Abstract
Purpose
Acrolein has been implicated in retinal pigment epithelium (RPE) cell death, and has been associated with diabetic retinopathy. Our purpose was to investigate the potential effect of high glucose in influencing acrolein-mediated RPE cytokine production and cell death. We investigated the influence of the acrolein effect on ARPE-19 cells in high glucose conditions and quantified the release of transforming growth factor β (TGFβ1 and 2) and vascular endothelial growth factor (VEGF). We assessed the ability of N-benzylhydroxylamine(NBHA) as well as TGFβ pathway inhibitors SIS3 and SB431542 to prevent this effect of acrolein on ARPE-19 cells.
Materials and methods
Confluent ARPE-19 cells were treated with acrolein and/or NBHA in both 5.5 and 18.8 mM glucose conditions. Cells were also pretreated with SIS3, a specific inhibitor of the SMAD3 pathway, and SB431542, a specific inhibitor of TGFβ signaling pathway, before treating them with acrolein. Viable cells were counted and ELISAs were performed to measure the cytokines TGFβ1 and 2, and VEGF released into the conditioned media.
Results
In ARPE-19 cells exposed to acrolein and hyperglycemia there was reduced cell viability and an increase in the cell media of VEGF, TGFβ1, and TGFβ2, which was reversed by NBHA. Acrolein/hyperglycemia-induced cell viability reduction and cytokine overproduction was also reduced by TGFβ pathway blockade.
Conclusions
We conclude that the effect of acrolein on the reduction of viability and VEGF increase by ARPE-19 cells in hyperglycemic media is conducted through the TGFβ signaling pathway. Our results suggest that benefits of sequestering acrolein by NBHA and the blockage of the TGFβ pathway by SB431542 and SIS3 offer suggestions as to potential useful pharmacological drug candidates for the prevention of diabetes-induced complications in the eye.
Keywords: Acrolein, NBHA, Age-related macular degeneration, TGFβ, VEGF and RPE
INTRODUCTION
The reactive aldehyde, acrolein (2-propenal), is formed environmentally and physiologically by oxidation. Environmentally, this oxidation is primarily from incomplete combustion of fossil fuels, wood, and plastic and during the heating of carbohydrates, vegetable oils, animal fats, and amino acids which occurs during cooking.1 Tobacco smoke, however, equals or exceeds the total human exposure to acrolein from all other environmental sources.2 Endogenous acrolein is formed from peroxidation of fats,3 including docosohexaenoic acid (DHA),4 the predominant unsaturated fatty acid in the retina,5 and from glucose auto-oxidative peroxidation.6 It is also formed from the oxidation of spermine7 and low-density lipoprotein (LDL).8 Acrolein interacts with biomolecules to form DNA adducts, amino acid adducts9 and protein cross links.10
In the retina, acrolein has been found adjacent to the retinal pigment epithelium (RPE) in the cones of rd1 mice undergoing oxidative damage,11 and acrolein-derived products have been found in Muller cells.12 Acrolein, or acrolein-derived products, have also been found in blood serum, hemoglobin13 and vitreous (Vidro, 2010, Ph.D. dissertation).14 Spermine, a precursor of acrolein, has been found in the vitreous of patients with proliferative retinopathy at a level that is 15 times that of those without diabetes.15 Zhang et al.13 found that the acrolein-derived hemoglobin product Nε(3-formyl-3,4-dehydropiperidino) lysine (FDP-lysine) measurement could be a marker for risk evaluation of proliferative diabetic retinopathy (PDR). The importance of the RPE in the pathogenesis of diabetic retinopathy relates not only to its role in maintaining the external blood-retinal barrier but also in its importance in the secretion into the retina of various factors known to play a role in angiogenesis such as vascular endothelial growth factor (VEGF),16 pigment epithelial-derived factor (PEDF)17 and transforming growth factor β (TGFβ).18,19 Exogenous exposure to acrolein by humans is well documented,20 as well as the fact that hyperglycemia leads to production of reactive molecules such as acrolein.6,21,22 Acrolein has been associated with diabetic nephropathy.23
The generation of oxidative stress in the eyes of patients with diabetes is known to result from an increase in the production of reactive oxygen species (ROS), presumably emanating from the mitochondrial respiratory chain.24 Recently, the oxidative stress marker heme oxygenase-1 has been demonstrated to increase in the retinas of diabetic rats compared to normal controls.25 We hypothesized that this increased oxidative environment, compared to that of the normoglycemic, might be more permissive to acrolein-induced damage to RPE cells, and that N-benzylhydroxylamine (NBHA), through its ability to sequester acrolein, would be effective in preventing these changes in viable cell number, and pro-angiogenic cytokine regulation. In the present study, experiments were also performed to assess the roles of the TGFβ receptor I (TGFβRI) and Smad3 in these acrolein-induced effects.
Acrolein-lysine adducts were found to be significantly higher in the urine of young type 1 diabetes patients,26 type 2 diabetes patients,27 and in the serum of patients with type 2 diabetes with end-stage renal disease as compared with normal controls.28 Our previous studies in normal glucose conditions concluded that NBHA and SIS3 are capable of reducing acrolein-mediated RPE cell death.29 We also reported that the action of acrolein in the reduction of cell viability and the increase of VEGF are partially mediated by TGFβ2.29 Acrolein, itself, though associated with diabetes complications and retinal degeneration in age-related macular degeneration has not been studied in the eyes of subjects with diabetes. We studied in vitro, using ARPE-19 cells cultured in low and high glucose media, the effect of acrolein on the production of TGFβ and VEGF, the ability of NBHA to prevent these effects of acrolein on cytokine release, its effect on cell viability, and explored the pathways by which acrolein causes an increase in VEGF synthesis.
MATERIALS AND METHODS
ARPE-19 Cell Culture
ARPE-19 cells (ATCC #CRL 2302) were received at passage 22. They were grown to confluence in T-75 flasks in DMEM (Mediatech No. 10-014-CV) (containing phenolred), with 18 mM glucose. This was supplemented with 10% FBS (Hyclone No. SH30088.03), 0.4% sodium bicarbonate (Sigma No. 55761), 100U/mL penicillin, and 100 µg/mL streptomycin (Mediatech 30-001-CI). The media was adjusted to pH 7.2, sterile-filtered and stored at 4°C until use. Cells were maintained at 37°C in a humidified atmosphere with 5% CO2. All media and treatments were warmed to 37°C before use. Growth media was changed every 3–4 days. At confluence, after 5–7 days of culture, they were passaged. At passage 24 they were subcultured into 24-well culture plates and maintained in the medium described above. Cells were used for testing after they had been confluent for 1 week. These cells were ovoid at confluence, and non-melanized, as described by Heimsath et al.30
Co-treatment of ARPE-19 Cells with Acrolein and NBHA
Experiments were performed with a postconfluent monolayer in 24-well plates. Acrolein (Sigma No. 01680) and NBHA (Sigma No. 13454) were dissolved in serum-free 5.5 or 18 mM glucose media immediately before use. Cells were serum-starved for 24 hours in either 5.5 or 18 mM glucose media before being treated in serum-free 5.5 or l8 mM glucose media with acrolein (12.5, 25, or 50 µM), NBHA (200 µM), or co-treated with 50 µM acrolein, plus 50, 100, or 200 µM NBHA for 72 hours. At this time, the conditioned media (CM) was collected and stored at −20°C until use. ARPE-19 cells in the wells were counted immediately. Additional acrolein studies were also done in DMEM without phenol red because of the potential for the estrogen-like effects of phenol red.31 Estrogen has been shown to increase cell proliferation in retinal vascular endothelial cells.32 No significant cell viability differences were found between ARPE-19 grown with, or without phenol red (data not shown).
Acrolein and Cell Transduction Pathway Inhibitors
Postconfluent 24-well plates of ARPE-19 were serum-starved in 5.5 or 18 mM glucose for 24 hours. They were then pre-treated33 for 24 hours with serum-free 5.5 or 18 mM glucose media containing SIS3 (Calbiochem No. 566405), a specific inhibitor of SMAD333,34 at a concentration of 2 µM (concentration per C. Nagineni personal communication). Cells were also pretreated35,36 for 24 hours with 10 µM SB431542 (Sigma No. S4317), a TGFβR1 receptor blocker,35,36 at an amount that would completely inhibit the pathway without being toxic.36 The inhibitors SIS3 and SB431542 were dissolved in DMSO at a concentration of 2 parts per 1000 which has been determined by control experiments to not affect either cell viability or VEGF concentration in the CM in this experimental protocol (data not shown). After this, the media containing the inhibitors was removed and the cells were then treated for 72 hours with serum-free 5.5 or 18 mM glucose media containing 50 µM acrolein. At this time, the CM was collected and stored at −20°C until use. ARPE-19 cells in the wells were counted immediately.
Viability of ARPE-19 Cells
Viable cells were counted using a tryphan blue dye exclusion method with a Neubauer Hemocytometer.
Cytokine Determination
VEGF 165 and 121 and TGFβ1 and 2 were quantified in the CM using Quantikine ELISA kits from R&D Systems (DVE00, DB100 and DB250, respectively) according to manufacturer's instructions.
Statistical Analysis
Shapiro-Wilks test was done on the data to determine normality, Bartletts test was used to determine homogeneity of variances and data were analyzed using either one or two-way ANOVA. A p-value ≤0.05 was considered to be statistically significant. In all cases, each experiment was performed at least in triplicate (n = 3).
RESULTS
Acrolein Decreases ARPE-19 Cell Viability in l8 mM Glucose
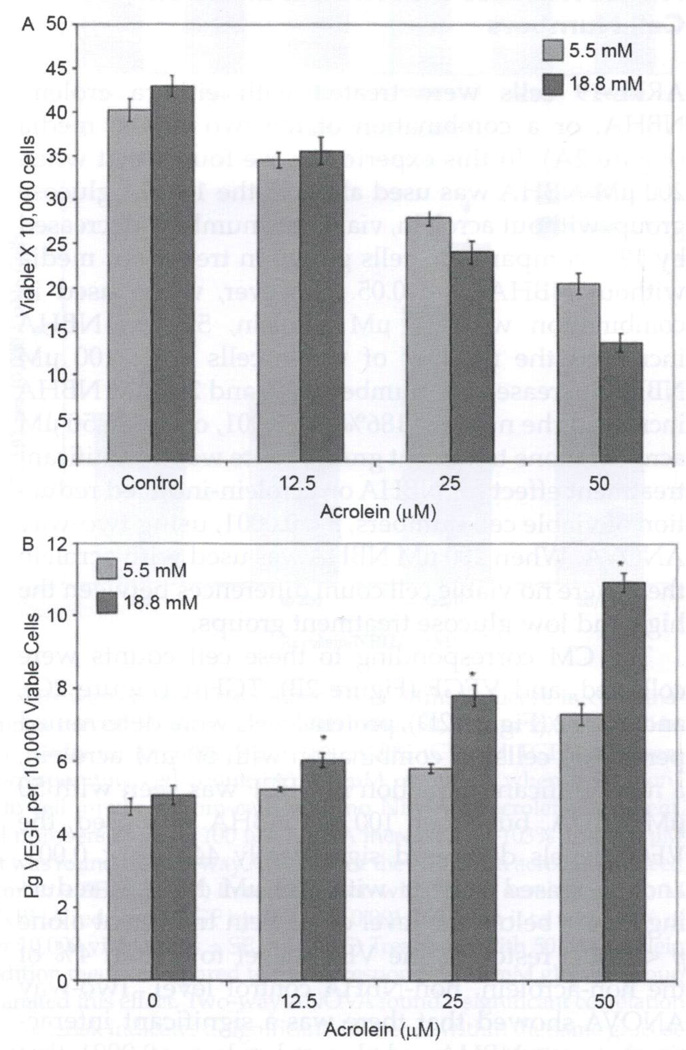
ARPE-19 cells were treated for 72 hours with increasing concentrations of acrolein (0, 12.5, 25 and 20 µM) in both normal (5 mM) and hyperglycemic (18 mM) treatment media (Figure 1A). With no acrolein in the treatment medium, there was no significant difference in viable cell number between the two glucose concentrations. However, with increasing acrolein concentration, differences in cell viability arose between the two glucose concentrations and two-way ANOVA was significant for the interaction of glucose and acrolein concentration, p < 0.05. There were 15% fewer viable cells in the 25 µM acrolein/18 mM glucose treatment condition than the equivalent 5.5 mM condition, p < 0.05. In addition, there were 34% fewer viable cells in the 18 mM glucose/50 µM acrolein than in the 5.5 mM glucose/50 µM acrolein group, p < 0.001 (Figure 1A).
FIGURE 1.
High glucose-enhances acrolein effects on ARPE-19 cell viability and VEGF levels. Confluent ARPE-19 cells were treated with either 5.5 or 18 mM glucose in combination with either 0, 12.5, 25, or 50 µM acrolein for 72 hours. Viable cell numbers (A) or VEGF (B) was determined for each treatment group. (A) 18 mM glucose increased cell loss at the 25 and 50 µM level of acrolein compared to cells treated with 5.5 mM glucose and an equivalent acrolein level, p < 0.05, p < 0.01. Two-way ANOVA was significant for the synergistic effect of glucose and acrolein on the loss of cell viability, p < 0.05. (B) 18 mM glucose increased the level of VEGF secreted into the conditioned media at each level of acrolein compared to cells treated with 5.5 mM glucose and an equivalent acrolein level. This reached significance at the 25 and 50 µM acrolein levels, p < 0.01, p < 0.001. Two-way ANOVA was significant for the synergistic effect of glucose and acrolein on the increase of VEGF, p < 0.0001. Single asterisk indicates a significant difference between 5.5 and 18 mM glucose at the same acrolein concentration (n = 3).
Acrolein Increases VEGF perViable ARPE-19 Cells in l8 mM Glucose
Confluent ARPE-19 cells were treated with increasing concentrations of acrolein in serum-free normal (5.5 mM) and high glucose (18 mM) media for 72 hours (Figure 1B). A two-way ANOVA was significant for this interaction between acrolein and glucose, p < 0.0001, and VEGF increase. This demonstrated a significant increase in VEGF secreted into the CM in the 18 mM glucose with the 25 or 50 µM acrolein group compared to the 5.5 mM glucose with the equivalent acrolein group. 25 µM acrolein/18 mM glucose had a 34% increase in VEGF per 10,000 cells compared to the 25 µM acrolein/5.5 nM glucose group, p < 0.01, and the 50 µM acrolein/18 mM glucose group had 50% more VEGF per 10,000 cells compared to the 50 µM acrolein/5.5 nM glucose group, p < 0.001 (Figure 1B).
NBHA Reduces the Acrolein Effect on Cell Numbers
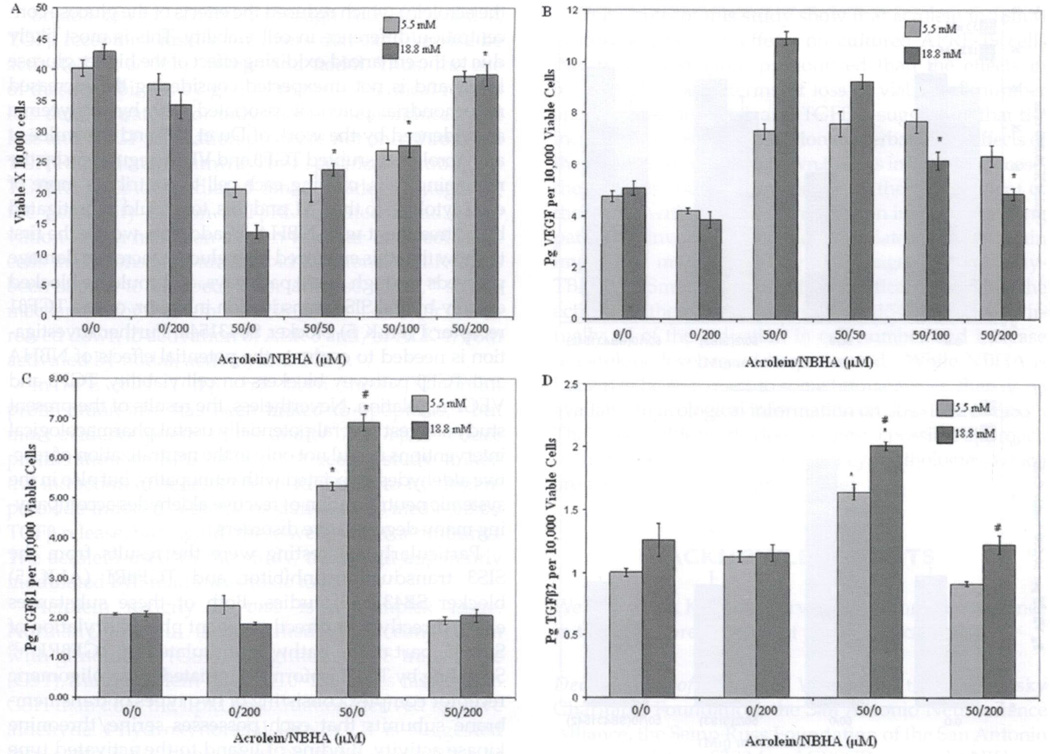
ARPE-19 cells were treated with eithera crolein, NBHA, or a combination of the two in the media (Figure 2A). In this experiment, we found that when 200 µM NBHA was used alone in the 18 mM glucose group, without acrolein, viable cell numbers decreased by 19% compared to cells grown in treatment media without NBHA, p < 0.05. However, when used in combination with 50 µM acrolein, 50 µM NBHA increased the number of viable cells 74%, 100 µM NBHA increased the number 103% and 200 µM NBHA increased the number 186%, p < 0.001, over the 50 µM acrolein-alone treatment group. There was a significant treatment effect for NBHA on acrolein-induced reduction of viable cell numbers, p < 0.0001, using two-way ANOVA. When 200 µM NBHA was used with acrolein there were no viable cell count differences between the high and low glucose treatment groups.
FIGURE 2.
NBHA inhibits the effects of acrolein. Confluent ARPE-19 cells were treated with either 5.5 or 18 mM glucose in combination with either no acrolein, 50 µM acrolein, 200 µM NBHA, or a combination of 50 µM acrolein with either 50, 100, or 200 µM NBHA for 72 hours. Viable cell numbers were counted (A) and from the cell media the proteins VEGF (B), TGFβ2 (C), and TGFβ1 (D) were determined per 10,000 cells. Protein levels were divided by their corresponding cell count. (A) 200 µM of NBHA when used alone in 18 mM glucose decreased viable cell numbers by 25% compared to cell numbers from cells with no NBHA or acrolein treatment, p < 0.05. 50 µM acrolein used with 50 µM NBHA increased viable cell numbers by 73%, 100 µM NBHA increased by 103% and 200 µM NBHA by 186%, p < 0.001 compared to acrolein alone. A treatment effect was found by two-way ANOVA for the NBHA on acrolein-induced reduction in cell number, p < 0.0001. Results are average viable cell number ± SE, n = 3. (B) In combination with 50 µM acrolein in 18 mM glucose, 100 µM NBHA reduced VEGF by 44%, p < 0.001 and 200 µM NBHA reduced VEGF by 56%, p < 0.001, bringing it to within 4% of the nonacrolein, non-NBHA group. Results are the mean pg VEGF per 10,000 viable cells ± SE, n = 3. (C) Treatment with 50 µM acrolein and 18mM glucose resulted in 230%, p < 0.001, more TGFβ1 in the condition media compared to the corresponding 18 mM glucose group without acrolein. Addition of 200 µM NBHA to the treating media eliminated this effect. Two-way ANOVA found a significant correlation between the acrolein/NBHA group and glucose levels, p < 0.0001. Single asterisk indicates a significant difference within the same glucose level; “#” indicates a significant difference between glucose levels with the same treatment. Results are the mean pg TGFβ1 per 10,000 viable cells ± SE (n = 3). (D) In the 18 mM glucose groups, 50 µM of acrolein resulted in a 75% increase, p < 0.05, of TGFβ2 levels per 10,000 cells which was reduced to control with a combination of 200 µM NBHA to the treatment media. Asterisk refers to a significant difference between acrolein effect within its own glucose level. Results are the mean pg TGFβ2 per 10,000 viable cells ± SE (n = 3).
The CM corresponding to these cell counts were collected, and VEGF (Figure 2B), TGFβ1 (Figure 2C), and TGFβ2 (Figure 2D), protein levels were determined per 10,000 cells. In combination with 50 µM acrolein, a nonsignificant reduction in VEGF was seen with 50 µM NBHA, but when 100 µM NBHA was used, the VEGF levels decreased significantly 44%, p < 0.001, and decreased further with 200 µM NBHA, reducing it 56% below the level of acrolein treatment alone p < 0.001, restoring the VEGF level to within 4% of the non-acrolein, non-NBHA control level. Two-way ANOVA showed that there was a significant interaction between NBHA and glucose levels, p < 0.0001, that accounted for 34% of the total variance of VEGF levels.
Acrolein and NBHA Affect l8 mM Glucose-induced TGFβ2 Regulation
Having established an optimal acrolein concentration, 50 µM, and an optimal NBHA concentration, 200 µM, for ARPE-19 cells, experiments were performed to determine the effect of acrolein on TGFβ2 levels (Figure 3D). Cells were treated with no acrolein or NBHA, 200 µM NBHA alone, 50 µM acrolein alone, or a combination of 50 µM acrolein and 200 µM NBHA in both high (18 mM) and low glucose (5.5 mM). In the 18 mM glucose groups, two-way ANOVA performed on results from ARPE-19 cells treated with 50 µM acrolein and 50 and 200 µM NBHA did not demonstrate a treatment effect of the NBHA (data not shown). However, the combination of 18 mM glucose and 50 µM acrolein did cause a 75% increase, p < 0.05, in TGFβ2 per 10,000 cells levels, which was eliminated with 200 µM NBHA co-treatment (Figure 2D). In the nonacrolein, non-NBHA groups, the 18 mM glucose group had 23% more TGFβ2 per 10,000 cells than the 5.5 mM group indicating that glucose levels alone play a significant role in the upregulation of TGFβ2 and enhance the acrolein effect.
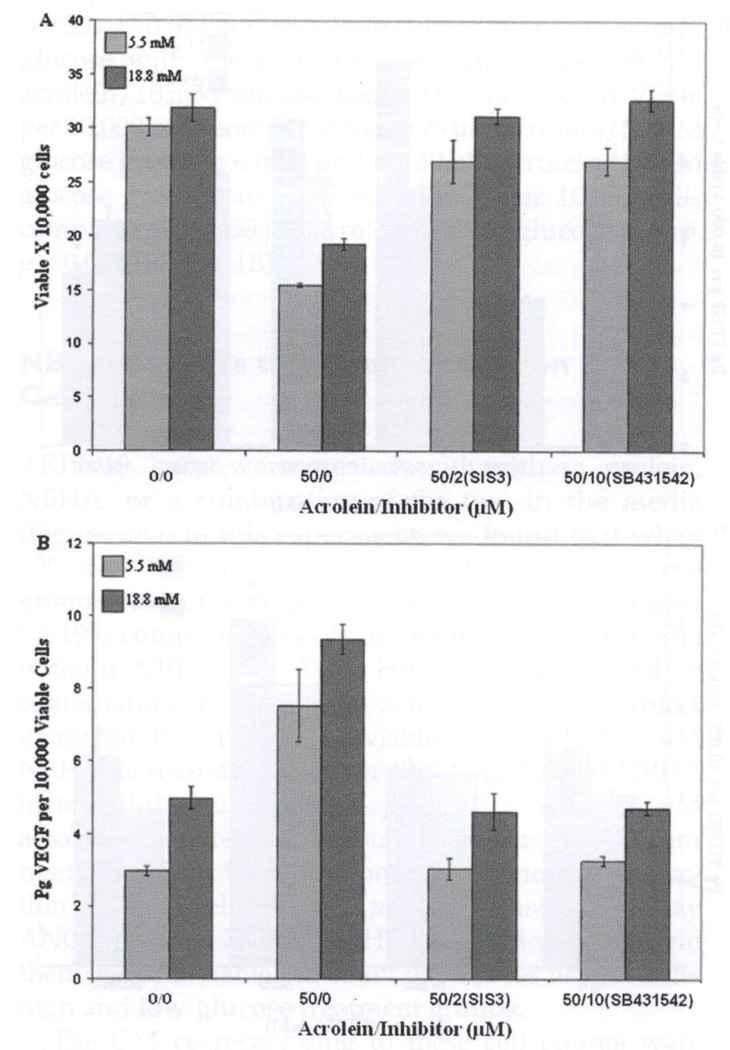
FIGURE 3.
SIS3 and SB431542 inhibits the effects of acrolein effects in high glucose. Treatment with the Smad3 inhibitor SIS3 or the TGFβ1 receptor blocker SB431542 was performed on confluent ARPE-19 cells before they were treated with acrolein in both low (5.5 mM glucose), or high (18 mM glucose) for 72 hours. (A) Pretreatment with SB431542 and SIS3 prevented acrolein-induced loss of cell viability in both 5.5 and 18 mM glucose. These blockers were equally effective in preventing 50 µM acrolein-induced viable cell loss and resulted in treated cell numbers equivalent to those without acrolein treatment in both glucose groups. Results are average viable cell number ± SE (n =3). (B) Pretreatment with SB431542 and SIS3 prevent acrolein-induced VEGF increases per 10,000 cells in the 5.5 and 18 mM glucose/50 µM acrolein treatment groups. These blockers were equally effective in preventing VEGF increase and resulted in VEGF per 10,000 cells found in the condition media equivalent to that without acrolein treatment in both glucose groups. ELISA for VEGF was performed on the conditioned media from each well, and the results were divided by the corresponding viable cell counts. Results are the mean pg VEGF per 10,000 viable cells ± SE (n =3).
A similar experiment was performed with ARPE-19 to examine the roles of acrolein and glucose in the regulation of TGFβ1 (Figure 2C). There was no difference in the low and high nonacrolein glucose treatment groups both with and without NBHA addition. However, in the presence of 50 µM acrolein, the 18 mM glucose cells had 30% more TGFβ1 in the CM, compared to the 5.5 mM group. The 18 mM glucose treatment with 50 µM acrolein increased TGFβ1 levels by 230%, p < 0.001, compared to 18 mM glucose levels in the nonacrolein group. This treatment effect was eliminated by the addition of 200 µM NBHA to the 18 mM glucose/50 µM acrolein treatment media. Results of two-way ANOVA show a significant difference between the acrolein/NBHA group and glucose levels, p < 0.0001.
The Effect of TGFβ Pathway Inhibition on Acrolein Effects in 18 mM Glucose
ARPE-19 cells in 5 and 18 mM glucose were pretreated with 2 µM SIS3 or 10 µM SB431542 for 24 hours before being treated with 50 µM acrolein for 72 hours. Figure 3A shows that these two blockers of TβR1 signaling were equally effective in preventing acrolein-induced loss of viable cells, both of them resulting in viable cell numbers not significantly different from controls or from each other. Pretreatment with these two inhibitors also prevented the acrolein-induced increase of VEGF (Figure 3B). Acrolein alone in 18 mM glucose increased the VEGF level 88%, p < 0.001, but this was prevented by both SIS3 and SB431542, restoring VEGF levels to the control level.
DISCUSSION
Acrolein has been associated with disorders including exacerbation of asthma, increased pulmonary edema, increased bronchial responsiveness,37 hemorrhagic cystitis,38 atherosclerosis,39 stroke,40,41 impaired immune function,42 renal failure,43 and Alzheimer's disease.44–46 Acrolein has also been associated with the RPE cell disruptions seen in age-related mascular degeneration.47–51 Acrolein adducts have been found in the serum and urine of both type 1 and type 2 diabetes patients,27,28,52 and recently Zhang et al.13 have found an association between the serum level of acrolein-derived FDP-lysine and the degree of diabetic retinopathy.
There have been several studies investigating substances that could mitigate the effects of acrolein-induced damage of cultured RPE cells. These substances include (R)-α lipoic acid,47 hydroxytyrosol,48 resveratrol,49 and vitamin E.50 All of these function as antioxidants and aid in mitochondrial protection. Hydroxylamines have been used in the amelioration of reactive aldehyde effects in neurodegeneration,52 damage to rat retinal precursor cells,53 and damage to mouse mammary cells.54 NBHA was successfully used in this study to prevent the toxic effects of acrolein on cultured ARPE-19 cells, and at 200 µM, NBHA was able to prevent loss of ARPE-19 cell viability caused by acrolein. NBHA also blocked increased production of TGFβ1, TGFβ2, and VEGF.
Previously, we have shown that acrolein at normal glucose levels (5.5 mM) is associated with decreased cell viability in ARPE-19 cells, which is reversed by NBHA, or the TGFβ pathway blocker SIS3. Furthermore, we found that acrolein increased levels of VEGF and TGFβ2 in the cell media of ARPE-19 cells, that the increase of TGFβ2 could be partially blocked by NBHA, and the increase in VEGF could be partially blocked by NBHA and the TGFβ pathway blocker SIS3 supporting the hypothesis that acrolein exerts its effects through modulation of the TGFβ pathway.29 We now demonstrate that acrolein has a greater effect on reducing ARPE-19 cells in higher than in normal glucose levels and that this reduction in cell number can be modulated by co-administration of NBHA (sequestering the acrolein) which reduced the effects of the glucose concentration difference in cell viability. This is most likely due to the enhanced oxidizing effect of the higher glucose levels and is not unexpected considering the increased mitochondrial potential associated with hyperglycemia as evidenced by the work of Du et al.55 and Brownlee et al.56 Acrolein disrupted TGFβ and VEGF regulation by the remaining cells, causing each cell to contribute more of each cytokine to the CM, and this, too, could be mitigated by co-treatment with NBHA. In addition, we are the first to show that this enhanced high glucose/acrolein damage proceeds through TGFβ pathways, and could be blocked equally by the SIS3 transduction inhibitor, or the TGFβ1 receptor I (ALK 5) blocker SB431542. Further investigation is needed to evaluate the potential effects of NBHA and TGFβ pathway blockers on cell viability, TGFβ and VEGF regulation. Nevertheless, the results of the present study suggest several potentially useful pharmacological interventions useful not only in the neutralization of reactive aldehydes associated with retinopathy, but also in the systemic neutralization of reactive aldehydes accompanying many degenerative disorders.
Particularly interesting were the results from the SIS3 transduction inhibitor and TGFβRI (ALK 5) blocker SB431542 studies. Both of these substances either directly or indirectly prevent phosphorylation of Smad3, part of the pathway stimulated by TGFβRI.33,35 Signaling by TGFβ isoforms is initiated by an oligomeric receptor complex consisting of two types of transmembrane subunits that each possesses serine/threonine kinase activity. Binding of ligand to the activated type II receptor (TβRII) promotes complex formation with the type I receptor (TβRI/ALK 5). TGFβRII then trans-phosphorylates TGFβRI/ALK 5 in a juxtamembrane region rich in glycine and serine residues (G–S region), leading to activation of TGFβRI/ALK 5 and propagation of TGFβ signaling by several signaling cascades. Signaling by TGFβ through the Smad pathway has been extensively characterized and is considered the canonical pathway.33,35
SB431542 (4-[4-(l,3-benzodioxol-5-yl)-5-(2-pyridinyl)-1H-imidazol-2-yl]benzamide) is a potent and selective inhibitor of ALK 4, 5, and 7, with selectivity for the kinase activity of ALK 5, and lesser selectivity for ALKs 4 and 7. It acts as a competitive ATP-binding site kinase inhibitor and has been shown to inhibit the in vitro phosphorylation of Smad3 and Smad2, but has no effect on Smad-independent pathways such as ERK, JNK, or p38 MAPK.57 SIS3 (specific inhibitor of Smad3) is a cell-permeable pyrrolopyridine compound that selectively inhibits TGFβ1-dependent Smad3 phosphorylation and Smad3-mediated cellular signaling with no effect on Smad2, p38 MAPK, ERK, or PI3K signaling.33 Smad3 is a receptor-activated molecule that, once phosphorylated by a member of the TGFβ receptor I family, i.e., ALK 4, 5, or 7, combines with Smad4 and the R-Smad/Smad4 complex then undergoes nuclear translocation and stimulates gene expression.
Smad3 can be activated through ALK 4, 5, and 7, TGFβ receptor I family members that can likewise be activated by activin, TGFβs and nodal. Thus, the use of these inhibitors, by giving virtually identical results has demonstrated not only that acrolein-induced cell loss and VEGF regulation proceeds not only through a TGFβRI-mediated pathway, but specifically through a TGFβRI receptor-mediated Smad3 pathway, negating the possibility of non-TGFβRI activated Smad3 activation which has been shown to occur in osteoblastic cells in response to parathyroid hormone.58 ALK 7 has been shown to be specific for adipose tissue,59 so the mechanism for acrolein reduction of cell number is narrowed down to activation of ALK 5 and/or ALK 4, both activated by nodal, activin, and TGFβ.
In RPE cells, TGFβ1 has been shown to inhibit proliferation and has been linked to apoptosis,60 but most evidence points to the ability of TGFβ to reduce proliferation of RPE cells.61–63 A recent study linked the induction of senescence in RPE cells to hydrogen-peroxide-induced subtoxic oxidative stress mediated by TGFβ release, but, again, these were subtoxic amounts. The acrolein used in the study, the action apparently modulated by Smad3 was toxic to RPE.
Acrolein toxicity in vivo is a complex issue. Normally, acrolein is detoxified by its conjugation with glutathione (GSH) via glutathione S-transferase (GST),64 and acrolein reacts 110–150 times faster with GSH than does the lipid peroxidation-derived reactive aldehyde 4-hydroxynonelal (HNE).1 It is suggested that some individuals may be more susceptible to acrolein toxicity due to impaired glutathione activity, especially in smokers.65 Reactive aldehydes like acrolein are also detoxified by aldehyde dehydrogenase (ALDH), a polymorphic enzyme responsible for the oxidation of aldehydes to carboxylic acids.66 Both of these mechanisms have been observed in cultured RPE cells in response to lipid-derived aldehydes.67 There is evidence, however, that reactive aldehydes can deactivate ALDH and lead to dopaminergic cell death as seen in Parkinson's disease.68 Diabetes has also been shown to impair the enzymatic disposal of HNE by both GST and ALDH in rat liver,69 and ALDH in rat retina has been shown to co-localize with lipid peroxidation products. Thus, it seems that acrolein defenses are present in the eye, but they can be disrupted by either the toxin they are meant to detoxify, or by genetic polymorphisms that render antioxidant defenses less than optimum.
Betts et al.70 demonstrated an increase in cell viability with increasing levels of exogenous VEGF in the CM. However, Betts et al. also show a decrease in ARPE-19 viability with increasing levels of VEGF, when the ginsenoside-Rb-1 is added to the CM. In this current work, we also show a decrease in ARPE-19 viability, this time in response to acrolein in the treating media, even though VEGF levels increased.
The results of this study show that acrolein in 18 mM glucose exerts toxic effects on cultured APRE-19 cells that were even more pronounced than the effects in 5.5 mM glucose in terms of loss of viable cell number and increases in VEGF and TGFB's, suggesting that the increased glucose concentration exacerbated the effects of the acrolein. It was also shown that, as in 5.5 mM glucose, these effects could be prevented by the co-treatment of these cells with NBHA. Investigation into the signaling pathways involved in acrolein-mediated toxicity again implicated members of the TGFB signaling pathway-TBRI and Smad3, because by specifically blocking the actions of these proteins with SB431542 and SIS3, virtually all of the reduction in cell number and increase in cytokine level could be prevented. While NBHA is known to be an irritant to some human tisues, there is no available toxicological information on SB431542 or SIS371. Their use in this work, does suggest a possible approach in the prevention, or treatment of eye pathologies, which are accompanied by high glucose.
ACKNOWLEDGMENTS
We also thank Kalpana Parvathaneni for her assistance in the initial preparation of this manuscript.
We thank the Kronkosky Charitable Foundation, the San Antonio Neuroscience Alliance, the Semp Russ Foundation of the San Antonio Area Foundation, STTM and CRSGP grants, the NIH and NIH Score Grant (GM08194) and the National Center for Research Resources- NIH Grant (5 G12RR013646-12), and the National Institute on Minority Health and Health Disparities- NIH Grant (G12MD007591) for their generous support of our work. The authors alone are responsible for the content and writing of this paper.
Footnotes
Declaration of interest: The authors report no conflicts of interest.
REFERENCES
- 1.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Bid Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Carmella SG, Chen M, Zhang Y, et al. Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol. 2007;20:986–990. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 4.Pan J, Keffer J, Emami A, et al. Acrolein-derived DNA adduct formation in human colon cancer cells: its role in apoptosis induction by docosahexaenoic acid. Chem Res Toxicol. 2009;22:798–806. doi: 10.1021/tx800355k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazan NG. Survival signaling in retinal pigment epithelial cells in response to oxidative stress: significance in retinal degenerations. Adv Exp Med Biol. 2006;572:531–540. doi: 10.1007/0-387-32442-9_74. [DOI] [PubMed] [Google Scholar]
- 6.Medina-Navarro R, Duran-Reyes G, Diaz-Flores M, et al. Glucose-stimulated acrolein production from unsaturated fatty acids. Hum Exp Toxicol. 2004;23:101–105. doi: 10.1191/0960327104ht416oa. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 8.Uchida K, Kanematsu M, Morimitsu Y, et al. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 9.Chung FL, Young R, Hecht SS. Formation of cyclic l,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 10.LoPachin RM, Barber DS, Gavin T. Molecular mechanisms of the conjugated alpha,β-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci. 2008;104:235–249. doi: 10.1093/toxsci/kfm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komeima K, Rogers BS, Lu L, et al. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yong PH, Zong H, Medina RJ, et al. Evidence supporting a role for N-(3-formyl-3,4-dehydropiperidino)lysine accumulation in Muller glia dysfunction and death in diabetic retinopathy. Mol Vis. 2010;16:2524–2538. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Lai Y, McCance DR, et al. Evaluation of N (epsilon)-(3-formyl-3,4-dehydropiperidino)lysine as a novel bio-marker for the severity of diabetic retinopathy. Diabetologia. 2008;51:1723–1730. doi: 10.1007/s00125-008-1071-3. [DOI] [PubMed] [Google Scholar]
- 14.Vidro-Kotchan E. The reactive aldehyde, acrolein, and factors associated with proliferative retinopathy. San Antonio, Texas: Department of Biology, University of Texas at San Antonio; 2010. [Google Scholar]
- 15.Nicoletti R, Venza I, Ceci G, et al. Vitreous polyamines spermidine, putrescine, and spermine in human proliferative disorders of the retina. Br J Ophthalmol. 2003;87:1038–1042. doi: 10.1136/bjo.87.8.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogt RR, Unda R, Yeh LC, et al. Bone morphogenetic pro-tein-4 enhances vascular endothelial growth factor secretion by human retinal pigment epithelial cells. J Cell Biochem. 2006;98:1196–1202. doi: 10.1002/jcb.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer BA, Flanders KC, Guérin CJ, et al. Transforming growth factor β 2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid and vitreous of the monkey eye. Exp Eye Res. 1994;59:323–333. doi: 10.1006/exer.1994.1114. [DOI] [PubMed] [Google Scholar]
- 19.Simó R, Villarroel M, Corraliza L, et al. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier-implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens JF, Maier CS. Acrolein: sources, metabolism, and bio-molecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994;43:836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 22.Ellis EA, Grant MB, Murray FT, et al. Increased NADH oxidase activity in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 1998;24:111–120. doi: 10.1016/s0891-5849(97)00202-5. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki D, Miyata T. Carbonyl stress in the pathogenesis of diabetic nephropathy. Intern Med. 1999;38:309–314. doi: 10.2169/internalmedicine.38.309. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 25.Curtis TM, Hamilton R, Yong PH, et al. Muller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products. Diabetologia. 2011;54:690–698. doi: 10.1007/s00125-010-1971-x. [DOI] [PubMed] [Google Scholar]
- 26.Tsukahara H, Sekine K, Uchiyama M, et al. Formation of advanced glycosylation end products and oxidative stress in young patients with type 1 diabetes. Pediatr Res. 2003;54:419–424. doi: 10.1203/01.PDR.0000076662.72100.74. [DOI] [PubMed] [Google Scholar]
- 27.Daimon M, Sugiyama K, Kameda W, et al. Increased urinary levels of pentosidine, pyrraline and acrolein adduct in type 2 diabetes. Endocr J. 2003;50:61–67. doi: 10.1507/endocrj.50.61. [DOI] [PubMed] [Google Scholar]
- 28.Noiri E, Yamada S, Nakao A, et al. Serum protein acrolein adducts: utility in detecting oxidant stress in hemodialysis patients and reversal using a vitamin E-bonded hemodialyzer. Free Radic Biol Med. 2002;33:1651–1656. doi: 10.1016/s0891-5849(02)01138-3. [DOI] [PubMed] [Google Scholar]
- 29.Vidro-Kotchan E, Yendluri BB, Le-Thai T, et al. NBHA reduces acrolein-induced changes in ARPE-19 cells: possible involvement of TGFβ. Curr Eye Res. 2011;36:370–378. doi: 10.3109/02713683.2010.549601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heimsath EG, Jr, Unda R, Vidro E, et al. ARPE-19 cell growth and cell functions in euglycemic culture media. Curr Eye Res. 2006;31:1073–1080. doi: 10.1080/02713680601052320. [DOI] [PubMed] [Google Scholar]
- 31.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grigsby JG, Parvathaneni K, Almanza MA, et al. Effects of tamoxifen versus raloxifene on retinal capillary endothelial cell proliferation. J Ocul Pharmacol Ther. 2011;27:225–233. doi: 10.1089/jop.2010.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-βl-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 34.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–1340. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- 35.Inman GJ, Nicolás FJ, Callahan JF, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 36.Laping NJ, Grygielko E, Mathur A, et al. Inhibition of transforming growth factor (TGF)-βl-induced extracellular matrix with a novel inhibitor of the TGF-β type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 37.Woodruff TJ, Wells EM, Holt EW, et al. Estimating risk from ambient concentrations of acrolein across the United States. Environ Health Perspect. 2007;115:410–415. doi: 10.1289/ehp.9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batista CK, Brito GA, Souza ML, et al. A model of hemorrhagic cystitis induced with acrolein in mice. Braz J Med Biol Res. 2006;39:1475–1481. doi: 10.1590/s0100-879x2006001100011. [DOI] [PubMed] [Google Scholar]
- 39.Park YS, Taniguchi N. Acrolein induces inflammatory response underlying endothelial dysfunction: a risk factor for atherosclerosis. Ann N Y Acad Sci. 2008;1126:185–189. doi: 10.1196/annals.1433.034. [DOI] [PubMed] [Google Scholar]
- 40.Saiki R, Nishimura K, Ishii I, et al. Intense correlation between brain infarction and protein-conjugated acrolein. Stroke. 2009;40:3356–3361. doi: 10.1161/STROKEAHA.109.553248. [DOI] [PubMed] [Google Scholar]
- 41.Tomitori H, Usui T, Saeki N, et al. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36:2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 42.Joshi-Barve S, Amancherla K, Patil M, et al. Acrolein, a ubiquitous pollutant and lipid hydroperoxide product, inhibits antiviral activity of interferon-alpha: relevance to hepatitis C. Free Radic Biol Med. 2009;47:47–54. doi: 10.1016/j.freeradbiomed.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakata K, Kashiwagi K, Sharmin S, et al. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun. 2003;305:143–149. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- 44.Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer's disease. J Neurosci Res. 2007;85:3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- 46.Williams TI, Lynn BC, Markesbery WR, et al. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer's disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Jia L, Liu Z, Sun L, et al. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by ®-alpha-lipoic acid. Invest Ophthalmol Vis Sci. 2007;48:339–348. doi: 10.1167/iovs.06-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, Sun L, Zhu L, et al. Hydroxytyrosol protects retinal pigment epithelial cells from acrolein-induced oxidative stress and mitochondrial dysfunction. J Neurochem. 2007;103:2690–2700. doi: 10.1111/j.1471-4159.2007.04954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheu SJ, Liu NC, Chen JL. Resveratrol protects human retinal pigment epithelial cells from acrolein-induced damage. J Ocul Pharmacol Ther. 2010;26:231–236. doi: 10.1089/jop.2009.0137. [DOI] [PubMed] [Google Scholar]
- 50.Feng Z, Liu Z, Li X, et al. α-Tocopherol is an effective Phase II enzyme inducer. protective effects on acrolein-induced oxidative stress and mitochondrial dysfunction in human retinal pigment epithelial cells. J Nutr Biochem. 2010;21:1222–1231. doi: 10.1016/j.jnutbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Shen JK, Dong A, Hackett SF, et al. Oxidative damage in age-related macular degeneration. Histol Histopathol. 2007;22:1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- 52.Wood PL, Khan MA, Moskal JR. The concept of “aldehyde load” in neurodegenerative mechanisms: cytotoxicity of the polyamine degradation products hydrogen peroxide, acrolein, 3-aminopropanal, 3-acetamidopropanal and 4-aminobutanal in a retinal ganglion cell line. Brain Res. 2007;1145:150–156. doi: 10.1016/j.brainres.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Wood PL, Khan MA, Kulow SR, et al. Neurotoxicity of reactive aldehydes: the concept of “aldehyde load” as demonstrated by neuroprotection with hydroxylamines. Brain Res. 2006;1095:190–199. doi: 10.1016/j.brainres.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida M, Tomitori H, Machi Y, et al. Acrolein toxicity: comparison with reactive oxygen species. Biochem Biophys Res Common. 2009;378:313–318. doi: 10.1016/j.bbrc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 55.Du XL, Edelstein D, Rossetti L, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Spl glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 57.Callahan JF, Burgess JL, Fornwald JA, et al. Identification of novel inhibitors of the transforming growth factor β1 (TGF-β1) type 1 receptor (ALK5) J Med Chem. 2002;45:999–1001. doi: 10.1021/jm010493y. [DOI] [PubMed] [Google Scholar]
- 58.Inoue Y, Canaff L, Hendy GN, et al. Role of Smad3, acting independently of transforming growth factor-β, in the early induction of Wnt-β-catenin signaling by parathyroid hormone in mouse osteoblastic cells. J Cell Biochem. 2009;108:285–294. doi: 10.1002/jcb.22252. [DOI] [PubMed] [Google Scholar]
- 59.Carlsson LM, Jacobson P, Walley A, et al. ALK7 expression is specific for adipose tissue, reduced in obesity and correlates to factors implicated in metabolic disease. Biochem Biophys Res Commun. 2009;382:309–314. doi: 10.1016/j.bbrc.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esser P, Heimann K, Bartz-schmidt KU, et al. Apoptosis in proliferative vitreoretinal disorders: possible involvement of TGF-β-induced RPE cell apoptosis. Exp Eye Res. 1997;65:365–378. doi: 10.1006/exer.1997.0341. [DOI] [PubMed] [Google Scholar]
- 61.Kishi H, Kuroda E, Mishima HK, et al. Role of TGF-β in the retinoic acid-induced inhibition of proliferation and melanin synthesis in chick retinal pigment epithelial cells in vitro. Cell Biol lnt. 2001;25:1125–1129. doi: 10.1006/cbir.2001.0795. [DOI] [PubMed] [Google Scholar]
- 62.Mitsuhiro MR, Eguchi S, Yamashita H. Regulation mechanisms of retinal pigment epithelial cell migration by the TGF-β superfamily. Ada Ophthalmol Scand. 2003;81:630–638. doi: 10.1111/j.1395-3907.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 63.Lee SC, Seong GJ, Kim SH, et al. Synthesized TGF-β s in RPE regulates cellular proliferation. Korean J Ophthalmol. 1999;13:16–24. doi: 10.3341/kjo.1999.13.1.16. [DOI] [PubMed] [Google Scholar]
- 64.Parent RA, Paust DE, Schrimpf MK, et al. Metabolism and distribution of [2,3-14C]acrolein in Sprague-Dawley rats. II. Identification of urinary and fecal metabolites. Toxicol Sci. 1998;43:110–120. doi: 10.1006/toxs.1998.2462. [DOI] [PubMed] [Google Scholar]
- 65.Palma S, Cornetta T, Padua L, et al. Influence of glutathione S-transferase polymorphisms on genotoxic effects induced by tobacco smoke. Mutat Res. 2007;633:1–12. doi: 10.1016/j.mrgentox.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Crabb DW, Matsumoto M, Chang D, et al. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc. 2004;63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- 67.Choudhary S, Xiao T, Srivastava S, et al. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol Appl Pharmacol. 2005;204:122–134. doi: 10.1016/j.taap.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 68.Rees JN, Florang VR, Anderson DG, et al. Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate. Chem Res Toxicol. 2007;20:1536–1542. doi: 10.1021/tx700248y. [DOI] [PubMed] [Google Scholar]
- 69.Traverso N, Menini S, Odetti P, et al. Diabetes impairs the enzymatic disposal of 4-hydroxynonenal in rat liver. Free Radic Biol Med. 2002;32:350–359. doi: 10.1016/s0891-5849(01)00811-5. [DOI] [PubMed] [Google Scholar]
- 70.Betts BS, Parvathaneni K, Yendluri BB, et al. Ginsenoside-Rb-1 induces ARPE-19 proliferation and reduces VEGF release. ISRN Ophthalmol. 2011;2011:6. doi: 10.5402/2011/184295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sigma-Aldrich. Material Safety Data Sheets. 2012 Available at: http://www.sigmaaldrich.com. [Google Scholar]