Abstract
Background
Matrix metalloproteinase-13 (Mmp-13) is an important enzyme for the modulation of bone turnover and gingival recession. Elevated levels of Mmp-13 are associated with alveolar bone resorption, periodontal ligament destruction, and gingival attachment loss, which are the clinical symptoms of periodontal disease. Continued evidence suggests periodontal disease contributes to oral tissue destruction and is linked to numerous systemic conditions. Triclosan is a long standing, proven antibacterial and anti-inflammatory agent found in the only FDA-approved dentifrice for the treatment of plaque and gingivitis.
Methods
This study examined the inhibitory effects of triclosan on lipopolysaccharide (LPS), parathyroid hormone (PTH) and prostaglandin E2 (PGE2) induced expression of Mmp-13 in UMR 106-01 cells, an osteoblastic osteosarcoma cell line. The cells were stimulated with PTH or PGE2 to induce Mmp-13 mRNA expression and Real Time RT-PCR was performed to determine gene expression levels. Western blot analysis assessed the presence or absence of protein degradation or inhibition of protein synthesis. Mmp-13 Promoter Reporter Assay was utilized to explore possible direct effects of triclosan on the Mmp-13 promoter.
Results
Triclosan significantly reduced PTH or PGE2 elevated expression of Mmp-13 in osteoblastic cells without affecting basal levels of the mRNA. Surprisingly, triclosan enhanced the expression of c-fos and amphiregulin mRNA. A promoter assay indicated triclosan directly inhibits the activation of the PTH-responsive minimal promoter of Mmp-13.
Conclusion
Our data appear to have identified a nuclear mechanism of action of triclosan which accounts for triclosan’s ability to inhibit PTH or PGE2 induced Mmp-13 expression in osteoblastic cells.
Keywords: Osteoblasts, Periodontal Diseases, Triclosan, Matrix Metalloproteinase 13
INTRODUCTION
Periodontal disease is characterized by a bacterially induced gingival inflammation, loss of gingival attachment, bone loss, and ultimately tooth loss1. Periodontal disease is quite complex involving the bacterial infection, the host immune response, and bone metabolism.2,3. periodontal disease has been linked to numerous systemic diseases4.
Triclosan (2,4,4′-tricloro-2′-hydroxydiphenyl ether) is a widely used broad spectrum antibacterial/anti-inflammatory agent5. Metalloproteinases, when out of balance, play a major role in connective tissue destruction associated with periodontal disease, arthritis, osteoporosis as well as other diseases6. The use of non-antibiotic tetracycline, which inhibits collagenases at the protein level, has become an effective adjunct treatment for the management of excess metalloproteinase activity in arthritis and periodontitis7,8.
Bone is a dynamic connective tissue consisting of a variety of cell types. Although considerably complex, osteoblasts are generally considered to be bone forming cells while osteoclasts are considered to be bone resorbing cells. Osteoblasts express receptors for parathyroid hormone (PTH) and prostaglandins as well as transmembrane cytokines including RANKL, which activates osteoclastogenesis in a paracrine manner. The secretion of type I collagen and specialized bone matrix proteins is a critical bone forming function of osteoblasts. 9,10 Osteoblasts also have a role in bone remodeling and are capable of contributing directly to the resorption of bone though the secretion of proteinases, in particular collagenase-3 (Mmp-13). Absence of Mmp-13 in knockout mice resulted in significant interstitial collagen accumulation and an increase in trabecular bone indicating its essential role in bone turnover.11,12.
A body of evidence has shown that MMP-13 is important in bone. The gene was shown to be expressed predominantly in ossifying centers during in vivo development of mice. 13 In postnatal rat calvariae, we have found that ample amounts of MMP-13 are detectable by immunohistochemistry from 1–14 days after birth14,15 which subsequently decline to become undetectable in normal adult bone. The staining is always in select areas, mostly associated with sites of active modeling. At the cellular level, it is associated with osteocytes and bone lining cells which have the appearance of osteoblasts. By in situ hybridization, Mmp-13 is expressed by the mineralizing hypertrophic chondrocytes and by trabecular osteoblasts in long bones of immature mice16. Similarly, in the developing human, MMP-13 appears to be specific for the skeleton and is expressed in hypertrophic chondrocytes, osteoblasts and periosteal cells. 17
The rat osteoblastic osteosarcoma line, UMR 106-01, responds to all bone resorbing hormones by synthesizing collagenase-3 (Mmp-13)18. PTH is the most effective bone resorbing agent for stimulating Mmp-13 production in these cells from the agents tested. PTH binds to its membrane receptor, which activates the G protein Gs. Activated Gs induces the conversion of ATP to cAMP, which activates the protein kinase A (PKA) signaling cascade and leads to the phosphorylation of the CREB transcription factor. CREB then stimulates c-fos transcription by binding the cAMP Response Element (CRE), and in turn, c-Fos, together with Runx2 at the RD site, bound to the AP-1 promoter site induces Mmp-13 transcription.
It has been previously shown that PTH-induced expression of Mmp-13 in osteoblasts requires the cooperative interaction between c-Fos, c-Jun, and Runx2 transcription factors on their cognate AP-1 and runt domain (RD) binding sites, respectively. These three transcription factors interact physically and cooperatively bind the AP-1 and RD binding sites within the Mmp-13 promoter 19,20. Our objective was to examine how triclosan may affect the expression of Mmp-13 in bone cells. Triclosan reduced expression of PTH and PGE2 induced Mmp-13 and we subsequently explored the mechanism of action.
MATERIALS AND METHODS
Materials
Rat PTH (1–34), 8-Bromo cAMP, Tri-Reagent™, RNA isolation reagents# Triclosan (TCN)* and PGE2 ** were used in this study. Primers used were Mmp-13,¥ β-actin, ¥ c-fos, ¥ and amphiregulin¥. Standard tissue culture media and reagents were used throughout experiments.£ Taq Man® Reverse Transcriptase reagents££ were used to prepare cDNA. SYBR Green PCR Core Reagents€ were used for cDNA amplification on a PTC-200 Real time PCR – DNA Engine Opticon.™ ***
#Sigma-Aldrich, St. Louis, MO
*Ciba-Geigy, Florham Park, NJ
**Assays Design, Ann Arbor, MI
¥ Integrated DNA Technologies, Inc, Coralville, IA
£ Mediatech, Manassas, VA
££Life Technologies, Carlsbad, CA
€Biosystems Warrington, UK
*** Bio-Rad, Hercule, CA
-
Sequence – rat B-actin F 5′-TCC TGA GCG CAA GTA CTC TGT G –3′
rat B-actin R 5′ CGG ACTCAT CGT ACT CCT GCT T -3′
rat MMP-13 F (845) 5′-GCC CTA TCC CTT GAT GCC ATT -3′
rat MMP–13 R (947) 5′-ACA GTT CAG GCT CAA CCT GCT G –3′
Other primers are listed in the supplementary materials of Qin L, Tamasi J, Raggatt L, Li X, Feyen JH, Lee DC, Dicicco-Bloom E, Partridge NC. J Biol Chem. 2005 Feb 4;280(5):3974–81. Amphiregulin is a novel growth factor involved in normal bone development and in the cellular response to parathyroid hormone stimulation.
Cell Culture
UMR 106-01 rat osteoblastic osteosarcoma cells were grown in medium containing Minimum Essential Medium (MEM), 5% FBS, 10 units penicillin/10 μg streptomycin, 1% non-essential amino acids (NEAA), and 25 mM HEPES. Cells were grown to 80% confluence in 10 cm2 wells. Cells were serum starved for 24 hours prior to collection and treated with the indicated agents at time zero. After 4 h of exposure, the cells were harvested from the plates with Tri-Reagent™ for the mRNA assays.
Isolation and Analysis of mRNA
RNA was isolated from the cells by adding 1 ml of Tri-Reagent™ per well. In addition, 0.2 ml of chloroform was added per ml of Tri-Reagent™ to ensure complete dissociation of nucleoprotein complexes. Following centrifugation, the layer containing RNA was transferred to a new RNase free tube. RNA was precipitated with 0.5 ml of isopropanol. The pellet was then washed with 75% ethanol, dried, and resuspended in 100 μl of RNase free water. The RNA concentration was determined on a spectrophotometer at 260 nm.
The cDNA was reverse transcribed from mRNA using the reagents in the Taq Man® Reverse Transcription kit. Real Time RT-PCR was performed to determine gene expression levels using the indicated primers. All samples were normalized to their own β-actin mRNA level.
Western blot analysis
UMR 106-01 cells were plated at 1.6 × 106 cells/100 mm dish. Cells were pre-treated with 0.003% triclosan or ethyl alcohol (ETOH) and then treated with 10−8 M PTH or control medium for 4 h. Cell lysates were then prepared in 500 μl lysis buffer (RECIPE?) and resolved on 10% SDS-PAGE, transferred to a PVDF membrane, and blotted with antibodies (Santa Cruz, CA) against c-Fos (sc-52), c-Jun(sc-45), Runx2 (PEBP2, sc-10758), and cdk2 (sc-163; loading control) at a dilution of 1:300. The second antibody (Santa Cruz, CA) was a goat-anti-rabbit polyclonal antibody (sc-2004) conjugated to horseradish peroxidase at a dilution of 1:10,000. The blots were developed with a commercial electrochemiluminescence (ECL) detection kit.
Mmp-13 Promoter Assay (CAT assay)
The −148 bp sequence of the rat collagenase-3 (Mmp-13) promoter was subcloned upstream of a CAT reporter gene in pSV0 (Promega, Madison, WI). The empty pSV0 plasmid was used as the negative control. The pSV2 plasmid was used as a positive control.
UMR 106-01 cells were plated at 4 × 105 cells/well in a 6-well plate in EMEM containing 5% fetal bovine serum. The following day, cells were transfected with 2 μg of DNA and 6 μl of GeneJammer (Agilent Technologies, La Jolla, CA) per well. After 48 h, the cells were treated with either control (ETOH), TCN (0.0007% or 7 ppm), or TCN + PTH-containing media for 12 h. CAT activity was measured by incubating 50 μl of cell lysate in duplicate in a 100 μl reaction volume consisting of 250 μM n-butyryl-coenzyme A and 23 mM [14C] chloramphenicol (0.125 μCi/assay). The values were normalized according to the protein concentration as determined by the Bradford method (Bio-Rad, Hercules, CA). A standard curve using purified CAT was performed in every experiment to determine the linear range of the enzyme assay.
Statistics
All experiments were performed in triplicate and the quantitative data are shown as means ± SD. A one way ANOVA was performed to determine significance and when found in experiments with more than two products a multiple comparison (Tukeys) test was also conducted. A significant separation was noted with p values less than or equal to 0.05.
RESULTS
Gene Expression
Preliminary experiments were needed to determine the optimal conditions for cell treatment and to confirm the response and behavior of the UMR cell line. To determine the best experimental design, method development initiated with the stimulation of UMR cells with 10−8 M PTH or 25 mg/ml LPS. At 4 h post –treatment, Mmp-13 expression was measured. PTH stimulation resulted in a 20-fold change from the ethanol (ETOH) control, consistent with published data from Partridge and colleagues, while LPS caused less than a 2-fold increase in the expression of Mmp-13. Therefore, we determined that LPS, while an important factor in periodontal disease, would not be useful for this research as a stimulating agent for this osteoblastic cell line (Fig 1).
Figure 1. Expression of Mmp-13 by UMR Cells when Stimulated with LPS or PTH.
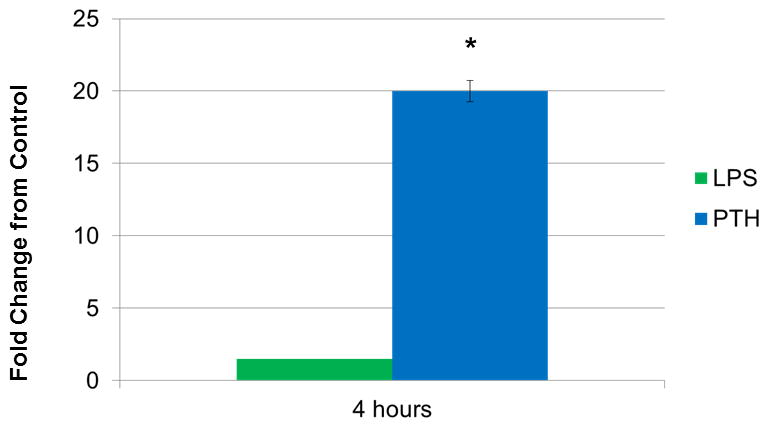
LPS was not found to be statistically significantly different from the ETOH control while the difference in the fold change between PTH and ETOH was statistically significant. *(P<0.05).
Triclosan had little effect on Mmp-13 expression in unstimulated cells. Cells treated with PTH or PGE2 together with triclosan had a lesser increase in Mmp-13 expression, showing that triclosan could block the expression of Mmp-13 by two different agents operating though two different G-protein-coupled receptors (Fig. 2a)
Figure 2. Expression of Mmp-13 by UMR Cells stimulated with PTH or PGE2 and TCN.
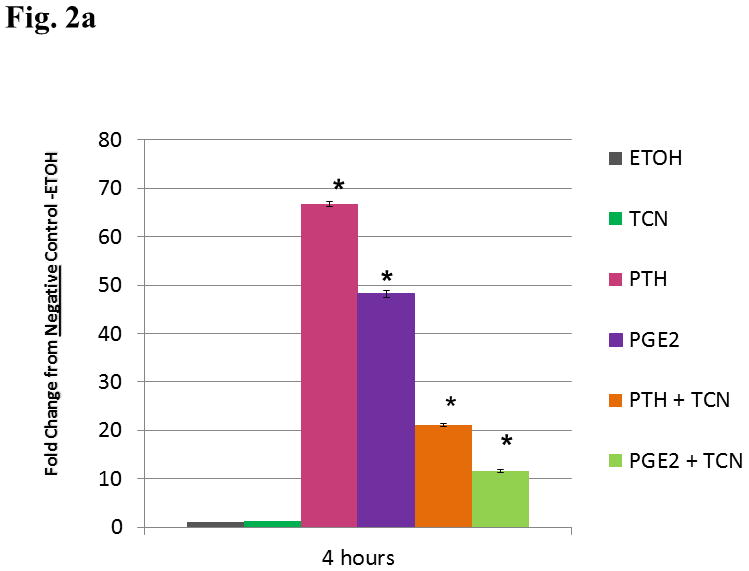
a. UMR cells were treated with ethanol vehicle, 0.003% triclosan, 10−8 M PTH, 10−6 M PGE2, PTH + triclosan or PGE2 + triclosan. At a 4 h time point expression levels of Mmp-13 mRNA were measured as fold stimulation. Statistical analysis found no significant difference between TCN treated cells and ETOH. Cells stimulated with PTH, PGE2, PTH + TCN, and PGE2 + TCN were significantly different from cells treated with ETOH or TCN alone.* (p<0.05) The addition of TCN to PTH stimulated cells produced a statistically significant decrease from the cells treated with PTH alone** (p< 0.05). The addition of TCN to PGE2 stimulated cells produced a statistically significant decrease from cells treated with PGE2 alone *** (p<0.05).
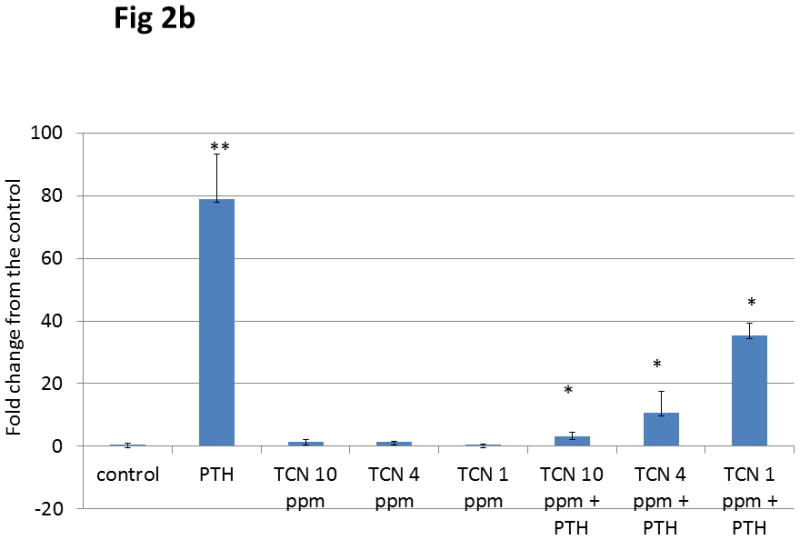
b. Dose Response with Triclosan UMR cells were treated with ethanol vehicle, 10−8 M PTH, and/or a dose response of TCN of 1, 4 or 10 ppm. Messenger RNA was analyzed after 4 h treatment. Results are depicted as the fold change from the ethanol vehicle. Means ± SD of triplicate measurements are indicated. PTH produced a 78.844 (± 14.45) as compared to negative control (ETOH). The addition of triclosan in the dose of 10 ppm, 4 ppm, and 1 ppm registered a 1.518 (± 0.702), 1.424 (± 0.162), 0.417 (± 0.239) fold change vs. ETOH respectively. The addition of triclosan (again at 10, 4 and 1 ppm) to PTH stimulated cells produced a 3.011 (± 1.498), 10.559 (± 6.869), 35.383 (± 3.934) fold change vs. ETOH respectively. PTH was found to be significantly different from the control **(p<0.05). The addition of TCN at all doses to PTH stimulated cells were found to be significantly different from PTH stimulated cells. *(p<0.05)
In a recent patent from Barnes et al., Anti-bone loss and anti-attachment loss effects of an oral composition, US 2012/0107843 A1, 5/3/2012, a dose response of triclosan demonstrated similar findings in the same osteoblastic cell line regardless of the dosage of triclosan. In all cases the addition of triclosan to unstimulated cells resulted in no increase of Mmp-13 expression as compared to the negative control. PTH stimulation produced a nearly 80 fold increase of Mmp-13 expression while the addition of triclosan from 1 ppm to 10 ppm significantly muted the Mmp-13 expression. (fig 2b)
We next tested 8-Bromo cAMP, a cell permeable cAMP analog, to investigate if the inhibitory effect of triclosan was at a post-receptor level. Results showed that 8-Bromo cAMP alone induced a robust increase in Mmp-13 expression while triclosan alone did not. Triclosan decreased the stimulation of Mmp-13 expression by 8-Bromo cAMP showing that triclosan’s effect was downstream of cAMP.
We compared the response to triclosan on the PTH primary response genes amphiregulin (AR) and c-fos. Cells were stimulated with 10−3M 8-Bromo cAMP, triclosan, or 8-Bromo cAMP + 0.003% triclosan in combination and the mRNA was extracted 4h post-treatment. The expression of c-fos increased 9.5, 5, and 21-fold compared to control with the respective treatments. Expression of Mmp-13 increased 2, 17, and 4-fold compared to control with the same treatments. Finally, AR expression paralleled the effects on c-fos and increased by 7, 5, and 12-fold compared to control with the three treatments. These experiments confirm our previous results showing that triclosan can reduce Mmp-13 expression in stimulated cells. In contrast, triclosan stimulated the expression of both c-fos and AR (Fig. 3).
Figure 3. Effect of 8-Br-cAMP and Triclosan on Mmp-13, c-fos, and AR.
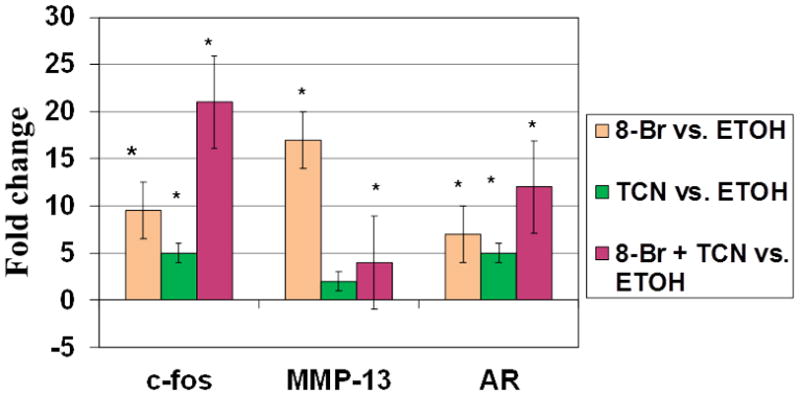
UMR cells were treated with ethanol vehicle, 0.003% triclosan, and/or 10 −3 M 8-Br-cAMP for 4 h. RNA was isolated and the fold-stimulation of Mmp-13, c-fos, and AR mRNAs determined. Results are depicted as the fold change from the ethanol vehicle. Means ± SD of triplicate measurements are indicated. All treatments that are significantly different from the cells treated with vehicle are shown by *(p<0.05).
To assess whether triclosan was inhibiting the expression of c-Fos protein either by degradation or inhibition of protein synthesis, western blot analysis was performed. As shown in Figure 4, c-Fos, c-Jun, Runx2, and cdk2 protein were present at 4 h post-treatment, indicating that triclosan did not have an effect on any of their protein levels. Therefore, we hypothesized that triclosan may be preventing the interaction between Fos/Jun and the AP-1 site, in the Mmp-13 promoter and its own promoter. This scenario would prevent PTH-mediated stimulation of Mmp-13 transcription and enhance c-fos transcription, since it would prevent feedback inhibition of the c-fos promoter by its own protein. To explore this hypothesis, a CAT assay was performed using −148 bp of the Mmp-13 promoter that contained the AP-1 and Runx2 binding sites. The promoter without stimulation showed basal activity (94 ± 24). The negative control plasmid with and without stimulation had minimal activity (17.4 ± 3.9 and 17.7 ± 4.0, respectively). Stimulation with PTH induced activity over baseline as expected (200 ± 24.6 vs. 94 ± 24.0, respectively). However, addition of triclosan with PTH reduced the activity to baseline levels (200 ± 24.6 vs. 97.5 ± 2.7, respectively). Stimulation with triclosan alone showed similar activity as the basal control (135 ± 3.7 vs 94) (Fig. 5).
Figure 4. Effect of Triclosan on Fos, RUNX2 and Jun Proteins.
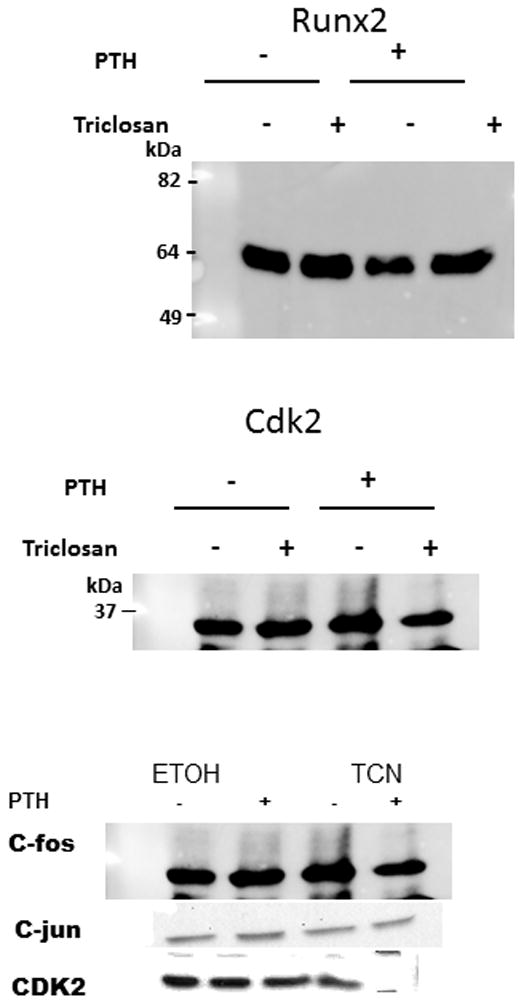
Western blot of c-Fos, RUNX2 and c-Jun. Cells were treated with either ethanol or 0.003% triclosan followed by 10−8 M PTH and harvested at 4 h. Cdk2 was used as a loading control.
Figure 5. Inhibition of PTH-Stimulated Mmp-13 Promoter by Triclosan.
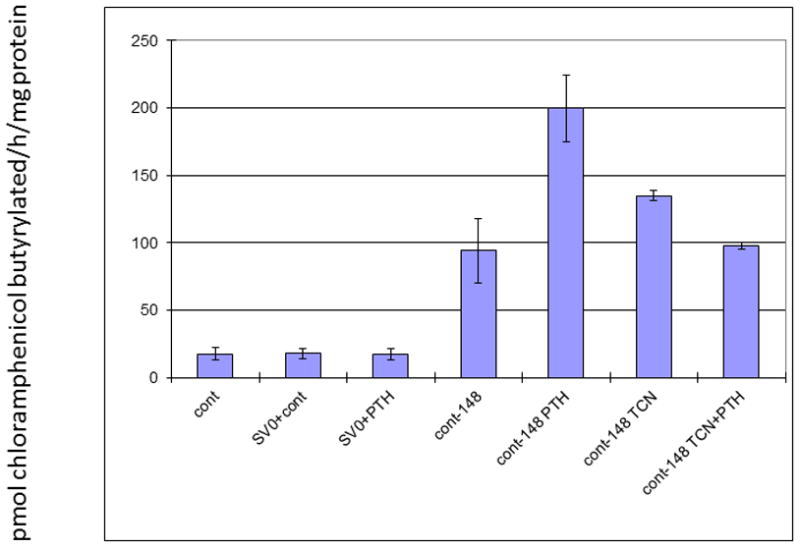
Cells were treated for 12 h with the indicated agents and then harvested for CAT activity. This was measured as pmol chloramphenicol butyrylated/h/mg protein24. n=3. The positive control at a level of 669 (±223) was too robust to be depicted on the y axis on this graph.
DISCUSSION
In this study, we found that the UMR 106-01 rat osteosarcoma cell line was responsive to both PTH and PGE2 with respect to stimulation of Mmp-13 expression and was not responsive to LPS. Expression levels reach a robust level at 4 h post-treatment. Triclosan significantly abrogated both the PTH and PGE2-mediated stimulation of Mmp-13 expression.
Both PTH and PGE2 stimulate Mmp-13 expression though the cAMP and PKA pathway. Since CREB mediates the transcription of c-Fos in the nucleus and the mRNA is translated in the cytoplasm before re-entering the nucleus to induce Mmp-13 transcription, c-fos expression is a primary response and Mmp-13 expression is a secondary response. Additionally, PTH and PGE2-mediated induction of AR expression is also a primary response though the PKA pathway.21
Since the PKA pathway involves several factors, there are a number of opportunities where triclosan can modulate the expression of Mmp-13. Our data suggest that triclosan does not exert its effect on the receptor or at the level of the membrane, since triclosan reduced the expression of Mmp-13 in both PTH and PGE2 stimulated osteoblasts. Additionally, it is unlikely that triclosan imparted an effect at the level of the receptor, since it would have to exert the same effect on two different receptors. Similarly, triclosan inhibited 8-Bromo cAMP-mediated stimulation, suggesting an intracellular effect. Using a systematic approach, key steps along the transduction pathway were investigated. We determined that triclosan had an effect downstream of cAMP by using 8-Bromo cAMP, a cell-permeable cAMP analog that is more resistant to phosphodiesterases than cAMP and preferentially activates cAMP-dependent protein kinase21. Addition of 8-Bromo cAMP significantly increased the expression of Mmp-13 as expected. Importantly, addition of triclosan significantly reduced 8-Bromo cAMP-mediated expression of Mmp-13, suggesting that triclosan functions at the level of cAMP or further downstream.
Since the free catalytic subunit of PKA translocates to the nucleus and exerts an effect on gene expression, several experiments were conducted to assess if triclosan acted within the nucleus. A previous study had shown that 14C-labeled triclosan was taken up by fibroblast cells and translocated to the nucleus. 23 To further investigate this observation, two primary response genes of the PKA regulated pathway, amphiregulin (AR) and c-fos, were investigated. Triclosan upregulated AR, which is a member of the epidermal growth factor (EGF) family and a potent growth factor for pre-osteoblasts. AR null mice display significantly less tibial trabecular bone than wild type mice21. AR is also known to lower Mmp-13 expression, similar to triclosan. Qin et al. reported that AR null mice have increased numbers of mature osteoblasts and osteoclasts, with a greater number of osteoclasts. Therefore, it is possible that AR exerts an inhibitory effect on osteoclastogenesis. Further exploration into the effects of AR as well as the role triclosan may play in the upregulation of AR is required.
Intriguingly, triclosan upregulated c-fos mRNA expression as well. This finding was surprising, since c-Fos is required for Mmp-13 expression and yet triclosan caused a reduction in Mmp-13 expression. Therefore, triclosan caused an upregulation of the primary response genes AR and c-fos. However, triclosan clearly inhibited the secondary response gene Mmp-13. This puzzling scenario led us to two questions. First, could triclosan be responsible for generalized protein degradation or the inhibition of c-Fos protein synthesis? To address this question, western blots were performed. The results indicated that triclosan did not inhibit c-Fos protein expression, and we concluded that triclosan does not cause generalized protein degradation or block c-Fos protein synthesis. Second, we assessed if triclosan could inhibit the binding of AP-1 to the Mmp-13 promoter. The CAT promoter/reporter assay using the −148 bp promoter of rat Mmp-13 that contained the AP-1 and Runx2 binding sites showed that triclosan may exert its effect through this region of the Mmp-13 promoter. These data suggested that triclosan may interfere with AP-1 and explain the reduction in Mmp-13 expression. Moreover, the ability of triclosan to upregulate the mRNA c-fos could also be due to preventing AP-1 binding to the negative feedback site in the c-fos promoter.
This study has potentially identified the specific mechanism of action that accounts for triclosan’s ability to inhibit Mmp-13 expression in an osteoblastic cell line. Other interesting findings were also observed and are worth noting. First, Mmp-13 expression in PTH or PGE2 stimulated cells can be reduced by triclosan, which is an effect similar to AR. Triclosan upregulates AR expression in this osteoblastic cell line. Moreover, the promoter/reporter assay suggests that triclosan may interfere with the Mmp-13 promoter region, which would provide a logical explanation as to why Mmp-13 transcription is reduced even in the presence of triclosan-enhanced c-fos transcription. It also suggests that triclosan inhibits the negative feedback at the AP-1 site of the c-fos gene.
Key Finding.
Triclosan blocks Mmp 13 expression in PTH stimulated osteoblasts by preventing the interaction between Fos/Jun and the AP-1 site, in the Mmp-13 promoter and its own promoter.
Acknowledgments
Research was funded by the Colgate Palmolive Company. Drs. Barnes and Xu were full time employees of the Colgate Palmolive Company at the time of the research. Dr. Barnes is currently the Associate Director of Clinical Research at the Colgate Technology Center in Piscataway, NJ. The remaining authors had no financial interest in the company beyond the funding provided for this work. We thank Rose Richter for assistance with formatting of the figures and statistics and Harsh Trivedi for his valuable critique.
This work is based on a thesis submitted to the graduate faculty, by Virginia Monsul Barnes, to the University of Medicine and Dentistry of New Jersey/Robert Wood Johnson Medical School/, in partial fulfillment of the requirements for the MS degree. Funding was provided by Colgate Palmolive Co.
Footnotes
There are no conflicts of interest with any of the authors.
The authors claim no conflict of interest with either the research or this publication.
References
- 1.Gaffar A, Volpe A. Inflammation, periodontal diseases and systemic health. Compendium. 2004;25(7 Suppl 1) [PubMed] [Google Scholar]
- 2.Xu T, Deshmukh M, Monsul-Barnes V, Trivedi H, Cummins D. Effectiveness of a triclosan/copolymer dentifrice on microbiological and inflammatory parameters. Compendium. 2004;25(Suppl 1) [PubMed] [Google Scholar]
- 3.Socransky SS, Haffajee AD. Microbiology of periodontal disease. In: Lindhe J, Karring T, Lang NP, editors. Clinical Periodontology and Implant Dentistry. 4. Blackwell Publishing Ltd; Oxford, England: 2003. pp. 106–149. [Google Scholar]
- 4.Scannapieco F. Periodontal inflammation: from gingivitis to systemic disease? Compendium. 2004;25(Suppl 1):7. [PubMed] [Google Scholar]
- 5.Volpe A, Petrone M, DeVizio W, Davies R. A review of plaque, gingivitis, calculus and caries clinical efficacy studies with a fluoride dentifrice containing triclosan and PVM/MA Copolymer. J Clin Dent. 1996;7:S1–14. [PubMed] [Google Scholar]
- 6.Jeffcoat M. The association between osteoporosis and oral bone loss. J Periodontol. 2005;76:2125–32. doi: 10.1902/jop.2005.76.11-S.2125. [DOI] [PubMed] [Google Scholar]
- 7.Golub L, Wolff M, Roberts S, Lee H-M, Leung M, Payonk G. Treating periodontal disease by blocking tissue destructive enzymes. JADA. 1994;125:163–169. doi: 10.14219/jada.archive.1994.0261. [DOI] [PubMed] [Google Scholar]
- 8.Caton JG, Ciancio SG, Blieden Tm, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planning in patients with adult periodontitis. J Periodontol. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 9.Baron R. Primer on the metabolic bone diseases and disorders of mineral metabolism. 5. American Society for Bone and Mineral Research; Washington, D.C: 2003. General principles of bone biology bone; pp. 1–8. [Google Scholar]
- 10.Delaisse JM, Engsig M, Everts V, et al. Proteinases in bone resorption: obvious and less obvious roles. Clinica Chimica Acta. 2000;291:223–234. doi: 10.1016/s0009-8981(99)00230-2. [DOI] [PubMed] [Google Scholar]
- 11.Inada M, Wang Y, Byrne MH, et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA. 2004;7:17192–7. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stickens D, Behonick DJ, Ortega N, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–95. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gack S, Vallon R, Schmidt J, et al. Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Diff. 1995;6:759–767. [PubMed] [Google Scholar]
- 14.Partridge NC, Scott DK, Gershan LA, et al. Collagenase production by normal and malignant osteoblastic cells. In: Novak JF, editor. Proceedings of the Osteosarcoma Research Conference 1991. Hogrefe and Huber Publishers; 1993. pp. 269–276. [Google Scholar]
- 15.Davis BA, Sipe B, Gershan LA, et al. Collagenase and tissue plasminogen activator production in developing rat calvariae: normal progression despite fetal exposure to microgravity. Calcif Tiss Int. 1998;63:416–422. doi: 10.1007/s002239900550. [DOI] [PubMed] [Google Scholar]
- 16.Tuckermann JP, Pittois K, Partridge NC, Merregaert J, Angel P. MMP-13 and Itm2a are marker genes of chondrogenic/osteoblastic cells in bone formation: Sequential temporal and spatial expression of Itm2a, alkaline phosphatase, MMP-13 and osteocalcin in the mouse. J Bone Min Res. 2000;15:1257–1265. doi: 10.1359/jbmr.2000.15.7.1257. [DOI] [PubMed] [Google Scholar]
- 17.Johansson N, Saarialho-Kere U, Airola D, et al. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev Dyn. 1997;208:387–397. doi: 10.1002/(SICI)1097-0177(199703)208:3<387::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Partridge NC, Jeffrey JJ, Ehlich LS, et al. Hormonal regulation of the production of collagenase and a collagenase inhibitor activity by rat osteogenic sarcoma cells. Endocrinology. 1987;120:1956–62. doi: 10.1210/endo-120-5-1956. [DOI] [PubMed] [Google Scholar]
- 19.D’Alonzo R, Selvamurugan N, Karsenty G, Partridge NC. Physical Interaction of the Activator Protein-1 Factors c-Fos and c-Jun with Cbfa1 for Collagenase-3 Promoter Activation. J Biol Chem. 2002;277:816–822. doi: 10.1074/jbc.M107082200. [DOI] [PubMed] [Google Scholar]
- 20.Hess J, Porte D, Munz C, Angel P. JBC, AP-1 and Cbfa/Runt Physically Interact and Regulate Parathyroid Hormone-dependent Mmp-13 Expression in Osteoblasts though a New Osteoblast-specific Element 2/AP-1 Composite Element. J Biol Chem. 2001;276:20029–20038. doi: 10.1074/jbc.M010601200. [DOI] [PubMed] [Google Scholar]
- 21.Qin L, Tamasi J, Raggatt L, et al. Amphiregulin is a novel growth factor involved in normal bone development and in the cellular response to parathyroid hormone stimulation. J Biol Chem. 2005;280:3974–3981. doi: 10.1074/jbc.M409807200. [DOI] [PubMed] [Google Scholar]
- 22.Meyer RB, Miller JP. Analogs of cyclic AMP and cyclic GMP: general methods of synthesis and the relationship of structure to enzymatic activity. Life Sci. 1974;14:1019. doi: 10.1016/0024-3205(74)90228-8. [DOI] [PubMed] [Google Scholar]
- 23.Mustafa M, Wondimu B, Hultenby K, Yucel-Lindberg T, Modeer T. Uptake, distribution and release of 14-C-triclosan in human gingival fibroblasts. J Pharm Sci. 2003;92:1648–1653. doi: 10.1002/jps.10429. [DOI] [PubMed] [Google Scholar]
- 24.Seed B, Sheen JY. A simple phase extraction assay for chloramphenicol acyltransferase activity. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]


