Abstract
Aims
To demonstrate the use of gadolinium (Gd)-labeled dendrimers as lymphatic imaging agents and establish the long-term biodistribution (90-day) of this type of agent in mice.
Materials & methods
A G5-Gd-BnDOTA dendrimer was prepared and injected into mice and monkeys for MR lymphangiography, and long-term biodistribution of the conjugate was studied.
Results
Administration of G5–Gd-BnDOTA in mice demonstrated a rapid uptake in the deep lymphatic system while injection in monkeys showed enhanced internal iliac nodes, indicating its general utility for lymphatic tracking. Biodistribution studies to 90 days showed that gadolinium conjugate is slowly being eliminated from the liver and other organs.
Conclusion
The use of G5–Gd-BnDOTA holds great promise for lymphatic imaging, but its slow clearance from the body might hamper its eventual clinical translation.
Keywords: biodistribution, dendrimers, G5–d-BnDOTA, lymphatic imaging, MRI contrast agent, PAMAM G5
Introduction
Dendrimers are a class of synthetic polymeric nanostructures composed of a central core, a repetitive branching interior and abundant terminal groups. They are highly branched, 3D, monodispersed structures with a precise nanoscale size. The increase in the growth of the dendrimer is defined as the “generation number”, with each new layer characterized by size, shape, molecular weight and the number of surface end groups. The surface functionalities can also be modulated to change the properties of the macromolecules. These unique features have made dendrimers attractive for a wide range of biomedical applications, including drug [1] and gene delivery [2,3], cancer treatment [4,5], and other theranostic agents [6].
One of the most important areas in biomedical research is diagnostic imaging, particularly MRI due to its high-sensitivity and lack of radiation exposure. The mechanisms of MRI detection are attributable to differences in proton densities, T1 or T2 relaxation times, and different water diffusion rates of water protons, thereby providing anatomical contrast. Hence, high-resolution images can be generated by the signals from water protons alone without ionizing radiation. To highlight the difference between normal and diseased tissue, and to improve the diagnostic accuracy of magnetic resonance (MR) images, exogenous gadolinium (Gd)-based contrast agents are often used. Gadolinium displays a high magnetic moment and a long electronic relaxation time. It effectively shortens the longitudinal relaxation time of adjacent water protons, thus providing increased signal contrast where it localizes. While all currently clinically approved Gd-based MR contrast agents are low molecular weight, the use of dendrimers as carriers for macromolecular contrast agents for MRI has been widely explored. Such agents have the potential to increase rotational correlation times of appended Gd(III) complexes resulting in enhanced relaxivities [7,8], while the large number of attached paramagnetic chelates on the macromolecular adduct increases the local concentration of the agent.
The behavior of the dendrimeric Gd(III) agents in vivo is affected by modification of the dendrimer properties, such as their size [9,10], core [11] and the exterior shell chemistry [12,13]. One concern about dendrimeric Gd(III) agents is the potential for retention in organs making them difficult to translate clinically. In our effort to prepare a clinically feasible MRI contrast agent for MR lymphangiography, we prepared a PAMAM G5 dendrimer conjugated to Gd-BnDOTA chelates. This is a modification of the PAMAM (G6)– Gd-BnDTPA conjugate developed by Kobayashi and coworkers that successfully demonstrated thoracic duct imaging after interstitial injection of a low dose of G5–Gd-BnDTPA into the paw of 35-kg pigs [14]. In an effort to design a more clinically applicable analog, a PAMAM G5–Gd-BnDOTA dendrimer was selected because of the greater inherent stability of the macrocyclic Gd-BnDOTA complex under in vivo challenge, its slightly smaller overall size, and an ability to unequivocally prepare a preformed 1:1 chelate:Gd(III) complex for dendrimer attachment, thereby resulting in a fully defined and reproducible conjugate for clinical manufacturing and regulatory purposes. We also used a diaminobutyl (DAB) core, instead of the ethylenediamine (EDA) core, since we hypothesized that the slightly longer spacer will produce fewer cavities or imperfections on the dendrimer surface by virtue of less packed branching, and consequently, a more reproducible batch-to-batch production.
Once prepared and fully characterized we planned to study the G5–Gd-BnDOTA conjugate in both small (mice) and large animal (monkey) models for visualization of their lymphatic systems as well as their ability to visualize blood vessels. In addition, we wanted to study the biodistribution of the fully formed conjugates radio-labeled with 153GdCl3 for accurate tissue quantitation. Our initial biodistribution studies indicated that the G5–Gd-BnDOTA was not completely cleared within 96 h in mice. We then wanted to determine if this agent would eventually clear out from the tissues, thus we extended our biodistribution studies to 90 days. Since the biological clearance rate of an agent is critical in order to determine the time-frame over which toxicity studies must be conducted, this information would be essential for any potential clinical development.
Materials & methods
All commercially available reagents were purchased from Sigma-Aldrich, and were used as received unless otherwise noted. The diaminobutyl (DAB) core PAMAM G5 den-drimer with primary amines on its surface was purchased as 20 wt% solution in methanol from Dendritech Inc. (MI, USA). The ligand S-2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane-tetraacetic acid (p-SCN-BnDOTA) was purchased from Macrocyclics, Inc. (TX, USA). 153GdCl3 was purchased from Eckert & Ziegler Isotope Products (CA, USA). Ultrafiltration membranes (Amicon-Ultra MWCO 30 kDa) were obtained from Millipore (MA, USA). The vacuum filtration system (Steriflip filter units, 0.22 µm) was purchased from Millipore. Reverse-phase HPLC analyses were performed on an Agilent 1200 Series instrument equipped with multi-wavelength detectors using a Zorbax Stable Bond C-18 column (4.6 × 50 mm, 3.5 µm) with a flow rate of 0.5 ml/min. A linear gradient of 5%B to 95%B over 10 min was used. Solvent A was 0.05% trifluoroacetic acid in water, solvent B was 0.05% TFA in acetonitrile. Electrospray ionization mass spectrometry (ESI-MS) were performed on an LC/MSD TrapXCl Agilent Technologies instrument. Size-exclusion HPLC (SE-HPLC) analyses were performed on an Agilent 1200 Series instrument equipped with multi-wavelength detectors using a TSK G3000SW column (7.5 mm ID × 30 cm, 10 um). Phosphate-buffered saline (1 x PBS) was used as the eluent with a flow rate of 1.0 ml/min. Dynamic light scattering (DLS) measurements were done on a ZetasizerNanoZS (Malvern Instruments, MD, USA) at 25°C. Elemental analyses were performed by Galbraith Laboratories, Inc. (TN, USA) using a combustion analysis method for C, H and N, an inductively coupled plasma-optical emission spectrometry (ICP-OES) method for S, and inductively coupled plasma-mass spectrometry (ICP-MS) method to determine the percentage of Gd(III).
Preparation of Gd-BnDOTA conjugate
The ligand p-SCN-BnDOTA (507 mg, 0.73 mmol) was suspended in water (1 ml) and the pH of the solution was slowly adjusted to 5.5 using 1M NaOH with mixing. An equimolar amount of GdCl3 solid (274 mg, 0.73 mmol) was added to the reaction mixture at room temperature and the pH was constantly adjusted to 5.5 using 1M NaOH solution. The reaction mixture was stirred at room temperature for 30 min at a constant pH of 5.5. The pH was then adjusted to 7.2–7.3 with 1M NaOH. The solution was vacuum-filtered and freeze-dried over 24 h to obtain Gd-p-SCN-BnDOTA, which was stored as a solid at −20°C. The absence of free Gd(III) ion was confirmed by a xylenol orange test [15]. LC–MS tR = 5.32 min (SAP isomer) and 5.56 min (TSAP isomer); m/z (ESI-MS +): calcd for [C24H31N5O8SGd]+ [M−1 + 2H+]+ 707.1, found 707.0. Anal. Calcd for C24H30N5O8SGd × 6 NaCl × 2 H2O: C, 26.4; H, 3.1; N, 6.4; S, 2.9; Gd, 14.4. Found: C, 26.6; H, 3.1; N, 6.4; S, 2.7; Gd, 14.4.
Conjugation of dendrimerG5 with Gd-BnDOTA
PAMAM dendrimer G5 (20 wt% solution in methanol, 90 mg, 3.21 µmol or 411 µmol of theoretical primary amino groups) was diluted to 10 mg/ml with 100 mM HEPES buffer (pH 8.6). The complex, Gd-p-SCN-BnDOTA (527 mg, 482µmol), was added to the dendrimer solution as a powder. The reaction mixture was stirred at 40°C for 72 h. The product was purified using an Amicon-Ultradiafiltration cell (30 kDa MWCO) against DI water. SE – HPLC of PAMAM dendrimer G5 (DAB core) starting material tR = 8.30 min (major peak) and tR = 7.20 min (minor peak, 10–12% aggregates), pH 3.5; SE-HPLC of G5–Gd-BnDOTA tR = 6.57 min in reference to thyroglobulin (670 kDa; tR = 5.01 min), L2 mAb (150 kDa; tR = 6.43 min), BSA (66 kDa; tR = 7.29 min), ovalbumin (44 kDa; tR = 8.01 min), and myoglobin (17 kDa; tR = 9.47 min). The number of chelates per dendrimer unit was obtained using the same method reported by Ali et al. [16]. Briefly, elemental analysis found for the G5 dendrimer conjugate is C 38.59%, Gd 13.0% or 38.90 total carbon atoms per gadolinium atom. The gadolinium complex formula C24H30N5O8SGd is equivalent 14.90 carbon atoms of dendrimer per gadolinium complex. The dendrimer formula C1264H2532N506O252 gives a ratio of 1264/14.90 or approximately 85 Gd chelates per dendrimer.
Molar relaxivity measurements
Stock solutions of 5 mM G5–Gd-BnDOTA conjugates were serially diluted to concentrations of 0.125 to 0.500 mM with PBS, pH 7.4. Relaxivity measurements were performed using a previous protocol [17] at ∼22°C using a 3-Tesla clinical scanner (Achieva 3.0 T, Philips Medical System, The Netherlands) using a manufacturer-supplied surface receiver coil (SENSE-Flex-M). A series of single slice 2D inversion recovery (IR) turbo spin echo images of the solutions were acquired with an acceleration factor of 8, an effective TE around 34 ms, and inversion recovery times (TI = 50, 100, 350, 750, 1250, 2500 and 5000 ms). The R1 values for each dilution were determined by fitting ROI intensity values from variable IR images using Igor Pro [18]. The longitudinal relaxivity, r1, was obtained from the slope of 1/T1 versus (Gd[III]) plots determined from region of interest measurements.
In vivo magnetic resonance studies
All procedures were performed in accordance with the NIH guidelines on the use of animals in research and were approved by the Animal Care and Use Committee of the National Cancer Institute.
In vivo magnetic resonance lymphangiography Studies
In vivo studies in mice
Six- to eight-week-old normal athymic (nu/nu) female mice (n = 4) were used to evaluate G5–Gd-BnDOTA agent’s ability to visualize the lymphatics. The mice were anesthetized using 2% isoflurane in O2 delivered using a Summit Anesthesia Solution vaporizer at a flow rate of approximately 1.0 ml/min. Intracutaneous injections of 10 µl each of 30 mM in Gd(III) of the G5–Gd-BnDOTA were administered in the middle phalanges of both upper extremities. Images were obtained using a 3-Tesla clinical scanner (Philips Achieva, Philips Medical System) equipped with an in-house parallel receiver coil array comprised of a modified Alderman-Grant resonators (38 mm o.d. × 75 cm). Dynamic MR images were obtained immediately after injections (<2 min) by repeating the 3D fast spoiled gradient recalled echo (3D-fSPGR) (Philips Achieva) approximately every 5 min for 30 min to monitor the lymphatic drainages in the axillary and lateral thoracic lymph nodes.
In vivo studies in monkeys
A detailed description of the contrast agent injection into the monkey was previously reported [19]. Briefly, 0.05 ml of 10 mM G5–Gd-BnDOTA dendrimer was injected into the proximal 1/3 vaginal mucosa in a macaque (weight ∼7 kg) to track the lymphatic drainage at 3 Tesla MRI.
In vivo magnetic resonance angiography studies in mice
Five 8-week-old normal athymic (nu/nu) female mice (n = 4) were used for testing the ability of G5–Gd-BnDOTA to visualize blood vessels. The mice were anesthetized using 2% isoflurane in O2 delivered using a Summit Anesthesia Solution vaporizer at a flow rate of approximately 1.0 ml/min. Intravenous injection of 100 µl each of 6 mM in Gd(III) of the G5–Gd-BnDOTA were administered in the tail vein. Images were obtained using a 3-Tesla clinical scanner (Philips Achieva, Philips Medical System) equipped with an in-house parallel receiver coil array comprised of a modified Alderman-Grant resonators (38 mm o.d. × 75 cm). Dynamic MR images were obtained immediately after injection (∼1 min) by repeating the 3D fast spoiled gradient recalled echo (3D–fSPGR) (Philips Achieva) every ∼3 min for 15 min to monitor the large blood vessels, and the bladder for evaluating urinary excretion.
Radiolabeling of G5–Gd-BnDOTA
PAMAM dendrimer G5 (20 wt% soln in methanol, 10 mg, 0.357 µmol, 45.7 µmol of theoretical primary amines) was diluted to 10 mg/ml with 100 mM HEPES buffer (pH 8.6). The ligand, p-SCN-BnDOTA (10µmol), and the cold Gd(III) complex, Gd-p-SCN-BnDOTA (91 µmol) was added to the reaction and stirred at 40°C for 24 h. The product was purified using an Amicon-Ultra diafiltration cell (30 kDa MWCO) with DI water. An aliquot of 1 mCi of 153GdCl3 adjusted to pH 5.5 using 5 M ammonium acetate buffer (pH 5.5) was added to the G5 den-drimer conjugate. Cold GdCl3 (0.25 ml, 100 mM) was then added and stirred at 40°C for 24 h. A solution of EDTA (0.1 M) was added to sequester the free Gd(III) present in the reaction. The product was purified using an Amicon-Ultra diafiltration cell (30 kDa MWCO) against PBS (pH 7.4). The recovered yield was 886 µCi in 900 µl. The radiochemical purity of the preparation was established by SE-HPLC analysis SE-HPLC: tR = 6.62 min reference to cold G5–Gd-BnDOTA (tR = 6.78 min) when analyzed with tandem in-line detectors.
Biodistribution of G5–153Gd/Gd-BnDOTA
All animal care and experimental protocols were approved by the National Cancer Institute Animal Care and Use Committee. The in vivo behavior of the G5–153Gd/Gd-BnDOTA was assessed using non-tumor-bearing athymic mice (Charles River Laboratories, MA, USA). 4- to 6-week-old female mice (n = 3–5 animals per time point) were injected with 1 µCi of G5–153Gd-BnDOTA (+100 µg cold G5–Gd-BnDOTA/animal) intravenously (i.v.) via tail vein in a volume of 0.2 ml. The mice were euthanized at 1, 4, 7, 16, 30, 60 and 90 days post-injection. The blood and major organs were collected, wet-weighed and counted in a γ-scintillation counter (2480 WIZ-ARD2, Perkin Elmer, CT, USA). The percentage of injected dose per gram (%ID/g) was determined for each tissue. The averages and standard deviations were determined.
Results
Synthesis & characterization
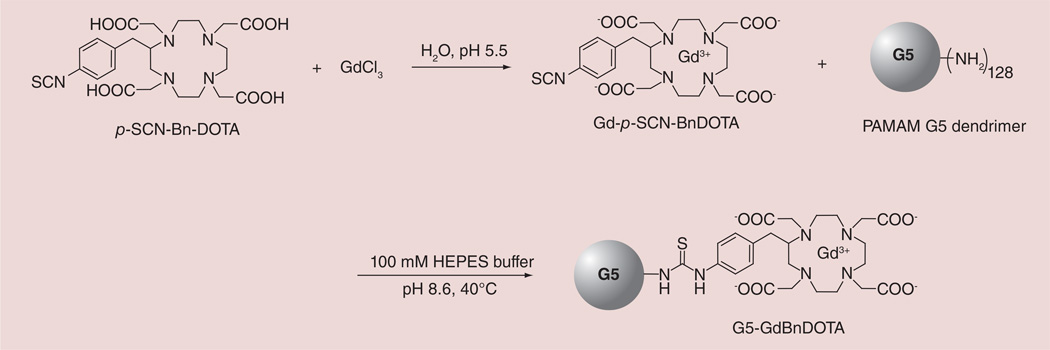
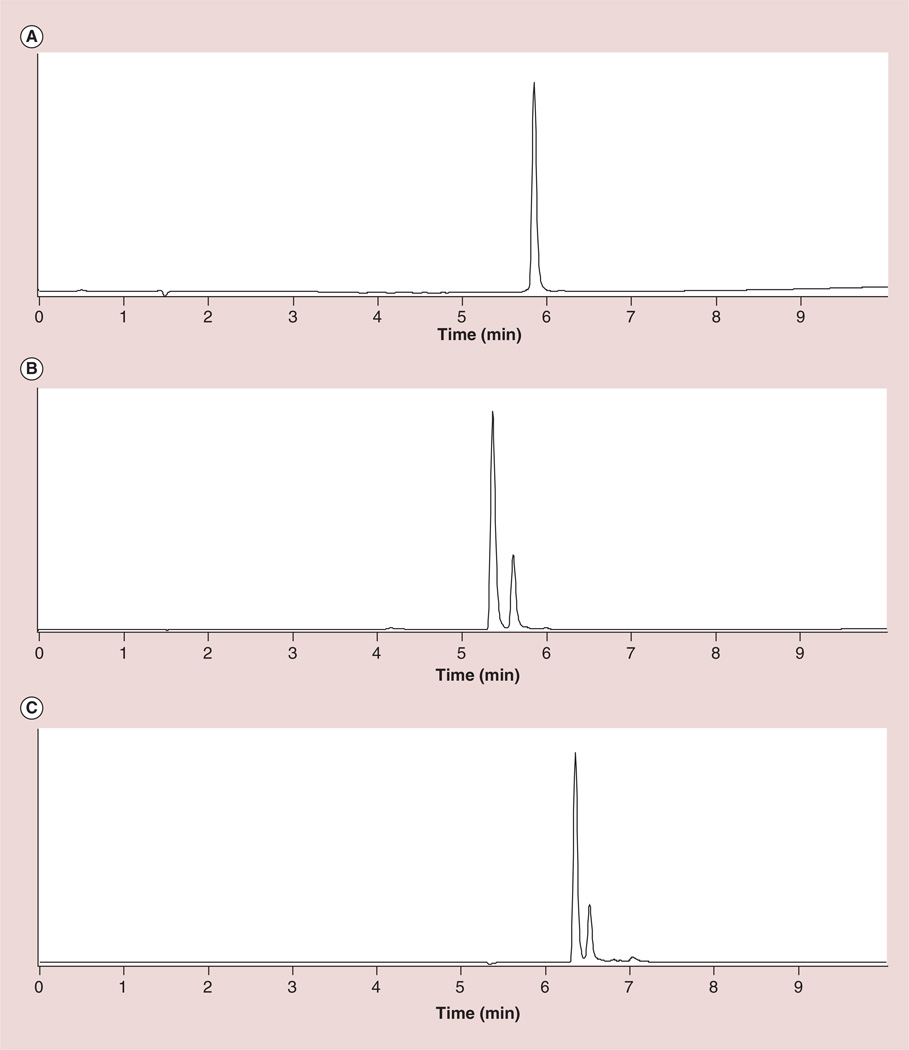
The preparation of G5–Gd-BnDOTA is outlined in Figure 1. The Gd complex was prepared by mixing an equimolar amount of GdCl3 and p-SCN-BnDOTA ligand in water. The isothiocyanates are known to hydrolyze slowly in water and rapidly at pH above 8, thus, the pH was carefully maintained near pH 5.5 at room temperature to prevent hydrolysis. The complex formation was monitored using LC–MS, by observing the appearance of Gd-p-SCN-BnDOTA (tR = 5.32 and 5.56 min) and the disappearance of the free ligand, p-SCN-BnDOTA (tR = 5.79 min) (Figure 2A & B). The two peaks observed in the complex formation both showed a characteristic Gd(III) isotope pattern, and a mass of 707.0, which corresponded to Gd-p-SCN-BnDOTA. These two peaks were attributed to the formation of two coordination isomers most common for DOTA-type complexes: the square antiprism (SAP) and the twisted square antiprism (TSAP). The two HPLC peaks were also observed for the complex, Y-p-NH2-BnDOTA, which were assigned to SAP and TSAP [20]. These isomers are in dynamic equilibrium in solution, and interconvert either by macrocyclic ring inversion or by pendant arm rotation [21]. In LnDOTA and LnDOTA-type complexes, it is well-established that the TSAP isomers have a faster water exchange (∼50-times faster) than the SAP isomers [22–24]. This has been ascribed to the more open coordination cage of the TSAP isomer, which has a reported twist angle of ∼30° between the 4N and 4O planes, whereas the SAP isomer has a ∼40° twist angle [25,26]. The more open structure of TSAP also accommodates the larger ionic radius of the early Ln(III) ions. Thus, as the ionic radius of the lanthanide decreases, the ratio of the SAP to TSAP isomer increases until around Er [27, 28]. The SAP isomer is the major isomer found for Gd-DOTA and Gd-BnDOTA derivatives. Through HPLC integration, the ratio of SAP and TSAP isomers (0.81:0.19) was measured and was found to be consistent with the reported values (Figure 2B) [27,28]. This ratio was also corroborated by comparison to the 1H NMR of Eu-p-NO2-BnDOTA, which represents a surrogate metal-chelate complex. The corresponding Gd complex cannot be observed using NMR because of the small paramagnetic shifts involved and the large peak width induced by the ion [29]. Hence the Eu (III) conjugate was prepared the same way as Gd–p-SCN-BnDOTA, and used for both NMR and HPLC analyses. The SAP:TSAP ratio of Eu-p-NO2-BnDOTA (0.82:0.18) measured through integration of the isomer signals from HPLC (Figure 2C) and the highly shifted axial proton peaks in the 1H NMR (not shown) are in good agreement with the literature [27, 28].
Figure 1.
Synthesis of G5–Gd-BnDOTA.
Figure 2. HPLC profile showing the lanthanide complex formation.
(A) p-SCN-BnDOTA ligand, (B) Gd–p-SCN-BnDOTA and (C) Eu–p-NO2-BnDOTA, the surrogate chelate complex.
Conjugation of Gd-p-SCN-BnDOTA complex to the G5 dendrimer was performed at a basic pH of 8.6 and at 40°C. Isothiocyanates form a stable product with primary amines at alkaline pH when the amine groups are mainly unprotonated, and due to the high reactivity of isothiocyanates to the amines, this strategy is very well adapted to the conjugation of ligands to dendrimers [30,31]. Addition of 1.2 equivalents of Gd-p-SCN-BnDOTA per equivalent of available den-drimer amino groups resulted in 85 Gd(III) chelates on the dendrimer surface out of a theoretical maximum of 128 (66% conjugation). This reaction mixture was stirred at 40°C for 3 days since we established that complete hydrolysis of Gd-p-SCN-BnDOTA occurred in 3 days under similar reaction conditions. Since one of our goals is to produce a G5–Gd-BnDOTA conjugate with the highest modification of the dendrimer primary amines, addition of Gd-p- SCN-BnDOTA was employed. However, two more additions of excess Gd(III) complex did not result in a higher dendrimer loading, according to SE-HPLC, and elemental analysis of Gd(III) content.
The average number of Gd(III) chelates conjugated on the dendrimer surface was assessed by elemental analysis, and by using the same calculation method reported by Ali and coworkers [16]. The total percentage carbon (from the G5 dendrimer and Gd conjugate) was found to be 38.59%, while the percentage gadolinium was found to be 13.0% from the elemental analysis. Merbach and coworkers also reported that the carbon percentage is the most accurate among C, H, N, O and S analysis [30]. By using their corresponding atomic weights, the ratio of total carbon to Gd was 38.90. The Gd complex has a molecular formula of C24H30N5O8SGd or 24 carbon atoms per gadolinium atom. Subtracting 24 C/Gd from 38.90 total C/Gd gives 14.90 dendrimer carbon per gadolinium. The G5 dendrimer (DAB core) has 1264 carbon atoms (from its molecular formula C1264H2532N506O252), which then gives a ratio of 1264:14.90 or 85 Gd chelates per dendrimer.
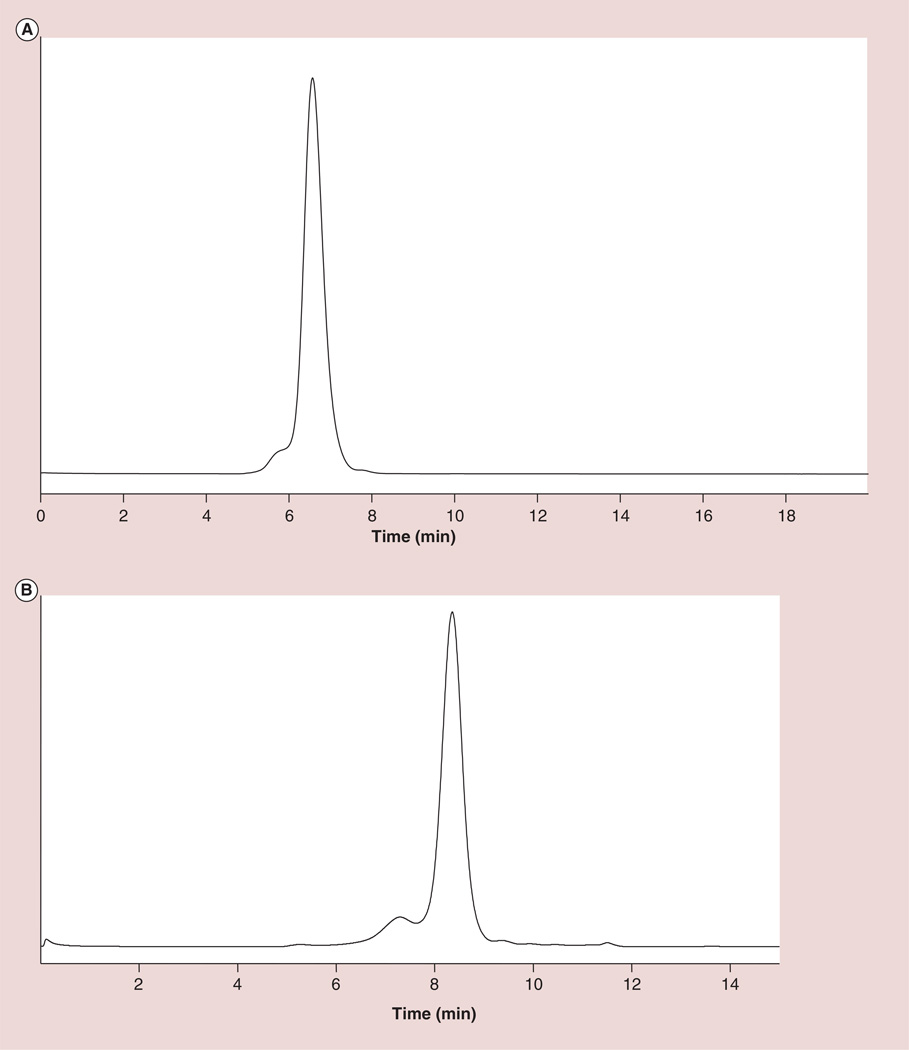
The SE-HPLC profile of the G5–Gd-BnDOTA conjugate (Figure 3A) showed a retention time of 6.57 min, which is near the molecular weight of a 150 kDa IgG standard. The shoulder in front of the main peak was due to the aggregated dendrimer already present in the starting material. The SE-HPLC profile of the starting material showed the presence of 10–12% aggregates (Figure 3B). Dynamic light scattering measurements gave an estimate of the hydrodynamic diameter of the dendrimer conjugate. Measurements were done in PBS (pH 7.4) at 25°C, and showed a diameter of 6.5 nm, a 1.6 nm increase from the hydrodynamic diameter of G5 dendrimer starting material experimentally measured to be 4.9 nm. This value compares well with that previously reported by Brechbiel’s group [32]. At 3 T and 23°C, the longitudinal relaxivity, r1, was found to be 12.98 ± 0.06 mM-1s-1.
Figure 3. Size-exclusion HPLC profile comparison of the G5-Gd conjugate and the starting material.
(A) G5–Gd-BnDOTA at pH 7.4, and (B) G5 starting material at pH 3.5.
In vivo lymphangiography studies
In vivo studies in mice
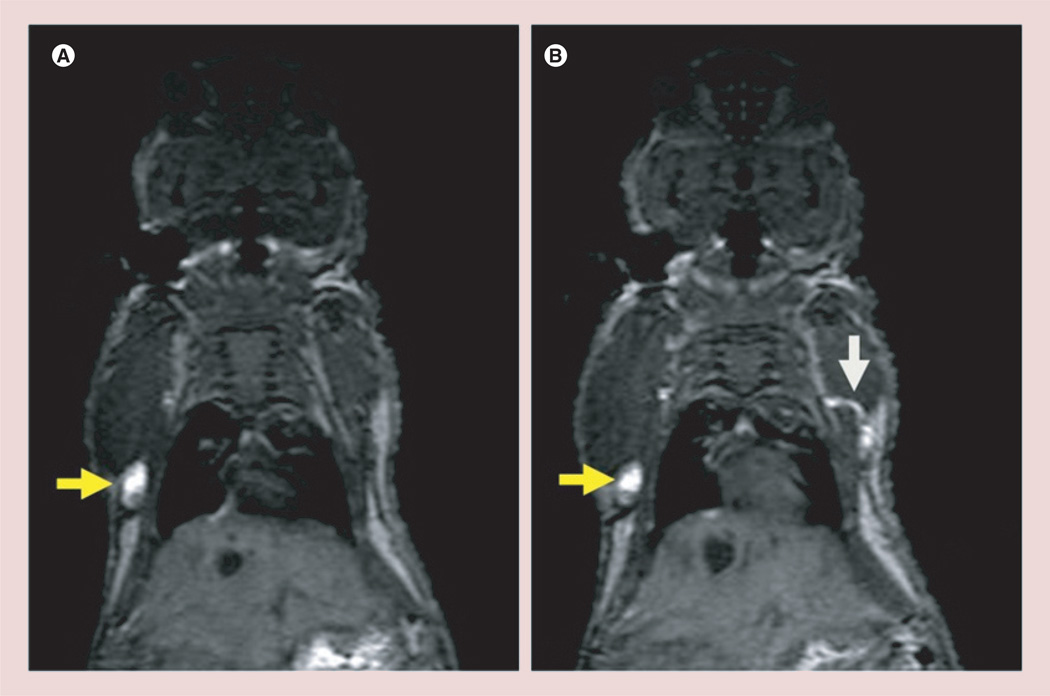
Dynamic contrast-enhanced MR images of the G5–Gd-BnDOTA (10 µl, 30 mM) at 15 min post-injection on the front paws of a female athymic mouse shows the axillary and lateral thoracic lymph nodes, as well as the connecting lymphatic ducts that drain the lymph fluids from the upper extremities, in all mice (Figure 4). Moreover, the deep lateral thoracic lymph node and the thoracic duct connecting it to the axillary lymph node were also visualized. These findings are consistent with previous studies done by Sena et al. [14].
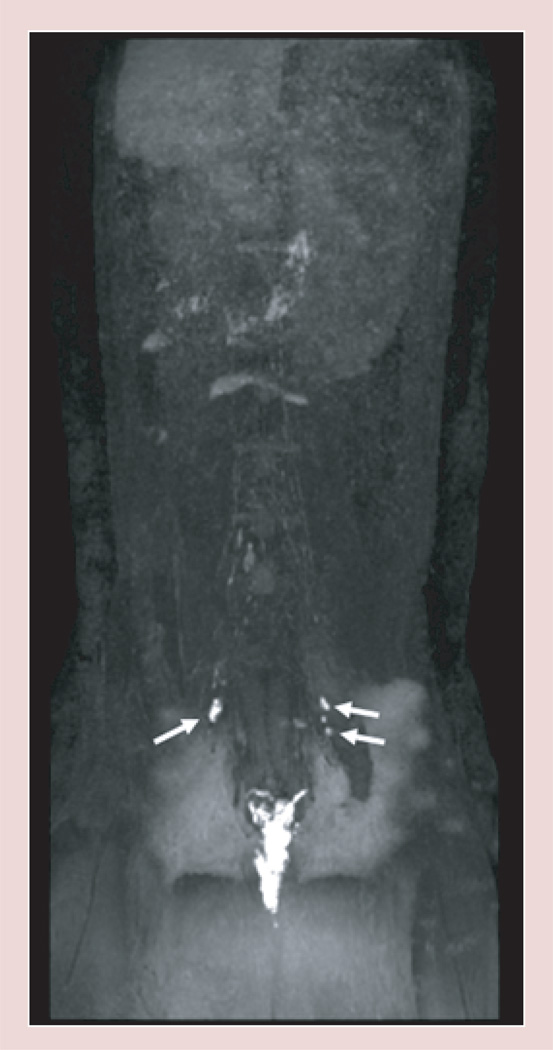
Figure 4. Selected consecutive cross-sectional contrast-enhanced T1-weighted MRIs demonstrating the lymphatic pathway with an intracutaneous injection of 15 µmol Gd/kg (10 µ/30 mM) G5–Gd-BnDOTA in the front paws of a female athymic mouse at 15 min post-injection.
Yellow arrows indicate the axillary lymph nodes, and the white arrow shows connecting lymphatic duct between the axillary node and the lateral thoracic lymph node.
In vivo studies in monkeys
The coronal maximum intensity projection T1 weighted MR image obtained 8 min after injection of G5–Gd-BnDOTA (50 µl, 10 mM) shows enhancing internal iliac nodes bilaterally (black/white arrows), as shown in Figure 5.
Figure 5. T1-weighted MRI of G5-Gd-BnDOTA after injection of 0.07 µmol Gd/kg (50µ/10mM) into the vaginal mucosa of a 7-kg monkey.
The white arrows show an enhanced image of the internal iliac nodes bilaterally.
In vivo angiography studies
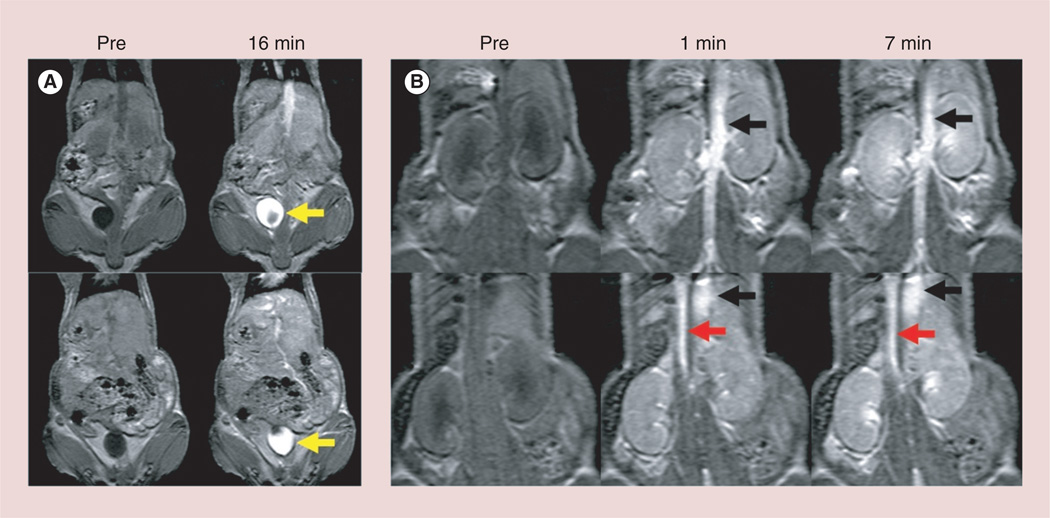
The major blood vessels (aorta and inferior vena cava) were clearly visualized immediately after intravenous injection of G5–Gd-BnDOTA (100 µl, 6 mM) in the tail vein of mice (Figure 6). The excreted contrast agent into the bladder was also visualized within 16 min post-injection.
Figure 6. Dynamic contrast-enhanced T1-weighted magnetic resonance angiography with intravenously injected 30 µmol Gd/kg of G5–Gd-BnDOTA (100 µl/6 mM) in a 20-g female athymic mice.
The aorta (red arrows) and inferior vena cava (black arrows) are clearly depicted immediately after injection of the contrast agent and last up to 16 min post-injection. Increasing amount of contrast agents are excreted into the bladder (yellow arrows). Images of two consecutive mice are shown top and bottom.
Radiolabeling of G5–Gd-BnDOTA
A153Gd (t½ = 241.6 d) radioactive chelate of G5–Gd-BnDOTA was prepared to assess the long-term biodistribution of the agent in vivo. Pre-metallation of the ligand, p-SCN-BnDOTA, under the conditions established for cold Gd (natural isotope abundance) with the radioactive 153Gd proved to be challenging because the complete formation of the complex cannot be absolutely confirmed. Instead, the free ligand (22% by mole of the dendrimer amines) and the cold Gd-BnDOTA (in excess) were conjugated to den-drimer G5 prior to radiolabeling. The radiolabeling of the dendrimer with 153Gd was performed using 5 M ammonium acetate buffer (pH 5.5) with stirring for 24 h at an elevated temperature of 40°C [33]. Cold GdCl3 was added to ensure complete complexation of remaining free ligand, and EDTA (0.1 M) was subsequently added to remove any non-incorporated free Gd(III) metal. Purification of the product gave the radiolabeled G5–153Gd/Gd-BnDOTA, with 1 radioHPLC peak detected corresponding to G5– Gd-BnDOTA conjugate, indicating the absence of free Gd-153. The HPLC was comparable to that of the cold G5–Gd-BnDOTA at a radiolabeling efficiency of 89%.
Biodistribution studies
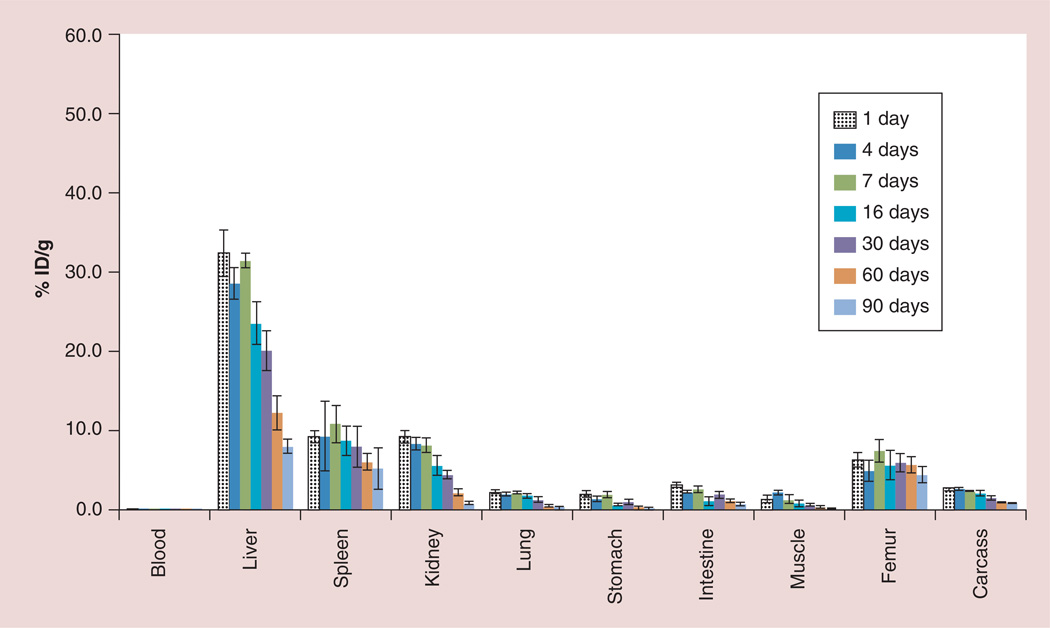
Biodistribution studies of the 153Gd-labeled G5–Gd-BnDOTA conjugate were monitored in non-tumor-bearing athymic mice (three to five mice per time point) for up to 90 days using 153Gd-spiked conjugate to establish the long-term disposition of G5–153Gd/ Gd-BnDOTA conjugates. Figure 7 shows the agent accumulated significantly in the liver at 32.3 ± 3.0% ID/g within 24 h, and less in the kidney and spleen (both at 9.2 ± 0.8% ID/g). The Gd(III) uptake was sustained in the liver for 7 days; however, the retained 153Gd steadily decreased to 20.1 ± 2.5% ID/g within 30 days, and reached 8.0 ± 0.9% ID/g, approximately fourfold lower, at 90 days post-injection. Most other tissues eliminated Gd(III) at a similar rate, except the femur, where Gd(III) uptake only decreased from approximately 5 to 4% ID/g at 90 days.
Figure 7. Long-term (90-day) biodistribution of G5–153Gd/Gd-BnDOTA in non-tumor-bearing athymic mice.
ID: Injected dose.
Discussion
A PAMAM G6 Gd-BnDTPA conjugate reported by Kobayashi and coworkers successfully demonstrated a low Gd(III) dose MRI of the thoracic duct of 35-kg pigs [14]. We extend this work toward human translation by designing and preparing a putatively more biologically stable G5–Gd-BnDOTA. We prepared a PAMAM G5 dendrimer (DAB core) conjugate using a preformed Gd–p-SCN-BnDOTA chelate via thiourea linkage. Pre-complexation of Gd(III) ensures that all of the ligands are metallated [34] , hence the absence of free chelating groups. This pre-complexation reaction is quite a challenging approach as the isothiocyanates are known to hydrolyze slowly in water and rapidly at pH above 8; while at low pH, the two diagonally opposing macrocyclic nitrogens remain protonated, as indicated by the first two protonation constants of the NH+ group in the tetraaza ring of DOTA [35,36], as well as the nitrobenzyl DOTA derivative (log K ∼9–11) [27]. For lanthanide chelates of DOTA, it has been elucidated that a diprotonated intermediate, Ln(H2DOTA)+, instantaneously forms upon complex formation resulting in a rapid dissociation of all protons from the carboxylates [36–39]. At this stage, the metal ion is bound to the four carboxylate groups and is outside of the coordination cage. The macrocyclic amine protons then dissociate and the lanthanide ion slowly penetrates the macrocycle cavity. The loss of the last nitrogen proton occurs mainly via the assistance of the OH− ions. The formation of the complex is characterized by an abrupt pH drop, followed by a slow decrease in pH until the pH definitively stabilizes (formation of the final stable complex). This is a very slow process and can take weeks to achieve at room temperature [39]. Increasing the solution pH, also increases the rate of complex formation (through the OH- ion assisted deprotonation). However, as we mentioned earlier, the isothiocyanate from the ligand hydrolyzes above pH 8, and in addition, hydroxylated species of Gd(III) start to form at pH above 6, while complex formation is slow below pH 4.5. Thus, we carefully adjusted and maintained the solution pH between 4.5 and 6 using 1 M NaOH, until no pH drop is observed indicating formation of the complex. We then confirmed the complete complex formation using LC-MS, by observing the appearance of the Gd(III) complex, with characteristic Gd(III) isotope pattern versus the disappearance of the free ligand.
It is also important to note that the macrocyclic cavity of DOTA also offers a unique rigidity to the gadolinium chelates formed and contributes to their slow rate of formation and dissociation, as well as to their high kinetic and thermodynamic stability [40]. This contrasts significantly to linear chelating agents, which are known to form and dissociate more rapidly [41]. This is of particular significance in agents such as dendrimers that have a longer biologic half-life as the opportunity for dissociation with resulting toxicities (e.g., nephrogenic sclerosis fibrosis) is increased.
Conjugation of Gd-p-SCN-BnDOTA complex to the G5 dendrimer resulted in 85 Gd-BnDOTA chelates attached per G5 dendrimer (total amino groups {n} = 128, 66% conjugation). A similar observation was reported by Nwe et al., where only 47% conjugation was achieved when the pre-complexed Gd-BnDOTA derivative was reacted with the terminal amines of PAMAM G5 (ethylenediamine core) [32]. Differences in the dendrimer cores (EDA vs DAB), reaction stoichiometries, and the starting dendrimer material quality [42,43] might have contributed to the discrepancies between our observed conjugation levels. Longitudinal relaxivity (r1) is a measure of the ability of the contrast agent to effectively relax water protons. It is determined by taking the slope of the longitudinal relaxation rate (1/T1) versus the Gd(III) concentration as described in Equation 1. The relaxivity values are also highly dependent on magnetic field and temperature.
| (Equation 1) |
The relaxivity values of the PAMAM G5 dendrimer conjugates are summarized in Table 1. The low MW Gd(III) MR agent, Magnevist, has a reported relaxivity of 4.2 mM−1s−1 (under similar conditions: 22°C and at 3 T magnetic field) [32]. The G5–Gd-BnDOTA conjugate has a relaxivity of 12.98 mM−1s−1, which is three-times higher than this low MW MR agent. This relaxivity enhancement is due to the slower rotational correlation time of macromolecules compared with low molecular weight agents. In comparison to previously reported G5 dendrimer-based Gd(III) chelates, the relaxivity measured for G5–Gd-BnDOTA in this work did not vary much for those conjugates measured at the same magnetic field and temperature (Table 1).
Table 1.
Comparison of relaxivities for different G5 dendrimericGd(III) chelates at 3 T and at physiological pH.
Intracutaneous injection of the G5–Gd-BnDOTA to the upper extremities of mice showed excellent delineation of the lymphatics within minutes post-injection. Injection into the vaginal mucosa of large animal models (monkeys), showed enhanced internal iliac nodes. This model is useful for tracking trans-mucosal drainage and transportation across membranes and is important in studying viral transmission routes of diseases such as HIV and detailed results will be reported separately. On the other hand, G5–Gd-BnDOTA can also visualize major blood vessels upon intravenous injection in the tail vein of mice.
The excellent ability of the G5–Gd-BnDOTA for MR lymphangiography and angiography prompted us to study its biodistribution for future toxicological studies. Our preliminary biodistribution studies of the 153Gd-labeled G5–Gd-BnDOTA conjugate were initially monitored in non-tumor-bearing athymic mice for up to 96 h. However, the Gd(III) agent was not cleared completely during this time range, with major uptake seen on the liver and spleen, and this retained gadolinium over a longer period in humans may lead to immunologic responses and/or localized inflammation at the injection site. Thus, we determined to accurately establish, for the first time to our knowledge, the long-term biodistribution and retention of a Gd-chelate dendrimer conjugate. We injected G5–153Gd/Gd-BnDOTA and quantified the major tissue uptake out to 90 days post-injection. The data suggests that an ultra-stable Gd-chelate dendrimer conjugate will clear continuously, albeit slowly, from most initial major deposition organs, other than the femur and possibly the spleen, and therefore the body, over several months. Whole body biological clearance can be estimated by virtue of harvesting all major organs including carcass (Figure 7). Whole body retention dropped from 68.1% ID/g at 1 day post-injection to 50% D/g at 16 days, 44% at 30 days, 29.5% at 60 days and 21% at 90 days post-injection. This implies that of the 68% sequestered in all body tissues at 1 day post-injection, the time taken for this residue to fall by half lies between 30 and 60 days, and this, in turn, implies that ten biological half-life retention periods for Gd(III) may be between 1 and 2 years post-injection.
Increasing sizes of macromolecular MR contrast agent may prolong their retention in certain organs. In fact, Kobayashi et al. reported that the higher generation PAMAM dendrimers conjugated to bifunctional Gd-BnDTPA were retained in the same organs longer than their lower generation counterparts [46,47]. In addition, other properties of dendrimer conjugates such as their overall hydrophobicities, molecular weights, stability, charges, plasticities, chemical structures, and the quality of the starting dendrimer material may also affect accumulation and retention of this class of agents in different organs of the body [11,48].
Liver accumulation of the dendrimer–Gd(III) agents is not unusual, as is reported in the literature. For instance, PAMAM G6 dendrimer (ammonia core) conjugated to a Gd-DTPA derivative stayed in the blood longer than its low generation counterpart, but also accumulated significantly in the liver at 35.9 ± 1.6% ID/g, while the lower generation PAMAM G5–Gd(III) conjugate accumulated in the liver at 30.9 ± 1.2% ID/g after 15 min of injection [47]. In a separate report, PAMAM G6 (ethylenediamine core) – (Gd-DTPA)256 derivative showed a lower liver accumulation of 17.1 ± 1.2% ID/g, but stayed in the blood longer compared with a PAMAM G6 (ammonia core)–(Gd-DTPA)192 derivative, which localized into the liver at 28.9 ± 1.2% ID/g at 15 min post-injection [11]. A Gd-153 labeled polypropyleniminediaminobutyl G4– GdDTPA derivative significantly accumulated in the liver (∼37% ID/g) rather than the kidney as compared with a PAMAM-based G4–GdDTPA derivative counterpart, which accumulated in the liver at ∼14% ID/g within 15 minutes after injection [46]. Accumulation of a dendrimer agent at 48 h post-injection was reported by Kobayashi et al. [48] for a polypropylenimine diaminobutyl (DAB) G4–GdDTPA derivative, which was found to accumulate in the liver with 36.8 ± 1.2% ID/g at 48 h post-injection, from 48 ± 3.5% ID/g within 15 min of injection. This agent was reported as a potential liver MRI contrast agent, with higher liver uptake attributable to the increased hydrophobicity of the DAB dendrimer framework, by virtue of its longer chains and lack of amide groups as compared with its PAMAM G4–GdDTPA (with an ethylenediamine core) counterpart. The latter accumulated in the liver at 23.6 ± 1.6% ID/g at 48 h post-injection. Accumulation of both agents in the bone also measured 4–5% ID/g within 15 min after injection. Another dendrimeric system reported by Margerum et al., showed liver retention of the neutral charged Gd–DO3A conjugated to PAMAM G5 (ammonia core) at 40% of the initial injected dose after 7 days, which was attributed to the increased molecular weight of the conjugate at ∼62 kDa [49]. It is worthy to mention that our data do not explicitly take dose effects into account and that if 100 µg were given to each animal, the observed tissue uptakes in the reticuloendothelial system might be lower, most likely due to saturation effects. Never-theless, the biodistribution seen in our work are likely to follow the same general patterns and are similar to other published data.
A summary of the liver accumulation of G5–Gd-BnDOTA is presented in Table 2. The level of the G5–Gd-BnDOTA used in this study was sustained in the liver for 7 days, which could be due to a combination of factors, such as surface charge, size and shape. As reported in the literature, accumulation of dendrimer agents in the liver occurs even for neutral and negatively charged Gd(III) chelates, as well as for a number of different generations higher than G3 and even for different chemistries of the dendrimeric core. It should be noted that the Gd(III) dose used for mice (weight ∼20 g) was 15 µmol Gd/kg, while larger animal models required lower injection dose yet were able to clearly visualize the lymphatic system. The injection dose for the 7 kg monkey used in this work is 0.07 µmol Gd/kg, while Sena et al., reported a 0.1 µmol Gd/kg dose for 35 kg pigs [14]. This is approximately 1% of the intravenous Gd(III) dose (taking the upper limit of the large animal model) usually employed with commercially available small molecule Gd(III) chelate; hence, the absolute Gd(III) amount that is deposited in organs is, in fact, much lower in lymphatic imaging when compared with conventional Gd(III) MRI.
Table 2.
Liver accumulation of G5–Gd-BnDOTA in non-tumor-bearing athymic mice.
| Days (n) | Liver uptake (% injected dose/g ± standard deviation) |
|---|---|
| 1 | 32.365 ± 2.954 |
| 4 | 28.553 ± 1.982 |
| 7 | 31.411 ± 0.907 |
| 16 | 23.538 ± 2.718 |
| 30 | 20.095 ± 2.512 |
| 60 | 12.289 ± 2.136 |
| 90 | 7.981 ± 0.912 |
To be able to use this type of agent within clinical settings, macromolecular MR contrast agents should ideally be eliminated from the body in an efficient and timely manner to minimize potential late toxicity caused by free, or even bound, Gd(III). Elimination via the renal system, through glomerular filtration, leaves low MW Gd(III) complexes in their intact form without undergoing cellular processing and this is the preferred route of clearance. Although Gd(III) complexed to the macrocyclic ligand DOTA is regarded as among the safest conjugates due to the complex’s high thermodynamic and kinetic stabilities, the observed long-term Gd uptake in the femur indicates that some Gd-153 deposition still occurred. This is probably due to the nature of the macromolecule itself as much as from putative decomplexation. The corresponding G5–153Gd-BnDTPA macromolecule showed 6.28 ± 0.88 – 4.92 ± 1.32%ID/g in femurs at 1–4 days post-injection, perhaps indicative of the relative amounts of Gd-153 femur deposition attributable to Gd-153 decomplexation versus from intact macrocycle complex deposition. To date, dendrimeric MRI agents have not been approved for use in humans by regulators and one aim of this study was to address the long-term quantitative disposition of the injected macrocycle by study of its 153Gd analog, with a view to guidance regarding possible forward toxicity issues. For instance, as a rule of thumb, toxicity studies should be performed for at least ten biologic half-lives which, in this case, would amount to over 1 year. This clearly presents some formidable obstacles and despite numerous positive descriptions of various Gd-dendrimers in preclinical studies, total body clearance remains an issue for clinical translation as it may for many proposed nanoparticle-based agents.
Conclusion
This article shows that the G5–Gd-BnDOTA conjugate can effectively visualize the lymphatics in both small and large animal models. The biodistribution profile shows retention of the conjugate in the liver and other organs, but is slowly being eliminated. Since G5–GdBn-DOTA is intended for use purely for lymphatic imaging at approximately 1% of the injected dose of Gd(III) typically used for currently approved MRI intravenous contrast agents, the Gd(III) tissue uptakes and retentions seen here might be acceptable in certain limited indications. However, the long-term biodistribution of 153Gd from these agents indicates that macromolecular dendrimer-based imaging and therapy agents may also be compromised by their off-target localization and retention properties.
Future perspective
PAMAM G5-Gd(III) agents showed great promise as lymphatic agent for MRI. However, the inefficient clearance of this agent from the body might prevent the eventual successful clinical translation of this MRI probe.
Executive summery.
Synthesis & characterization
The amino terminal group of PAMAM G5 dendrimer (diaminobutyl core) was covalently attached to the Gd(III) complex of p-SCN-BnDOTA via a thiourea linkage, which resulted in 85 Gd-BnDOTA chelates attached per G5 dendrimer.
In vivo imaging studies
We have demonstrated that G5-Gd-BnDOTA can effectively visualize the lymphatics in both mice and monkeys, as well as the major blood vessels in mice using a low dose of Gd(III).
Biodistribution studies
Long-term biodistribution (90 days) of G5-Gd-BnDOTA radiolabeled with Gd-153 showed that the agent is being cleared from the liver and other organs, albeit very slowly.
Conclusion
The slow clearance of the G5-Gd-BnDOTA from the body implies that for toxicity studies, ten biological half-life retention periods for Gd(III) may be between 1 and 2 years post-injection.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, NIH, under Contract no. HHSN261200800001E. This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Dutta T, Garg M, Jain NK. Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur. J. Pharm. Sci. 2008;34(2–3):181–189. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Martinez FC, Ocana AV, Perez-Carrion MD, Cena V. Dendrimers as vectors for genetic material delivery to the nervous system. Curr. Med. Chem. 2012;19(29):5101–5108. doi: 10.2174/0929867311209025101. [DOI] [PubMed] [Google Scholar]
- 3.Posadas I, Guerra FJ, Cena V. Nonviral vectors for the delivery of small interfering RNAs to the CNS. Nanomedicine. 2010;5(8):1219–1236. doi: 10.2217/nnm.10.105. [DOI] [PubMed] [Google Scholar]
- 4.Wolinsky JB, Grinstaff MW. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv. Drug Deliv. Rev. 2008;60(9):1037–1055. doi: 10.1016/j.addr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 5.El Kazzouli S, El Brahmi N, Mignani S, et al. From metallodrugs to metallodendrimers fornanotherapy in oncology: a concise overview. Curr. Med. Chem. 2012;19(29):4995–5010. doi: 10.2174/0929867311209024995. [DOI] [PubMed] [Google Scholar]
- 6.Lo ST, Kumar A, Hsieh JT, Sun XK. Dendrimer nanoscaffolds for potential theranosticsof prostate cancer with a focus on radiochemistry. Mol. Pharm. 2013;10(3):793–812. doi: 10.1021/mp3005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiener EC, Brechbiel MW, Brothers H, et al. Dendrimer-based metal-chelates - a new class ofmagnetic-resonance-imaging contrast agents. Magn. Reson. Med. 1994;31(1):1–8. doi: 10.1002/mrm.1910310102. •• First publication of the use of dendrimer as an MRI contrast agent
- 8.Adam G, Neuerburg J, Spuntrup E, et al. Dynamic contrast-enhanced MR-imaging of the upperabdomen - enhancement properties of gadobutrol, gadolinium-DTPA-polylysine, and gadolinium-DTPA-cascade polymer. Magn. Reson. Med. 1994;32(5):622–628. doi: 10.1002/mrm.1910320511. [DOI] [PubMed] [Google Scholar]
- 9.Langereis S, de Lussanet QG, van Genderen MHP, et al. Evaluation of Gd(III)DTPA-terminated poly(propylene imine) dendrimers as contrast agents for MR imaging. NMR Biomed. 2006;19(1):133–141. doi: 10.1002/nbm.1015. [DOI] [PubMed] [Google Scholar]
- 10.Yordanov AT, Kobayashi H, English SJ, et al. Gadolinium-labeled dendrimers as biometric nanoprobes to detect vascular permeability. J. Mat. Chem. 2003;13(7):1523–1525. [Google Scholar]
- 11.Kobayashi H, Sato N, Kawamoto S, et al. Comparison of the macromolecular MR contrast agents with ethylenediamine-core versus ammonia-core generation-6 polyamidoamine dendrimer. Bioconjug. Chem. 2001;12(1):100–107. doi: 10.1021/bc000075s. [DOI] [PubMed] [Google Scholar]
- 12.Swanson SD, Kukowska-Latallo JF, Patri AK, et al. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic resonance contrast enhancement. Int. J. Nanomed. 2008;3(2):201–210. [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi H, Kawamoto S, Saga T, et al. Positive effects of polyethylene glycol conjugation to generation-4 polyamidoamine dendrimers as macromolecular MR contrast agents. Magn. Reson. Med. 2001;46(4):781–788. doi: 10.1002/mrm.1257. [DOI] [PubMed] [Google Scholar]
- 14.Sena LM, Fishman SJ, Jenkins KJ, et al. Magnetic resonance lymphangiography with a nano-sized gadolinium-labeled dendrimer in small and large animal models. Nanomedicine. 2010;5(8):1183–1191. doi: 10.2217/nnm.10.70. •• Describes the use of gadolinium (Gd)-dendrimer conjugates as MRI contrast agents for lymphatic imaging agents in small and large animal models using a low dose of Gd(III)
- 15.Barge A, Cravotto G, Gianolio E, Fedeli F. How to determine free Gd and free ligand in solution of Gd chelates. A technical note. Contrast Media Mol. Imaging. 2006;1(5):184–188. doi: 10.1002/cmmi.110. [DOI] [PubMed] [Google Scholar]
- 16.Ali MM, Woods M, Caravan P, et al. Synthesis and relaxometric studies of a dendrimer-based pH-responsive MRI contrast agent. Chem. Eur. J. 2008;14(24):7250–7258. doi: 10.1002/chem.200800402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nwe K, Bernardo M, Regino CAS, Williams M, Brechbiel MW. Comparison of MRI properties between derivatized DTPA and DOTA gadolinium-dendrimer conjugates. Bioorg. Med. Chem. 2010;18(16):5925–5931. doi: 10.1016/j.bmc.2010.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WaveMetrics. www.wavemetrics.com.
- 19.Smedley J, Turkbey B, Bernardo ML, et al. Tracking the luminal exposure and lymphatic drainagepathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlesinger J, Koezle I, Bergmann R, et al. An Y-86-labeled mirror-image oligonucleotide: influence of Y-DOTA isomers on the biodistribution in rats. Bioconjug. Chem. 2008;19(4):928–939. doi: 10.1021/bc700453h. [DOI] [PubMed] [Google Scholar]
- 21.Hoeft S, Roth K. Structure and dynamics of lanthanoid tetraazacyclododecanetetraacetate DOTA complexes in solution. Chemische Berichte-Recueil. 1993;126(4):869–873. [Google Scholar]
- 22.Aime S, Barge A, Bruce JI, et al. NMR, relaxometric, and structural studies of the hydration and exchange dynamics of cationic lanthanide complexes of macrocyclic tetraamide ligands. J. Am. Chem. Soc. 1999;121(24):5762–5771. [Google Scholar]
- 23.Dunand FA, Aime S, Merbach AE. First O-17 NMR observation of coordinated water on both isomers of [Eu(DOTAM)(H2O)]3+: a direct access to water exchange and its role in the isomerization. J. Am. Chem. Soc. 2000;122(7):1506–1512. [Google Scholar]
- 24.Woods M, Kovacs Z, Zhang SR, Sherry AD. Towards the rational design of magnetic resonance imaging contrast agents: isolation of the two coordination isomers of lanthanide DOTA-type complexes. Angew Chem. Int. Ed. Engl. 2003;42(47):5889–5892. doi: 10.1002/anie.200352234. • First report of pre-complexation of Gd(III) chelate prior to dendrimer conjugation
- 25.Aime S, Botta M, Ermondi G. NMR-study of solution structures and dynamics of lanthanide (III) complexes of DOTA. Inorg Chem. 1992;31(21):4291–4299. [Google Scholar]
- 26.Jacques V, Desreux JF. Quantitative 2-dimensional EXSY spectroscopy and dynamic behavior of a paramagnetic lanthanide macrocyclic chelate - YbDOTA (DOTA = 1,4,7,10-tetraazacyclododecane-N,N’,N”,N”’-tetraacetic acid) Inorg. Chem. 1994;33(18):4048–4053. [Google Scholar]
- 27.Woods M, Kovacs Z, Kiraly R, et al. Solution dynamics and stability of lanthanide(III) (S)-2-(p-nitrobenzyl)DOTA complexes. Inorg. Chem. 2004;43(9):2845–2851. doi: 10.1021/ic0353007. [DOI] [PubMed] [Google Scholar]
- 28.Aime S, Botta M, Fasano M, et al. Conformational and coordination equilibria on DOTA complexes of lanthanide metal ions in aqueous solution studied by H-1-NMR spectroscopy. Inorg. Chem. 1997;36(10):2059–2068. doi: 10.1021/ic961364o. [DOI] [PubMed] [Google Scholar]
- 29.Inagaki F, Miyazawa T. NMR analyses of molecular-conformations and conformational equilibria with the lanthanide probe method. Prog. Nucl. Magn. Reson. Spectrosc. 1980;14:67–111. [Google Scholar]
- 30.Laus S, Sour A, Ruloff R, Toth E, Merbach AE. Rotational dynamics account for pH-dependent relaxivities of PAMAM dendrimeric, Gd-based potential MRI contrast agents. Chem. Eur. J. 2005;11(10):3064–3076. doi: 10.1002/chem.200401326. [DOI] [PubMed] [Google Scholar]
- 31.Wiener EC, Konda S, Shadron A, Brechbiel M, Gansow O. Targeting dendrimer-chelates to tumors and tumor cells expressing the high-affinity folate receptor. Invest. Radiol. 1997;32(12):748–754. doi: 10.1097/00004424-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Nwe K, Bryant LH, Brechbiel MW. Poly(amidoamine) dendrimer based MRI contrast agents exhibiting enhanced relaxivities derived via metal preligation techniques. Bioconjug. Chem. 2010;21(6):1014–1017. doi: 10.1021/bc1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biricova V, Laznickova A, Laznicek M, Polasek M, Hermann P. Radiolabeling of PAMAM dendrimers conjugated to a pyridine-N-oxide DOTA analog with In-111: optimization of reaction conditions and biodistribution. J. Pharm. Biomed. Anal. 2011;56(3):505–512. doi: 10.1016/j.jpba.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Nwe K, Xu H, Regino CAS, et al. A new approach in the preparation of dendrimer-based bifunctional diethylenetriaminepentaacetic acid MR contrast agent derivatives. Bioconjug. Chem. 2009;20(7):1412–1418. doi: 10.1021/bc900057z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desreux JF, Merciny E, Loncin MF. Nuclear magnetic-resonance and potentiometric studies of the protonation scheme of 2 tetraaza tetraacetic macrocycles. Inorg. Chem. 1981;20(4):987–991. [Google Scholar]
- 36.Burai L, Fabian I, Kiraly R, Szilagyi E, Brucher E. Equilibrium and kinetic studies on the formation of the lanthanide(III) complexes, [Ce(DOTA)](−) and [Yb(DOTA)] (−) (H(4)DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) J. Chem. Soc. Dalton. 1998;(2):243–248. [Google Scholar]
- 37.Toth E, Brucher E, Lazar I, Toth I. Kinetics of formation and dissociation of lanthanide(III)-DOTA complexes. Inorg. Chem. 1994;33(18):4070–4076. [Google Scholar]
- 38.Wu SL, Horrocks WD. Kinetics of complex-formation by macrocyclic polyaza polycarboxylate ligands - detection and characterization of an intermediate in the Eu3+-DOTA system by laser-excited luminescence. Inorg. Chem. 1995;34(14):3724–3732. [Google Scholar]
- 39.Moreau J, Guillon E, Pierrard JC, et al. Complexing mechanism of the lanthanide cations Eu3+, Gd3+, and Tb3+ with 1,4,7,10-tetrakis(carboxymethyl)-1,4,7,10-tetraazacyclododecane (DOTA) - characterization of three successive complexing phases: study of the thermodynamic and structural properties of the complexes by potentiometry, luminescence spectroscopy, and EXAFS. Chem. Eur. J. 2004;10(20):5218–5232. doi: 10.1002/chem.200400006. [DOI] [PubMed] [Google Scholar]
- 40.Wang XY, Jin TZ, Comblin V, et al. A kinetic investigation of the lanthanide DOTA chelates - stability and rates of formation and of dissociation of a macrocyclic gadolinium(Iii) polyaza polycarboxylic mri contrast agent. Inorg. Chem. 1992;31(6):1095–1099. [Google Scholar]
- 41.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 1999;99(9):2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 42.Mullen DG, Desai A, van Dongen MA, et al. Best practices for purification and characterization of PAMAM dendrimer. Macromolecules. 2012;45(12):5316–5320. doi: 10.1021/ma300485p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesniak WG, Kariapper MST, Nair BM, et al. Synthesis and characterization of PAMAM dendrimer-based multifunctional nanodevices for targeting alpha(v)beta(3) integrins. Bioconjug. Chem. 2007;18(4):1148–1154. doi: 10.1021/bc070008z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q, Li KA, Wen SH, et al. Targeted CT/MR dual mode imaging of tumors using multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials. 2013;34(21):5200–5209. doi: 10.1016/j.biomaterials.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Lim J, Turkbey B, Bernardo M, et al. Gadolinium MRI contrast agents based on triazine dendrimers: relaxivity and in vivo pharmacokinetics. Bioconjug. Chem. 2012;23(11):2291–2299. doi: 10.1021/bc300461r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi H, Kawamoto S, Jo SK, et al. Macromolecular MRI contrast agents with small dendrimers: Pharmacokinetic differences between sizes and cores. Bioconjug. Chem. 2003;14(2):388–394. doi: 10.1021/bc025633c. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi H, Sato N, Hiraga A, et al. 3D–micro-MR angiography of mice using macromolecular MR contrast agents with polyamidoamine dendrimer core with reference to their pharmacokinetic properties. Magn. Reson. Med. 2001;45(3):454–460. doi: 10.1002/1522-2594(200103)45:3<454::aid-mrm1060>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi H, Kawamoto S, Saga T, et al. Novel liver macromolecular MR contrast agent with a polypropylenimine diaminobutyl dendrimer core: comparison to the vascular MR contrast agent with the polyamidoamine dendrimer core. Magn. Reson. Med. 2001;46(4):795–802. doi: 10.1002/mrm.1259. [DOI] [PubMed] [Google Scholar]
- 49.Margerum LD, Campion BK, Koo M, et al. Gadolinium(III) DO3A macrocycles and polyethylene glycol coupled to dendrimers - effect of molecular weight on physical and biological properties of macromolecular magnetic resonance imaging contrast agents. J. Alloys Compd. 1997;249(1–2):185–190. [Google Scholar]