Abstract
Methamphetamine (METH) exposure can produce hyperthermia that might lead to toxicity and death. Modafinil is a wake-promoting compound that is also been prescribed off-label to treat METH dependence. Modafinil has shown neuroprotective properties against METH harmful effects in animal models. The goal of the present study was to test if the prevention of hyperthermia might play a role on the neuroprotective actions of modafinil against METH toxicity using various ambient temperatures. METH was administered to female C57BL/6 mice in a binge regimen: 4 × 5 mg/kg , 2h apart; modafinil (90mg/kg) was injected twice, 1h before first and fourth METH injections. Drugs were given at cold ambient temperature (14 °C) or hot ambient temperature (29 °C). Body temperature was measured during treatments. Brains were dissected out six days after treatments and processed for TH, DAT, GFAP and c-Fos immunohistochemistry. Exposure to hot ambient temperature exacerbated METH toxicity evidenced by sriatal reductions in TH and DAT and increased GFAP immmunoreactivity. Modafinil counteracted reductions in TH and DAT, but failed to block astroglial activation. At both ambient temperatures tested modafinil did induce increments in GFAP, but the magnitude was significantly lower than the one induced by METH. Both drugs induced increases in c-Fos positive nuclei; modafinil did not block this effect. Our results suggest that protective effects of modafinil against METH-induced neurotoxicity may be dependent, in part, to its hypothermic effects. Nevertheless, modafinil maintained some protective properties on METH-induced alterations in the striatum at different ambient temperatures.
Keywords: modafinil, methamphetamine, dopamine, astroglia, striatum, toxicity, body temperature, hyperthermia, hypothermia
Introduction
Methamphetamine (METH) is a psychostimulant with a high potential for abuse and addiction. METH use can be lethal due to hyperthermia (Schep et al., 2010). In clinical emergency settings, elevations in body temperature are a common presenting symptom with few treatment options (Matsumoto et al., 2014). Additionally, METH addicts show several signs of neuropathology that include biochemical and structural abnormalities (Cadet et al., 2014). Neurotoxic effects of METH abuse have been reported in several regions of the mammalian brain, most notably in the striatum (Cadet et al., 2014). For example, reduced dopamine transporter (DAT) binding (Volkow et al., 2001), and low dopamine (DA), tyrosine hydroxylase (TH), and DAT levels (Wilson et al., 1996) were found in the caudate nucleus of METH addicts. These abnormalities could be related to mechanisms that include the reversal of DAT transport, resulting in large DA efflux from dopaminergic terminals (Gruner et al., 2009). In preclinical studies, animals given repeated moderate to large doses of METH experienced significant loss of DA and serotonin (5-HT) in the striatum, cortex, and olfactory bulb (Deng et al., 2007). In addition, METH decreased striatal DAT binding as well as TH and DAT immunoreactivity in animal models (Bowyer et al., 2008; Deng et al., 1999; O"Callaghan and Miller, 1994). Neuroinflammation is also critical to the neurotoxicity of METH (Krasnova and Cadet, 2009). Specifically, glial activation is known to increase the production of inflammatory signaling molecules such as TNF-alpha and the formation of reactive molecules related to oxidative stress (Ladenheim et al. 2000; Flora et al. 2002; Block and Hong 2005).
Protection against METH- or amphetamine-induced DA depletion by compounds such as haloperidol and dizocilpine, have been ascribed to the blockade of hyperthermia resulting from administration of these compounds at room temperature (Albers and Sonsalla, 1995; Miller and O"Callaghan, 1994). In both rat and mouse, hyperthermia potentiates DA and TH depletion and the astrocytosis caused by METH or amphetamine exposure, while hypothermia can protect against these effects (Bowyer et al., 1993; Miller and O"Callaghan, 1994; O"Callaghan and Miller, 1994). However, mice given high doses of METH are not always completely protected against DA depletion during exposure to a cold environment (Ali et al., 1994). These findings suggest that increased body temperature may represent an important contributing event, but not the sole factor responsible for METH-induced toxicity.
Modafinil is a drug approved for the pharmacological management of narcolepsy, and is currently used off-label to treat psychostimulant dependence with promising results (Mereu et al., 2013). Although it has stimulant-like effects in humans and animals, it has a complex pharmacology that is clearly different from those of the catecholaminergic stimulants like d-amphetamine and methylphenidate. Despite the fact that modafinil has modest micromolar affinity for DAT (Madras et al., 2006; Zolkowska et al., 2009) and low affinity for norepinephrine or 5-hydroxytryptamine transporters (NET and SERT) (Madras et al., 2006; Minzenberg and Carter, 2008), positron emission tomography experiments in humans have revealed that therapeutic doses of modafinil occupy a substantial proportion of striatal DAT sites in human subjects and increase synaptic dopamine concentrations (Volkow et al., 2009). Compared to classical psychostimulants such as cocaine or amphetamine, the sites of action and the behavioral effects of modafinil appear to be different (Mereu et al., 2013). In addition, modafinil influences GABAergic, glutamatergic, noradrenergic, serotoninergic, histaminergic, and orexinergic systems (Mizenberg and Carter, 2008). Moreover, modafinil enhances electrotonic coupling by increasing the effectiveness of gap junction communication between neurons (Urbano et al., 2007; Garcia-Rill et al., 2007). Interestingly, modafinil can also protect against METH-induced DA depletion and reductions in TH and DAT levels in the striatum (Raineri et al., 2011). Furthermore, modafinil also attenuated METH-induced hyperthermia and glial activation in striatal tissue (Raineri et al., 2012). This protective effect of modafinil may be secondary to its effects as a DAT blocker (Zolkowska et al., 2009; Raineri et al., 2011; 2012). Nevertheless, the protective properties of modafinil could also be attributed to a direct effect on thermoregulatory responses to METH treatment (i.e. by inducing hypothermia) independent of its presumed mechanisms of action (Raineri et al., 2012).
The present study was conducted to test the hypothesis that prevention of hyperthermia might play an important role on the neuroprotective actions of modafinil against METH toxicity using various ambient temperatures. We were interested in examining whether modafinil maintains its protective properties in a high temperature environment (at 29°C), which is known to enhance METH-induced hyperthermia and toxicity (Bowyer et al., 1992, 1994; Miller and O"Callaghan, 1994, Riddle et al., 2006). To further explore the effect of ambient temperature on modafinil actions and the link between METH-induced toxicity and temperature regulation, we also administered these drugs in a cold ambient environment (14°C), a manipulation that is known to protect against METH-induced toxicity (Bowyer et al, 1993; 1994).
The immediate early gene c-Fos and its encoded protein, the transcription factor Fos, are expressed in brain regions that are physiologically activated by a variety of stimuli and, therefore, is useful as a marker of functional activity in the brain (Sagar et al., 1988). METH exposure can cause substantial alterations in c-Fos expression in the rodent brain (Martin et al., 2012; Cadet et al., 2013). Indeed, it has been suggested that c-Fos induction in response to toxic doses of METH may be involved in protective mechanisms against METH-induced neurotoxicity (Deng et al., 1999). Modafinil can also affect c-Fos expression in several brain areas (Gozzi et al., 2012). Thus, we investigated if modafinil and METH would differently alter c-Fos expression in the striatum at different environmental temperatures.
Material and Methods
1.1. Animals
Female C57BL/6 mice (20–25 g) from the Central Animal Facility at the School of Pharmacy, Universidad de Buenos Aires (UBA), were housed in a light- and temperature-controlled room. Mice had free access to food and water. Principles of animal care were followed in accordance with “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council, 2003) and approved by Universidad de Buenos Aires authorities (Protocol Number: A5801-01) using OLAW and ARENA directives (NIH, Bethesda, USA).
1.2. Pharmacological reagents
Drugs were purchased from either Sigma (St. Louis, MO) or Tocris (Ellisville, MO). Modafinil (racemic mixture of R- and S-enantiomers) was generously donated by Laboratorios Beta S.A. (Argentina). (+)-Methamphetamine hydrochloride was purchased from Sigma (St Louis, MO).
1.3. Pharmacological and Physiological Procedures
METH was administered in a binge regimen: 4 × 5 mg/kg i.p. 2h apart (calculated as METH in the free-base form). The METH regimen used in this study was chosen according to previous studies (Raineri et al., 2012). Modafinil (90 mg/kg, dissolved in DMSO-Arabic gum 5% in sterile saline solution) was injected twice, 60 min before the first and fourth METH injections. Control groups received the same volume of sterile saline and DMSO Arabic gum /saline, using the same schedule as METH and modafinil injections. Drugs were injected at a volume of 10 ml/kg of body weight.
1.4. Body Temperature Monitoring
The ambient room temperature during METH “binge” treatment for Experiment-1 (cold ambient temperature) was 14 ± 1 °C, and for Experiment-2 (hot ambient temperature) was 29 ± 1°C. Mice were placed in a 14 °C or 29 °C environment 1 hour prior to METH exposure and remained there until 3 hours after the last dose of METH.
Core body (rectal) temperature was measured using a Bat-10 thermometer equipped with a RET-3 mouse rectal probe (Physitemp, Inc., Clifton, NJ, USA), as previously described (Raineri et al., 2012). Core temperatures were recorded immediately prior to the initiation of the experiments (baseline readings), 1 hour after every dose of METH and up to 3 hours after the last dose of METH. Previous results from other laboratories indicate that core body temperatures determined at 1 hour post-injection of METH are representative of the peak changes in temperature (Bowyer et al., 2001). An additional temperature reading was taken (16 hours after last METH injection) after animals had returned to the animal facility (standard temperature).
1.5. Tissue processing for histochemical studies
Animals were deeply anesthetized 6 days after the last METH injection with ketamine-xylazine (0.5 ml/kg i.p.; 1.4 ml/kg i.p., respectively) and then transcardially perfused with 30 ml of phosphate buffer saline (PBS) 10 mM (pH 7.4) followed by 80 ml of ice-cold paraformaldehyde 4% (Sigma, USA) diluted in 0.1 M (pH 7.4) PBS. Brains were dissected and placed in the same fixative solution for 16 hours at 4 °C. Then, brains were placed in PBS/sucrose 30% for cryoprotection until coronal sections were cut throughout the striatum.
c-Fos is part of a family of immediate early genes (IEGs) which are activated transiently and rapidly in response to a variety of different stimuli. Thus, for c-Fos immunohistochemistry, animals were killed one hour after the last METH injection.
1.6. Immunohistochemistry
Immunostaining for TH, GFAP and c-Fos were performed on 25 µm free-floating coronal serial slices from striatal sections. During all staining procedures 0.1 M PBS, 0.15% Triton X-100 (PBS-T) was used for diluting all immunoreagents and for washing after incubating with antibodies. Sections were incubated in 0.6% H2O2 (3% in the case of GFAP immunoassay), followed by 2% normal goat serum (NGS) in PBS-T and exposed to anti-TH antiserum (1:1000, Pel Freeze Biologicals, USA), anti-GFAP antibody (1:500, Sigma, USA) or anti-cFos antiserum (1:800, Sigma, USA) overnight at 48 °C. After washing, sections were incubated for 2 hr in biotinylated goat antirabbit IgG antiserum (1:500, Sigma, USA) followed by the avidin-biotin peroxidase complex (1:125, Vectastain, ELITE ABC kit, Vector Laboratories). Chromogenic reactions were induced with 0.5 mg/ml DAB, and 0.015% H2O2. Sections were then rinsed, mounted on gelatin-coated slides, air-dried, dehydrated, cleared, and coverslipped. The same protocol was applied for DAT immunostaining using DAT monoclonal primary antibody (1:500, Millipore, USA), and HRP-conjugated anti-rat antibody (1:1000, Sigma, USA).
The GFAP-immunoreactive area was quantified as follows: light microscopic examination of sections revealed GFAP-positive staining throughout the striatum. Data were collected with the help of mapping software (Mercator Pro; Explora Nova, France). The immunoreactive area was determined using a semiautomatic system that allowed tagging the percent of stained area within 14 probes (236 × 140 µm area of the striatum) randomly selected by the Mercator software in the outlined striatum as previously described (Raineri et al., 2012). Percent of stained area was calculated by generating an average count for each treated subject. In order to differentiate specific staining from background, a color threshold was used in the process. Numbers of Fos-labeled neurons were counted in the dorsal striatum from 4 stained sections per animal within three probes of 236 × 140 um2 with the help the mapping software Mercator Pro (Explora Nova, France). Neurons were selected and counted as c-Fos positive if the intensity of the nuclear Fos labeling was above background (either black or dark brown).
TH and DAT levels in the striatum were quantified as follows: histological sections stained with anti-TH and anti-DAT antibodies were observed under light microscopy with a CCD camera for image acquisition. For measurement of integrated optical density, slice images were obtained with a 10X objective under standard conditions (magnification, brightness). Unequal brightness distribution (shading) was corrected by subtracting a background-image obtained with the a posteriori shading correction plug-in to all images (NIH ImageJ). Then, a threshold level of optical density was applied according to the average of the automatic values of threshold obtained for the saline treated group of animals in order to avoid including non-specific staining. Integrated density was then measured in the dorsolateral striatum from striatal TH or DAT from 4 stained sections per animal with an ellipsoidal region of interest (613,400 µm2) using ImageJ software.
1.7. Statistical analysis
InfoStat software (www.infostat.com.ar) was used for statistical comparisons. Statistics were performed using two-way (treatment X temperature) ANOVA followed by Bonferroni post hoc tests. For temperature recordings, data were analyzed using Repeated Measures two-way (time X treatment) ANOVA with Fisher LSD test. Data were transformed when required and Kruskal Wallis ANOVA on Ranks was performed when data did not comply with the assumptions of parametric tests. Differences were considered significant if p<0.05.
2. Results
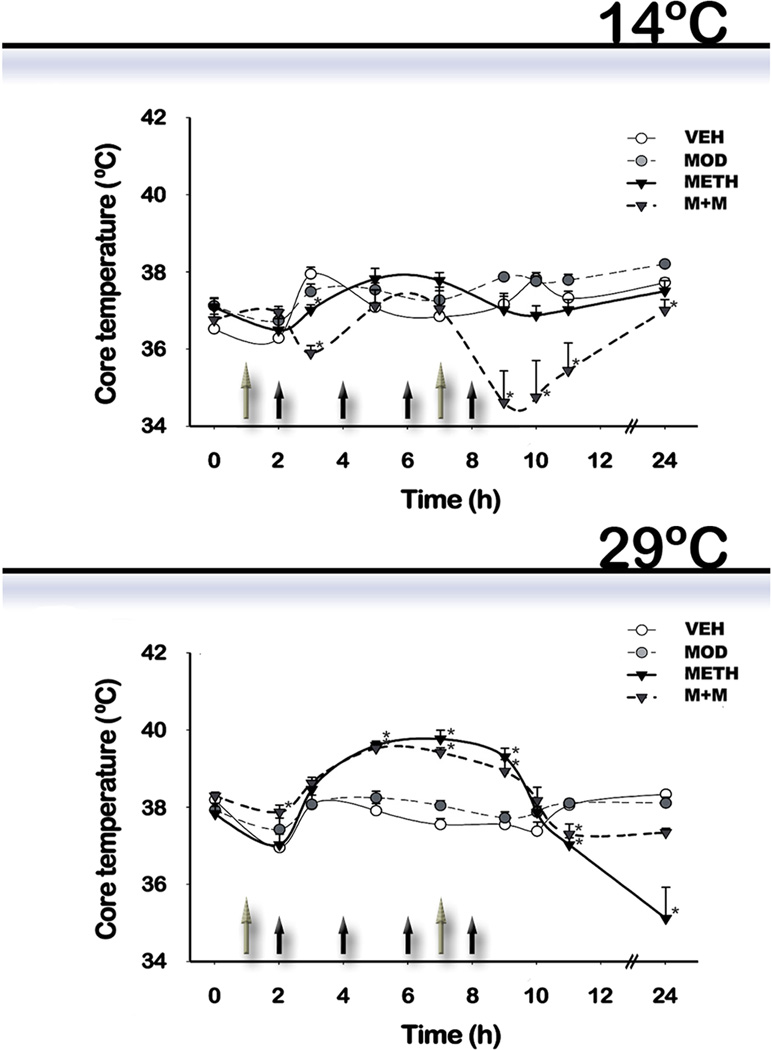
3.1. Effects of modafinil and methamphetamine on body core temperature of mice exposed to different ambient temperatures
For experiment #1, mice were treated in a room maintained at a temperature of 14 ± 1 °C. For experiment # 2, pharmacological treatments were administered in a room maintained at 29 ± 1°C. Core body temperature was measured 1 hr before the first modafinil injection and at different intervals thereafter (Figure 1). Repeated measures Two-way ANOVA showed that treatments and time produced a significant change when animals were treated at low ambient temperature 14°C [F(treatment) (3,159) = 4.88, p=0.0029, F(time) (7,159) =12.80, p<0.0001] vs. elevated ambient temperature 29°C treatment [F(treatment) (3,191) = 4.8; p=0.0029, F(time) (7,191) =31.5; p<0.0001], and that there was an interaction between the two factors in both cases [14°C: F(treatment x time) (21,159) = 11.71, p<0.0001; 29°C: F(treatment x time) (21,191) =10.57; p<0.0001]. Post hoc tests indicated that: 1) METH-treated mice showed an hyperthermic response when treated at 29°C, which was significantly different from the vehicle (VEH) group at the second, third, and last METH injections (p<0.05), and a hypothermic response 3hours after the last METH injection and thereafter (p<0.05). At 14°C, a significant difference was found when METH groups were compared to VEH (1 hr after the second METH injection); no hyperthermic response was observed at any time; 2) Modafinil administration by itself had no effect on body temperature at any time or environmental temperature; and 3) Modafinil co-administration with METH (M+M group) was not able to prevent METH-induced hyperthermia (p<0.05 vs. VEH, p>0.05 vs. METH) at 29°C, while at 14°C a hypothermic response was observed 1 hr after the first and last METH injection and thereafter (p<0.05).
Figure 1. Effects of modafinil (MOD) and METH administration on core body temperature evaluated at 14 and 29 °C ambient temperatures.
Mice were treated at 14 and 29 °C ambient temperatures Mice body temperatures were recorded 1 hr before the first MOD injection and at different intervals thereafter. Black filled arrows indicate the time of METH injection while grey filled arrows indicate the time of MOD injection. Data are presented as mean core temperature (°C) ± SEM (n = 5–10) at the indicated times from animals treated with VEH (white circle), MOD (grey circle, dotted line), METH (black inverted triangle), or M+M (dark grey inverted triangle, dotted line). Repeated measures two-way ANOVA followed by Fisher’s LSD. *: pˊ0.05 vs. VEH.
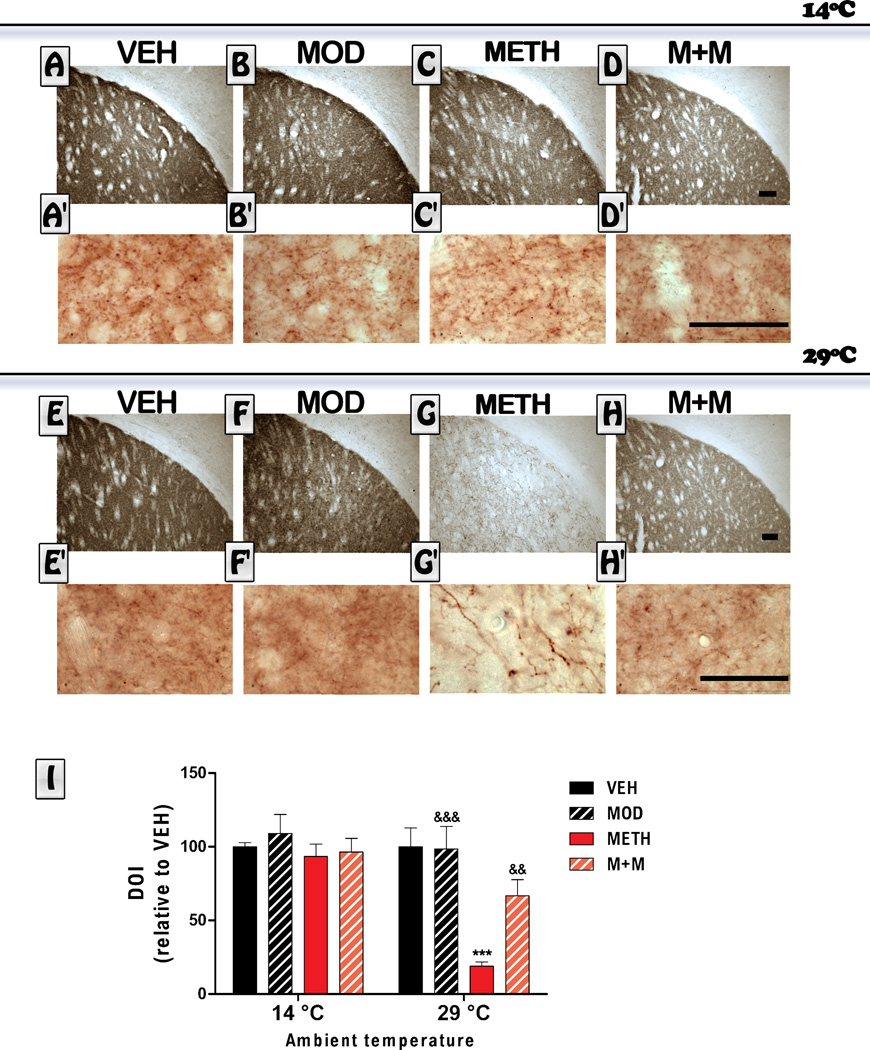
3.2. TH and DAT immunoreactivity
Mice underwent a repeated dosing schedule that has been previously shown to cause significant dopaminergic toxicity in response to METH (Raineri et al., 2012). In order to assess the integrity of dopaminergic terminals, we examined TH and DAT immunoreactivity in the dorsolateral region of the striatum. Representative photomicrographs and quantification of TH staining in the striatal sections are shown in Figure 2. Mice exposed to METH in a cold environment did not show any significant difference in TH immunoreactiviy relative to the VEH treated group. As expected, mice exposed to METH in a hot environment did show a marked reduction in TH immunoreactivity 6 days after treatments were administered. ANOVA indicated a significant treatment effect [F(treatment) (3,55) = 9.76, p<0.0001, a temperature effect F [(temperature) (1,55) =18.54; p<0.0001], and that there was an interaction between the two factors [(treatment x temperature) (3,55) = 6.61, p<0.001]. Post hoc tests indicated that, at 29°C, modafinil co-administration was able to prevent the METH-induced decrease in TH immunoreactivity levels (p<0.01). Also, we only observed a change in the morphology of TH-positive fibers only in the METH group (Figure 2). We found that some TH-positive fibers become thicker (see Figure 2G’). This is consistent with previous data showing altered TH fibers in the striatum after partial dopaminergic lesions (Song and Haber, 2000).
Figure 2. Effects of modafinil (MOD) on METH-induced changes in striatal Tyrosine hydroxylase (TH) immunoreactivity evaluated at 14 and 29 °C ambient temperatures.
Representative images of the striatum from brains of animals treated with either VEH (A, E), MOD (B, F), METH (C, G) or M+M (D, H), scale bar: 100 µm, and their respective higher magnification photomicrographs (Aˊ–Hˊ), scale bar: 50 µm. Mice were euthanized 6 days after the last METH injection and brains were processed for TH immunoreactivity. Average integrated density (relative to VEH) was determined in a region of interest located/placed in the dorsolateral striatum. Values are expressed as mean ± SEM (n = 5–10). Two way ANOVA followed by Bonferroni. ***: p<0.001 vs. VEH-29°C; &&: p<0.01 and &&&: p<0.001 vs. METH-29°C.
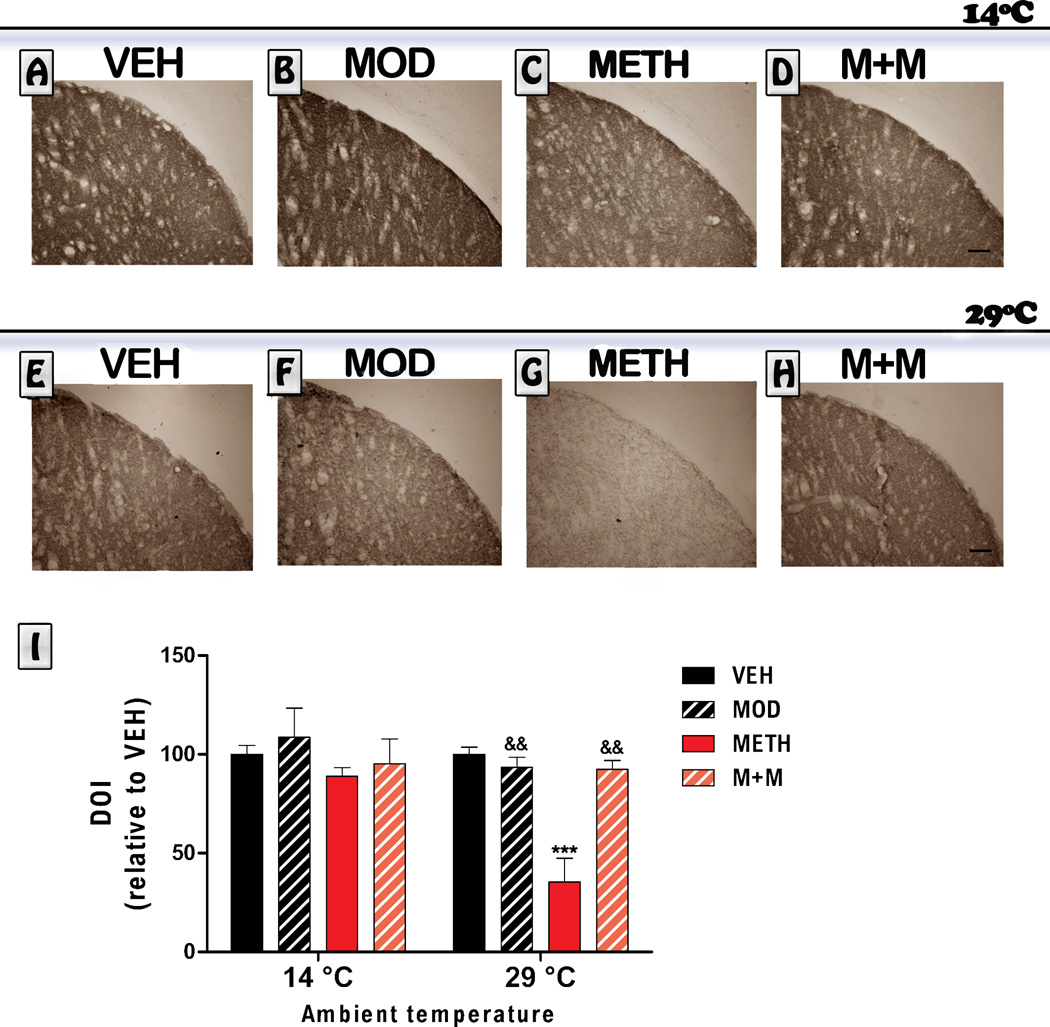
As shown in Figure 3, we obtained similar results for DAT immunoreactivity. Mice exposed to METH treatment in a cold environment did not show any significant change in DAT immunoreactiviy levels. However, mice exposed to a hot environment and treated with METH did show a marked reduction in DAT immunoreactivity 6 days after treatment , Kruskal Wallis [H = 19.11, p<0.01]. Modafinil co-administration (M+M group) prevented the METH-induced decrease in DAT immunoreactivity levels in animals exposed to high temperature environment (p<0.01).
Figure 3. Effects of modafinil (MOD) on METH-induced changes in striatal DAT immunoreactivity evaluated at 14 and 29 °C ambient temperatures.
Representative images of the striatum from brains of animals treated with either VEH (A, E), MOD (B, F), METH (C, G) or M+M (D, H), scale bar: 100 µm. Mice were euthanized 6 days after the last METH injection and brains were processed for DAT immunoreactivity. Average integrated density (relative to VEH) was determined in a region of interest located in the dorsolateral striatum. Values are expressed as mean ± SEM (n = 5–10). Kruskal-Wallis. ***: p<0.001 vs. VEH-29°C; &&: p<0.01 vs. METH-29°C.
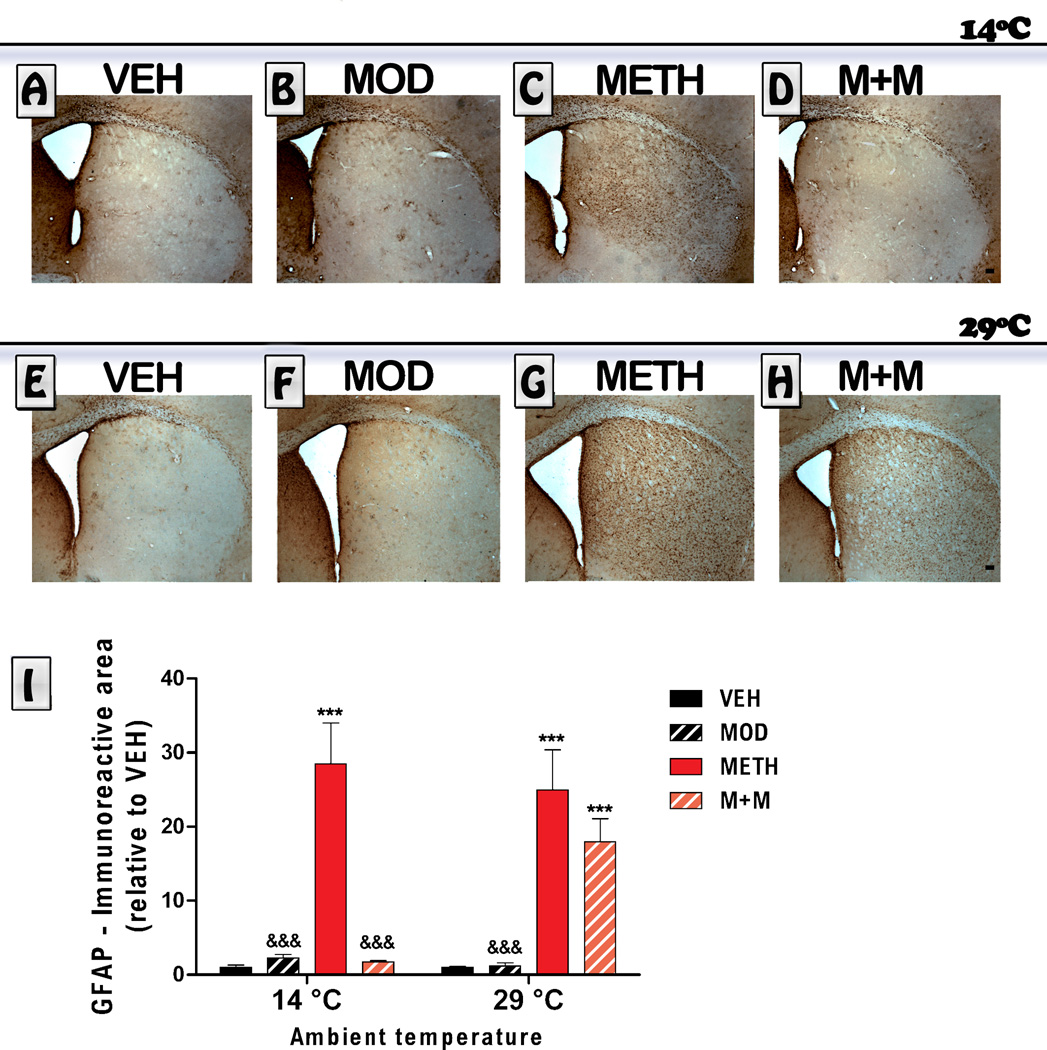
3.3. GFAP immunoreactivity
METH administration is known to increase GFAP immunoreactivity, which is considered a standard marker of astrogliosis (O´Callaghan et al., 1995). Astrogliosis was evaluated by immunohistochemistry in the striatum of mice 6 days post METH. Representative sections from all treatment groups are shown in Figure 4. ANOVA indicated a significant treatment effect [F(treatment) (3,48) = 85.85, p<0.0001, a temperature effect F [(temperature) (1,48) =5.78; p<0.05], and that there was an interaction between the two factors [(treatment x temperature) (3,48) = 20.59, p<0.0001]. A clear increase in the GFAP-immunoreactive area was observed in METH-treated mice at 14°C (p<0.0001) and 29°C (p<0.0001). Modafinil blocked the METH-induced astroglial activation (p<0.05) in animals treated at 14°C, but failed to counteract astrogliosis induced by METH in animals treated at 29°C (pˊ0.05). Modafinil treatment by itself induced an increase in the immunoreactive area for GFAP compared to VEH values in both environments (hot and cold, VEH vs Modafinil, p<0.001), but the increase in immunoreactive area was significantly lower than the one observed for the METH group (METH vs Modafinil, p<0.001).
Figure 4. Effects of modafinil (MOD) on METH-induced changes in striatal astroglial activation evaluated at 14 and 29 °C ambient temperatures.
Representative images of the striatum from animals treated with either VEH (A, E), MOD (B, F), METH (C, G) or M+M (D, H), scale bar: 100 µm. Mice were euthanized 6 days after the last METH injection and brains were processed for GFAP immunoreactivity. The immunoreactive area was determined in a region of interest located in the dorsolateral striatum (relative to VEH). Values are expressed as mean ± SEM (n = 5–11). Two way ANOVA followed by Bonferroni. ***: p<0.001 vs. VEH of same ambient temperature; &&:& p<0.001 vs. METH of same ambient temperature.
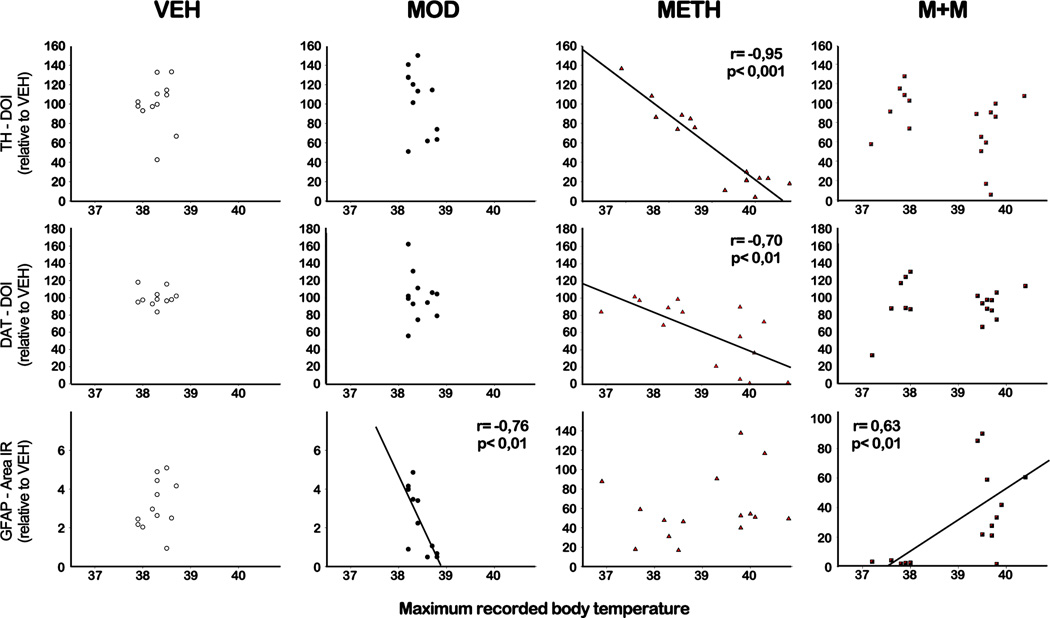
3.4. Correlational analysis of body temperature and toxicity markers in the striatum
The toxic consequences of METH are evidenced by long-term decreases in the expression of the rate-limiting enzyme for DA synthesis, TH, the DAT, and increases in glial activation in the striatum, both in animal models and in human METH addicts (Cadet et al., 2014). It has been shown that core temperature can influence METH neurotoxicity (Xie et al., 2000). Further analysis of these effects were conducted using a Pearson’s correlation coefficient between the maximum recorded temperature reached by the animals over the course of the experiment and the level of TH/DAT immunoreactivity or GFAP immunoreactive area in the striatum. Analysis of the relationship between the maximum recorded temperature and subsequent changes in dopaminergic neuronal markers (summarized in Figure 5) showed that these variables were correlated in METH treated animals. For TH and DAT levels, our results showed a negative correlation between maximum temperature and TH/DAT immunoreactivity in the METH group (TH, r= −0,95, p<0.001; DAT, r= −0,70, p<0.01), but there was no correlation with GFAP immunoreactive area. As for data obtained from the MOD treated group, there was a negative correlation between the maximum body temperature reached during treatment and GFAP striatal immunoreactivity (r= −0,76, p<0.01). Finally, the group that received the combination of METH with MOD showed a positive correlation between body temperature and GFAP striatal immunoreactivity (r= −0,63, p<0.01).
Figure 5. Correlation analysis between core body temperature and toxicity markers immunoreactive levels in the striatum.
Pearson correlation analysis was performed between maximum core body temperatures reached by each mouse during the treatment at 14 and 29 °C ambient temperatures and TH (upper panel), DAT (middle panel) or GFAP (lower panel) immunoreactivity. The Pearson r coefficient is shown on the graphs when p<0.05.
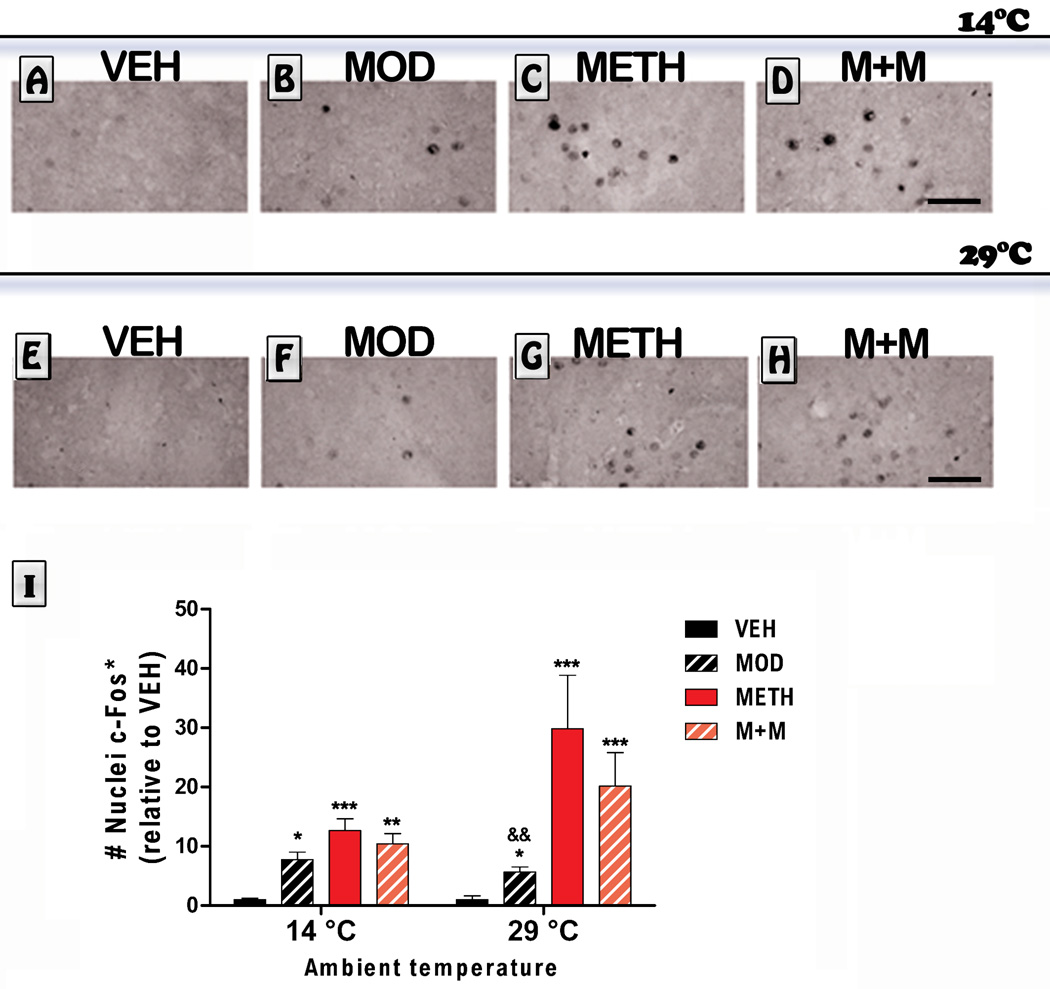
3.5. c-Fos positive nuclei
We also measured striatal c-Fos immunoreactivity in mice treated with modafinil and METH at different temperatures. The overall ANOVA was significant for treatment effect [F (3,45) = 31.18,p <0.0001]. As shown in Figure 6, the modafinil, METH, and the group that received the combination of modafinil and METH (M+M) showed an increase in the number of Fos-labeled neurons in the dorsal striatum at 14°C (MOD: p<0.05, METH: p<0.001 and M+M: p<0.01 vs. VEH). Also, these groups exhibited an increase in the number of Fos-labeled neurons in the dorsal striatum at 29°C (MOD: p<0.05, METH: p<0.001 and M+M: p<0.001 vs. VEH). Post hoc tests also indicated that the number of c-Fos-labeled neurons in the MOD group was significantly lower compared to the METH-treated group (p<0.01 vs. METH).
Figure 6. Effects of modafinil (MOD) on METH-induced striatal c-Fos immunoreactivy evaluated at 14 and 29 °C ambient temperatures.
Representative images of the striatum from animals treated with either VEH (A, E), MOD (B, F), METH (C, G) or M+M (D, H), scale bar: 50 µm. Mice were euthanized 1 h after the last METH injection and brains were processed for c-Fos immunoreactivity in a region of interest located in the dorsolateral striatum. Values are expressed as mean ± SEM (relative to VEH), (n = 5–11). Two way ANOVA followed by Bonferroni. *: p<0.05, **: p<0.01, ***: p<0.001 vs. VEH of same ambient temperature; &&: p<0.01 vs. METH of same ambient temperature.
Discussion
The present study provides evidence indicating that changes in body temperature induced by exposure to either low or high environmental temperatures can affect modafinil and METH actions on striatal dopaminergic and glial markers. Our results show that modafinil maintained protective properties against METH-induced striatal toxicity in mice exposed at different ambient temperatures. Under the present experimental conditions, increased ambient temperature exacerbated METH toxicity (compared to responses obtained at 14 °C), which was evidenced by marked reductions in TH and DAT immmunoreactivity. Interestingly, modafinil counteracted the METH-induced striatal reduction in TH and DAT immunoreactivity, but failed to modulate astroglial activation in a hot environment. Acute increases in body temperature in response to METH are well documented and may be related to its neurotoxic effects (Bowyer et al., 1992, 1994; Miller and O"Callaghan, 1994, Riddle et al., 2006). However, the exact nature of the relationship between METH and the increase in body temperature remains enigmatic. For example, in mice treated with reserpine, which produces a hypothermic state, no protection against METH-induced toxicity was obtained (Albers and Sonsalla, 1995).
Interestingly, a number of neuroprotective agents tested against METH toxicity were shown to lack significant effects on METH-induced hyperthermia (reviewed in Matsumoto et al., 2014). For example, that is the case for minocycline (Zhang et al., 2006), memantine (a NMDA antagonist) (Chipana et al. 2008) or calcitriol (a vitamin D metabolite) (Cass et al., 2006) that proved to have neuroprotective effects in animals treated with METH without affecting METH-induced hyperthermia.
Thus, it seems likely that the increases in body temperature may represent an important contributing, but not the sole, factor responsible for METH-induced toxicity. Our current findings agree with the concept that hyperthermia contributes to the neurotoxicity response, such that exposure of the animals to the hot ambient temperature exhibited reductions in dopaminergic terminal markers. Even if correlation analyses do not necessarily imply causality, it is of interest to discuss the correlation analysis performed in our study. Indeed, we did find a negative correlation of the maximum temperature reached in these experiments with the level of TH and DAT immunoreactitvy, but only in METH-treated subjects. The negative correlation suggests that, in METH treated subjects, body temperature may be useful as a predictor of the effects of METH toxic on dopaminergic markers. In the group that received the combination of modafinil and METH, we found only a positive correlation linking GFAP and temperature, suggesting that maximum body temperatures might predict levels of astrocyte activation. It should be noted that this is the only group that experienced hypothermia during treatment at 14°C.
Exposure to the cold environment failed to produce a significant decrease in TH or DAT in the striatum in all treatment groups. Still, mice treated with METH and exposed to the cold environment exhibited marked increases in astroglia reactivity that was blocked by modafinil administration. Results from our study suggest that some actions of METH, probably linked to glial activation, may still be present in the absence of hyperthermia. Our group has previously shown that modafinil was able to block astrocyte activation induced by METH (administered in the animal facility at standard temperature) (Raineri et al., 2012). Also, our current results are in agreement with results from a recent study that showed that modafinil was able to attenuate astrocyte hypertrophy induced by sleep deprivation in the rat hippocampus (Sahu et al., 2013). Also, it should be noted that at both ambient temperatures tested here modafinil did induce an increase in glial activation level, but the magnitude of this increase was significantly lower than the astroglial activation induced by METH. It is of interest to note the observation that activation of glial cells can lead either to toxic or to protective events (Milligan and Watkins, 2009). Although no specific effect of modafinil has been described that may affect the function of glial cells, the possibility exists that this compound, under some circumstances, might be able to attenuate the negative effects of glial pro-inflammatory mediators, thereby protecting neurons from insults such as METH toxicity.
As previously discussed, it has been shown that core temperature can influence METH neurotoxicity and that the expression of DA neurotoxicity after amphetamines (METH and amphetamines) is critically dependent on DAT function (Pu et al., 1994; Fumagalli et al., 1998). It is possible that, at least in part, increased core temperature enhances METH-induced DA neurotoxicity by amplifying a DAT-dependent neurotoxic cascade (Callahan et al., 2001). Other findings indicate that relatively small, physiologically relevant changes in temperature significantly alter DAT function and intracellular METH accumulation, suggesting that the effect of temperature on METH-induced DA neurotoxicity is mediated, at least in part, at the level of the DAT (Xie et al., 2000). Animals that do not express DAT, a molecular gateway to DA terminals for neurotoxicants, do not exhibit astrogliosis after METH or 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) administration (Gainetdinov et al. 1997). Interestingly, mice with increased DAT display potentiated astrogliosis when given MPTP (Gainetdinov et al. 1997; Donovan et al., 1999).
Although the effects of modafinil on midbrain DA neuronal activity remain inconsistent, modafinil acts as a weak DAT inhibitor (Mereu et al., 2013). Modafinil, by partially blocking the DAT, may have interfered with METH-induced hyperthermia and associated toxicity on dopaminergic markers. Alternatively, elevations in core temperature could have enhanced neurotoxicity via alternate mechanisms. For example, core temperature elevations could lead to increased formation of reactive oxidative species (Cubells et al., 1994; Cadet and Brannock, 1998), or amplify other molecular events that are thought to underlie the mechanism of action of METH neurotoxicity (Krasnova and Cadet, 2009).
It has been reported that METH-induced hyperthermia and neurotoxicity can be blocked by lowering ambient temperature to 5°C, which is related to reduction in free radical production (Bowyer et al, 1993; 1994). Our results also suggests that cooling would be beneficial in preventing the acute toxic effects of METH, which is in agreement with previous data reported by Namiki et al. (2005). These authors showed that the lethal effects induced by METH and morphine were attenuated by immediate cooling after drug administration (Namiki et al., 2005). It is also of interest to discuss our results showing that modafinil administered to METH-treated animals at low ambient temperature significantly decreased body temperature in mice that received the combination of both drugs. These results are in agreement with a previous report from our laboratory (Raineri et al., 2012) showing that modafinil administration lowered body temperature in animals that received both modafinil and METH at standard temperature (Raineri et al., 2012). Also, a recent report showed that high doses of modafinil can induce significant hypothermia in mice (Okuro et al., 2010). So, to some extent, it seems that modafinil could have the ability to induce hypothermia. In the present study, at a low temperature environment (14°C), an environment that could facilitate hypothermia, animals that received the combination of both drugs showed a hypothermic response probably due to the facts discussed above.
Overall, these data suggest that modafinil might modulate thermoregulation by either interfering with heat loss mechanisms or with heat generating mechanisms in physiological or pathological conditions.
The effects of METH administration on immediate-early gene (IEGs) expression have been well described and include induction of numerous IEGs in several brain regions (Krasnova and Cadet, 2009). Specifically, it has been shown that METH exposure can cause substantial alterations in c-Fos expression in rats (Martin et al., 2012; Cadet et al., 2013). Modafinil can also affect c-Fos expression in several brain areas (Fiocchi et al., 2009; Gozzi et al., 2012). Accordingly, in the present study, we found that at high and low ambient temperatures both modafinil and METH significantly increased c-Fos expression in the striatum. Modafinil did not block METH-induced c-Fos increases at any temperature, suggesting that the protective actions of modafinil are not linked to a differential activation of c-Fos in the striatum. Also, it needs to be noted that, in animals exposed at high temperatures, modafinil induced significantly lower c-Fos activation compared to METH-treated animals. These data suggest that thermoregulatory responses induced in a hot environment contributed to METH-induced increases in neuronal activation in the striatum.
In summary, reductions in striatal dopaminergic markers, such as TH and DAT levels, and an increase in astroglial activation were used here as the benchmark determinants of METH-induced neurotoxicity. We conclude that environment-induced changes in body temperature can influence the neuroprotective effects of modafinil and appear to account for some of its actions. Nevertheless, modafinil maintained its neuroprotective properties against METH-induced alterations in the striatum at different ambient temperatures. Results from the present study suggest that even under rather demanding environmental conditions (high or cold ambient temperatures) modafinil was still able to interfere with METH harmful effects as it was previously shown for METH-induced changes in biochemical markers (Raineri et al., 2011; 2012; Zolkowska et al., 2009) and behavioral functions (Zolkowska et al., 2009; Gonzalez et al., 2014, Reichel et al., 2014). Moreover, we found that modafinil (by itself) did not alter the level of any dopaminergic marker in the striatum even at high ambient temperatures. Also, our results showed that cooling the environment has a beneficial effect on METH-induced toxicity. We also found that modafinil, under specific circumstances, can induce a hypothermic response. Thus, it might be interesting to further explore if these actions on temperature regulation exerted by modafinil can be considered of therapeutic value. Additionally, further studies will also need to investigate whether modafinil could influence glial cell reactivity as a component of its protective actions.
ACKNOWLEDGMENTS
Dr. Bisagno has been authorized to study drug abuse substances in animal models by A.N.M.A.T. (National Board of Medicine Food and Medical Technology, Ministerio de Salud, Argentina). Dr. Betina Gonzalez is a recipient of a Postdoctoral Award from Fundación Bunge y Born. This work is supported by grants PIP 11420100100072, PICT 2012-0924, PICT 2012-1769, Argentina, and by NIH awards P20 GM103425 and P230 GM110702 to the Center for Translational Neuroscience.
Footnotes
CONFLICT OF INTEREST
Authors also report no conflict of interest, financial or otherwise, related directly or indirectly to this work.
REFERENCES
- Ali SF, Newport GD, Holson RR, Slikker W, Jr, Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658(1–2):33–38. doi: 10.1016/s0006-8993(09)90007-5. [DOI] [PubMed] [Google Scholar]
- Albers DS, Sonsalla PK. Methamphetamine-Induced Hyperthermia and Dopaminergic Neurotoxicity in Mice: Pharmacological Profile of Protective and Nonprotective Agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Holson RR. Further Studies of the Role of Hyperthermia in Methamphetamine Neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Bowyer JF, Holson RR, Miller DB, O"Callaghan JP. Phenobarbital and dizocilpine can block methamphetamine-induced neurotoxicity in mice by mechanisms that are independent of thermoregulation. Brain Res. 2001;919(1):179–183. doi: 10.1016/s0006-8993(01)03051-7. 16. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260(2):817–824. [PubMed] [Google Scholar]
- Bowyer JF, Gough B, Slikker W, Jr, Lipe GW, Newport GD, Holson RR. Effects of a cold environment or age on methamphetamine-induced dopamine release in the caudate putamen of female rats. Pharmacol Biochem Behav. 1993;44(1):87–98. doi: 10.1016/0091-3057(93)90284-z. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Robinson B, Ali S, Schmued LC. Neurotoxic-related changes in tyrosine hydroxylase, microglia, myelin, and the blood-brain barrier in the caudate-putamen from acute methamphetamine exposure. Synapse. 2008;62:193–204. doi: 10.1002/syn.20478. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32(2):117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91–107. doi: 10.1007/s00401-013-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Ladenheim B, Saint-Preux F, Lehrmann E, De S, Becker KG, Brannock C. Genome-wide profiling identifies a subset of methamphetamine (METH)-induced genes associated with METH-induced increased H4K5Ac binding in the rat striatum. BMC Genomics. 2013;14:545. doi: 10.1186/1471-2164-14-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BT, Cord BJ, Yuan J, McCann UD, Ricaurte GA. Inhibitors of Na(+)/H(+) and Na(+)/Ca(2+) exchange potentiate methamphetamine-induced dopamine neurotoxicity: possible role of ionic dysregulation in methamphetamine neurotoxicity. J Neurochem. 2001;77:1348–1362. doi: 10.1046/j.1471-4159.2001.00341.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:261–271. doi: 10.1196/annals.1369.023. [DOI] [PubMed] [Google Scholar]
- Chipana C, Torres I, Camarasa J, Pubill D, Escubedo E. Memantine protects against amphetamine derivatives-induced neurotoxic damage in rodents. Neuropharmacology. 2008;54:1254–1263. doi: 10.1016/j.neuropharm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14(4):2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Jayanthi S, Cadet JL. Methamphetamine administration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol Psychiatry. 2007;61:1235–1243. doi: 10.1016/j.biopsych.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Tsao LI, Cadet JL. Null mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxicity. J Neurosci. 1999;19:10107–10115. doi: 10.1523/JNEUROSCI.19-22-10107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Miner LL, Perry MP, Revay RS, Sharpe LG, Przedborski S, Kostic V, Philpot RM, Kirstein CL, Rothman RB, Schindler CW, Uhl GR. Cocaine reward and MPTP toxicity: alteration by regional variant dopamine transporter overexpression. Brain Res Mol Brain Res. 1999;73(1–2):37–49. doi: 10.1016/s0169-328x(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Fiocchi EM, Lin YG, Aimone L, Gruner JA, Flood DG. Armodafinil promotes wakefulness and activates Fos in rat brain. Pharmacol Biochem Behav. 2009;92(3):549–557. doi: 10.1016/j.pbb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Maragos W, Hennig B, Toborek M. Methamphetamine-induced TNF-alpha gene expression and activation of AP-1 in discrete regions of mouse brain: potential role of reactive oxygen intermediates and lipid peroxidation. Neuromolecular Med. 2002;2(1):71–85. doi: 10.1385/NMM:2:1:71. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci. 1998;18(13):4861–4869. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69(3):1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30(11):1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González B, Raineri M, Cadet JL, García-Rill E, Urbano FJ, Bisagno V. Modafinil improves methamphetamine-induced object recognition deficits and restores prefrontal cortex ERK signaling in mice. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.02.002. pii: S0028-3908(14)00055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Colavito V, Seke Etet PF, Montanari D, Fiorini S, Tambalo S, Bifone A, Zucconi GG, Bentivoglio M. Modulation of fronto-cortical activity by modafinil: a functional imaging and fos study in the rat. Neuropsychopharmacology. 2012;37(3):822–837. doi: 10.1038/npp.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner JA, Marcy VR, Lin YG, Bozyczko-Coyne D, Marino MJ, et al. The roles of dopamine transport inhibition and dopamine release facilitation in wake enhancement and rebound hypersomnolence induced by dopaminergic agents. Sleep. 2009;32:1425–1438. doi: 10.1093/sleep/32.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379–340. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol. 2000;58(6):1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319(2):561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Seminerio MJ, Turner RC, Robson MJ, Nguyen L, Miller DB, O"Callaghan JP. Methamphetamine-induced toxicity: An updated review on issues related to hyperthermia. Pharmacol Ther. 2014 May 14; doi: 10.1016/j.pharmthera.2014.05.001. pii: S0163-7258(14)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Jayanthi S, McCoy MT, Brannock C, Ladenheim B, Garrett T, Lehrmann E, Becker KG, Cadet JL. Methamphetamine causes differential alterations in gene expression and patterns of histone acetylation/hypoacetylation in the rat nucleus accumbens. PLoS Oneb. 2012;7(3):e34236. doi: 10.1371/journal.pone.0034236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu M, Bonci A, Newman AH, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology. 2013;229(3):415–434. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O"Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270(2):752–760. [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33(7):1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Namiki M, Mori T, Sawaguchi T, Ito S, Suzuki T. Underlying mechanism of combined effect of methamphetamine and morphine on lethality in mice and therapeutic potential of cooling. J Pharmacol Sci. 2005;99(2):168–176. doi: 10.1254/jphs.fpj05004x. [DOI] [PubMed] [Google Scholar]
- O"Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270(2):741–751. [PubMed] [Google Scholar]
- Okuro M, Fujiki N, Kotorii N, Ishimaru Y, Sokoloff P, Nishino S. Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice. Sleep. 2010;33(7):930–942. doi: 10.1093/sleep/33.7.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu C, Fisher JE, Cappon GD, Vorhees CV. The effects of amfonelic acid, a dopamine uptake inhibitor, on methamphetamine-induced dopaminergic terminal degeneration and astrocytic response in rat striatum. Brain Res. 1994;649(1–2):217–224. doi: 10.1016/0006-8993(94)91067-7. [DOI] [PubMed] [Google Scholar]
- Raineri M, Gonzalez B, Goitia B, Garcia-Rill E, Krasnova IN, Cadet JL, Urbano FJ, Bisagno V. Modafinil abrogates methamphetamine-induced neuroinflammation and apoptotic effects in the mouse striatum. PLoS One. 2012;7(10):e46599. doi: 10.1371/journal.pone.0046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri M, Peskin V, Goitia B, Taravini IR, Giorgeri S, Urbano FJ, Bisagno V. Attenuated methamphetamine induced neurotoxicity by modafinil administration in mice. Synapse. 2011;65(10):1087–1098. doi: 10.1002/syn.20943. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006;8(2):E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Gilstrap MG, Ramsey LA, See RE. Modafinil restores methamphetamine induced object-in-place memory deficits in rats independent of glutamate N-methyl-d-aspartate receptor expression. Drug Alcohol Depend. 2014;134:115–122. doi: 10.1016/j.drugalcdep.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sahu S, Kauser H, Ray K, Kishore K, Kumar S, Panjwani U. Caffeine and modafinil promote adult neuronal cell proliferation during 48 h of total sleep deprivation in rat dentate gyrus. Exp Neurol. 2013;248:470–481. doi: 10.1016/j.expneurol.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Schep LJ, Slaughter RJ, Beasley DM. The clinical toxicology of metamfetamine. Clin Toxicol (Phila) 2010;48:675–694. doi: 10.3109/15563650.2010.516752. [DOI] [PubMed] [Google Scholar]
- Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci. 2000;20:5102–5114. doi: 10.1523/JNEUROSCI.20-13-05102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Leznik E, Llinás RR. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci U S A. 2007;104(30):12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301(11):1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2(6):699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Xie T, McCann UD, Kim S, Yuan J, Ricaurte GA. Effect of temperature on dopamine transporter function and intracellular accumulation of methamphetamine: implications for methamphetamine-induced dopaminergic neurotoxicity. J Neurosci. 2000;20(20):7838–7845. doi: 10.1523/JNEUROSCI.20-20-07838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]