Abstract
Obesity is associated with insulin resistance and type 2 diabetes; molecular mechanisms promoting energy expenditure may be utilized for effective therapy. Src-associated-in-mitosis-of-68kDa (Sam68) is potentially significant because knockout (KO) of Sam68 leads to markedly-reduced adiposity. Here we sought to determine the mechanism by which Sam68 regulates adiposity and energy homeostasis. We firstly found in Sam68-KO mice a significantly-reduced body weight with the difference explained entirely by decreased adiposity. Interestingly, these effects were not mediated by a difference in food intake, but rather associated with enhanced physical activity. When fed high-fat diet, Sam68-KO mice gained much lesser body weight and fat mass as compared to wild-type (WT) littermates and displayed an improved glucose and insulin tolerance. The brown adipose tissue (BAT), inguinal and epididymal depots are smaller and their adipocytes less hypertrophy in Sam68-KO mice than in WT littermates. The BAT of Sam68-KO mice exhibited reduced lipid stores and expressed higher levels of Ucp1 and key thermogenic and fatty-acid-oxidation genes. Similarly, depots of inguinal and epididymal white adipose tissue (WAT) in Sam68-KO mice appeared browner, their multilocular Ucp1-positive cells were much more abundant, and the expression of Ucp1, Cidea, Prdm16 and Ppargc1a genes was greater as compared to WT controls, suggesting that loss of Sam68 also promotes WAT browning. Furthermore, in all fat depots of Sam68-KO mice, the expression of M2 macrophage markers were upregulated and M1 markers downregulated. Thus Sam68 plays a crucial role in the control of thermogenesis and may be targeted to combat obesity and associated disorders.
Introduction
Obesity, characterized by abnormal or excessive fat accumulation due to energy intake exceeding expenditure, has become globally epidemic and associated with an array of medical conditions, including insulin resistance, type 2 diabetes, and cardiovascular disease (Bornfeldt and Tabas 2011). In the body, white adipose tissue (WAT) is major lipid depot that contains unilocular white adipocytes for the store of vast amounts of nutrients as lipids. Brown adipose tissue (BAT), on the other hand, is a key site of energy expenditure through heat production (thermogenesis). The brown adipocytes display multilocular lipid droplet structures and express uncoupling protein 1 (Ucp1) that, when activated, short circuits the electrochemical gradient of ATP production and generates heat (Cypess et al. 2009). Because heat production is an important component of energy expenditure, targeting the molecular and cellular mechanisms controlling thermogenesis could be an effective strategy for prevention and treatment of obesity.
Recently, important advancement has been made in the identification of clusters of Ucp1 expressing adipocytes with thermogenic capacity in the WAT (Vitali et al. 2012; Wu et al. 2012). These cells, called beige or brite fat cells, are defined by their multilocular lipid droplet morphology, high mitochondrial content and the expression of a core set of brown fat-specific genes (e.g., Ucp1, Cidea and Pgc1alpha). The accumulation of beige adipocytes in WAT is referred to as ‘browning’, which includes the induction of Ucp1 expression and a gene program that gives rise to uncoupled respiration and heat production. Thus, both brown and beige adipocytes are specialized in energy expenditure through thermogenesis (Saito et al. 2009); and evidence suggests that their activities contribute to weight loss and improvement of diabetes in animals and correlate with leanness in humans (Feldmann et al. 2009; Harms and Seale 2013). At present, a number of genes that activate brown adipocytes and/or drive the induction of beige cells have been reported (Wang et al. 2013; Cohen et al. 2014), however, many of them remain to be validated (Nedergaard and Cannon 2014). Identifying novel factors that induce the white-to-brown transition will not only contribute to a better understanding of the comprehensive regulation of energy homeostasis but also provide potential molecular targets for intervention of obesity and its metabolic consequences.
In addition to environmental temperature and nutrient intake, inflammation is increasingly recognized as a significant regulator of energy homeostasis (Rao et al. 2014). In fact, obesity is now considered a state of chronic low-grade systemic inflammation (Solinas 2012). In obese animals, fat tissues display a substantial degree of inflammation (Shoelson et al. 2006), secrete inflammatory cytokines (Hotamisligil GS et al. 1993), and harbor various immune cells (Osborn and Olefsky 2012a). More recently, studies have shown that adipose tissue macrophages derived from lean mice express markers of M2 or ‘alternative activated’ macrophages. In contrast, obese states are concomitant with M1 or ‘classically activated’ macrophages (Lumeng et al. 2007). Interestingly, M2 macrophages in the adipose tissues have been shown to secrete catecholamines to induce thermogenic gene expression in BAT and induce lipolysis in WAT (Nguyen et al. 2011; Qiu et al. 2014), whereas M1 macrophages polarization is pro-inflammatory (Eguchi et al. 2013).
Sam68 (Src-associated in mitosis of 68 kDa), also known as KHDRBS1 (KH domain containing, RNA binding, signal transduction associated 1), is a member of the STAR (signal-transducer-and-activator-of-RNA) family of RNA-binding proteins (Richard 2010; Sette 2010). It is involved in a number of cellular processes including RNA processing (Bielli et al. 2011; Iijima et al. 2011), growth factor receptor and kinase signaling (Huot et al. 2009; Ramakrishnan and Baltimore 2011), transcription (Zhou et al. 2009; Fu et al. 2013), cell cycle regulation and apoptosis (Lukong and Richard 2003; Bielli et al. 2014). Most strikingly, Sam68-null (Sam68-KO) mice exhibit a markedly-reduced adiposity (Huot et al. 2012) and the underlying mechanism is not completely understood. In this study, we demonstrate that loss of Sam68 in mice enhances adipose thermogenic program and triggers WAT browning, which contribute to their lean phenotype, resistance to induction of obesity, and improved glucose metabolism.
Materials and Methods
Animals
Sam68+/− mice on C57BL background were provided by Dr. Stéphane Richard (McGill University, Montreal, Canada) (Richard S et al. 2005) and bred to obtain Sam68−/− (Sam68-KO) mice and WT littermate controls. All breeding, maintenance, and experimental procedures were approved by the Institutional Animal Care and Use Committee of Northwestern University and were conducted at the University's Center for Comparative Medicine. Mice were maintained on a 12-hour/12-hour light/dark cycle with food and water provided ad libitum. Genotypes were determined by PCR of tail DNA using primers listed in the Supplemental Table S1. For all the experiments in this report, male mice were used. For diet-induced-obesity studies, mice were fed with high-fat diet (HFD) (42 kcal % fat with high sucrose, 88137, Harlan Teklad) for 12 weeks.
Nuclear magnetic resonance (NMR) analysis of body compositions
Body compositions were analyzed with Echo MRI Whole Body Composition Analyzer (Echo Medical Systems) in the Comprehensive Metabolic Core of Northwestern University following standard operation procedures.
Open field test of general activity
The open field arena (56 x 56 cm) was used to assess ambulation and anxiety levels in the Behavioral Phenotyping Core of Northwestern University. Briefly, a mouse is placed in the center of the arena and its ambulation activity is collected by the LimeLight software for 20 minutes. The software provides the total distance traveled as well as the percentage of time/distance within different parts of the arena. An anxious mouse will spend more of its time along the perimeter of the arena. A hyperactive mouse will have a large ambulation score.
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
For GTTs, mice were fasted overnight. Glucose (2 g/kg) was administered intraperitoneally (i.p.), and then blood glucose levels were measured at 0, 15, 30, 60, and 120 min with a blood glucose meter (Bayer Contour, Whippany, NJ). For ITT, mice were fasted for 6 h. Insulin (0.7 U/kg) was i.p. administered, and blood glucose was measured at 0, 15, 30, 60, and 120 min.
Histology
Fat tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Five-micrometer sections were cut, stained with hematoxylinand eosin (H&E), and photographed under a light microscope. Immunohistochemical staining was performed with anti–Ucp1 antibodies (Abcam, Cambridge, MA) as described by Rosen laboratory (Kong et al. 2014).
Western blotting
Fat tissues were collected and immediately frozen in liquid nitrogen. Proteins were extracted with RIPA buffer (Cell Signaling Technology, Inc., Boston, MA) supplemented with complete protease inhibitor cocktail (Roche, Indianapolis, IN). For Western blotting analyses, protein samples were subjected to SDS-PAGE under reducing conditions, transferred, and blotted with antibodies to IRS1 (Millipore, Temecula, CA), phospho-IRS1(Tyr612) (Abcam Inc, Cambridge, MA), Akt and phospho-Akt(Ser473) (Cell Signaling Technology, Inc., Boston, MA), and HSP90 (Santa Cruz Biotechnology, Inc. Dallas, Texas).
Quantitative real-time RT-PCR (qRT-PCR)
RNA extraction and qRT-PCR were performed as we described previously (Tang et al. 2009). Primer sequences are listed in the Supplemental Table S1.
Statistics
All values are reported as mean ± SEM. Unpaired two-tailed Student's t tests were used for comparisons between 2 groups. Two-way ANOVA with repeated measures were used for comparisons of 2 factors at multiple levels in the time course studies. p<0.05 was considered statistically significant.
Results
Loss of Sam68 protects against diet-induced obesity
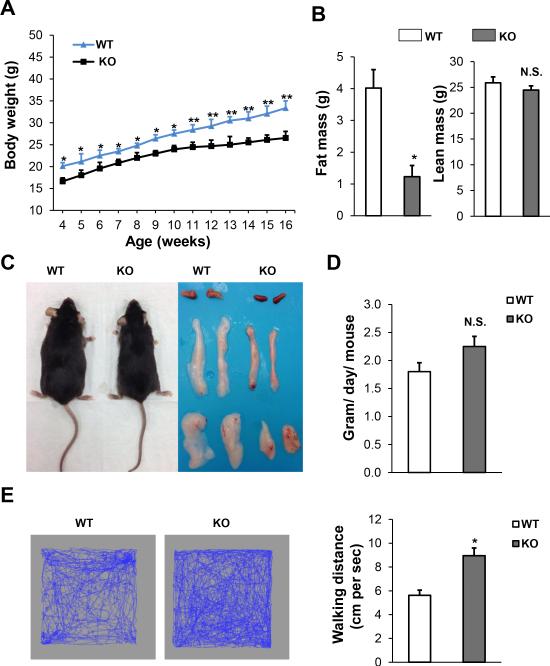
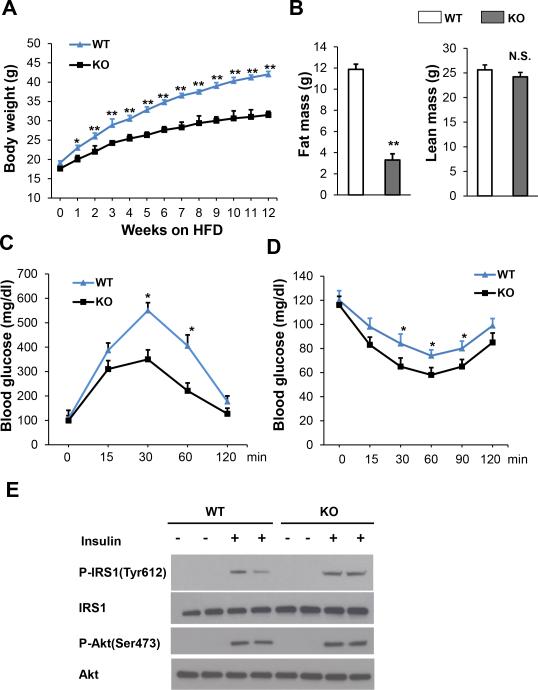
We initiated our investigation by measuring the body weight and composition in Sam68-KO mice and their WT littermates. Sam68-KO mice displayed a significantly reduced body weight and fat mass with the difference explained entirely by decreased adiposity, as lean mass was normal (Figure 1A & 1B). Further analyses showed that BAT, inguinal and epididymal depots are smaller in Sam68-KO mice (Figure 1C), and their adipocytes show less hypertrophy than those of control mice (Figure 4A), without any weight changes in liver and spleen (Supplement Figure S1). The effect on adiposity was not mediated by food intake, which did not differ between the two genotypes (Figure 1D), but rather was associated with enhanced physical activity (Figure 1E). Taken together, these data suggest that the Sam68-KO mice are lean probably because of a significant increase in physical activity not related to feeding behavior. Next, we chose the Sam68-KO mice and WT controls that were matched for body weights and fed them with high-fat diet (HFD). Notably, the increase in body weight and fat mass was significantly attenuated in Sam68-KO mice as compared to WT controls (Figure 2A & 2B).
Figure 1. Loss of Sam68 decreases adiposity in mice on regular chow.
(A) Body weight of WT mice and Sam68KO mice (n=6–10/group, *p<0.05, **p<0.01). (B) Body compositions, fat mass (left panel) and lean mass (right panel), were assessed by NMR in 6-month–old mice (n=6–10/group, *p < 0.05). (C) Gross morphology of 6-month–old WT and Sam68-KO mice and BAT, inguinal and epididymal fat pads. (D) Food intake was measured daily for 3 days in 4-month–old mice and averaged (n=6/group, N.S., not significant). (E) Representative (left and middle panels) and quantification (right panel) of general activity of 6-month–old WT and Sam68-KO mice by open field test (n=4/group, *p<0.05).
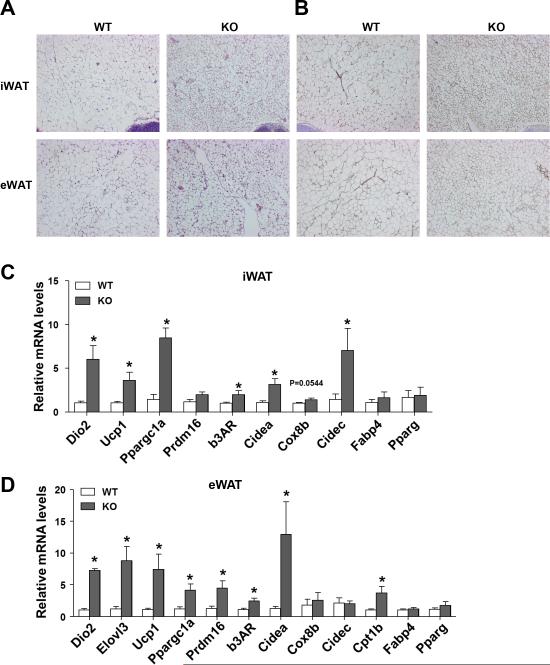
Figure 4. Ablation of Sam68 enhances the browning signature of WAT.
Inguinal and epididymal WATs were isolated from male 10-month–old WT and Sam68-KO mice. (A-B) H&E staining (A) and immunohistochemical detection of Ucp1 (brown) (B) in paraffin-embedded sections. Original magnification: 100X. (C-D) qRT-PCR quantification of mRNA expression in inguinal WAT (C) and epididymal WAT (D). Data are normalized to the 36B4 mRNA (n=5/group, *p<0.05).
Figure 2. Loss of Sam68 attenuates diet-induced obesity (DIO) and improves insulin sensitivity.
Male 8-week–old WT and Sam68-KO mice were firstly matched with body weight and then fed with HFD for 12 weeks, followed by assessments of body compositions, GTT, ITT, and insulin signaling in adipose tissues. (A) Serial measurements of body weight (n=6/group, *p<0.05, **p<0.01). (B) Body compositions by NMR (left panel, fat mass; right panel, lean mass. n=6/group, **p < 0.01, N.S., Not significant). (C) GTT and (D) ITT (n=6, *p<0.05). (E) Western blotting analyses of WAT fat depots from WT and Sam68-KO mice 30 min after i.p. injection of insulin.
Loss of Sam68 results in an improved insulin sensitivity
Diet-induced obesity is frequently associated with glucose imbalance and progressive metabolic dysfunction. Notably, Sam68-KO mice on HFD showed improved glucose and insulin tolerance to a degree consistent with their suppressed body weight (Figure 2C & 2D). In addition, we assessed the effect of Sam68 on insulin signaling and found that the insulin-induced phosphorylation of IRS-1 (Tyrosine 612) and Akt (Serine 473) was augmented in the WAT of Sam68−/− mice (Figure 2E). These findings indicate that the Sam68-KO animals are protected against HFD-induced glucose intolerance and insulin resistance.
Sam68 is required for the normal thermogenesis in BAT
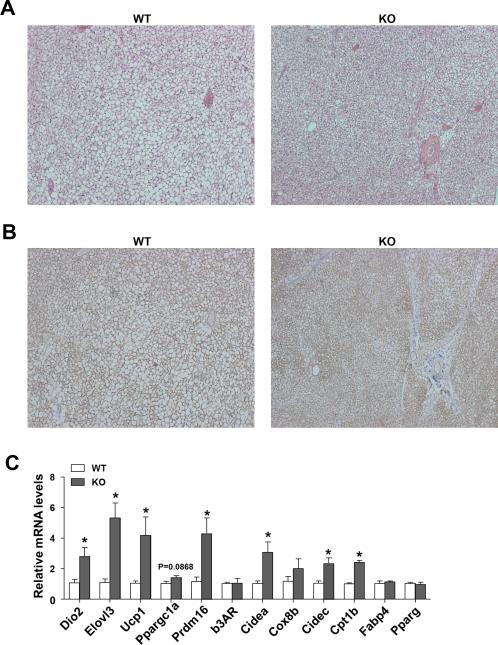
Since adipose tissue is an important organ that contributes to glucose homeostasis. BAT has been confirmed recently to play a crucial a role in glucose metabolism (Stanford et al. 2013), thus we evaluated whether Sam68 expression is required for BAT structure and function. The Sam68-KO mice have smaller brown adipose depots (Figure 1C) and reduced lipid stores in BAT at room temperature (Figure 3A). BAT from Sam68-KO mice maintained at 24°C expressed higher levels of Ucp1 than BAT from control mice (Figure 3B & Supplemental Figure S2). This phenotypic change was accompanied by increased expression of key thermogenic (e.g. Ucp1, Prdm16, Dio2, Cidea and Elovl3), as well as fatty acid oxidation (e.g. Cidec, Cpt1b) genes, but without a concomitant increase in mitochondrial genes (e.g. b3AR, Cox8b) and adipose tissue marker genes (e.g. Fabp4, Pparg) (Figure 3C). Thus, genetic inactivation of Sam68 leads to enhanced BAT structure, augmented Ucp1 activation, and elevated thermogenic gene expression.
Figure 3. Sam68 is required for the normal thermogenesis in BAT.
BAT was isolated from male 10-month–old WT and Sam68-KO mice. (A) H&E staining of paraffin-embedded sections. Original magnification: 100X. (B) Immunohistochemical detection of Ucp1 (brown). Original magnification: 100X. (C) qRT-PCR quantification of mRNA expression. Data are normalized to the mRNA level of ribosomal protein 36B4 (n=5/group, *p<0.05).
Ablation of Sam68 mice enhances the browning signature of WAT
Interestingly, depots of inguinal and epididymal WAT of Sam68-KO mice appeared browner than their WT counterparts (Figure 1C & 4A). Additionally, the weight of various fat depots was lighter in the KO mice as compared to control littermates (data not shown). Furthermore, multilocular Ucp1 positive cells were much more abundant and Ucp1 total protein levels were higher in the iWAT and eWAT of Sam68-KO mice than in WT control mice (Figure 4B & Supplemental Figure S2). Consistently, the expression of Ucp1, Cidea, Prdm16, and Ppargc1a was increased in the WAT from the Sam68-KO mice (Figure 4C). Taken together, these results suggest that deletion of Sam68 promotes adipose browning.
Sam68-KO mice favors M2 macrophage anti-inflammatory phenotypes
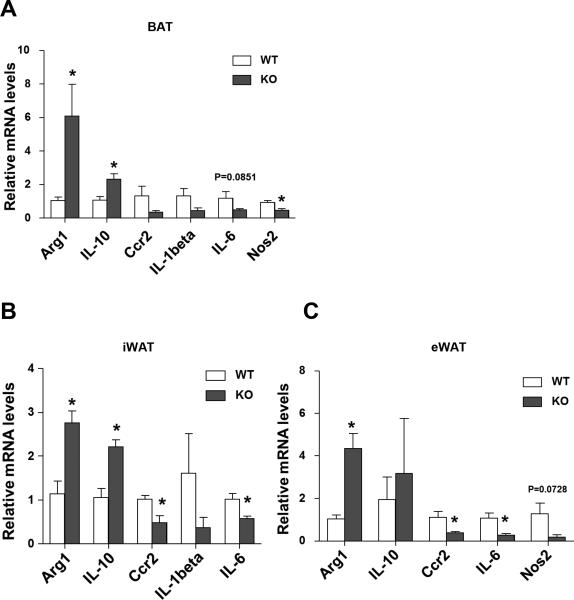
Obesity initiates a state of low-grade inflammation and fibrosis, which ultimately predisposes to the progression to insulin resistance and type 2 diabetes (Shoelson et al. 2006; Sun et al. 2011; Osborn and Olefsky 2012b). In particular, a high degree of pro-inflammatory macrophages infiltrates into the adipose tissue when obese. Thus, we looked at the expression of macrophage markers in different fat depots. As expected, the mRNA levels of M2 markers (e.g., Arg1, IL-10) were upregulated, while the M1 markers (e.g., Ccr2, IL-1beta, and IL-6) were decreased in all fat depots (Figure 5). These data suggest that loss of Sam68 also leads to macrophages switching to augment thermogenic gene expression in BAT and promote browning in WAT.
Figure 5. Sam68-KO mice favors M2 macrophage anti-inflammatory phenotypes.
RNAs were isolated from the adipose tissues of male 10-month–old WT and Sam68-KO mice. qRT-PCR was performed and the values were normalized to 36B4 (n=5, *p<0.05) for assessments of the inflammatory gene expression in (A) BAT, (B) inguinal WAT, and (C) epididymal WAT.
Discussion
In this study, we for the first time report that ablation of Sam68 in mice lead to an increase in the adipose thermogenic gene expression and WAT browning, revealing a critical role of Sam68 in the control of thermogenesis and energy expenditure. We further demonstrate that Sam68-KO mice are resistant to diet-induced obesity and display an improved insulin sensitivity. Thus Sam68 provides a novel link between energy homeostasis and obesity.
Sam68 is the prototype of the STAR family of RNA-binding proteins. It has been shown to modulate various cellular signaling and link the signaling to RNA processing and gene expression (Vogel and Richard 2012). Sam68 is expressed abundantly in most tissues (unpublished observations), and its structural characteristics permit multiple types of post-translational modifications, compatible with its involvement in many cellular functions in different tissue types (Lukong and Richard 2003; Zhou et al. 2009; Iijima et al. 2011; Ramakrishnan and Baltimore 2011) and the diverse phenotypes of Sam68-deficient mice (Vogel and Richard 2012). The Sam68-null mice appear grossly normal and have a normal life span, however, display a higher rate of perinatal death, male infertility, female delayed mammary gland development, attenuated age-related osteoporosis, and reduced capacity for motor coordination (Vogel and Richard 2012). Most strikingly, these mice demonstrate a reduced adiposity and increased energy expenditure, which has been associated with a decreased adipose differentiation (Huot et al. 2012); however the underlying mechanism remains incompletely understood. In this study, we have characterized the fat tissues in the Sam68-KO mice and revealed a previously unrecognized function of Sam68 that suppresses browning and thermogenesis. Recently, much have been learned in the types of adipocytes and the core gene program of thermogenesis (van der Lans et al. 2013). Our data identify Sam68 as a crucial upstream mechanism in the process and are also consistent with the notion that energy expenditure through thermogenesis plays a significant role in the body weight control.
Our results suggest that Sam68 suppresses thermogenesis, at least in part, through control of the physical activity and repression of the M2 anti-inflammatory cytokine expression. While physical activity is known to be a robust means to increase thermogenesis and energy expenditure, the important role of anti-inflammatory mediators is recognized only recently. For example, the M2 macrophages in the adipose tissues have been shown to participate in thermogenesis via secretion of catecholamines (Nguyen et al. 2011; Qiu et al. 2014). We found a significantly upregulated M2 macrophage signature as well as a downregulated M1 macrophage signature in the adipose tissues of Sam68-KO mice. Interestingly, Sam68 has recently been shown to promote NF-kB activation by TNF-alpha and modulate NF-kB selection of target promoters in mouse embryonic fibroblasts and the activated T cells, respectively (Ramakrishnan and Baltimore 2011; Fu et al. 2013). Since NF-kB is an important regulator of macrophage polarization (Porta et al. 2009), it is conceivable that NF-kB might be involved in the alteration of M2-M1 balance in the Sam68-KO mice. Taken together, our data suggest that Sam68 acts to coordinate the activities of multiple tissues to suppress heat generation. Furthermore, the role of Sam68 on gene expression program may be cell-type and biological-context dependent. Whereas the levels of adipose genes PPARgamma and Fabp4 are unaltered in the adipose tissues of Sam68-KO mice, their expressions are downregulated in the 3T3-L1 cells after knockdown of Sam68 and culture in adipose differentiation condition (Huot et al. 2012). Ongoing studies are underway to dissect the relative contributions from brown fat cells, beige cells, and macrophages as well as the molecular underpinnings how Sam68 regulates the mRNA expression in these different cell types.
In summary, our study demonstrates that Sam68 plays a crucial role in adipose thermogenesis, WAT browning, and body weight control. Thus, targeting Sam68 in relevant tissues may provide a potential strategy to combat obesity and associated metabolic disorders.
Supplementary Material
Acknowledgements
This work was supported by the Northwestern University Mouse Histology and Phenotyping Laboratory and a Cancer Center Support Grant (NCI CA060553).
Funding
This work was supported by the National Institute of Health (R01 Grants HL093439 and HL113541 to G.Q.); American Heart Association (Grant# 13SDG17120011 to J.Z.); and the Natural Science Foundation of China (Grant# 81100084 to M.C.).
Footnotes
Declaration of interest
None
Reference
- Bielli P, Busa R, Di Stasi SM, Munoz MJ, Botti F, Kornblihtt AR, Sette C. The transcription factor FBI-1 inhibits SAM68-mediated BCL-X alternative splicing and apoptosis. EMBO Rep. 2014;15:419–427. doi: 10.1002/embr.201338241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielli P, Busa R, Paronetto MP, Sette C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocr Relat Cancer. 2011;18:R91–R102. doi: 10.1530/ERC-11-0041. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Levy Julia D, Zhang Y, Frontini A, Kolodin Dmitriy P, Svensson Katrin J, Lo James C, Zeng X, Ye L, Khandekar Melin J, et al. Ablation of PRDM16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng Y- H, Doria A, et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi J, Kong X, Tenta M, Wang X, Kang S, Rosen ED. Interferon Regulatory Factor 4 Regulates Obesity-Induced Inflammation Through Regulation of Adipose Tissue Macrophage Polarization. Diabetes. 2013;62:3394–3403. doi: 10.2337/db12-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 Ablation Induces Obesity and Abolishes Diet-Induced Thermogenesis in Mice Exempt from Thermal Stress by Living at Thermoneutrality. Cell Metabolism. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Fu K, Sun X, Zheng W, Wier EM, Hodgson A, Tran DQ, Richard S, Wan F. Sam68 modulates the promoter specificity of NF-kappaB and mediates expression of CD25 in activated T cells. Nat Commun. 2013;4:1909. doi: 10.1038/ncomms2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, BM. S. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Huot M-É, Vogel G, Zabarauskas A, Ngo Chau T-A, Coulombe-Huntington J, Majewski J, Richard S. The Sam68 STAR RNA-Binding Protein Regulates mTOR Alternative Splicing during Adipogenesis. Molecular Cell. 2012;46:187–199. doi: 10.1016/j.molcel.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Huot ME, Brown CM, Lamarche-Vane N, Richard S. An adaptor role for cytoplasmic Sam68 in modulating Src activity during cell polarization. Mol Cell Biol. 2009;29:1933–1943. doi: 10.1128/MCB.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T, Wu K, Witte H, Hanno-Iijima Y, Glatter T, Richard S, Scheiffele P. SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell. 2011;147:1601–1614. doi: 10.1016/j.cell.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, Wang X, Yu S, Lo JC, Tseng YH, et al. IRF4 is a key thermogenic transcriptional partner of PGC-1alpha. Cell. 2014;158:69–83. doi: 10.1016/j.cell.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukong KE, Richard S. Sam68, the KH domain-containing superSTAR. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2003;1653:73–86. doi: 10.1016/j.bbcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012a;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012b;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen Khoa D, Odegaard Justin I, Cui X, Tian X, Locksley Richard M, Palmiter Richard D, Chawla A. Eosinophils and Type 2 Cytokine Signaling in Macrophages Orchestrate Development of Functional Beige Fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan P, Baltimore D. Sam68 is required for both NF-kappaB activation and apoptosis signaling by the TNF receptor. Mol Cell. 2011;43:167–179. doi: 10.1016/j.molcel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S. Reaching for the stars: Linking RNA binding proteins to diseases. Adv Exp Med Biol. 2010;693:142–157. [PubMed] [Google Scholar]
- Richard S, Torabi N, Franco GV, Tremblay GA, Chen T, Vogel G, Morel M, Cléroux P, Forget-Richard A, Komarova S, et al. Ablation of the Sam68 RNA binding protein protects mice from age-related bone loss. PLoS Genet. 2005;1:74–87. doi: 10.1371/journal.pgen.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High Incidence of Metabolically Active Brown Adipose Tissue in Healthy Adult Humans: Effects of Cold Exposure and Adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette C. Post-translational regulation of star proteins and effects on their biological functions. Adv Exp Med Biol. 2010;693:54–66. doi: 10.1007/978-1-4419-7005-3_4. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G. Molecular pathways linking metabolic inflammation and thermogenesis. Obes Rev. 2012;13(Suppl 2):69–82. doi: 10.1111/j.1467-789X.2012.01047.x. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lans AAJJ, Hoeks J, Brans B, Vijgen GHEJ, Visser M, xEb, lle GW, Vosselman MJ, Hansen J, xF, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. The Journal of Clinical Investigation. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G, Richard S. Emerging roles for Sam68 in adipogenesis and neuronal development. RNA Biol. 2012;9:1129–1133. doi: 10.4161/rna.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu R, Wang F, Hong J, Li X, Chen M, Ke Y, Zhang X, Ma Q, Wang R, et al. Ablation of LGR4 promotes energy expenditure by driving white-to-brown fat switch. Nat Cell Biol. 2013;15:1455–1463. doi: 10.1038/ncb2867. [DOI] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhu Y, Cheng M, Dinesh D, Thorne T, Poh KK, Liu D, Botros C, Tang YL, Reisdorph N, et al. Regulation of vascular contractility and blood pressure by the E2F2 transcription factor. Circulation. 2009;120:1213–1221. doi: 10.1161/CIRCULATIONAHA.109.859207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.