Abstract
Emerging infectious diseases from animals pose significant and increasing threats to human health; places of risk are simultaneously viewed as conservation and emerging disease ‘hotspots’. The One World/One Health paradigm is an ‘assemblage’ discipline. Extensive research from the natural and social sciences, as well as public health have contributed to designing surveillance and response policy within the One World/One Health framework. However, little research has been undertaken that considers the lives of those who experience risk in hotspots on a daily basis. As a result, policymakers and practitioners are unable to fully comprehend the social and ecological processes that catalyze cross-species pathogen exchange. This study examined local populations’ comprehension of zoonotic disease. From October 2008-May 2009 we collected data from people living on the periphery of Kibale National Park, in western Uganda. We administered a survey to 72 individuals and conducted semi-structured, in-depth interviews with 14 individuals. Results from the survey showed respondents had statistically significant awareness that transmission of diseases from animals was possible compared to those who did not think such transmission was possible (χ2 = 30.68, df=1, p<0.05). However, individual characteristics such as gender, occupation, location, and age were not significantly predictive of awareness. Both quantitative and qualitative data show local people are aware of zoonoses and provided biomedically accurate examples of possible infections and corresponding animal sources (e.g., worm infection from pigs and Ebola from primates). Qualitative data also revealed expectations about the role of the State in managing the prevention of zoonoses from wildlife. As a result of this research, we recommend meaningful discourse with people living at the frontlines of animal contact in emerging disease and conservation hotspots in order to develop informed and relevant zoonoses prevention practices that take into account local knowledge and perceptions.
Keywords: Uganda, Kibale National Park, One World/One Health, Mixed Methods, Zoonoses Awareness
Introduction
The accelerating rate of emerging infectious disease poses significant and increasing health risks for people and animals worldwide (Gibbs, 2005; Wilcox & Gubler, 2005). An estimated 70% of emerging and re-emerging human infections are zoonotic (originate in animals) (Jones et al., 2008). Scholars and practitioners agree that emerging zoonotic disease is a product of entangled global flows, interconnections, and feedback loops among human, animal and ecological actors, as evidenced by the increased visibility and uptake of “EcoHealth,” “One Health,” “Conservation Medicine,” and most recently “One World/One Health.” Inherent in each of these assembled fields is a mandate to implement interdisciplinary epistemologies that unite biomedical and social sciences in order to understand human, animal, and ecological health and wellbeing. Embedded within these fields is emerging disease discourse trained at places and people in the Global South. It is here that entangled global flows and feedback are seen as viscerally intense, almost atavistic, and therefore are likely sites of the next emerging disease (Daszak, 2006; Jones et al., 2008; Wolfe, Dunavan, & Diamond, 2007). This visioning practice extends the idea of a “hotspot” from a tool to target conservation resources to the practice of predicting the source of the next global pandemic.
In the African context, research into emerging infections from animal sources implicates nonhuman primate (‘primate’ hereafter) bushmeat hunting as the primary catalyst of new diseases. This paradigm reflects a bias towards understanding and explaining the etiology of HIV/AIDS (Chomel, 2007; LeBreton et al., 2006; Wallis & Lee, 1999; Wolfe et al., 2004; Wilkie, 2006). Moreover, this attention on HIV/AIDS and bushmeat hunting makes conservation a common sense approach to protect global health. This happens through the dual use of the term “hotspot.”
A “hotspot” originally referred to a location with high biodiversity and wildlife density that was under significant threat of degradation or destruction as a consequence of human activities (Myers et al., 2000). It was developed to aid conservation practitioners in targeting resources to those places on the globe with the highest conservation-based return on investment (Myers et al., 2000). The idea of hotspots as an analytic in emerging zoonotic disease literature gained traction through a seminal piece by Jones et al. (2008). By mapping locations of emerging diseases from the 1960s on, Jones et al. demonstrated the spatial overlap between locations of emerging zoonotic diseases and biodiversity hotspots, and thus trained the researcher’s attention on the spatial relationship between biodiversity hotspots and emerging zoonoses. The work presented herein is critically situated within the hotspot context, and contributes to the One World/One Health literature by engaging individuals living in emerging zoonotic disease and biodiversity hotspots.
The idea for this study arose out of our desire to promote the voices of individuals at risk of zoonotic disease. We were skeptical that awareness of zoonoses had filtered “down” to the individuals for whom such knowledge was most relevant. Post-colonial theory informed our study design. We designed creative data collection materials that we hoped would allow us to draw out and present narratives and knowledge from “exoticized others” in a way that preserved the richness and dynamism of lived experiences (Ferguson, 2006; Said, 1978; Spivak, 1988).
Nightingale (2003) is a feminist scholar with a post-colonial lens whose work on natural resource management in Nepal served as a guide for our methods. Her 2003 article “A feminist in the forest: situated knowledges and mixing methods in natural resource management” explains how a mixed methods research design can be used to “interrogate the partiality of knowledge” (p. 78) by exploring “the silences and incompatibilities that become evident when data sets produced by diverse methodologies are brought together” (p. 80). The intent of her project was not simply to build data integrity across methods, but to explore those spaces where different data reveal different types of knowledge, which can subsequently enrich results.
We modeled our case study after Nightingale’s piece; she worked to understand the variety and partiality of knowledge, and did so through careful and creative application of multiple methodologies. We also mixed data collection and analytic methods to generate a case study capable of illuminating gaps that persist in purely quantitative analyses, yet retained quantitative methods in order to identify, describe, and possibly, generalize zoonotic disease awareness and knowledge. These mixed methods allowed us to test our skepticism that knowledge and awareness of zoonotic disease moved from global to local spheres.
This case study was conducted as part of a long-term disease ecology research project based near Kibale National Park, in western Uganda. Our aim was to uncover how people at the forefront of the “human-animal interface” in an emerging disease and conservation hotspot comprehend zoonotic disease. We operationalized our research question of “comprehension” through qualitative and quantitative approaches. We sought information from individuals regarding awareness, knowledge of specific diseases or symptoms, examples of transmission routes, and suggestions for preventing spillover events using both semi-structured interviews and closed-ended surveys. We positioned our findings in the context of our hypothesis that zoonoses knowledge and awareness is fixed at the global level. Finally, we argued for the role of mixed-methods research approaches that enable deep engagement with frontier populations as the way forward for One World/One Health research, practice and policy development.
Case Study: The Kibale Hotspot
Kibale National Park (KNP) in western Uganda is a focal point of biodiversity conservation and human livelihood conflict. Human population growth in the region is among the highest in Africa (Hartter, 2007) and pressures on wildlife through habitat loss and degradation make this region of the world a hotspot for disease emergence (Goldberg, Paige, & Chapman, 2012). Three outbreaks of Ebola and one of Marburg Hemorrhagic Fever Virus have been recorded in the region since 2000 (Polonsky et al., 2014). Humans already bear a high disease burden (e.g. HIV, malaria, respiratory illness) (Kabarole District Health Statistics Office, 2014). Approximately 20% of the resident population report risky contact with animals (Paige et al., 2014), making the population susceptible to novel zoonotic pathogens.
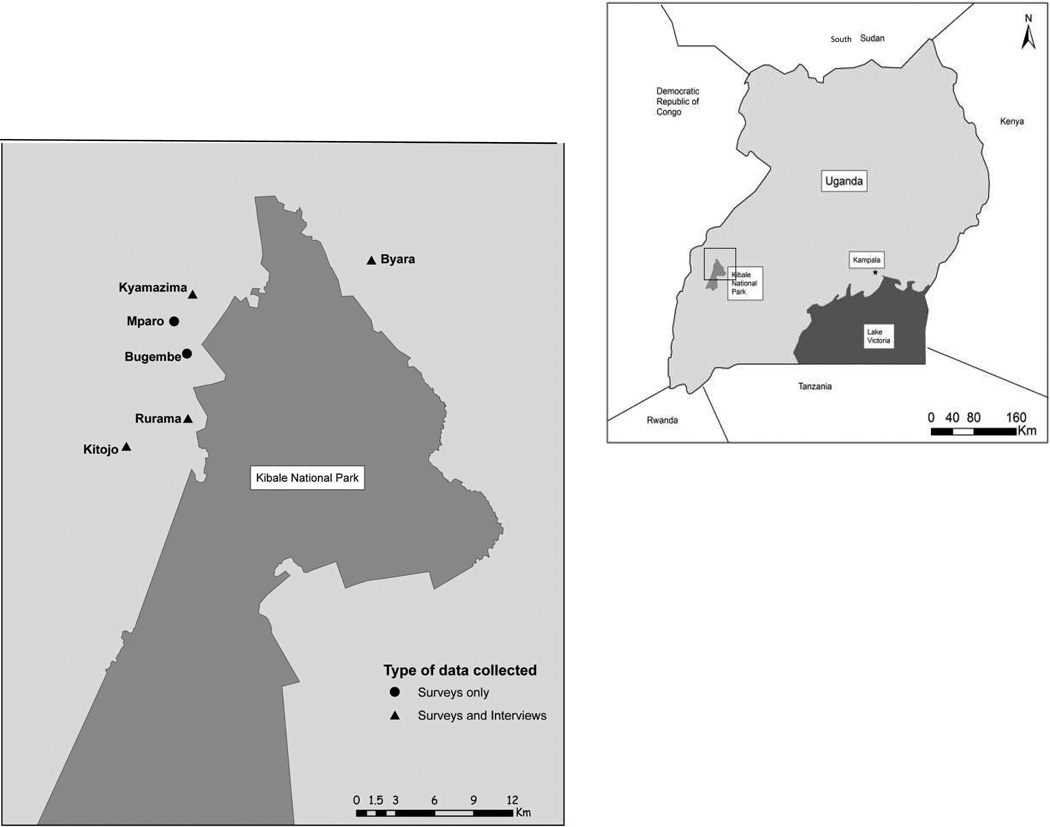
Consisting of 795 km2, KNP, near the foothills of the Rwenzori Mountains (Fig. 1), boasts the highest biomass of colobine primates in the world and the largest chimpanzee population in Uganda. Thirteen primate species, including the endangered red colobus (Piliocolobus tephrosceles) and approximately ten Chimpanzee (Pan troglydytes) communities fuel long-term biological research and ecotourism to KNP.
Fig. 1.
Map of study locations
Our case study is situated along the northwestern periphery of KNP (Fig. 1). This area is a mosaic of swamps, household compounds, trading centers, pastures, tea fields, crops, paths, roads, and forest fragments. Forest fragments outside of KNP are relevant locales for a study on awareness of zoonotic disease given regular interaction between people and animals (domestic and wild) in these landscapes. Utilization of the forest fragments to access natural resources place people and wildlife in shared spaces on a daily basis (Goldberg, Gillespie, & Rwego, 2008; Naughton-Treves, 1997; Naughton-Treves, Kammen, & Chapman, 2007). Crop-raiding by wildlife extends shared spaces from the fragment into fields, and hunting by dogs brings wildlife carcasses into the household compound (Goldberg, Paige, & Chapman, 2012). Because of their small areas, proximity to human settlements, and permeable borders, forest fragment systems enable intense and frequent interaction and contact between people and wildlife (Paige et al., 2014).
Decentralization of land management in the mid-1990s meant decision-making regarding communal and private lands shifted from the national to local level. Therefore, each forest fragment is impacted by human use in a unique way: "Governed by the local council by-laws and by the social norms and culture, communities have developed their own sets of rules to regulate forest and wetland use" (Hartter & Ryan, 2010, p. 822). However, as land management was decentralized, wildlife management was not. Communities determine the use of resources derived from forest fragments to support human livelihood, but are unclear about the status of the wildlife residing within the fragments. Because fragments are not protected areas, are the wildlife in those fragments considered unprotected? Are a select species protected while others are not? Or are all animal wildlife the auspices of the Uganda Wildlife Authority (UWA), whereas the trees, land, and water are the responsibility, and therefore, resources for local residents? Unclear expectations about fragment and wildlife management, coupled with daily interaction between people and wildlife in a space of ambiguity inform our study into how people with potentially risky animal contacts comprehend the risk of zoonotic disease.
Methods
We used a mixed-methods approach for this study. Two types of data were simultaneously collected, analyzed, compared, and contrasted. Qualitative data were collected through semi-structured interviews and a pile-sorting exercise, and quantitative data from a cross-sectional survey.
Qualitative semi-structured interviews and pile-sorting
We used purposive sampling in order to capture variation in occupation, age, and sex among respondents to generate rich and varied qualitative data. We confined our sampling frame to those same communities where we were implementing the survey so that our comparison of mixed-method data would be valid. Additionally, because we were sensitive to research burden on each community, we deliberately planned for small sample sizes for both the interviews and the survey.
We used semi-structured interviews as a way to generate data that was broad and participant-driven. After capturing participant health priorities, the interview topics progressively narrowed to focus on local knowledge of zoonoses (Box 1). Our goal was to ensure that researcher emphasis on zoonoses would not overwhelm or undermine participant health concerns and priorities so as to avoid diminishing participants’ health priorities.
Box 1: Interview Guide.
Health-Individual
-
1
How do you rank your health overall?
-
2
What is the most important health issue for you? Why? What can be done about that?
-
3
How do you define “health”?
-
4
What do you and your family do to stay healthy?
-
5
What else could be done to help you and your family stay healthy?
Health-Community
-
6
Overall, Do you think the community is healthy?
-
7
What do you think is the most important health issue for the community? Tell me more about that. [probe for broad, structural issues]
-
8
What could be done to improve health of the community?
Health-Past and Future
-
9
What do you think people suffered from 50 years ago? Why has it changed/stayed the same?
-
10
What do you see for health in the future? Will people suffer from the same diseases as today?
Health and Animals
-
11
How do people classify animals? (Show pictures of different kinds of animals and ask participant to put them into groups) What are the names for each group? Can you please describe each? How did you decide to put animals in these groups?
-
12
Do you think diseases can go from animals to people? Which animals? Which diseases? Why do you think those animals can share diseases with people? How does that happen?
-
13
Are you concerned about diseases from animals? Why or why not? What could be done to prevent that? (If they haven’t mentioned primates, ask about them specifically.)
-
14
Can animals (wildlife and domestic animals) get sick?
-
15
Do you think diseases can go from people to animals?
-
16
If so, Which animals and which diseases? Why do you think some diseases may go from people to animals? How does that happen?
-
17
Are you concerned about animals getting diseases from people? Why or why not? What could be done to prevent that? (If they haven’t mentioned primates, ask about them specifically.)
-
18
Do you think that diseases between people and animals were there 50 years ago? If yes, tell me more. Which diseases and which animals? (If they haven’t mentioned monkeys or apes, ask about them specifically.)
We used a pile sorting exercise to transition from the solemn atmosphere of individual and community health needs into one that was interactive and engaging (Quintiliani, Campbell, Haines, & Webber, 2008). Interviewees were handed a stack of 18 color photos of animals and asked to classify the animals, leaving the classification criteria up to the respondent (Box 2). Photos included animals familiar to respondents, like goats, pigs, dogs, and were usually classified as domestic animals. Less familiar animals, such as hippos, bats, elephants, and multiple bird and primate species were also included and were classified differently with each interview. The act of engaging with the photos and discussing animals lightened the atmosphere. The exercise inevitably attracted children and other adults to interact with the photos, as well as the interviewers and interviewees.
Box 2: Species included in the pile sorting exercise.
Goat (Capra hircus)
Pig (Sus scrofa)
Dog (Canis lupis familiaris)
Bat (Eidolon helvum)
Pelican (Pelecanus onocrotalus)
Red-tailed Guenon (Cercopithecus ascanius)
Black-and-white Colobus (Colobus guereza)
Agama (Agama agama)
Hippo (Hippopotamus amphibious)
Cape buffalo (Syncerus caffer caffer)
Baboon (Papio anubis)
Elephant (Loxodonta africana)
Chimpanzee (Pan troglydytes troglydytes)
Lion (Panthera leo)
Bushbuck (Tragelaphus scriptus)
Ankole cow (Box bovis)
Chicken (Gallus domesticus)
Bird (Cyanomitra obscura)
Interviews lasted approximately one hour, with 20–30 minutes devoted to discussing zoonoses. All interviews were conducted in the local language (Rutooro) by one highly-trained female field assistant. They took place in a variety of settings –field, household compound, and schoolyard. All interviews were audio-recorded with a digital voice recorder. At the end of each interview, respondents could choose their “thank you” gift; all interviewees opted for one color-printed photograph of the respondent along with his or her family. The following day, the photo was printed and returned to the respondent, and deleted from the camera and computer memory.
Interviews were translated, transcribed and hand-coded immediately after data collection. The same field assistant who conducted the interviews performed translations from Rutooro to English. Once transcribed, the text was carefully reviewed by the researcher and field assistant. Instances where the meaning of phrases was unclear were resolved through reviewing the recording and a second translation. Transcripts were hand-coded in Microsoft Word (Redmond, WA) and then re-read across themes to uncover further relationships among responses and codes (Jackson, 2001). During multiple re-readings, codes were iteratively added or revised as new constructs emerged (Glaser, 1967). Codes were sorted into three organizational levels. “Themes” is the most general level, followed by “category” and then “content.” These groupings reflect tiered and networked information that crystallized through the multiple cycles of deep data immersion.
Quantitative zoonoses knowledge survey
While semi-structured interviews were underway, we implemented the zoonoses knowledge survey. Survey respondents were identified through random sampling of households that relied on forest fragments for subsistence resources. This process involved enumerating and mapping all households within 0.5km of a forest fragment, and then randomly selecting households until a minimum of 6 households per fragment were identified. The process was repeated across 6 forest fragment communities (Fig. 1).
Once all households were selected, one adult (typically the head of household or the spouse) was surveyed. A household information survey included location, age, gender, cultural group and occupation of each respondent. The zoonotic disease survey sought information on the respondent’s awareness of zoonoses, and if aware, sought examples of diseases and corresponding animal hosts.
Survey data were also orally obtained in the local language and were manually recorded on paper forms. The survey was administered by two highly trained field assistants (one male and one female) from the local area. Employing local field assistants improved access to study communities and reduced (but by no means eliminated) bias introduced by the presence of outsiders. All households received gifts for participation in the form of soap, water purification tablets, or ectoparasite treatment for their domestic animals.
Quantitative data were analyzed to identify individual characteristics associated with zoonotic disease awareness. We conducted a t-test on knowledge of zoonoses (Can people contract diseases from animals? Yes/No). We calculated basic summary statistics of the frequency of diseases mentioned and corresponding animal hosts. We performed univariate logistic regression to determine if demographic characteristics of individuals (age, cultural group, sex, location, occupation) influenced knowledge of zoonoses, and a stepwise backwards logistic regression to identify if any significant predictor variables remained when all were controlled for. We conducted univariate logistic regression in place of chi-square tests as we were interested in knowing the nature of the relationship between characteristics and awareness of zoonoses, not just if there was a significant difference between different demographic groups and knowledge of zoonoses. Logistic regression can suggest relationship trends whereas chi-square tests only indicate if a relationship exists, but not if there is a possible positive or negative effect (Hosmer & Lemeshow, 2000).
Ethics
Ethical approval for research was granted by Uganda Wildlife Authority, the Uganda National Council for Science and Technology (Ref # NS 221), The University of Washington Institutional Review Board (#32300), and Local Council Leaders. All participation was voluntary and all participants provided oral informed consent prior to data collection.
Results
Qualitative Results
Eleven semi-structured interviews and pile-sorting exercises were conducted with a total of 14 respondents across four of the six study sties. Six women and eight men were interviewed with ages that ranged from 14–76. Respondents included an herbalist, a nursery school teacher, a health care provider, a pupil, an employee of a local non-governmental organization, and subsistence farmers. Semi-structured interviews spanned a wide range of health geography topics, but the findings presented here are limited to three themes: 1) awareness of zoonotic disease; 2) examples of potential transmission pathways and corresponding animal hosts; and 3) suggestions for interventions.
Domestic Zoonoses and Transmission Routes
Domestic animals were the most frequent species mentioned as a potential reservoir for a zoonotic disease 93% (n=13). Only one respondent stated that zoonoses from domestic animals were impossible, and that was because of the role of vaccination in protecting domestic animal health, and consequently, human health. Some respondents described zoonotic diseases using biomedical terms, and some used local terms, which were translated into the biomedical term by the field assistant. The majority of examples provided were biomedically plausible. We note instances where the example is biomedically inaccurate. Below, information is organized and presented by species.
-
-
Pigs (Sus scrofa): Pigs were the most frequently cited animal responsible for infectious disease. Five individuals described a scenario that involved stepping in pig feces without foot protection and contracting a worm or parasite. Consuming contaminated pork was implicated as a source of worms. Pigs were also implicated as sources of fever or influenza.
Chickens (Gallus domesticus): Chickens were frequently implicated in zoonotic diseases. One respondent named coccidiosis as a zoonotic disease that was caused by consuming undercooked chicken. (While it is not recommended to consume animals that died from infection, the parasite responsible for coccidiosis in chickens is species specific and does not infect humans). Two individuals cited chickens as sources of bird flu. Two respondents reported that consuming water that was shared with chickens could result in a zoonotic infection‥
- Dogs (Canis lupis familiaris): Three participants named rabies as a zoonotic disease from dogs. This is relevant as rabies is an endemic problem in the region.
-
-Cows (Box bovis): Two respondents identified cows as a source of sleeping sickness. One person said unboiled milk caused of brucellosis and bovine TB. Another participant suggested consuming contaminated beef was linked to parasite infection. These examples are supported by the scientific literature.
-
-
Goats (Capra hircus): Four respondents suggested goats could cause a zoonotic disease, but none mentioned a specific illness or symptom. Instead, one respondent suggested illness could result from “shared breath” that occurs when people and domestic animals share sleeping spaces. (It is possible that respiratory illness can be exchanged between goats and humans, but we have yet to discover an airborne pathogen that is capable of infecting both humans and goats. Therefore this example was new to us.)
Wildlife Zoonoses and Transmission Routes
Respondents also reported wildlife associated with zoonotic disease. Snakes, buffalo, and elephants were each mentioned once. Elephants were reported to cause elephantiasis (which is a common misconception), and consuming improperly cooked buffalo meat was said to lead to helminth infection. Snakes were not linked to specific illness; instead, their bites were linked to imminent death.
We were especially interested in respondents’ perceptions of the role of primates in zoonoses. All respondents said “yes” -that people were susceptible to primate zoonoses; half of the respondents (7/14) named a specific disease or symptom. Primate species specifically named as a zoonotic source included:
-
–Baboons (Papio anubis); Baboons were most frequently mentioned primate. Four people reported baboons as a source of zoonotic disease; one mentioned fever while the other three did not indicate what type of illness or symptom would the result from the infection. Two respondents provided an example of how one could be exposed to an infectious disease from a baboon; both examples were based on crop-raiding scenarios.You may find that a baboon has eaten your maize. It has eaten part of it, part of it is left. You then take the maize home and roast it without realizing that your teeth are going to overlap with where the baboon’s teeth were. Then you may end up contracting a disease which a baboon may have.” (58y/o female farmer)
-
–
Black-and-white colobus (Colobus guereza) & Red-tailed guenons (Cercopithecus ascanius); One respondent mentioned black and white colobus and red-tailed guenons as causing fever or being a source of infection with parasites by stepping on feces with bare feet.
-
–
Chimpanzees (Pan troglodytes schweinfurthii); Two respondents linked the etiology of HIV to human interaction with chimpanzees. One of those described human-chimpanzee interaction as a sexual contact, reminiscent of early (inaccurate) explanations of the pandemic’s emergence. The same respondent described a second scenario of exposure that involved indirect contact with leftover banana that a chimpanzee had eaten; similar to the baboon example above. A third respondent indicated fever was one symptom that could arise from exposure to a chimp-borne illness.
-
–Red colobus (Piliocolobus rufomitratus tephrosceles); Two respondents reported red colobus exposure could lead to a zoonotic infection. Both respondents were from the same study community and both shared the following narrative:In this community we hear people telling us that about two or three years ago, ‘a red colobus bit my two children and they died.’ We don’t know if was poison from their teeth or a disease from their blood, but yes, they also have diseases.” (45y/o male drug shop owner)
This is the only example in which red colobus was specifically mentioned as a potential source of primate zoonoses. Also, in this same study site, many residents aired grievances against red colobus, even to the extent of hunting them while we were present. For example, during data collection, residents attacked a red colobus on the grounds of a primary school. It was chased into a classroom and killed; leaving blood on the floor of the classroom around children’s seating areas. This event is relevant because this was the only location where interview responses included a hostile overtone and described intense, aggressive contact with primates that resulted in potentially high-risk exposure. We note the documented presence of several potentially zoonotic viruses in the red colobus population inside KNP and are concerned about the risk of novel primate zoonoses to residents here (Bailey et al., 2014; Lauck et al., 2013a; Lauck et al., 2013b; Lauck et al., 2011; Sibley et al., in press). While we remained open to witnessing all types of human-primate interaction, and even established participant observation methods that aimed to capture this type of potentially sensitive behavior, this is the only event of this type that we were able to witness and record.
-
–Non-specific primate species: Even without discussing specific symptoms and species involved, respondents were interested in discussing risks in more generalized terms, and typically contextualized the risk of exposure to primate zoonoses in terms of daily activities. For example, four respondents mentioned possible waterborne transmission routes that all followed a similar formula:
Additionally, 3 respondents reported that food could be contaminated through cropraiding, and as such, crop raiding was an indirect route of transmission for primate zoonoses.… monkeys could urinate in your water source. Then you go fetch that water unknowingly. Then you drink that water boiled or un-boiled. That is how you can get diseases from monkeys.” (70 y/o female midwife)
Suggested Interventions
Towards the end of the interviews, respondents were asked about ways to interrupt the transmission of zoonotic diseases from both domestic animals and wildlife. Suggestions for preventing diseases from domestic animals was through protecting feet from feces (3/14), sheltering livestock in structures separate from homes (5/14), and avoiding “unknown meat” (3/14). The majority of respondents suggested the use of vaccines by both individuals and the government for livestock and wildlife (6/14). For example,
“owners of livestock should engage themselves in spraying at least once a week and they should vaccinate all animals at least twice a month” (20 y/o female farmer)
The government should make up plans of vaccinating even wild animals so that if there are diseases from animals they can stop that. They should vaccinate both people and animals!” (58 y/o female farmer)
When discussing ways to prevent zoonoses from wildlife, respondents focused on the role of the government. Ten respondents made recommendations government involvement and focused suggestions on vaccinating wildlife, removing crop-raiding primates from fragments, and erecting and maintaining barriers between KNP and adjacent gardens to keep people and wildlife separate. The quote below is from one respondent recommending practices to be taken by the Uganda Wildlife Authority.
The game rangers (i.e., UWA) should engage themselves in research so that they notice when diseases have affected wildlife and if need be, they vaccinate the animals so that zoonoses cannot affect people’s health. Then animals which are crop raiders, they should put them in the national park and keep guarding them so they don’t destroy people’s food which, also affects human health. The government should put in place community health education especially on how to care for their animals because so many people around sleep with chickens, goats inside the house. …. People should start studying about animals and their drugs so there can be a permanent doctor for animals and then governments should keep their animals so that they cannot have contact with people.” (42 y/o female local nurse)
Respondents that place responsibility for interrupting zoonotic transmission on the government all looked to the Uganda Wildlife Authority as the operational body that could put their suggestions into practice.
Quantitative Results
Alongside the semi-structured interviews, a total of 72 participants were surveyed across six study sites. The majority of respondents were female; were Mutooro; and were subsistence farmers. The modal age was 28 years, with an age range of 14–85 years. Sixty (83.3%) respondents reported that diseases could spread from animals to people (χ2 = 30.68, df=1, p<0.05). We asked those respondents who reported “Yes” to also provide an example of a disease/infection and its animal source. Of the sixty who reported that zoonoses were possible, ten (16.7%) gave the example of worm infections from pigs, seven (11.7%) mentioned fevers from cows, and five (8.3%) mentioned Ebola from monkeys (Table 1). Interestingly, monkeys were the only wildlife mentioned from the quantitative data collected.
Table 1.
Distribution of reported animals and corresponding zoonotic infections or symptoms
| Animal | Zoonotic symptom/infection |
Count | % of all zoonoses examples |
|
|---|---|---|---|---|
| Pigs | Worms | 12 | 20.0 | |
| Unknown infection | 3 | 5.0 | ||
| Fever | 2 | 3.3 | ||
| Cholera | 1 | 1.7 | ||
| Total | 18 | 30.0 | ||
| Monkeys | Ebola | 5 | 8.2 | |
| Monkeypox | 3 | 5.0 | ||
| Unknown infection | 3 | 5.0 | ||
| Fever | 2 | 3.3 | ||
| Cough | 1 | 1.7 | ||
| Worms | 1 | 1.7 | ||
| Total | 15 | 25.0 | ||
| Domestic Animals | Fever | 6 | 10.0 | |
| Cough | 3 | 5.0 | ||
| Total | 9 | 15.0 | ||
| Cows | Fever | 7 | 11.7 | |
| Worms | 1 | 1.7 | ||
| Total | 8 | 13.3 | ||
| Any Animal | Unknown Infection | 4 | 6.7 | |
| Fever | 1 | 1.7 | ||
| Worms | 2 | 3.3 | ||
| Total | 7 | 11.7 | ||
| Birds | Flu | 1 | 1.7 | |
| Goats | Cough | 1 | 1.7 | |
| Mosquitos | Fever | 1 | 1.7 | |
| Total | 60 | 100.0 | ||
Univariate logistic regression was performed to determine the possible relationship between individual characteristics (age, cultural group, sex, location, occupation) of respondents to awareness of zoonotic disease. The only significant predictor variable was cultural group. Bakiga and “Other” cultural groups had significantly higher odds of zoonotic disease awareness at the 95% level compared to Batooro (OR: 1.306; CI 0.197–25.898; p-value = 0.0443). We also conducted a logistic regression, which included all independent variables in a stepwise additive multivariable logistic model, allowing the model to calculate the predictor variables with the best fit and dropping those lacked adequate explanatory power. The resulting model with the best fit contained only gender and cultural group as the best predictors of zoonotic disease awareness (df=1, AIC = 62.267, p=0.039), however, none of the predictor variables themselves were statistically significant.
Discussion
The results of our case study demonstrate that people living in an emerging disease hotspot are aware of zoonotic disease, and are able to confidently and (biomedically) accurately describe livestock zoonoses. Our initial suspicion that knowledge of zoonoses had yet to ‘trickle down’ to frontier populations, or those populations most at risk of exposure and infection, was misplaced. Qualitative and quantitative data were largely complementary. Both data indicate that domestic animals, especially pigs, were perceived as the most ready source of zoonoses. While pigs predominated as the domestic animal of risk, primates were frequently invoked as the riskiest wildlife species. Even though respondents were non-specific about specific illnesses that could be acquired from primates, descriptions of possible routes of pathogen transmission were plausible. Those who did mention specific illnesses associated with primates (Ebola, HIV/AIDS) were correct as primates have been implicated in Ebola outbreaks, and HIV is a mutation of a primate retrovirus. Additionally, the implications of being infected by a soil-transmitted helminth, as compared to Ebola or HIV are greatly divergent. Ebola and HIV are much more likely to result in significant impact on an individual’s morbidity and mortality. This suggests that people may perceive zoonotic diseases that result from wildlife infections as much more impactful than those that may be acquired through domestic animal sources.
Respondents also informed the researchers of potentially new routes of transmission. We were not aware that crop-raiding primates would leave behind half-eaten food; nor that people may consume food that remains after a crop-raiding event. The example of baboon and human teeth overlapping on a piece of maize was new to the researchers, and impressionable. While most of the examples of zoonoses, corresponding animal sources, and transmission routes were biomedically plausible, a handful of responses were unlikely. For example, one respondent reported HIV was the result of sexual contact between a human and chimpanzee, a frequent refrain from news media in the late 1980s, which echoed common stereotypes about central Africa during that era (Jarosz, 1992). Additionally, elephantiasis, also known as lymphatic filariasis is actually caused by a parasite (Wuchereria bancrofti) and is transmitted through mosquitos, not through contact with elephants. The term ‘elephantiasis’ refers to the manifestation of the infection in the body, as in thickening of skin, along with pain and major swelling in limbs.
Interview data illustrated the perceived role of the Uganda Wildlife Authority in protecting human health in the context of wildlife zoonoses. Interview respondents framed their suggestions for the prevention of zoonoses through the use of vaccines and medicine for wildlife and the removal of primates from forest fragments. None of the respondents suggested avoidance of forest fragments, planting of less palatable crops, culling primates, or sacrificing portions of food crops to crop-raiding primates. While forest fragments are not officially protected, they are considered ‘forest’ and there is usually informal management over those spaces. Regardless, respondents never suggested fragment owners, or the community that relies on the fragments, as having a role in preventing the transmission of potential wildlife zoonoses. The silence around local management of fragments and the recommendation that the government take the lead in managing primate zoonotic risk suggests that external resources would be necessary to support interventions to limit exposure to potential primate zoonoses. This type of insight was possible through immersion with the interview data.
Previous studies that explored local perceptions of zoonotic risk focus on populations involved in the bushmeat trade. LeBreton et al. (2006) suggest cultural health belief models may explain risk perceptions behind bushmeat hunting and minimal use of preventive measures (p. 362). Here, we present information gathered from people who are also at ‘high risk’ of zoonotic disease, given the frequency with which zoonoses emerge in the area, the high frequency of direct contact between humans and primates (Paige et al., 2014), and the discovery of new, possibly zoonotic, retroviruses in red colobus: the same primate species that resides in forest fragments. We found that people in this region were also aware of zoonoses, especially with respect to livestock and primates. However, the responsibility for prevention of zoonoses from wildlife was laid with the state, which is in contrast to the conclusion of LeBreton et al (2006). The discrepancy was not about cultural health belief models, as respondents provided biomedically and ecologically viable transmission scenarios, it was about economic and political structures that constrain agency and shape expectations.
While the qualitative and quantitative findings had the fortunate outcome of triangulating well, our goal in using mixed methods was to interrogate ‘silent spaces.’ Our quantitative methods generated information that demonstrated awareness of zoonotic disease, and a handful of ‘culprit’ animals. Qualitative data illustrated the context of risk. By considering qualitative data in an ethnographic sense; by opening our notebooks to those bits of data that fall outside traditional methods, we were able to witness and log a very significant event. We were present for the death of a primate, one whose species is known to carry potentially zoonotic agents. Had we adhered strictly to the survey or the interview protocol, the opportunity to capture a significant piece of data would have been missed. Moreover, the use of qualitative methods enabled the elevation of the voices of research “subjects” who are the individuals facing risk of zoonotic infection on a daily basis. One has an ethical and scientific obligation to reach out to those who are most susceptible to zoonotic infection and unpack, not only knowledge, awareness, and experience with zoonoses, but also the corresponding structural context within which individual human lives are situated. The silence that could have persisted with purely quantitative data was addressed through reading across qualitative data and allowing issues of animal ‘ownership’ and expectations to emerge, alongside the hopes about the role of the state in preventing the next zoonotic pandemic.
Conclusion
The work presented here contributes to the One World/One Health literature through empirical engagement with individuals living in emerging disease “hotspots.” Research from the natural sciences has contributed to the One World/One Health paradigm by focusing primarily on post-hoc biophysical explanations of disease emergence or field-based biological surveillance methods. Such contributions have had a dramatic impact on our collective knowledge regarding the biology and etiology of many zoonotic diseases, especially HIV, hemorrhagic fevers, SARS, Nipah virus, and influenzas. Social science literatures present fascinating work detailing perceptions of risk (Setbon & Raude, 2009), explanations of emergence (Degeling & Kerridge, 2013, Briggs & Nichter, 2009), and optimal modeling approaches that integrate community prevention practices (Leach & Scoones, 2013). The social sciences have contributed to the theoretical literature exploring the bio-political and moral implications that hinge upon the “epidemiological gaze” of global surveillance, response, and preparedness discourse and practice (Craddock, Giles-Vernick, & Gunn, 2010; Hinchliffe, Allen, Lavau, Bingham & Carter, 2013; Hinchliffe, & Bingham, 2008). While the biophysical and social sciences function in primarily separate spheres, the One World/One Health research design pushes the disparate sciences together as biological explanations for health and disease are contextualized by social, psychological, and ecological experiences. The work presented here not only adds to the One World/One Health theoretical framework, but also includes important findings about the extent of awareness of zoonotic disease in a “frontier” population, coupled with knowledge regarding modes of transmission, and opportunities for intervening. Our data suggest that the way forward in One World/One Health practice is to go beyond human and animal biological surveillance and incorporate the human social and structural context that opens up potential zoonotic pathogen pathways.
Research Highlights.
Describes local zoonotic disease awareness and transmission knowledge
Participants were significantly aware of cross species disease exchange
Pigs and primates were considered the most likely source of a zoonotic disease
Examples of zoonotic disease transmission routes were typically plausible
One Health practitioners should leverage existing zoonoses knowledge
Acknowledgements
Kibale EcoHealth Project Field Staff: Edith Mbabazi Abwooli, Joseph Abooke Byaruhanga, John Atwooki Rusoke, Patrick Akiiki Katurama, Annet Abooke Nyamwija, Alice Akiiki Mbabazi. This work was funded by NIH grant TW009237 as part of the joint NIH-NSF Ecology of Infectious Disease program and the UK Economic and Social Research Council; SBP was funded by the NSF Doctoral Dissertation Improvement Grant and the Thomas Francis Jr. Global Health Fellowship of the University of Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey AL, Lauck M, Weiler A, Sibley SD, Dinis JM, Bergman Z, O’Connor DH. High genetic diversity and adaptive potential of two simian hemorrhagic fever viruses in a wild primate population. PLoS One. 2014;9:e90714. doi: 10.1371/journal.pone.0090714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C, Nichter M. Biocommunicability and the biopolitics of pandemic threats. Medical Anthropology. 2009;28:189–198. doi: 10.1080/01459740903070410. [DOI] [PubMed] [Google Scholar]
- Chomel BB. Wildlife, exotic pets, and emerging zoonoses. Emerging Infectious Diseases. 2007;13:6–11. doi: 10.3201/eid1301.060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock S, Giles-Vernick T, Gunn JL, et al. Influenza and public health: Learning from past pandemics. Abingdon: Earthscan; 2010. [Google Scholar]
- Daszak P. Risky behavior in the Ebola zone. Animal Conservation. 2006;9:366–367. doi: 10.1111/j.1469-1795.2006.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Degeling C, Kerridge I. Hendra in the news: public policy meets public morality in times of zoonotic uncertainty. Social Science & Medicine. 2013;82:156–163. doi: 10.1016/j.socscimed.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. Global shadows: Africa in the neoliberal world order. Durham: Duke University Press; 2006. [Google Scholar]
- Gibbs EP. Emerging zoonotic epidemics in the interconnected global community. The Veterinary Record. 2005;157:673–679. doi: 10.1136/vr.157.22.673. [DOI] [PubMed] [Google Scholar]
- Glaser BG, Strauss AL. The discovery of grounded theory: Strategies for qualitative research. Chicago: Aldine; 1967. [Google Scholar]
- Goldberg TL, Gillespie TR, Rwego IB. Health and disease in the people, primates and domestic animals of Kibale National Park: Implications for conservation. In: Wrangham R, Ross E, editors. Science and conservation in African forests: The benefits of long-term research. Cambridge: Cambridge University Press; 2008. pp. 75–87. [Google Scholar]
- Goldberg TL, Paige S, Chapman CA. The Kibale EcoHealth Project: Exploring connections among human health, animal health, and landscape dynamics in western Uganda. In: Aguirre AA, Daszak P, Ostfeld RS, editors. Conservation medicine: Applied cases of ecosystem health. New York: Oxford University Press; 2012. pp. 452–465. [Google Scholar]
- Hinchliffe S, Allen J, Lavau S, Bingham N, Carter S. Biosecurity and the topologies of infected life: From borderlines to borderlands. Transactions of the Institute of British Geographers. 2013;38:531–543. [Google Scholar]
- Hartter J, Ryan SJ. Top-down or bottom-up? Decentralization, natural resource management, and usufruct rights in the forests and wetlands of Western Uganda. Land Use Policy. 2010;27:815–826. [Google Scholar]
- Hartter J. Landscape change around Kibale National Park, Uganda: Impacts on land cover, land use, and livelihoods. Gainesville: University of Florida; 2007. (Unpublished doctoral dissertation) [Google Scholar]
- Hinchliffe S, Bingham N. Securing life: the emerging practices of biosecurity. Environment and Planning A. 2008;40:1534–1551. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. Hoboken: John Wiley & Sons; 2000. [Google Scholar]
- Jackson P. Making sense of qualitative data. In: Limb M, Dwyer C, editors. Qualitative methodologies for Geographers: Issues and debates. London: Arnold; 2001. pp. 199–214. [Google Scholar]
- Jarosz J. Constructing the dark continent: Metaphor as geographic representation of Africa. Geografiska Annaler. Series B, Human Geography. 1992;74:105–115. [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabarole District Health Statistics Office. Quarterly Infectious Disease Statistics from the District Health Office to the Ministry of Health, Uganda (October – December, 2013) Fort Portal, Uganda. 2014 [Google Scholar]
- Lauck M, Switzer WM, Sibley SD, Hyeroba D, Tumukunde A, Weny G, O’Connor DH. Discovery and full genome characterization of two highly divergent simian immunodeficiency viruses infecting black-and-white colobus monkeys (Colobus guereza) in Kibale National Park, Uganda. Retrovirology. 2013;10:107. doi: 10.1186/1742-4690-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauck M, Sibley SD, Lara JM, Purdy A, Khudyakov Y, Hyeroba D, Goldberg TL. A novel hepacivirus with an unusually long and intrinsically disordered NS5A protein in a wild Old World primate. Journal of Virology. 2013;87:8971–8981. doi: 10.1128/JVI.00888-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauck M, Hyeroba D, Tumukunde A, Weny G, Lank SM, Chapman CA, Goldberg TL. Novel, divergent simian hemorrhagic fever viruses in a wild Ugandan red colobus monkey discovered using direct pyrosequencing. PLoS One. 2011;6:e19056. doi: 10.1371/journal.pone.0019056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach M, Scoones I. The social and political lives of zoonotic disease models: narratives, science and policy. Social Science & Medicine. 2013;88:10–17. doi: 10.1016/j.socscimed.2013.03.017. http://dx.doi:10.1016/j.socscimed.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Prosser AT, Tamoufe U, Sateren W, Mpoudi-Ngole E, Diffo JLD, Wolfe ND. Patterns of bushmeat hunting and perceptions of disease risk among central African communities. Animal Conservation. 2006;9:495–495. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Naughton-Treves L. Farming the forest edge: Vulnerable places and people around Kibale National Park, Uganda. Geographical Review. 1997;87:27–46. [Google Scholar]
- Naughton-Treves L, Kammen DM, Chapman C. Burning biodiversity: Woody biomass use by commercial and subsistence groups in western Uganda's forests. Biological Conservation. 2007;134:232–241. [Google Scholar]
- Nightingale A. A feminist in the forest: Situated knowledges and mixing methods in natural resource management. ACME: An International E-Journal for Critical Geographies. 2003;2:77–90. [Google Scholar]
- Paige S, Frost SD, Gibson MA, Jones JH, Shankar A, Switzer WM, Goldberg TL. Beyond bushmeat: Animal contact, injury, and zoonotic disease risk in western Uganda. EcoHealth. 2014 doi: 10.1007/s10393-014-0942-y. http://dx.doi.org/10.1007/s10393-014-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky JA, Wamala JF, de Clerck H, Van Herp M, Sprecher A, Porten K, Shoemaker T. Emerging filoviral disease in Uganda: Proposed explanations and research directions. American Journal of Tropical Medicine and Hygiene. 2014;90:790–793. doi: 10.4269/ajtmh.13-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintiliani LM, Campbell MK, Haines PS, Webber KH. The use of the pile sort method in identifying groups of healthful lifestyle behaviors among female community college students. Journal of the American Dietetic Association. 2008;108:1503–1507. doi: 10.1016/j.jada.2008.06.428. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. (Version 3.0.3) [Software] 2013 Available from http://www.R-project.org. [Google Scholar]
- Said EW. Orientalism. 1st ed. New York: Pantheon Books; 1978. [Google Scholar]
- Setbon M, Raude J. Population response to the risk of vector-borne diseases: lessons learned from socio-behavioural research during large-scale outbreaks. Emerging Health Threats Journal. 2009;2:e6. doi: 10.3134/ehtj.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley SD, Lauck M, Bailey AL, Hyeroba D, Tumukunde A, Weny G, Friedrich T. Discovery and characterization of distinct simian pegiviruses in three wild African Old World monkeys. PLoS ONE. 9:e98569. doi: 10.1371/journal.pone.0098569. http:dx.doi.org/10.1371/journal.pone.0098569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak G. Can the subaltern speak? In: Nelson C, Grossberg L, editors. Marxism and the interpretation of culture. Chicago: University of Illinois Press; 1988. pp. 271–313. [Google Scholar]
- Wallis J, Lee DR. Primate conservation: the prevention of disease transmission. International Journal of Primatology. 1999;20:803–826. [Google Scholar]
- Wilcox B, Gubler D. Disease ecology and the global emergence of zoonotic pathogens. Environmental Health and Preventive Medicine. 2005;10:263–272. doi: 10.1007/BF02897701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie D. Bushmeat: A disease risk worth taking to put food on the table? Animal Conservation. 2006;9:370–371. [Google Scholar]
- Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe ND, Switzer WM, Carr JK, Bhullar VB, Shanmugam V, Tamoufe U, Heneine W. Naturally acquired simian retrovirus infections in central African hunters. Lancet. 2004;363:932–937. doi: 10.1016/S0140-6736(04)15787-5. [DOI] [PubMed] [Google Scholar]