Abstract
We previously reported that preculture of fibroblasts (FBs) and endothelial cells (ECs) prior to cardiomyocytes (CMs) improved the structural and functional properties of engineered cardiac tissue compared to culture of CMs alone or co-culture of all three cell types. However, these approaches did not result in formation of capillary-like cords, which are precursors to vascularization in vivo. Here we hypothesized that seeding the ECs first on Matrigel and then FBs 24 h later to stabilize the endothelial network (sequential preculture) would enhance cord formation in engineered cardiac organoids. Three sequential preculture groups were tested by seeding ECs (D4T line) at 8%, 15% and 31% of the total cell number on Matrigel-coated microchannels and incubating for 24 h. Cardiac FBs were then seeded (32%, 25% and 9% of the total cell number, respectively) and incubated an additional 24 h. Finally, neonatal rat CMs (60% of the total cell number) were added and the organoids were cultivated for seven days. Within 24 h, the 8% EC group formed elongated cords which eventually developed into beating cylindrical organoids, while the 15% and 31% EC groups proliferated into flat EC monolayers with poor viability. Excitation threshold (ET) in the 8% EC group (3.4 ± 1.2 V cm−1) was comparable to that of the CM group (3.3 ± 1.4 V cm−1). The ET worsened with increasing EC seeding density (15% EC: 4.4 ± 1.5 V cm−1; 31% EC: 4.9 ± 1.5 V cm−1). Thus, sequential preculture promoted vascular cord formation and enhanced architecture and function of engineered heart tissues.
1. Introduction
The goal of cardiac tissue engineering is to build heart tissues that can serve as models for pathophysiological discovery or as functional replacements for damaged myocardium. The engineered tissues should thus not only resemble the structure of native myocardium, but also be vascularized to enable proper oxygenation and integration with the blood supply of the host.
Cardiac tissue consists of three major cell types: cardiomyocytes (CMs), which compose about a third of the total cell mixture, and the non-myocytes (fibroblasts, FBs and endothelial cells, ECs), which make up the remainder. CMs are responsible for generating contractile force (Banerjee et al 2006, Nag 1980) while FBs secrete extracellular matrix (ECM) proteins and paracrine factors (Banerjee et al 2006, Kuzuya and Kinsella 1994). ECs line coronary vasculature to provide a barrier to thrombus formation and can form capillary tubes which are precursors to neovascularization (Seghezzi et al 1998). A host of other non-myocyte cell types also exist in the heart including erythrocytes, leukocytes, smooth muscle cells and pericytes (Banerjee et al 2006, Nag 1980), though in much smaller numbers. Pericytes are responsible for stabilizing capillary sprouts in vivo through the deposition of perivascular ECM components (Armulik et al 2005, von Tell et al 2006), and it was recently suggested that these pericytes may express many of the same markers as mesenchymal stem cells (MSCs) (Crisan et al 2008). Initial attempts at cardiac tissue engineering tended to remove non-myocytes from heart cell isolates (Bursac et al 1999), whereas recent work shows that their inclusion can enhance the function of engineered tissue (Caspi et al 2007, Iyer et al 2009a, Levenberg et al 2005, Naito et al 2006).
We previously showed that pre-culturing EC and FB in microchannels for two days prior to seeding CM (termed ‘preculture’) resulted in beating cardiac organoids resembling native myofibers (Iyer et al 2009a). These organoids displayed superior functional and structural properties to organoids engineered using purified CMs (‘enriched CMs’) or organoids engineered by mixing all three cell types (‘simultaneous tri-culture’) (Iyer et al 2009a). However, ECs within the precultured organoids did not organize themselves into capillary-like structures, and instead were found evenly distributed throughout the tissue (Iyer et al 2009a, 2009b). This was surprising, since human umbilical vein endothelial cells (HUVEC) and human dermal microvascular endothelial cells (HMVEC) grown on collagen gels in 3D have been shown to form capillary-like sprouts (Matsumoto et al 2007, Nor et al 1999, Wright et al 2002). Human embryonic stem-cell (hESC)-derived ECs have been shown to spontaneously form cords when seeded on Matrigel (Levenberg et al 2002). The formation of these capillary sprouts by ECs is a key step toward neovascularization (Seghezzi et al 1998), which motivated us to further explore if we could enhance cord formation in our engineered tissues.
We hypothesized that seeding FBs 24 h after ECs would stabilize the immature sprouts formed by ECs, and that these stabilized vascular networks would result in improved functional properties of engineered cardiac tissue. To test this hypothesis, we sequentially cultured ECs to allow vascular sprouting to occur, followed by FBs 24 h later to aid in stabilization of the cords by deposition of perivascular matrix (Armulik et al 2005, Levenberg et al 2005, von Tell et al 2006). We tested the effect of EC number (8%, 15% and 31% of the total cell number) on cord formation, to determine the optimal percentage of ECs for engineering cardiac tissues containing stable vascular cords. We made use of microfabricated poly(ethylene glycol) (PEG) channels to answer this question using microtissues. Thus, microfabrication enabled us to efficiently use cells and culture media compared to the regular mm-scale tissue culture.
2. Methods
2.1. Microfabrication and sterilization of PEG templates
The technique for PEG template microfabrication has been previously described (Iyer et al 2009a). Briefly, liquid PEG diacrylate monomer was mixed with 0.5% v/v hydroxy methyl propiophenone photoinitiator (HMPP, Sigma) to create a pre-polymer solution. The pre-polymer was crosslinked around a polypropylene mesh (used as a master for the microchannels) by exposure to a UVB light source (UVL-21, ultraviolet products) for 30 s at a distance of 1 cm through a circular mask, resulting in PEG discs patterned with three-dimensional microchannels of 100–200 µm in diameter and 3–1 mm in length. The discs were sterilized then soaked in CM/FB medium (composition described in section 2.2.1) for 24–18 h. Discs were coated with Matrigel® matrix (Beckton–Dickinson) and placed in 96-well titer dishes at 4 °C for 24 h (see supplemental figure 1 available at stacks.iop.org/BF/4/035002/mmedia). The discs were then placed at 37 °C for 1 h prior to cell seeding to allow the Matrigel® to undergo gelation.
2.2. Cells
2.2.1. Neonatal rat heart cell isolation
The procedure for neonatal rat heart cell isolation is described extensively in our previous work (Radisic et al 2003, 2004a, 2004b, 2006). Briefly, neonatal (1–2 day-old) Sprague-Dawley rats were euthanized according to the procedure approved by the University of Toronto Committee on Animal Care. The hearts were quartered and the cells were isolated by serial enzymatic digest. The supernatant was collected and centrifuged at low speed (750 RPM or 94 × g, to remove red blood cell contamination) for 4 min. The resulting cell suspension was either resuspended in culture medium and pre-plated into T75 flasks for two 1 h intervals to separate the non-adherent cells (enriched CM) from the adherent cells (non-myocytes), or was used directly as a whole heart isolate (see supplemental figure 1 available at stacks.iop.org/BF/4/035002/mmedia and section 2.3.1). Primary cardiac FBs used in experiments were obtained by passaging the cells adhered to the T75 flask during the pre-plating step. The CM and FB were cultured in the same medium, termed ‘CM/FB medium’, consisting of Dulbecco’s modified eagle medium (DMEM) with 4.5 g L−1 glucose, 4 mM L-glutamine, 10% certified fetal bovine serum (FBS), 100 U mL−1 penicillin, 100 µg mL−1 streptomycin, and 10 mM 4–2-hydroxyethyl-1-piperazineethanesulfonic acid buffer (HEPES, Gibco/Invitrogen).
2.2.2. Cell lines
D4T ECs (kind gift of P Zandstra), an embryoid body-derived mouse EC line (Choi et al 1998), were maintained in EC medium consisting of Iscove’s modified Dulbecco’s medium (IMDM), 5% certified FBS, 100 U mL−1 penicillin, and 100 µg mL−1 streptomycin (Gibco/Invitrogen). Cells were passaged at 70% confluence every 5–7 days.
2.3. Experimental design
2.3.1. Modulating EC seeding density
For sequential preculture organoids (supplemental figure 1(A) available at stacks.iop.org/BF/4/035002/mmedia), the total cell number seeded was 2 × 105 cells per PEG disc as in our previous studies (Iyer et al 2009a, 2009b). ECs were seeded first (to enable cord formation), followed by seeding of FBs 24 h later (to enable cord stabilization through ECM deposition), and enriched CMs 48 h later (to form cardiac tissue). The fraction of CMs was fixed at 60%, as determined to be favorable for organoid formation in our previous studies (Iyer et al 2009b). The fractions of ECs and FBs in the remaining 40% of non-myocytes were varied, to form three experimental groups: (i) 8% ECs + 32% FBs, (ii) 15% ECs + 25% FBs and (iii) 31% of ECs + 9% of FBs. In all groups, the total number of CMs, ECs and FBs was maintained at 2 × 105 cells disc−1. Organoids based on enriched CMs (supplemental figure 1(B)) and the whole heart cell isolates (supplemental figure 1(C), both available at stacks.iop.org/BF/4/035002/mmedia) served as controls. The experimental design is described in supplemental figure 1 (available at stacks.iop.org/BF/4/035002/mmedia) and table 1.
Table 1.
Percentages of cells seeded in the sequential preculture experiments.
| Group | EC (applied at 0 h) | FB (applied after 24 h) | Enriched CM (applied at 48 h) |
|---|---|---|---|
| Sequential preculture 8% | 8% (16 × 103) | 32% (64 × 103) | 60% (1.2 × 105) |
| Sequential preculture 15% | 15% (31 × 103) | 25% (49 × 103) | 60% (1.2 × 105) |
| Sequential preculture 31 % | 31% (62 × 103) | 9% (18 × 103) | 60% (1.2 × 105) |
2.4. Assessments
2.4.1. Live/dead staining
Live/dead staining was performed as previously described (Iyer et al 2009a). Briefly, the PEG discs were incubated in a solution of propidium iodide and carboxyfluorescein diacetate-succinimidyl ester for 30–45 min at 37 °C. As a result, non-viable cell nuclei were labeled red while viable cell cytoplasms were labeled green. The tissues were imaged on a fluorescence microscope (Leica DMIRE2).
2.4.2. Cryosectioning and immunofluorescence
For immunostaining, organoids were cryosectioned and immunostained as described previously (Iyer et al 2009a). Briefly, discs were fixed in 4% paraformaldehyde (PEA) for 1 h and immersed in a 30% w/v solution of sucrose for 1–2 h. The discs were placed face down in cryomolds, embedded in O.C.T medium, and snap frozen in liquid nitrogen for 30 s. The discs were stored at −80 °C for at least 1 h before cryosectioning in a cryostat operating at an ambient and specimen temperature of −24 °C. 10 µm thick face sections were mounted on positively charged specimen slides (VWR) and stored at −20 °C for 24 h. The specimens were thawed at room temperature, fixed in acetone, and again air dried before blocking with 10% normal goat serum (NGS) or normal horse serum (NHS) in PBS and stained with primary antibodies (at 4 °C for 24–48 h) and secondary antibodies (at room temperature for 1 h). Primary antibodies included rabbit or mouse anti-cardiac troponin I (Chemicon, 1:150), rabbit anti-PECAM-l/CD31 (Abeam, 1:50), and mouse anti-vimentin-Cy3 (Sigma, 1:50). FITC-conjugated goat anti-rabbit (Vector Laboratories, 1:100) or goat anti-mouse antibody was used as a secondary antibody for troponin I and CD31. DAPI was used as a nuclear counterstain (final concentration of 1 µg mL−1) and was added to the secondary antibody solution. A drop of mounting medium was added to the specimen and a glass coverslip was placed on top.
Fluorescence microscopy was carried out on an inverted fluorescence microscope (Olympus America, Model # IX81) controlled by QED Imaging In Vitro Software version 3.1.0 (Media Cybernetics Inc.). Cell fractions were determined by manual counting, which was performed by two blinded, independent observers. Numbers of CMs, non-myocytes, or ECs were determined by counting the number of troponin I-positive, vimentin-positive, or CD31 -positive cells in each fluorescence image. These were normalized to the total cell number per image, as determined by DAPI counterstaining, and plotted as average percentages over several images. Elongation of CMs was determined by measuring the aspect ratio (the ratio of cell length to cell width) of troponin I-positive cells (n = 5 images/group). The analysis was done by drawing lines on the axes of the cells and measuring the length of the line by ImageJ.
2.4.3. Cell tracking
Endothelial cells were fluorescently labeled to facilitate cell tracking of cords, as previously described (Iyer et al 2009b). Briefly, ECs were incubated for 30 min at 37 °C and 5% CO2 in a 5 µM solution of CellTracker™ Red CMPTX (Molecular Probes Cat. no C34552) made up in serum-free medium. The cells were centrifuged and washed twice prior to seeding in microchannels. The organoids were imaged by fluorescence microscopy at 24 h (prior to FB seeding) and 48 h (after FB seeding) to monitor the presence and morphology of vascular cords.
2.4.4. Functional properties
Contractile function of cardiac organoids was measured using either an S88X or an S48 grass stimulator (Grass Technologies/Astro-Med Inc) as described previously (Iyer et al 2009a, Radisic et al 2004a). Briefly, the excitation threshold (ET, measured in V cm−1) of the tissues was determined by subjecting the tissues to monophasic, square pulses of 2 ms pulse width and measuring the minimum amplitude at which synchronous contractions could be observed. Maximum capture rate (MCR, measured in Hz) was determined at 200% of ET by measuring the maximum frequency of synchronized contractions. Thus, a lower ET and higher MCR implied a more electrically responsive tissue. If the organoids could not be excited by an electric field strength of up to 10 V cm−1, they were deemed as non-beating (denoted n/a). We also quantified the success rate, defined as the ratio of the number of beating samples to the total number of samples tested for contractile function. A success rate of 1.0 implied all tested samples could be induced to contract, while a value less than 1.0 implied fewer samples were able to contract.
2.4.5. Statistics and data representation
Results are presented as means ± standard deviation. Statistically significant differences, unless otherwise indicated, are denoted with horizontal bars. All statistical calculations were performed using SigmaStat 3.0 (SPSS Inc). One-way ANOVA in conjunction with the Holm–Sidak posthoc test was used to compare three or more groups or two groups versus control while t-test was used for pairwise comparisons. Normality of data and equality of variance were checked for all comparisons. Where normality failed, one-way ANOVA on ranks was performed using a Mann–Whitney test. Where equality of variance failed, ANOVA on ranks in conjunction with Dunn’s test and multiple pairwise comparisons was performed. A p-value < 0.05 was considered statistically significant for all tests. Sample numbers were n = 3–5 biological replicates for all analyses. For cell number/viability quantitation and image analyses, data were pooled from n = 3–5 biological samples, and n = 9–12 pooled images were counted and analyzed from a representative set of images for each sample.
3. Results and discussion
3.1. The 8% EC group yielded beating organoids with vascular-like cords
We tested the hypothesis that sequential preculture of ECs prior to FBs and CMs would enable the formation of vascular cords in cardiac organoids. To this end, different numbers of ECs (table 1) were first seeded on Matrigel-coated PEG microchannels to allow for capillary-like sprouts to form within 24 h, as previously reported (Levenberg et al 2002, Wright et al 2002). The two EC densities at the lower end of the spectrum (8% and 15%) were chosen because they were in agreement with seeding densities previously reported to support cord formation (Levenberg et al 2002, Matsumoto et al 2007, Nor et al 1999, Wright et al 2002). The value of 31% ECs served as an effective control for comparison to our previous studies (Iyer et al 2009b).
We have previously conducted culture medium screen assays to compare the effects of EC medium (IMDM + 5% FBS + 1% pen/strep) with those of CM/FB medium (DMEM + 10% FBS + 1% HEPES + 1% pen/strep) on cell viability, density and proliferation. These medium screen data are published in our previous work (Iyer et al 2009a), and we have shown that there were no significant differences in proliferation, cell density, or cell viability when ECs were grown in EC medium versus CM/FB medium (Iyer et al 2009a). Thus, we chose the CM/FB medium for the tri-culture studies.
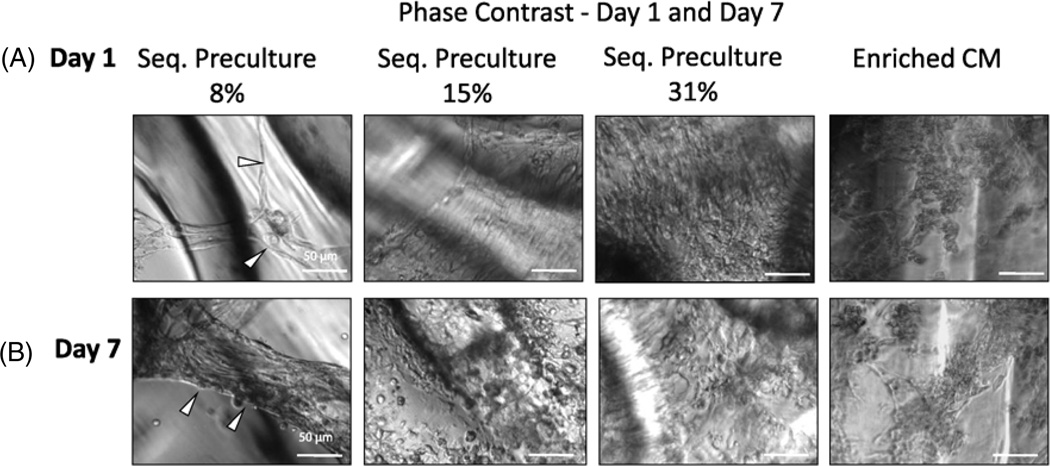
As shown in phase contrast micrographs (figure 1(A)), we observed marked differences in morphology among the groups at 24 h after seeding the ECs on Matrigel. As hypothesized, ECs formed cords on Matrigel prior to the addition of FBs, and the 8% EC group was the only group which showed evidence of capillary cord-like sprouts that eventually integrated into healthy cardiac organoids resembling cardiac myofibres (figure 1(A), white arrows). In the groups with higher EC seeding densities, most of the cells had proliferated into flat cell layers. Figure 1(B) shows phase contrast images of the cardiac organoids on day 7 of cultivation after adding FB and CM. The 8% EC group also formed three-dimensional organoids (white arrows) characterized by elongated cells while the higher EC seeding percentage groups (15% ECs, 31% ECs) remained as a flat layer of cells. As the EC seeding percentage increased we noted fewer spontaneous contractions on day 7 and a higher number of rounded cells.
Figure 1.
EC cord formation is dependent on the percentage of ECs in sequential preculture (phase contrast). Sequential preculture containing 8% ECs (sequential preculture 8%) was performed to allow for capillary cord formation by ECs. The enriched CM fraction was kept constant at the percentage determined most beneficial for regular pre-culture in previous studies (60% enriched CM) and the EC seeding percentage was varied from 8% to 31%. (A) Phase contrast microscopy at 24 h following EC seeding. Sequential preculture 8% group displayed evidence of cords (white arrows) while the other groups showed EC proliferation into flat cell layers. (B) Phase contrast, microscopy after seven days of cultivation. Cardiac FB and enriched CM were added at 24 and 48 h following EC seeding, respectively, and the organoids were cultivated for seven additional days. Sequential preculture 8% formed cylindrical organoids (white arrows) with overall elongated cell morphology, while the other groups remained as two-dimensional cell layers consisting of rounded cells. Enriched CMs also appeared mostly rounded. n = 3–5 samples per group.
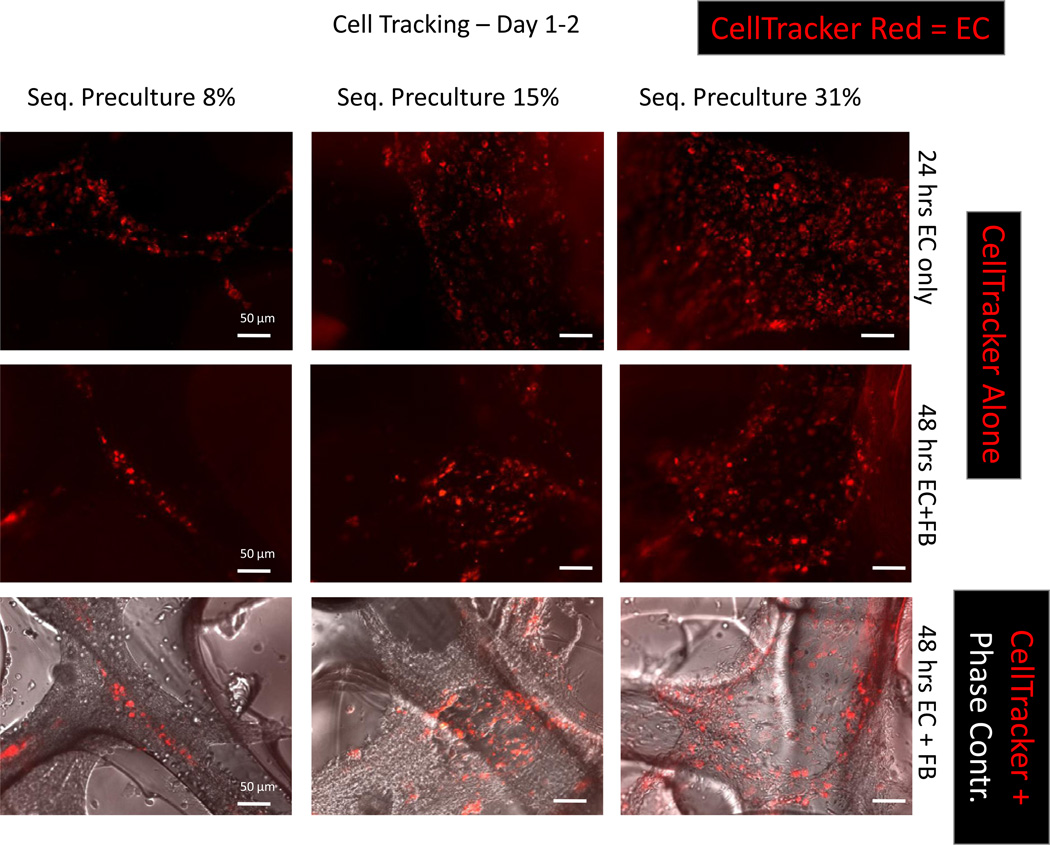
3.2. Cords are stabilized over 48 h in the 8% EC group
We further investigated whether the cords retained their structure after seeding the FBs. This was assessed by performing cell tracking on the EC cords by labeling the ECs with a CellTracker red dye prior to seeding. As shown in figure 2, the cords in the 8% EC group retained their cord-like organization even after FBs were seeded and did not proliferate into EC monolayers. This was in contrast to the 15% and 31% EC groups, where flat monolayers of cells were visible at 48 h (figure 2, red labeled cells).
Figure 2.
Cell tracking on cords demonstrates persistence of EC cords after FB seeding in the sequential preculture 8% group. Primary ECs were incubated with CellTracker Red and grown for 24 h on Matrigel®-coated microchannels to allow for cord formation. Primary cardiac FBs were then seeded and the cords were grown for an additional 24 h. At 48 h cords were imaged under both fluorescence and phase contrast microscopy. Cords formed in the sequential preculture 8% group after 24 h, whereas the ECs proliferated into flat monolayers at all other seeding densities (top row). After seeding the FBs, the cords appear to be stabilized in the sequential preculture 8% group. In the 15% and 31% groups, CellTracker Red-labeled ECs are found scattered throughout the structure (scale bar: 50 /xm). n = 3–5 samples per group.
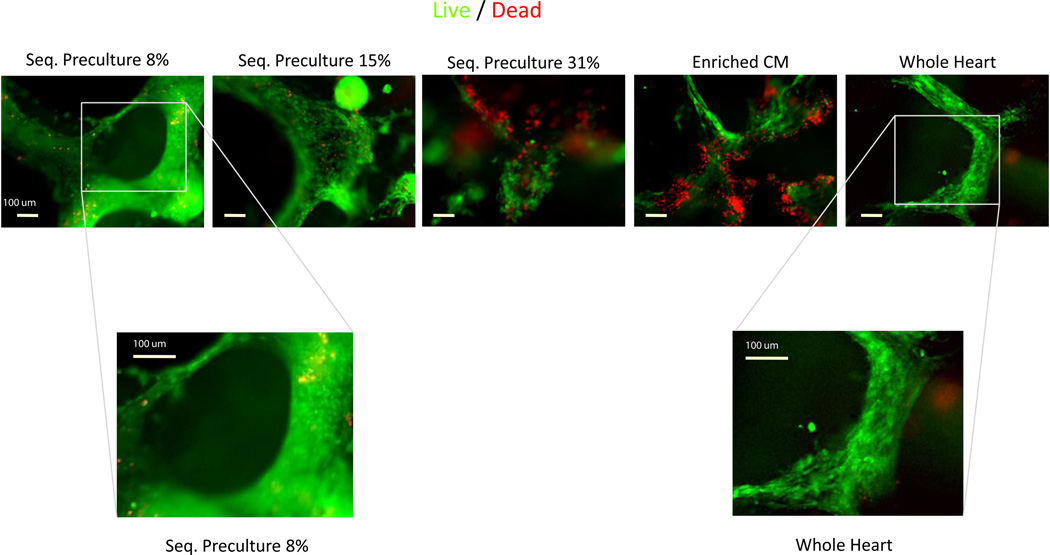
3.3. Morphology and viability of the 8% EC group organoids resemble organoids grown using whole heart cell isolates
Live/dead staining performed at day 7 on the sequential preculture groups (figure 3), seeded with varying EC percentages (8%, 15% and 31%), showed similar morphological features to phase contrast microscopy and cell tracking studies (figures 1 and 2). We also performed live/dead staining on organoids engineered using CMs and the whole heart isolate as a baseline condition (figure 3). Due to the non-specific nature of live-dead staining (figure 3), it was not possible to determine exactly the phenotypes of necrotic cells. The 8% EC group which formed the cords was also the group which formed the most viable cardiac organoids. In contrast, the other groups showed marked areas of rounded cells, and the integrity of the organoids became more fragmented as the EC seeding percentage increased to 31%. The majority of cells in the 8% EC cord-forming group showed high viability (figure 3, green cells showing CFDA expression). However, as the EC seeding density increased to 31 %, the number of dead cells (figure 3, nuclei stained red with propidium iodide) increased and became comparable to the CM group, which displayed the greatest amount of cell death (figure 3, red stained nuclei). Cells in the whole heart isolate group formed fibrillar structures which appeared elongated, viable and healthy, and bore a very similar morphology to the 8% EC group which also contained vascular-like cords (figure 3, right panel).
Figure 3.
Viability of sequential preculture is the highest in the 8% group. Organoids were stained with CFDA-SE (green) and propidium iodide (red) to label live cells and dead cell nuclei, respectively, after seven days of sequential preculture. The group which formed cords (8%) had the fewest number of dead cells and the highest cell viability as well as elongated organoids densely populated with cells. Viability appeared to decrease with increasing EC density and was lowest in the enriched CM group. Higher magnification images for the sequential preculture 8% and whole heart isolate groups showed similar cell morphology in the two groups. n = 3–5 samples per group.
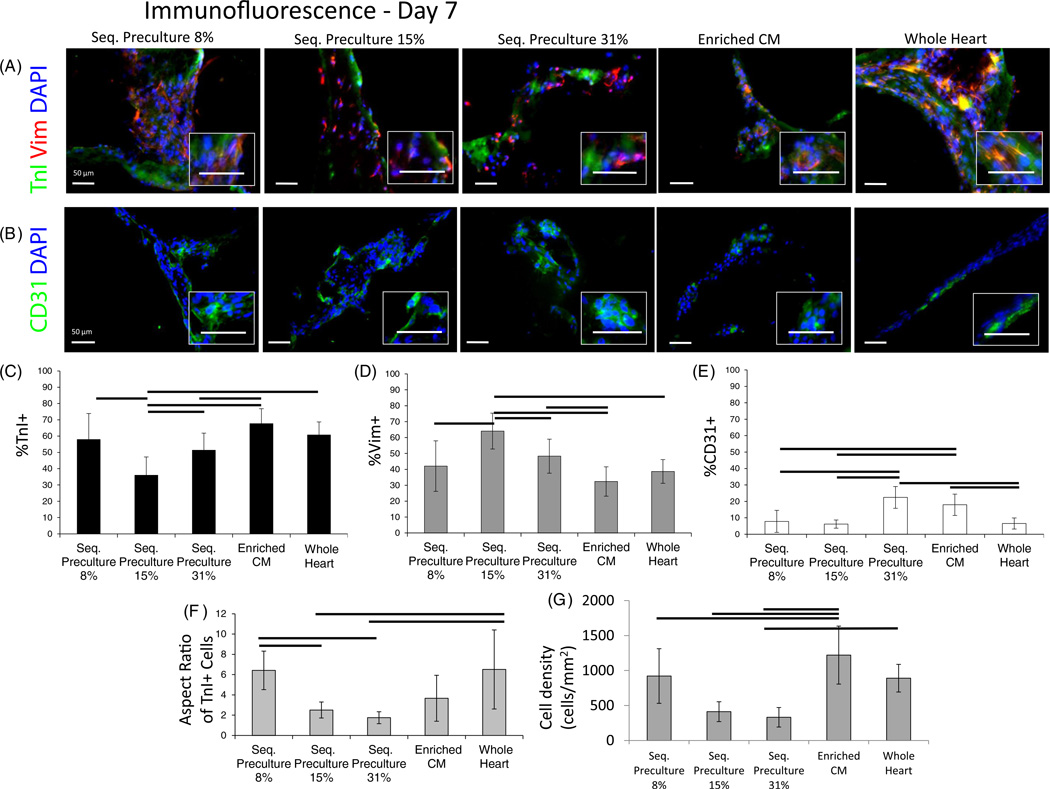
3.4. Elongation of troponin I-positive cells is most prevalent in the 8% EC and whole heart isolate groups
Immunostaining (figure 4(A)) for troponin I (TnI), vimentin (Vim) and CD31 was performed in all groups to provide information on the cell composition of organoids at day 7 of cultivation, using CM organoids and whole heart isolate organoids as baseline controls. The 8% EC group formed the most elongated organoids with a compact arrangement of cells as compared to the 15% and 31% EC groups where the cell density appeared sparse. The presence of cardiac marker TnI was markedly more abundant in the 8% EC group, CM group and the whole heart isolate group. Notably, the TnI-positive cells were most elongated in the 8% EC and whole heart isolate groups (figure 4(F), P < 0.0001 one-way ANOVA). The cell density was overall highest in the enriched CM group and comparable among the sequential preculture 8% and whole heart groups, while cell densities were overall lowest in the 15% and 31% sequential preculture groups (figure 4(G), P < 0.05 ANOVA on ranks). Commensurate with the lower number of Tnl-positive cells and higher numbers of ECs in the 15% and 31% EC groups, there were also more non-myocytes (Vim-positive cells) in these groups. Meanwhile the presence of Vim was lower in the 8% EC group, the CM group, and the whole heart isolate group as compared to the 15% and 31% EC groups.
Figure 4.
Immunofluorescence microscopy for Troponin-I, Vimentin, and CD31 in sequential preculture. Immunofluorescence data were obtained from frozen sections of organoids in order to assess phenotype, cell distribution and tissue morphology. (A) Troponin-I (green) was used to label cardiomyocytes and Vimentin (red) was used to label non-myocytes (fibroblasts and endothelial cells collectively). (B) Staining for CD31 was used to image endothelial cells independently. DAPI (blue) was used as a counterstain for cell nuclei in all images. Troponin I presence appeared highest in the group which formed cords (8%), while Vimentin presence increased as the EC seeding percentage increased. Organoids were overall more elongated in the group with the cords and similar in morphology to the whole heart isolate group (see high magnification insets). (C)–(E) Manual counting of images was performed to assess the relative contribution of each cell type to the final cellular composition of the organoids. Percentages of (C) Troponin I-positive, (D) Vimentin-positive and (E) CD31-positive cells. (C) The percentage of Troponin I-positive cells was high in the sequential preculture 8% group which formed cords, the enriched CM group and the whole heart isolate group, and was lowest in the sequential preculture 15% and sequential preculture 31% groups. (D) The opposite trend was seen in the Vimentin-positive percentages, with lower non-myocyte percentages in the sequential preculture 8% group, enriched CM group, and whole heart isolate group compared to high percentages in the sequential preculture 15% and sequential preculture 31 % groups, suggesting non-myocyte proliferation and/or possible myocyte death in these groups. (E) The CD31-positive fraction was in the range from 5% to 10% in the sequential preculture 8%, sequential preculture 15%, and whole heart isolate groups, but nearly double in the sequential preculture 31% and enriched CM groups. n = 3–5 samples per group for experimental samples, n = 9–11 images counted per group. (F) Aspect ratio of Troponin I-positive cells showed more elongated cardiomyocytes in the sequential preculture 8% and whole heart isolate groups (n = 5 images counted per group). (G) High cell density was found in the sequential preculture 8%, enriched CM and whole heart isolate groups.
We expected the higher TnI presence and lower Vim presence in the CM group since we have previously reported that the percentage of CMs after two preplating steps was 81% ± 14%, as determined by analytical flow cytometry (Iyer et al 2009a). Since these cells are derived from a primary source, consisting of a heterogeneous population, the purity of CMs used in the present study is consistent with previous results (Naito et al 2006). CD31 staining (figure 4(B)) correlated well with the percentages of ECs, with an increase in EC seeding percentage correlating to an increase in the number of CD31-positive cells on day 7. The 8% EC group and the CM group also displayed lower percentages of CD31 -positive cells as compared to the 15% and 31% EC groups. Overall, we noted that, qualitatively, the cell density, distribution of Tnl-positive, Vim-positive, and CD31-positive cells, and cell elongation were most similar between the 8% EC group which formed cords and the whole heart cell isolate group.
As shown in figure 4(C), the percentage of CM on day 7, as expected, was the highest in the CM group (~70%). This high value was expected since the enriched CMs contain 81% ± 14% Tnl-positive cells after two preplating steps (Iyer et al 2009a), and the fraction of CMs decreases with time due to proliferation of non-myocytes and some CM death. The percentage of CMs was maintained at day 7 in 8% EC and 31% EC groups, but not in the 15% EC group (figure 4(C)). Interestingly, organoids engineered from the whole heart isolate also attained a final percentage of ~60% CMs, similar to the 8% and 31% EC groups.
As expected, the trends in the percentage of Vim-positive cells (figure 4(D)) were exactly the inverse of the trends seen in the Tnl-positive cells. The 15% EC and 31% EC groups contained 50–70% non-myocytes after seven days of culture, presumably due to proliferation of non-myocytes. The CM group, 8% EC group, and the whole heart isolate group all attained similar final percentages of non-myocytes (between 30% and 40%).
To determine the endothelial contribution to the non-myocyte percentages, the number of CD31 -positive cells was also quantified from immunofluorescence data (figure 4(E)). In all sequential preculture groups, the final percentages of ECs on day 7 increased with increasing the seeding number of ECs, as expected. The final percentages of CD31-positive cells were similar (between 5% and 10%) in the 8% EC, 15% EC, and whole heart isolate groups, but were higher (around 20%) in the 31% EC and CM groups. Considering that the percentages of Vim-positive cells were higher than the percentages of CD31-positive cells, it is likely that the majority of non-myocytes present in the organoids were FBs and that the FBs were also primarily responsible for any non-myocyte proliferation that may have taken place. As with the TnI and Vim positive cells, the 8% EC group and the whole heart isolate group had similar numbers of CD31 -positive cells, and appeared similar in terms of myocyte and non-myocyte composition. Aspect ratios of TnI-positive cells (figure 4(F)) were highest in the 8% EC and whole heart isolate groups (~6) compared to the other groups (~2–3). Cell densities (figure 4(G)) were also highest in these two groups as well as the enriched CM group (~1000 cells/mm2) compared with the other groups where cell densities were less than half of this number (~300–500 cells/mm2), in line with qualitative observations.
3.5. Excitation threshold is lowest in the 8% EC group and increases with EC seeding percentage, while MCR is comparable among all sequential preculture groups
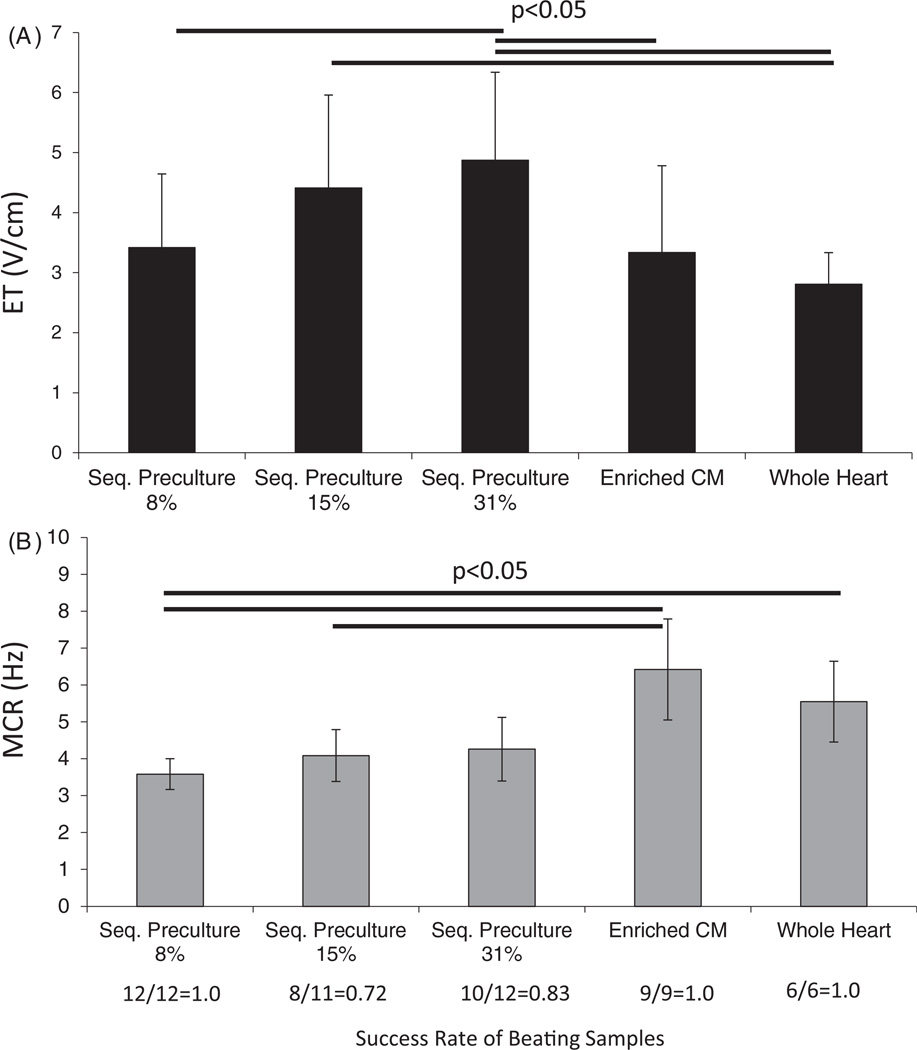
Functional data were obtained by measuring the ET (figure 5(A)) and MCR (figure 5(B)) of the organoids grown for seven days. The ET was best (lowest) in the 8% EC group and tended to increase as the EC seeding percentage increased (figure 5(A)). For the 8% EC group, the ET was 3.4 V cm−1, which was comparable to the CM group (3.3 V cm−1), and lower than both the 15% (4.4 V cm−1) and the 31% EC group (4.9 V cm−1). All of the sequential preculture groups had comparable MCRs (~4 Hz). However, the group with the highest MCR was the CM group (6.4 Hz).
Figure 5.
Functional properties of sequential preculture. (A) Excitation threshold (ET). (B) Maximum capture rate (MCR). Functional properties were measured for sequential preculture organoids by varying the electric field strength and frequency of stimulation. The ET was found to be lowest in the sequential preculture 8% group with the cords and the whole heart isolate group. MCRs, however, were highest in the enriched CM group and the whole heart isolate group, and comparable among all of the sequential preculture groups, n = 3–5 samples per group.
We also noted that the success rate of beating samples was highest (1.0) in the sequential preculture 8% group and the enriched CM group, where all samples could be induced to contract in the presence of an electric field. However in the 15% and 31% EC groups, the success rates were 0.7 and 0.8, respectively, suggesting poorer overall functional properties in these groups.
The use of sequential preculture containing 8% ECs presents a possible strategy for improving the structural organization and functional outcome of engineered heart tissue by enabling vascular-sprouting and stabilization. Our data indicate that the cell population consisting of 8% ECs, 32% FBs and 60% CMs supports both the formation of vascular cords and establishment of contractile function. Since increasing the EC seeding density above 8% worsened both the cell viability and functional properties (ET) of the organoids, this EC percentage may represent an upper limit beyond which functionality and viability may be compromised due to EC overgrowth and CM death.
Despite the success of the 8% EC group, the MCR was still higher in the CM and whole heart isolate groups. The whole heart isolate, in particular, represented a physiological mixture of cells where the cellular percentages present in the heart were not modified in any way. In effect, this was an excellent baseline condition for our studies, the one we were seeking to emulate through the use of sequential preculture so that the cellular composition and organization would mimic that of the native heart.
We used microfabricated substrates to facilitate the optimization of cell culture conditions that would require significant cell and culture media resources if performed on a mm-scale. Here we provide feasibility data, demonstrating that the results of the microscale studies can be scaled-up to mm-sized constructs. Scale-up of preculture conditions from microtemplate studies was possible by volumetrically scaling up the amount of Matrigel and cells in the 8% group used in 100 µm diameter microchannels and applying this to the macroscale geometry of a cylindrical collagen sponge of 2 mm height, 7 mm diameter. ECs were seeded first, followed by FB 24 h later and CM 48 h later resulting in a beating cardiac construct after nine days in culture (supplemental figure 2 and supplemental video 1, available at stacks.iop.org/BF/4/035002/mmedia). Further detailed studies of the mm-scale tissue properties are a subject of future work.
A confounding factor with the use of primary cells for cardiac tissue engineering is that the precise ratios of cells in the whole heart isolate can vary depending on the isolation conditions and viability of the starting population. Thus, we believe that the 8% EC group, which has defined seeding percentages of ECs, FBs and CMs, and allows for cord formation and stabilization, represents a better strategy for cardiac tissue engineering studies and is easily amenable to non-primary cell sources as well. As an example, the optimized cell percentages arrived at in this study (that is, 8% ECs, 32% FBs, and 60% CMs) combined with sequential preculture could be applied to hESC-derived or induced pluripotent stem cell-derived ECs, FBs, and CMs and employed in engineering pre-vascularized, human, patient-specific heart tissues with properties similar to those in the native heart. Such an engineered heart tissue could provide a physiological-like heart tissue model for drug development and disease modeling studies as well as a source of engineered cardiac patches for patient therapy.
Upon enzymatic isolation, the native heart isolate contains ~47% CM, 49% FB, 2–3% EC and 2–3% smooth muscle cells (Naito et al 2006). After one pre-plating step the suspension contains ~63% CM, 33% FB, ~3% EC and ~3% smooth muscle cells as we and others have reported (Brown et al 2008, Iyer et al 2009a, Naito et al 2006). One more pre-plating step enriches the CMs to 81% ± 14% at the expense of FB while EC and smooth muscle cell percentage remains largely unaffected. In the enriched CM group and the whole heart group, the formation of vascular cords was not observed by EC although these cells made up 20% of the cells in the enriched CM group on day 7 in culture (figure 4(E)).
The vascular cords in the sequential preculture organoids did not have open lumens. In vivo, sprouting angiogenesis occurs by tip cell proliferation, migration and cord formation, followed by lumen formation through vacuole propagation (Fantin et al 2010, Wang et al 2010, Kamei et al 2006, Lubarsky and Krasnow 2003). In this study the last stage vacuole propagation and lumen formation was not yet achieved.
No cord formation was observed at all at EC densities of 15% and 31%. At higher densities, EC monolayer formation was observed. One possible explanation for formation of cords in 8% EC group could be secretion of autocrine growth factors (e.g. VEGF), which at the given cell density could be present at such concentration to promote cord formation rather than proliferation alone. One limitation of this study is that we could not determine the exact number of EC in the 8% group participating in the sprouting process, nor did we test a lower EC seeding density than 8%.
Cell tri-culture is now commonly used to pre-vascularize engineered cardiac tissues in vitro. Asakawa et al (2010) created an in vitro pre-vascular network by co-culturing ECs between cell sheets that were stacked to form three-dimensional tissues. In another example using hESC-derived CMs, Murry and colleagues (Stevens et al 2009a, 2009b) created scaffold-less tissues, and Levenberg and colleagues (Caspi et al 2007, Lesman et al 2010) created tissues on porous scaffolds using CMs, ECs and mesenchymal cells such as FBs. Their work collectively points to the fact that integration of the engineered cardiac tissue with the host myocardium was enhanced in the case of tri-culture (Iyer et al 2009b, Lesman et al 2010). In those cases, the primitive vessels in the engineered tissue functionally integrated with the host coronary vasculature and enabled graft survival. Recent evidence suggests that these randomly distributed capillary-like structures anastomose with the host vasculature through wrapping and tapping anastomosis, where the implant vasculature wraps around the host vasculature, followed by basement membrane dissolution and connection of the implant endothelium to the host endothelium (Cheng et al 2011). Human MSCs from the bone marrow were demonstrated to be instrumental in stabilizing the engineered blood vessels giving rise to perivascular cells (Au et al 2008). The work presented here addresses the in vitro component of pre-vascularization and demonstrates that the sequential seeding is beneficial for cord formation and stabilization. Achieving a more organized vascular structure in vitro is critical for the efficient vascular integration in vivo (Koffler et al 2011).
Still, much remains to be investigated—that is, while preculture of ECs and FBs prior to CMs has improved the structure and function of engineered cardiac tissue, the underlying mechanisms are yet to be explored. A combination of soluble factors, secreted by the FBs and ECs during the preculture period, as well as ECM deposited by these cells, may be responsible for the improved survival and function of CMs. Future work will be focused on identifying these soluble factors and their downstream targets in order to further elucidate the mechanisms at play.
4. Summary
Sequential preculture of ECs was investigated in an attempt to improve formation of vascular-like precursors known as cords. By fixing the seeding percentage of CMs at 60% and sequentially adding ECs, FBs (24 h later) and CMs (48 h later), and modulating the percentage of ECs (8%, 15% and 31% of the total cell number), capillary cords were found to form within 24 h only at the lowest seeding percentage of 8%. The 8% EC group eventually developed into functional cardiac organoids with ET comparable to organoids engineered using CMs and the whole heart cell isolates. The higher percentages (15% EC and 31 % EC) groups showed evidence of EC overgrowth and the formation of flat EC monolayers, resulting in cardiac organoids with inferior morphology and functional properties. The viability decreased with an increasing EC density. Morphological similarities and similar final percentages of CMs were noted between the 8% EC group and the whole heart cell isolate group, suggesting that the 8% EC group may be optimal for achieving a cellular composition physiologically similar to that of the native heart. Thus, sequential preculture with 8% ECs may aid in engineering heart tissues that not only emulate the electrophysiological properties of the native myocardium, but also enhance the vascularization potential of the tissue.
Supplementary Material
Acknowledgments
The authors wish to thank Peter Zandstra for the use of the D4T cell line and the following funding sources: National Institutes of Health (R01HL076485, P41 EB002520), NSERC Strategic grant (STPGP 381002-09), NSERC-CIHR Collaborative Health Research grant (CHRPJ 385981-10), NSERC Discovery grant (RGPIN 326982-10) and Discovery Accelerator Supplement (RGPAS 396125-10), Ontario Centres of Excellence International Scholarship, the Ontario Graduate Scholarship in Science and Technology (OGSST) and the Ontario Graduate Scholarship (OGS).
Footnotes
Online supplementary data available from stacks.iop.org/BF/4/035002/mmedia
References
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Asakawa N, Shimizu T, Tsuda Y, Sekiya S, Sasagawa T, Yamato M, Fukai F, Okano T. Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials. 2010;31:3903–3909. doi: 10.1016/j.biomaterials.2010.01.105. [DOI] [PubMed] [Google Scholar]
- Au P, Tarn J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann. New York Acad. Sci. 2006;1080:76–84. doi: 10.1196/annals.1380.007. [DOI] [PubMed] [Google Scholar]
- Brown MA, Iyer RK, Radisic M. Pulsatile perfusion bioreactor for cardiac tissue engineering. Biotechnol. Prog. 2008;24:907–920. doi: 10.1002/btpr.11. [DOI] [PubMed] [Google Scholar]
- Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am. J. Physiol. 1999;277:H433–H444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ. Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- Cheng G, Liao S, Kit Wong H, Lacorre DA, di Tomaso E, Au P, Fukumura D, Jain RK, Munn LL. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood. 2011;118:4740–4749. doi: 10.1182/blood-2011-02-338426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer RK, Chiu LL, Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J. Biomed. Mater. Res. A. 2009a;89:616–631. doi: 10.1002/jbm.a.32014. [DOI] [PubMed] [Google Scholar]
- Iyer RK, Chui J, Radisic M. Spatiotemporal tracking of cells in tissue-engineered cardiac organoids. Tissue Eng. Regen. Med. 2009b;3:196–207. doi: 10.1002/term.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo . Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- Koffler J, Kaufman-Francis K, Shandalov Y, Egozi D, Pavlov DA, Landesberg A, Levenberg S. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc. Natl Acad. Sci. USA. 2011;108:14789–14794. doi: 10.1073/pnas.1017825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuya M, Kinsella JL. Induction of endothelial cell differentiation in vitro by fibroblast-derived soluble factors. Exp. Cell Res. 1994;215:310–318. doi: 10.1006/excr.1994.1347. [DOI] [PubMed] [Google Scholar]
- Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng. Part A. 2010;16:115–125. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S, et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yung YC, Fischbach C, Kong HJ, Nakaoka R, Mooney DJ. Mechanical strain regulates endothelial cell patterning in vitro . Tissue Eng. 2007;13:207–217. doi: 10.1089/ten.2006.0058. [DOI] [PubMed] [Google Scholar]
- Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:172–178. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am. J. Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol. Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl Acad. Sci. USA. 2004a;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am. J. Physiol. Heart Circ. Physiol. 2004b;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc. Natl Acad. Sci. USA. 2009a;106:16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng. Part A. 2009b;15:1211–1222. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp. Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Leach L, Shaw PE, Jones P. Dynamics of vascular endothelial-cadherin and beta-catenin localization by vascular endothelial growth factor-induced angiogenesis in human umbilical vein cells. Exp. Cell Res. 2002;280:159–168. doi: 10.1006/excr.2002.5636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.