Abstract
BACKGROUND
Tobacco assessment and cessation support are not routinely included in cancer care. An automated tobacco assessment and cessation program was developed to increase the delivery of tobacco cessation support for cancer patients.
METHODS
A structured tobacco assessment was incorporated into the electronic health record at Roswell Park Cancer Institute to identify tobacco use in cancer patients at diagnosis and during follow-up. All patients who reported tobacco use within the past 30 days were automatically referred to a dedicated cessation program that provided cessation counseling. Data were analyzed for referral accuracy and interest in cessation support.
RESULTS
Between October 2010 and December 2012, 11,868 patients were screened for tobacco use, and 2765 were identified as tobacco users and were referred to the cessation service. In referred patients, 1381 of those patients received only a mailed invitation to contact the cessation service, and 1384 received a mailing as well as telephone contact attempts from the cessation service. In the 1126 (81.4%) patients contacted by telephone, 51 (4.5%) reported no tobacco use within the past 30 days, 35 (3.1%) were medically unable to participate, and 30 (2.7%) declined participation. Of the 1381 patients who received only a mailed invitation, 16 (1.2%) contacted the cessation program for assistance. Three questions at initial consult and follow-up generated over 98% of referrals. Tobacco assessment frequency every 4 weeks delayed referral in <1% of patients.
CONCLUSIONS
An automated electronic health record-based tobacco assessment and cessation referral program can identify substantial numbers of smokers who are receptive to enrollment in a cessation support service.
Keywords: tobacco, smoking, cancer, cessation, oncologist, electronic health record, electronic medical record, clinical efficiency
INTRODUCTION
The adverse health effects of smoking are well documented,1 and smoking by patients with cancer is associated with increased overall mortality, cancer recurrence, treatment-related toxicity, and the risk of developing a second primary cancer.2–10 The routine assessment of tobacco use and the provision of tobacco cessation support are advocated by the American Society of Clinical Oncology, the American Association for Cancer Research, the Joint Commission for the Accreditation of Hospitals, the Oncology Nursing Society, and the National Cancer Institute.11–18 However, few institutions have implemented procedures that both assess tobacco use and refer tobacco users to cessation support services. Ideally, tobacco assessment and evidence-based cessation support are needed to treat tobacco use in cancer patients.
Cancer clinics are strained with the demands of increasingly complex cancer care that reduces time to provide counseling for tobacco cessation.19 Information on tobacco use in cancer patients is often based on tobacco assessments that are sporadic, nonstandardized, and reliant on the disposition of individual practitioners, resulting in poor tobacco use documentation and inconsistent delivery of tobacco cessation efforts.20,21 Tobacco assessment in clinical practice and in clinical trials needs to be strengthened and conducted in an efficient manner.21,22 Smoking cessation among cancer patients may be enhanced substantially if a clinically efficient model could be developed to accurately identify tobacco use, provide cessation support, and minimize the clinical burden associated with assessment and cessation.
An institutional committee at Roswell Park Cancer Institute (RPCI) developed a standardized tobacco assessment and dedicated tobacco cessation program to provide evidence-based cessation support for all cancer patients who are at risk for continued tobacco use. The objective of the current study was to evaluate whether automated assessment and referral could increase enrollment by cancer patients in a tobacco cessation support service.
MATERIALS AND METHODS
Methods
An institutional committee consisting of physicians (surgical, medical, and radiation oncologists), nurses, psychologists, and information technologists developed a standardized tobacco assessment and cessation program as a part of routine clinical care for all cancer patients in a setting in which there was no prior structured tobacco cessation program. The specific objective was to design a tobacco cessation program based on Public Health Service (PHS) guidelines23 that could be administered in a clinically efficient and reproducible manner to large numbers of cancer patients. PHS guidelines are based on the “5 As” (ask, advise, assess, assist, and arrange), and the RPCI program was developed to provide an automated method to address the “5 As” through structured tobacco assessments and cessation support.
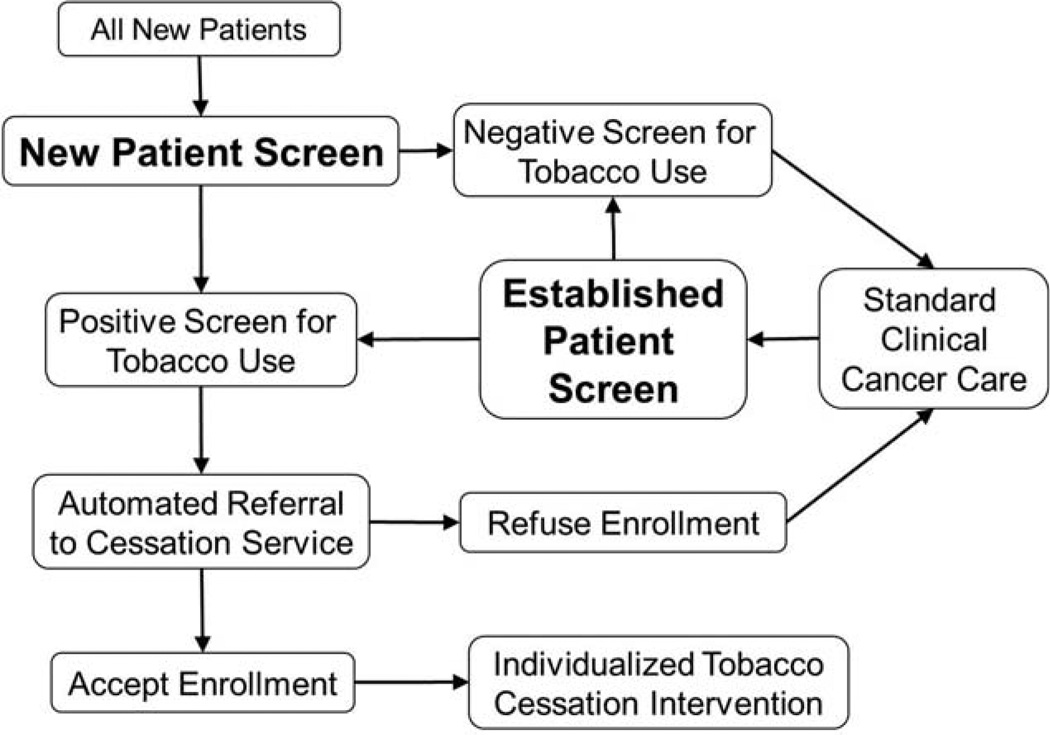
Specific questions were designed and placed in the electronic health record (EHR) at RPCI for nurses to ask cancer patients about tobacco use during outpatient clinic visits at the initial consult, during treatment, and during follow-up care after cancer treatment. The clinical schema used to assess tobacco is provided in Figure 1. A new patient screen, which was presented at the initial consult, was used to obtain information on current and prior tobacco use. An established patient screen, which was presented at all follow-up evaluations, was used to obtain information only on tobacco use between clinic visits. Questions were designed for coherence with North American Quitline Consortium guidelines,24 and some questions were suggested by published recommendations for cancer patients25; however, the administration of questions by nurses was designed to accommodate patients with cancer, because most cancer patients would return to the clinic on a regular basis for several months or years for treatment. The new patient screening questions and the established patient screening questions that were used to assess tobacco use are provided in Table 1.
Figure 1.
The algorithm for new patient screening and established patient screening is illustrated.
TABLE 1.
New Patient and Established Patient Screening Questionsa
New patient screening questions
|
Tobacco use categories are definedb
|
For current tobacco users
|
For former tobacco users
|
Established patient screening questions
|
Questions 2, 3, 10, 15, 16, and 18 in the new patient screen and questions 19, 20, 21, and 23 in the established patient screen are used to generate automatic referrals through the electronic health record to a dedicated cessation program.
After answering questions 1 through 3, patients are divided into specific tobacco use categories. Current tobacco users were asked questions 4–9 and 15–18. Former tobacco users were asked questions 10–18. Never tobacco users were asked questions 17–18.
GlaxoSmithKline, Research Triangle Park, NC.
Pfizer, New York, NY.
All patients who reported tobacco use within the past 30 days were automatically referred to the dedicated institutional tobacco cessation service. In addition, patients who reported using a cessation pharmacotherapy (such as nicotine, varenicline, or bupropion) were automatically referred for cessation support. Referral was determined at the time of patient screening based on self-reported, annotated tobacco use variables. The referral was generated automatically in the EHR if patients reported tobacco use within the past 30 days (as indicated by the screening questions in Table 1). Tobacco cessation support consisted primarily of motivational counseling and was delivered outside of the clinic by a dedicated tobacco cessation service. The cessation service attempted up to 5 telephone calls to contact each patient, and call times were varied to increase the likelihood of contacting patients; however, no further attempts were made if the patient was not contacted within 5 calls. When a patient was contacted, they were informed by the cessation service of the adverse effects of tobacco on cancer treatment and that tobacco cessation support was being provided as an institutional standard of care. The cessation service re-evaluated current tobacco use, prior quit attempts, willingness to quit, and social and environmental factors and used this information to develop an individualized tobacco cessation treatment plan. Nearly all cessation support was delivered by telephone, but counselors would occasionally meet cancer patients during a clinic visit if requested by the patient. Although there were no specific guidelines for the length of time spend on telephone calls, an interim analysis of the first 808 referrals demonstrated a median of 8 minutes was spent counseling per telephone call. Over the timeframe reported in this article, resources were limited to an average of 1.5 full-time–equivalent tobacco cessation specialists. Consequently, approximately 50% of the referred patients (1381 of 2765) received only a mailing sent to their home that discussed the benefits of cessation with an invitation to contact the RPCI tobacco cessation service. The cessation program was started in the thoracic clinic and was expanded to reach all clinics by July 2012. Preference for telephone-based contact was given to patients with thoracic cancers and patients who were new to the institute, but new referrals and contacts were refreshed each week. Thus, approximately 50% of referrals from each week had telephone-based contact attempts, and the remaining referrals received a standard mailing alone.
Pharmacotherapy was discussed with appropriate patients, and recommendations were provided for nicotine-replacement therapy according to PHS guidelines; other forms of pharmacotherapy (varenicline or bupropion) were not discussed unless patients requested them. The treating physician was then informed of the recommendation, and a prescription was provided at the discretion of the treating physician. Nicotine replacement was recommended if patients reported a current habit of 10 cigarettes per day. There was no program to provide free nicotine replacement from the institution, but the cessation service would work with the patient to identify methods to help obtain nicotine replacement through insurance, community resources like the New York State Quitline, or personal purchase options.
The cessation program was designed to facilitate clinical cessation support for cancer patients. Consequently, patients were not required to complete lengthy assessments or to commit to a specific cessation plan at the time of first contact by the cessation service. Data were reflective of the design and implementation of a structured, EHR-based clinical tobacco assessment with automatic referral of appropriate cancer patients to a dedicated clinical tobacco cessation program.
Data Analysis
The study was approved by the Institutional Review Board at RPCI. The results describe referral patterns, response to the cessation support program, interest in cessation support, and the efficiency of referral questions for capturing appropriate patient referrals.
RESULTS
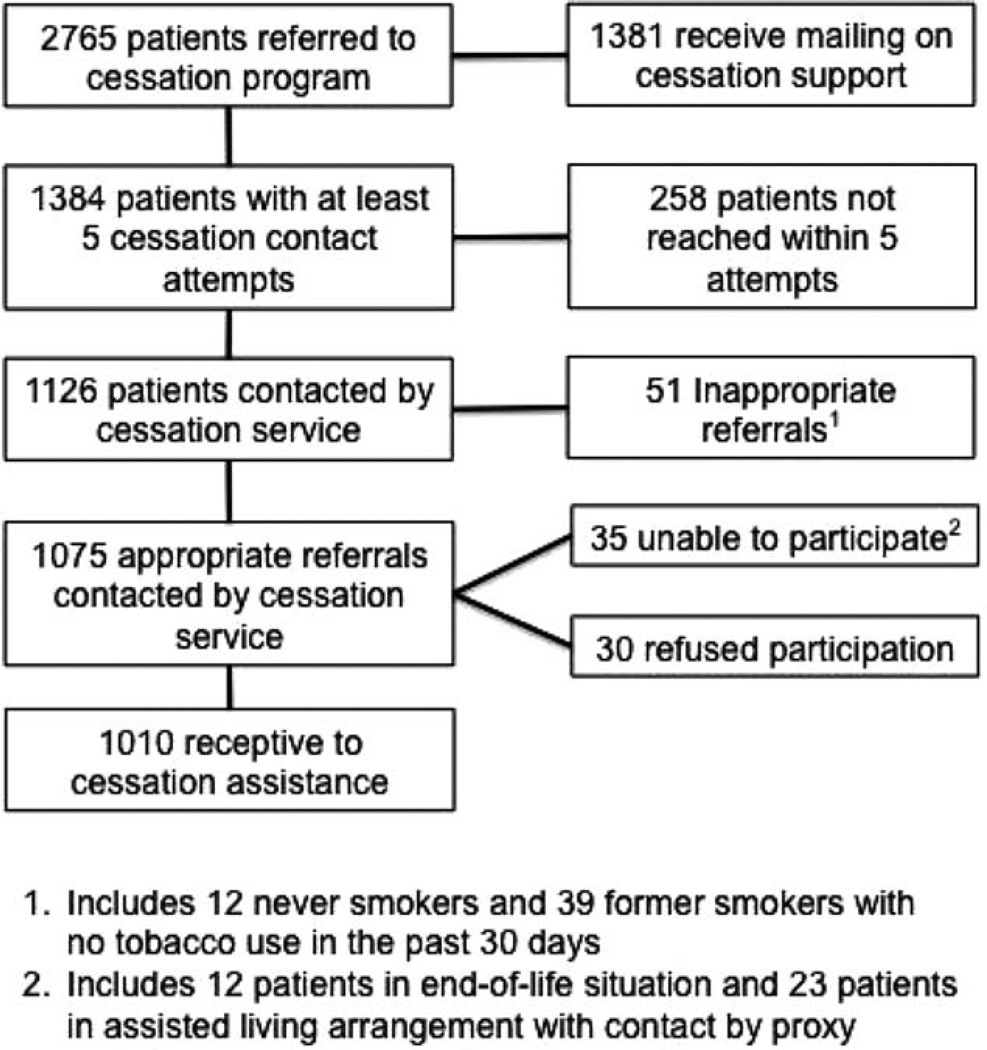
Between October 2010 and December 2012, 11,868 patients were screened for tobacco use, 2765 cancer patients screened positive, and all patients were referred to the cessation service (Fig. 2). The program was initiated in the thoracic clinic and rolled out institute-wide beginning in July 2012. The cessation service made at least 5 telephone contact attempts for 1384 patients (50.1%), and the remaining 1381 patients received only the standard mailing on tobacco cessation, which included information about tobacco cessation and how to contact the RPCI cessation service. Of 1384 cancer patients with at least 5 contact attempts, 1126 patients (81.3%) were successfully contacted. Among the 1126 successfully contacted patients, 51 (4.5%) reported to the cessation counselor that they had never smoked or had not smoked in the past 30 days, suggesting a low error rate associated with the tobacco assessment. Among the 1381 cancer patients who were sent only the tobacco cessation mailing, only 16 patients (1.2%) contacted the cessation program for assistance.
Figure 2.
Patient referral characteristics and first contact outcomes are illustrated for patients who were referred to the cessation service.
All successfully contacted patients who confirmed tobacco use within the past 30 days were informed about the tobacco cessation program and were offered free enrollment. In total, 1075 cancer patients who used tobacco within the past 30 days were offered cessation support, but 35 patients (3.3%) were unable to participate in the program because of end-of-life care or because no direct contact with the patient was available (such as assisted-living arrangements). Only 30 cancer patients (2.8%) refused to use the tobacco cessation support program.
Interim analyses were performed for quality-control purposes. Initially, tobacco assessments were performed with a frequency of up to once per week; however, both nurses and patients voiced significant complaints about the clinical burden associated with assessment on this schedule. Consequently, assessments were made no more frequently than every 2 weeks for a trial period. After 428 cessation referrals were generated, the referrals were analyzed to assess the effect of implementing assessments every 4 weeks instead of every 2 weeks. This interim analysis demonstrated that assessments every 4 weeks instead of every 2 weeks would delay only 3 of 428 referrals (0.7%). Thereafter, assessments were performed no more frequently than every 4 weeks, thereby reducing the clinical burden associated with more frequent assessments.
The initial questionnaire included several detailed questions on cigarette use as well as other noncigarette forms of tobacco use, such as cigars, pipes, and smokeless tobacco. However, completion of the initial questionnaire was time consuming and was resisted by clinicians and patients. A final questionnaire (Table 1) included a single question on other noncigarette forms of tobacco use that included 6 potential referral questions on the new patient screen and 4 potential referral questions on the established patient screen.
A second interim analysis was performed after 808 referrals to identify the frequency with which each screening question generated a referral (Table 2). Smokers were categorized as current tobacco users (patients who reported using tobacco every day or some days) or former tobacco users (patients who did not report using tobacco every day or some days but did report tobacco use within the past 30 days). The purpose of this distinction was to determine whether identifying patients using self-reported current tobacco use (every day or some days) was sufficient to identify patients at risk for tobacco use. Table 2 indicates that the question, “Do you now smoke cigarettes every day, some days, or not at all” was the highest yield question on the new patient screen, generating 83.1% of total new patient referrals; however, the question, “About how long has it been since you last smoked a cigarette, even a puff” was critical to identifying patients who used tobacco within the past 30 days, generating 10.1% of new patient referrals. Cumulatively, questions 2, 3, and 10 in the new patient screen captured 98.8% of referrals, but very few patients were captured using questions 15, 16, and 18. Questions 19, 20, and 21 in the established patient screen were responsible for 98.3% of referrals.
TABLE 2.
Referral Efficacy for Specific Tobacco-Assessment Questions on the New Patient and Established Patient Screensd
| Percentage of Total Referrals | |||
|---|---|---|---|
| Current Tobacco Users |
Former Tobacco Users |
Percentage of Total NPS Referrals Generated |
|
| NPS Referral Question | |||
| 2. Do you now smoke cigarettes every day, some days, or not at all? (Automatic referral if “everyday” or “some days”) | 93.7 | NA | 83.1 |
| 3. Do you currently use any other tobacco products such as cigars, pipes, chewing tobacco, snuff, dip, SNUS, clove cigarettes, kreteks, or bidis? (Automatic referral if “yes”) | 6.3 | NA | 5.6 |
| 10. About how long has it been since you last smoked a cigarette, even a puff? (Referral generated if tobacco use within the past 30 days) | NA | 89 | 10.1 |
| 15. About how long has it been since you last smoked/used other tobacco products such as cigars, cigarillos, little cigars, pipe tobacco, or used chewing tobacco, snuff, dip, or SNUS even once? (Automatic referral if tobacco use within the past 30 days) | NA | 1.4 | 0.2 |
| 16. Are you currently using any of the following methods or strategies to try to quit? (Automatic referral if any nicotine replacement or other pharmacotherapy used by patient) | NA | 2.7 | 0.3 |
| 18. Are you interested in stopping tobacco use or speaking with our tobacco cessation specialist? (Automatic referral if “yes”) | NA | 6.8 | 0.8 |
| EPS Referral Question | |||
| 19. Since your last visit to (the Institute) or within the past 30 days, have you smoked a cigarette, even 1 or 2 puffs? (Automatic referral if “yes”) | — | — | 89.7 |
| 20. Since your last visit to (the Institute), have you used any other tobacco products such as cigars, pipes, chewing tobacco, snuff, dip, SNUS, clove cigarettes, kreteks, or bidis? (Automatic referral if “yes”) | — | — | 3.4 |
| 21. Are you currently using any of the following methods or strategies to try to quit? (Automatic referral if any nicotine replacement or other pharmacotherapy used by patient) | — | — | 5.1 |
| 23. Would you like to speak with our tobacco cessation specialist? (Automatic referral if “yes”) | — | — | 1.7 |
Abbreviations: EPS, established patient screen; NA, not applicable; NPS, new patient screen.
Data are from the first 808 referred patients. Questions are numbered in relation to the labels from Table 1.
DISCUSSION
A standardized tobacco assessment in the EHR with automatic referral to a dedicated tobacco cessation service can be achieved with low error rates, low clinician burden, and high patient receptiveness. Three questions asked at the initial consult and 3 questions asked at follow-up clinic visits with patients appear to identify a high proportion of cancer patients who use tobacco. However, specifically assessing tobacco use within the past 30 days identifies approximately 10% of cancer patients who are at risk for continued tobacco use. These data suggest that tobacco assessments can be performed every 4 weeks with minimal delay in the implementation of cessation support. The data also clearly document that a mailing alone is a far less effective method for attracting cancer patients to participate in cessation support. Collectively, this program demonstrates that cessation support can be delivered to a high volume of cancer patients using structured tobacco assessments and automated cessation referrals.
The adverse health effects of tobacco on chronic disease risk and mortality are well documented.1 Tobacco use by cancer patients is further associated with several adverse outcomes, including increased cancer recurrence, treatment-related morbidity, second malignancy, cancer-related mortality, and overall mortality in both tobacco-related and non–tobacco related cancers.2–10 Recognizing the importance of addressing tobacco use in cancer patients, several national and international organizations have advocated for tobacco control in cancer patients.11–17 Unfortunately, tobacco assessments and cessation support are not routinely provided by oncologists. In a recent study of 160 patients with head/neck or lung cancers, only 27.2% of smokers were offered pharmacotherapy and behavioral treatments.26 Less than 10% of inpatient hospital wards treating patients with head/neck or lung cancer provide inpatient cessation support.19 Approximately 60% of cancer patients who smoke do not receive any form of cessation assistance.27–29 A large survey of thoracic oncologists demonstrated that, although > 90% believe that tobacco affects cancer outcomes and that tobacco cessation should be a standard part of cancer care, only 40%provide tobacco cessation assistance.30 Collectively, these data demonstrate that significant advances are needed to increase access to tobacco cessation assistance for cancer patients. The automated screening and automatic referral program at RPCI is able to refer all cancer patients who are at risk for tobacco use to a dedicated tobacco cessation support program. In this program, tobacco-using cancer patients were highly receptive to cessation support through a standard institutional program that used automatic referrals to a dedicated cessation service. In contrast, very few patients responded to a standard mailing. These observations support the effectiveness of the systematic assessment and referral of cancer patients who use tobacco to a dedicated tobacco cessation support program.
Much research in tobacco cessation focuses on increasing the efficacy of tobacco cessation in smaller subgroups rather than the implementation of a clinically efficient mechanism to screen and treat large numbers of cancer patients. The implementation of meaningful-use initiatives will require the assessment of tobacco use and evidence of clinical follow-up. During the timeframe of this report, our program provided cessation support through mailings or telephone-based counseling to approximately 1300 patients annually with an average of 1.5 full-time–equivalent cessation counselors. Although this program was not staffed with sufficient resources to make telephone contact with all tobacco cessation referrals, these data suggest that telephone-based counseling could be provided to a large patient volume with relatively sparse resources. Clinical efficiency may be facilitated by implementing 3 standard assessment questions at the initial consult and at follow-up visits. Although minimizing questions will limit the ability to accurately identify nuances of prior tobacco use, it will facilitate the rapid identification of large numbers of cancer patients who use tobacco and will promote efficient referral to dedicated tobacco cessation resources. Tobacco assessments every 4 weeks may adequately identify patients for referral to tobacco cessation support services. Attempts at repeated questions on a daily or weekly basis produced significant clinical burden, as manifested by resistance from both patients and nursing staff. Substantial work is needed to better evaluate the potential effectiveness of this approach in both academic and community centers. However, the data demonstrate that some cessation support can be implemented with promising clinical efficiency and patient receptiveness.
There are several limitations to these results. The most significant limitation is that our program was designed to provide clinical tobacco cessation support to a large numbers of cancer patients given fixed tobacco cessation resources. The results do not establish that patients will take advantage of the program and cease to smoke or that cessation will alter cancer treatment outcomes. We have yet to understand major predictors of cessation efficacy. It has been demonstrated that patients with cancer who smoke also may misrepresent tobacco use,31–33 and self-reported accuracy may be limited further by telephone-based counseling. There is a need for additional data on institutional programs that place emphasis on the adverse effects of tobacco on cancer treatment outcomes to potentially “entice” patients to participate. This “enticement” may transiently enhance interest in participation. Certainly, the unexpectedly low refusal rate (2.6%) contrasts with published data suggesting larger refusal rates in cancer patients.26,34
The clinical implications of these results are important. Behavioral economics suggest that a smaller effect in a larger population may result in a dramatically better outcome than a larger effect in a much smaller population. Clinicians should routinely provide cessation support, but most oncologists do not routinely provide tobacco cessation assistance.30 Substantial work remains not only to improve tobacco cessation treatment efficacy, but also to improve access to structured tobacco cessation support. Automated referral to a dedicated cessation program that actively contacts patients, rather than reliance on patient-based cessation enrollment initiatives, may substantially increase overall tobacco cessation through increased patient participation, as evidenced herein and in other recent studies.35 The RPCI program provides a potentially useful model for screening patients for tobacco use and delivering cessation support to patients with cancer in a busy clinical oncology setting.
Acknowledgments
FUNDING SUPPORT
This work was supported in part by the Roswell Park Alliance Foundation and the American Cancer Society (MRSG-11-031-01-CCE, to G.W.W.).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Martin C. Mahoney has received compensation as a member of the Pfizer Speaker’s Bureau, he has served as a paid plaintiff expert witness in cases brought against tobacco manufacturers, and he serves on the editorial board of CA-A Cancer Journal for Clinicians. K. Michael Cummings has received grants from Pfizer Inc. to help establish hospital-based cessation services; he has served as a paid expert witness in litigation against cigarette manufacturers; and he is employed at the Medical University of South Carolina, where his job is to help establish tobacco cessation services for cancer patients. Richard J. O’Connor has received compensation from the US Food and Drug Administration as a consultant on their tobacco products scientific advisory committee (Tobacco Constituents Subcommittee), from Johns Hopkins University for consulting on international tobacco product design, and from the University of Massachusetts for consulting on questionnaire design for smokeless tobacco products; he has received lecture fees from the University at Buffalo for a lecture at the Research Institute on Additions on tobacco harm reduction; he has received compensation from the Centers for Disease Control and Prevention for preparation of a monograph chapter on smokeless tobacco products and from the Society for Research on Nicotine and Tobacco for an article on tobacco product surveillance; he has received compensation from the Connecticut Academy of Science and Engineering as a grant reviewer; and he is a member of the Committee on Scientific Standards for Studies of Modified-Risk Tobacco Products at the Institute of Medicine.
REFERENCES
- 1.US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 2.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132:401–410. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 3.Dal Maso L, Zucchetto A, Talamini R, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–2194. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 4.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305:2548–2555. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modesitt SC, Huang B, Shelton BJ, Wyatt S. Endometrial cancer in Kentucky: the impact of age, smoking status, and rural residence. Gynecol Oncol. 2006;103:300–306. doi: 10.1016/j.ygyno.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Duffy SA, Taylor JM, Terrell JE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 7.van den Belt-Dusebout AW, de Wit R, Gietema JA, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25:4370–4378. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- 8.Gajdos C, Hawn MT, Campagna EJ, Henderson WG, Singh JA, Houston T. Adverse effects of smoking on postoperative outcomes in cancer patients. Ann Surg Oncol. 2012;19:1430–1438. doi: 10.1245/s10434-011-2128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery database study. Ann Thorac Surg. 2009;88:362–370. doi: 10.1016/j.athoracsur.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Bittner N, Merrick GS, Galbreath RW, et al. Primary causes of death after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2008;72:433–440. doi: 10.1016/j.ijrobp.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 11.American Society of Clinical Oncology (ASCO) ASCO policy statement update: tobacco control-reducing cancer incidence and saving lives. J Clin Oncol. 2003;15:2777–2786. doi: 10.1200/JCO.2003.04.154. [DOI] [PubMed] [Google Scholar]
- 12.Viswanath K, Herbst RS, Land SR, Leischow SJ, Shields PG. Tobacco and cancer: an American Association for Cancer Res. policy statement. Cancer Res. 2010;70:3419–3430. doi: 10.1158/0008-5472.CAN-10-1087. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network (NCCN) Patient and Caregiver Resources: Quitting Smoking for Cancer Survivors. [Accessed October 10, 2013]; Available at: http://www.nccn.org/patients/resources/survivorship/smoking.aspx. [Google Scholar]
- 14.Joseph AM, Knapp JM, Nichol KL, Pirie PL. Determinants of compliance with a national smoke-free hospital standard. JAMA. 1995;274:491–494. [PubMed] [Google Scholar]
- 15.Tobacco cessation and quality cancer care. J Oncol Pract. 2009;5:2–5. doi: 10.1200/JOP.0913501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan G, Schnoll RA, Alfano CM, et al. National Cancer Institute conference on treating tobacco dependence at cancer centers. J Oncol Pract. 2011;7:178–182. doi: 10.1200/JOP.2010.000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toll BA, Brandon T, Gritz ET, Land SR, Warren GW, Herbst RA. Assessing and stopping cancer patients’ tobacco use: an American Association for Cancer research policy statement. Clin Cancer Res. 2013;19:1941–1948. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oncology Nursing Society. Oncology Nursing Society Position on Nursing Leadership in Global and Domestic Tobacco Control. [Accessed October 10, 2013]; Available at: http://www.ons.org/Publications/Positions/Tobacco/ [Google Scholar]
- 19.Mazza R, Lina M, Invernizzi G, et al. The gap between tobacco treatment guidelines, health service organization, and clinical practice in comprehensive cancer centres [serial online] J Oncol. 2011:145617. doi: 10.1155/2011/145617. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis [serial online] BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Land SR. Methodologic barriers to addressing critical questions about tobacco and cancer prognosis. J Clin Oncol. 2012;30:2030–2032. doi: 10.1200/JCO.2012.41.7402. [DOI] [PubMed] [Google Scholar]
- 22.Peters EN, Torres E, Toll BA, et al. tobacco assessment in actively accruing National Cancer Institute (NCI) Cooperative Group clinical trials. J Clin Oncol. 2012;30:2869–2875. doi: 10.1200/JCO.2011.40.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 24.Campbell HS, Ossip-Klein D, Bailey L, Saul J North American Quitline Consortium. Minimal dataset for quitlines: a best practice. Tob Control. 2007;16(suppl 1):i16–i20. doi: 10.1136/tc.2007.019976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gritz ER, Dresler CM, Sarna L. Smoking, the missing drug interaction in clinical trials. Cancer Epidemiol Biomarkers Prev. 2005;14:2287–2293. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 26.Cooley ME, Emmons KM, Haddad R, et al. Patient-reported receipt of and interest in smoking-cessation interventions after a diagnosis of cancer. Cancer. 2011;117:2961–2969. doi: 10.1002/cncr.25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostroff JS, Jacobsen PB, Moadel AB, et al. Prevalence and predictors of continued tobacco use after treatment of patients with head and neck cancer. Cancer. 1995;75:569–576. doi: 10.1002/1097-0142(19950115)75:2<569::aid-cncr2820750221>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 28.Schnoll RA, Rothman RL, Newman H, et al. Characteristics of cancer patients entering a smoking cessation program and correlates of quit motivation: implications for the development of tobacco control programs for cancer patients. Psychooncology. 2004;13:346–358. doi: 10.1002/pon.756. [DOI] [PubMed] [Google Scholar]
- 29.Cooley ME, Sarna L, Kotlerman J, et al. Smoking cessation is challenging even for patients recovering from lung cancer surgery with curative intent. Lung Cancer. 2009;66:218–225. doi: 10.1016/j.lungcan.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warren GW, Marshall JR, Cummings KM, et al. Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. J Thorac Oncol. 2013;8:543–548. doi: 10.1097/JTO.0b013e318288dc96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hald J, Overgaard J, Grau C. Evaluation of objective measures of smoking status—a prospective clinical study in a group of head and neck cancer patients treated with radiotherapy. Acta Oncol. 2003;42:154–159. doi: 10.1080/02841860310005020. [DOI] [PubMed] [Google Scholar]
- 32.Warren GW, Arnold SM, Valentino JP, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012;103:45–48. doi: 10.1016/j.radonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khuri FR, Kim ES, Lee J, et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2001;10:823–829. [PubMed] [Google Scholar]
- 34.Schnoll RA, Rothman RL, Lerman C, et al. Comparing cancer patients who enroll in a smoking cessation program at a comprehensive cancer center with those who decline enrollment. Head Neck. 2004;26:278–286. doi: 10.1002/hed.10368. [DOI] [PubMed] [Google Scholar]
- 35.Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458–464. doi: 10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]