Abstract
Purpose
To test the hypothesis that blood flow autoregulation in the optic nerve head has less reserve to maintain normal blood flow in the face of blood pressure-induced ocular perfusion pressure decrease than a similar magnitude intraocular pressure-induced ocular perfusion pressure decrease.
Materials and Methods
Twelve normal nonhuman primates were anesthetized by continuous intravenous infusion of pentobarbital. Optic nerve blood flow was monitored by laser speckle flowgraphy. In the first group of animals (n=6), the experimental eye intraocular pressure was maintained at 10 mmHg using a saline reservoir connected to the anterior chamber. The blood pressure was gradually reduced by a slow injection of pentobarbital. In the second group (n=6), the intraocular pressure was slowly increased from 10 mmHg to 50 mmHg by raising the reservoir. In both experimental groups, optic nerve head blood flow was measured continuously. The blood pressure and intraocular pressure were simultaneously recorded in all experiments.
Results
The optic nerve head blood flow showed significant difference between the two groups (P = 0.021, repeat measures analysis of variance). It declined significantly more in the blood pressure group compared to the intraocular pressure group when the ocular perfusion pressure was reduced to 35 mmHg (P<0.045) and below. There was also a significant interaction between blood flow changes and the ocular perfusion pressure treatment (P=0.004, adjusted Greenhouse & Geisser univariate test), indicating the gradually enlarged blood flow difference between the two groups was due to the ocular perfusion pressure decrease.
Conclusions
The results show that optic nerve head blood flow is more susceptible to an ocular perfusion pressure decrease induced by lowering the blood pressure compared with that induced by increasing the intraocular pressure. This blood flow autoregulation capacity vulnerability to low blood pressure may provide experimental evidence related to the hemodynamic pathophysiology in glaucoma.
Introduction
Blood flow autoregulation denotes an intrinsic ability of an organ or a tissue to maintain constant blood perfusion in the face of a range of blood pressure changes and to deliver appropriate oxygen and glucose under altered metabolic activities. Impaired autoregulatory capacity may leave tissues vulnerable to perfusion pressure changes and potentially harmful tissue under- or over-perfusion.1–4 This pathological mechanism has been proposed to contribute to the initiation and/or progression of glaucomatous optic neuropathy,5–9 i.e., the optic nerve head (ONH) can no longer maintain normal blood flow (BF) when ocular perfusion pressure (OPP) fluctuates.
The OPP has been defined as the difference between arterial blood pressure (BP) entering the eye and the intraoocular pressure (IOP).10–13 While increased IOP has been cited as a risk factor contributing to the pathogenesis of glaucoma,14, 15 not until recently have population based epidemiological studies provided clinical evidence that BP-related OPP reduction is associated with the prevalence and incidence of glaucoma.16–19,20,21,22–27 With or without abnormally elevated IOP, BP in glaucoma patients often has greater nocturnal reductions or is lower than normal.23, 24, 28–30 However, while there is a consensus that a lower OPP is important, there is no agreement on the exact effects of BP-related OPP change on BF in ONH versus that induced by IOP in glaucoma.
Previous studies have demonstrated that the performance of autoregulation in normal ocular tissues may vary depending on whether the OPP is modulated by BP or IOP.4, 31–33 For example, the autoregulation system of the rabbit choroidal circulation functions better if the OPP is altered by IOP while BP is held constant rather than vice versa.34 Similarly, the human choroidal circulation is better regulated when OPP is increased by BP during isometric exercise than if OPP is decreased by raising IOP.35 Tested in rats, higher IOP is needed to attenuate ocular BF in animals with higher BP.36 The results of these studies indicate a complicated interaction between BP and IOP on BF regulation.
In the ONH, where the major pathological change develops in glaucoma,37 the autoregulation system tolerates OPP decreases induced by IOP elevation better than that in the choroid of humans.33 In nonhuman primates,38 the same magnitude of IOP increment caused significantly more ONH BF decrease if the systemic BP was low than if the BP was high.38 In both studies, however, the BF responses to either BP or IOP change were studied at different ranges of OPPs, whether the BF responds at the same level of OPP change remains unclear. This current study was undertaken to compare the ONH BF responses to either BP- or IOP-induced OPP decrease in two groups of normal nonhuman primates. It was hypothesized that BP exerts an important role in local BF control by maintaining basal vascular tone.14, 39, 40 Thus, in the face of BP-induced OPP decrease, ONH autoregulation has less reserve to maintain normal perfusion than the same extent of IOP-induced OPP decrease. These results may provide experimental evidence to elucidate the roles of BP and IOP in BF regulation in the ONH, and potentially hemodynamic related pathological mechanisms in glaucoma.
Material and Methods
Animals
All experimental methods and animal care procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the local Institutional Animal Care and Use Committee. In total, 12 adult rhesus monkeys (Macaca mulatta; female; 11.2 ± 3.9 years old) were used in this study.
Anesthesia and general preparation
Prior to testing, each animal was sedated by an intramuscular injection of ketamine/xylazine (15 mg/kg and 0.8 mg/kg). The animal was then intubated and breathed air in a prone position on a table. The anesthesia was maintained thereafter by a continuous infusion of pentobarbital (6–9 mg/kg, IV, Nembutal®, AKORN). Each mL Nembutal contains 50 mg pentobarbital sodium, 40% propylene glycol and 10% alcohol. Pentobarbital was used because, unlike the volatile gas anesthetics, it has minimal impact on autoregulation.4, 41–44 The head of the animal was fixed with a head-rest and a bite-bar to keep the face forward. The body temperature was maintained at approximately 37°C with a heating pad. Heart rate and oxygen saturation were monitored continuously (Propaq Encore model 206EL; Protocol Systems, Inc., Beaverton, OR). A superficial branch of the tibial artery in a lower leg was cannulated with a 27-gauge needle, which was connected to a pressure transducer (BLPR2, WPI, NH) for continuous recording of mean arterial BP on a four-channel amplifier system (Lab-Trax-4/24T, WPI, NH). End tidal carbon dioxide level (EtCO2) was monitored continuously (RSP-300, Kent Scientific Co.).
Proparacaine (0.5%) was administered topically and an eyelid speculum was used to keep the eyelids open. Pupils were dilated with 1.0% tropicamide. The anterior chamber was cannulated through the corneoscleral rim with a 27-gauge needle connected to a bottle filled with sterile saline, and IOP was controlled by positioning the reservoir bottle at a pre-calibrated height. A plano-powered, rigid, gas-permeable contact lens was used to maintain optical transparency and corneal hydration.
BF measurement with Laser Speckle Flowgraphy
A laser speckle flowgraphy device (LSFG, Softcare, Iizuka, Japan) was used to measure the BF in the ONH. The principles of the laser speckle technique and its application in our lab to measure ONH BF in nonhuman primates has been described in previous publications.38, 45–47
In brief, a fundus camera-based LSFG device was used to define an area centered on the ONH, which has dimensions of about 3.8mm × 3mm (W × H) and an estimated depth of tissue penetration up to 1 mm at 810 nm laser wavelength.48 After switching on the laser (λ = 830 nm, maximum output power, 1.2 mW), a speckle pattern appears due to random interference of the scattered light from the illuminated area, which was continuously imaged by a charge coupled device (700 × 480 pixel) at a frequency of 30 frames per second. Offline analysis software computed the mean blur rate (MBR) of the speckle images. MBR is a squared ratio of mean intensity to the standard deviation of light intensity, which varies in time according to the velocity of blood cell movement and correlates well with capillary BF within the ONH.49, 50 A composite MBR map representing arbitrary BF distributed within the ONH was generated from each of the series images. After eliminating the area within the images corresponding to large blood vessels, the time course of capillary BF (A.U.) in the ONH was generated.
Experimental protocols
In the two experimental groups, the effects of a slow BP decrease (Exp 1) and a slow IOP increase (Exp 2) on the ONH BF were studied. The third experiment (Exp 3) assessed the effect of pentobarbital compounds on the retinal blood vessel diameter in 3 additional monkeys and 9 rats.
Exp 1. ONH BF during slow BP decrease
In 6 animals, the IOP of the experimental eye was maintained at 10 mmHg manometrically under general anesthesia by continuous infusion of pentobarbital as described above. While the beat-to-beat BP recording was monitored continuously, Euthasol (390 mg sodium pentobarbital and 50 mg sodium phenytoin, 10% ethyl alcohol, 18% propylene glycol, 3,688 µg rhodamine B, and 2% benzyl alcohol per milliliter, Virbac, AH Inc. TX) diluted by 3 times with balanced salt solution was slowly injected intravenously. The speed of injection was manually controlled to keep the BP decrease as slow as possible. During the injection, ONH BF was recorded continuously until the BP was reduced to 10 mmHg. The minimal dose that induced the initial BP decrease varied among animals. In general, approximately ½ ml Euthasol (containing 25 mg pentobarbital) was given before the BP started to decline. At the end of the experiment, the animal was euthanized by an overdose of Euthasol.
Exp 2. ONH BF during slow IOP increase
In one eye of each animal (n=6), the IOP was set at 10 mmHg for at least 5 minutes using a saline reservoir connected to the anterior chamber and then manually increased at a rate of approximately 0.4 (n=3) or 1 mmHg per second (n=3) until IOP reached 50 mmHg. The speed control for the IOP elevation was approximated by slowly raising the reservoir attached to a sliding track while following actual manometer pressure reading shown on the screen by one operator. While another person counted the time lapse, the speed was adjusted to be as even as possible. Ten seconds before and as IOP rose, ONH BF was measured by the LSFG; BP and IOP were recorded simultaneously.
The reason for two different rates of IOP elevation being used was to match the average and highest speeds of BP decrease in Exp 1, which varied individually and during the time course of the slow pentobarbital injection. A previous study51 found that different rates of OPP change may cause different autoregulation responses (see Discussion).
Data analysis of Exp 1 and Exp 2
For each test, the OPP = [(BP – 5 mmHg) – IOP] was calculated over the entire time course, where the 5 mmHg was used to correct the height difference between the tested eye and the level where BP was measured. OPP was then binned every 5 mmHg starting from the highest to the lowest. The corresponding BF change within the range of each binned OPP group relative to the baseline value was calculated and averaged across all experiments.
Exp 3. Peripapillary retinal vessels diameter changes during slow BF decrease
In Exp 1 (BP group), since the pentobarbital had to be given at a higher dose than that used in Exp 2 (IOP group) to pharmacologically reduce the BP, the difference in dose and the difference of ingredients between the two solutions used in the two groups varied. Therefore, a direct vasomotor effect of the drug on the upstream arterioles in the ONH supplying these capillaries needs to be considered. However, since the vessel diameter in the ONH cannot be reliably measured, Exp 3 was designed to examine such an effect by measuring the blood vessel diameter in the peripapillary region (Figure 1) at the same dose used in the BP group (by body weight) assuming the two sets of blood vessels share the same physiological properties (most importantly, the same response to pentobarbital). In order to reduce the use of higher order animals, the preliminary results were obtained in 9 rats and then confirmed in 3 monkeys.
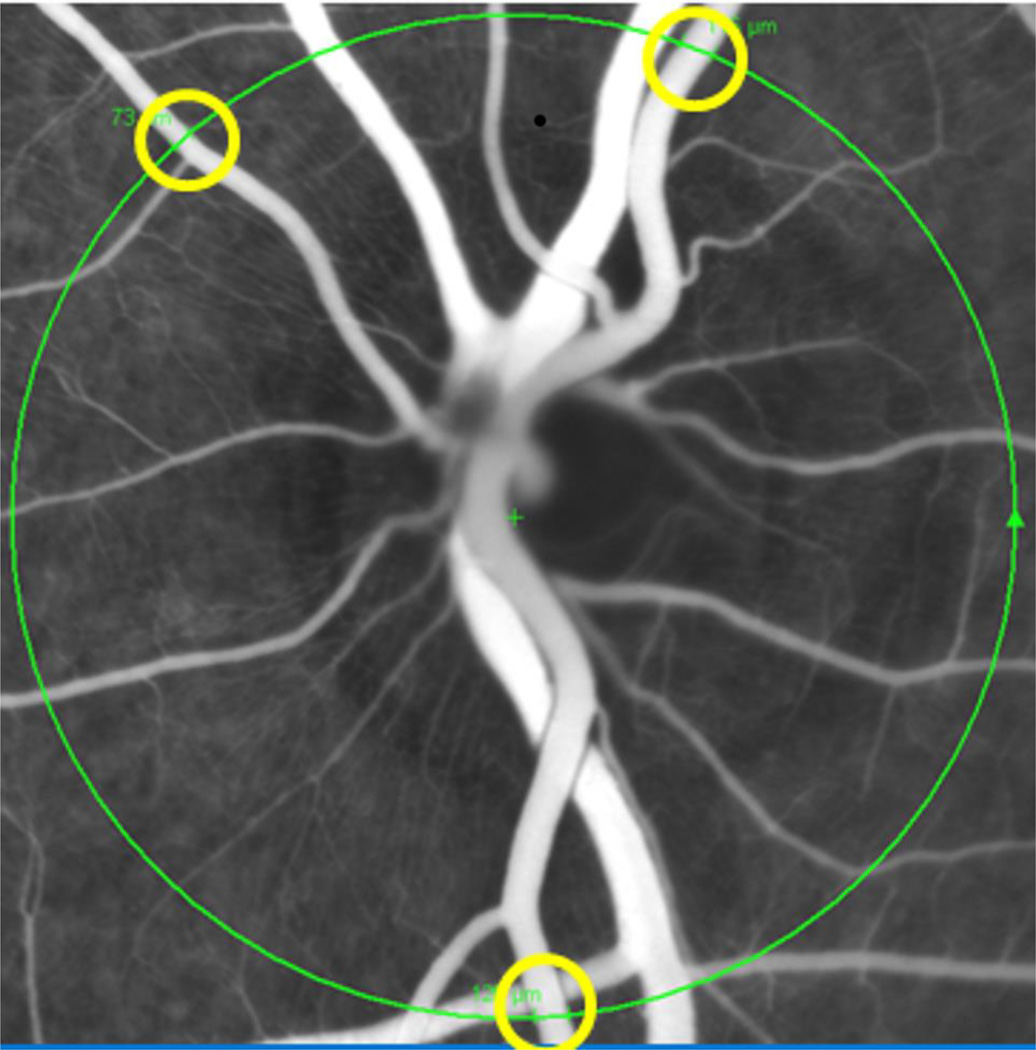
Figure 1.
The confocal scanning laser ophthalmoscopy (CSLO) image in fluorescence angiography (FA) mode shows one of a series of simultaneous CSLO/SD-OCT circular scans (large circle) during slow intravenous injection of pentobarbital in a monkey. The diameter of the retinal arteries across the circle (the smaller rings) was measured on screen offline. Using the instrument’s real-time eye tracking software enabled arteriole diameter measurements to be made at identical locations over time from the CSLO-FA series (at the position of the circular SD-OCT scan).
Male adult albino rats (Sprague Dawley® Rat, Charles River Laboratories Inc., Wilmington, MA) were initially anesthetized by intramuscular injection of ketamine/xylazine. One of the femoral arteries was cannulated for continuous BP registration. The monkeys were anesthetized with the same anesthesia protocol as applied in Exp 1. A Spectralis™ HRA+OCT instrument (Heidelberg Engineering, GmbH, Heidelberg, Germany) was then used to image the vessels in the posterior retina. A circular peripapillary SDOCT scan centered on the ONH was obtained by focusing on the large retinal vessels in the left eye (Fig 1). The SDOCT was then switched into fluorescence angiography (FA) mode; contrast sensitivity was adjusted and followed by intravenous injection of fluorescein dye (Fluorescite®, Alcon Laboratories, Inc, Fort Worth, Texas), approximately 0.1 ml for a rat and 0.5 ml for a monkey to increase the visibility of the blood vessels. As soon as the injection was finished, sequential peripapillary circular scans were repeated approximately every 15 seconds for 4 minutes in FA/OCT combined mode. Use of the combined mode allows measuring the vascular diameter at the same location on the blood vessels in all sequential images. Starting at the onset of the second image, pentobarbital (Euthasol) at the dose of 200 mg/kg body weight as used in Exp 1 was injected slowly (I.V.) in both the rats and monkeys.
The series of SDOCT images were analyzed offline. With the help of SDOCT software, three arteries with best focus across the SDOCT circular scan in each eye were selected (Fig 1). The diameter of the arteries at every 15 second time points after pentobarbital injection was measured and averaged. The blood vessel diameter changed from baseline at every 5 mmHg range of OPP decrement was averaged across the group.
Two-way analysis of variance for repeated measures (RM-ANOVA with adjusted Greenhouse & Geisser univariate test) was used to evaluate the BF differences between IOP and BP groups and Fisher LSD test (post-hoc) for the mean BF comparison between baseline (OPP 50–60 mmHg) and different OPP levels in each group. Probability <0.05 was considered as the critical level for rejecting a null hypothesis.
Results
Exp 1. ONH BF during slow BP decrease
The baseline BP (before the injection of Euthasol) was 81.2 ± 11.3 mmHg (mean +/− SE). After the injection began, the BP often remained at the baseline level for several seconds or minutes. The BP then started to decline at varied rates after approximately less than ¼ of the total dose was given. It declined from baseline to the lowest level (< 10 mmHg) often at varied speeds. This decline was 0.27 ± 0.12 mmHg/sec on average, which was calculated based on the BP difference between baseline and the lowest level divided by the total time (seconds) between the two selected BP. The highest speed of BP decline was calculated in a same manner by determining the time period with fastest BP decline for each animal (0.90 ± 0.37 mmHg/sec on average).
Offline analysis of the LSFG images showed that the ONH BF gradually decreased as the BP declined. The ONH BF decrease was not significant compared with the average BF baseline value (OPP between 50 and 60 mmHg) until the OPP was ≤ 38.3 ± 2.4 mmHg (Fig 2).
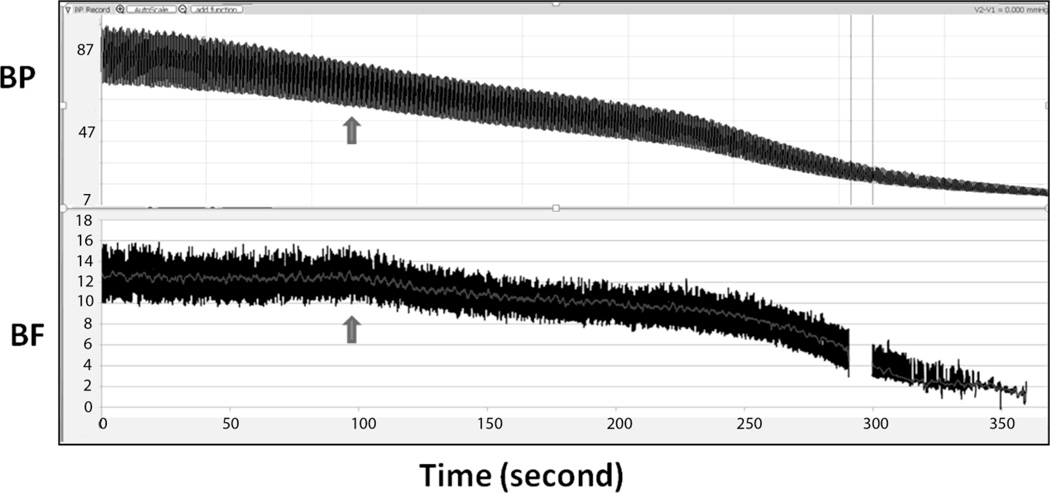
Figure 2.
In this typical example from Exp 1, the mean BP (top panel) was 71 mmHg at the starting point and declined to approximately 11 mmHg 6–7 minutes after pentobarbital administration. The BF (bottom panel) remained unchanged for the first ~100 sec and then started decline (arrow). The corresponding OPP from where the BF started decline was approximately 43 mmHg. The gap of BF at approximately 290 seconds was due to a technical interruption during recording.
Exp 2. ONH BF during slow IOP increase
As the reservoir input pressure was slowly increased by raising the reservoir, the IOP increased almost simultaneously (Fig 3). The average BP during slow IOP increase was 80.5 ± 6.5 mmHg, which was not significantly different from the baseline average in the BP group (P = 0.71). Analysis of the LSFG data showed that BF decreased gradually as the IOP increased. The BF decrease was not significant compared with the average baseline value until the OPP was ≤ 27.7 ± 0.18 mmHg (P < 0.05, see Fig 4).
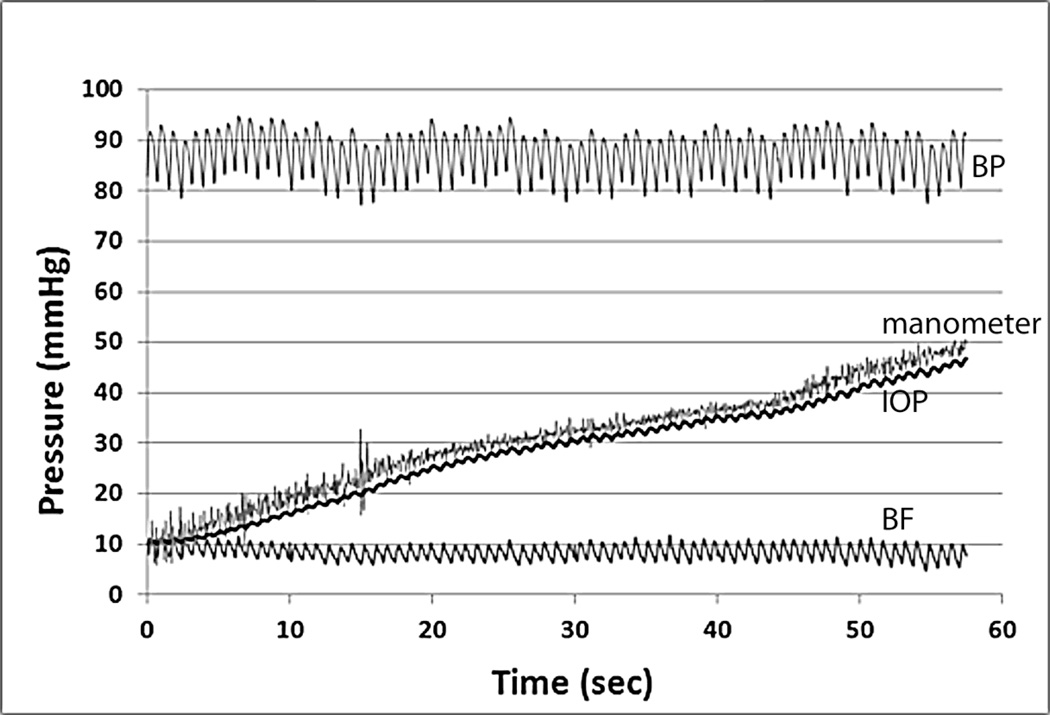
Figure 3.
The graph shows a representative test of slow IOP increase from Exp 2. From the top to bottom are the recordings of BP (top), manometer pressure and IOP (middle two traces), and ONH BF (bottom). While the IOP increased continuously, the BP remained constant. Note the ONH BF recorded by LSFG showed almost no change during the IOP elevation in this example.
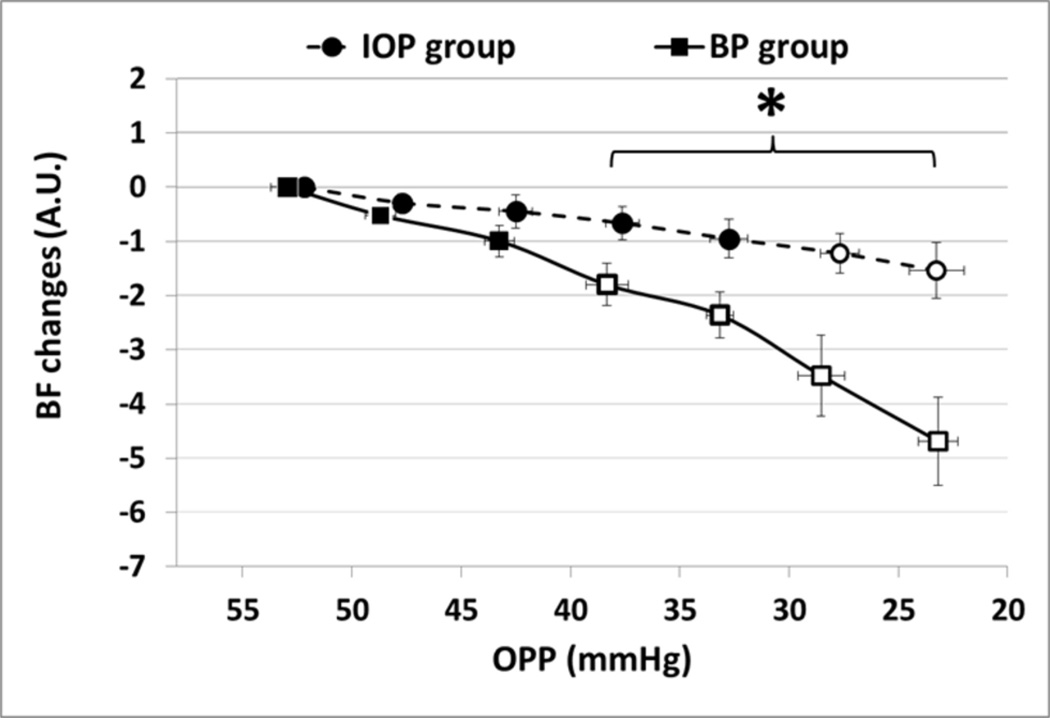
Figure 4.
The figure summarizes the ONH BF changes relative to the baseline value (OPP >50 mmHg) during every 5 mmHg of OPP decline in the BP group (squares, n=6) and the IOP group (circles, n=6). Open circles and squares indicate significant BF change compared with the baseline average for each group (RM-ANOVA P<0.05); an asterisk indicates significant difference between the two groups. Error bar: (±SE)
Comparison between the two experimental groups
RM-ANOVA showed the BF changed significantly more in the BP group than that in the IOP group (P = 0.021). There was also a significant interaction between BF changes and the OPP treatment (P=0.004, adjusted Greenhouse & Geisser univariate test): i.e., the BF difference between the two groups became significantly enlarged due to the OPP lowering. The BF declined significantly more in the BP group compared to the IOP group when the OPP was 35 mmHg (P<0.045) and below.
Exp 3. Peripapillary retinal vessels diameter changes during slow BP decrease
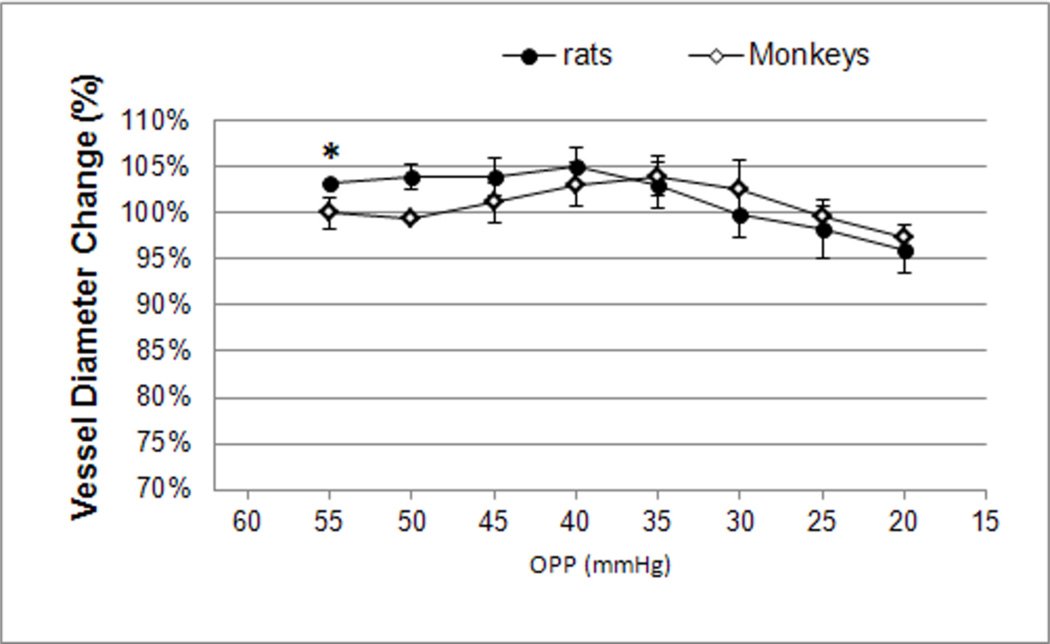
The peripapillary arterial blood vessel diameters averaged from three vessels in each eye showed no change during each 5 mmHg OPP decrement from baseline (OPP 50mmH) in monkeys (n=3). There were neither significant diameter changes in rats (n=9), except immediately after the administration of pentobarbital in the OPP 55 mmHg range (P=0.04, see Figure 5).
Figure 5.
The average diameter of peripapillary retinal arteries in rats (circles) and monkeys (diamonds) was plotted against the OPP every 5 mmHg decrement after the pentobarbital compound (Euthasol) injection. The asterisk indicates a signficant diameter increase from pre-injection baseline (100%) in rats. Error bar: SE.
Discussion
By comparing the ONH BF change at the same nominal OPP level induced by either lowering BP or increasing IOP in nonhuman primates, the current study demonstrated that ONH BF decreased significantly more if the OPP was reduced by lowering the BP than by increasing the IOP.
This observation, in good agreement with our previous report,38 indicates that a sufficient level BP is an important factor for effective BF autoregulation in the ONH. The results are also in line with similar studies in other ocular tissues and species. For example, in the rabbit choroid BF is regulated better during an IOP-induced OPP decrease than that by BP at the same OPP levels.4, 34 It takes a greater IOP elevation to reduce the choroidal BF to a given rate than it does by lowering BP.34 In humans, if the OPP was manipulated higher by altering the BP (isometric exercise), the BF compensated better than when OPP was lowered by increasing IOP in the human choroid.35 By measuring both retinal function and ocular BF in rats, higher BP has been found to have a protective effect on retinal function and this has been considered to be mediated by improved BF.36 Collectively, despite differences in tissues, species and experimental conditions, these earlier results and the current results suggest that higher BP enhances BF autoregulation and lower BP weakens BF autoregulation.
There are several possible mechanisms underlying the significantly greater ONH BF decrease during the BP-induced OPP decrease. First, it may be related to a stretch-dependent mechanism.14 Studies have demonstrated that the effective triggering and full-scale reaction of pressure-induced autoregulation depends on both transmural pressure and flow-induced shear stress.39, 40 The two factors are essential for the blood vessels to maintain a basal myogenic tone, also known as “vasodilative reserve”, to provide further vascular contraction or dilatation to adjust the local BF. While both lowering BP and increasing IOP resulted in the same OPP decrease and transmural pressure change, lowering BP had a likely stronger effect weakening the shear stress because it significantly reduced cardiac output and BF velocity compared with that achieved by increasing the IOP.14 Thus, even with a mathematically equal decrease of OPP, achieved by either lowering BP or increasing IOP, a greater decline in the ONH BF could be attributed to a reduced vasodilative reserve during the BP lowering. However, this theory is limited to the mechanical regulation in the arterioles or the resistance vessels. The ONH BF is also regulated via metabolic mechanisms, possibly also at the capillary level. Previous studies have suggested that capillaries may assist arteries and veins in the regulation of ONH BF accomplished by the contractile mural cell of capillaries including the pericyte52–55 and endothelium.52, 56 Thus, it is possible that the significantly greater ONH BF decrease during the BP-induced OPP decrease reflects differences between conditions in metabolic mechanisms of autoregulation, at least in part.
Several additional factors that may have potential impact on the results of this study should also be considered. In a previous study of ONH BF response to IOP increase in human subjects,51 a slower IOP increase induced a wider autoregulation range than that of a faster IOP increase.51 The authors speculated that if the IOP elevated too fast, it did not allow the vascular system to fully autoregulate. Thus, an ideal experimental condition should alter the OPP in both groups at the same speed. However, since the speed of this pharmacologically induced BP decline was too difficult (if not impossible) to control precisely, the OPP was reduced at a varied speed within and between individual animals. To minimize this potential influence, we adjusted the speed of change in the IOP group to match that in the BP group. As shown in the results, the average speed of the OPP change in the BP group was slightly slower than that in the IOP group, suggesting that the significantly greater decrease of ONH BF in the BP group was unlikely to be the result of a higher speed of BP decrease. However, it cannot be ruled out that if a sufficient recovery time was provided periodically during the course of OPP decrease, the BF decrease may have had the same magnitude under both conditions (BP and IOP).
Another caveat is the quantitative difference of pentobarbital and the ingredients in the two different pentobarbital formulations (Nembutal vs. Euthasol) administered in the two groups. If the pentobarbital or the ingredients in the solutions has a dose-dependent vasoconstrictor effect on the ONH blood vessels, the significantly more decreased BF in the BP group might be related to the dose differences. In the literature, the majority of studies have shown that pentobarbital either abolishes or attenuates induced vasoconstriction in non-ocular tissues57–63 and the vascular tone in the anterior uvea and optic nerve.64 However, a few studies show a direct effect of vasoconstriction in vivo on the rat hindquarter resistance vessels.65, 66 To specifically evaluate the effect of pentobarbital and/or the ingredients on the vessels’ diameter at the dose and the route administered in this study, the peripapillary retinal vessel diameter was determined as a surrogate to the vessels from where the capillary BF derives, which assumes both vessels share same physiological properties (Exp 3). The result showed mild vasodilation, instead of vasoconstriction after pentobarbital administration until OPP was reduced to 20 mmHg. This vasodilative effect by pentobarbital has been previously reported in the rabbit ONH as well.64 However, whether the pentobarbital has additional unknown dose-dependent effects on pathways influencing the performance of autoregulation in the ONH and/or whether high dose administration of pentobarbital to reduce BP has other systemic vascular effects are unclear.
It should also be noted that this study was designed to compare ONH BF at identical (nominal) levels of OPP. However, the calculated OPP may not represent the exact pressure difference between the arterial pressure entering the ONH and venous pressure leaving the ONH67, 68 because the former was estimated from systemic BP measured in a leg and the latter was estimated from a surrogate (IOP). Thus, the BF comparisons might not be at precisely “equal OPP” levels between the two experiments since the underlying assumptions could differ under each condition. However, the potential error is not expected to account for the full scale of BF differences observed between the two groups at each OPP level. In addition, a potential effect of systemic response in the BP group on the ONH autoregulation should also be considered.
Bearing in mind these limitations and complexity, the quantitative difference in the response of ONH BF to decreased OPP depending on achieved by whether lowered BP or increased IOP, parallels the clinical findings in glaucoma and may have potential implications. Population based epidemiological studies typically estimate OPP in a similar manner to that in this study, and find that the prevalence, incidence and progression of glaucoma are closely associated with low OPP, as opposed to IOP alone. In these patients, their systole18, 69 and diastole20, 27, 69 are lower and the risk to develop glaucoma is several times higher than the normal population. According to these studies, the diastolic OPP could be as low as 45 mmHg,27 or even lower if taking the nocturnal BP dip into account.28–30 The current study suggests that the ONH BF is particular susceptible to the BP associated OPP decrease. In addition, because the diurnal fluctuation of mean BP in the glaucomatous patients can be as high as ±22.8 mmHg whilst the IOP fluctuates by an average ±5.3 mmHg,30 it adds additional risks for the BP-related OPP decrease than the IOP-related OPP decrease, which may accelerate the development and progression in glaucoma.
In summary, ONH BF has been found to be more susceptible to an OPP decrease induced by lowering the BP as compared with that induced by increasing the IOP in the current experimental setups. The mechanism behind this change can be partly attributed to the reduced vasodilative reserve which depends upon the normal BP. This may reflect the potential mechanism of perfusion deficit in the ONH of glaucomatous patients with lower BP.
Acknowledgments
The authors thank for the technical assistance by Miss Chelsea Piper, manuscript preparation by Dr. Simon Thompson and critical review by Dr. Jeff Kiel.
Funding: R01EY019939 (Wang, L); R01EY019327 (Fortune, B); Legacy Good Samaritan Foundation, Portland OR.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Reference
- 1.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- 2.Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, Robertson CS. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus. 2008;25:E7. doi: 10.3171/FOC.2008.25.10.E7. [DOI] [PubMed] [Google Scholar]
- 3.Aaslid R. Cerebral autoregulation and vasomotor reactivity. Front Neurol Neurosci. 2006;21:216–228. doi: 10.1159/000092434. [DOI] [PubMed] [Google Scholar]
- 4.Kiel JW. The Ocular Circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 5.Cioffi GA. Vascular anatomy of the anterior optic nerve. In: Pillunat LE, Harris A, Anderson DR, Greve EL, editors. Current Concepts on Ocular Blood Flow in Glaucoma. The Hague: Kugler Publications; 1999. p. 45. [Google Scholar]
- 6.Hayreh SS. The 1994 Von Sallman Lecture. The optic nerve head circulation in health and disease. Exp Eye Res. 1995;61:259–272. doi: 10.1016/s0014-4835(05)80121-6. [DOI] [PubMed] [Google Scholar]
- 7.Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 8.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Venkataraman ST, Flanagan JG, Hudson C. Vascular reactivity of optic nerve head and retinal blood vessels in glaucoma--a review. Microcirculation. 2010;17:568–581. doi: 10.1111/j.1549-8719.2010.00045.x. [DOI] [PubMed] [Google Scholar]
- 10.Bill A. The uveal venous pressure. Arch Ophthalmol. 1963;69:780–782. doi: 10.1001/archopht.1963.00960040786021. [DOI] [PubMed] [Google Scholar]
- 11.Bill A. Some aspects of the ocular circulation. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1985;26:410–424. [PubMed] [Google Scholar]
- 12.Morgan WH, Yu DY, Cooper RL, Alder VA, Cringle SJ, Constable IJ. Retinal artery and vein pressures in the dog and their relationship to aortic, intraocular, and cerebrospinal fluid pressures. Microvasc Res. 1997;53:211–221. doi: 10.1006/mvre.1997.2010. [DOI] [PubMed] [Google Scholar]
- 13.Westlake WH, Morgan WH, Yu DY. A pilot study of in vivo venous pressures in the pig retinal circulation. Clin Experiment Ophthalmol. 2001;29:167–170. doi: 10.1046/j.1442-9071.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- 14.Bevan JA, Hwa JJ. Myogenic tone and cerebral vascular autoregulation: the role of a stretch-dependent mechanism. Ann Biomed Eng. 1985;13:281–286. doi: 10.1007/BF02584245. [DOI] [PubMed] [Google Scholar]
- 15.Maumenee AE. Causes of optic nerve damage in glaucoma. Robert N. Shaffer lecture. Ophthalmology. 1983;90:741–752. doi: 10.1016/s0161-6420(83)34493-6. [DOI] [PubMed] [Google Scholar]
- 16.Memarzadeh F, Ying-Lai M, Chung J, Azen SP, Varma R. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2010;51:2872–2877. doi: 10.1167/iovs.08-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell P, Lee AJ, Rochtchina E, Wang JJ. Open-angle glaucoma and systemic hypertension: the blue mountains eye study. J Glaucoma. 2004;13:319–326. doi: 10.1097/00061198-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–1293. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 21.Orzalesi N, Rossetti L, Omboni S. Vascular risk factors in glaucoma: the results of a national survey. Graefes Arch Clin Exp Ophthalmol. 2007;245:795–802. doi: 10.1007/s00417-006-0457-5. [DOI] [PubMed] [Google Scholar]
- 22.Caprioli J, Coleman AL. Blood pressure, perfusion pressure, and glaucoma. Am J Ophthalmol. 2010;149:704–712. doi: 10.1016/j.ajo.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Leske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr Opin Ophthalmol. 2009;20:73–78. doi: 10.1097/ICU.0b013e32831eef82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa VP, Arcieri ES, Harris A. Blood pressure and glaucoma. Br J Ophthalmol. 2009;93:1276–1282. doi: 10.1136/bjo.2008.149047. [DOI] [PubMed] [Google Scholar]
- 25.Nemesure B, Wu SY, Hennis A, Leske MC. Factors related to the 4-year risk of high intraocular pressure: the Barbados Eye Studies. Arch Ophthalmol. 2003;121:856–862. doi: 10.1001/archopht.121.6.856. [DOI] [PubMed] [Google Scholar]
- 26.Hennis A, Wu SY, Nemesure B, Leske MC. Hypertension, diabetes, and longitudinal changes in intraocular pressure. Ophthalmology. 2003;110:908–914. doi: 10.1016/S0161-6420(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 27.Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–1826. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 28.Meyer JH, Brandi-Dohrn J, Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol. 1996;80:864–867. doi: 10.1136/bjo.80.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham SL, Drance SM, Wijsman K, Douglas GR, Mikelberg FS. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology. 1995;102:61–69. doi: 10.1016/s0161-6420(95)31053-6. [DOI] [PubMed] [Google Scholar]
- 30.Choi J, Kim KH, Jeong J, Cho HS, Lee CH, Kook MS. Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2007;48:104–111. doi: 10.1167/iovs.06-0615. [DOI] [PubMed] [Google Scholar]
- 31.He Z, Vingrys AJ, Armitage JA, Bui BV. The role of blood pressure in glaucoma. Clin Exp Optom. 2011;94:133–149. doi: 10.1111/j.1444-0938.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 32.Schmidl D, Garhofer G, Schmetterer L. The complex interaction between ocular perfusion pressure and ocular blood flow - Relevance for glaucoma. Exp Eye Res. 2010;93:141–155. doi: 10.1016/j.exer.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Schmidl D, Boltz A, Kaya S, Werkmeister R, Dragostinoff N, Lasta M, et al. Comparison of choroidal and optic nerve head blood flow regulation during changes in ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2012;53:4337–4346. doi: 10.1167/iovs.11-9055. [DOI] [PubMed] [Google Scholar]
- 34.Kiel JW, van Heuven WA. Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1995;36:579–585. [PubMed] [Google Scholar]
- 35.Polska E, Simader C, Weigert G, Doelemeyer A, Kolodjaschna J, Scharmann O, et al. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest Ophthalmol Vis Sci. 2007;48:3768–3774. doi: 10.1167/iovs.07-0307. [DOI] [PubMed] [Google Scholar]
- 36.He Z, Nguyen CT, Armitage JA, Vingrys AJ, Bui BV. Blood pressure modifies retinal susceptibility to intraocular pressure elevation. PLoS One. 2012;7:e31104. doi: 10.1371/journal.pone.0031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 38.Liang Y, Downs JC, Fortune B, Cull GA, Cioffi GA, Wang L. Impact of Systemic Blood Pressure on the Relationship between Intraocular Pressure and Blood Flow in the Optic Nerve Head of Non-Human Primates. Invest Ophthalmol Vis Sci. 2009;50:2154–2160. doi: 10.1167/iovs.08-2882. [DOI] [PubMed] [Google Scholar]
- 39.Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res. 2012;49:375–389. doi: 10.1159/000338747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henrion D. Pressure and flow-dependent tone in resistance arteries. Role of myogenic tone. Arch Mal Coeur Vaiss. 2005;98:913–921. [PubMed] [Google Scholar]
- 41.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 42.Werner C, Lu H, Engelhard K, Unbehaun N, Kochs E. Sevoflurane impairs cerebral blood flow autoregulation in rats: reversal by nonselective nitric oxide synthase inhibition. Anesth Analg. 2005;101:509–516. doi: 10.1213/01.ANE.0000160586.71403.A4. [DOI] [PubMed] [Google Scholar]
- 43.Kremser PC, Gewertz BL. Effect of pentobarbital and hemorrhage on renal autoregulation. Am J Physiol. 1985;249:F356–F360. doi: 10.1152/ajprenal.1985.249.3.F356. [DOI] [PubMed] [Google Scholar]
- 44.Preckel MP, Leftheriotis G, Ferber C, Degoute CS, Banssillon V, Saumet JL. Effect of nitric oxide blockade on the lower limit of the cortical cerebral autoregulation in pentobarbital-anaesthetized rats. Int J Microcirc Clin Exp. 1996;16:277–283. doi: 10.1159/000179186. [DOI] [PubMed] [Google Scholar]
- 45.Fujii H, Nohira K, Yamamoto Y, Ikawa H, Hjura T. Evaluation of blood flow by laser speckle image sensing, Part 1. Applied Optics. 1987;26:5321–5325. doi: 10.1364/AO.26.005321. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010;88:723–729. doi: 10.1111/j.1755-3768.2009.01586.x. [DOI] [PubMed] [Google Scholar]
- 47.Liang Y, Fortune B, Cull G, Cioffi GA, Wang L. Quantification of dynamic blood flow autoregulation in optic nerve head of rhesus monkeys. Exp Eye Res. 2010;90:203–209. doi: 10.1016/j.exer.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Koelle JS, Riva CE, Petrig BL, Canstoun SD. Depth of tissue sampling in the optic nerve head using laser Doppler flowmetry. Lasers Med Sci. 1993;8:49–54. [Google Scholar]
- 49.Wang L, Cull GA, Piper C, Burgoyne CF, Fortune B. Anterior and posterior optic nerve head blood flow in nonhuman primate experimental glaucoma model measured by laser speckle imaging technique and microsphere method. Invest Ophthalmol Vis Sci. 2012;53:8303–8309. doi: 10.1167/iovs.12-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugiyama T, Utsumi T, Azuma I, Fujii H. Measurement of optic nerve head circulation: comparison of laser speckle and hydrogen clearance methods. Jpn J Ophthalmol. 1996;40:339–343. [PubMed] [Google Scholar]
- 51.Riva CE, Hero M, Titze P, Petrig B. Autoregulation of human optic nerve head blood flow in response to acute changes in ocular perfusion pressure. Graefes Arch Clin Exp Ophthalmol. 1997;235:618–626. doi: 10.1007/BF00946937. [DOI] [PubMed] [Google Scholar]
- 52.Haefliger IO, Meyer P, Flammer J, Luscher TF. The vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology? Surv Ophthalmol. 1994;39:123–132. doi: 10.1016/0039-6257(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 53.Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010 May 21;2:5. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson DR, Davis EB. Glaucoma, capillaries and pericytes. 5. Preliminary evidence that carbon dioxide relaxes pericyte contractile tone. Ophthalmologica. 1996;210:280–284. doi: 10.1159/000310726. [DOI] [PubMed] [Google Scholar]
- 56.Haefliger IO, Flammer J, Luscher TF. Heterogeneity of endothelium-dependent regulation in ophthalmic and ciliary arteries. Invest Ophthalmol Vis Sci. 1993;34:1722–1730. [PubMed] [Google Scholar]
- 57.Sanchez-Ferrer CF, Marin J, Salaices M, Rico ML, Munoz-Blanco JL. Interference of pentobarbital and thiopental with the vascular contraction and noradrenaline release in human cerebral arteries. Gen Pharmacol. 1985;16:469–473. doi: 10.1016/0306-3623(85)90006-0. [DOI] [PubMed] [Google Scholar]
- 58.Offenbartl K, Flint L. Pentobarbital attenuates arteriolar constrictor and dilator responses to hemorrhage. Prog Clin Biol Res. 1988;264:385–390. [PubMed] [Google Scholar]
- 59.Kolh P, Lambermont B, Ghuysen A, Tchana-Sato V, Dogne JM, D'Orio V, et al. Comparison of the effects of propofol and pentobarbital on left ventricular adaptation to an increased afterload. J Cardiovasc Pharmacol. 2004;44:294–301. doi: 10.1097/01.fjc.0000133050.11105.c2. [DOI] [PubMed] [Google Scholar]
- 60.Edvinsson L, McCulloch J. Effects of pentobarbital on contractile responses of feline cerebral arteries. J Cereb Blood Flow Metab. 1981;1:437–440. doi: 10.1038/jcbfm.1981.48. [DOI] [PubMed] [Google Scholar]
- 61.Bulow J, Henriksen O, Amtorp O. Inhibitory effect of pentobarbital anesthesia on venous stasis induced arteriolar vasoconstriction in the dog hindleg. Acta Physiol Scand. 1984;122:307–311. doi: 10.1111/j.1748-1716.1984.tb07514.x. [DOI] [PubMed] [Google Scholar]
- 62.Abildgaard U. Pentobarbital inhibits the vasoconstrictor response to renal venous pressure elevation in the dog kidney. Acta Physiol Scand. 1985;124:625–627. doi: 10.1111/j.1748-1716.1985.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 63.Wetzel RC, Martin LD. Pentobarbital attenuates pulmonary vasoconstriction in isolated sheep lungs. Am J Physiol. 1989;257:H898–H903. doi: 10.1152/ajpheart.1989.257.3.H898. [DOI] [PubMed] [Google Scholar]
- 64.Bill A, Stjernschantz J. Cholinergic vasoconstrictor effects in the rabbit eye: vasomotor effects of pentobarbital anesthesia. Acta Physiol Scand. 1980;108:419–424. doi: 10.1111/j.1748-1716.1980.tb06553.x. [DOI] [PubMed] [Google Scholar]
- 65.Teranishi Y, Tsuru H, Shimomura H, Amano T, Matsubayashi H. Compensatory vasoconstrictor effects of sodium pentobarbital on the hindquarters of conscious normotensive control and lumbar-sympathectomized Wistar rats. Auton Neurosci. 2000;82:130–136. doi: 10.1016/S0165-1838(00)00102-8. [DOI] [PubMed] [Google Scholar]
- 66.Teranishi Y, Iriuchijima J. Sympathetic vasoconstrictor tone induced by pentobarbital anesthesia in hindquarters of rats. Jpn J Physiol. 1992;42:171–178. doi: 10.2170/jjphysiol.42.171. [DOI] [PubMed] [Google Scholar]
- 67.Costa VP, Harris A, Anderson D, Stodtmeister R, Cremasco F, Kergoat H, et al. Ocular perfusion pressure in glaucoma. Acta ophthalmologica. 2013 doi: 10.1111/aos.12298. In press. [DOI] [PubMed] [Google Scholar]
- 68.Costa VP, Anderson D, Harris A. Surrogates for ocular perfusion pressure are not perfect-authors reply. Acta ophthalmologica. 2014 doi: 10.1111/aos.12381. In press. [DOI] [PubMed] [Google Scholar]
- 69.Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]