Abstract
Women often exhibit larger hormonal and subjective responses to opioid receptor antagonists than men, but the biological mechanisms mediating this effect remain unclear. Among women, fluctuations in estradiol (E2) and progesterone (P4) across the menstrual cycle (MC) affect the endogenous opioid system. Therefore, the goal of the current study was to compare acute naltrexone response between women in the early follicular phase of the MC (low E2 and P4), women in the luteal phase of the MC (high E2 and P4), and men. Seventy healthy controls (n = 46 women) participated in two morning sessions in which they received 50 mg naltrexone or placebo in a randomized, counterbalanced order. Women were randomized to complete both sessions in either the early follicular (n = 23) or luteal phase of the MC. Serum cortisol, prolactin, and luteinizing hormone (LH), salivary cortisol, and subjective response were assessed upon arrival to the laboratory and at regular intervals after pill administration. In luteal and early follicular women but not men, naltrexone (vs. placebo) increased serum cortisol and prolactin levels from baseline; however, the naltrexone-induced increases in these hormones were significantly greater in luteal women than early follicular women. Additionally, only luteal women demonstrated an increase from baseline in salivary cortisol levels and the severity of adverse drug effects in response to naltrexone. In sum, the results indicate that luteal phase women are more sensitive to acute hormonal and subjective effects of naltrexone than early follicular women and men. These findings may have important implications for the use of naltrexone in women.
Keywords: naltrexone, menstrual cycle, cortisol, prolactin, luteinizing hormone, subjective response, sex differences, endogenous opioid system
1.0 Introduction
The opioid receptor antagonist naltrexone is an approved pharmacotherapy in the treatment of alcohol and opioid dependence and is currently under investigation for use in other addictive behaviors (Comer et al., 2013; King et al., 2012). Several studies have reported a high degree of individual variability in treatment response to naltrexone, which has been theorized to contribute to naltrexone’s modest efficacy in treating alcohol use disorders (Kranzler and Van Kirk, 2001; Ray et al., 2010; Rosner et al., 2010; Streeton and Whelan, 2001). As other opioid receptor antagonists are also effective in the treatment of alcohol use disorders (e.g., nalmefene; Mann et al., 2013) and are commonly used as probes of hormonal function (e.g., naloxone), understanding factors that contribute to individual differences in response to opioid receptor antagonists is a priority. While most studies of individual differences have focused on examining how genetic variability may contribute to opioid receptor antagonist responsivity (Chamorro et al., 2012; Ray et al., 2010), sex differences in response to opioid receptor antagonists have also been reported, with women showing greater cortisol and prolactin responses, more adverse subjective effects, and better treatment outcomes than men (al’Absi et al., 2004; Epperson et al., 2010; Epstein and King, 2004; King et al., 2013; Lovallo et al., 2012b; Roche et al., 2010). Women’s greater sensitivity to opioid receptor antagonists suggests an underlying sex difference in the endogenous opioid system, but the biological factors underlying these effects have not yet been characterized.
The gonadal hormones estradiol (E2) and progesterone (P4) influence endogenous opioid activity in order to regulate various reproductive functions across the menstrual cycle (MC) and estrus cycle in female mammals (Eckersell et al., 1998). In a stereotypical MC, circulating E2 levels are lowest during the early follicular phase, highest during the late follicular phase, and reach a relatively steady, intermediate level throughout the luteal phase; while P4 levels are low throughout the follicular phase, rise after ovulation, and peak during the mid-luteal phase. Across several species, E2 and P4 levels are positively associated with opioid transmission at the hypothalamic and systemic levels, and therefore may affect response to opioid receptor-binding drugs (Bernardi et al., 2006; Eckersell et al., 1998; Foradori et al., 2005; Stomati et al., 1997; Wardlaw et al., 1982; Wehrenberg et al., 1982). Despite this evidence, few studies have examined the effects of MC phase on subjective (e.g., mood) or hormonal responses to opioid receptor antagonists. Studies examining such outcome measures often enrolled women without recording MC phase, included only men in the design, or tested women in one broadly defined MC phase without hormonal confirmation, the latter of which fails to account for hormonal changes within each MC phase.
The endogenous opioid system regulates the hypothalamic-pituitary-adrenal (HPA) axis, hypothalamic-pituitary-gonadal (HPG) axis, and tuberoinfundibular dopamine (TIDA) system and thus affects the secretion of several hormones into systemic circulation, including cortisol, luteinizing hormone (LH), and prolactin, respectively. Anatomical and functional data suggests the HPA and HPG axes are tonically inhibited by endogenous opioids at the level of the hypothalamus (Baker and Herkenham, 1995; Dudas and Merchenthaler, 2004). Accordingly, opioid receptor antagonists acutely disinhibit these axes and increase the secretion of cortisol from the adrenal cortices and LH from the anterior pituitary (Mendelson and Mello, 2009). Opioids directly regulate the hypothalamic TIDA neurons that tonically inhibit prolactin secretion from the anterior pituitary (Durham et al., 1996; Fitzsimmons et al., 1992). In support, naltrexone and nalmefene administration acutely increases circulating prolactin levels (al’Absi et al., 2004; Bart et al., 2005). While MC effects on subjective and hormonal response to naltrexone and nalmefene have not been thoroughly investigated, LH response to naloxone has been shown to be larger in late follicular and mid luteal phase women (i.e., high E2 and/or P4 level) than early follicular phase or post-hysterectomy women (i.e., low E2 and P4; Quigley and Yen, 1980; Shoupe et al., 1985).
In sum, endogenous opioid activity increases as E2 and P4 levels rise (Foradori et al., 2005; Stomati et al., 1997; Wardlaw et al., 1982; Wehrenberg et al., 1982) and, importantly, the opioid tone produced by E2 and P4 circulation may be necessary for hormonal response to opioid receptor antagonism (Shoupe et al., 1985; Stomati et al., 1997). Furthermore, hormonal response to opioid receptor antagonists may be an effective proxy measure of central endogenous opioid activity, as hypothalamic and striatal opioid transmission is positively associated with greater cortisol response to naloxone (Wand et al., 2011). Thus, in the present study, we examined whether MC phase affects acute cortisol, prolactin, LH, and subjective responses to naltrexone within women, and also compared women’s responses to men. We hypothesized that women in the luteal phase of the MC possess heightened endogenous opioid tone compared to early follicular phase women and men and, therefore, would be more sensitive to an acute dose of naltrexone and exhibit greater hormonal and subjective responses.
2.0 Materials and Methods
2.1 Participants
Participants were recruited through advertisements on internet sites, print flyers, and by word-of-mouth referrals. Interested participants filled out an online survey and completed an initial phone screen to determine eligibility. Subjects were eligible if they were 18–35 years old, were of good general physical and mental health, had a BMI ≥ 17 or ≤ 35 kg/m2 (mild thinness to moderate obesity) and did not work overnight shifts, which can affect diurnal hormone levels. Women were eligible if they had not been pregnant or lactating in the last 6 months and had a regularly occurring MC of 22 – 36 days (Fehring et al., 2006). Women on hormonal birth control were not considered for the study because hormonal contraceptives may affect basal cortisol levels and cortisol response to naltrexone (Roche et al., 2013). The age of 35 was chosen as the higher limit because numerous studies suggest a decline in reproductive function and increased variability in MC length and MC-related hormone levels after this age (Dunson et al., 2002; Klein et al., 1996; Lenton et al., 1988; Reame et al., 1996). The age of 18 was the lower limit because naltrexone has not been approved for use in persons under this age.
Eligible participants were invited for an in-laboratory screening session. The laboratory screening consisted of a general physical examination by the study nurse, administration of a diagnostic psychiatric interview, and completion of several questionnaires, which included the Beck Depression Inventory, State–Trait Anxiety Inventory, Childhood Trauma Questionnaire (Bernstein et al., 2003), and health and substance use history questionnaires. These questionnaires were chosen because depression, anxiety, and history of trauma and substance abuse disorders may affect hormonal function and response pharmacological challenge (al’Absi et al., 2008; Burnett et al., 1999; Hubert and de Jong-Meyer, 1992; Lovallo et al., 2012a; Mangold and Wand, 2006). Individuals were excluded from participation if they had a current or past major medical condition (e.g., cardiovascular, hepatic, neurological, endocrine, etc.) or psychiatric disorder (i.e., any DSM-IV Axis I disorder), including drug or alcohol dependence. They were also excluded for current drug or alcohol abuse, regular smoking behavior (> 5 cigarettes/week), or use of a medication that may affect HPA axis or opioidergic functioning. Additionally, subjects with blood hepatic indices outside normal limits or a positive urine toxicology obtained during the screening (cocaine, opiates, benzodiazepines, amphetamine, barbiturates, and PCP) were excluded from participation. Women also completed the pain, negative affect, and behavioral change subscales of the Moos Menstrual Distress Questionnaire for premenstrual symptoms (MDQ; Moos, 1968), provided a urine sample to test for pregnancy and a blood sample to measure hormone and liver enzyme levels, and completed a calendar-based interview to determine average MC length and to estimate the onset of next menses. Only female subjects who were not pregnant and did not report severe premenstrual symptoms (MDQ score of under 76) were enrolled in the study.
One hundred and fourteen individuals completed the in-laboratory screening session. Of those, 24 were excluded from participation due to one of the following reasons: having a BMI outside of the cutoff range (n = 9), meeting criteria for a psychiatric disorder (n = 7), unable to complete a blood draw (n = 4; either due to the nurse being unable to perform a blood draw or to the participant being unwilling to have blood drawn), having a medical disorder that may affect hormone function or medication responsivity (n = 2), being outside the age range after presenting a driver’s license (n = 1), or having outside normal limits liver enzyme levels (n = 1). Of the 90 individuals eligible to participate in the study after the screening session, 20 were classified as enrollment failures (i.e., did not complete an experimental session) due to one of the followed reasons: voluntarily withdrew (n = 13; e.g., moved out of area, new job, or no longer interested), unable to be contacted by the study staff (n = 3), unable to have an indwelling IV line inserted by the research nurse (n = 3), or removed from study due to development of a medical condition after enrollment (n = 1).
2.2 Procedure
The study was approved by The University of Chicago Institutional Review Board and in accordance with the Declaration of Helsinki.
Seventy subjects (n=24 male) participated in this double-blind, placebo-controlled human laboratory study. Men and women completed two sessions in which they received either 50 mg oral naltrexone or placebo in a randomized, counterbalanced order. Women were randomized to complete both sessions in either the early follicular (n=23) or luteal (n=23) phase of their MC. Menstrual cycle phase was confirmed by hormone measurements, as detailed in the Measures section below. The early follicular and luteal phases of the MC were selected because 1) they are one of the few periods the MC where E2 and P4 levels remain relatively stable for a prolonged period of time (i.e., several days), which increases the likelihood that women would be scheduled and tested during the correct phase in both sessions, and 2) they are ideal natural representations of “low” and “high” E2/P4 states, which should theoretically also produce detectably different states of low and high endogenous opioid activity. Women in both groups were instructed to contact the study staff on the first day of menstruation in order to schedule their experimental session. Participants in the early follicular group had sessions scheduled after the onset of menses and before the predicted E2 and LH surge of the late follicular phase [on average, day 5.3 ± 3.5 (mean ± SD) of the MC]. Participants in the luteal group had sessions scheduled to take place during the mid-luteal phase (MC day 21.9 ± 2.8). Luteal session dates were determined by using a combination of self-reported MC length and the results of the calendar-based MC length interview. The luteal phase is highly stable within and between women and typically lasts 12–14 days (Fehring et al., 2006); therefore, the final 13 days of the subjects’ predicted MC was considered the luteal phase. In order to test women at the same approximate day of their MC for each session and to allow the effects of naltrexone to sufficiently wash out between sessions, women were tested only once per cycle (i.e., each woman completed her second session approximately one month after her first session, regardless of group). To maintain a similar interval between experimental sessions across men and women, men had their sessions scheduled at least two weeks apart. On average, there were 21.1 ± 8.0 days (mean ± SD) between sessions for men, 52.0 ± 34.0 days between sessions for early follicular women (n = 18/23 completed their second session within two cycles from their first experimental session), and 48.6 ± 27.2 days between sessions for luteal women (n = 20/23 completed their second session within two cycles from their first experimental session).
Participants were instructed to refrain from alcohol and other drugs for at least 48 hrs before their session, and to fast and abstain from caffeine use prior to arrival on the testing day. Participants arrived at the laboratory at 0830 h, provided breath samples for alcohol and carbon monoxide measurement to confirm recent (12 hr) alcohol and smoking abstinence, and self-reported 48-hour drug abstinence, verified by a negative urine drug toxicology screen. Female participants’ urine samples were additionally tested for pregnancy and LH surge/ovulation (both required to be negative to continue with the experimental session). Subsequent to urine testing, participants consumed a light meal (20% daily calories, based on sex, age, and body weight). At 0900 h, the subject completed baseline subjective questionnaires. Immediately afterward, the research nurse inserted an indwelling IV into the antecubital vein and provided the subject with a cotton Salivette (Sarstedt AG & Company, Numbrecht, Germany) in order to obtain baseline blood and saliva samples, respectively. At 0930 h, the participant ingested the naltrexone or placebo capsule. Subjective measures and blood samples were repeated at 60, 90, 120, 150, and 180 min after pill administration. Saliva samples were also obtained at the 60, 120, and 180 min timepoints. Participants remained seated throughout the experiment on a comfortable couch and during intervals when measures were not being collected (i.e., between timepoints), the participants were free to relax and watch movies or read magazines from a pre-selected list that was screened for general interest content without violent, sexual, or language content that might be excessively stimulating or distressing. At 1130 h, the participant consumed a light snack (20% daily calories) because hunger could potentially affect mood and fasting-related changes in glucose levels could affect acute cortisol reactivity (Dallman, 2003). At 1230 h, after the 180 min timepoint, the research nurse removed the IV catheter and the subject was discharged. The second session was identical to the first with the exception of the contents of the pill (naltrexone or placebo). At the end of the second session, subjects were debriefed and compensated $150 for participation.
2.3 Measures
2.3.1 Hormone Assays
In order to measure circulating levels of several hormones (described below), blood samples were obtained at baseline and 60, 90, 120, 150, and 180 min after pill administration. Blood samples were stored at 2 – 8 degrees C for ≤ 48 hrs after collection. On the day of processing samples were held for up to two hrs at 20–25 degrees C to clot and normalize the serum separator tubes, then spun at 500G for 9 min at 2 – 8 degrees C using a refrigerated centrifuge. The supernatant was removed to a polypropylene tube for assay and storage. Serum assays were chemiluminimmunoassays performed on a Siemens Immulite 2000 automated analyzer. All serum processing and assays were performed at the University of Chicago Ligand Assay Core Laboratory of the Diabetes Research and Training Center.
Saliva samples for free cortisol determination were stored in a −80°C freezer and then later spun down at 2600 rpm for 20 min at 4 degrees C using a refrigerated centrifuge. Samples were assayed using a Salimetrics high-sensitivity enzyme immuno-assay kit that was standardized and validated at The University of Chicago Clinical Research Center Core Laboratory.
2.3.2 Menstrual Cycle Phase Confirmation
To confirm MC status in women, baseline levels of serum E2, P4, LH, and follicle stimulating hormone (FSH) were assayed. Estradiol and P4 concentrations were determined using a solid-phase, enzyme-labeled chemiluminescent competitive immunoassay with intra-assay coefficients of variation (CV) between 4.3 – 9.9% and 7 – 17.4%, and inter-assay CV between 6.7 – 16% and 9.5 – 21.7%, respectively. LH and FSH concentrations were determined using a solid-phase, two-site chemiluminescent immunometric assay with intra-assay CV between 3.04 – 13.1% and 2.9 – 4.2%, and inter-assay CV between 6.1 – 26.3% and 4.1 – 7.9%, respectively.
The reference ranges provided by (Vankrieken, 2000) were used to determine acceptable within-phase hormone levels for the early follicular and luteal phases. Early follicular women were confirmed with E2 levels from non-detectable (ND) – 84 pg/mL, P4 from ND – 1.13 ng/mL, LH from 1.1 – 11.6 mIU/mL, and FSH from 3.0 – 14.4 mIU/mL. Luteal women were confirmed with E2 levels from 27 – 246 pg/mL, P4 from 0.95 – 21.0 ng/mL, LH from ND – 14.7 mIU/mL, and FSH from 1.2 – 9.0 mIU/mL. Seven women were excluded from data analysis due to having hormone levels outside the listed reference ranges in one or both sessions. Thus, the analyzed early follicular and luteal groups consisted of n = 19 and n = 20 women, respectively.
2.3.3 Hormone Dependent Measures
To measure hormonal response to naltrexone, serum levels of cortisol, prolactin, and LH and salivary cortisol levels were assayed for men and women from samples obtained at baseline and post-pill timepoints. Both serum and salivary cortisol levels were measured because each form represents a different aspect of cortisol concentration. Approximately 95% of secreted cortisol is bound to corticosteroid-binding globulin (CBG) or serum albumin, with only ~5% being “free cortisol” (Lewis et al., 2005). Salivary cortisol has been shown to be an accurate measurement of free, or unbound, cortisol, while serum cortisol levels are representative of total circulating levels (i.e., bound + unbound). While these measures are often highly correlated, they can also be dissociable. In women, gonadal hormones can increase corticosteroid-binding globulin CBG levels, which can in turn reduce free cortisol and increase total cortisol (Hellhammer et al., 2009; Kajantie and Phillips, 2006). Serum cortisol concentrations were determined using a solid-phase, enzyme-labeled chemiluminescent competitive immunoassay with intra-assay CV between 6.1 – 7.4% and inter-assay CV between 6.8 – 9.4%. Prolactin and LH concentrations were determined using a solid-phase, two-site chemiluminescent immunometric assay with intra-assay CV between 2.7 – 3.4% and 3.04 – 13.1%, and inter-assay CV between 4.0 – 5.3% and 6.1 – 26.3%, respectively. Salivary cortisol (i.e., unbound cortisol) was determined using a high-sensitivity enzyme immuno-assay kit with inter- and intra-assay coefficients of variation were 6.88% and 7.12%, respectively.
2.3.4 Subjective Measures
Subjective response to naltrexone was assessed at baseline and at each post-medication timepoint with an opioid receptor antagonist-specific adverse effect scale (Epstein and King, 2004), which was summarized as the total score of 17 common opioid receptor antagonist side effects with each item rated from none (1) to extreme (4). A modified version of the Menstrual Distress Questionnaire - Today form (MDQ-T; Moos, 1968; pain, negative affect, and behavioral change subscales) was administered at baseline in order to assess the severity of menstrual-related symptoms on the day of the session. The modified version of the MDQ-T form was summarized as the total score of the 6 items of the pain subscale, the 8 items of the negative affect subscale, and 2 items from the behavioral change subscale, with individual items rated from 1 (no experience of the symptom) to 6 (severe or partially disabling).
2.4 Statistical Analysis
Demographic, substance use, personality measures, and baseline levels of all dependent measures were compared among men, and early follicular and luteal women by repeated measure ANOVAs, t-tests, and Chi-square tests, as appropriate. To assess differences in sensitivity to an acute dose of naltrexone, hormonal and subjective data were transformed to represent area under the curve with respect to the increase from baseline levels (AUCi; Pruessner et al., 2003). As described by Pruessner et al., (2003), the AUCi variable is useful in describing the sensitivity of a system to a stimuli by emphasizing the changes across time in a variable, and is calculated by first obtaining the total AUC for a variable using the trapezoidal method and then subtracting the area between ground and baseline across all time points from this value. The ability of AUCi to account for baseline differences is important in the current study, as significant differences baseline in prolactin, LH, and adverse effect values were observed between groups. Negative AUCi values indicate an overall decrease in subjective or hormonal levels from baseline, whereas positive values indicate an overall increase from baseline. All hormonal and subjective dependent measures were analyzed using a repeated measures ANOVA with group (Men, Early Follicular, or Luteal) as a between subjects factor and dose (naltrexone or placebo) as within subjects factor. Partial eta squared (ηp2) was calculated as effect size estimates for significant effects of the main dependent measures (i.e., AUCi response to naltrexone). Significant interactions were explored using planned comparisons for simple effects post hoc testing. If a significant interaction was observed, two sets of post hoc comparisons were made: 1) comparing naltrexone AUCi levels between groups and 2) comparing naltrexone and placebo AUCi levels within groups. Finally, Pearson product-moment correlation coefficients were calculated to examine the relationship between hormonal and subjective response to naltrexone.
3.0 Results
3.1 Participant Characteristics and Baseline Measures
Demographic and personality measures were similar between men, luteal, and early follicular women (Table 1). As expected, there were differences between luteal and early follicular women on MC-related hormones (Table 2). Luteal women had greater E2 (F(1, 37) = 76.0, p < 0.0001) and P4 (F(1, 37) = 116.9, p < 0.0001) levels, while early follicular women had greater FSH levels (F(1, 37) = 38.4, p < 0.0001). Baseline differences in hormonal and subjective dependent measures were also evident between groups (Table 2). Luteal and early follicular women had higher baseline prolactin (F(2, 60) = 7.8, p < 0.001; Post Hoc: p’s < 0.05) and LH levels than men (F(2, 60) = 6.6, p = 0.01; Post Hoc: p’s < 0.05). For the subjective measure, early follicular women reported greater adverse effect scores than both men and luteal women (F(2, 60) = 5.2, p < 0.01; Post Hoc: p’s < 0.05). There were no significant within-group baseline differences between naltrexone and placebo sessions for any measure and the baseline MC-related hormone levels from each session were highly correlated within subjects (r’s 0.57 –0.88, p’s < 0.0001), suggesting participants were being tested at similar hormonal states in each experimental session.
Table 1.
Demographic, drug use, and personality characteristics
| Men | Early Follicular | Luteal | |
|---|---|---|---|
| General Characteristics | |||
| Age (yrs) | 24.8 (3.9) | 24.4 (3.6) | 24.1 (4.2) |
| Body Mass Index (kg/m2) | 24.0 (3.0) | 24.3 (4.3) | 23.0 (3.4) |
| Education (yrs) | 15.6 (2.2) | 15.6 (1.6) | 15.5 (1.9) |
| Race: % caucasian | 54 | 68 | 70 |
| Drug Use Characteristics | |||
| Drinking Days/Week | 1.3 (0.99) | 1.3 (0.77) | 1.0 (0.79) |
| Drinks/Drinking Day | 2.4 (1.3) | 2.1 (1.0) | 2.4 (1.4) |
| Smoked in last yr (%) | 25 | 37 | 25 |
| Cigarettes/Week in smokers | 1.8 (1.9) | 0.71 (0.28) | 1.1 (0.50) |
| Personality Characteristics | |||
| Beck Depression Inventory | 4.1 (5.2) | 1.7 (3.8) | 2.4 (2.5) |
| State-Trait Anxiety Index (T-score) | |||
| -Trait score | 58.9 (4.3) | 57.2 (2.6) | 58.0 (3.2) |
| -State score | 63.3 (1.6) | 64.5 (2.4) | 64.8 (3.6) |
| Childhood Trauma Questionnaire | 14.1 (4.3) | 13.3 (4.1) | 13.9 (3.9) |
| Menstrual Distress Questionnaire | - | 36.1 (12.99) | 35.4 (35.4) |
Data indicate mean (SD) for continuous variables and % for categorical variables.
There were no significant differences between groups on any characteristic.
Table 2.
Baseline hormone and subjective measures
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Early Follicular | Luteal | |||||
| Nalt | Pla | Nalt | Pla | Nalt | Pla | |
| Salivary Cortisol (ug/mL) | 0.40 (0.06) | 0.36 (0.07) | 0.45 (0.08) | 0.43 (0.11) | 0.26 (0.03) | 0.28 (0.04) |
| Serum Cortisol (ug/dL) | 13.9 (0.8) | 13.0 (1.0) | 13.0 (1.0) | 13.5 (1.1) | 11.1 (0.8) | 12.6 (1.0) |
| Prolactin (ng/mL) | 8.7 (0.7)a | 8.2 (0.8)a | 12.4 (1.2) | 11.6 (1.0) | 13.7 (1.5) | 13.7 (1.6) |
| LH (mIU/mL) | 2.8 (0.2)a | 2.7 (0.3)a | 4.6 (0.4) | 5.1 (0.6) | 4.8 (0.8) | 4.3 (0.6) |
| E2 (pg/mL) | - | - | 34.0 (3.6)b | 35.2 (3.3)b | 124.8 (15.1) | 145.0 (16.5) |
| P4 (ng/mL) | - | - | 0.43 (0.1)b | 0.35 (0.02)b | 8.4 (0.8) | 7.2 (0.7) |
| FSH (mIU/mL) | - | - | 6.0 (0.4) | 5.9 (0.4) | 3.2 (0.3)c | 3.3 (0.3)c |
|
| ||||||
| Adverse Effects | 4.4 (0.5) | 3.6 (0.5) | 6.3 (0.7)d | 6.2 (0.9)d | 4.2 (0.6) | 3.8 (0.5) |
| Menstrual Distress Questionnaire – Today Form | - | - | 22.1 (1.4) | 22.9 (1.6) | 19.6 (1.0) | 20.0 (1.1) |
Data indicate mean (SEM). Nalt = naltrexone and Pla = placebo.
Men had lower baseline PRL (p’s < 0.05) and LH levels (p’s < 0.05) than women.
Eary follicular women had lower E2 and P4 levels than luteal women (p’s < 0.0001).
Luteal women had lower FSH levels than early follicular women (p < 0.0001).
Early follicular women reported significantly higher adverse effect scores than men and luteal women (p’s < 0.05).
3.2 Hormonal and Subjective Response to Naltrexone
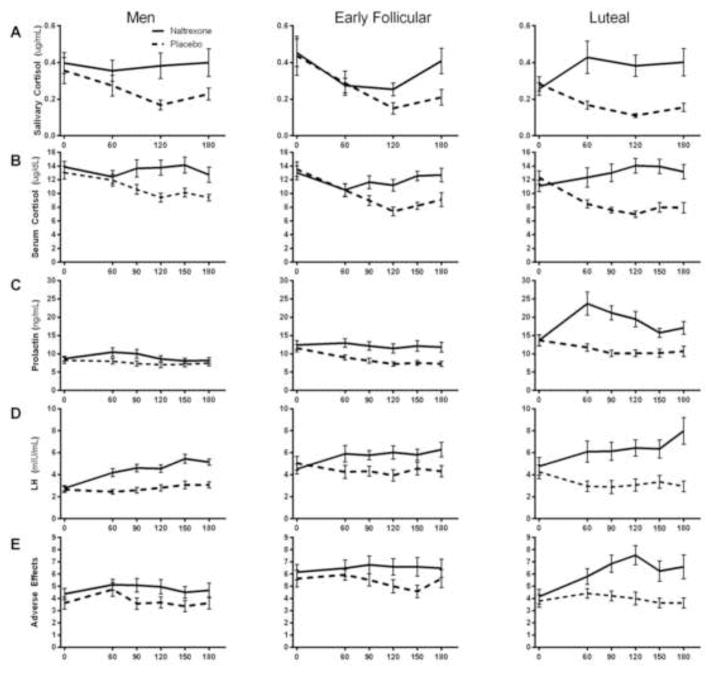
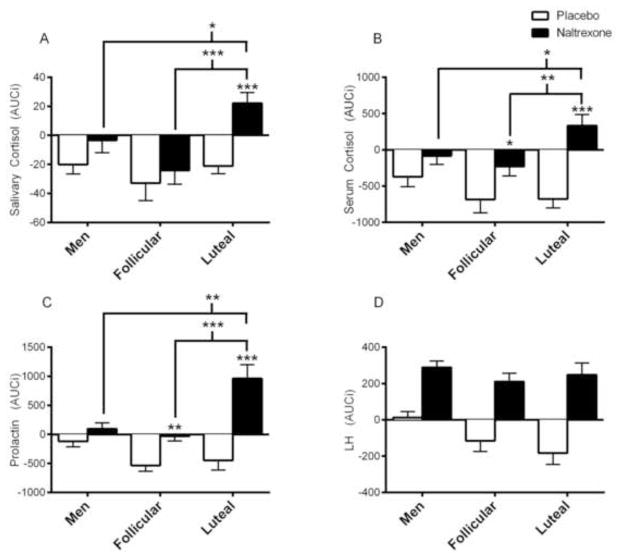
For illustrative purposes, raw hormonal and subjective responses to naltrexone and placebo across each timepoint are displayed in Figure 1. Covarying for length of time between sessions did not affect any of the results presented below. Naltrexone’s effects on salivary cortisol (Figure 2A; Dose x Group, F(2, 60) = 3.1, p < 0.05; ηp2 = 0.10), serum cortisol (Figure 2B; Dose x Group, F(2, 60) = 4.1, p < 0.05; ηp2 = 0.12), and prolactin (Figure 2C; Dose x Group, F(2, 60) = 12.4, p < 0.0001; ηp2 = 0.29) differed between groups, as measured by AUCi. In luteal women, but not early follicular women or men, naltrexone increased salivary cortisol from baseline compared with placebo (Post Hoc: p < 0.001). Additionally, naltrexone (vs. placebo) significantly increased serum cortisol and prolactin in both early follicular and luteal women, but not men (Post Hoc: Serum Cortisol: p’s < 0.05; Prolactin: p’s < 0.01). However, luteal women’s serum cortisol and prolactin naltrexone responses were significantly greater than those of both men (Post Hoc: p’s < 0.05) and early follicular women (Post Hoc: p’s < 0.01). Naltrexone (vs. placebo) increased LH levels across all groups (Dose, F(1,60) = 75.7, p < 0.0001; ηp2 = 0.56) and this pattern of response did not differ between groups (Figure 2D; Dose x Group, p = 0.26). In the overall sample, salivary cortisol, serum cortisol, and prolactin response to naltrexone were highly correlated (r’s > 0.50, p’s < 0.0001); yet, LH response to naltrexone was not related to other hormone responsivity (r’s = −0.19 – 0.08). At the group level, salivary cortisol, serum cortisol, and prolactin response to naltrexone were correlated in men and luteal women (r’s > 0.38, p’s < 0.05), but not in early follicular women.
Figure 1. Raw hormone and subjective levels across all time points.
The figures illustrate the raw levels salivary cortisol (A), serum cortisol (B), prolactin (C), and LH levels (D), and adverse effects (E) in men, early follicular women, and luteal women. The X-axis represents minutes after pill administration. Salivary cortisol was measured at baseline and three post-pill timepoints, while serum cortisol, prolactin, LH, and adverse effects were collected at baseline and four post-pill timepoints. Hormone levels are reported as mean raw data ± SEM.
Figure 2. AUCi of hormonal response to naltrexone.
Naltrexone significantly elevated salivary cortisol (A), serum cortisol (B), and prolactin (C) from baseline to a greater extent in luteal phase women than men and follicular women. However, naltrexone similarly increased LH in all groups (D). Post Hoc: Asterisks above brackets indicate between groups differences of naltrexone response, non-bracketed asterisks above a bar graph indicate within group differences of naltrexone vs. placebo responses; *p < 0.05, **p < 0.01, ***p < 0.001. Hormone levels are reported as mean AUCi ± SEM.
Subjective response to naltrexone also significantly differed between groups, as measured by the adverse effects questionnaire (Figure 3; Dose x Group, F(2, 60) = 3.4, p < 0.05; ηp2 = 0.10). In luteal women, but not in men and early follicular women, naltrexone increased the severity of adverse effects from baseline (Post Hoc: p < 0.01). Subjective response to naltrexone was not correlated with any measure of hormonal response to naltrexone (r’s = 0.02 – 0.19).
Figure 3. AUCi of subjective response to naltrexone.
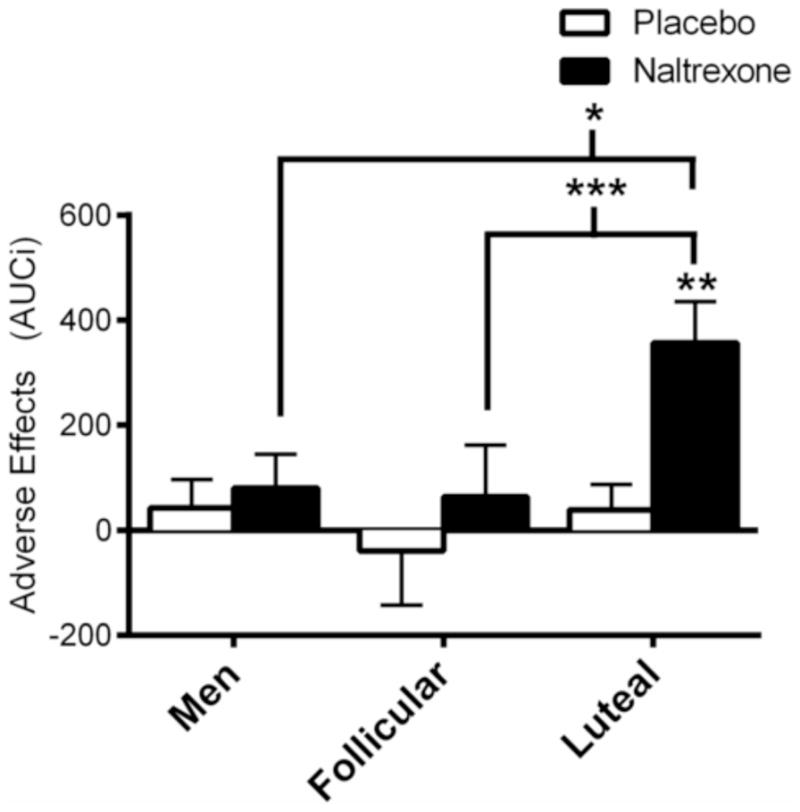
Naltrexone significantly elevated the severity of adverse effects from baseline to a greater degree in luteal women than early follicular women and men. Post Hoc: Asterisks above brackets indicate between groups differences of naltrexone response, non-bracketed asterisks above a bar graph indicate within group differences of naltrexone vs. placebo responses; *p < 0.05, **p < 0.01, ***p < 0.001. Subjective data are reported as mean AUCi ± SEM.
4.0 Discussion
The current study results indicate that MC phase significantly affects women’s acute subjective and hormonal response to naltrexone. Luteal phase women demonstrated larger cortisol (serum and saliva) and prolactin responses to naltrexone and more sensitivity to its adverse subjective effects than early follicular women and men. While early follicular women did show significant naltrexone-induced increases in serum cortisol and prolactin compared with placebo, the magnitude and direction of their cortisol, prolactin, and adverse effect responses to naltrexone did not significantly differ from those displayed by men. These findings suggest that the endogenous opioid system is affected by MC phase and also that sex differences in acute response to naltrexone may only be readily apparent when testing normally-cycling women during certain times of the MC.
Luteal women’s heightened cortisol and prolactin response to naltrexone relative to early follicular women may be due to differences in hypothalamic endogenous opioid activity, particularly β-endorphin and dynorphin. The β-endorphin and dynorphin neurons projecting to the hypothalamic nuclei that regulate the HPA axis and TIDA system, respectively, show increased activity during the luteal phase and in response to E2 and P4 administration (Baker and Herkenham, 1995; Bernardi et al., 2006; Durham et al., 1996; Fitzsimmons et al., 1992; Foradori et al., 2005; Stomati et al., 2002; Wardlaw et al., 1982; Wehrenberg et al., 1982). Importantly, recent evidence suggests that increased hypothalamic β-endorphin activity is associated with augmented cortisol response to naloxone (Wand et al., 2011). Therefore, in luteal women, increased hypothalamic opioid transmission may result in heightened inhibition of TIDA neurons and the HPA axis and, in turn, greater naltrexone-induced disinhibition of these systems. These results also lend further support to the notion that hormonal response to opioid receptor antagonism is a useful proxy measure of central endogenous opioid activity.
In contrast to our findings with cortisol and prolactin, naltrexone significantly increased LH levels in all groups. These findings support prior studies demonstrating naloxone, naltrexone, and nalmefene increase LH secretion in men and luteal women (Ellingboe et al., 1982; Graves et al., 1993; Quigley and Yen, 1980). While our results are contrary to several studies that found naloxone failed to increase LH levels during the early follicular phase (Quigley and Yen 1980), they support one study that reported naltrexone increases LH in early follicular women (Teoh et al., 1988). This may provide evidence that naltrexone is a more potent disinhibitor of the HPG axis than naloxone, potentially due to the former’s longer half-life and greater opioid receptor affinity, particularly at kappa opioid receptors (Toll et al., 1998).
The differences in the severity of opioid antagonist-related adverse effects between groups at baseline and in response to naltrexone may also reflect altered endogenous opioid activity. As E2 and P4 levels decrease toward the end of the luteal phase, so do circulating levels of endogenous opioids (Giannini et al., 1990; Wehrenberg et al., 1982). This reduction in opioid tone has been theorized to result in minor opioid withdrawal and contribute to the development of premenstrual syndrome (PMS), which begins in the late luteal phase and can last several days into menstruation (Giannini et al., 1984; Rapkin, 2003; Straneva et al., 2002). Peripheral β-endorphin levels are negatively correlated with the number of PMS-related symptoms (Giannini et al., 1984, 1990) and PMS-related symptomatology can bear resemblance to minor opioid withdrawal (Halbreich and Endicott, 1981). Furthermore, women with premenstrual symptoms do not demonstrate LH or cortisol responses to naloxone, both of which are suggestive of reduced endogenous opioid tone (Facchinetti et al., 1988, 1990; Rapkin et al., 1996). Thus, the increased severity of opioid withdrawal-like symptoms at baseline and the lack of subjective response to naltrexone observed in the early follicular group may be due to an attenuated endogenous opioid tone related to decreased E2 and P4 levels. Conversely, naltrexone may antagonize a relatively heightened opioid tone in luteal women, precipitating a minor opioid withdrawal-like state and increasing the reported severity of naltrexone’s adverse effects.
Finally, an interesting pattern of results emerged after exploring the relationships between the various hormonal responses to naltrexone. In the overall sample, serum cortisol, salivary cortisol, and prolactin responses to naltrexone were highly correlated, which may indicate some degree of overlap between the endogenous opioid circuitry regulating these hormones and again provide support for the use of cortisol and prolactin response to naltrexone as a proxy measure of central endogenous opioid activity. However, at the within-group level, these hormonal responses were not associated in early follicular women, which in turn may be related to the finding that naltrexone increased serum cortisol in this group without affecting salivary cortisol. While salivary and serum cortisol are often correlated, some results suggest that high levels of gonadal hormones can disassociate the two measures by augmenting production of CBG, which subsequently increases variability in cortisol levels by producing a higher ratio of serum cortisol to salivary cortisol (Hellhammer et al., 2009; Kajantie and Phillips, 2006). Therefore, the finding that salivary and serum cortisol were correlated in luteal women, but unassociated in early follicular women, is contrary to what would be expected and requires further research to explain. Also of note, and in again contrast to prior findings (O’Malley et al., 2002), we did not detect a relationship between subjective and hormonal responses to naltrexone, which may suggest that the endogenous opioid systems responsible for naltrexone’s adverse effects are distinct from those governing TIDA, HPA axis, and HPG axis functioning. For example, the endogenous opioid system is prominent in the gastrointestinal system and could feasibly contribute to naltrexone-induced nausea without relating to hormonal responsivity (Holzer, 2009).
Though the present study has several strengths, such as carefully assessing MC phase through multiple cycles and confirming gonadal hormone levels at each session, there are some limitations that merit consideration. First, while the study design incorporated a within-subjects component (i.e., naltrexone vs placebo), which allowed us to effectively examine whether women were responsive to naltrexone within a given MC phase, a fully within subject, crossover design would have increased confidence in the obtained results by minimizing between-subject variability in hormone levels. Furthermore, we are confident that six serum samples over three hours were sufficient to accurately capture cortisol and prolactin responses to naltrexone, but this may not be true for LH response. Luteinizing hormone is released in a highly pulsatile manner that varies over the MC, with rapid, low amplitude pulses during the early follicular phase and low frequency, high amplitude pulses across the luteal phase (Filicori et al., 1986). Because of this, LH response to naltrexone may not have been fully captured (e.g., missing a peak and capturing a valley), particularly in luteal women, and our LH results should be viewed with some caution. Additionally, while studying healthy regularly-cycling young women was essential in order to establish the relationship between MC phase and naltrexone responsivity, it is possible that these results do not describe the relationship between gonadal hormonal levels and responsiveness in women who have irregular MCs, are over 35, are post-menopausal/hysterectomy, or are using hormonal contraception. Future studies should characterize responses in these groups of women, as they represent a sizeable percentage of the population who may be eligible for naltrexone pharmacotherapy or participation in laboratory studies administering naltrexone. Similarly, women were tested in the early follicular and luteal phases because they provide stable periods of low and high E2/P4 states, respectively. While prior studies have suggested that heightened endogenous opioid activity is also observable at other high E2/P4 MC phases (e.g., late follicular phase; Quigley and Yen, 1980; Shoupe et al., 1985), future studies are needed to examine whether the current results generalize to an independent sample and across a broader MC range. Finally, administering a single dose of naltrexone, rather than titrating naltrexone using a repeated, daily dosing schedule, may limit the current results’ clinical implications and generalizability. However, it is presently unclear whether the acute effects of a single naltrexone dose differ before and after repeated dosing. Additional research is needed to elucidate the effects of chronic naltrexone administration on hormone function and to determine whether the results of the current study are reproducible after repeated naltrexone dosing.
In summary, the current study indicated that luteal women are overall more sensitive to the acute subjective and hormonal effects of naltrexone than men and early follicular women. Naltrexone is an approved pharmacotherapy for heroin and alcohol dependence and is under investigation for the treatment other addictions (Comer et al., 2013; King et al., 2012). Along with nalmefene and naloxone, naltrexone is also commonly used to probe endogenous opioid, dopamine, HPA axis, and HPG axis functioning (Bart et al., 2005; Mendelson and Mello, 2009; Wand et al., 2011). Therefore, the current study’s finding that women display altered acute sensitivity to naltrexone based on their respective MC phase may have several clinical and experimental implications. These results suggest that sex and MC phase should be accurately assessed and controlled for when designing studies measuring subjective and hormonal response to naltrexone or other opioidergic drugs. Many past studies measuring hormonal response to opioid receptor antagonism did not examine sex as a factor and the majority of these studies did not record MC phase data at all, or only used retrospective self-reports collected on the day of the experimental session without hormonal verification. The latter method may produce inaccurate self-report data due to the high within- and between-subject variability of MC length (Fehring et al., 2006). For example, while many studies have reported opioid receptor antagonism acutely increases cortisol in mixed samples of men and women, most did not compare response between sexes, as described by Lovallo et al., (2012b). In support of the current results, the studies that did compare sexes indicated that men have no, or minimal, cortisol response to naltrexone, while women are highly responsive (Klein et al., 2000; Lovallo et al., 2012b; Roche et al., 2010); yet, none of these studies a priori assessed MC phase. Furthermore, many studies have examined genetic influences on naltrexone responsitivity, particularly variation in the mu opioid receptor gene (OPRM1; Anton et al., 2008; Chamorro et al., 2012; Ray et al., 2010). Despite numerous studies of this relationship, as well as several reports that hormonal response naloxone is affected by OPRM1 (Chong et al., 2005; Hernandez-Avila et al., 2003; 2007) or that sex may mediate OPRM1 effects on pain responsivity and drug reinforcement (Fillingim et al., 2005; Hasvik et al., 2014; Ray et al., 2006), only a few laboratory studies have explored sex by OPRM1 effects on response to naltrexone, with mixed results (Ray et al., 2006; Setiawan et al., 2011). As such, future studies examining the relationship between OPRM1 or other genetic variants and naltrexone response should consider sex as a variable in their design.
The current results may also have future implications for the use of naltrexone as a pharmacotherapy for the treatment of addiction in women. The HPA axis, HPG axis, and TIDA system become dysregulated in various drug addictions and their dysfunction may contribute to early relapse and the maintenance of addiction (al’Absi, 2006; Back et al., 2010). For example, during early abstinence, smokers and alcoholics show impaired HPA axis basal function and reactivity to a stressor, and the degree of this dysfunction is predictive of relapse (al’Absi, 2006; Sinha et al., 2011). The HPA axis has been theorized as a target for naltrexone pharmacotherapy and naltrexone’s ability to increase basal cortisol levels during alcoholism treatment is associated with a reduced risk of relapse (Kiefer et al., 2006). If naltrexone’s ability to rapidly normalize hormone levels is a protective factor against early relapse, then the results of the current study may support beginning treatment during the luteal phase of the MC; however, this phase was also associated with heightened acute sensitivity to the aversive effects of naltrexone, which might affect medication adherence. At this point, conclusions regarding clinical practice are premature given that the current study administered a single, acute dose of naltrexone to healthy individuals. Future studies examining naltrexone’s intermediate- and long-term effects in at-risk or drug-dependent populations are needed to replicate the current results, and then, if the results remain, an examination of the impact of MC phase on response to early naltrexone pharmacotherapy would be warranted.
Highlights.
Naltrexone response differed between men, early follicular women, and luteal women
Luteal women had the largest cortisol and prolactin response to naltrexone
Luteal women reported more severe adverse subjective effects to naltrexone
Response to naltrexone may vary across the menstrual cycle in women
Women with high estradiol and progesterone levels were most responsive to naltrexone
Acknowledgments
We extend our appreciation to Brian Prendergast, PhD, Helen Kim, MD, Harriet de Wit, PhD, Daniel McGehee, PhD, and Peggy Mason, PhD, for their advice and assistance during the design of the study. We also thank the study research nurses Alina Schneider, RN, MS, and Barbara Grimsley, RN, for their roles in medical supervision, participant screening and recruitment, and data collection; Royce Lee, MD, for his medical supervision; Paul Rue, Neal Scherberg, Jessica Camp, and the staffs of the Ligand Assay Core Laboratory of the Diabetes Research and Training Center and the Clinical Research Center Core Laboratory for their roles in the storage and processing of biological samples; and Sarah Martini, BA, Chloe Kern, BA, Constantine Trela, BA, Michael Palmeri, BS, and Patrick McNamara, BA, for their roles in participant recruitment, data collection, and database management.
Role of the funding source
This study was supported by Grants R01-DA016834 (AK) and 1F31DA030073 (DR) from the National Institute of Drug Abuse, and UL1 RR024999 (AK) from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health.
Footnotes
Conflicts of Interest
Drs. Roche and King have no conflicts of interest to disclose, financial or otherwise.
Contributions
DR designed and ran the study, analyzed the data, and prepared the manuscript. AK assisted with study design and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Hatsukami D, Westra R. Blunted opiate modulation of hypothalamic-pituitary-adrenocortical activity in men and women who smoke. Psychosom Med. 2008;70:928–935. doi: 10.1097/PSY.0b013e31818434ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, et al. An evaluation of μ-opioid receptor (oprm1) as a predictor of naltrexone response in the treatment of alcohol dependence: Results from the combined pharmacotherapies and behavioral interventions for alcohol dependence (combine) study. Archives of general psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug and alcohol dependence. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RA, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. The Journal of comparative neurology. 1995;358:518–530. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, Kreek MJ. Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology. 2005;30:2254–2262. doi: 10.1038/sj.npp.1300811. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Pluchino N, Pieri M, Begliuomini S, Lenzi E, Puccetti S, Casarosa E, Luisi M, Genazzani AR. Progesterone and Medroxyprogesterone Acetate Effects on Central and Peripheral Allopregnanolone and Beta-Endorphin Levels. Neuroendocrinology. 2006;83:348–359. doi: 10.1159/000095400. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child abuse & neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Burnett FE, Scott LV, Weaver MG, Medbak SH, Dinan TG. The effect of naloxone on adrenocorticotropin and cortisol release: evidence for a reduced response in depression. Journal of Affective Disorders. 1999;53:263–268. doi: 10.1016/s0165-0327(98)00127-x. [DOI] [PubMed] [Google Scholar]
- Chamorro AJ, Marcos M, Mirón-Canelo JA, Pastor I, González-Sarmiento R, Laso FJ. Association of μ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addiction biology. 2012;17:505–512. doi: 10.1111/j.1369-1600.2012.00442.x. [DOI] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2005;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Comer SD, Mogali S, Saccone PA, Askalsky P, Martinez D, Walker EA, Jones JD, Vosburg SK, Cooper ZD, Roux P, Sullivan MA, Manubay JM, Rubin E, Pines A, Berkower EL, Haney M, Foltin RW. Effects of acute oral naltrexone on the subjective and physiological effects of oral d-amphetamine and smoked cocaine in cocaine abusers. Neuropsychopharmacology. 2013;38:2427–2438. doi: 10.1038/npp.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF. Fast glucocorticoid feedback favors ‘the munchies’. Trends Endocrinol Metab. 2003;14:394–396. doi: 10.1016/j.tem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Dudas B, Merchenthaler I. Close anatomical associations between β-endorphin and luteinizing hormone-releasing hormone neuronal systems in the human diencephalon. Neuroscience. 2004;124:221–229. doi: 10.1016/j.neuroscience.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Human Reproduction. 2002;17:1399–1403. doi: 10.1093/humrep/17.5.1399. [DOI] [PubMed] [Google Scholar]
- Durham R, Johnson J, Moore K, Lookingland K. Evidence that D 2 receptor-mediated activation of hypothalamic tuberoinfundibular dopaminergic neurons in the male rat occurs via inhibition of tonically active afferent dynorphinergic neurons. Brain research. 1996;732:113–120. doi: 10.1016/0006-8993(96)00501-x. [DOI] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingboe J, Veldhuis JD, Mendelson JH, Kuehnle JC, Mello NK. Effect of endogenous opioid blockade on the amplitude and frequency of pulsatile luteinizing hormone secretion in normal men. J Clin Endocrinol Metab. 1982;54:854–857. doi: 10.1210/jcem-54-4-854. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Toll B, Wu R, Amin Z, Czarkowski KA, Jatlow P, Mazure CM, O’Malley SS. Exploring the impact of gender and reproductive status on outcomes in a randomized clinical trial of naltrexone augmentation of nicotine patch. Drug and alcohol dependence. 2010;112:1–8. doi: 10.1016/j.drugalcdep.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AM, King AC. Naltrexone attenuates acute cigarette smoking behavior. Pharmacol Biochem Behav. 2004;77:29–37. doi: 10.1016/j.pbb.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Martignoni E, Fioroni L, Sances G, Genazzani AR. Opioid control of the hypothalamus-pituitary-adrenal axis cyclically fails in menstrual migraine. Cephalalgia. 1990;10:51–56. doi: 10.1046/j.1468-2982.1990.1001051.x. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Martignoni E, Sola D, Petraglia F, Nappi G, Genazzani A. Transient failure of central opioid tonus and premenstrual symptoms. The Journal of reproductive medicine. 1988;33:633. [PubMed] [Google Scholar]
- Fehring RJ, Schneider M, Raviele K. Variability in the Phases of the Menstrual Cycle. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2006;35:376–384. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Filicori M, Santoro N, Merriam GR, Crowley WF., Jr Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. Journal of Clinical Endocrinology & Metabolism. 1986;62:1136–1144. doi: 10.1210/jcem-62-6-1136. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wallace MR. The A118G single nucleotide polymorphism of the μ-opioid receptor gene ( OPRM1) is associated with pressure pain sensitivity in humans. The Journal of Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons M, Olschowka J, Wiegand S, Hoffman G. Interaction of opioid peptide-containing terminals with dopaminergic perikarya in the rat hypothalamus. Brain research. 1992;581:10–18. doi: 10.1016/0006-8993(92)90338-a. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146:1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- Giannini AJ, Martin DM, Turner CE. Beta-endorphin decline in late luteal phase dysphoric disorder. The International Journal of Psychiatry in Medicine. 1990;20:279–284. doi: 10.2190/JRQJ-XTX9-CQPF-HD70. [DOI] [PubMed] [Google Scholar]
- Giannini AJ, Price WA, Loiselle RH. β-Endorphin withdrawal: a possible cause of premenstrual tension syndrome. International Journal of Psychophysiology. 1984;1:341–343. doi: 10.1016/0167-8760(84)90028-x. [DOI] [PubMed] [Google Scholar]
- Graves G, Kennedy T, Weick R, Casper R. Endocrinology: The effect of nalmefene on pulsatile secretion of luteinizing hormone and prolactin in men. Human Reproduction. 1993;8:1598–1603. doi: 10.1093/oxfordjournals.humrep.a137898. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Endicott J. Possible involvement of endorphin withdrawal or imbalance in specific premenstrual syndromes and postpartum depression. Medical Hypotheses. 1981;7:1045–1058. doi: 10.1016/0306-9877(81)90100-6. [DOI] [PubMed] [Google Scholar]
- Hasvik E, Schistad EI, Grøvle L, Haugen AJ, Røe C, Gjerstad J. Subjective health complaints in patients with lumbar radicular pain and disc herniation are associated with a sex-OPRM1 A118G polymorphism interaction: a prospective 1-year observational study. BMC Musculoskeletal Disorders. 2014;15:161. doi: 10.1186/1471-2474-15-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Covault J, Wand G, Zhang H, Gelernter J, Kranzler HR. Population-specific effects of the Asn40Asp polymorphism at the [mu]-opioid receptor gene (OPRM1) on HPA-axis activation. Pharmacogenetics and genomics. 2007;17:1031–1038. doi: 10.1097/FPC.0b013e3282f0b99c. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Wand G, Luo X, Gelernter J, Kranzler HR. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the i-opioid receptor locus (OPRM1) American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2003;118:60–65. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- Holzer P. Opioid receptors in the gastrointestinal tract. Regulatory Peptides. 2009;155:11–17. doi: 10.1016/j.regpep.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert W, de Jong-Meyer R. Saliva Cortisol Responses to Unpleasant Film Stimuli Differ between High and Low Trait Anxious Subjects. Neuropsychobiology. 1992;25:115–120. doi: 10.1159/000118819. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Naber D, Wiedemann K. Hypothalamic-pituitary-adrenocortical axis activity: a target of pharmacological anticraving treatment? Biol Psychiatry. 2006;60:74–76. doi: 10.1016/j.biopsych.2005.11.023. [DOI] [PubMed] [Google Scholar]
- King AC, Cao D, O’Malley SS, Kranzler HR, Cai X, Matthews AK, Stachoviak RJ. Effects of Naltrexone on Smoking Cessation Outcomes and Weight Gain in Nicotine-Dependent Men and Women. Journal of Clinical Psychopharmacology. 2012;32:630–636. doi: 10.1097/JCP.0b013e3182676956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Cao D, Zhang L, O’Malley SS. Naltrexone reduction of long-term smoking cessation weight gain in women but not men: a randomized controlled trial. Biol Psychiatry. 2013;73:924–930. doi: 10.1016/j.biopsych.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LC, Jamner LD, Alberts J, Orenstein MD, Levine L, Leigh H. Sex differences in salivary cortisol levels following naltrexone administration. Journal of Applied Biobehavioral Research. 2000;5:144–153. [Google Scholar]
- Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. The Journal of Clinical Endocrinology & Metabolism. 1996;81:1038–1045. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcoholism, clinical and experimental research. 2001;25:1335–1341. [PubMed] [Google Scholar]
- Lenton EA, Sexton L, Lee S, Cooke ID. Progressive changes in LH and FSH and LH: FSH ratio in women throughout reproductive life. Maturitas. 1988;10:35–43. doi: 10.1016/0378-5122(88)90129-6. [DOI] [PubMed] [Google Scholar]
- Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin Chim Acta. 2005;359:189–194. doi: 10.1016/j.cccn.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biol Psychiatry. 2012a;71:344–349. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, King AC, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: Studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology. 2012b;37:1922–1928. doi: 10.1016/j.psyneuen.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold DL, Wand GS. Cortisol and Adrenocorticotropic Hormone Responses to Naloxone in Subjects With High and Low Neuroticism. Biological psychiatry. 2006;60:850–855. doi: 10.1016/j.biopsych.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Mann K, Bladstrom A, Torup L, Gual A, van den Brink W. Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene. Biol Psychiatry. 2013;73:706–713. doi: 10.1016/j.biopsych.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Endocrine effects of opioid antagonists. Opiate Receptors and Antagonists. 2009:581–604. [Google Scholar]
- Moos RH. The development of a menstrual distress questionnaire. Psychosomatic medicine. 1968;30:853–867. doi: 10.1097/00006842-196811000-00006. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Quigley ME, Yen SSC. The role of endogenous opiates on lh secretion during the menstrual cycle. Journal of Clinical Endocrinology & Metabolism. 1980;51:179–181. doi: 10.1210/jcem-51-1-179. [DOI] [PubMed] [Google Scholar]
- Rapkin A. A review of treatment of premenstrual syndrome and premenstrual dysphoric disorder. Psychoneuroendocrinology. 2003;28(Suppl 3):39–53. doi: 10.1016/s0306-4530(03)00096-9. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Shoupe D, Reading A, Daneshgar KK, Goldman L, Bohn Y, Brann DW, Mahesh VB. Decreased Central Opioid Activity in Premenstrual Syndrome: Luterinizing Hormone Response to Naloxone. Journal of the Society for Gynecologic Investigation. 1996;3:93–98. doi: 10.1016/1071-5576(95)00045-3. [DOI] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9:13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- Ray R, Jepson C, Patterson F, Strasser A, Rukstalis M, Perkins K, Lynch KG, O’Malley S, Berrettini WH, Lerman C. Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology (Berl) 2006;188:355–363. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- Reame NE, Kelche R, Beitins I, Yu M, Zawacki CM, Padmanabhan V. Age effects of follicle-stimulating hormone and pulsatile luteinizing hormone secretion across the menstrual cycle of premenopausal women. The Journal of Clinical Endocrinology & Metabolism. 1996;81:1512–1518. doi: 10.1210/jcem.81.4.8636360. [DOI] [PubMed] [Google Scholar]
- Roche DJ, Childs E, Epstein AM, King AC. Acute HPA axis response to naltrexone differs in female vs. male smokers. Psychoneuroendocrinology. 2010;35:596–606. doi: 10.1016/j.psyneuen.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, King AC, Cohoon AJ, Lovallo WR. Hormonal contraceptive use diminishes salivary cortisol response to psychosocial stress and naltrexone in healthy women. Pharmacol Biochem Behav. 2013;109:84–90. doi: 10.1016/j.pbb.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010:CD001867. doi: 10.1002/14651858.CD001867.pub3. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SM, Gianoulakis C, Palmour RM, Benkelfat C, Leyton M. The Effect of Naltrexone on Alcohol’s Stimulant Properties and Self-Administration Behavior in Social Drinkers: Influence of Gender and Genotype. Alcoholism: Clinical and Experimental Research. 2011;35:1134–1141. doi: 10.1111/j.1530-0277.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- Shoupe D, Montz FJ, Lobo RA. The effects of estrogen and progestin on endogenous opioid activity in oophorectomized women. Journal of Clinical Endocrinology & Metabolism. 1985;60:178–183. doi: 10.1210/jcem-60-1-178. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomati M, Bernardi F, Luisi S, Puccetti S, Casarosa E, Liut M, Quirici B, Pieri M, Genazzani AD, Luisi M, Genazzani AR. Conjugated equine estrogens, estrone sulphate and estradiol valerate oral administration in ovariectomized rats: effects on central and peripheral allopregnanolone and β-endorphin. Maturitas. 2002;43:195–206. doi: 10.1016/s0378-5122(02)00205-0. [DOI] [PubMed] [Google Scholar]
- Stomati M, Bersi C, Rubino S, Palumbo M, Comitini G, Genazzani AD, Santre M, Petraglia F, Genazzani AR. Neuroendocrine effects of different estradiol-progestin regimens in postmenopausal women. Maturitas. 1997;28:127–135. doi: 10.1016/s0378-5122(97)00073-x. [DOI] [PubMed] [Google Scholar]
- Straneva PA, Maixner W, Light KC, Pedersen CA, Costello NL, Girdler SS. Menstrual cycle, beta-endorphins, and pain sensitivity in premenstrual dysphoric disorder. Health Psychology. 2002;21:358. [PubMed] [Google Scholar]
- Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36:544–552. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- Teoh SK, Mendelson JH, Mello NK, Skupny A. Alcohol effects on naltrexone-induced stimulation of pituitary, adrenal, and gonadal hormones during the early follicular phase of the menstrual cycle. Journal of Clinical Endocrinology & Metabolism. 1988;66:1181–1186. doi: 10.1210/jcem-66-6-1181. [DOI] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O’Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr. 1998;178:440–466. [PubMed] [Google Scholar]
- Vankrieken L. In: IMMULITE reproductive hormone assays: multicenter reference range data. Vankriken L, editor. Diagnostic Products Corporation; 2000. [Google Scholar]
- Wand GS, Weerts EM, Kuwabara H, Frost JJ, Xu X, McCaul ME. Naloxone-induced cortisol predicts mu opioid receptor binding potential in specific brain regions of healthy subjects. Psychoneuroendocrinology. 2011;36:1453–1459. doi: 10.1016/j.psyneuen.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw SL, Wehrenberg WB, Ferin M, Antunes JL, Frantz AG. Effect of sex steroids on beta-endorphin in hypophyseal portal blood. J Clin Endocrinol Metab. 1982;55:877–881. doi: 10.1210/jcem-55-5-877. [DOI] [PubMed] [Google Scholar]
- Wehrenberg WB, Wardlaw SL, Frantz AG, Ferin M. beta-Endorphin in hypophysealfportal blood: variations throughout the menstrual cycle. Endocrinology. 1982;111:879–881. doi: 10.1210/endo-111-3-879. [DOI] [PubMed] [Google Scholar]