Abstract
Background
Advanced non-small cell lung cancer (NSCLC) patients were treated as part of a Phase I dose escalation and expansion study evaluating a true human monoclonal antibody targeting IL-1α (Xilonix), which is intended to modulate the malignant phenotype—inhibiting tumor growth, spread and offering relief of symptoms.
Methods
Sixteen NSCLC patients were included. Patients failed a median of 4 chemotherapy regimens, including 10/16 failing anti-EGFR therapy. Disease progression was evaluated using a multi-modal approach: tumor response, patient reported outcomes (EORTC-QLQC30), and lean body mass (LBM). Patients received infusions every two or three weeks until progression, and were followed 24 months to assess survival.
Results
There were no infusion reactions, dose-limiting toxicities, or deaths due to therapy. Albeit not statistically significant, there was a trend in IL-6 (−2.6±18.5 (0.1 [−2.8-2.4]), platelet counts (−11±54 (−4[−36.0-1.0]), CRP (−3.3±30.2 (0.4 [−10.7-1.8]) and LBM (1.0±2.5 (0.4 [−0.5-2.6]). Self-reported outcomes revealed reductions in pain, fatigue and improvement in appetite. Median survival was 7.6 (IQR 4.4-11.5) months, stratification based on prior anti-EGFR therapy revealed a median survival of 9.4 months (IQR 7.6-12.5) for those pretreated (N=10) versus a survival of 4.8 months (IQR 4.3-5.7) for those without (N=6, logrank p=0.187).
Conclusion
Xilonix was well tolerated, with gains in LBM and improvement in symptoms suggesting a clinically important response. Although not statistically significant, the survival outcomes observed for patients with and without prior anti-EGFR therapy raises intriguing questions about the potential synergy of IL-1α blockade and anti-EGFR therapy. Further study for this agent in NSCLC is warranted.
Introduction
There is an urgent need for therapies to treat non-small cell lung cancer (NSCLC)—which represents 80% of all malignancies affecting the lung and is the leading cause of cancer death worldwide(1). However, new therapies to target lung cancer are hampered by the diverse molecular and histological variants of the disease. While targeted therapies are emerging agents to address this need—limited survival benefits, rapid resistance and significant side effects speak to ongoing limitations with these approaches so far. New disease modifying agents are needed to control NSCLC and preferably also improve patient health and symptoms during therapy. Ideally, new therapies would also complement or synergize with the emerging array of targeted approaches.
Xilonix is a true human antibody that neutralizes interleukin-1 alpha (IL-1α), a key inflammatory signaling cytokine that drives tumor growth and metastasis through various potential mechanisms. IL-1α is present on the surface of monocytes and platelets. Interactions between cell surface associated IL-1α and the vascular endothelium lead to activation and upregulation of adhesions molecules, which is a vital first step not only for white blood cell diapedesis, but also circulating tumor cell metastasis(2,3).
Many tumor types also express IL-1α. The presence of this cytokine has been associated with higher histologic tumor grades and worsened clinical outcomes, including an increased likelihood of recurrence after surgery and decreased survival(4,5,6).
Finally, IL-1α is expressed constitutively in epithelial cells of various tissues including the skin, gastrointestinal tract, and lung(7). This preformed cytokine is released upon necrotic cell death and acts as a danger-associated molecular pattern (DAMP) or ―alarmin‖(8). Release of this DAMP in response to injury, or tissue hypoxia, results in infiltration of the surrounding area by macrophages and neutrophils, with subsequent release of vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), chemotactic factors (IL-8, MCP-1), and IL-6(9, 10)—all of which are necessary for wound healing in a physiologic state(11). In the setting of refractory cancer however, this wound healing response may actually be beneficial to the tumor, allowing for neoangiogenesis, growth, metastasis, and escape from immune surveillance through the action of myeloid derived suppressor cells(12).
Herein we report findings for a cohort of patients with NSCLC treated with Xilonix. These findings are consistent with anti-tumor activity and systemic effects of therapy reported in other patient populations, such as colorectal cancer(13). In addition, the possibility that EGFR inhibition may render tumors susceptible to immune modulating therapy suggests further approaches for optimizing anti-EGFR and provides clues to the mechanism of anti-tumor activity seen with anti-IL-1α antibody therapy.
Materials and Methods
Eligibility
This was an open label, phase I dose escalation and expansion cohort, using Xilonix in patients with advanced cancers. The subset of patients reported herein had metastatic NSCLC that was refractory to all standard therapies. Patients were ≥ 18 years of age, ECOG 0, 1, or 2 and had adequate hematologic, renal, and hepatic function. Patients with serious uncontrolled medical disorders or active infections were excluded. MD Anderson’s Institutional Review Board approved the study and informed consent was obtained from each patient.
Treatment
A 3+3 design was used for dose escalation, and subjects received Xilonix via intravenous infusion every 3 weeks (one cycle) through four dose levels—0.25 mg/kg, 0.75 mg/kg, 1.25 mg/kg, and 3.75 mg/kg; and 3.75 mg/kg every 2 weeks in the highest dose level. Patients were not pre-treated with steroids or anti-histamines. Patients could continue on study until disease progression (radiographic or clinical), unless they elected to leave study earlier or toxicities warranted discontinuation.
Radiographic Assessment of Tumors
Tumor assessments using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were conducted every 8 weeks for 7 of 16 NSCLC patients(14). Due to the mechanism of action of the agent, which is immunomodulatory, 9 of 16 NSCLC patients were assessed every 10 weeks using the Immune Related Response Criteria (irRC) in an effort to determine if this criteria more accurately captured the anti-neoplastic effect of Xilonix(15).
Metabolic Assessments
In addition to tumor restaging, body composition measurements, including change in lean body mass (LBM), were performed using dual-energy X-ray absorptiometry (DEXA). Patients underwent DEXA scans at screening and after eight weeks on study. Quality of life was assessed with the EORTC-QLQ C30(16). Resting energy expenditure (REE) was assessed using a handheld, indirect calorimeter (MedGem by Healthtech, Golden CO).
Xilonix
Xilonix (MABP1), is a true human monoclonal antibody specific for human interleukin-1α (IL-1α). The background, pharmacokinetics, and pharmacodynamics of this true human antibody have been previously described by Hong et al(17).
Statistical Analysis
An intent-to-treat analysis was performed for the safety endpoint and included all enrolled patients with NSCLC who received at least one dose of Xilonix. All patients with evaluable data on baseline and week 8 measures were included in the efficacy analysis assessing change at follow-up. Baseline, demographics, and safety variables were summarized by descriptive statistics, continuous variables were reported as mean ± standard deviation (SD), or median and interquartile range (IQR) if non-normal. Categorical variables were reported as number of cases (n) and percentage.
Repeated measure analysis was performed to compare change (from baseline to week 8) on efficacy variables between patients with and without anti-EGFR pretreatment. Survival analysis was performed by the Kaplan–Meier product-limit method. Overall survival (OS) was defined was the duration between enrollment data and date of death. Patients alive at end of follow-up were censored. Time to progression was measured from enrollment date to documented radiologic review date. The disease control rate was computed as the percent of patients having best-response rating of stable disease. The log-rank test was used to compare cumulative survival and progression free survival (PFS) across groups. SAS 9.2 software (SAS Institute Inc., Cary, NC) was used for analysis.
Results
Population Characteristics
Sixteen patients with NSCLC patients were enrolled between March 15 2010 and July 30, 2012 as part of a Phase I dose escalation and expansion(17). Their median age was 58.5 (IQR 51.5 to 70.3) years, and 62.5% (10/16) were women. The majority of patients (9 [56.3%]) had an ECOG performance status (PS) of 1, while 6 (37.5%) had PS 0, and one (6.2%) had PS 2. The patients had failed an average of 3.9±1.6 (median=4) prior systemic regimens. EGFR and KRAS mutational coding sequence, prior to enrollment, was available for 14 and 12 patients respectively. Only 1 of 14 (7%) the patients was positive for EGFR mutation, and KRAS mutation was observed in 8 of 12 (67) patients (table 1).
Table 1.
Characteristics of patients with NSCLC according to Anti-EGFR pretreatment
| All NSCLC Patients (n=16) |
Anti-EGFR pretreated (n=10) |
No Anti-EGFR pretreatment (n=6) |
p | |
|---|---|---|---|---|
| Age, (Mean±SD (Median, [IQR])), year |
59.3±13.7 (58.5 [51.5- 70.3]) |
58.9±16.9 (57.0 [49.3- 74.0]) |
59.8±7.0 (60.0 [54.0- 63.8]) |
0.96 |
| Male, n(%) | 6 (37%) | 3 (30%) | 3 (50%) | 0.44 |
| Female, n(%) | 10 (63%) | 7 (70%) | 3 (50%) | |
| ECOG status | ||||
| 0 | 6 (38%) | 3 (30%) | 3 (50%) | 0.44 |
| 1 | 9 (56%) | 6 (60%) | 3 (50%) | 0.71 |
| 2 | 1 (6%) | 1 (10%) | 0 | 1.00 |
| EGFR Mutation status | ||||
| +ve | 1 (6%) | 1 (10%) | 0 (0%) | 1.00 |
| −ve | 13 (86%) | 7 (70%) | 6 (100%) | 0.25 |
| Not done | 2 (13%) | 2 (20%) | 0 (100%) | 0.50 |
| KRAS Mutation status | ||||
| +ve | 8 (50%) | 5 (50%) | 3 (50%) | 1.00 |
| −ve | 4 (25%) | 3 (30%) | 1 (17%) | 1.00 |
| Not done | 4 (25%) | 2 (20%) | 2 (33%) | 0.60 |
| Prior Chemotherapies (Mean±SD (Median, [IQR])) |
3.4±2.4 (3.0 [2.0-4.3]) |
3.6±2.3 (3.5 [2.3-4.8]) |
3.2±2.6 (3.0 [2.3-3.0]) |
0.58 |
ECOG: Eastern Cooperative Oncology Group, IQR: inter-quartile range
Ten patients had received erlotinib therapy prior to entering study. Preliminary indications that these patients had positive outcomes prompted a stratified sub-analysis based on erlotinib prior therapy status. Baseline and clinical characteristics of the overall population and prior or no-prior erlotinib therapy is therefore presented.
Adverse Events
The most common adverse events (AE) reported in the NSCLC cohort were fatigue (8.5%), anorexia (6.8%), dyspnea (3.4%), and nausea (3.4%). Most AEs (90%) were grade 1 or 2. The adverse events greater than or equal to Grade II and at least possibly related to drug were fatigue (1.7%) and thrombocytopenia (1.7%). The other grade 3 AEs were headache (1.7%), nausea (1.7%), hospitalization for shortness of breath (1.7%), and focal seizure (1.7%). There was one serious adverse event (SAE), pneumonia that was reported as possibly related to drug.
More than 100 intravenous infusions were administered without a single infusion reaction. No patient had a dose discontinued, delayed, or reduced for toxicity. Also there were no deaths or discontinuations due to treatment related toxicity.
Pharmacokinetic Data
A single patient was treated with 0.75 mg/kg; three received 3.75 mg/kg with a 3 week dosing cycle, and the remaining 12 patients were treated at 3.75 mg/kg with 2 week cycles. The average serum concentrations on days 0 (60 minutes post-infusion), 8 and 15 (for patients in 3 week cycle) were 87.9, 17.3 and 14.1 μg/ml respectively (Table 2). Post-dose antibody concentration did not vary significantly across the dosing cycles (cycle 1 day 1 through cycle 3 day 1, p=0.22). The serum half-life was approximately 3 days.
Table 2.
Pharmacokinetic data
| Time Point | n | MABp1 concentrations (Average±SD ug/ml) |
Maximum concentration (ug/ml) |
Coefficient of variation (CV) |
|---|---|---|---|---|
| Cycle 1 Day 1 | 16 | 72.7±17.4 | 92.0 | 24% |
| Cycle 1 Day 8 | 14 | 15.1±6.4 | 26.9 | 44% |
| Cycle 2 Day 1 | 15 | 76.8±22.1 | 118.6 | 29% |
| Cycle 2 Day 8 | 14 | 16.2±10.5 | 35.0 | 65% |
| Cycle 3 Day 1 | 14 | 82.5±23.6 | 120.7 | 29% |
| Cycle 3 Day 8 | 12 | 15.5±8.8 | 32.8 | 57% |
Pharmacodynamic Parameters
Baseline and follow-up plasma interleukin-6 (IL-6) data were available for 15 of the 16 patients.. For the entire group baseline serum IL-6 levels were 13.4±18.1 pg/ml. In the patients with anti-EGFR prior therapy the baseline was 13.9±23.7 pg/ml (n=9) versus 12.5±4.4 pg/ml (n=6) in the no pretreatment group (p=0.20). After treatment, patients pre-treated with anti-EGFR showed a reduction of −8.2±20.7 (median −1.8) pg/ml in serum IL-6 levels, whereas non-pre-treated patients had an increase of 5.9±11.6 (2.0 [−1.2-8.1]) pg/ml (p=0.16).
Platelet counts declined during the study period, with a mean change from baseline to week 8 in the overall population of −11,000±5,400 per microliter (p=0.23). The anti-EGFR pre-treated and no pretreatment groups mean changes were −15,000±3,000 (p=0.22) per microliter and −6,000±8,300 (p=0.81) per microliter, respectively. A mean reduction of −14.9±27.4 mg/dL in CRP levels was seen in the anti-EGFR pre-treatment group while patients with no pre-treatment had a gain of 15.2±26.9 mg/dL (p=0.21).
Lean Body Mass and Resting Energy Expenditure
Baseline and week 8 DEXA assessments were available for 14 of 16 patients. Overall, patients had a baseline mean lean body mass of 41.4±8.9 kg, which increased to 42.5±9.5 kg during Xilonix™ therapy, representing a mean increase of 1.0±2.5 kg (p=0.22). For patients with anti-EGFR pre-treatment, a mean lean body mass increase of 1.7±2.6 kg was observed (n=8, p=0.15), while an increase of only 0.2±2.4 kg was noted for non-pretreated patients (n=6, p=1.00).
Results from indirect calorimetry recorded a median reduction in REE of −100±103 kcal/day for the anti-EGFR pre-treated group after 8 weeks of therapy with Xilonix (p=0.08). On the contrary, a significant REE increase of 208±227 kcal/day was reported in the non-pretreated group during Xilonix therapy (p=0.25).
Quality of Life
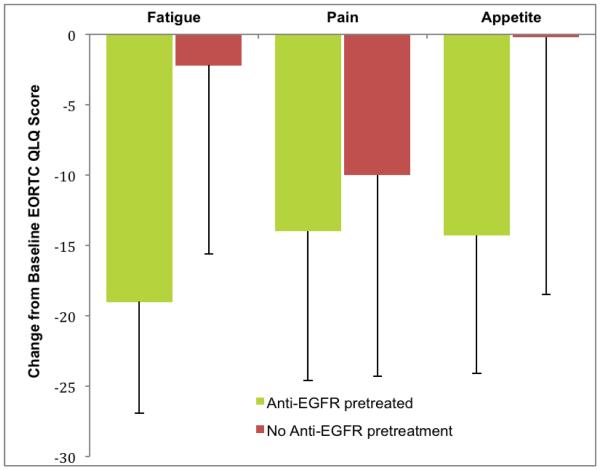
Assessment of quality of life at baseline and 8 week was performed using cancer specific EORTC QLQ C-30 questionnaire. Complete survey data for both time points was available for 12 patients (7 patients with Anti-EGFR pretreatment and 5 without). Patients with anti-EGFR pretreatment reported improved scores for functional and symptom scales from baseline to 8 weeks, while modest improvement, or worsening was observed in the no pretreatment group. Mean and median values for all seven domains, as well as p-values, are presented in table 4.
Table 4.
EORTC scores at baseline and week 8, according to anti-EGFR pretreatment
| EORTC Scales | Anti-EGFR pretreatment (n=7) | No Anti-EGFR pretreatment (n=5) | ||
|---|---|---|---|---|
| EORTC Score Average±SD (median) |
Signed rank p for Change |
EORTC Score Average±SD (median) |
Signed rank p for Change |
|
| Baseline physical function | 69.5±29.8 (73.3) | 74.7±8.7 (73.3) | ||
| Week 8 physical function | 73.3±21.4 (80.0) | 0.50 | 70.7±22.9 (80.0) | 1.00 |
| Baseline role function | 61.9±39.3 (83.3) | 56.7±22.4 (66.7) | ||
| Week 8 role function | 76.2±25.2 (83.3) | 0.25 | 63.3±29.8 (66.7) | 0.75 |
| Baseline emotional function | 73.8±30.6 (83.3) | 76.7±7.0 (75.0) | ||
| Week 8 emotional function | 78.6±18.5 (75.0) | 0.81 | 76.7±12.4 (75.0) | 1.00 |
| Baseline social function | 71.4±24.9 (66.7) | 46.7±18.3 (33.3) | ||
| Week 8 social function | 76.2±18.9 (66.7) | 0.69 | 56.7±14.9 (66.7) | 0.50 |
| Baseline fatigue score | 52.4±34.4 (55.6) | 44.4±26.1 (33.3) | ||
| Week 8 fatigue score | 33.3±15.7 (33.3) | 0.13 | 42.2±18.3 (33.3) | 1.00 |
| Baseline pain score | 35.7±42.4 (16.7) | 40.0±43.5 (33.3) | ||
| Week 8 pain score | 21.4±28.4 (0.0) | 0.31 | 30.0±34.2 (16.7) | 0.75 |
| Baseline appetite score | 23.8±37.1 (0.0) | 33.3±33.3 (33.3) | ||
| Week 8 appetite score | 9.5±16.3 (0.0) | 0.50 | 33.3±23.6 (33.3) | 1.00 |
EORTC: European Organization for Research and Treatment of Cancer
Tumor Responses
Tumor burden was assessed using RECIST methods in 7 patients and through irRC in 9. Although there were no objective responses by RECIST or irRC criteria the number of observations suggested cytotoxic activity and objective tumor responses in patients treated with anti-IL-1α therapy.
Patient 006 had a "mixed response" with some lesions increasing in size and some decreasing, and was on trial for 15 weeks. Patient 015 had cavitation of lesions on PET scan, and was on trial for 45 weeks (Figure 3). Patient 056 was diagnosed with a metastatic brain lesion approximately 5 days after C1D1, and was subsequently discontinued. A follow-up chest X-ray revealed a reduction of target lesions and the patient was restarted on therapy, continuing for a total of 12 weeks, until discontinuation due to brain involvement. Patient 074 was on trial for 16 weeks with stable disease. Patient 076 was on trial for 18 weeks with stable disease. Overall 19% (3/16) patients had SD ≥4 months. Individual patient level demographic, clinical, and genotype characteristics are presented in table 5.
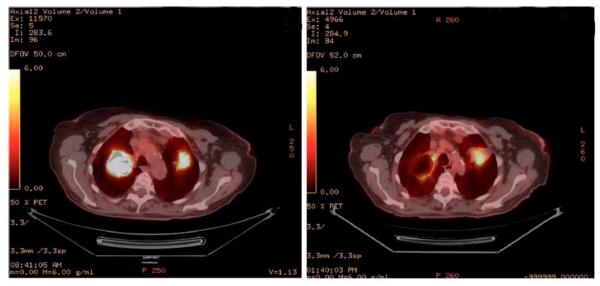
Figure 3.
Cavitation of a pulmonary lesion in a stage IV non-small cell lung cancer patient seen on PET scan. The left panel shows high metabolic activity in bilateral pulmonary lesions. The right panel shows that each of these lesions had reduced metabolic activity. Response was seen after 3 cycles of Xilonix administered at 3.75 mg/kg every 3 weeks. This patient continued on study for >45 weeks.
Table 5.
Characteristics of patients included in the study
| Subject ID | Gender | Race | Age (years) |
Anti-EGFR Pretreatment |
Response Assessment Method |
ECOG PS |
Best Response |
EGFR Mutation Status |
KRAS Mutation Status |
Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| CA-01-0006 | Female | Black | 75 | Yes | RECIST | 1 | SD | Not done | KRAS −ve | 15 weeks on trial with mixed tumor response, 8% decrease target lesions |
| CA-01-0015 | Female | White | 83 | Yes | RECIST | 0 | SD | EGFR −ve | KRAS −ve | 45 weeks on trial, cavitation of lesions on PET scan, 3% decrease target lesions |
| CA-01-0017 | Female | White | 60 | Yes | RECIST | 0 | PD | EGFR −ve | KRAS +ve | |
| CA-01-0063 | Male | White | 34 | Yes | RECIST | 1 | PD | EGFR −ve | KRAS +ve | |
| CA-01-0068 | Female | Asian | 49 | Yes | RECIST | 1 | PD | EGFR −ve | Not done | |
| CA-01-0069 | Male | White | 71 | Yes | irRC | 1 | PD | EGFR −ve | KRAS +ve | |
| CA-01-0070 | Male | Hispanic | 54 | Yes | irRC | 2 | PD | EGFR +ve | KRAS −ve | |
| CA-01-0072 | Female | White | 76 | Yes | irRC | 1 | PD | Not done | Not done | |
| CA-01-0074 | Female | Hispanic | 50 | Yes | irRC | 1 | SD | EGFR −ve | KRAS +ve | 16 weeks SD |
| CA-01-0075 | Female | Asian | 37 | Yes | irRC | 0 | PD | EGFR −ve | KRAS −ve | |
| CA-01-0056 | Female | White | 52 | No | RECIST | 0 | SD | EGFR −ve | KRAS +ve | 12 weeks,6% decrease target lesions after 2 doses/6 weeks |
| CA-01-0066 | Female | White | 70 | No | RECIST | 1 | PD | EGFR −ve | Not done | |
| CA-01-0073 | Male | White | 63 | No | irRC | 0 | PD | EGFR −ve | KRAS +ve | |
| CA-01-0076 | Male | White | 57 | No | irRC | 1 | SD | EGFR −ve | KRAS −ve | 18 weeks SD, 8.6% decrease target lesions |
| CA-01-0077 | Female | White | 64 | No | irRC | 0 | PD | EGFR −ve | Not done | |
| CA-01-0079 | Male | White | 53 | No | irRC | 1 | PD | EGFR −ve | KRAS +ve |
Progression-Free Survival
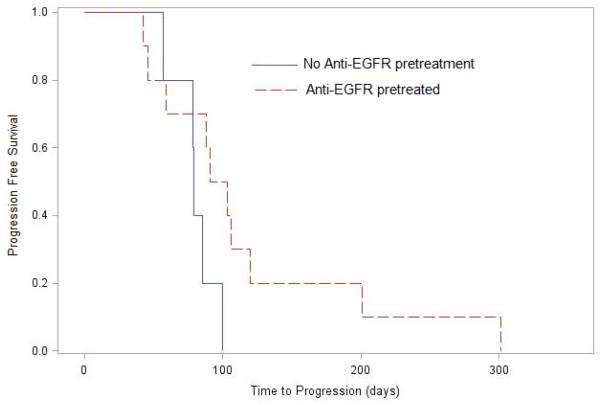
Patients remained on study for an average of 109±69 (median 95) days. The duration on study in the anti-EGFR pretreatment group was (120±84 (median 97) days) compared to no pretreatment patients (91±27 (median 92) days) (P=0.63). The median PFS time was 57 days in the overall group;, 97 days in patients with anti-EGFR pretreatment, and 78 days in patients without pre-treatment (log-rank p=0.099, figure 2A). Five of the 16 NSCLC patients had stable disease for median 16 weeks (range 6 to 45 weeks) as best response.
Figure 2A.
Kaplan-Meier curves comparing of progression free survival (PFS) in patients with anti-EFGR pretreatment (broken red line) and no anti-EFGR pretreatment (solid black line). The median time to PFS was 97.0 (IQR 66 to 116) days and 78.5 (78 to 83) days respectively (Log-rank p=0.099).
Overall Survival
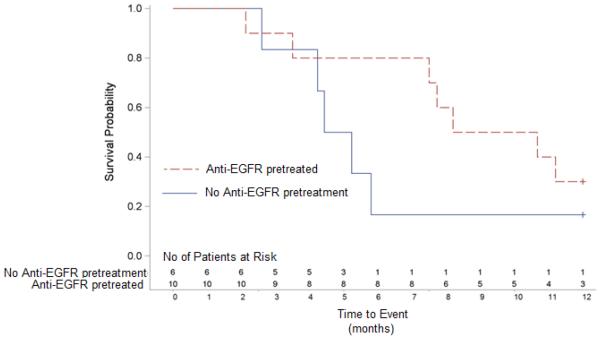
The median overall survival (OS) in the 16 patients with NSCLC was 7.6 (IQR 4.4 - 11.5) months. Survival in pretreated patients compared to those without anti-EGFR pretreatment (9.4 [IQR 7.6 to 12.5] months versus 4.8 [IQR 4.3 - 5.7] months, log-rank p=0.187) and is shown in figure 2B.
Figure 2B.
Kaplan–Meier curves comparing overall survival between anti-EGFR pretreated patients (broken red line) and with no pretreatment (solid black line). Patients with anti-EGFR pretreatment tended toward a longer survival (median 9.4 [IQR 7.6 to 12.5] months vs. 4.8 [IQR 4.3 - 5.7] months respectively, log-rank p=0.187).
Discussion
Xilonix was very well tolerated by NSCLC patients, with the validated QoL questionnaire showing clinically significant reductions in pain, fatigue and improved appetite. Self-reporting of improvements in health were corroborated by objective measures, confirming our previous report(6). The primary limitation of this report however, is the small number of patients which made any comparisons statistically difficult. These finding are largely hypothesis generating.
The pharmacodynamic endpoints chosen were those expected to be linked to IL-1α physiology and on the basis of their potential correlation with clinical performance and survival in cancer patients. Each of the pharmacodynamic measures in this study—serum IL-6(18), CRP, platelet levels(19), REE(20) and LBM(17)—have been reported elsewhere as predictors of survival. Similarly, self-reported measures of fatigue, pain and appetite loss are known prognosticators for survival(21).
Although the numbers were small and the p-value not significant, reductions in REE and increased LBM were consistent with decreased tumor metabolic demands and/or a correction in metabolic dysregulation from anti-IL-1α therapy. The anti-tumor activity suggested by these pharmacodynamics measures was supported by conventional radiology.
Pharmacodynamic and self-reported measures trended towards improvement in patients that had received prior therapy with erlotinib. Moreover, an intriguing observation suggesting survival benefit was observed with patients that had received prior treatment with—but had progressed on—anti-EGFR therapy. Median overall survival for those previously treated with anti-EGFR therapy was 9.4 months compared to only 4.8 months for non-pretreated patients. Given the small numbers of patients we cannot rule out other factors that could have accounted for this apparent difference, however one hypothesis is a synergistic interaction between anti-EGFR pre-treatment and anti-IL-1α therapy.
Understanding the interaction between anti-EGFR and anti-IL-1α therapy may provide direction to improving targeted and immunological approaches to cancer therapy. A clue to this interaction may be provided by the observation of subtle but radiologically evident tumor cytotoxicity in several patients. Antibody directed cytotoxicity may explain some of the cytotoxic effect, since IL-1α is often highly expressed on tumor cells, but this may not explain the unique pharmacodynamics and clinical benefits seen with erlotinib pre-treated patients. Previous reports of erlotinib’s effect on modifying crucial pathways that are involved in recognition and killing of tumor cells by cytotoxic T lymphocytes, may be relevant to explain these observations(22,23).
The first step in host immune control of malignant disease is the specific recognition of tumor cells. Cytotoxic T lymphocytes survey for malignant cells by engaging class I HLA molecules on the tumor cell surface, analyzing for the presence of tumor-related antigens(24,25).
Observations over the past several decades that reduced class I expression correlates with disease stage has provided some of the most compelling evidence for the existence of host immune surveillance against tumors. Tumor-associated antigens present on class I HLA molecules result in detection of tumor cells by host cytotoxic T lymphocytes. Over time, an outgrowth of tumor cell clones occurs that lack significant HLA expression, or, in other words, clones grow that are not recognized and avoid being destroyed by cytotoxic lymphocytes(26). Hence the correlation between disease stage and loss of class I expressing tumor.
While the first step is recognition, the second step in control of malignant disease is mediating tumor cell killing. A critical mechanism for sensitizing NSCLC tumors to killing has been recently suggested that involves EGFR inhibition.
Hermann and others have reported that EGFR signaling in tumor cells turns down expression of class I HLA, and that an EGFR inhibitor can be used to increase surface expression of class I molecules(22,23). The ability of anti-EGFR therapy to facilitate class I expression on tumor cells may thus be critically important for facilitating recognition of tumor cells by cytotoxic T lymphocytes.
Patients that have progressed on erlotinib therapy, may have tumors with upregulated class I HLA expression(27,28), which would prime tumor cells for recognition and killing by cytotoxic T lymphocytes. However, negative immunoregulatory actions of myeloid suppressors and T regulatory subsets in the tumor microenvironment may undermine the potential for cell-mediated control of the tumor during erlotinib treatment, resulting in disease progression on erlotinib therapy. These immunoregulatory cells can be recruited initially through the release of IL-1α from necrotic tumors or the surrounding tissue(29), and can be perpetuated by mediators that are downstream of IL-1α, such as IL-6(30). In diseases characterized by sterile inflammation, such as cancer, elevated serum IL-6 levels indeed may be a surrogate for increased IL-1 signaling(31). At the level of the tumor microenvironment, increases in IL-6 production also occur secondary to EGFR blockade(32,33), which further feeds the cycle of immunosuppression due to inflammation.
Serum IL-6 levels have been shown to be a prognostic indicator for worsened survival in some tumors(34). IL-6 has also been identified as a potential target in the treatment for the symptoms of cancer associated cachexia(35). The concept of this inflammatory cytokine contributing to the development of drug resistance however, is relatively new and certainly intriguing. Recent pre-clinical trials though, have suggested that induction of IL-6 may be one of the key mechanisms in the development of resistance to anti-EGFR therapies (36,37).
Treatment with anti-IL-1α therapy is expected to not only decrease systemic inflammation, but also to alter the inflammatory milieu of the tumor, reducing the presence or activity of myeloid suppressor and T regulatory cells that abrogate cytotoxic T lymphocyte–mediated tumor clearance(38). With erlotinib sensitization of tumors, anti-IL-1α therapy was better enabled to boost host immune control of the disease. While this effect was not great enough in the EGFR-pretreated patients to provide wide observations of radiographic responses, it may have been substantive enough to improve progression free survival as well as overall survival.
Considering the rapid development and widespread use of anti-EGFR agents, the discovery that anti-EGFR failure can be exploited to enhance immune modulating anti-tumor therapy is of urgent relevance to management of hitherto refractory patients. The use of anti-IL-1α monoclonal antibody therapy in these patients maybe a new approach in EGFR treatment failed patients. Further study in NSCLC with larger patient numbers with anti-IL-1α monotherapy and in the context of anti-EGFR agents or anti-EGFR refractory patients is warranted.
Supplementary Material
One Sentence Summary.
IL-1α blockade may help overcome resistance to anti-EGFR therapy by inhibiting the immunosuppressive effects of tumor-associated inflammation.
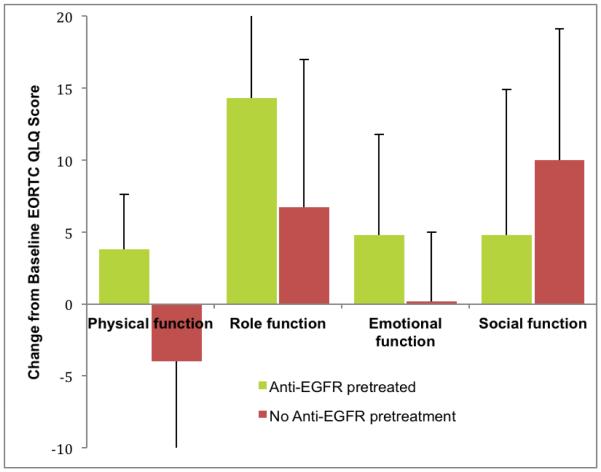
Figure 1A.
Change in EORTC functional score from baseline to week 8 according to Anti-EGFR pretreatment groups. Physical function, role function, emotional function and social function categories are defined and assessed through a questionnaire. The EORTC questionnaire is in supplementary materials.
Figure 1B.
Change in EORTC symptom score from baseline to week 8 according to Anti-EGFR pretreatment groups. Lower score for appetite shows reduction in appetite loss.
Table 3.
Pharmacodynamic parameters at baseline and week 8
| All NSCLC Patients (n=16) |
Anti-EGFR pretreatment (n=10) |
No Anti-EGFR pretreatment (n=6) |
|
|---|---|---|---|
| Screening IL-6 (pg/ml) | 13.4±18.1 (10.3 [4.4-14.1]) | 13.9±23.7 (5.8 [2.3-12.9]) | 12.5±4.4 (12.8 [10.7-14.2]) |
| Week 8 IL-6 (pg/ml) | 10.8±11.8 (6.3 [3.9-13.8]) | 5.7±5.4 (4.4 [2.8-5.4]) | 18.4±15.0 (12.1 [9.5-21.5]) |
| IL-6 change (pg/ml) | −2.6±18.5 (0.1 [−2.8-2.4]) | −8.2±20.7 (−1.8 [−3.0-1.0]) | 5.9±11.6 (2.0 [−1.2-8.1]) |
| Screening platelet count (1000/ul) |
221±64 (220 [188-260]) | 224±51 (242 [206-262]) | 215±86 (203 [178-223]) |
| Week 8 platelet count (1000/ul) |
209±85 (204 [154-265]) | 209±66 (218 [202-262]) | 209±116 (168 [124-296]) |
| Platelet count change (1000/ul) |
−11±54 (−4 [−36.0-1.0]) | −15±30 (−4.0 [−33.0-0.0]) | −6±83 (−2.0 [−50.5-1.5]) |
| Screening CRP (mg/dL) | 20.5±39.5 (7.0 [1.2-20.2]) | 26.5±50.4 (1.7 [0.6-25.5]) | 11.0±7.1 (10.6 [7.0-14.8]) |
| Week 8 CRP(mg/dL) | 17.2±24.4 (8.6 [1.8-14.4]) | 11.6±24.5 (2.1 [1.6-4.9]) | 26.1±24.0 (14.4 [9.8-32.5]) |
| CRP change (mg/dL) | −3.3±30.2 (0.4 [−10.7-1.8]) | −14.9±27.4 (0.2 [−17.5-0.6]) | 15.2±26.9 (6.7 [−0.4-21.9]) |
| Screening LBM (kg) | 41.4±8.9 (41.2 [36.1-44.8]) | 38.7±9.2 (37.1 [35.0-42.5]) | 45.0±7.6 (43.5 [41.0-50.0]) |
| Week 8 LBM (kg) | 42.5±9.5 (40.7 [35.8-47.7]) | 40.4±10.4 (38.4 [35.2-43.5]) | 45.2±8.1 (44.7 [40.4-47.7]) |
| LBM change (kg) | 1.0±2.5 (0.4 [−0.5-2.6]) | 1.7±2.6 (1.4 [0.1-2.7]) | 0.2±2.4 (0.1 [−0.6-1.9]) |
Data summarized as mean±SD (median [inter-quartile range])
Acknowledgments
Funding:
This study was funded by XBiotech USA, Inc.
Footnotes
Supplementary Materials
Supplementary Materials and Methods S1. EORTC Questionnaire
Conflicts of Interest:
DSH reports grants from Xbiotech during the conduct of the study. RK reports funding for research.
MS and PM are employees of XBiotech with stock options. JS is an employee of XBiotech with stock options, and patents related to anti-IL-1
Ethical approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent:
Informed consent was obtained from all individual participants included in the study.
References and Notes
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2014;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Thornton, et al. Platelet interleukin-1a drives cerebrovascular inflammation. Blood. 2010;115(17):3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
- 3.Sabrkhany, et al. The role of blood platelets in tumor angiogenesis. Biochemica Biophysica Acta. 2011;1815:189–196. doi: 10.1016/j.bbcan.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Tjomsland V, et al. Interleukin 1α sustains the expression of inflammatory factors in human pancreatic cancer microenvironment by targeting cancer-associated fibroblasts. Neoplasia. 2011 Aug;13(8):664–75. doi: 10.1593/neo.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomimatsu S, Ichikura T, Mochizuki H. Significant correlation between expression of interleukin-1alpha and liver metastasis in gastric carcinoma. Cancer. 2001 Apr 1;91(7):1272–6. doi: 10.1002/1097-0142(20010401)91:7<1272::aid-cncr1128>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Singer CF, et al. Interleukin 1 system and sex steroid receptor expression in human breast cancer: interleukin 1alpha protein secretion is correlated with malignant phenotype. Clin Cancer Res. 2003 Oct 15;9(13):4877–83. [PubMed] [Google Scholar]
- 7.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013 Dec 12;39(6):1003–18. doi: 10.1016/j.immuni.2013.11.010. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012 Aug;11(8):633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salven, et al. Interleukin-1α promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002 Jul 18;:1471–73. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- 10.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007 Dec;82(6):1375–81. doi: 10.1189/jlb.0607338. Epub 2007 Aug 20. [DOI] [PubMed] [Google Scholar]
- 11.Rock KL, et al. The Sterile Inflammatory Response. Annu Rev Immunol. 2010 Mar;28:321–42. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012 Feb;167(2):195–205. doi: 10.1111/j.1365-2249.2011.04515.x. doi: 10.1111/j.1365-2249.2011.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello CA. Interleukin-1α neutralisation in patients with cancer. Lancet Oncol. 2014 May;15(6):552–3. doi: 10.1016/S1470-2045(14)70164-0. doi: 10.1016/S1470-2045(14)70164-0. Epub 2014 Apr 17. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD, et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 16.Quinten C, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–71. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 17.Hong DS, et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014 May;15(6):656–66. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 18.Yeh KY, Li YY, Hsieh LL, et al. Analysis of the effect of serum interleukin-6 (IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancer. Jpn J Clin Oncol. 2010 Jun;40(6):580–7. doi: 10.1093/jjco/hyq010. doi: 10.1093/jjco/hyq010. Epub 2010 Mar 1. [DOI] [PubMed] [Google Scholar]
- 19.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012 Feb 16;366(7):610–8. doi: 10.1056/NEJMoa1110352. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravasco P, Monteiro-Grillo I, Camilo M. Colorectal cancer: intrinsic characteristics modulate cancer energy expenditure and the risk of cachexia. Cancer Invest. 2007 Aug;25(5):308–14. doi: 10.1080/07357900701208873. [DOI] [PubMed] [Google Scholar]
- 21.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009 Sep;10(9):865–71. doi: 10.1016/S1470-2045(09)70200-1. doi: 10.1016/S1470-2045(09)70200-1. Epub 2009 Aug 18. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann F, et al. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res. 64:215–220. doi: 10.1158/0008-5472.can-2522-2. [DOI] [PubMed] [Google Scholar]
- 23.Mimura K, et al. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int. J. Cancer. 2004;128:390–401. doi: 10.1002/ijc.25613. T. [DOI] [PubMed] [Google Scholar]
- 24.So T, et al. Haplotype loss of HLA class I antigen as an escape mechanism from immune attack in lung cancer. Cancer Res. 2005 Jul 1;65(13):5945–52. doi: 10.1158/0008-5472.CAN-04-3787. [DOI] [PubMed] [Google Scholar]
- 25.Corthay A. Does the immune system naturally protect against cancer? Front Immunol. 2014 May 12;5:197. doi: 10.3389/fimmu.2014.00197. doi: 10.3389/fimmu.2014.00197. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. doi:10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trivedi S, Concha-Benavente F, Srivastava RM, Jie HB, Gibson SP, Schmitt NC, Ferris RL. Immune biomarkers of anti-EGFR monoclonal antibody therapy. Ann Oncol. 2014 Jul 4;:mdu156. doi: 10.1093/annonc/mdu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollack BP, Sapkota B, Cartee TV. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res. 2011;17:4400–4413. doi: 10.1158/1078-0432.CCR-10-3283. [DOI] [PubMed] [Google Scholar]
- 29.Voronov E, Carmi Y, Apte RN. The role IL-1 in tumor-mediated angiogenesis. Front Physiol. 2014 Mar 28;5:114. doi: 10.3389/fphys.2014.00114. doi: 10.3389/fphys.2014.00114. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009 Apr 15;182(8):4499–506. doi: 10.4049/jimmunol.0802740. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim B, et al. The Interleukin-1α Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front Immunol. 2013 Nov 20;4:391. doi: 10.3389/fimmu.2013.00391. doi: 10.3389/fimmu.2013.00391. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fletcher EV, et al. EGFR inhibition induces proinflammatory cytokines via NOX4 in HNSCC. Mol Cancer Res. 2013 Dec;11(12):1574–84. doi: 10.1158/1541-7786.MCR-13-0187. doi: 10.1158/1541-7786.MCR-13-0187. Epub 2013 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giles KM, et al. Axl mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor erlotinib. Mol Cancer Ther. 2013 Nov;12(11):2541–58. doi: 10.1158/1535-7163.MCT-13-0170. doi: 10.1158/1535-7163.MCT-13-0170. Epub 2013 Sep 11. [DOI] [PubMed] [Google Scholar]
- 34.Stone RL, et al. Paraneoplastic Thrombocytosis in Ovarian Cancer. N Engl J Med. 2012;366:610–8. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narsale AA, Carson JA. Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care. 2014 Dec;8(4):321–7. doi: 10.1097/SPC.0000000000000091. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Z, et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15535–40. doi: 10.1073/pnas.1009472107. doi: 10.1073/pnas.1009472107. Epub 2010 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res. 2014 May 15;20(10):2714–26. doi: 10.1158/1078-0432.CCR-13-2613. doi: 10.1158/1078-0432.CCR-13-2613. Epub 2014 Mar 18. [DOI] [PubMed] [Google Scholar]
- 38.Qin Y, Ekmekcioglu S, Liu P, Duncan LM, Lizée G, Poindexter N, Grimm EA. Constitutive aberrant endogenous interleukin-1 facilitates inflammation and growth in human melanoma. Mol Cancer Res. 2011 Nov;9(11):1537–50. doi: 10.1158/1541-7786.MCR-11-0279. doi: 10.1158/1541-7786.MCR-11-0279. Epub 2011 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.