Abstract
Heart failure constitutes a significant source of morbidity and mortality in the United States, and its incidence and prevalence continue to grow, increasing its burden on the health care system. Renal dysfunction in patients with heart failure is common and has been associated with adverse clinical outcomes. This complex interaction is characterized by a pathophysiological disequilibrium between the heart and the kidney, in which cardiac malfunction promotes renal impairment, which in turn feeds back, resulting in further deterioration of cardiovascular function. Multiple neurohumoral and hemodynamic mechanisms are involved in this cardiorenal dyshomeostasis, including resistance to compensatory cardiac natriuretic peptides, leading to sodium retention, volume overload, and organ remodeling. Previous studies in animal models of heart failure have demonstrated that renal denervation promotes a robust natriuresis and diuresis as well as increased response of endogenous and exogenous natriuretic agents. With the recent development of minimally invasive renal denervation in humans, it is possible to suggest that this technique may become effective and important in the management of renal sodium and water metabolism in heart failure.
Keywords: natriuresis, diuresis, natriuretic peptides, sympathetic nervous system
Introduction
Approximately 5.1 million Americans suffer from heart failure, and over 800,000 new cases are diagnosed each year in the United States. Its incidence approaches 10 per 1000 in those older than 65 years of age and accounts for 1 million hospitalizations and 3 million office visits annually.1 In patients diagnosed with heart failure, sudden cardiac death occurs at 6–9 times the rate of the general population. One in eight deaths have heart failure mentioned on their death certificate, accounting for over 300,000 deaths annually.1 The direct and indirect costs of this epidemic of heart failure in the United States in 2012 approximated $30 billion, and it is anticipated that these costs will continue to rise over the next decade.1 Globally, the demographics are similar, with an estimated 5.7 million diagnosed with heart failure each year.2 The average prevalence of heart failure worldwide is 2% to 2.5% and increases to more than 10% in octogenarians.3
Pathophysiologic Considerations in Heart Failure
Myocardial injury and/or changes in left ventricular loading leads to activation of the sympathetic nervous system (SNS), resulting in increased availability of norepinephrine at the adrenergic nerve endings via increased production and decreased uptake.4 This response is primarily designed to enhance cardiac output via increase in myocardial contractility and heart rate. Furthermore, this catecholamine surge also leads to systemic vasoconstriction, leading to maintenance of systemic blood pressure and restoration of left ventricular filling. At the level of the kidneys, this sympathetic excess leads to activation of the renin–angiotensin–aldosterone system (RAAS), renal arterial vasoconstriction, and increased proximal tubular sodium reabsorption.5 In the long term, this chronic sympathetic stimulus promotes volume overload, abnormal left ventricular remodeling, and worsening of cardiac function.4 With progressive reduction in cardiac performance and subsequent renal underperfusion, cardiorenal dyshomeostasis ensues.6 This progressive dysregulation is secondary to multiple factors acting in concert. These include hemodynamic derangements with increased renal venous congestion,7,8 leading to a reduction in the arteriovenous pressure gradient across the kidney, as well as decrements in mean arterial pressure and renal perfusion pressure, all of which tend to promote glomerular underfilling and consequent reductions in glomerular filtration rate. Concomitantly, there is the pathophysiologic activation of various neurohumoral mechanisms, which promote marked sodium and water retention, volume overload, and adverse cardiovascular and renal remodeling.6 Under these conditions, a critical factor contributing to progressive cardiorenal dysfunction is the reduced renal action of compensatory cardiac natriuretic peptides, which are activated to maintain sodium and water hemostasis.6 This phenomenon is related to important counter-regulatory factors including the RAAS, antidiuretic hormone, endothelin, and various other inflammatory and vasoactive substances.6 Prominent among these mechanisms is the excessive stimulation of the systemic and renal SNS.5,9–11 This review focuses on the role of renal nerves in sodium water homeostasis in heart failure and renal denervation as a potential novel therapeutic modality in cardiac dysfunction.
Functional Role of the Renal Nerves
Afferent and efferent renal nerves, which are localized within the adventitia of the renal arteries, are involved in the physiologic and pathophysiologic regulation of systemic hemodynamics and renal function.5,9–11 The afferent renal fibers are activated by mechano-and chemosensitive receptors within the kidney,5,11 and their signals, in turn, are integrated in the midbrain for the overall control of systemic and regional efferent sympathetic tone.5,11
In the kidney, the efferent innervation involves the renal vasculature, the renal tubules, and the juxtaglomerular cells, all of which actively control renal hemodynamics, tubular sodium reabsorption, and the secretion of renin, for the overall regulation of sodium and water balance.11 Through these three mechanisms, renal sympathetic excess in heart failure promotes marked sodium and water retention and progressive cardiovascular and renal deterioration.5,6,10,11
Renal Denervation in Experimental Heart Failure
Studies by Villarreal et al.9 have examined the effects of bilateral renal denervation, or sham denervation, on postprandial sodium excretion in conscious dogs with experimental high-output heart failure produced by a large infrarenal arteriovenous (AV) fistula (Fig. 1). After ingestion of a meal containing 125 mg of sodium, the total sodium excretion and the factional sodium excretion were strikingly twofold higher in the renal denervated AV fistula dogs compared to the sham-denervated controls (Fig. 1). The plasma levels of cardiac natriuretic peptides and plasma renin activity (Fig. 2), as well systemic hemodynamics (data not shown), were similar in the two subsets of animals. In a different investigation in the AV fistula canine model, it was also demonstrated that in contrast to dogs with intact renal nerves, chronic renal denervation was associated with a significantly increased natriuresis and diuresis in response to acute infusion of synthetic cardiac natriuretic peptides.10
Figure 1.
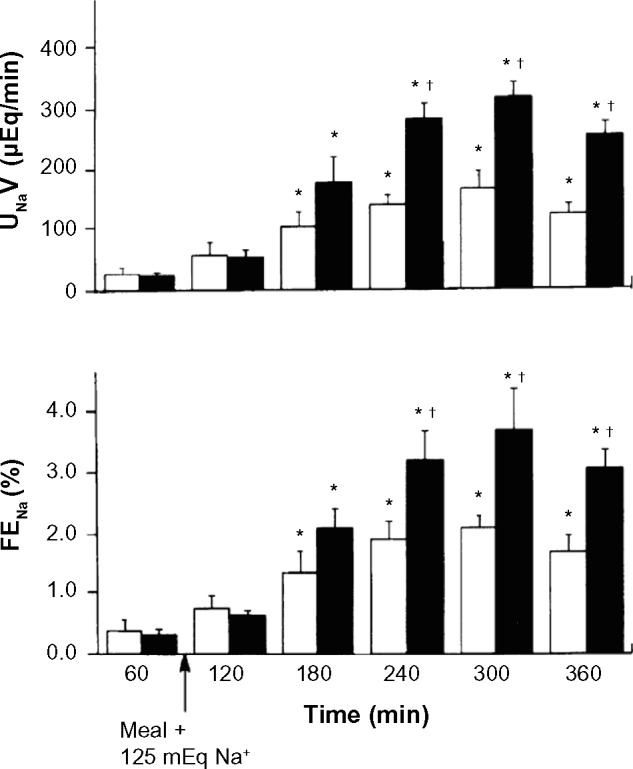
Effects of high-sodium meal in dogs with arteriovenous fistula and chronic compensated high-output heart failure. Values are means ± SE; n = 5 dogs with bilateral renal denervation (closed bars) and 4 dogs with intact renal nerves (open bars). UNaV, urinary sodium excretion; FENA, fractional excretion of sodium. *P < 0.05 vs controls before meals (60 minutes). +P < 0.05 between groups. Reproduced with permission from Villarreal D, Freeman RH, Johnson RA, et al. Effects of renal denervation on postprandial sodium excretion in experimental heart failure. Am J Physiol. 1994;26:R1599–R1604.
Figure 2.
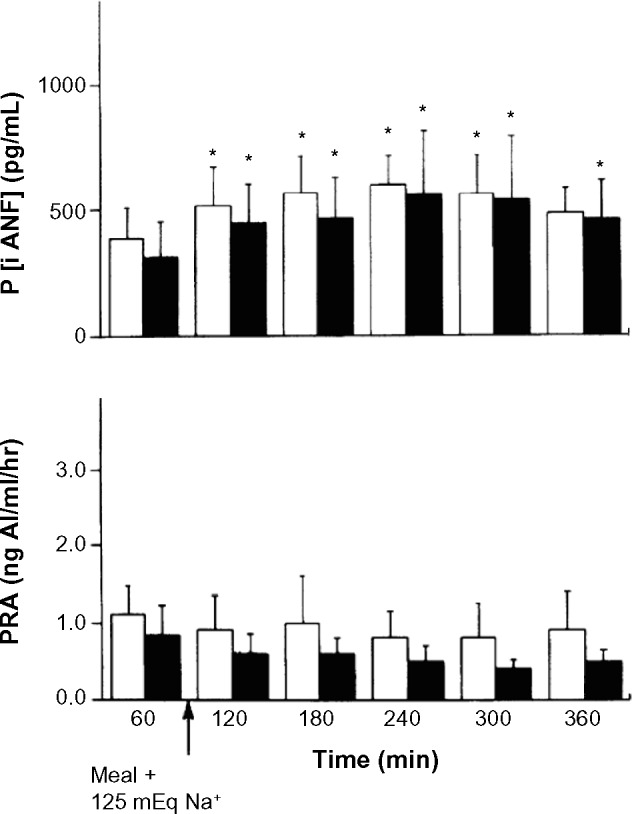
Effects of high-sodium meal in dogs with arteriovenous fistula and chronic compensated high-output heart failure. Values are means ± SE; n = 5 dogs with bilateral renal denervation (closed bars) and 4 dogs with intact renal nerves (open bars). P [iANF], plasma concentration of immuroreactive atrial natriuretic factor; PRA, plasma rennin activity. *P < 0.105 vs controls before meals (60 minutes). Reproduced with permission from Villarreal D, Freeman RH, Johnson RA, et al. Effects of renal denervation on postprandial sodium excretion in experimental heart failure. Am J Physiol. 1994;26:R1599–R1604.
Other investigators in different models of cardiac dysfunction have obtained similar results.5,12 In a rat model of heart failure produced by coronary artery ligation and myocardial infarction, the group of Souza et al.12 demonstrated that, compared to sham denervated rats, bilateral renal denervation was associated with increased sodium and water excretion during a intravenous load of saline.12 Thus, in the aggregate, the available information indicates that the renal nerves exert an important modulatory role on sodium and water metabolism in heart failure, and renal denervation facilitates sodium excretion and the renal expression of both endogenous and exogenous natriuretic and diuretic agents, including the family of cardiac peptides.5,9,10,12
Bilateral Renal Denervation in Humans
The experimental information in animal models of heart failure5,9,10,12 becomes highly relevant in view of the recently developed minimally invasive endovascular technique for renal denervation in humans.11 In this procedure and under fluoroscopic control, the renal arteries are cannulated, and once the ablation device is positioned, radio frequency energy is applied according to a predetermined algorithm, which prompts thermal heat to penetrate the adventitia and ablate both afferent and efferent renal nerves.13
This technique was initially developed to examine the role of afferent and efferent renal denervation on blood pressure reduction in patients with refractory hypertension.13 Although the procedure is well tolerated and appears to be devoid of significant side effects,14 it continues to be refined in order to maximize the completeness and duration of renal denervation.11 Indeed, in the SYMPLICITY HTN-3 trial, the effectiveness of the technique of renal denervation in primary refractory hypertension in humans has been questioned, and it has been suggested that the expertise in the performance of the procedure was uneven across the various participating centers, which led to conflicting results.15
In the context of human heart failure, the REACH- Pilot study examined the safety of bilateral renal denervation in seven patients with compensated class III or IV NYHA systolic heart failure with an average left ventricular ejection fraction (LVEF) of 43% and in optimal medical regimen.14 In this open-labeled, nonrandomized pilot study, at 6 months of follow-up post bilateral renal denervation, arterial blood pressure and renal function remained stable and unchanged from predenervation values.14 Although detailed sodium and water balance studies were not performed, loop diuretics were reduced or stopped in four out of the seven patients, suggesting that renal denervation may have facilitated the achievement of euvolemia.14 Moreover, all patients symptomatically improved, with a significant increase in exercise tolerance.14 This latter observation may be relevant in the context of a recent unblinded, nonrandomized report indicating that bilateral renal denervation in patients with resistant hypertension significantly reduced LV mass and improved diastolic function and left ventricular ejection fraction at 6 months post procedure.16
Summary and Future Perspectives
Heart failure is associated with the activation of the renal SNS, which in turn contributes to the progression of cardiorenal dysfunction characterized by poor prognosis and clinical outcomes.1–3,6 Available information indicates that experimental renal denervation in animal models of heart failure promotes robust natriuresis and diuresis as well as an increased response to endogenous and exogenous natriuretic agents.9,10 In view of preliminary information suggesting that minimally invasive renal denervation in humans with heart failure is safe and well tolerated,14 it is expected that forthcoming clinical trials, with optimal renal nerve ablation techniques, will determine the positive impact of renal denervation as a novel and effective treatment of the heart failure syndrome.
Acknowledgments
The authors wish to acknowledge the expert technical assistance of Stephanie Harper.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
FUNDING: This work was supported in part by the Veteran Affairs Research Program (Merit Review), the American Heart Association, the Joseph C. George Research Award, and the Hendricks Research Award. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
All authors contributed substantially and significantly to the literature search, writing and editing of this manuscript. Dr. Daniel Villarreal conducted many of the experiments reported in this article. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics 2009 update A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . The Global Burden of Disease – 2004 Update. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- 3.Sanderson JE, Tse TF. Heart failure: a global disease requiring a global response. Heart. 2003;89(6):585–6. doi: 10.1136/heart.89.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114:1815–26. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 5.Dibona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 6.Kshatriya S, Kozman H, Siddiqui D, et al. The kidney in heart failure: friend or foe? Am J Med Sci. 2012;344(3):228–32. doi: 10.1097/MAJ.0b013e318242a631. [DOI] [PubMed] [Google Scholar]
- 7.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 8.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–96. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villarreal D, Freeman RH, Johnson RA, Simmons JC. Effects of renal denervation on postprandial sodium excretion in experimental heart failure. Am J Physiol. 1994;26:R1599–604. doi: 10.1152/ajpregu.1994.266.5.R1599. [DOI] [PubMed] [Google Scholar]
- 10.Villarreal D, Freeman RH, Johnson RA. Neurohumoral modulators and sodium balance in experimental heart failure. Am J Physiol. 1993;264:H1187–93. doi: 10.1152/ajpheart.1993.264.4.H1187. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Hering D, Sata Y, et al. Renal denervation: current implications and future perspectives. Clin Sci. 2014;126:41–53. doi: 10.1042/CS20120581. [DOI] [PubMed] [Google Scholar]
- 12.Souza DRB, Mill JG, Cabral AM. Chronic experimental myocardial infarction produces antinatriuresis by a renal nerve dependent mechanism. Braz J Biol Res. 2004;73:285–93. doi: 10.1590/s0100-879x2004000200017. [DOI] [PubMed] [Google Scholar]
- 13.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 14.Davies JE, Manisty CH, Petraco R, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162:189–92. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt DL, Kandzari DE, O’Neill WW, et al. SYMPLICITY HTN-3 Investigators A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 16.Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–9. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]


