore, it has been shown to mediate leucine based regulation of protein synthesis by being itself negatively regulated by leucine. Anthony et al. were able to use a GCN2 knockout experimental set up in mice to demonstrate some of the interplay between leucine and GCN2. In wild type animals which had functional GCN2 activity, leucine deprivation caused loss of both liver and lean body mass in the animals, but no mortality was observed (30). Due to the fact that GCN2 mediates leucine's interaction with EIF2, among other initiation factors, knockout mice were unable to respond appropriately to leucine restriction with the repression of EIF2. Observed in these animals was a loss of skeletal muscle only, while other tissues were largely spared, as well as significantly increased mortality in animals. Decoupling leucine sensitivity from EIF2 regulation in these animals appears to alter the host response to the lack of BCAAs from a systemic reduction of protein synthesis towards an unhealthy depletion of peripheral tissue mass. Furthermore, leucine has been implicated in the stimulation of protein synthesis through EIF2(31), which strongly suggests that there is a regulatory element towards branched chain amino acid activity in protein synthesis beyond their use as substrates. Together, these findings suggest that leucine is an important metabolite which is capable of regulating protein synthesis in an mTOR-dependent pathway that is likely mediated through EIF proteins. When leucine is scarce, the appropriate response appears to involve a reduction in overall protein synthesis and a distributed minor loss of lean body mass with minimal impact on the host. The loss of GCN2 function, which lead to an unregulated activation of EIF2 in the knock-out animals in spite of the absence of leucine led to an abnormal loss of skeletal muscle protein and metabolic complications that are strikingly similar to that observed hypermetabolism after burns and trauma. The addition of leucine has been shown to stimulate EIF4E activity in skeletal muscle following burn injury in rats, albeit there was little evidence of EIF2 activity (32), and has been implicated in increased protein synthesis following burn. With regards to burn, trauma and sepsis-related stress, it is worth noting that the GCN2/EIF2 regulatory pathway described above is similar in function to other EIF families, which have been shown to be regulated by a much wider array of cellular functions than leucine deprivation, including apoptosis related factors (33) Furthermore, the regulatory structure that governs the mediation of protein synthesis through amino acid sensing is not completely elucidated (34). While evidence suggests that branched chain amino acids, and in particular, leucine, play an important role in linking metabolic functions to protein synthesis, however, one also needs to take into consideration the fact that these amino acids may affect protein degradation rates as well. The same pathways discussed above are in fact involved in apoptosis and protein catabolism. The net effect of BCAAs on protein turnover appears to vary depending on the tissue and the specific protein as well.
For example, although BCAAs stimulate protein translation and increase protein synthesis in skeletal muscle, they do not increase total protein production in liver (27). The effect in liver seems much more specific; for example, administration of leucine to fasted rats increases the translation of ribosomal proteins L26, S4 and S8 by enhancing polysome size, where their translation occurs. This contrasts with β-actin and albumin, for which there is no change in polysomal association of their mRNAs (24). These three ribosomal mRNAs have a terminal oligopyrimidine (TOP) sequence at the 5’ end, which is common for proteins involved in the translational apparatus (27). Association of TOP mRNAs with the polysomes requires phosphorylation of p70S6K, and rapamycin selectively suppresses their translation through inhibition of mTOR activity induced by leucine. These results suggest that BCAAs, particularly leucine, have a regulatory role in liver which is distinct from skeletal muscle. This is also reflected by the fact that liver does not oxidize BCAAs, in contrast to skeletal muscle. In cases of severe injury and/or infection, hepatic BCAA uptake increases in order to meet the increased demand of amino acids for protein synthesis imposed by the acute phase response (35). This increased BCAA uptake may also affect fundamental processes of translation and transcription which also impact on the acute phase response, but have not yet been fully explored.
Branched Chain Amino Acid Modulation of Insulin Signaling and Glucose Uptake
The effects of amino acids on glucose homeostasis that have been reported in the literature are extremely variable, ranging from increasing glucose sensitization to inducing insulin resistance. The effects are in fact highly dependent on several considerations, including (but not limited to) the constituents of the regimen administered, the presence of other factors such as insulin or lipids, the organ or cell type in which the results are obtained and the presence of underlying disease, such as hepatitis, cirrhosis or diabetes.
Firstly, a series of in vitro studies in which amino acid mixtures were supplemented to muscle and liver cells indicated that amino acids reduce insulin induced glucose uptake (29). In neonatal rat thigh skeletal muscle cells, in vitro, a balanced mixture of amino acids reduced insulin-stimulated glucose transport and this reduction was related to increased mTOR activity. Incubation of the cells with amino acids potentiated the activation of mTOR/p70S6 kinase pathway by insulin causing deactivation of phosphatidylinositol 3-kinase (PI3-kinase). This deactivation effect was associated with Ser/Thr phosphorylation of IRS-1 resulting in impaired recruitment of PI3-kinase to IRS-1 and subsequent reduction in cellular glucose uptake (36). This study also confirmed a prior in vitro study in which amino acids modulated the action of insulin in hepatoma and muscle cells bidirectionally; acting as positive signals for protein anabolism while limiting glucose transport (28). Activation of mTOR/p70S6 pathway was also associated with increases in glycogen synthesis. In human muscle cells, amino acids were shown to stimulate mTOR/p70S6 kinase pathway and transiently inhibit glycogen synthase kinase-3 (GSK-3), thereby stimulating glycogen synthesis (37).
In insulin free conditions, contrary to what was observed before, amino acids, particularly BCAAs, enhanced glucose uptake and utilization. In isolated rat skeletal muscle cells, leucine specifically increased glucose transport through a PI3-kinase and protein kinase C (PKC) dependent, albeit through an mTOR independent mechanism (38). This was also confirmed in skeletal muscle of cirrhotic rats where glucose metabolism was impaired. Leucine, and more potently isoleucine, improved glucose tolerance independently from insulin as a result of increased GLUT4 and GLUT1 translocation to plasma membrane. Additionally, glycogen synthase (GS) activity was also augmented by leucine (not by isoleucine) through an mTOR dependent mechanism. Taken together these data suggested that leucine and isoleucine supplementation in cirrhotic patients may partially substitute for insulin and therefore alleviate the impaired glucose homeostasis (39). Insulin resistance is frequently observed in patients with chronic liver disease, such as cirrhosis and prolonged hepatitis C. In the case of cirrhosis, insulin sensitivity is impaired independent of the etiology of the disease or clinical and nutritional state of the patients (40). In the case of hepatitis C virus (HCV); virus driven core protein is shown to cause degradation of the insulin receptor substrate 1 and 2 (IRS-1, IRS-2) as well as suppression of insulin signaling cascade through reduction of PI3k and Akt phosphorylation (41). If not properly managed, development of insulin resistance leads to various complications of hepatic or extrahepatic origin throughout the course of these diseases (42). In both of these conditions, the effectiveness of BCAA supplementation for improvement of glucose tolerance was demonstrated clinically (43-45). However, the molecular details of how BCAAs can help maintain glucose homeostasis in such diverse pathologies as well as whether there are any conditions where BCAAs aggravate the pathology instead of ameliorating remains to be investigated. Another aspect that has been the subject of many studies was the individual effects of three BCAAs and which one is most beneficial therapeutically.
Differential effects of each BCAA on whole body glucose clearance and utilization mechanisms were reported in rats. Oral administration of BCAAs showed that isoleucine significantly reduces glucose tolerance whereas valine increases it and leucine has no effect. The glucose-lowering effect of isoleucine was due to insulin independent glucose uptake in the skeletal muscle. Isoleucine did not induce glycogen synthesis, however, leucine and valine stimulated glycogen synthesis (46). The hypoglycemic effect of isoleucine was investigated further, where isoleucine significantly inhibited glucose production in hepatocytes associated with a decline in mRNA levels for two key gluconeogenic enzymes, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (G6Pase). Decreased activity of G6Pase was also reported. Therefore, it is established that isoleucine exerts an overall hypoglycemic effect involving a reduction in gluconeogenesis in liver in addition to an increase in skeletal muscle glucose uptake (47). This effect was also confirmed in db/db mice which model severe type-2 diabetes (48). However, when BCAAs were delivered in a balanced mixture, the specific hypoglycemic effect of isoleucine subsided. Glucose levels in plasma following a glucose tolerance test did not change much in response to BCAA administration, although a boost was still observed for the glucose transport system and primary enzyme (liver type glucokinase; L-GK) for glucose utilization in liver (49). The fact that the BCAA mixture is not as effective as isoleucine alone for improving glucose uptake may be due to confounding effects of the other two BCAAs, especially leucine, on glucose transport as well as insulin secretion. While supplementation of isoleucine improved glucose clearance, a similar effect was observed for the deprivation of leucine. A diet deficient in leucine was shown to improve whole-body insulin sensitivity as well as increased insulin signaling in liver under both normal and insulin resistant conditions, in vivo (50). An increase in the relative amounts of isoleucine and valine accompanies leucine deprivation, therefore whole-body insulin sensitivity may be the result of the shifted balance of these metabolites. As exemplified by these cases, combinations of BCAAs may have various effects on insulin signaling and anabolic responses, and a comprehensive understanding of the way BCAAs may crosstalk, which is currently lacking, would be important to optimize BCAA supplementation regimens.
Another confounding factor that needs a thorough assessment for the use of BCAA supplementation regimens is the consumption of dietary lipids along with the amino acids. Firstly, isoleucine was shown to exert its hypoglycemic and insulin sensitizing effect even under the metabolic burden of a high fat diet (HFD) (48). On the contrary, leucine also improves glucose metabolism in animals on HFD, but is ineffective in animals on a normal diet. Zhang et al. (51) conducted a study where they doubled the amount of dietary leucine by giving leucine containing drinking water to mice. Leucine water, in addition to normal chow diet, had no effect. However, for animals on HFD, leucine improved glucose metabolism by increasing insulin sensitivity and decreasing glucagon secretion as well as cholesterol levels. Macotela et al. (52) confirmed these findings and further showed that doubling leucine reduces adipose tissue inflammation and hepatic steatosis induced by HFD, while improving insulin signaling in muscle, liver and fat. In addition to individual effects of leucine and isoleucine, combination of other amino acids was also investigated. Fortification of all ketogenic amino acids (KAAs) in HFD (KAAs: leucine, isoleucine, valine, lysine and threonine), improved diet-induced hepatic steatosis and improved glucose tolerance in mice (53). All of these studies favor the supplementation of BCAAs to prevent the detrimental effects of HFD on glucose homeostasis; however a recent metabolomics study challenges this view. Principal component analysis of more than 100 analytes measured in the plasma from lean versus obese subjects revealed that, rather than lipid related markers, BCAAs were more strongly associated with insulin resistance (54). BCAAs and aromatic amino acids forms an independent cluster including by-products of BCAA catabolism such as glutamine, alanine, C3 and C5 acylcarnites suggesting that; not BCAAs themselves, but an altered flux through BCAA catabolism pathway forms the actual association with insulin resistance. Furthermore, gastric bypass surgery, which is an established treatment for excessive obesity, is shown to improve insulin sensitivity and glucose homeostasis in parallel with a dramatic decline in circulating BCAAs and BCAA-related metabolites (55, 56).
Overall, there is no consensus on under what conditions BCAAs are useful for the regulation of glucose homeostasis. As the further molecular details unfold with future studies, it may be possible to better predict these responses and consequently formulate better regimens for the use of BCAA supplementation in critical illness. What would help to build a solid understanding about the mechanisms BCAAs work is to use a systems approach to investigate molecular events as a whole, rather than observing perturbations in limited number of representative markers. Only then, it would be possible to form standardized regimens for different scenarios applicable in clinic.
Activation of mTOR and ROS Scavenging by Branched Chain Amino Acids
Besides modulating protein translation, mTOR has a major impact on oxygen consumption, choice of substrates for energy production, and defense capacity against reactive oxidants (57). A BCAA supplementation study in mice showed increased average life-span, increased mitochondrial biogenesis, and upregulation of expression of genes involved in scavenging of reactive oxygen species (ROS). Furthermore, these changes appeared to be largely mediated by increased mTOR activity (10). In essence, by acting as the indicators of available nutrient supply, BCAAs regulate the critical cellular decisions, such as selecting the correct substrate for energy production based on available resources and determining whether initiating new protein synthesis action is feasible or not. Since BCAA availability in the liver is effectively controlled through the skeletal muscle, this mechanism appears to serve as a way for the liver to take measure of the robustness of its supply lines during crisis.
Under conditions of stress, preserving mitochondrial function is critical for maintaining cell and tissue viability. For example, excessive ROS production by dysfunctional mitochondria has been implicated in organ failure induced by inflammation and hypoxia following trauma (58). It has been suggested that BCAAs behave as evolutionarily conserved modulators of life-span through exerting control on mitochondrial biogenesis, cellular energy metabolism and ROS scavenging systems (59). Two studies in patients with liver disease indicated that BCAAs markedly reduce oxidative stress and positively affect protein synthesis (11, 60). As we have discussed earlier, BCAAs are implicated in the mTOR signaling pathway, while impacting on other anabolic regulators such as insulin, and on the cellular translational machinery. Studies also suggest that BCAAs are being utilized as markers of amino acid availability, and have different effects in liver and muscle. From this, it is clear that BCAAs have a much greater role in regulating whole body metabolism than their role as protein synthesis substrates would suggest. Their ability to interact with anabolic pathways in order to stimulate glucose uptake, protein synthesis, and kinase signaling pathways indicates that they may be a critical line of communication between muscle tissues and central metabolism in the liver, and therefore may be worthwhile as therapeutic agents.
Conclusions
SIRS and related injury-induced systemic inflammatory responses have not seen much improvement in terms of clinical treatment options, primarily due to the complexity and redundancy in the regulatory pathways and in the inter-organ relationships involved. Thus, traditional approaches that attempt to target a single pathway or molecule have largely failed. It is also noteworthy that mediators and signaling aspects of the inflammatory response have received the bulk of the attention in SIRS research. On the other hand, many of the deleterious effects of SIRS are metabolic in nature: loss of lean body mass, a negative nitrogen balance, and energetic failure.
Some of the most promising nutritional supplements that are currently being explored are BCAAs. Despite being essential amino acids (therefore they cannot be endogenously made on demand), they play a major role in the communication between muscle and liver. BCAAs, and their reversible degradation products, BCKAs, are differentially regulated in the muscle and liver, with the production of BCKAs from BCAAs occurring almost exclusively in the muscle, and the final irreversible catabolism of BCKAs occurring in the liver. The fact that the liver is responsible for the final breakdown suggests that energy derived from these amino acids is intended for the liver, and yet the fact that hepatic cells have extremely limited capacities for the breakdown of BCAAs directly indicates that the role of BCAAs within hepatic cells is not solely as substrate. Indeed, studies have shown that within the muscle, where BCAA breakdown to BCKA is encouraged, the addition of BCAAs will increase total protein production. In contrast, hepatic exposure to BCAAs causes a significantly different response, whereby they promote the synthesis of translational proteins, increasing the liver's capacity for overall protein production. Furthermore, leucine has been shown to promote mitochondrial biogenesis and reactive oxygen defenses, both of which enhance the liver's capacity for energy production. Since the liver is incapable of directly influencing BCAA concentration, BCAAs may serve as signals that are controlled by the muscle. These signals may reflect the nutritional state of the host, and therefore influence the metabolic output of the liver during periods of stress. The interplay of BCAA supplementation between the liver and muscle highlights the importance of a systems approach towards the study of both the efficacy of nutritional interventions, and the structure of the regulatory architecture they seek to alter. Too much BCAA supplementation may induce anabolic activity in the liver at the expense of peripheral tissues, while too little may create a metabolic response appropriate for starvation, both of which could potentially be detrimental to patient recovery. Investigation of the mechanisms of action of BCAAs thus represents an opportunity for Systems Biology to tackle challenges inherent to the multi-organ response that governs the mammalian inflammatory response to severe injury and infection.
Acknowledgements
The authors gratefully acknowledge the financial support from NIH grant GM082974.
References
- 1.Beal AL, Cerra FB. Multiple organ failure syndrome in the 1990s. Systemic inflammatory response and organ dysfunction. Jama. 1994;271:226–233. [PubMed] [Google Scholar]
- 2.Reddell L, Cotton BA. Antioxidants and micronutrient supplementation in trauma patients. Current Opinion in Clinical Nutrition & Metabolic Care. 2012;15:181. doi: 10.1097/MCO.0b013e32835076df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawson CM, Miller KR, Smith VL, McClave SA. Appropriate Protein and Specific Amino Acid Delivery Can Improve Patient Outcome: Fact or Fantasy? Current gastroenterology reports. 2011;1-8 doi: 10.1007/s11894-011-0201-0. [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL. Metabolic support in sepsis and multiple organ failure: More questions than answers [horizontal ellipsis]. Critical care medicine. 2007;35:S436. doi: 10.1097/01.CCM.0000278601.93369.72. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Toigo G, Ciocchi B, Situlin R, Iscra F, Gullo A, Guarnieri G. Metabolic response to injury and sepsis: changes in protein metabolism. Nutrition. 1997;13:52–57. doi: 10.1016/s0899-9007(97)00206-2. [DOI] [PubMed] [Google Scholar]
- 6.Vermeulen MAR, van de Poll MCG, Ligthart-Melis GC, Dejong CHC, van den Tol MP, Boelens PG, van Leeuwen PAM. Specific amino acids in the critically ill patient-Exogenous glutamine/arginine: A common denominator? Critical care medicine. 2007;35:S568. doi: 10.1097/01.CCM.0000278600.14265.95. [DOI] [PubMed] [Google Scholar]
- 7.Luiking YC, Poeze M, Dejong CH, Ramsay G, Deutz NE. Sepsis: An arginine deficiency state? Critical care medicine. 2004;32:2135. doi: 10.1097/01.ccm.0000142939.81045.a0. [DOI] [PubMed] [Google Scholar]
- 8.Latifi R. Nutritional therapy in critically ill and injured patients. The Surgical clinics of North America. 2011;91:579. doi: 10.1016/j.suc.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 9.De Bandt J-P, Cynober L. Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis. The Journal of nutrition. 2006;136 doi: 10.1093/jn/136.1.308S. [DOI] [PubMed] [Google Scholar]
- 10.D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba M, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell metabolism. 2010;12:362–434. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima H, Miwa Y, Shiraki M, Gomi I, Toda K, Kuriyama S, Nakamura H, Wakahara T, Era S, Moriwaki H. Oral branched-chain amino acid supplementation improves the oxidized/reduced albumin ratio in patients with liver cirrhosis. Hepatology Research. 2007;37:765–770. doi: 10.1111/j.1872-034X.2007.00123.x. [DOI] [PubMed] [Google Scholar]
- 12.Tomoyoshi O, Yasuhito T, Fuminaka S, Etsuro O, Izumi H, Haruhiko N, Atsunaga K, Seijiro M, Masayuki E, Yoshito T, Kenji S, Masashi M. Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis. Hepatology Research. 2008;38 doi: 10.1111/j.1872-034X.2008.00319.x. [DOI] [PubMed] [Google Scholar]
- 13.Lundholm K, Scherstén T. Incorporation of Leucine into Human Skeletal Muscle Proteins: A Study of Tissue Amino Acid Pools and their Role in Protein Biosynthesis. Acta Physiologica Scandinavica. 1975;93:433–441. doi: 10.1111/j.1748-1716.1975.tb05833.x. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan J, Popovitch JR, Tapley DF. Leucine transport by rat liver mitochondria in vitro. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1969;173:532–539. doi: 10.1016/0005-2736(69)90017-0. [DOI] [PubMed] [Google Scholar]
- 15.Hasselgren P, P. P. S. H. C. W. B. W. F. J. E. CUrrent concepts of protein turnover and amino acid transport in liver and skeletal muscle during sepsis. Archives of Surgery. 1988;123:992–999. doi: 10.1001/archsurg.1988.01400320078016. [DOI] [PubMed] [Google Scholar]
- 16.Gardiner K, Barbul A. Review: Intestinal Amino Acid Absorption During Sepsis. Journal of Parenteral and Enteral Nutrition. 1993;17:277–283. doi: 10.1177/0148607193017003277. [DOI] [PubMed] [Google Scholar]
- 17.Shimomura Y, Honda T, Goto H, Nonami T, Kurokawa T, Nagasaki M, Murakami T. Effects of liver failure on the enzymes in the branched-chain amino acid catabolic pathway. Biochemical and Biophysical Research Communications. 2004;313:381–385. doi: 10.1016/j.bbrc.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Chang TW, Goldberg AL. The metabolic fates of amino acids and the formation of glutamine in skeletal muscle. Journal of Biological Chemistry. 1978;253:3685–3693. [PubMed] [Google Scholar]
- 19.Bonen A, Heynen M, Hatta H. Distribution of monocarboxylate transporters MCT1-MCT8 in rat tissues and human skeletal muscle. Appl Physiol Nutr Metab. 2006;31:31–39. doi: 10.1139/h05-002. [DOI] [PubMed] [Google Scholar]
- 20.Halestrap AP. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 21.Hutson SM, Sweatt AJ, LaNoue KF. Branched-chain amino acid metabolism: implications for establishing safe intakes. The Journal of nutrition. 2005;135:1557S–1564S. doi: 10.1093/jn/135.6.1557S. [DOI] [PubMed] [Google Scholar]
- 22.Shimomura Y, Murakami T, Nakai N. Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. The Journal of .. 2004 doi: 10.1093/jn/134.6.1583S. [DOI] [PubMed] [Google Scholar]
- 23.Holecek M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition. 2010;26:482–490. doi: 10.1016/j.nut.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Reiter AK, Anthony TG, Anthony JC, Jefferson LS, Kimball SR. The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. The International Journal of Biochemistry & Cell Biology. 2004;36:2169–2179. doi: 10.1016/j.biocel.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Kimball SR, Jefferson LS. Signaling Pathways and Molecular Mechanisms through which Branched-Chain Amino Acids Mediate Translational Control of Protein Synthesis. The Journal of Nutrition. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 26.Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine Regulates Translation Initiation in Rat Skeletal Muscle Via Enhanced eIF4G Phosphorylation. The Journal of Nutrition. 2004;134:1704–1710. doi: 10.1093/jn/134.7.1704. [DOI] [PubMed] [Google Scholar]
- 27.Anthony TG, Anthony JC, Yoshizawa F, Kimball SR, Jefferson LS. Oral Administration of Leucine Stimulates Ribosomal Protein mRNA Translation but Not Global Rates of Protein Synthesis in the Liver of Rats. The Journal of Nutrition. 2001;131:1171–1176. doi: 10.1093/jn/131.4.1171. [DOI] [PubMed] [Google Scholar]
- 28.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. Journal of Clinical Investigation. 1998;101:1519. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair KS, Short KR. Hormonal and Signaling Role of Branched-Chain Amino Acids. The Journal of Nutrition. 2005;135:1547S–1552S. doi: 10.1093/jn/135.6.1547S. [DOI] [PubMed] [Google Scholar]
- 30.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 31.Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, Rupassara SI, Garlick PJ. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab. 2011;301:E1236–1242. doi: 10.1152/ajpendo.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr. 2004;134:1704–1710. doi: 10.1093/jn/134.7.1704. [DOI] [PubMed] [Google Scholar]
- 33.Su N, Kilberg MS. C/EBP Homology Protein (CHOP) Interacts with Activating Transcription Factor 4 (ATF4) and Negatively Regulates the Stress-dependent Induction of the Asparagine Synthetase Gene. Journal of Biological Chemistry. 2008;283:35106–35117. doi: 10.1074/jbc.M806874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20:436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimball SR, Orellana RA, O'Connor PMJ, Suryawan A, Bush JA, Nguyen HV, Thivierge MC, Jefferson LS, Davis TA. Endotoxin induces differential regulation of mTOR-dependent signaling in skeletal muscle and liver of neonatal pigs. American Journal of Physiology - Endocrinology And Metabolism. 2003;285:E637–E644. doi: 10.1152/ajpendo.00340.2002. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. Journal of Biological Chemistry. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong JL, Bonavaud SM, Toole BJ, Yeaman SJ. Regulation of glycogen synthesis by amino acids in cultured human muscle cells. Journal of Biological Chemistry. 2001;276:952–956. doi: 10.1074/jbc.M004812200. [DOI] [PubMed] [Google Scholar]
- 38.Nishitani S, Matsumura T, Fujitani S, Sonaka I, Miura Y, Yagasaki K. Leucine promotes glucose uptake in skeletal muscles of rats. Biochemical and Biophysical Research Communications. 2002;299:693–696. doi: 10.1016/s0006-291x(02)02717-1. [DOI] [PubMed] [Google Scholar]
- 39.Nishitani S, Takehana K, Fujitani S, Sonaka I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2005;288:G1292–G1300. doi: 10.1152/ajpgi.00510.2003. [DOI] [PubMed] [Google Scholar]
- 40.Müller M, Willmann O, Rieger A, Fenk A, Selberg O, Lautz H, Bürger M, Balks H, von zur Mühlen A, Schmidt F. Mechanism of insulin resistance associated with liver cirrhosis. Gastroenterology. 1992;102:2033. doi: 10.1016/0016-5085(92)90329-w. [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. The American journal of pathology. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawaguchi T, Sata M. Importance of hepatitis C virus-associated insulin resistance: therapeutic strategies for insulin sensitization. World journal of gastroenterology: WJG. 2010;16:1943. doi: 10.3748/wjg.v16.i16.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyake T, Abe M, Furukawa S, Tokumoto Y, Toshimitsu K, Ueda T, Yamamoto S, Hirooka M, Kumagi T, Hiasa Y. Long-term Branched-chain Amino Acid Supplementation Improves Glucose Tolerance in Patients with Nonalcoholic Steatohepatitis-related Cirrhosis. Internal medicine (Tokyo, Japan) 2012;51:2151. doi: 10.2169/internalmedicine.51.7578. [DOI] [PubMed] [Google Scholar]
- 44.Kawaguchi T, Nagao Y, Matsuoka H, Ide T, Sata M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. International journal of molecular medicine. 2008;22:105. [PubMed] [Google Scholar]
- 45.Kawaguchi T, Izumi N, Charlton MR, Sata M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology. 2011;54:1063–1070. doi: 10.1002/hep.24412. [DOI] [PubMed] [Google Scholar]
- 46.Doi M, Yamaoka I, Fukunaga T, Nakayama M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochemical and Biophysical Research Communications. 2003;312:1111–1117. doi: 10.1016/j.bbrc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 47.Doi M, Yamaoka I, Nakayama M, Sugahara K, Yoshizawa F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. American Journal of Physiology- Endocrinology And Metabolism. 2007;292:E1683–E1693. doi: 10.1152/ajpendo.00609.2006. [DOI] [PubMed] [Google Scholar]
- 48.Ikehara O, Kawasaki N, Maezono K, Komatsu M, Konishi A. Acute and chronic treatment of L-isoleucine ameliorates glucose metabolism in glucose-intolerant and diabetic mice. Biological and Pharmaceutical Bulletin. 2008;31:469–472. doi: 10.1248/bpb.31.469. [DOI] [PubMed] [Google Scholar]
- 49.Higuchi N, Kato M, Miyazaki M, Tanaka M, Kohjima M, Ito T, Nakamuta M, Enjoji M, Kotoh K, Takayanagi R. Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. Journal of cellular biochemistry. 2011;112:30–38. doi: 10.1002/jcb.22688. [DOI] [PubMed] [Google Scholar]
- 50.Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu Y-H. Increasing Dietary Leucine Intake Reduces Diet-Induced Obesity and Improves Glucose and Cholesterol Metabolism in Mice via Multimechanisms. Diabetes. 2007;56:1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 52.Macotela Y, Emanuelli B, Bång AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR. Dietary Leucine - An Environmental Modifier of Insulin Resistance Acting on Multiple Levels of Metabolism. PloS one. 2011;6:e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noguchi Y, Nishikata N, Shikata N, Kimura Y, Aleman JO, Young JD, Koyama N, Kelleher JK, Takahashi M, Stephanopoulos G. Ketogenic essential amino acids modulate lipid synthetic pathways and prevent hepatic steatosis in mice. PLoS ONE. 2010;5:e12057. doi: 10.1371/journal.pone.0012057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newgard C, An J, Bain J, Muehlbauer M, Stevens R, Lien L, Haqq A, Shah S, Arlotto M, Slentz C, Rochon J, Gallup D, Ilkayeva O, Wenner B, Yancy W, Eisenson H, Musante G, Surwit R, Millington D, Butler M, Svetkey L. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell metabolism. 2009;9:311–337. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melnik BC. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World Journal of Diabetes. 2012;3:38. doi: 10.4239/wjd.v3.i3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newgard CB. Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell metabolism. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schieke SM, Phillips D, McCoy JP, Aponte AM, Shen R-F, Balaban RS, Finkel T. The Mammalian Target of Rapamycin (mTOR) Pathway Regulates Mitochondrial Oxygen Consumption and Oxidative Capacity. Journal of Biological Chemistry. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 58.Kozlov AV, Bahrami S, Calzia E, Dungel P, Gille L, Kuznetsov AV, Troppmair J, Kozlov A. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Annals of intensive care. 2011;1:41. doi: 10.1186/2110-5820-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valerio A, D'Antona G. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: an evolutionary perspective. Aging (Albany NY) 2011 doi: 10.18632/aging.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohno T, Tanaka Y, Sugauchi F, Orito E, Hasegawa I, Nukaya H, Kato A, Matunaga S, Endo M. Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis. Hepatology Research. 2008;38:683–688. doi: 10.1111/j.1872-034X.2008.00319.x. [DOI] [PubMed] [Google Scholar]
- 61.Manelli J, Garabedian M, Ounis N, Houvenaeghel M, Ottomani A, Bimar J. Effects on muscular and general proteolysis in burn patients of a solution enriched with branched amino acids] 1984:256. doi: 10.1016/s0750-7658(84)80116-1. [DOI] [PubMed] [Google Scholar]
- 62.Yu YM, Wagner DA, Walesreswski J, Burke JF, Young VR. A kinetic study of leucine metabolism in severely burned patients. Comparison between a conventional and branched-chain amino acid-enriched nutritional therapy. Annals of surgery. 1988;207:421. doi: 10.1097/00000658-198804000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King P, Power D. Branched chain amino/keto acid supplementation following severe burn injury: a preliminary report. Clinical Nutrition. 1990;9:226–230. doi: 10.1016/0261-5614(90)90024-m. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-de-Lorenzo A, Ortiz-Leyba C, Planas M, Montejo JC, Nunez R, Ordonez FJ, Aragon C, Jimenez FJ. Parenteral administration of different amounts of branch-chain amino acids in septic patients: clinical and metabolic aspects. Critical care medicine. 1997;25:418. doi: 10.1097/00003246-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Griffiths RD, Jones C, Allan Palmer T. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition*. Nutrition. 1997;13:295–302. [PubMed] [Google Scholar]
- 66.Marchesini G, Marzocchi R, Noia M, Bianchi G. Branched-chain amino acid supplementation in patients with liver diseases. The Journal of nutrition. 2005;135:1596S–1601. doi: 10.1093/jn/135.6.1596S. [DOI] [PubMed] [Google Scholar]
- 67.Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clinical Gastroenterology and Hepatology. 2005;3:705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 68.Ken S, Masanori Y, Takashi M, Kazuki T, Takeo T, Jun M, Rumi M, Akinobu T, Hideaki U, Yoshihiko M. Beneficial effects of supplementation of branched-chain amino acids on postoperative bacteremia in living-donor liver transplantation recipients. Liver Transplantation. 2011;17:1073–1080. doi: 10.1002/lt.22324. [DOI] [PubMed] [Google Scholar]


