Abstract
Tamoxifen (Tam) treatment is a first-line endocrine therapy for estrogen receptor α (ERα) positive breast cancer patients. Unfortunately, resistance frequently occurs and is often related with overexpression of the membrane tyrosine kinase receptor HER2. This is the rationale behind combined treatments with endocrine therapy and novel inhibitors that reduce HER2 expression and signaling and thus inhibit Tam-resistant breast cancer cell growth. In this study we show that activation of Farnesoid X Receptor (FXR), by the primary bile acid chenodeoxycholic acid (CDCA) or the synthetic agonist GW4064, inhibited growth of Tam-resistant breast cancer cells (termed MCF-7 TR1), which was used as an “in vitro” model of acquired Tam-resistance. Our results demonstrate that CDCA treatment significantly reduced both anchorage-dependent and anchorage–independent epidermal growth factor (EGF)-induced growth in MCF-7 TR1 cells. Furthermore, results from Western blot analysis and real-time RT-PCR revealed that CDCA treatment reduced HER2 expression and inhibited EGF-mediated HER2 and p42/44 MAPK phosphorylation in these Tam-resistant breast cancer cells. Transient transfection experiments, using a vector containing the human HER2 promoter region, showed that CDCA treatment down-regulated basal HER2 promoter activity. This occurred through an inhibition of NF-κB transcription factor binding to its specific responsive element located in the HER2 promoter region as revealed by mutagenesis studies, electrophoretic mobility shift assay and chromatin immunoprecipitation analysis.
Collectively, these data suggest that FXR ligand-dependent activity, blocking HER2/MAPK signaling, may overcome antiestrogen resistance in human breast cancer cells, and could represent a new therapeutic tool to treat breast cancer patients that develop resistance.
Keywords: FXR, Breast cancer, Tamoxifen resistance, HER2, NF-κB
Introduction
Administration of the selective estrogen receptor modulator Tamoxifen (Tam), to block ERα activity, is still a first-line endocrine therapy for the management of all stages of ERα-positive breast cancer patients (Fisher et al., 1998; Gradishar, 2004). Unfortunately, not all patients who have ERα-positive tumors respond to Tam (de novo resistance), and a large number of patients who do respond will eventually develop disease progression or recurrence while on therapy (acquired resistance), limiting the efficacy of the treatment.
Multiple mechanisms are responsible for the development of endocrine resistance. Among these are the loss of ERα expression or function (Encarnacion et al., 1993), alterations in the balance of regulatory cofactors, increased oncogenic kinase signaling (Blume-Jensen and Hunter, 2001), and altered expression of growth factor signaling pathways (Arpino et al., 2004; Schiff et al., 2004; Sabnis et al., 2005; Staka et al., 2005). For instance, a number of preclinical and clinical studies suggest that both de novo and acquired resistance to Tam in breast cancer cells can be associated with elevated levels of the membrane tyrosine kinase HER2 (c-ErbB2, Her2/neu) (Chung et al., 2002; Meng et al., 2004; Shou et al., 2004; Gutierrez et al., 2005).
The HER2 gene codes for a 185 kDa receptor, a member of the EGFR (HER1) family of transmembrane tyrosine kinases, which also includes HER3 and HER4, mainly involved in signal transduction pathways that regulate cell growth and differentiation. This receptor has no ligand of its own, but is activated by hetero-oligomerization with other ligand-activated receptors (Yarden, 2001). The HER2 gene is amplified and/or overexpressed in 20–25% of ERα-positive breast cancers (Slamon et al., 1989), and clinical observations indicate that tumors with high levels of HER2 have poor outcome when treated with Tam (Osborne et al., 2003; Kirkegaard et al., 2007).
The mechanisms by which HER2 overexpression mediates Tam resistance result from an intimate crosstalk between ERα and growth factor receptors intracellular kinase cascades, such as Ras/MAPK signaling, that in turn can promote growth and progression in breast cancer cells, negating the inhibitory effects of Tam on nuclear ERα activity (Arpino et al., 2008). Overexpression of the HER2 gene is not attributed solely to amplification of the HER2 gene copy number, but can also occur from a single-copy gene due to deregulation events at the transcriptional level (Hurst, 2001).
Thus an analysis of new mechanisms controlling HER2/neu receptor gene expression could be important to enhance strategies to reverse Tam resistance in breast cancer patients.
FXR, a member of the nuclear receptor superfamily of ligand-dependent transcription factors, is mainly expressed in the liver and the gastrointestinal tract where it regulates expression of genes involved in bile acids, cholesterol and triglyceride metabolism (Forman et al., 1995; Makishima et al., 1999; Parks et al., 1999). Recently this receptor was also detected in different nonenterohepatic compartments including breast cancer tissue and breast cancer cell lines (Bishop-Bailey, 2004; Swales et al., 2006; Journe et al., 2008; Catalano et al., 2010). For instance, FXR activation inhibits breast cancer cell proliferation and negatively regulates aromatase activity reducing local estrogen production (Swales et al., 2006), while other authors have reported that FXR activation stimulates MCF-7 cell proliferation but only in steroid-free medium (Journe et al., 2008). However, the functions of the bile acid sensor FXR in breast cancer tissue are still not completely understood, and there are no data regarding its role in the endocrine resistant breast cancer phenotype. Thus, we have investigated whether activated FXR may modulate the growth of human MCF-7 Tam-resistant breast cancer cells, a model which was developed to mimic “in vitro” the occurrence of acquired Tam-resistance.
Here we demonstrate that a specific FXR ligand chenodeoxycholic acid (CDCA) or its synthetic agonist GW4064 inhibited Tam-resistant breast cancer cell proliferation and EGF-induced growth, by reducing expression levels of the tyrosine kinase receptor HER2. This occurs through an FXR-mediated inhibition of NF-κB binding on the human HER2 promoter region.
Results
FXR expression in Tam-resistant breast cancer cells
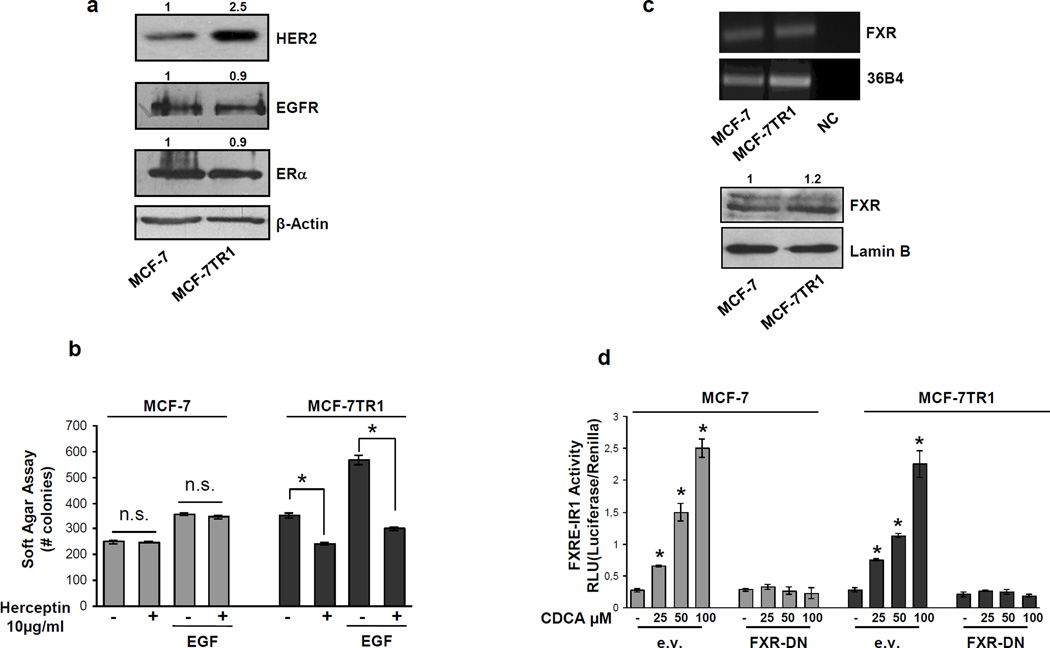
Acquired resistance to Tam has been associated with elevated levels of the membrane tyrosine kinase HER2 (Knowlden et al., 2003; Nicholson et al., 2004; Gutierrez et al., 2005). In agreement with these reports, we found a marked increase in the levels of total HER2 protein content in Tam-resistant MCF-7TR1 compared to MCF-7 cells, while no differences were seen in the expression of EGFR and ERα (Figure 1a). We therefore evaluated anchorage-independent growth of MCF-7 and MCF-7TR1 cells after treatment with Herceptin, a humanized monoclonal antibody directed against the extracellular domain of HER2. Cells were plated in soft agar, treated with EGF in the presence or absence of Herceptin and after 14 days colonies were counted (Figure 1b). Herceptin had no effect on MCF-7 growth while significantly inhibited anchorage-independent growth of MCF-7TR1 cells in basal conditions as well as upon EGF treatment. These data confirm that the HER2 overexpression found in the MCF-7TR1 cells rendering them more sensitive to the inhibitory effect of this selective HER2-targeted agent.
Figure 1.
FXR expression and activation in MCF-7 and MCF-7TR1 cells. (a) Western blot analysis of HER2, EGFR, ERα in total protein extracts from MCF-7 and MCF-7TR1 cells; β-Actin was used as loading control. (b) Soft Agar growth assay in MCF-7 and MCF-7TR1 cells plated in 0.35% agarose and treated with EGF 100ng/ml in the presence or absence of Herceptin (10µg/ml). After 14 days of growth colonies > 50 µm diameter were counted. n.s. (nonsignificant); *p<0.05 compared to vehicle or EGF.
(c) Total RNA was extracted from MCF-7 and MCF-7TR1 cells, reverse transcribed and cDNA was subjected to PCR using primers specific for FXR or 36B4 (upper panel); NC: negative control, RNA sample without the addition of reverse transcriptase. Nuclear proteins were extracted from MCF-7 and MCF-7TR1 and then western blotting analysis was performed using anti-FXR antibody. Lamin B was used as loading control (lower panel). (d) MCF-7 and MCF-7TR1 cells were transiently transfected with a FXR responsive reporter gene (FXRE-IR1) with either empty vector (e.v.) or FXR-DN (dominant negative) expression plasmid. After transfection cells were treated for 24h with vehicle (−) or increasing doses of CDCA (25–50–100µM) and then luciferase activity was measured. Results represent the mean ± SD of three different experiments each performed in triplicate. *p<0.05 compared to vehicle. Numbers on top of the blots represent the average fold change versus control of MCF-7 cells normalized for β-Actin.
Next, we evaluated the expression of FXR in MCF-7 and MCF-7TR1 breast cancer cells. Our results revealed the presence of FXR mRNA (as a band of 362 bp; Figure 1c, upper panel) and protein (as a band of ~60 kDa; Figure 1c, lower panel) in both MCF-7 and MCF-7TR1 cells. To assess the ability of FXR to be transactivated by the most effective FXR activator CDCA, we transiently transfected cells with a FXR-responsive reporter gene (FXRE-IR1) followed by treatment with increasing doses of CDCA. The specificity of the system was tested by cotransfecting the cells with a dominant negative FXR (FXR-DN) plasmid. As shown in Figure 1d, CDCA treatment induced a dose-dependent FXR activation in both cell lines and expression of the dominant negative FXR completely abrogated the CDCA-induced transactivation.
FXR activation inhibits Tam-resistant breast cancer cell growth
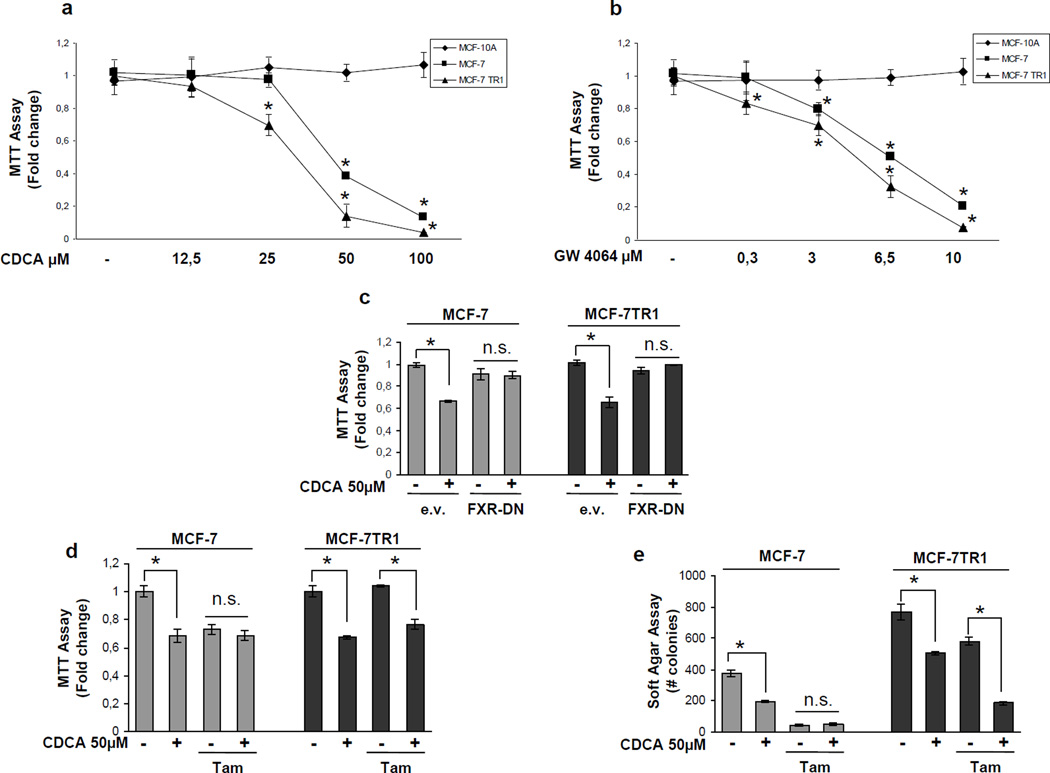
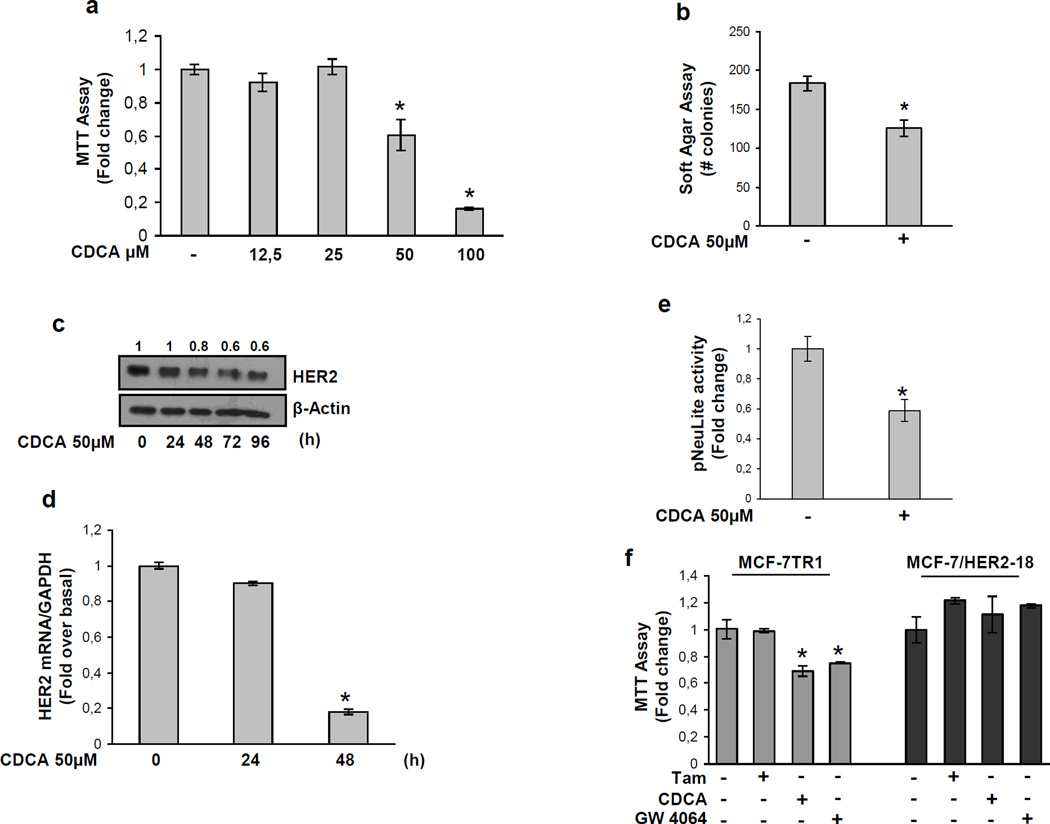
We examined, by MTT growth assays, the effects of increasing doses of CDCA and GW4064, a synthetic FXR agonist. Treatment with both ligands reduced cell proliferation in a dose-dependent manner in MCF-7 and in MCF-7TR1 cells, while had no effects on normal breast epithelial cells MCF-10A (Figure 2a and b). Similar results in growth inhibition were also obtained in another Tam-resistant breast cancer cell line termed MCF-7TR2 (Supplemental Figure 2a and b). It is worth note that the inhibitory effects exerted by activated FXR on cell proliferation were significant at lower dose in MCF-7TR1 cells compared to MCF-7 cells, as evidenced by IC50 values exhibited by MCF-7TR1 cells for both ligands (Table 1). The antiproliferative effects exerted by CDCA were completely reversed in the presence of a FXR-DN plasmid supporting the specific involvement of the FXR (Figure 2c).
Figure 2.
FXR ligands effects on breast cancer cells proliferation. MTT growth assays in MCF-10A, MCF-7 and MCF-7TR1 cells treated with vehicle (−) or increasing doses of CDCA (12,5–25–50–100 µM) (a) or GW4064 (0,3-3–6,5–10 µM) (b) for 7 days. Cell proliferation is expressed as fold change ± SD relative to vehicle treated cells, and is representative of three different experiments each performed in triplicate. (c) MCF-7 and MCF-7TR1 cells, transiently transfected with either empty vector (e.v.) or FXR-DN vector plasmids, were treated with vehicle (−) or CDCA 50µM for 4 days before testing cell viability using MTT assay. Results are expressed as fold change ± SD relative to vehicle treated cells, and are representative of three different experiments each performed in triplicate. (d) MTT growth assay in MCF-7 and MCF-7TR1 cells treated with vehicle (−) or CDCA 50µM in the presence or not of Tam 1µM for 4 days. Results are expressed as fold change ± SD relative to vehicle treated cells, and are representative of three different experiments each performed in triplicate. (e) Soft Agar growth assay in MCF-7 and MCF-7TR1 cells plated in 0.35% agarose and treated as above indicated. After 14 days of growth colonies > 50 µm diameter were counted. n.s. (nonsignificant); *p<0.05 compared to vehicle or Tam.
Table 1.
IC50 of CDCA and GW4064 for MCF-7 and MCF-7 TR1 cells on anchorage-dependent growth
| IC50 (µmol/L) CDCA |
95% confidence interval |
p | IC50 (µmol/L) GW 4064 |
95% confidence interval |
p | |
|---|---|---|---|---|---|---|
| MCF-7 | 46 | 42.2–50.1 | 6.04 | 5.44–6.70 | ||
| MCF-7 TR1 | 31 | 28.6–33.9 | <0.0001 | 4.47 | 3.6–5.49 | 0.008 |
Next we tested the effects of CDCA in the presence of Tam on cell growth (Figure 2d). As expected, with antiestrogen treatment cell viability was significantly reduced in MCF-7 cells, while MCF-7TR1 cells growth was unaffected, confirming the Tam-resistant phenotype. Interestingly, combined treatment with CDCA and Tam reduced growth of MCF-7TR1 cells compared to treatment with Tam alone, but showed no additive effects in MCF-7 cells (Figure 2d). The ability of CDCA and Tam to inhibit Tam-resistant growth was also confirmed using anchorage-independent growth assays (Figure 2e). These results suggest that FXR activation can interfere with the cellular mechanisms by which MCF-7TR1 cells escape antihormonal treatments.
CDCA reduces HER2 expression and signalling in MCF-7 TR1 cells
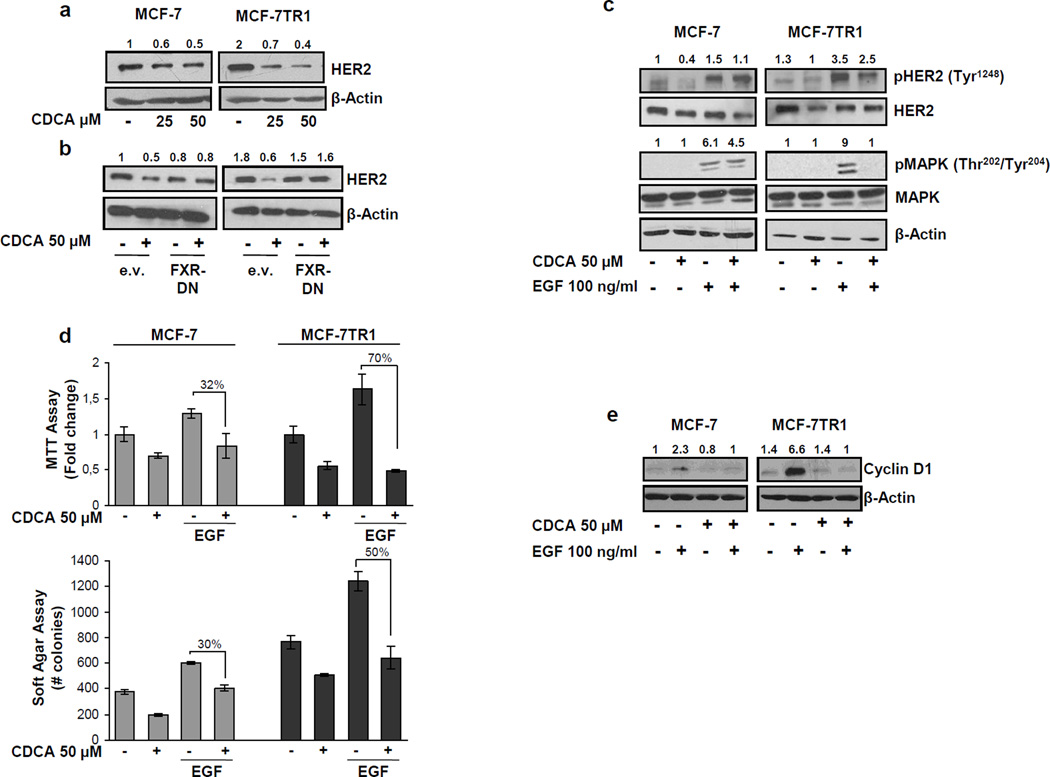
To understand the mechanisms associated with CDCA-mediated inhibition of Tam-resistant growth in breast cancer cells, we evaluated the possible role of FXR ligands in modulating HER2 expression. As shown in Figure 3a, treatment with CDCA at 25 and 50µM down-regulated HER2 protein expression in both cell lines, but with higher reduction seen in MCF-7TR1 cells. Similar results were also observed after treatment with GW4064 (data not shown). A reduction in HER2 levels was also found upon CDCA treatment in MCF-7TR2 cells (Supplemental Figure 2c). No differences were found in EGFR expression upon CDCA treatment (Supplemental Figure 3), confirming that activated FXR specifically target HER2 expression in breast cancer cells. In the presence of a FXR-DN expression vector the HER2 down-regulation was completely abrogated, confirming FXR involvement in CDCA-induced effects on HER2 (Figure 3b). Next, we questioned if these HER2 decreased levels could modify the responsiveness of breast cancer cells after growth factor stimulation. Thus we investigated the effects of short-term stimulation with EGF, in the presence of CDCA treatment, on phosphorylation levels of HER2 and MAPK, the main downstream effectors of the growth factor signalling pathway. EGF treatment increased phosphorylation of both HER2 and MAPK, even though in higher extent in MCF-7TR1 cells. However, pretraetment with CDCA reduced EGF-induced phosphorylation of HER2 in both cell lines and drastically prevented MAPK activation in MCF-7TR1 cells (Figure 3c). In addition, data obtained from MTT (Figure 3d upper panel) as well as soft agar (Figure 3d lower panel) growth assays, revealed that CDCA treatment inhibited EGF-induced growth by 70% in anchorage-dependent and 50% in anchorage-independent assays in MCF-7TR1 cells. CDCA was less effective in MCF-7 cells. These results well correlated with the down-regulatory effect of CDCA on EGF-induced cyclin D1 expression particularly in MCF-7TR1 cells (Figure 3e).
Figure 3.
Effects of CDCA on HER2 expression and its transduction pathways in MCF-7 and MCF-7TR1 cells. (a) MCF-7 and MCF-7TR1 cells were treated for 24h with vehicle (−) or CDCA 25 and 50µM before lysis. Equal amounts of total cellular extract were analyzed for HER2 levels by Western blotting. β-Actin was used as loading control. (b) Cells were transiently transfected with either empty vector (e.v.) or FXR-DN plasmids and then treated with vehicle (−) or CDCA 50µM for 24h and HER2 levels were evaluated by Western blotting. β-Actin was used as loading control. (c) Immunoblot analysis showing phosphorylated HER2 (pHER2 Tyr1248) and MAPK (pMAPK Thr202/Tyr204), total HER2, total MAPK in MCF-7 and MCF-7TR1 cells pretreated for 24h with CDCA 50µM and then treated for 10 min with EGF 100ng/ml. β-Actin was used as loading control. (d) MTT growth assay (upper panel) and soft agar assay (lower panel) in cells treated with CDCA 50µM with or without EGF 100ng/ml for 4 days and 14 days respectively. The MTT assay results are expressed as fold change ± SD relative to vehicle treated cells, and are representative of three different experiments each performed in triplicate. The soft agar assay values are represented as a mean of colonies number >50 µm diameter counted at the end of assay. Percentages of inhibition induced by CDCA versus EGF treatment alone are showed. (e) Cells were treated for 24h with vehicle (−) or EGF 100ng/ml in the presence or not of CDCA 50µM before lysis and then cellular extracts were analyzed for cyclin D1 levels by Western Blot analysis. β-Actin was used as loading control. Numbers on top of the blots represent the average fold change versus control of MCF-7 cells normalized for β-Actin.
Activated FXR inhibits the binding of NF-kB to HER2 promoter region
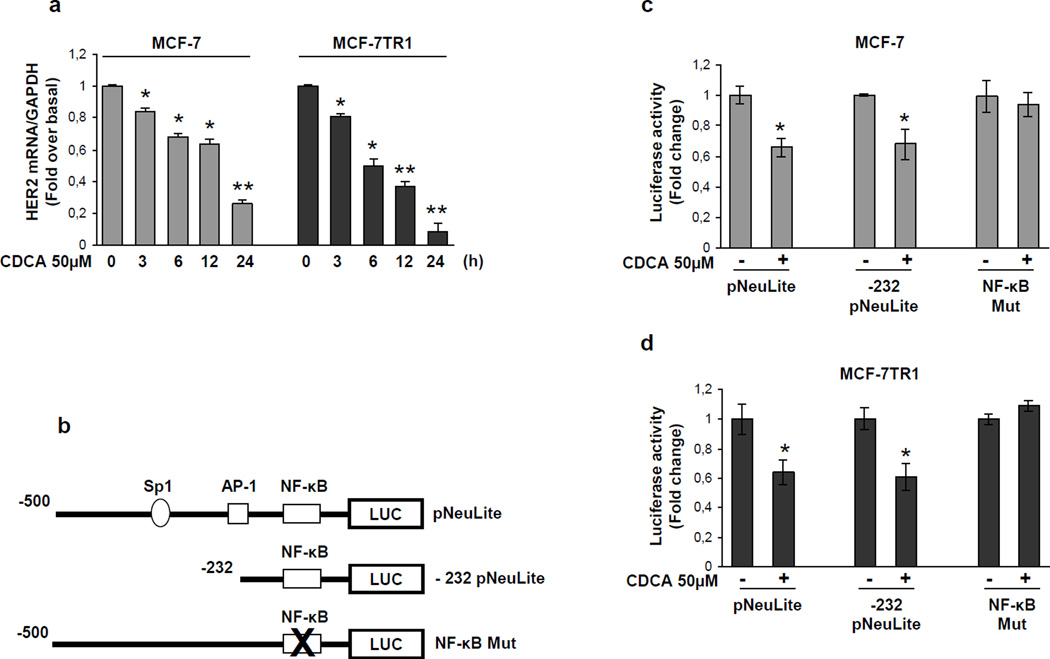
To explore if HER2 down-regulation relies on transcriptional mechanisms we evaluated, using real-time RT-PCR, HER2 mRNA levels after treatment with CDCA for different times. Exposure to CDCA exhibited a time-dependent reduction in HER2 mRNA levels in both MCF-7 and MCF-7TR1 cells (Figure 4a). Also, transcriptional activity of a reporter plasmid containing the human HER2 promoter region (pNeuLite) was significantly reduced with CDCA treatment in both cell lines (Figure 4c and d).
Figure 4.
Effects of CDCA on human HER2 promoter activity. (a) mRNA HER2 content, evaluated by real time RT-PCR, after treatment with vehicle or CDCA 50µM as indicated. Each sample was normalized to its GAPDH mRNA content. *p<0.05 and ** p<0.001 compared to vehicle. (b) Schematic map of the human HER2/neu promoter region constructs used in this study. All of the promoter constructs contain the same 3’ boundary. The 5’ boundaries of the promoter fragments varied from −500 (pNeuLite) to −232 (−232 pNeuLite). A mutated NF-κB binding site is present in NF-κB mut construct. HER2 transcriptional activity in MCF-7 (c) and MCF-7TR1 (d) cells transfected with promoter constructs are shown. After transfection, cells were treated in the presence of vehicle (−) or CDCA 50µM for 6h. The values represent the means ± SD of three different experiments each performed in triplicate. *p<0.05 compared to vehicle.
The human HER2 promoter contains multiple consensus sites for several transcription factors including Sp1, as well as AP-1 (activator protein-1) and NF-κB (nuclear factor-κB) the well known effectors of FXR transrepression (He et al., 2006; Vavassori et al., 2009) (Figure 4b). To indentify the region within the HER2 promoter responsible for CDCA inhibitory effects a HER2 promoter deleted construct (−232pNeuLite) activity was tested (Figure 4b). We observed that the responsiveness to CDCA was still maintained suggesting that the region from −232 to +1 containing the NF-κB motif might be involved in transrepression mechanisms exerted by activated FXR (Figure 4c and d). Thus we next performed site directed mutagenesis on the NF-κB domain (NF-κB Mut) within the HER2 promoter (Figure 4b). Mutation of this domain abrogated CDCA effects (Figure 4c and d). These latter results demonstrate that the integrity of NF-κB binding site is necessary for FXR modulation of HER2 promoter activity in breast cancer cells.
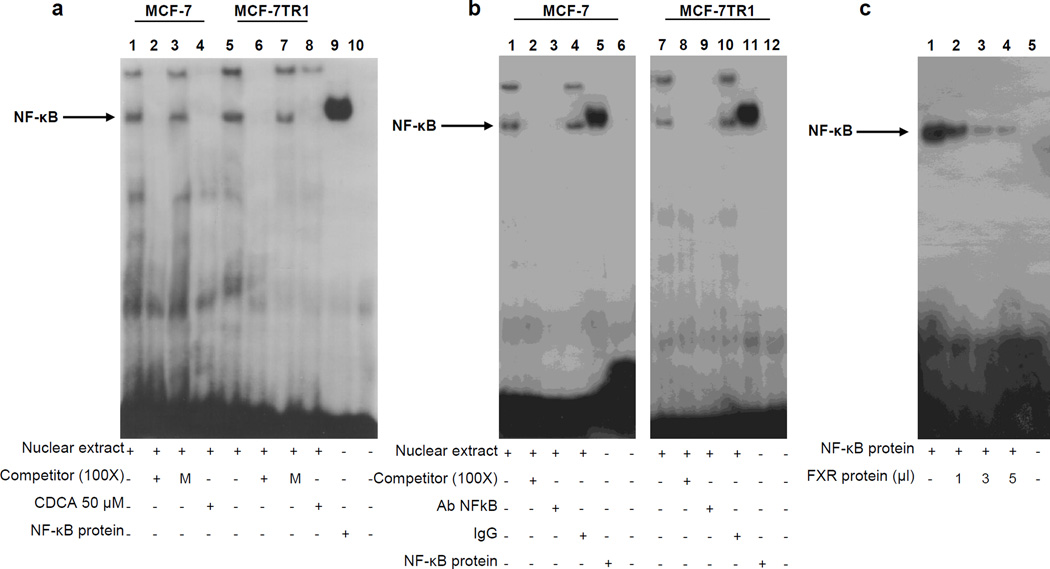
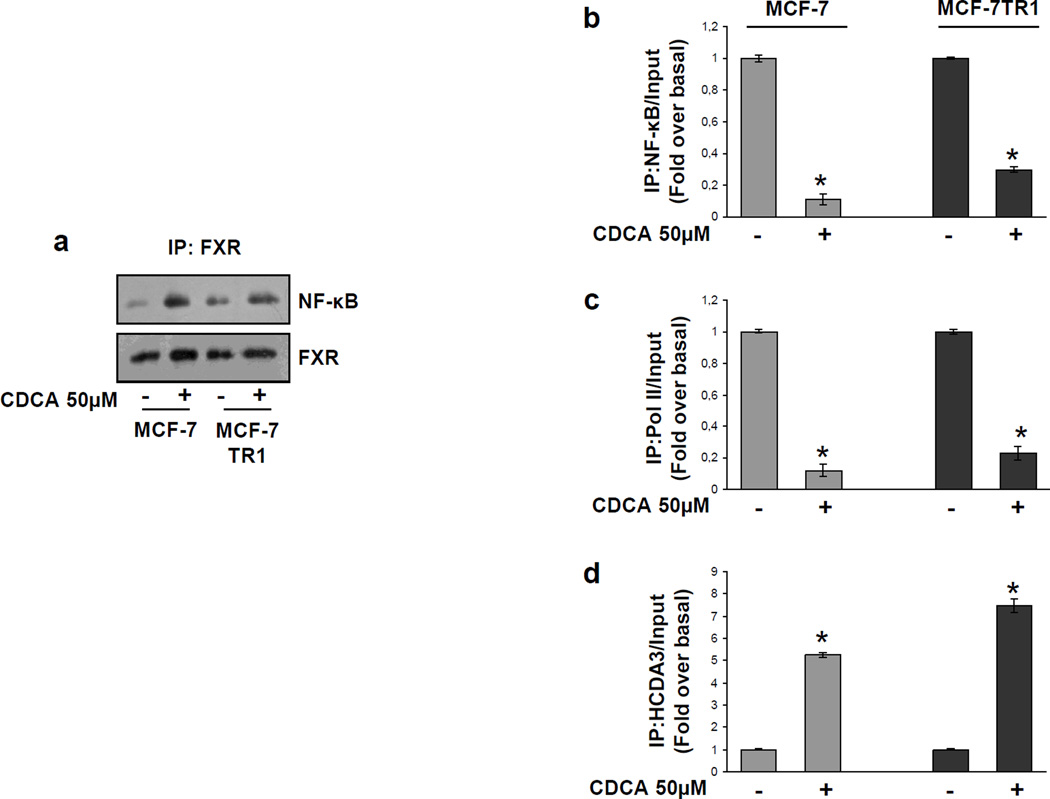
The specific role of the NF-κB motif in the transcriptional regulation of HER2 by CDCA was investigated using EMSA experiments. We observed the formation of a complex in nuclear extracts from MCF-7 and MCF-7TR1 cells, using synthetic oligodeoxyribonucleotides corresponding to the NF-κB motif (Figure 5a, lanes 1 and 5) which was abrogated by incubation with 100-fold molar excess of unlabeled probe (Figure 5a, lanes 2 and 6) demonstrating the specificity of the DNA binding complex. This inhibition was no longer observed when mutated oligodeoxyribonucleotide was used as competitor (Figure 5a, lanes 3 and 7). Interestingly, treatment with CDCA strongly decreased the DNA-binding protein complex compared with control samples (Figure 5a, lanes 4 and 8). The inclusion of an anti-NF-κB antibody in the reaction immunodepleted the specific band confirming the presence of NF-κB in the complex (Figure 5b, lanes 3 and 9). Non specific IgG did not affect NF-κB complex formation (Figure 5b, lanes 4 and 10). Recombinant NF-κB protein revealed a complex migrating at the same level as that of nuclear extracts from cells (Figure 5a, lane 9; Figure 5b, lanes 5 and 11). Of note the CDCA-induced reduction in the DNA-binding complex was no longer observed utilizing as probe synthetic oligodeoxyribonucleotides corresponding to the AP-1 and Sp1 motifs (Supplemental Figure 1a and b). To better define the role of FXR in the inhibition of NF-κB binding on HER2 promoter, a competition assay using recombinant NF-κB protein and increasing amounts of in vitro-translated FXR protein (1, 3 and 5µl) was carried out. A dose dependent reduction in the NF-κB complex was seen (Figure 5c, lanes 1–4), suggesting that physical interaction between these two transcription factors may inhibit the binding of NF-κB to human HER2 promoter region. To further test this possibility we performed coimmunoprecipitation studies using nuclear protein fractions from MCF-7 and MCF-7TR1 cells treated with CDCA. As shown in Figure 6a, the formation of a FXR and NF-κB complex was detected in untreated cells, and this association was enhanced with FXR ligand treatment.
Figure 5.
Electrophoretic mobility shift assay of the NF-κB binding site in the HER2 promoter region. (a) Nuclear extracts from MCF-7 and MCF-7TR1 cells were incubated with a double-stranded NF-κB specific sequence probe labeled with [γ32P]ATP and subjected to electrophoresis in a 6% polyacrylamide gel (lanes 1 and 5). Competition experiments were performed adding as competitor a 100-fold molar excess of unlabeled probe (lanes 2 and 6) or a 100-fold molar excess of unlabeled oligonucleotide containing a mutated NF-κB RE (lanes 3 and 7). Lanes 4 and 8, nuclear extracts from CDCA (50µM) treated MCF-7 and MCF-7TR1 cells respectively incubated with probe. Lane 9, NF-κB protein. Lane 10, probe alone. (b) Nuclear extracts from MCF-7 and MCF-7TR1 cells were incubated with a double-stranded NF-κB specific sequence probe labeled with [γ32P]ATP (lanes 1 and 7) or with a 100-fold molar excess of unlabeled probe (lanes 2 and 8). Nuclear extracts incubated with anti-NF-κB (lanes 3 and 9) or IgG (lanes 4 and 10). Lanes 5 and 11, NF-κB protein. Lanes 6 and 12, probe alone. (c) Lane 1, NF-κB protein. Lanes 2, 3 and 4, NF-κB protein incubated with increasing doses (1, 3 and 5 µl) of transcribed and translated in vitro FXR protein. Lane 5, probe alone.
Figure 6.
FXR inhibits NF-κB recruitment to HER2 promoter. (a) MCF-7 and MCF-7 TR1 cells were treated with vehicle (−) or CDCA 50µM for 1h before lysis. FXR protein was immunoprecipitated using an anti-FXR polyclonal antibody (IP:FXR) and resolved in SDS-PAGE. Immunoblotting was performed using an anti-NF-κB (p65 subunit) monoclonal antibody and anti-FXR antibody. MCF-7 and MCF-7TR1 cells were treated in the presence of vehicle (−) or CDCA 50 µM for 1h, then cross-linked with formaldehyde, and lysed. The precleared chromatin was immunoprecipitated with anti-NF-κB (b), anti-RNA Pol II (c) and anti-HDCA3 (d) antibodies. 5µl volume of each sample and input were analyzed by real time PCR using specific primers to amplify HER2 promoter sequence including the NF-κB site. Similar results were obtained in multiple independent experiments. * p<0.01 compared to vehicle.
Moreover, to confirm the involvement of NF-κB in CDCA-mediated HER2 down regulation at the promoter level, ChIP assays were performed. Using specific antibodies against NF-κB and RNA-polymerase II, protein-chromatin complexes were immunoprecipitated from cells cultured with or without CDCA for 1h. The resulting precipitated DNA was then quantified using real time PCR with primers spanning the NF-κB binding element in the HER2 promoter region. NF-κB recruitment was significantly decreased upon CDCA treatment in both cell lines (Figure 6b). This result was well correlated with a lower association of RNA-polymerase II to the HER2 regulatory region (Figure 6c).
To further confirm the transcriptional repression mediated by activated FXR, we also evaluated the histone deacetylase 3 (HDAC3) association on the NF-κB responsive sequence within the HER2 promoter. CDCA stimulation enhanced the recruitment of HDAC3 to this NF-κB promoter site (Figure 6d).
HER2 downregulation underlies the ability of FRX ligands to inhibit breast cancer cell growth
We evaluated the effects of CDCA on cell growth in the ERα-negative and HER2-overexpressing breast cancer cells SKBR3. Treatment with CDCA inhibited SKBR3 anchorage-dependent growth in a dose dependent manner (Figure 7a), and reduced colony growth in anchorage-independent assay (Figure 7b). Indeed, we found, after 48h of treatment with CDCA, a marked decrease in both HER2 protein and mRNA levels (Figure 7c and d). In these cells HER2 promoter activity was similarly reduced with CDCA treatment (Figure 7e).
Figure 7.
Effects of FXR ligand on SKBR3 breast cancer cells. (a) MTT proliferation assay of SKBR3 cells treated with vehicle (−) or increasing doses of CDCA (12,5–25–50–100 µM) for 7 days. Results are expressed as fold change ± SD relative to vehicle treated cells, and are representative of three different experiments each performed in triplicate. (b) Soft Agar growth assay in SKBR3 cells plated in 0.35% agarose and treated with vehicle (−) or CDCA 50 µM. After 14 days of growth colonies > 50 µm diameter were counted. (c) SKBR3 cells were treated as indicated with vehicle (−) or CDCA 50µM before lysis. Equal amounts of total cellular extract were analyzed for HER2 levels by Western blotting. β-Actin was used as loading control. Numbers on top of the blots represent the average fold change relative to control normalized for β-Actin. (d) mRNA HER2 content, evaluated by real time RT-PCR, after treatment with vehicle (−) or CDCA 50µM as indicated. Each sample was normalized to its GAPDH mRNA content. (e) SKBR3 cells were transiently transfected with pNeuLite construct. After transfection cells were treated in the presence of vehicle (−) or CDCA 50 µM for 24h and the promoter activity was evaluated. The values represent the means ± SD of three different experiments each performed in triplicate. * p<0.05 compared to vehicle. (f) MTT growth assay in MCF-7TR1 and MCF-7/HER2-18 cells treated with vehicle (−), CDCA 50µM and GW4064 3µM in the presence or not of Tam 1µM for 4 days. Results are expressed as fold change ± SD relative to vehicle treated cells, and are representative of three different experiments each performed in triplicate. * p<0.05 compared to Tam.
Finally, we explored the ability of FXR ligands to inhibit proliferation using as additional model Tam-resistant derivative cell line engineered to stably overexpress HER2 (MCF-7/HER2-18). As expected Tam-resistant growth in these cells was not affected by both CDCA and GW4064 treatments (Figure 7f). All together these results well evidence how FXR-mediated down-regulation of HER2 at transcriptional level is fully responsible for inhibiting breast cancer cell proliferation.
Discussion
In this study we show for the first time that the activated Farnesoid X Receptor down-regulates HER2 expression in ERα-positive breast cancer cells resistant to Tam. This occurs through the inhibition of NF-κB binding to its responsive element located in the human HER2 promoter region, and results in a significant reduction of Tam-resistant growth.
The HER2/neu transmembrane kinase receptor is a signalling amplifier of the HER family network, since activation of membrane tyrosine receptors (EGFR, HER3 and HER4) by their respective ligands determines the formation of homodimeric and heterodimeric kinase complexes into which this receptor is recruited as a preferred partner (Yarden, 2001). Multiple lines of evidences suggest a role of HER2 in the pathogenesis of breast carcinoma (Allred et al., 1992; Glockner et al., 2001), and clinical data suggest that breast tumors expressing elevated level of HER2 show a more aggressive phenotype and worse outcome when treated with Tam (Arpino et al., 2004; De Laurentiis et al., 2005). Thus, inhibitory agents targeting HER2, such as the monoclonal antibody trastuzumab (Herceptin), have been explored to improve hormonal treatment or delay emergence of endocrine resistance in estrogen-dependent breast tumors (Johnston, 2009). However, even though an increased response rate is obtained when trastuzumab is used in combination with chemotherapeutic agents (Seidman et al., 2001; Slamon et al., 2001) patients can still develop resistance (Slamon et al., 2001). All these observations highlight the importance of discovering new therapeutic tools interfering with HER2-driven signalling to overcome therapy resistance.
Here we have demonstrated that treatment of breast cancer cells resistant to Tam with the FXR natural ligand CDCA resulted in a reduction of HER2 protein expression. Similar results were also obtained in the ERα-negative and HER2-overexpressing SKBR3 breast cancer cells suggesting that it may represent a general mechanism not related to cell specificity. Moreover, it assumes more relevance in Tam-resistant breast cancer cells which are strongly dependent on HER2 activity for their growth. The complete abrogation of FXR-mediated HER2 down-regulation with expression of a FXR dominant negative vector, along with the effects exerted by the synthetic FXR agonist GW4064, clearly demonstrated that activated-FXR is involved in the regulation of HER2 expression. Furthermore, quantitative RT-PCR analysis demonstrated that HER2 mRNA levels were significantly decreased in both MCF-7 and MCF-7TR1 cells treated with CDCA, suggesting that the FXR-induced HER2 down-regulation arises via transcriptional mechanisms. Therefore, in our study we focused on the molecular mechanisms by which FXR mediates repression of HER2 gene expression, and on the biological consequences of FXR activation on antiestrogen-resistant growth of breast cancer cells.
FXR acts mainly by regulating the expression of target genes by binding either as a monomer or heterodimer with the retinoid X receptor (RXR) to FXR response elements (FXREs) (Laffitte et al., 2000; Ananthanarayanan et al., 2001; Claudel et al., 2002; Kalaany and Mangelsdorf, 2006;). Human HER2 promoter did not display any FXREs, thus it is reasonable to hypothesize that FXR induced down-regulation of HER2 promoter activity may occur through its interaction with other transcriptional factors. For instance, it has been described the trans-repression mechanisms for FXR-mediated inhibition of endothelin (ET)-1 expression in vascular endothelial cells (He et al., 2006). In addition, it has also been demonstrated that FXR negatively regulates IL-1β expression by stabilizing the nuclear corepressor NCoR on the NF-κB sequence within the IL-1β promoter (Vavassori et al., 2009). Several recognition elements are present within the HER2 proximal promoter (Ishii et al., 1987; Hurst, 2001) and among these functional motifs we have identified both AP-1 and NF-κB response elements as potential targets of FXR. We have demonstrated by functional studies and site specific mutagenesis analysis that the integrity of the NF-κB sequence is a prerequisite for the downregulatory effects of the FXR ligand on HER2 promoter activity. These results were supported by EMSA experiments which revealed a marked decrease in a specific DNA binding complex in nuclear extracts from MCF-7 and MCF-7TR1 cells treated with CDCA. In vitro competition studies showed that FXR protein was able to inhibit the binding of NF-κB to its consensus site on the HER2 promoter. Furthermore, we observed a reduced recruitment of both NF-κB and RNA polymerase II in CDCA treated cells, concomitant with an enhanced recruitment of HDAC3 supporting a negative transcriptional role for FXR in modulating HER2 expression.
The physiological relevance of these effects is pointed out by proliferation studies showing that FXR activation reduced breast cancer cell growth, but did not affect the proliferation of the nontumorogenic breast epithelial MCF-10A cell line. MCF-7TR1 cells exhibited lower IC50 values for both ligands compared with parental MCF-7 cells, suggesting an higher sensitivity of the Tam resistant cells to the effects of FXR ligands. This suggestion is also well supported by the results obtained from growth assays showing that combined treatment with CDCA and Tam significantly reduced Tam-resistant growth in MCF-7TR1 cells, compared to Tam alone, but had no additive effects in MCF-7 parental cells. Moreover, FXR ligands failed to inhibit tam-resistant growth in MCF-7/HER2-18 cells in which HER2 expression is not driven by its own gene promoter activity. These latter results provided evidences that the down-regulation of HER2 expression at transcriptional level underlies the ability of activated FXR to inhibit tam-resistant growth in breast cancer cells.
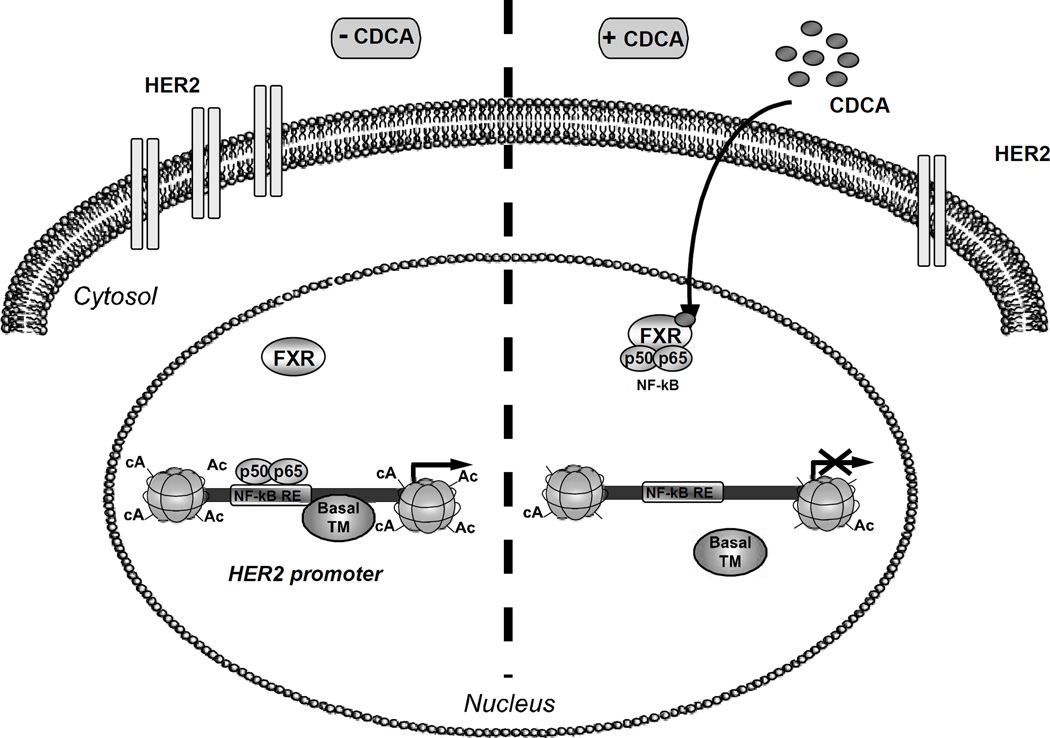
Previous in vitro studies showed that enhanced EGFR/HER2 expression together with activation of downstream signalling pathways such as p42/44 MAPK are involved in acquired Tam resistance (Knowlden et al., 2003; Nicholson et al., 2004). Our studies showed that CDCA treatment significantly reduced the ability of EGF to activate its signal transduction cascade in MCF-7TR1 cells, inhibiting both HER2 and MAPK phosphorylation. In addition, FXR activation was associated with a marked inhibition in EGF-induced growth, concomitant with a reduction in cyclin D1 expression in Tam resistant breast cancer cells. All together these data demonstrate, as graphically represented in Figure 8, that activated FXR, by preventing the binding of NF-κB to its response element located in the HER2 promoter sequence, abrogates HER2 expression and signalling, resulting in an inhibition of Tam resistant growth in breast cancer cells.
Figure 8.
Proposed working model of the FXR-mediated regulation of HER2 expression in Tam-resistant breast cancer cells. In the absence of CDCA, HER2 expression is regulated by several serum factors, including NF-κB, acting through a regulatory region in HER2 promoter and enabling gene transcription. Upon CDCA treatment, FXR binds NF-κB inhibiting its recruitment on the response element located in the proximal HER2 promoter, causing displacement of RNA polymerase II with consequent repression of HER2 expression.
Deciphering the molecular mechanisms responsible for the development of hormonal resistance is essential for establishing the most appropriate hormone agent according to tumor characteristics and for defining the optimal sequence of endocrine therapies. Moreover, this knowledge is critical for development of new therapeutic approaches able to either overcome or prevent endocrine resistance in breast cancer patients. Over the last years, significant survival benefits for breast cancer were derived from the use of combined treatment of endocrine therapies with new targeted therapies in endocrine responsive breast cancer (Johnston, 2009). In this scenario the sequencing or the combination of tamoxifen with FXR ligands may represent an important research issue to explore as an alternative therapeutic strategy to treat breast cancer patients whose tumors exploit HER2 signaling to escape Tam treatment.
Materials and Methods
Reagents and antibodies
DMEM, L-glutamine, penicillin, streptomycin, FBS, MTT, 4-Hydroxytamoxifen, CDCA and EGF were from Sigma (Milan, Italy). TRIzol by Invitrogen (Carlsbad, CA). FuGENE 6 by Roche (Indianapolis, IN). TaqDNA polymerase, RETROscript kit, Dual Luciferase kit, TNT master mix, and NF-κB protein were from Promega (Madison, WI). SYBR Green Universal PCR Master Mix by Biorad (Hercules, CA). Antibodies against FXR, β-actin, Cyclin D1, p65, ERα, EGFR and Lamin B by Santa Cruz Biotechnology (Santa Cruz, CA), MAPK, phosphorylated p42/44 MAPK (Thr202/Tyr204), phosphorylated HER2 (Tyr1248) from Cell Signaling Technology (Beverly, MA), HER2 from NeoMarker (Fremont, CA). ECL system and Sephadex G-50 spin columns from Amersham Biosciences (Buckinghamshire, UK). [γ32P]ATP from PerkinElmer (Wellesley, MA). Herceptin was form Genentech (San Francisco, CA).
Plasmids
The plasmid pNeuLite containing human HER2/neu promoter region was kindly provided by Dr. Mien-Chie Hung (University of Texas M.D. Anderson Cancer Center, Houston, TX, USA) (Xing et al., 2000). The FXR responsive reporter gene (FXRE-IR1) and FXR-DN (dominant negative) expression plasmids were provided from Dr. T.A. Kocarek (Institute of Environmental Health Sciences, Wayne State University, USA) (Kocarek et al., 2002).
The −232 pNeuLite construct was generated by PCR using as template the pNeuLite plasmid with the following primers: forward 5’-GATAAGTGTGAGAAC GGCTGCAGGC- 3’ and reverse 5’-GGGCAGATCTGGTTTTCCGGTCCCAATGGA- 3’. The amplified DNA fragment was digested with BglII and KpnI and ligated into pGL2-Basic vector. The desired deletion was confirmed by DNA sequencing.
Site-directed mutagenesis
The pNeuLite promoter plasmid-bearing NF-κB–responsive element mutated site (NF-κB mut) was created by site-directed mutagenesis using Quick Change kit (Stratagene, La Jolla, CA) according to manufacturer’s method. We used as template the pNeuLite plasmid and the following mutagenic primers (mutations are shown as lowercase letters): 5’- AGAGAGGGAGAAAGTGAAGCTaatcGTTGCCGACTCCCAGACTTCG- 3’ and 5’- CGAAGTCTGGGAGTCGGCAACgattAGCTTCACTTTCTCCCTCTCT- 3’. The desired mutation was confirmed by DNA sequencing.
Cell culture
MCF-7 cells were cultured in DMEM containing 10% FBS. MCF-7TR1 and MCF-7TR2 cells were generated in the laboratory of Dr. Fuqua as previously described (Vivacqua et al., 2009),and were routinely maintained with 10−6 M (MCF-7TR1) and 10−7 M (MCF-7 TR2) of 4-hydroxytamoxifen. SKBR3 cells were cultured in phenol red-free RPMI medium containing 10% FBS. MCF-10A normal breast epithelial cells were grown in DMEM-F12 medium containing 5% horse serum. MCF-7-C18 HER2 were kindly provided by Dr. Schiff (Baylor College of Medicine, Houston, TX, USA) and maintained as described (Shou et al., 2004). Before each experiment, cells were grown in phenol red-free medium, containing 5% charcoal-stripped FBS for 2 days and then treated as described.
Cell proliferation assays
Cell proliferation was assessed using MTT growth assay and soft agar anchorage-independent as described (Barone et al., 2009; Giordano et al., 2010). The IC50 values were calculated using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA) as described (Herynk et al., 2006). In a set of experiments cells were transiently transfected with the FXR-DN plasmid for 24h before starting with the treatments.
Immunoprecipitation and immunoblot analysis
Cells were treated as indicated before lysis for total protein extraction (Catalano et al., 2010). Nuclear extracts were prepared as described (Morelli et al., 2004). For coimmunoprecipitation experiments, we used 1 mg of nuclear protein extract and 2µg of FXR polyclonal antisera, followed by protein A/G precipitation. Equal amounts of cell extracts and coimmunoprecipitated protein were subjected to SDS-PAGE, as described (Catalano et al., 2010).
RT-PCR and Real-time RT-PCR assays
FXR gene expression was evaluated by the reverse transcription-PCR method using a RETROscript kit. The cDNAs obtained were amplified by PCR using the following primers: forward 5’-CGAGCCTGAAGAGTGGTACTGTC-3’ and reverse 5’-CATTCAGCCAACATTCCCATCTC-3’ (FXR); forward 5’-CTCAACATCTCC CCCTTCTC-3’ and reverse 5’- CAAATCCCATATCCTCGT -3’ (36B4).
The PCR was performed for 35 cycles for hFXR (94°C 1 min, 65°C 1 min, 72°C 1 min) and 18 cycles for 36B4 (94 °C for 1 min, 58 °C for 1 min, and 72 °C for 1 min) as described (Catalano et al., 2010).
Analysis of HER2 gene expression was performed by Real-time RT–PCR. Total RNA (2µg) was reverse transcribed with the RETROscript kit; 5µl of diluted (1:3) cDNA were analysed in triplicates by real-time PCR in an iCycler iQ Detection System (Bio-Rad, USA) using SYBR Green Universal PCR Master Mix following the manufacturer’s recommendations. Negative control contained water instead of cDNA was used. Each sample was normalized on its GAPDH mRNA content. Primers used for the amplification were: forward 5’-CACCTACAACACAGACACGTTTGA-3’ and reverse 5’-GCAGACGAGGGTGCAGGAT-3’ (HER2); forward 5’-CCCACTCCTCCACCTTTGAC-3’ and reverse 5’-TGTTGCTGTAGCCAAATTCGTT-3’ (GAPDH). The relative gene expression levels were calculated as described (Sirianni et al., 2007).
Transient transfection assays
MCF-7 and MCF-7TR1 cells were transiently transfected using the FuGENE 6 reagent with FXR reporter gene (FXRE-IR1) in the presence or absence of FXR-DN plasmid. In a set of experiments MCF-7, MCF-7TR1 and SKBR3 cells were transfected with different HER2 promoter constructs for 24h followed by treatments, and luciferase activity was assayed as described (Catalano et al., 2010).
Electrophoretic mobility shift assays (EMSA)
Nuclear extracts from cells, treated or not for 3h with CDCA 50µM, were prepared as previously described (Andrews and Faller, 1991). The DNA sequences used as probe or as cold competitors are the following (nucleotide motifs of interest are underlined and mutations are shown as lowercase letters): NF-κB, 5’-AAGTGAAGCTGGGAGTTGCCGACTCCCAGA-3’; mutated NF-κB, 5’-AAGTGAAGCTaatcGTTGCCGACTCCCAGA-3’; AP-1, 5’-AGGGGGCAGAGTCAC CAGCCTCTG-3’; mutated AP-1, 5’-AGGGGGCAtcaTCACCAGCCTCTG-3’; Sp1 5’-ATCCCGGACTCCGGGGGAGGGGGC-3’; mutated Sp1, 5’-ATCCCGGACCTCattG GGAGGGGGC-3’. In vitro transcribed and translated FXR protein was synthesized using the T7 polymerase in the rabbit reticulocyte lysate system. Probe generation and the protein-binding reactions were carried out as previously described (Catalano et al., 2010). For experiments involving anti-NF-κB (p65) antibody, the reaction mixture was incubated with this antibody at 4°C for 12h before addition of labelled probe.
Chromatin immunoprecipitation assays
Cells were treated with CDCA 50µM or left untreated for 1h and then DNA/protein complexes were extracted as previously described (Catalano et al., 2010). The precleared chromatin was immunoprecipitated with specific anti NF-κB (p65), anti HDAC3 or anti polymerase II antibodies. A normal mouse serum IgG was used as negative control. A 5µl volume of each sample and input DNA were used for real time PCR using the primers flanking NF-κB sequence in the human HER2 promoter region: 5’-TGAGAACGGCTGCAGGCAAC-3’ and 5’-CCCACCAACTGCATTCCAA-3’. Real-time PCR analysis was performed as described above. Final results were calculated using the ΔΔCt method, using input Ct values instead of the GAPDH mRNA. The basal sample was used as calibrator.
Statistical analyses
Each datum point represents the mean ± S.D. of three different experiments. Data were analyzed by Student’s t test using the GraphPad Prism 4 software program. P<0.05 was considered as statistically significant.
Supplementary Material
Acknowledgements
This work was supported by AIRC grants, MIUR Ex 60% 2009, PRIN 2009. NIH/NCI R01 CA72038 and RP101251 from the Cancer Prevention and Research Institute of Texas (SAWF).
References
- Allred DC, Clark GM, Molina R, Tandon AK, Schnitt SJ, Gilchrist KW, et al. Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992;23:974–979. doi: 10.1016/0046-8177(92)90257-4. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpino G, Green SJ, Allred DC, Lew D, Martino S, Osborne CK, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin Cancer Res. 2004;10:5670–5676. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone I, Cui Y, Herynk MH, Corona-Rodriguez A, Giordano C, Selever J, et al. Expression of the K303R estrogen receptor-alpha breast cancer mutation induces resistance to an aromatase inhibitor via addiction to the PI3K/Akt kinase pathway. Cancer Res. 2009;69:4724–4732. doi: 10.1158/0008-5472.CAN-08-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Bailey D. FXR as a novel therapeutic target for vascular disease. Drug News Perspect. 2004;17:499–504. doi: 10.1358/dnp.2004.17.8.863693. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- Catalano S, Malivindi R, Giordano C, Gu G, Panza S, Bonofiglio D, et al. Farnesoid X receptor, through the binding with steroidogenic factor 1-responsive element, inhibits aromatase expression in tumor Leydig cells. J Biol Chem. 2010;285:5581–5593. doi: 10.1074/jbc.M109.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH. Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer. 2002;97:306–312. doi: 10.1002/ijc.1614. [DOI] [PubMed] [Google Scholar]
- Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V, et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest. 2002;109:961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- Encarnacion CA, Ciocca DR, McGuire WL, Clark GM, Fuqua SA, Osborne CK. Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res Treat. 1993;26:237–246. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- Giordano C, Cui Y, Barone I, Ando S, Mancini MA, Berno V, et al. Growth factor-induced resistance to tamoxifen is associated with a mutation of estrogen receptor alpha and its phosphorylation at serine 305. Breast Cancer Res Treat. 2010;119:71–85. doi: 10.1007/s10549-009-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockner S, Lehmann U, Wilke N, Kleeberger W, Langer F, Kreipe H. Amplification of growth regulatory genes in intraductal breast cancer is associated with higher nuclear grade but not with the progression to invasiveness. Lab Invest. 2001;81:565–571. doi: 10.1038/labinvest.3780265. [DOI] [PubMed] [Google Scholar]
- Gradishar WJ. Tamoxifen--what next? Oncologist. 2004;9:378–384. doi: 10.1634/theoncologist.9-4-378. [DOI] [PubMed] [Google Scholar]
- Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, et al. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res. 2006;98:192–199. doi: 10.1161/01.RES.0000200400.55539.85. [DOI] [PubMed] [Google Scholar]
- Herynk MH, Beyer AR, Cui Y, Weiss H, Anderson E, Green TP, et al. Cooperative action of tamoxifen and c-Src inhibition in preventing the growth of estrogen receptor-positive human breast cancer cells. Mol Cancer Ther. 2006;5:3023–3031. doi: 10.1158/1535-7163.MCT-06-0394. [DOI] [PubMed] [Google Scholar]
- Hurst HC. Update on HER-2 as a target for cancer therapy: the ERBB2 promoter and its exploitation for cancer treatment. Breast Cancer Res. 2001;3:395–398. doi: 10.1186/bcr329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Imamoto F, Yamanashi Y, Toyoshima K, Yamamoto T. Characterization of the promoter region of the human c-erbB-2 protooncogene. Proc Natl Acad Sci U S A. 1987;84:4374–4378. doi: 10.1073/pnas.84.13.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SR. Enhancing the efficacy of hormonal agents with selected targeted agents. Clin Breast Cancer. 2009;9(Suppl 1):S28–S36. doi: 10.3816/CBC.2009.s.003. [DOI] [PubMed] [Google Scholar]
- Journe F, Laurent G, Chaboteaux C, Nonclercq D, Durbecq V, Larsimont D, et al. Farnesol, a mevalonate pathway intermediate, stimulates MCF-7 breast cancer cell growth through farnesoid-X-receptor-mediated estrogen receptor activation. Breast Cancer Res Treat. 2008;107:49–61. doi: 10.1007/s10549-007-9535-6. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, McGlynn LM, Campbell FM, Muller S, Tovey SM, Dunne B, et al. Amplified in breast cancer 1 in human epidermal growth factor receptor - positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res. 2007;13:1405–1411. doi: 10.1158/1078-0432.CCR-06-1933. [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Shenoy SD, Mercer-Haines NA, Runge-Morris M. Use of dominant negative nuclear receptors to study xenobiotic-inducible gene expression in primary cultured hepatocytes. J Pharmacol Toxicol Methods. 2002;47:177–187. doi: 10.1016/S1056-8719(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Laffitte BA, Kast HR, Nguyen CM, Zavacki AM, Moore DD, Edwards PA. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem. 2000;275:10638–10647. doi: 10.1074/jbc.275.14.10638. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci U S A. 2004;101:9393–9398. doi: 10.1073/pnas.0402993101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli C, Garofalo C, Sisci D, del Rincon S, Cascio S, Tu X, et al. Nuclear insulin receptor substrate 1 interacts with estrogen receptor alpha at ERE promoters. Oncogene. 2004;23:7517–7526. doi: 10.1038/sj.onc.1208014. [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JM. Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocr Relat Cancer. 2004;11:623–641. doi: 10.1677/erc.1.00778. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- Sabnis GJ, Jelovac D, Long B, Brodie A. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res. 2005;65:3903–3910. doi: 10.1158/0008-5472.CAN-04-4092. [DOI] [PubMed] [Google Scholar]
- Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331S–336S. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–2595. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- Sirianni R, Chimento A, Malivindi R, Mazzitelli I, Ando S, Pezzi V. Insulin-like growth factor-I, regulating aromatase expression through steroidogenic factor 1, supports estrogen-dependent tumor Leydig cell proliferation. Cancer Res. 2007;67:8368–8377. doi: 10.1158/0008-5472.CAN-06-4064. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Staka CM, Nicholson RI, Gee JM. Acquired resistance to oestrogen deprivation: role for growth factor signalling kinases/oestrogen receptor cross-talk revealed in new MCF-7X model. Endocr Relat Cancer. 2005;12(Suppl 1):S85–S97. doi: 10.1677/erc.1.01006. [DOI] [PubMed] [Google Scholar]
- Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006;66:10120–10126. doi: 10.1158/0008-5472.CAN-06-2399. [DOI] [PubMed] [Google Scholar]
- Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Lappano R, De Marco P, Sisci D, Aquila S, De Amicis F, et al. G protein-coupled receptor 30 expression is up-regulated by EGF and TGF alpha in estrogen receptor alpha-positive cancer cells. Mol Endocrinol. 2009;23:1815–1826. doi: 10.1210/me.2009-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X, Wang SC, Xia W, Zou Y, Shao R, Kwong KY, et al. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat Med. 2000;6:189–195. doi: 10.1038/72294. [DOI] [PubMed] [Google Scholar]
- Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(Suppl 2):1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.