Abstract
Context:
The advantages of allograft anterior cruciate ligament reconstruction (ACLR), which include shorter surgical time, less postoperative pain, and no donor site morbidity, may be offset by a higher risk of failure. Previous systematic reviews have inconsistently shown a difference in failure prevalence by graft type; however, such reviews have never been stratified for younger or more active patients.
Objective:
To determine whether there is a different ACLR failure prevalence of autograft compared with allograft in young, active patients.
Data Sources:
EMBASE, MEDLINE, Cochrane trials registry.
Study Selection:
Comparative studies of allograft versus autograft primary ACL reconstruction in patients <25 years of age or of high-activity level (military, Marx activity score >12 points, collegiate or semiprofessional athletes).
Study Design:
Systematic review with meta-analysis.
Level of Evidence:
Level 3.
Data Extraction:
Manual extraction of available data from eligible studies. Quantitative synthesis of failure prevalence and Lysholm score (outcomes in ≥3 studies) and I2 test for heterogeneity. Assessment of study quality using CLEAR NPT and Newcastle-Ottawa Scale (NOS).
Results:
Seven studies met inclusion criteria (1 level 1; 2 level 2, 4 level 3), including 788 patients treated with autograft tissue and 228 with various allografts. The mean age across studies was 21.7 years (64% male), and follow-up ranged between 24 and 51 months. The pooled failure prevalence was 9.6% (76/788) for autografts and 25.0% (57/228) for allografts (relative risk, 0.36; 95% CI, 0.24-0.53; P < 0.00001; I2 = 16%). The number needed to benefit to prevent 1 failure by using autograft was 7 patients (95% CI, 5-10). No difference between hamstrings autograft and patella tendon autograft was noted. Lysholm score was reported in 3 studies and did not differ between autograft and allograft.
Conclusion:
While systematic reviews comparing allograft and autograft ACLR have been equivocal, this is the first review to examine young and active patients in whom allograft performs poorly.
Keywords: anterior cruciate ligament reconstruction, allograft, autograft, young age, revision
While there is consensus that anterior cruciate ligament reconstruction (ACLR) is the best treatment to provide near normal laxity after anterior cruciate ligament (ACL) rupture in an active person, the superior graft choice, fixation method, and surgical technique continue to be debated. Autograft tissue continues to be the most common choice overall, with regional variations favoring bone–patella tendon–bone (BPTB) over hamstrings in some parts of North America17 and vice versa internationally.11 In contrast, the use of allograft tissue is less common. Allograft is preferred by 11% of surgeons in international survey11 and 22% in the United States.17
There are advantages and disadvantages to each graft choice. Disadvantages of using autograft tissue include donor site morbidity, such as weakness and loss of knee flexion with hamstring autograft,53 weakness of the quadriceps mechanism with BPTB,53 variable graft sizes with hamstring tendon,47 and patella fracture or anterior knee pain with BPTB.53 Potential advantages of decreased operative time, consistent graft sizes, and lack of donor site morbidity make allograft tissue an attractive option for surgeons. However, increased cost, delayed incorporation of allograft tissue as compared with autograft,68 and possible disease transmission54 are potential disadvantages.
One previous meta-analysis of level 2 and 3 studies comparing primary BPTB autograft and BPTB allograft ACLR found a 5.03 times higher odds of graft rupture for patients undergoing allograft ACLR. However, if irradiated or chemically processed allografts were excluded, they found no statistically significant difference.34 Another meta-analysis reported a 5% failure prevalence for autografts compared with a 14% failure prevalence for allografts (P < 0.01).63 Two systematic reviews comparing autograft with allograft ACLR did not find a statistically significant difference in failure prevalence between autograft and allograft ACLR.9,21 While some studies have reviewed failure prevalence of autograft ACLR and allograft ACLR in patients with a higher level of activity, until recently, there has not been a comparison of allograft and autograft ACLR in young patients.3,5 In a large prospective, multisite cohort study, Kaeding et al30 demonstrated a higher revision prevalence for allograft that was most clinically significant in younger patients. From these data, for example, a 14-year-old was estimated to have a 22% risk of revision with allograft compared with a 6.6% chance for autograft.
The purpose of the current systematic review is to determine whether there is a difference in failure prevalence between allograft and autograft ACLR in young and highly active patients.
Materials and Methods
Literature Search
A literature search of the EMBASE, MEDLINE, and Cochrane trials registry databases (from 1980 to the fourth week of October 2014) was conducted using keywords in combination “auto$”, “allo$”, and “anterior cruciate ligament” for EMBASE and “autog*”, “allog*”, and “anterior cruciate ligament” for MEDLINE and Cochrane. The only limit for the search was humans for all databases.
All titles and abstracts were reviewed, and if the study design was comparative and included any clinically relevant outcome (see criteria below), the full article was retrieved for the selection process. Systematic reviews from our search were retrieved, and their references were reviewed for any additional studies that could be included. An automatic alert option for MEDLINE was used that alerted the author by email if any articles were newly available through the database, which satisfied the search keywords in combination. This option was not available in EMBASE.
Eligibility Criteria
For inclusion, a study had to be a therapeutic study design comparing allograft with autograft isolated ACL reconstruction, and either prospective or retrospective (level of evidence [LOE] 1, 2, and 3). The primary outcome of the study had to be failure of ACLR with an acceptable definition such as revision, magnetic resonance imaging (MRI) confirmation of rupture, and Lachman 2+ or instrumented laxity measurement >5 mm side-to-side. Each study had to meet all inclusion criteria including: (1) appropriate study population (competitive athletes [active military, mean Marx score >12, varsity (college), semiprofessional, or professional] or patients <25 years old or stratified age groups for outcomes, if older patients included), (2) correct procedure (unilateral primary ACLR); (3) correct intervention being studied (autograft compared with allograft); (4) any relevant outcomes included (patient-reported outcomes, physical examination, reoperation, or failure); (5) minimum follow-up duration (2 years); and (6) minimum study size (15 patients in each treatment arm). Any study that failed to meet all of the above inclusion criteria was excluded. All case series (LOE 4) were excluded. Average follow-up of 2 years was not sufficient for inclusion. A study was also excluded if data from the same patients were included in another study with longer follow-up, in favor of the latter study. Abstracts presented at conferences but not published in peer-reviewed literature were also excluded. Concurrent meniscal or articular cartilage surgery was not an inclusion/exclusion criteria.
Study Selection
Two reviewers screened the titles and abstracts generated by the literature search for eligibility. If there was any uncertainty or ambiguity regarding eligibility, the study was included for full-text review. The reviewers independently assessed each full report to determine whether inclusion criteria were met. Disagreements were resolved by discussion with the senior author, when necessary. Journal, author name, and institution were not masked at any stage.
Data Extraction
Two reviewers extracted relevant data from each included study and recorded them into worksheet tables. Data collected in the worksheets included first author, journal and year in which the study was published, level of evidence, number of patients, follow-up duration, source of the autograft and allograft, allograft sterilization method if known, percentage of failures for each group, and study definition of graft failure. A comments section was included for any other relevant data particular to each study. All abstracted outcome data were entered into a meta-analysis software package (RevMan version 5.1; The Cochrane Collaboration) for pooled analysis.
Assessment of Risk of Bias in Eligible Studies
The checklist to evaluate a report of a nonpharmacological trial (CLEAR NPT8) was used to evaluate the quality of included randomized controlled trials (RCTs). The CLEAR NPT is a validated quality assessment tool used to examine the adequacy of 10 key elements of an RCT. The Newcastle-Ottawa Scale86 (NOS) was used to evaluate the quality of eligible prospective and retrospective cohort studies. The NOS assesses each study on 3 domains: selection, comparability, and outcome. Two reviewers independently assessed the methodological quality of eligible studies. Any disagreements were resolved with consensus discussion.
Statistical Analysis
Descriptive statistics were calculated with categorical data presented as frequency with percentages and continuous data as mean ± SD. Weighted means with their corresponding SDs were calculated for all parameters. Pooled risk ratios (RRs) were calculated for dichotomous outcomes, while mean differences were calculated for continuous outcomes. Ninety-five percent CIs were reported for all point estimates. The Cochrane χ2 test for homogeneity (ie, Q test, P < 0.10) was used to test for heterogeneity, while the I2 test was used to quantify heterogeneity.12 To assess for potential publication bias, we constructed a funnel plot for each outcome analyzed (see Appendix Figure 1, available at http://sph.sagepub.com/content/by/supplemental-data).
We pooled data from eligible studies using a random effects model because of the anticipated heterogeneity across studies with respect to surgical technique, allograft/autograft type, and allograft sterilization method. We planned an a priori subgroup analysis of graft failure prevalence based on sterilization method (irradiation vs no irradiation), autograft type (BPTB vs quadrupled hamstring [QHS]), and level of evidence (1 and 2 vs 3). In circumstances where only a median and interquartile range were provided by the study, established statistical methods were used to obtain imputed means and SDs.26 In one case, the author was contacted directly via email correspondence to provide SDs where imputation was not possible.2
Results
Literature Search
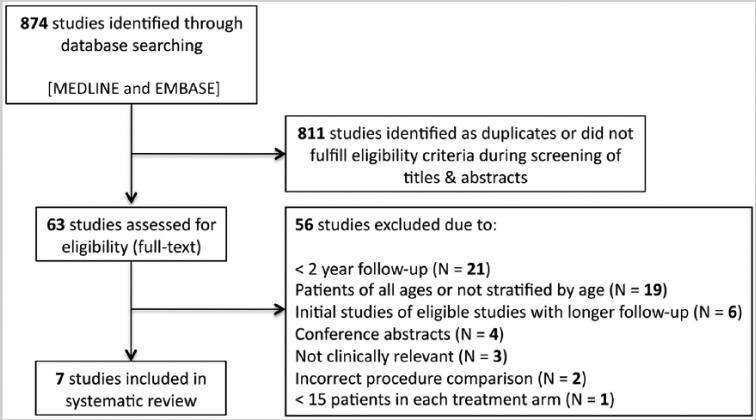
The computed literature search identified 874 studies for review (Figure 1). After review of each study title and/or abstract, 63 studies were retrieved for full-text review. Six studies were excluded because data from the same patients were published in other studies with longer duration of follow-up.4,6,22,69,72,77 Four studies were excluded because they were conference abstract presentations without peer-reviewed publication.44,57,71,74 Nineteen articles were excluded because they included patients of all ages and did not stratify their outcomes by age.5,10,18,23-25,32,38,42,49,56,61,62,66,76,78-80,83 Twenty-one studies were excluded because duration of follow-up was less than 2 years.1,13,14,31,35,39-41,43,51,52,58,65,70,73,75,84,87,89-91 Three studies were excluded because they did not report on failure (2 reported on muscle strength and 1 on transplantation safety).37,50,55 One study was excluded for not meeting the minimum treatment arm size of 15 patients.16 Two studies did not satisfy the correct procedure requirement.46,81 Seven studies fulfilled the inclusion criteria, including 1 RCT7 (level 1), 2 prospective cohort studies30,59 (level 2), and 4 retrospective cohort studies2,3,19,20 (level 3).
Figure 1.
Flow diagram summarizing the literature search, screening, and selection process.
General Study Characteristics
A total of 1016 participants were enrolled in the 7 eligible studies, including 463 treated with QHS autograft, 325 treated with BPTB autograft, and 228 treated with various allografts. All 7 studies that met inclusion criteria were conducted in the United States and enrolled patients undergoing ACLR between 1998 and 2012. Four studies involved a single surgeon.2,3,7,19 The mean age of participants across all studies was 21.7 years. Four of the studies only included patients younger than 25 years.2,19,20,59 Two studies included patients older than 25 years; however, the results were stratified by age.3,30 The last study had an average age of 28.6 years, but still met the criteria for inclusion as the study participants were military cadets.7 Patient sex was reported in 5 studies2,7,19,20,59 of the 7, among which 281 of 442 patients (64%) were men. The mean follow-up was reported for 4 studies2,7,19,20 and ranged from 24 to 51 months. The other 3 studies did not report a mean follow-up. All studies reported the graft failure prevalence after ACLR. The specific definitions used to identify graft failure in each included study are listed in Table 1, along with other baseline patient characteristics.
Table 1.
Baseline characteristics for all eligible studies
| First Author | Journal | Year | LOE | Sample Size (% ) | Minimum Follow-up, mo | Autograft Type | Allograft Type/Sterilization Method | Definition of Graft Failure | Autograft Failure, % | Allograft Failure, % | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Barber | Arthroscopy | 2014 | III | 81 (49) | 24 | BPTB | BPTB; no chemical processing or irradiation | Subsequent ACL revision surgery; 2+ Lachman; positive pivot-shift; side-to-side KT difference >5 mm | 9.4 | 7.1 | Allografts from MTF (Edison, NJ) |
| Barrett | AJSM | 2011 | III | 224 (NR) | 24 | BPTBQHS | Tibialis posterior or BPTB; not specified | 2+ Lachman; positive pivot shift; side-to-side difference >5 mm | BPTB: 11.8QHS: 25 | 29.2 | Did not comment on allograft type or sterilization processDivided based on age (< or >25 years) |
| Bottoni | OJSM | 2014 | I | 97 (89) | 120 | QHS | Tibialis posterior; aseptically processed and fresh frozen without terminal irradiation | Requiring ACL revision surgery | 8.3 | 26.5 | Allografts from MTF (Edison, NJ)US Military cadets |
| Ellis | Arthroscopy | 2012 | III | 79 (38) | 24 | BPTB | BPTB; patented BioCleanse formula (RTI Biologics) or 1.0-1.3 rad (AlloSource) | Requiring ACL revision surgery | 3 | 35 | Allografts from 2 separate tissue banks [failures] including: RTI Biologics [3/7] and AlloSource [4/7] |
| Engelman | AJSM | 2014 | III | 73 (55) | 24 | QHS | Tibialis anterior (n = 11) or tibialis posterior (n = 23) or peroneal tendon (n = 4); patented BioCleanse formula (RTI Biologics) or <2 mrad irradiation (JRF, AlloSource, MTF) | Requiring ACL revision surgery and/or MRI confirmed ACL graft failure | 11.43 | 28.95 | Allografts from 4 separate tissue banks (failures) including: RTI Biologics (8/11), AlloSource (2/11), JRF (0/11), MTF (1/11) |
| Kaeding | Sports Health | 2011 | II | 340 (NR) | 24 | QHS | Predominantly fresh frozen tibialis anterior, tibialis posterior, Achilles tendon, or BPTB; some irradiated <2.5 mrad | Requiring ACL revision surgery within 2 years of primary ACL reconstruction | 6.3 | 18.9 | Ad hoc analysis could not identify tissue bank, allograft type, or processing/irradiation status as a significant variable for retear Included revisions (10.3%) |
| Pallis | AJSM | 2012 | II | 122 (75) | 24 | BPTB QHS |
Unavailable | Requiring ACL revision surgery | BPTB: 11.5QHS: 13.3 | 43.8 | No information on allograft type or sterilizationUS Military cadets |
ACL, anterior cruciate ligament; AJSM, American Journal of Sports Medicine; BPTB, bone–patellar tendon–bone; JRF, Joint Restoration Foundation; LOE, level of evidence; MRI, magnetic resonance imaging; MTF, Musculoskeletal Tissue Foundation; NR, not reported; OJSM, Orthopaedic Journal of Sports Medicine; QHS, quadrupled hamstring.
Graft Choice and Treatment
Graft choice was decided by the patient, after a discussion with the surgeon regarding the risks and benefits of both options in 5 studies.2,3,19,20,30 However, 1 study mentioned that the authors did not recommend allografts to their patients prior to 2002, although they enrolled from 1998 to 2009.19 The study of military cadets59 did not comment on graft choice decision as the ACLR was performed prior to matriculation and therefore prior to enrollment in their study. In the RCT,7 patients were randomly assigned to treatment groups.
Two of the 7 included studies used fresh frozen allografts that did not undergo chemical processing or irradiation.2,7 Chemical processing using BioCleanse (RTI Biologics) or irradiation with <2 mrad20 or 1.0 to 1.3 rad19 was used in 2 studies. Another study30 predominantly used fresh frozen allografts; however, some patients were also treated with irradiated grafts (<2.5 mrad). The 2 remaining studies3,59 did not specify how allografts were treated.
Surgical Technique
Drilling of the femoral tunnel was carried out using the transtibial technique in 3 studies,2,3,7 the 2-incision rear-entry technique in 1 study,19 the anteromedial portal technique in 1 study,20 and a combination of techniques in 1 study.30 Participants had undergone ACLR prior to enrollment in 1 study and technique was not specified.59
Study Quality
The only RCT included in the current review reported adequate allocation concealment; however, there was some uncertainty regarding the generation of the allocation sequence and whether the intention-to-treat principle was used for statistical analysis. In general, the cohort studies (prospective and retrospective) had well-matched cohort and control groups (within studies). They were comparable with respect to important demographic variables (ie, age) and surgical technique (within studies). The complete results of the methodological quality assessment using the CLEAR NPT and NOS are presented in Tables 2 and 3, respectively.
Table 2.
Methodological quality assessment for the one eligible randomized controlled trial included in the study using the CLEAR NPT guidelines
| Primary Author (Study Year) |
CLEAR NPT Criterion* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Y/N/U | 2 Y/N/U | 3 Y/N/U | 4 Y/N/U | 5 Y/N/U | 6 Y/N/U | 7 Y/N/U | 8 Y/N/U | 9 Y/N/U | 10 Y/N/U | Explanation (if Needed) | |
| Bottoni (2014) | U | Y | Y | Y | Y | Y | Y | Y | Y | U | 1. No description of allocation sequence generation10. Uncertain whether intention-to-treat principle was followed |
CLEAR NPT, checklist to evaluate a report of a nonpharmacological trial; N, no; U, unclear; Y, yes.
1. Adequate generation of allocation sequence. 2. Treatment allocation concealed. 3. Details of each intervention available. 4. Expertise similar in each arm. 5. Participant adherence assessed. 6. Adequate participant blinding. 7. Care providers blinded. 8. Outcome assessors adequately blinded. 9. Similar follow-up between groups. 10. Used intention-to-treat analysis.
Table 3.
Methodological quality assessment of the 6 eligible (prospective and retrospective) included cohort studies using the Newcastle-Ottawa Scale
| Newcastle-Ottawa Scale Criterion |
||||||||
|---|---|---|---|---|---|---|---|---|
| Primary Author (Study Year) [Level of Evidence] | Selection |
Comparability |
Exposure/Outcome |
|||||
| 1 | 2 | 3 | 4 | 1 | 1 | 2 | 3 | |
| Barber (2014) [III] | * | * | * | * | * | 0 | * | * |
| Barret (2011) [III] | * | * | * | * | * | * | * | * |
| Ellis (2012) [III] | * | * | * | * | ** | 0 | * | * |
| Engelman (2014) [III] | * | * | * | * | ** | * | * | 0 |
| Kaeding (2011) [II] | * | * | * | * | ** | * | * | * |
| Pallis (2012) [II] | * | * | * | * | * | * | * | * |
Star (*) = item present. Maximum 1 star (*) for the Selection and Outcome components. Maximum 2 stars (**) for the Comparability component.
Primary Outcome
Failure
The overall pooled graft failure prevalence across all patients included in this review was 13.9% (133/1016). The pooled graft failure prevalence for patients undergoing QHS autograft, BPTB autograft, and allograft was 9.5% (44/463), 9.8% (32/325), and 25.0% (57/228), respectively. The combined failure prevalence of all autografts was 9.6% (76/788). A quantitative synthesis of all 7 studies demonstrated a statistically significant difference in the overall risk of graft failure favoring patients undergoing ACLR with autograft compared with allograft (7 studies; RR, 0.36; 95% CI, 0.24-0.53; P < 0.00001; I2 = 16%) (Figure 2). The number of patients undergoing ACLR who needed to be treated to benefit (NNTB) with autograft to prevent 1 episode of graft failure was 7 patients (95% CI, 5-10). The funnel plot did not demonstrate evidence of publication bias.
Figure 2.
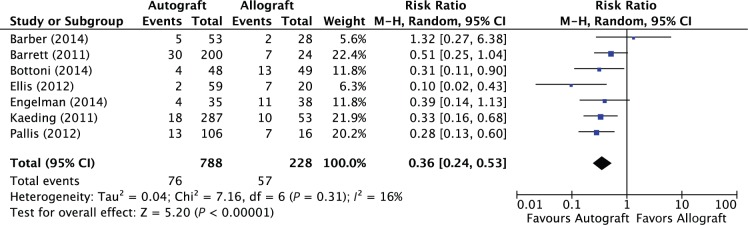
Forest plot illustrating results of the pooled analysis for graft failure prevalence in patients who underwent anterior cruciate ligament reconstruction using autograft versus allograft.
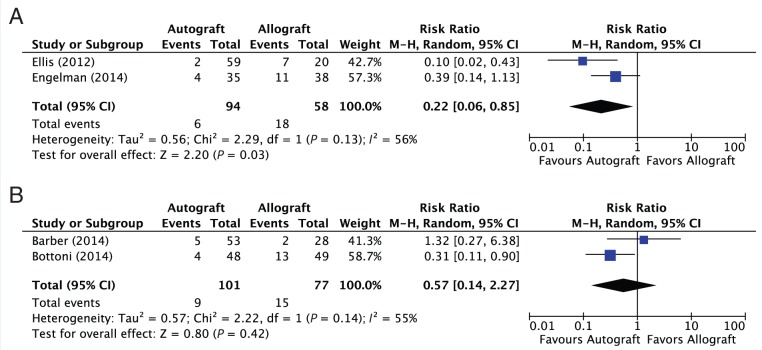
The prevalence of graft failure in patients receiving irradiated (low dose) versus nonirradiated allografts was 31.0% (18/58) and 19.5% (15/77), respectively. A subgroup analysis comparing graft failure prevalence between autografts and allografts with and without irradiation demonstrated a statistically significant difference favoring autografts over allografts with irradiation (2 studies; RR, 0.22; 95% CI, 0.06-0.85; P < 0.03; I2 = 56%) (Figure 3A). No statistically significant difference in graft failure was seen between autografts and allografts without irradiation (2 studies; RR, 0.57; 95% CI, 0.14-2.27; P < 0.42) (Figure 3B).
Figure 3.
(A) Forest plot depicting the pooled results for graft failure prevalence in patients who underwent anterior cruciate reconstruction using autograft versus irradiated allograft. (B) Forest plot depicting the pooled results for graft failure prevalence in patients who underwent anterior cruciate ligament reconstruction using autograft versus nonirradiated allograft.
An additional subgroup analysis was performed based on autograft type (QHS and BPTB) used versus allograft for ACLR. There was a statistically significantly difference in graft failure that favored the QHS (5 studies; RR, 0.42; 95% CI, 0.28-0.63; P < 0.0001; I2 = 7%) (see Appendix Figure 2A) and BPTB (4 studies; RR, 0.37; 95% CI, 0.17-0.81; P < 0.01; I2 = 57%) (see Appendix Figure 2B) groups when compared independently with allograft ACLR.
Last, there was a statistically significant difference in the overall graft failure prevalence when the results from level 1 or level 2 studies were pooled alone (3 studies; RR, 0.31; 95% CI, 0.19-0.49; P < 0.00001; I2 = 0%) (see Appendix Figure 3A) and when level 3 studies were pooled alone (4 studies; RR, 0.41; 95% CI, 0.18-0.93; P < 0.03; I2 = 51%) (see Appendix Figure 3B). We noted greater precision around the pooled point estimate of higher level studies (level 1 or 2).
Secondary Outcomes
Lysholm Score
A quantitative synthesis of the included studies did not demonstrate a statistically significant difference in the postoperative Lysholm scores among patients undergoing ACLR with an allograft compared with an autograft (3 studies; mean difference, 1.87 points; 95% CI, −0.44 to 4.18; P < 0.11). This is illustrated in Figure 4.
Figure 4.
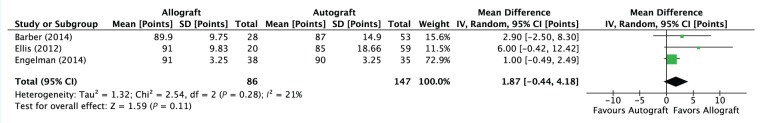
Forest plot summarizing the pooled analysis for mean difference in Lysholm score in patients who underwent anterior cruciate ligament reconstruction using autograft versus allograft.
Other Patient-Reported Outcomes
Although a formal quantitative synthesis could not be performed on the Tegner activity scale,7,19 International Knee Documentation Committee2,20 (IKDC), Single Assessment Numeric Evaluation7 (SANE), and Cincinnati score2 because of the small number of reporting studies, none individually reported a statistically significant outcome.
Discussion
This systematic review identified a clear difference in failure prevalence favoring primary ACLR performed with autograft tissue over allograft tissue in young (≤25 years of age) or highly active patients. The relationship was consistent whether all studies were included (level 3) or only those of highest quality, and demonstrated little publication bias. From these summary data, among patients younger than 25 years, for every 7 patients treated with autograft instead of allograft tissue, 1 failure would be prevented.
A lack of data among included studies on other outcomes, including patient-reported outcome measures, precluded meta-analysis of any outcome other than failure in our review. Three studies reported postoperative Lysholm scores, but quantitative synthesis of these data did not reveal any differences of statistical or clinical significance.
The earliest included study30 was published in 2011 and served as hypothesis-generating for this review. Those results have been confirmed with the inclusion of 6 subsequent studies, many of which were published in the most recent calendar year. Previously published meta-analyses comparing allograft and autograft ACLR, however, offer mixed conclusions.9,21,27,33,34,63,82,88 Although a higher rerupture prevalence for allograft was reported in some,34,63,88 no previous analysis used age stratification or age criteria for inclusion. Some authors of prior systematic reviews on this topic have noted this limitation in the literature.9,15,48 The small absolute difference30 in revision prevalence between allograft and autograft in older patients may explain why previous systematic reviews that have included studies of patients over a large age range produced mixed results.
There are potential confounding factors in the relationship between age and graft choice. Registry data28 have suggested that surgeons who use allograft tissue are more likely to be low volume and not fellowship trained. Although there is limited current evidence for a relationship between surgery volume and outcome in ACLR,45 precedence exists in other areas of orthopaedic surgery.64,85 The volume of surgeries performed at the centers in each of the included studies from this review is unknown.
One of the largest controversies in allograft ACLR relates to the treatment of the tissue. Some have suggested that the studies that have shown greater failure with allograft tissue either did not include sterilization method or used irradiated or chemical sterilization methods that could lead to higher failure. In our review, we noted a difference between irradiated allograft and autograft tissue that achieved statistical significance, and also a difference between nonirradiated grafts and autograft but that did not achieve statistical significance. Caution should be used in this interpretation, however, as this synthesis included a very small number of studies. Furthermore, the 2 studies included in this review that used irradiated grafts were small and both were level 3. Other literature has supported better clinical outcomes with irradiated grafts,60 and 1 recent systematic review of soft tissue grafts that was not stratified by age showed no difference between nonirradiated allografts and autograft ACLR.36 Considering the difference we have demonstrated between allograft and autograft in the young or highly active population, we believe the burden of proof remains on the fresh-frozen allograft user to demonstrate safety in a high-level clinical study.
Delayed revascularization and recellularization of allograft tissue in vivo may be one explanation for our study’s findings. Animal models have demonstrated delayed revascularization67 and poorer performance of allograft ACLR.29 Delayed revascularization has also been demonstrated in humans using contrast-enhanced MRI in allograft ACLR compared with autograft ACLR at 6-month follow-up.55,91
Disadvantages of this study must be considered, many of which relate to the available data on this topic. Our inclusion criteria was for young patients and those with high activity level, but not specifically for other factors that may increase risk of failure such as those with poor rehabilitation or muscular control. Two of the included studies7,19 used only revision as the definition of failure, and this may have biased the results. We acknowledge there is no consensus definition of failure.
Conclusion
The differences in failure prevalence that we observed between allograft and autograft reconstruction among young and highly active patients should provide caution to those involved in the orthopaedic care of these patients. There is a paucity of data in this patient population to determine whether this difference between autograft and allograft persists based on allograft sterilization methods.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Aslan A, Ozer O, Baydar ML, Yorgancigil H, Ozerdemoglu RA, Aydogan NH. Anterior cruciate ligament injuries: does surgical treatment with autograft versus allograft option affect the clinical results? [in Turkish]. Turk J Trauma EmergSurg. 2012;18:153-161. [DOI] [PubMed] [Google Scholar]
- 2. Barber FA, Cowden CH, 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone-patellar tendon-bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30:483-491. [DOI] [PubMed] [Google Scholar]
- 3. Barrett AM, Craft JA, Replogle WH, Hydrick JM, Barrett GR. Anterior cruciate ligament graft failure: a comparison of graft type based on age and Tegner activity level. Am J Sports Med. 2011;39:2194-2198. [DOI] [PubMed] [Google Scholar]
- 4. Barrett G, Stokes D, White M. Anterior cruciate ligament reconstruction in patients older than 40 years: allograft versus autograft patellar tendon. Am J Sports Med. 2005;33:1505-1512. [DOI] [PubMed] [Google Scholar]
- 5. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26:1593-1601. [DOI] [PubMed] [Google Scholar]
- 6. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case-control study. Am J Sports Med. 2009;37:2362-2367. [DOI] [PubMed] [Google Scholar]
- 7. Bottoni CR, Smith EL, Raybin SG, et al. Autograft vs allograft ACL reconstructions: a prospective, randomized clinical study with minimum 10 year follow-up. Orthop J Sports Med. 2014;2(suppl 2):2325967114S00043. [DOI] [PubMed] [Google Scholar]
- 8. Boutron I, Moher D, Tugwell P, et al. A checklist to evaluate a report of a nonpharmacological trial (CLEAR NPT) was developed using consensus. J Clin Epidemiol. 2005;58:1233-1240. [DOI] [PubMed] [Google Scholar]
- 9. Carey JL, Dunn WR, Dahm DL, Zeger SL, Spindler KP. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg Am. 2009;91:2242-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang SK, Egami DK, Shaieb MD, Kan DM, Richardson AB. Anterior cruciate ligament reconstruction: allograft versus autograft. Arthroscopy. 2003;19:453-462. [DOI] [PubMed] [Google Scholar]
- 11. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37:201-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256-266. [PubMed] [Google Scholar]
- 13. Collette M, Dupont B, Peters M. Reconstruction of the anterior cruciate ligament with a free graft of the patellar tendon: allograft versus autograft [in French]. Acta Orthop Belg. 1991;57(suppl 2):54-60. [PubMed] [Google Scholar]
- 14. Crawford DC, Hallvik SE, Petering RC, et al. Post-operative complications following primary ACL reconstruction using allogenic and autogenic soft tissue grafts: increased relative morbidity risk is associated with increased graft diameter. Knee. 2013;20:520-525. [DOI] [PubMed] [Google Scholar]
- 15. Cvetanovich GL, Mascarenhas R, Saccomanno MF, et al. Hamstring autograft versus soft-tissue allograft in anterior cruciate ligament reconstruction: a systematic review and meta-analysis of randomized controlled trials. Arthroscopy. 2014;30:1616-1624. [DOI] [PubMed] [Google Scholar]
- 16. Dahm DL, Wulf CA, Dajani KA, Dobbs RE, Levy BA, Stuart MA. Reconstruction of the anterior cruciate ligament in patients over 50 years. J Bone Joint Surg Br. 2008;90:1446-1450. [DOI] [PubMed] [Google Scholar]
- 17. Duquin TR, Wind WM, Fineberg MS, Smolinski RJ, Buyea CM. Current trends in anterior cruciate ligament reconstruction. J Knee Surg. 2009;22:7-12. [DOI] [PubMed] [Google Scholar]
- 18. Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res. 2008;466:2238-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellis HB, Matheny LM, Briggs KK, Pennock AT, Steadman JR. Outcomes and revision rate after bone-patellar tendon-bone allograft versus autograft anterior cruciate ligament reconstruction in patients aged 18 years or younger with closed physes. Arthroscopy. 2012;28:1819-1825. [DOI] [PubMed] [Google Scholar]
- 20. Engelman GH, Carry PM, Hitt KG, Polousky JD, Vidal AF. Comparison of allograft versus autograft anterior cruciate ligament reconstruction graft survival in an active adolescent cohort. Am J Sports Med. 2014;42:2311-2318. [DOI] [PubMed] [Google Scholar]
- 21. Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38:189-199. [DOI] [PubMed] [Google Scholar]
- 22. Gorschewsky O, Browa A, Vogel U, Stauffer E. Clinico-histologic comparison of allogenic and autologous bone-tendon-bone using one-third of the patellar tendon in reconstruction of the anterior cruciate ligament [in German]. Der Unfallchirurg. 2002;105:703-714. [DOI] [PubMed] [Google Scholar]
- 23. Gorschewsky O, Klakow A, Riechert K, Pitzl M, Becker R. Clinical comparison of the Tutoplast allograft and autologous patellar tendon (bone–patellar tendon–bone) for the reconstruction of the anterior cruciate ligament: 2- and 6-year results. Am J Sports Med. 2005;33:1202-1209. [DOI] [PubMed] [Google Scholar]
- 24. Guo L, Yang L, Duan XJ, et al. Anterior cruciate ligament reconstruction with bone–patellar tendon–bone graft: comparison of autograft, fresh-frozen allograft, and γ-irradiated allograft. Arthroscopy. 2012;28:211-217. [DOI] [PubMed] [Google Scholar]
- 25. Harner CD, Olson E, Irrgang JJ, Silverstein S, Fu FH, Silbey M. Allograft versus autograft anterior cruciate ligament reconstruction: 3- to 5-year outcome. Clin Orthop Relat Res. 1996;(324):134-144. [DOI] [PubMed] [Google Scholar]
- 26. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inacio MC, Paxton EW, Maletis GB, et al. Patient and surgeon characteristics associated with primary anterior cruciate ligament reconstruction graft selection. Am J Sports Med. 2012;40:339-345. [DOI] [PubMed] [Google Scholar]
- 29. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176-185. [DOI] [PubMed] [Google Scholar]
- 30. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katz LM, Battaglia TC, Patino P, Reichmann W, Hunter DJ, Richmond JC. A retrospective comparison of the incidence of bacterial infection following anterior cruciate ligament reconstruction with autograft versus allograft. Arthroscopy. 2008;24:1330-1335. [DOI] [PubMed] [Google Scholar]
- 32. Kleipool AE, Zijl JA, Willems WJ. Arthroscopic anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft or autograft. A prospective study with an average follow up of 4 years. Knee Surg Sports Traumatol Arthrosc. 1998;6:224-230. [DOI] [PubMed] [Google Scholar]
- 33. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41:2439-2448. [DOI] [PubMed] [Google Scholar]
- 34. Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:292-298. [DOI] [PubMed] [Google Scholar]
- 35. Kustos T, Balint L, Than P, Bardos T. Comparative study of autograft or allograft in primary anterior cruciate ligament reconstruction. Int Orthop. 2004;28:290-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29:1113-1122. [DOI] [PubMed] [Google Scholar]
- 37. Landes S, Nyland J, Elmlinger B, Tillett E, Caborn D. Knee flexor strength after ACL reconstruction: comparison between hamstring autograft, tibialis anterior allograft, and non-injured controls. Knee Surg Sports Traumatol Arthrosc. 2010;18:317-324. [DOI] [PubMed] [Google Scholar]
- 38. Lawhorn KW, Howell SM, Traina SM, Gottlieb JE, Meade TD, Freedberg HI. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28:1079-1086. [DOI] [PubMed] [Google Scholar]
- 39. Leal-Blanquet J, Alentorn-Geli E, Tuneu J, Valenti JR, Maestro A. Anterior cruciate ligament reconstruction: a multicenter prospective cohort study evaluating 3 different grafts using same bone drilling method. Clin J Sports Med. 2011;21:294-300. [DOI] [PubMed] [Google Scholar]
- 40. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26:41-49. [DOI] [PubMed] [Google Scholar]
- 41. Lephart SM, Kocher MS, Harner CD, Fu FH. Quadriceps strength and functional capacity after anterior cruciate ligament reconstruction. Patellar tendon autograft versus allograft. Am J Sports Med. 1993;21:738-743. [DOI] [PubMed] [Google Scholar]
- 42. Li H, Tao H, Cho S, Chen S, Yao Z, Chen S. Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: a comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am J Sports Med. 2012;40:1519-1526. [DOI] [PubMed] [Google Scholar]
- 43. Liu HG, Chen SX, Zhao CD, Ding LJ, Situ J. Comparative study on reconstruction of anterior cruciate ligaments with allografts and hamstring tendon under arthroscopy [in Chinese]. Zhongguo Gu Shang. 2008;21:267-269. [PubMed] [Google Scholar]
- 44. Lupetti E, Goretti C, Belluati A, Colombelli A. Allograft versus autograft in first planting ACL reconstruction. J Orthop Traumatol. 2011;12:S157. [Google Scholar]
- 45. Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91:2321-2328. [DOI] [PubMed] [Google Scholar]
- 46. Ma YT, Song SF, Zeng F, et al. Arthroscopic anterior and posterior cruciate ligament reconstruction: autograft versus allograft. J Clin Rehabil Tissue Eng Res. 2011;15:4726-4730. [Google Scholar]
- 47. Mariscalco MW, Flanigan DC, Mitchell J, et al. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) Cohort Study. Arthroscopy. 2013;29:1948-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mascarenhas R, Erickson BJ, Sayegh ET, et al. Is there a higher failure rate of allografts compared with autografts in anterior cruciate ligament reconstruction: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:364-372. [DOI] [PubMed] [Google Scholar]
- 49. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 suppl):S58-S66. [DOI] [PubMed] [Google Scholar]
- 50. Mauro CS, Irrgang JJ, Williams BA, Harner CD. Loss of extension following anterior cruciate ligament reconstruction: analysis of incidence and etiology using IKDC criteria. Arthroscopy. 2008;24:146-153. [DOI] [PubMed] [Google Scholar]
- 51. Mehta VM, Mandala C, Foster D, Petsche TS. Comparison of revision rates in bone-patella tendon-bone autograft and allograft anterior cruciate ligament reconstruction. Orthopedics. 2010;33:12. [DOI] [PubMed] [Google Scholar]
- 52. Moghtadaei M, Farahini H, Jahansouz A, Mokhtari T, Nabi R. Comparative study of treatment results for anterior cruciate ligament reconstruction with allograft and autograft. Shafa Orthop J. 2013;1(3):13-16. [Google Scholar]
- 53. Mohtadi NG, Chan DS, Dainty KN, Whelan DB. Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2011;(9):CD005960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16:559-565. [DOI] [PubMed] [Google Scholar]
- 55. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24:1038-1044. [DOI] [PubMed] [Google Scholar]
- 56. Noh JH, Yi SR, Song SJ, Kim SW, Kim W. Comparison between hamstring autograft and free tendon Achilles allograft: minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19:816-822. [DOI] [PubMed] [Google Scholar]
- 57. Otis D, Bava ED, Barber FA. A comparison of patellar tendon allograft to patellar tendon autograft anterior curciate ligament reconstruction in athletes under 25 years of age. Arthroscopy. 2012;28:e38. [Google Scholar]
- 58. Ozenci AM, Inanmaz E, Ozcanli H, et al. Proprioceptive comparison of allograft and autograft anterior cruciate ligament reconstructions. Knee Surg Sports Traumatol Arthrosc. 2007;15:1432-1437. [DOI] [PubMed] [Google Scholar]
- 59. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40:1242-1246. [DOI] [PubMed] [Google Scholar]
- 60. Park SS, Dwyer T, Congiusta F, Whelan DB, Theodoropoulos J. Analysis of irradiation on the clinical effectiveness of allogenic tissue when used for primary anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43:226-235. [DOI] [PubMed] [Google Scholar]
- 61. Peterson RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a 5-year follow-up. Arthroscopy. 2001;17:9-13. [DOI] [PubMed] [Google Scholar]
- 62. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21:774-785. [DOI] [PubMed] [Google Scholar]
- 63. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15:851-856. [DOI] [PubMed] [Google Scholar]
- 64. Ravi B, Jenkinson R, Austin PC, et al. Relation between surgeon volume and risk of complications after total hip arthroplasty: propensity score matched cohort study. BMJ. 2014;348:g3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rihn JA, Irrgang JJ, Chhabra A, Fu FH, Harner CD. Does irradiation affect the clinical outcome of patellar tendon allograft ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2006;14:885-896. [DOI] [PubMed] [Google Scholar]
- 66. Saddemi SR, Frogameni AD, Fenton PJ, Hartman J, Hartman W. Comparison of perioperative morbidity of anterior cruciate ligament autografts versus allografts. Arthroscopy. 1993;9:519-524. [DOI] [PubMed] [Google Scholar]
- 67. Scheffler SU, Schmidt T, Gangey I, Dustmann M, Unterhauser F, Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24:448-458. [DOI] [PubMed] [Google Scholar]
- 68. Scheffler SU, Unterhauser FN, Weiler A. Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16:834-842. [DOI] [PubMed] [Google Scholar]
- 69. Shelton WR, Papendick L, Dukes AD. Autograft versus allograft anterior cruciate ligament reconstruction. Arthroscopy. 1997;13:446-449. [DOI] [PubMed] [Google Scholar]
- 70. Shino K, Nakata K, Horibe S, Inoue M, Nakagawa S. Quantitative evaluation after arthroscopic anterior cruciate ligament reconstruction. Allograft versus autograft. Am J Sports Med. 1993;21:609-616. [DOI] [PubMed] [Google Scholar]
- 71. Song EK, Seon JK, Kim H. Prospective comparative study of ACL reconstruction between using hamstring autograft and soft tissue allograft. Orthop J Sports Med. 2014;2(suppl 2):2325967114S00118. [Google Scholar]
- 72. Spindler KP, Huston LJ, Wright RW, et al. The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med. 2011;39:348-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stringham DR, Pelmas CJ, Burks RT, Newman AP, Marcus RL. Comparison of anterior cruciate ligament reconstructions using patellar tendon autograft or allograft. Arthroscopy. 1996;12:414-421. [DOI] [PubMed] [Google Scholar]
- 74. Stuart K, Getelman MH. ACL reconstruction in patients under age 25: outcome and failure rates of autograft and allograft (SS-11). Arthroscopy. 2013;29(6):e6. [Google Scholar]
- 75. Sun K, Tang JW, Xu Q, et al. A prospective study of the anterior cruciate ligament reconstruction: allograft versus autograft [in Chinese]. Zhonghua Wai Ke Za Zhi. 2004;42:989-992. [PubMed] [Google Scholar]
- 76. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17:464-474. [DOI] [PubMed] [Google Scholar]
- 77. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. ACL reconstruction with BPTB autograft and irradiated fresh frozen allograft. J Zhejiang Univ Sci B. 2009;10:306-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone–patellar tendon–bone autograft versus allograft. Arthroscopy. 2009;25:750-759. [DOI] [PubMed] [Google Scholar]
- 79. Sun K, Zhang J, Wang Y, et al. Arthroscopic anterior cruciate ligament reconstruction with at least 2.5 years’ follow-up comparing hamstring tendon autograft and irradiated allograft. Arthroscopy. 2011;27:1195-1202. [DOI] [PubMed] [Google Scholar]
- 80. Sun K, Zhang J, Wang Y, et al. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: a prospective, randomized controlled study. Am J Sports Med. 2011;39:1430-1438. [DOI] [PubMed] [Google Scholar]
- 81. Tian S, Zhang J, Wang Y, et al. A prospective study on anterior cruciate ligament reconstruction with patellar tendon autograft versus gamma irradiated allograft [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24:282-286. [PubMed] [Google Scholar]
- 82. Tibor LM, Long JL, Schilling PL, Lilly RJ, Carpenter JE, Miller BS. Clinical outcomes after anterior cruciate ligament reconstruction: a meta-analysis of autograft versus allograft tissue. Sports Health. 2010;2:56-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Victor J, Bellemans J, Witvrouw E, Govaers K, Fabry G. Graft selection in anterior cruciate ligament reconstruction—prospective analysis of patellar tendon autografts compared with allografts. Int Orthop. 1997;21:93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang JS, Bai LH, Wang Y, Wang GB, He M. Comparative research of anterior cruciate ligament reconstruction with allo- or auto-graft hamstring. J Dalian Med Univ. 2010;32:422-425. [Google Scholar]
- 85. Wasserstein D, Henry P, Kreder H, Paterson M, Jenkinson R. Risk factors for re-operation and mortality following the operative treatment of tibial plateau fractures in Ontario, 1996-2009 [published online September 17, 2014]. J Orthop Trauma. 10.1097/BOT.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 86. Wells GA, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed March 5, 2015.
- 87. Yang L, Guo L, Dai C, et al. Bone–patellar tendon–bone graft in anterior cruciate ligament reconstruction: allograft versus autograft [in Chinese]. Zhonghua Wai Ke Za Zhi. 2007;45:82-85. [PubMed] [Google Scholar]
- 88. Yao LW, Wang Q, Zhang L, et al. Patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Eur J Orthop Surg Traumatol. 2015;25:355-365. [DOI] [PubMed] [Google Scholar]
- 89. Yi Long Z, Ning L, Zhi-Huai L, et al. Hamstring tendon autograft versus tendon allograft for reconstruction of anterior cruciate ligament. J Clin Rehabil Tissue Eng Res. 2011;15:5743-5746. [Google Scholar]
- 90. Zhang L, Liu JS, Sun J, Li ZY, Ma J. Comparison of the clinical outcome of anterior cruciate ligament reconstruction using allograft anterior tibialis and autologous hamstring tendon [in Chinese]. Zhongguo Gu Shang. 2009;22:166-169. [PubMed] [Google Scholar]
- 91. Zheng XF, Huang HY, Zhang Y, Li PY, Yin QS. Arthroscopic anterior cruciate ligament reconstruction using bone-patellar tendon-bone allograft, bone–patellar tendon–bone autograft and semitendinosus tendon autograft: a comparison of therapeutic effects. J Clin Rehabil Tissue Eng Res. 2009;13:3903-3906. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.