Abstract
High-intensity interval exercise (HIIE) has gained popularity in recent years for patients with cardiovascular and metabolic diseases. Despite potential benefits, concerns remain about the safety of the acute response (during and/or within 24 hours postexercise) to a single session of HIIE for these cohorts. Therefore, the aim of this study was to perform a systematic review to evaluate the safety of acute HIIE for people with cardiometabolic diseases. Electronic databases were searched for studies published prior to January 2015, which reported the acute responses of patients with cardiometabolic diseases to HIIE (≥80% peak power output or ≥85% peak aerobic power, VO2peak). Eleven studies met the inclusion criteria (n = 156; clinically stable, aged 27–66 years), with 13 adverse responses reported (~8% of individuals). The rate of adverse responses is somewhat higher compared to the previously reported risk during moderate-intensity exercise. Caution must be taken when prescribing HIIE to patients with cardiometabolic disease. Patients who wish to perform HIIE should be clinically stable, have had recent exposure to at least regular moderate-intensity exercise, and have appropriate supervision and monitoring during and after the exercise session.
Keywords: exercise prescription, cardiovascular disease, metabolic disease, acute risk
Introduction
Exercise prescription is an important adjunct to medical management in the treatment of cardiometabolic diseases.1 While the traditional approach of prescribing moderate-intensity continuous exercise has been associated with improved health outcomes and a low incidence of adverse events,2 there is growing evidence for a dose–response relationship between exercise intensity and all-cause mortality, suggesting that higher-intensity exercise may afford greater benefit.3 However, this needs to be balanced against the increased risk of adverse responses with acute high-intensity exercise,4 which is especially relevant for individuals with cardiometabolic diseases.
The guidelines of the American College of Sports Medicine,1 the American Heart Association,5,6 and the Exercise and Sports Science Australia7 for exercise prescription recommend moderate aerobic exercise (40%–60% of peak aerobic power, VO2peak) for most patients (outpatients) with cardiovascular disease (CVD), while the same organizations recommend up to vigorous-intensity aerobic exercise (84% of VO2 reserve or heart rate reserve and 70%–89% maximal heart rate, HRmax) in patients with type 2 diabetes mellitus (T2DM).8–10
Despite the well-documented benefits from exercise training for patients with cardiometabolic diseases, 60% of adults11 and up to 77% of adults with T2DM12 do not meet the minimum-recommended level of physical activity (at least 30 minutes of moderate-intensity aerobic exercise most days of the week). Lack of time is cited as one of the main barriers for participation in exercise programs.13,14 Novel approaches to exercise prescription have therefore been developed to reduce the time required to achieve health and fitness benefits. One such exercise prescription strategy is high-intensity interval exercise (HIIE), which involves brief bursts of intense exercise (usually ≥90% of VO2peak) interspersed by periods of rest/low-intensity exercise. This contrasts with the longer-duration, continuous moderate-to-vigorous exercise intensity (<85% of VO2peak) traditionally applied.15
HIIE training has been shown to improve a broad range of cardiovascular risk factors, including VO2peak,16,17 insulin sensitivity and glycemic control,18,19 high-density lipoprotein levels,20 body mass index and waist circumference,20 systolic and diastolic blood pressure,21 and left ventricular (LV) structure and function,16 although not in all cases.17,22,23 For comprehensive reviews of the effects of HIIE training on clinical and physiological outcomes, refer Kessler et al.15, Gibala et al.24, Weston et al.25, and Hawley and Gibala.26 Importantly, several studies have found that HIIE produces superior physical, clinical, and functional benefits compared to moderate-intensity continuous exercise (MICE),16,27 raising the question of whether HIIE should be utilized more broadly in clinical practice. To date, there are ongoing concerns about the safety of HIIE, which need to be evaluated before recommendations can be made regarding its use in clinical exercise programs.
While a recent study found that significant adverse responses (cardiac arrest or myocardial infarction [MI]) occurred rarely during HIIE in individuals with cardiometabolic diseases (1 event per 23,182 hours of exercise), this was more frequent than that observed during MICE (1 event per 129,456 hours of exercise).28 The acute cardiovascular response to a session of HIIE may underlie this discrepancy in event rates; however, a systematic review of the cardiovascular response during, as well as the associated event rates of, HIIE is yet to be undertaken in patients with cardiometabolic disease One previous review has examined whether high-intensity interval training should be included in the exercise rehabilitation of patients with chronic heart failure (CHF).29 However, this review focused only on CHF and only three articles were included in the review. A second review3 examined the effects of intermittent exercise, not HIIE, versus continues exercise in patients with CHF. The intensities were varied and the focus was not on high-intensity exercise. Accordingly, the purpose of the current review is to examine the published literature to determine the incidence of adverse responses during or immediately following a single session of HIIE in people with cardiometabolic diseases to assess its safety and appropriateness for inclusion in exercise programs for this population group.
Materials and Methods
The review was conducted based on the guidelines set out by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.30
Search strategy
Medline, Embase, and CINAHL electronic databases were searched for exercise trials ending January 2015, using the search strategy described in detail in Supplementary Table 1. In brief, keyword and category-based searches (including full terms and abbreviations) were performed, which included the following: (i) exercise type: high-intensity interval training and high-intensity intermittent training, HIIE, and high-intensity intermittent exercise, interval training, interval exercise, repeated sprint training, and sprint interval training; (ii) intensity: high-intensity or vigorous exercise/training; and (iii) duration: acute bout, single bout, and exercise training. Categories (i)–(iii) were also combined using “AND”, limited to humans, reported in English, and performed in adults aged ≥18 years. In addition, reference lists of publications meeting the inclusion criteria were manually searched to identify any relevant studies not found through electronic searches. At least two authors independently assessed the suitability of each study for inclusion.
Inclusion and exclusion criteria
Studies with the following criteria were included: (i) the study’s cohort had one or more of the following conditions/diseases: CVD including, but not restricted to, hypertension, coronary artery disease (CAD), ischemic heart disease, and CHF or metabolic disease including obesity, metabolic syndrome, insulin resistance, impaired glucose tolerance, and T2DM; and (ii) reported any adverse events, defined as an unexpected exercise-related response resulting in ill-health, abnormal changes in cardiovascular response, or death of an individual31 or other measures that may compromise safety (including electrocardiogram [ECG] changes and biomarkers for myocardial damage) during or within the 24-hour period following a single session of HIIE. In this instance, HIIE was defined as repeated bouts of exercise (of any mode) of any duration performed at ≥80% peak power output or ≥85% VO2peak.
Studies were excluded if they did not report measures of blood pressure, heart rate, or measures that indicate myocardial damage or adverse responses (such as vasovagal episodes) during or immediately after exercise. Authors who did not report an adverse event to HIIE were contacted to ascertain whether an adverse response was observed and not reported or whether an adverse response was simply not observed. In the case of more than one publication arising from the same study or patient cohort, only the largest study was included.
Risk of bias
The Cochrane Collaboration’s tool for assessing risk of bias was used to assess the methodological quality and risk of bias of the included studies in the systematic review.32 Individual quality items were investigated using a descriptive component approach that included items such as the method used to allocate participants into comparable groups; blinding of participants/personnel of interventions and outcomes; measurement of outcomes in a standard, reliable, and valid way; completeness of outcome data for each main outcome (including attrition and exclusions from the analysis); selective reporting, and any other sources of bias. A summary of risk of bias in each study is provided in Table 1. Any disagreement or uncertainty was resolved by discussion and consensus. Using this approach, each study was allocated a risk of bias rating.
Table 1.
Risk of bias.
| STUDY | ADEQUATE SEQUENCE GENERATION | ALLOCATION CONCEALMENT | BLINDING OF PARTICIPANTS, PERSONNEL, AND OUTCOME ASSESSORS | INCOMPLETE OUTCOME DATA ADDRESSED | FREE OF SELECTIVE OUTCOME REPORTING | FREE OF OTHER SOURCES OF BIAS |
|---|---|---|---|---|---|---|
| Gayda et al. 201233 | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Meyer et al. 201134 | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Guiraud et al. 201335 | ? | ? | ✓ | × | ✓ | ✓ |
| Normandin et al. 201336 | n/a | n/a | ✓ | ✓ | ✓ | ✓ |
| Tomczac et al. 201137 | n/a | n/a | ✓ | ✓ | ✓ | ✓ |
| Guiraud et al. 201038 | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Guiraud et al. 201139 | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Currie et al. 201240 | × | ? | ✓ | ✓ | ✓ | ✓ |
| Whyte et al.41 | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Gillen et al. 201242 | n/a | n/a | ✓ | ✓ | ✓ | ✓ |
| Tjonna et al. 201143 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Notes: ✓ indicates ‘yes’- low risk of bias. ? indicates unclear – not enough information provided in the publication. × indicates ‘no’ – high risk of bias. n/a indicates this assessment was not applicable for this study type.
In view of the heterogeneity in study design, interventions, age of patients, medication use, cardiometabolic disease status, and outcome measures, we provide a descriptive review rather than a meta-analysis of results.
Results
A total of 282 studies matching the search criteria were found in the literature search. The titles and abstracts of these publications were screened and 243 articles were excluded, as they did not meet the inclusion criteria (Fig. 1). The full texts of 39 articles were retrieved. An additional 28 studies were excluded after reading the full text as they did not meet the inclusion criteria. Eleven articles were accepted for the systematic review (Table 2). The primary aim of 4 out of the 11 studies was to examine different aspects of the cardiovascular response following a single bout of HIIE. Although the cardiovascular response postexercise was not the main scope of the current review, these studies were included as they also reported whether patients exhibited adverse response during HIIE.
Figure 1.
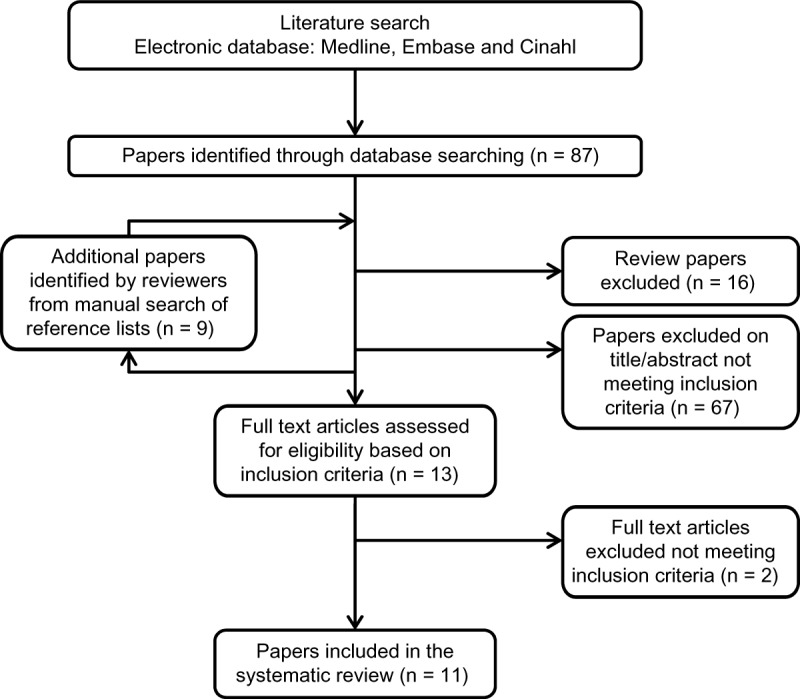
Identification, screening, and selection of studies reporting adverse responses to acute high-intensity interval exercise (HIIE) in people with cardiometabolic disease.
Table 2.
Studies’ protocol.
| REFERENCE | AGE (Y) BMI (km m−2), VO2peak (ml/kg/min) | EXCLUSIONS CRITERIA | INCLUSIONS CRITERIA | EXERCISE PROTOCOL |
|---|---|---|---|---|
| Gayda et al. 201233 Study design: Randomised crossover Participants: CHF n = 13 |
Age: 59 BMI: 29.5 VO2peak: 17.3 |
• Any contraindications to exercise • Fixed-rate pacemaker or ICD devices with HR limits <exercise target HR • Major cardiovascular event (<3 months) • Chronic atrial fibrillation CHF secondary to significant uncorrected primary valvular disease, congenital heart disease or obstructive cardiomyopathy |
• Age ≥18 y • LVEF <40% • Stage CHF • NYHA class I to III • Stable optimal medical therapy, including BB and ACE inhibitors or ARB • Ability to perform a maximal cardiopulmonary exercise test |
Exercise protocol: The following separated by 1 week: • MICE at 60% PPO for 22 mins • HIIE of 2 × 8 min sets consisting of 30 sec at 100% PPO interspersed with 30 sec passive recovery; 4 min passive recovery between sets. Supervision: • Exercise physiologist • Nurse • Cardiologist |
| Meyer et al. 201134 Study design: Randomised crossover Participants: CHF n = 20 |
Age: 60 BMI: 30.1 VO: 17.2 2peak |
• As Gayada et al.31 | • As Gayada et al.31 |
Exercise protocol: • 4 single HIIE sessions consisting of 30 sec or 90 sec at 100% of PPO with active (50% peak aerobic power) or passive recovery between bouts (1:1 ratio). Supervision: • N/R |
| Guiraud et al. 201335 Study design: Randomised crossover Participants: CHF n = 18 |
Age: 53 BMI: 26.9 VO: N/R 2peak |
• As Gayada et al.27 | • As Gayada et al.31 |
Exercise protocol: • MICE at 60% of PPO for 22 mins • HIIE consisted of a warm-up for 2 min at 50% of PPO, followed by 2 × 8 min sets consisting of 30 sec at 100% of PPO interspersed by 30 sec of passive recovery Supervision: • N/R |
| Normandin et al. 201336 Study design: Randomised crossover Participants: CHF n = 20 |
Age: 61 BMI: 29.9 VO2peak: 0.99–1.1 L/min. |
• As Gayada et al.27 | • As Gayada et al.31 • Medications: ACE inhibitors, ARBs, BB, digoxin, furosemide, oral hypoglycaemic agents, insulin, spironolactone |
Exercise protocol: • Identical to Guirard et al. 201329 Supervision: • Exercise physiologist • Cardiologist |
| Tomczak et al. 201137 Study design: Single HIIE protocol Participants: CHF n = 12 (9 completed) |
Age: 49 BMI: 29 VO2peak: 27.3 |
• No exclusion criteria stated | • NYHA I and II, • LVEF <50%, • Clinically stable • No changes in symptoms or medications for at least 3 months (no report of exact medications) • Normal sinus rhythm |
Exercise protocol: • HIIE 4 × 4 min sets at 95% HRmax interspersed with 3 min active recovery (walking 50–70% HRmax). Supervision: • Exercise physiologist • Cardiologist |
| Guiraud et al. 201038 Study design: Randomised crossover Participants: CHD n = 19 |
Age: 65 BMI: 28 VO2peak: 27.1 |
• Acute coronary syndrome (<3 months) • Significant resting ECG abnormality, severe arrhythmias • History of CHF • Uncontrolled hypertension • Recent (<3 months) bypass surgery • Percutaneous coronary inter vention (<6 months) • LV EF (<45%) • Pacemaker • Recent modification of medication (<2 wk) • Musculoskeletal conditions making exercise difficult or contraindicated |
• History of ≥ 70% arterial diameter narrowing of at least one coronary artery • Previous MI • Perfusuion defect on seta MIBI exercise test • Medications: Anti platelet agents, BB, CCB, ACE inhibitors, ARB, statins, nitrates |
Exercise protocol: • HIIE consisting of 15 sec or 60 sec at 100% of maximal aerobic power with active (50% peak aerobic power) or passive recovery between bouts (1:1 ratio) Supervision: Supervision: • Exercise physiologist • Cardiologist |
| Guiraud et al. 201139 Study design: Randomised crossover Participants: CHD n = 19 |
Age: 62 BMI: 27 VO2peak: 28.4 |
• As Guiraud et al. 201036 | • As Guiraud et al. 201036 |
Exercise protocol: • MICE at 70% PPO, mean exercise time of 28.7 min • HIIE consisting of a 10 min warm-up (50 PPO), followed by 2 × 10 min sets composed of repeated phase 15 sec work at 100% PPO 15 sec active recovery (1:1 ratio), 5 min cool-down. Total time of 35 min Supervision: • Exercise physiologist • Nurse • Cardiologist |
| Currie et al. 201240 Study design: Randomised crossover Participants: CAD n = 10 |
Age: 66 BMI:26.8 VO2peak: 28.6 |
• Smoking (<3 months), • Non-cardiac surgical procedure (<2 months), • MI or CABG (<2 months) • PCI within (<1 month), • New York Heart Association class II–IV symptoms of CHF • Valve stenosis • Severe COPD • Symptomatic PAD • Unstable angina • Uncontrolled hypertension • Uncontrolled ventricular dysrhythmia • Premenopausal women • Pregnancy • Musculoskeletal abnormality • Insulin dependent diabetes |
• CAD with stenosis ≥50%, • Previous MI • Percutaneous coronary intervention, • Or CABG • Positive exercise test. • Medications: Anti platelet agents, BB, CCB, ACE inhibitors, ARB, statins, nitrates |
Exercise protocol: • MICE at 55% PPO for 30 min • HIIE consisting of 10 × 1 min sets at 80% PPO, with 1 min active recovery at 10% PPO Supervision: • Supervised in university clinic |
| Whyte et al. 201341 Study design: Randomised crossover Participants: Overweight/obese n = 10 |
Age: 26.9 BMI:29.9 VO2peak: 42 |
• Uncontrolled hypertension. • History of CHD • Family history of early cardiac death • Diabetes |
• Age: 18–40 • BMI: 25–35 • ≤2h/wk regular physical activity |
Exercise protocol: • SIT: 4 × 30 sec “all-out” sprint interspersed with 4.5 min active recovery at 30 W and 4 min cool down period at 30 W • Continues sprint exercise (200 sec): same volume of work (kJ) as SIT • Control: no exercise Supervision: • N/R |
| Gillen et al. 201242 Study design: Crossover design Participants: T2DM n = 7 |
Age: 62 BMI: 30.5 VO2peak: N/R |
• Taking insulin • History of end-stage liver or kidney disease. • Evidence of neuropathy, retinopatathy • Uncontrolled hypertension • CVD • Other contraindication to exercise |
• Sedentary lifestyle • Fasting glucose ≥7.0 mmol/L and/or 2-h oral glucose tolerance test blood glucose ≥11.1 mmol/l. • Medications: Metformin, gliclazide, pioglitazone, sitagliptin, repaglinide. |
Exercise protocol: • 10 × 60 sec sets interspersed with 60 sec of rest. • intensity corresponded to 90% of workload maximum and elicited 85 of maximal HR. Supervision: • N/R |
| Tjonnaetal. 201143 Study design: Training study Participants: Mets n = 28 AIT= 11 CME = 8 Con = 9 |
Age: 52.3 BMI: ~30 VO2peak: 34 |
Not reported | • People with MetS (WHO criteria) • Sedentary lifestyle • Medications: BB, CCB, ACE inhibitors, ARB, statins, metformin, Acetylsalicylic acid |
Exercise protocol: • Acute effects of exercise, before and after 16 wk training, 3 x/week. • AIT consisting of a warm-up for 10 min at 70% HRmax, with × 4 min sets at 90–95% HRmax with 3 min recovery at 70% HRmax. 5 min cool down. • CME consisting of 47 min at 70% HRmax (equal volume) • Control consisting of advice from family doctor Supervision: • N/R |
Abbreviations: ACE, angiotensin converting enzyme; AIT, aerobic interval training; ARB, angiotensin receptor blockers; BB, beta blockers; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery diseases; CCB, calcium channel blockers; CHD, coronary heart disease; CHF, chronic heart failure; CME, continues moderate exercise; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ECG, electrocardiography; HUE, high intensity interval exercise; HR, heart rate; ICD, implantable cardioverter-defibrillator; LV, left ventricular; LVEF, left ventricular ejection fraction; MetS, metabolic syndrome; Ml, myocardial infarction; MICE, moderate intensity interval exercise; n, number of participants; N/R, Not reported; NYHA, New York Heart Association classification; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PPO, peak power output; SIT, sprint interval training; T2DM, type 2 diabetes mellitus.
Description of the included studies
Eight studies examined the effects of HIIE on patients with CVD. Of these, five studies involved patients with CHF,33–37 and three studies involved patients with coronary heart disease (CHD) or CAD.38–40 In addition, three studies examined the effects of HIIE on patients with metabolic diseases, including obesity,41 metabolic syndrome,20 and T2DM.42 The sample size of the studies ranged between n = 7 and n = 28. As determined by the Cochrane Collaboration’s tool for assessing risk of bias, the majority of studies included in the systematic review reported a moderate risk of bias (Table 1).
Patients
In total, 173 patients took part in the 11 studies in which 156 patients performed HIIE (age range: 27–66 years). Studies only included patients who were clinically stable and treated with appropriate medications. In addition, the studies included a wide range of exclusion criteria including both relative and absolute contraindications (Table 2).
Study protocols
Some protocols (4 of 11 studies) for patients with CVD involved exercise on a cycle ergometer. The protocols for patients with CHF included several sets (3–10) of 30 seconds’ high-intensity exercise followed by 30 seconds of active34 or passive33,35,36 recovery. Meyer et al.34 examined the effects of four different HIIE protocols on exercise time in patients with CHF: interval durations of 30 seconds or 90 seconds, with active versus passive recovery between bouts of exercise. In contrast, the protocol used by Tomczak et al.37 included exercise on a treadmill consisting of four bouts of 4-minute duration at 90%–95% of HRmax with 3-minute recovery at 70% of HRmax. Similar to the protocols for patients with CHF, the protocols for patients with CAD and CHD included 15–60 seconds of HIIE followed by a similar duration of active or passive recovery (1:1 ratio).38–40
In contrast to the protocols used in patients with CVD, the protocol used in young overweight/obese patients included four sets of 30 seconds’ “all out” sprint with 4.5 minutes’ active recovery in between sets.41 In patients with T2DM, Gillen et al.42 used a protocol that included 10 bouts of 60 seconds’ duration at ~90% of maximal workload interspersed with 60-second recovery (1:1 ratio).
Supervision level
Five studies did not report the professional qualifications of the person responsible for supervising the exercise sessions. Those that did indicated that the HIIE intervention took place at medical or university centers, which are likely to be equipped to deal with severe adverse events, and under the supervision of a cardiologist, an exercise physiologist, and/or a nurse.33,36–40
Adverse responses
Tomczak et al.37 reported that HIIE did not have a negative effect on LV function and two further studies reported no adverse responses during HIIE.33,35 Four studies did not report whether they observed adverse responses or not during HIIE39,40,42,43; however, the authors of these studies were contacted and they confirmed that no adverse responses were observed during the study (personal correspondence). Overall, 13 adverse responses (8%) were reported in the 156 individuals who completed the 11 identified studies. Adverse responses included a vasovagal reaction (n = 2),38 nausea (n = 1),41 ventricular bigeminy (n = 1),37 a single asymptomatic episode of atrial tachycardia (n = 1),34 and aphasia and dyspraxia as a result of suspected transient cerebral ischemia (n = 1).36 One study reported one case of myocardial ischemia, as evident by ST depression >2 mm.34 Guiraud et al.38 reported that three individuals exhibited ST depression and developed mild angina during HIIE. These three individuals also developed myocardial ischemia during a maximal graded exercise test. A similar study also reported that three participants had demonstrable myocardial ischemia during HIIE.39
Primary and secondary end points
Five studies assessed aspects of safety as the primary aims/end points. One study found that the blood pressure response to HIIE was similar to that to moderate continuous exercise.33 Two studies reported that HIIE had no negative effects on cardiac troponin T, C-reactive protein, or brain natriuretic peptide in patients with CHD and CHF.36,39 The primary aim of two studies34,38 was to examine the effects of different HIIE protocols on exercise time to exhaustion in patients with CHD and CHF. No abnormal hemodynamic responses to exercise were observed and it was reported that short-duration exercise (15–30 seconds), with passive recovery between bouts (1:1 ratio), was more tolerable than longer exercise intervals (60–90 seconds), based on patients being able to maintain higher exercise percentage of VO2peak. In addition, passive recovery between exercise bouts increased time to exhaustion compared to active recovery.34,38
In the other six studies (Table 3), safety was a secondary end point. Guiraud et al.35 reported that HIIE reduced the occurrence of premature ventricular contraction (24-hour Holter ECG recording), compared to both MICE, in patients with CHF with no adverse events during the exercise. Two studies examined the effect of HIIE on endothelial function in patients with metabolic syndrome and CAD. Neither study reported adverse responses during, nor post, exercise and HIIE improved endothelial function to a similar or greater extent than MICE.40,43 Similarly, the other two studies did not report an adverse response during or post-HIIE, and the primary end point was to examine the effects of HIIE on glucose control and insulin sensitivity in obese individuals and those with T2DM.41,42
Table 3.
Acute effects of HIIE.
| REFERENCE | BP AND HR DURING EXERCISE | BIOCHEMICAL MARKERS OF MUSCLE/MYOCARDIAL DAMAGE | ADVERSE RESPONSE | MAIN FINDINGS | MAIN FINDINGS IN REGARD TO SAFETY |
|---|---|---|---|---|---|
| Gayda et al. 201233 | Normal BP response to exercise | N/R | • No adverse responses. • No significant ventricular arrhythmias and/or abnormal BP response during exercise. |
• Stroke volume, cardiac output and arterio-venous difference were similar between CME and HIIE. | • HIIE was well tolerated and safe • No significant arrhythmias or abnormal BP changes. |
| Meyer et al. 201234 | N/R | N/R | • No significant ventricular arrhythmias occurred. • N = 1 asymptomatic atrial tachycardia, which remitted spontaneously after 60 sec. • N = 1, 2 mm ST depression. |
• Short duration 30 sec, compared to 90 sec, interspersed with passive recovery interval is better tolerated than active recovery. | • No safety issues. • HIIE is tolerated in stable patients with CHF. |
| Guiraud et al. 201335 | N/R | N/R | • No adverse responses. | • HIIE increases vagal modulation. • Post-exercise ave HR was lower after HIIE vs CON or CME • Incidence of premature ventricular contractions were reduced post HIIE vs CON and CME. |
• HIIE is safe for patients with CHF. |
| Normandin et al. 201336 | HR similar between CME and HIIE | Serum cardiac troponin T, CRP or BNP did not change after HIIE. | • N = 1: Female age 77 documented ischemic cardiomyopathy. 12–24h after HIIE developed aphasia and dyspraxia (3 hrs) diagnosed with transitorycerebral ischemia. Full recovery after treatment with clopidogrel. • No other adverse events were recorded. • No significant ventricular arrhythmias and/or abnormal BP response. |
• Serum cardiac troponin T unchanged after HIIE. • No significant increase in CRP and BNP postexercise. |
• HIIE appears to be safe. • HIIE completed without occurrence of arrhythmias, myocardial injury, or cardiac decompensation. |
| Tomczak et al. 201137 | HR = 96% HRmax no control group for comparison | N/R | • N = 1# withdrew due to claustrophobia from outcome measure. • N = 1# dizziness during exercise testing (terminated at 64% predicted HRmax), but completed remaining exercise tests symptom free. • N = 1 developed ventricular bigeminy during HIIE. |
• LV function was maintained or improved immediately post HIIE. | • HIIE is safe in a supervised medical setting. • HIIE does not increase CHF symptoms. |
| Guiraud et al. 201038 | N/R | N/R | • N = 2 vagal reaction following HIIE. • N = 3 Myocardial ischemia (ST segment depression) on ECG and developed mild angina during HIIE. |
• Passive recovery between sets resulted in longer time to exhaustion while maintaining high time spent at high %Vo2max.(br/)• 15 sec intervals may be more appropriate than 60 sec intervals. | • HIIE appears to be safe in stable, ft, well-selected patients with CHD. |
| *Guiraud et al. 201139 | N/R | No difference in cardiac troponin T between CME and HIIE. | • N = 3 myocardial ischemia (ST segment depression <2 mm) during HIIE. | • Participants reported HIIE as the preferred exercise compared to CME • HIIE was safe in patients who exercise regularly |
• HIIE appears to be safe for selected stable patients with CHD who exercise regularly. |
| *Currie et al. 201240 | HR: was similar between HIIE and CME | N/R | • No adverse responses | • Endothelial-dependent dilation was improved post HIIE | • No conclusion in regard to the safety of HIIE. |
| Whyte et al. 201341 | N/R | N/R | • N = 1 nausea during HIIE. Patient did not complete the session. | • Extended sprint increased insulin sensitivity both immediately and 24 h post-exercise. • SIT increased insulin sensitivity 24h after exercise only compared to control. |
• No conclusion in regard to the safety of HIIE. • Authors indicated that safety studies are needed. |
| *Gillen et al. 201242 | N/R | N/R | • No adverse responses | • HIIE improved glycaemic control in patients with T2DM. | • No conclusion in regard to the safety of HIIE. |
| *Tjonna et al. 201143 | N/R | N/R | • No adverse responses | • Endothelial function improved post-exercise • BP and blood glucose were reduced after HIIE. |
• No safety data were provided. |
Notes:
Indicates studies that did not report an adverse event and were contacted to clarify whether an adverse event occurred and was not reported in the study.
Indicates that the physiological response was not included as an adverse response caused as a result of HIIT.
Abbreviations: BNP, Brain natriuretic peptide; BP, blood pressure; CHD, coronary heart disease; CHF, chronic heart failure; CME, continues moderate exercise; CON, control; CRP, C reactive proteins; ECG, electrocardiography; HIIE, high intensity interval exercise; LV, left ventricular; N/R, not reported; SIT, sprint interval training; CVD, cardiovascular disease; HR, heart rate; T2DM, type 2 diabetes mellitus.
Discussion
The current systematic review found that adverse responses to HIIE among patients with cardiometabolic diseases enrolled in closely supervised studies were around 8%. However, caution should be taken when translating these findings to all patients with cardiometabolic disease due to the small sample size, high level of supervision involved, and strict inclusion and exclusion criteria applied. Larger randomized-controlled studies that are powered to assess safety and include clinically complex and older patients are required before a more definitive statement on the safety of HIIE in patients with cardiometabolic disease can be made.
Increased physical activity levels can improve functional capacity, quality of life, improve clinical outcomes, and potentially reduce mortality in people with cardiometabolic diseases.44,45 However, exercise may also transiently increase the risk of cardiovascular events, especially at high intensities in patients with CVD.4 An important aim of clinical exercise prescription is therefore to optimize physiological adaptations without placing the patient at risk of exercise-induced events.
To enable inclusion of HIIE in a clinical exercise setting, the safety of the protocol must be established. It is crucial to demonstrate that any additional health benefit of HIIE training outweighs any additional risk and that translation of such programs from a highly supervised/research-based environment to the general population is practically possible. In this review, we focused on the acute response to HIIE in patients with cardiometabolic diseases and not the long-term adaptation that is associated with HIIE training.
Sample size and patients selection
To our knowledge, there are no large-scale trials that have examined the safety of acute HIIE, as a primary end point, for patients with CVD and/or metabolic diseases. The sample size of the few studies that have examined the acute response of these patients to HIIE is relatively small (7–28 patients; Table 2). Studies of this size are underpowered to determine the risk of adverse responses that occur relatively infrequently in patients who are medically stable.2 The small sample size across studies makes it difficult to (i) reach a meaningful conclusion with regard to the physiological/clinical response to the intervention and (ii) generalize the results to the wider population with cardiometabolic conditions.
Furthermore, the studies reviewed included patients who were carefully screened, were clinically stable, and were receiving appropriate medication. Each study included a wide range of exclusion criteria (Table 2) to reduce the risk associated with high-intensity exercise.4 As a result, patients, and in particular those with CVD/CHF, who attend clinical exercise programs may represent a higher-risk cohort than the populations studied to date. It is also important to note that studies of the effects of HIIE training have also used a selected group of patients, with inclusion/exclusion criteria similar to those identified in the current review.16,46,47 This has a particular relevance for CHF as the median age at the time of diagnosis is approximately 76 years of age,48 while the age range of patients with CHF in the HIIE studies is 49–61 years (Table 2). Clearly, the current patient cohorts reported in this review do not represent the vast majority of patients in the community.
HIIE protocols
There are endless combinations of exercise parameters that can be prescribed in HIIE. Accordingly, it is important to identify the optimal protocol/s for each clinical population. It has been shown that short exercise intervals (15–30 seconds), followed by 15–30 seconds’ passive recovery (1:1 ratio), may be better tolerated compared to longer interval times (60–90 seconds). Furthermore, in patients with CHD and CHF, passive recovery seems to be superior to active recovery in terms of total exercise time to exhaustion and to be equal in terms of work at >85% of VO2peak.34,38 As such, it appears that for patients with CVD, the combination of short intervals with passive recovery is better tolerated and, thus, may increase long-term exercise participation. However, it remains to be determined which HIIE protocol elicits superior long-term improvements in clinically relevant end points in these populations.
One study prescribed four bouts of 4-minute duration at 90%–95% of HRpeak with 3-minute active recovery between intervals,37 which is similar to the protocols used by Rognmo et al.17 and Wisloff et al.16 However, given the absence of a control group, it is hard to assess whether this protocol is superior to other protocols in terms of safety. It is important to acknowledge that in all studies, the rate of adverse responses was low, and the HIIE sessions were well tolerated overall across all populations (Table 3). From a practical point of view, the selection of a HIIE protocol should be similar to the selection of any other exercise training protocols and should include consideration of risk versus benefits. It should also be based on case-by-case consideration of exercise prescription and not a “one-size fits all” model.
Supervision level
There is general agreement across the studies reviewed that HIIE should be performed in facilities that have both the equipment and the expertise to handle severe adverse responses. Most studies reported that the exercise was performed in medical or university centers and under the supervision of a cardiologist, an exercise physiologist, and/or a nurse.33,36–39 As such, it is still unclear whether HIIE should be performed in community facilities or at home, where equipment and/or medical personnel may not be available to deal with adverse events. Due to the potential risk of HIIE4 and the lack of detailed trials investigating the “actual” risk of this type of exercise as a primary end point, caution needs to be taken and it is therefore recommended that an experienced clinical exercise physiologist should supervise this type of exercise prescription. In addition, extra care must be taken in monitoring signs and symptoms during and post-HIIE, especially in sedentary individuals. We recommend that blood pressure be observed regularly during and postexercise. In addition, it may be beneficial to use a 12-lead ECG in patients with cardiometabolic diseases before, during, and post-HIIE to monitor arrhythmias and myocardial ischemia as these are the most prevalent adverse events reported during exercise in these populations (Table 3).
Adverse responses
Exercise at mild, moderate, and vigorous (up to 80% of peak capacity) intensities are safe for appropriately screened patients with cardiometabolic diseases.1 This is important as the higher the exercise intensity, the higher is the increase in the myocardial oxygen demand (as measured by the rate–pressure product: heart rate × systolic blood pressure), which may increase the risk of myocardial ischemia and MI.49 Indeed, it has been reported that 4.4% of patients who exhibited acute MI were performing heavy physical exertion (>6 METs [metabolic equivalent]) in the hour prior to the event and that the risk for acute MI in the subsequent hour after heavy physical exertion is 5.9 times higher compared to light or no exertion.50 Furthermore, men with low levels of habitual activity have a 56% increase in relative risk of cardiac arrest during or immediately postexercise, compared with that at other times and the relative risk is reduced to 5% among men at the highest level of habitual activity.51 As such, it appears prudent that individuals should perform moderate-intensity physical activity, on a regular basis, before they engage in HIIE.
HIIE appears to have no adverse effect on LV function37; indeed, long-term HIIE training may in fact improve LV function.16 In addition, most studies reported no adverse responses during or following HIIE.33,37,39,40,42,43 However, overall 13 adverse responses were reported across the studies that were included in the current review. Adverse responses were related to myocardial ischemia, arrhythmias, vagal reactions, and nausea. One patient experienced aphasia and dyspraxia as a result of transient cerebral ischemia 12–24 hours postexercise; however, it was not clear whether this response was related to the exercise. It is important to note that although the rate of adverse events was 8% during or following HIIE, most of the adverse responses were mild in nature. This emphasizes the importance of close supervision during and following this form of exercise.
Although the current review did not assess the incidence of adverse response/events during HIIE training, it is important to note that the event rates that were reported during training interventions are generally low.16,27,46 However, it has recently been reported that the rates of complications (cardiac arrest) during and immediately post-HIIE training was 1 per 23,182 hours compared to 1 per 129,456 hours of moderate-intensity exercise, suggesting a five times higher rate of cardiac arrest during HIIE compared to that during MICE.28 As such, caution must be taken and detailed screening assessments must be performed before prescribing HIIE to patients with cardiometabolic disease.
Limitations
The current review has some potential limitations. First, the number of patients (n = 156) who participated in HIIE is relatively small and as such, more research should be conducted before it will be possible to conclusively advocate for or against this form of exercise to be included in routine clinical practice. Second, due to the low number of available studies reporting adverse responses to a single bout of HIIE, we included studies on patients with a variety of cardiometabolic disease, including CHF, CHD, CAD, obesity, T2DM, and metabolic syndrome. Given the heterogeneity of both the patient population and the HIIE protocols used, the results on adverse responses should be translated with caution. This further highlights the requirement for more studies to evaluate the safety of HIIE protocols in patients with cardiometabolic disease.
In conclusion, the current review indicates that the incidence of adverse responses during or within 24 hours post-HIIE in patients with cardiometabolic diseases is around 8%, which is somewhat higher compared to the previously reported risk during MICE. As such, caution must be taken when prescribing HIIE to patients with cardiometabolic disease. Patients who wish to perform HIIE should be clinically stable, have had recent exposure to at least regular moderate-intensity exercise, and have appropriate supervision and monitoring during and after the exercise session.
Supplementery Materials
Supplemental Table 1. Search strategy for systematic review.
Notes: Medical Subject Heading for Medline (mp) = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier. * = any character.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
FUNDING: IL was supported by a Future Leader Fellowship (ID 100040) from the National Heart Foundation of Australia. NS and AM were supported by the Australian Government’s Collaborative Research Networks (CRN) program. The authors confirm that the funders had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiment: IL, CSS, AJM. Analyzed the data: IL, NKS, CSS. Wrote the first draft of the manuscript: IL CSS. Contributed to the writing of the manuscript: IL, CSS, NKS, SC, AJMcA, CC, AJM. Jointly developed the structure and arguments for the paper: IL, CSS, NKS, SC, AJMcA, CC, AJM. Made critical revisions and approved final version: IL, CSS, NKS, SC, AJMcA, CC, AJM. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.ACSM . Exercise prescription for patients with cardiovascular and cerebrovascular disease. In: Pescatello LS, Arena R, Riebe D, et al., editors. ACSM’s Guidlines for Exercise Testing and Prescription. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014. pp. 236–59. [Google Scholar]
- 2.Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation. 2003;107(1):e2–5. doi: 10.1161/01.cir.0000048890.59383.8d. [DOI] [PubMed] [Google Scholar]
- 3.Ismail H, McFarlane JR, Nojoumian AH, Dieberg G, Smart NA. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: a systematic review and meta-analysis. JACC Heart Fail. 2013;1(6):514–22. doi: 10.1016/j.jchf.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Thompson PD, Franklin BA, Balady GJ, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism, American Heart Association Council on Clinical Cardiology, American College of Sports Medicine Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–68. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher GF, Balady G, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 6.Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 7.Selig SE, Levinger I, Williams AD, et al. Exercise & sports science Australia position statement on exercise training and chronic heart failure. J Sci Med Sport. 2010;13(3):288–94. doi: 10.1016/j.jsams.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 8.ACSM . Exercise prescription for other clinical populations. In: Thompson WR, Gordon NF, Pescatello LS, editors. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. pp. 225–71. [Google Scholar]
- 9.Colberg SR, Sigal RJ, Fernhall B, et al. American College of Sports Medicine, American Diabetes Association Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–67. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hordern MD, Dunstan DW, Prins JB, Baker MK, Singh MA, Coombes JS. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. J Sci Med Sport. 2012;15(1):25–31. doi: 10.1016/j.jsams.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Center for Disease Control and Prevention . State Indicator Report on Physical Activity. Atlanta, GA: U.S. Department of Health and Human Services; 2010. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speight J, Browne J, Holmes-Truscott E, et al. Diabetes MILES – Australia2011 Survey Report. Melbourne, Australia: Diabetes Australia. Vic; 2011. [Google Scholar]
- 13.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34(12):1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Stutts WC. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN. 2002;50(11):499–507. [PubMed] [Google Scholar]
- 15.Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012;42(6):489–509. doi: 10.2165/11630910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 17.Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11(3):216–22. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 18.Little JP, Gillen JB, Percival ME, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111(6):1554–60. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 19.Cocks M, Shaw CS, Shepherd SO, et al. Sprint interval and moderate-intensity continuous training have equal benefits on aerobic capacity, insulin sensitivity, muscle capillarisation and endothelial eNOS/NAD(P)Hoxidase protein ratio in obese men. J Physiol. 2015 doi: 10.1113/jphysiol.2014.285254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjønna AE, Lee SJ, Rognmo Ø, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–54. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciolac EG. High-intensity interval training and hypertension: maximizing the benefits of exercise? Am J Cardiovasc Dis. 2012;2(2):102–10. [PMC free article] [PubMed] [Google Scholar]
- 22.Moholdt TT, Amundsen BH, Rustad LA, et al. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am Heart J. 2009;158(6):1031–7. doi: 10.1016/j.ahj.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Warburton DE, McKenzie DC, Haykowsky MJ, et al. Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol. 2005;95(9):1080–4. doi: 10.1016/j.amjcard.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 24.Gibala MJ, Little JP, Macdonald MJ, et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(pt 5):1077–84. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weston M, Taylor KL, Batterham AM, Hopkins WG. Effects of low-volume high-intensity interval training (HIT) on fitness in adults: a meta-analysis of controlled and non-controlled trials. Sports Med. 2014;44(7):1005–17. doi: 10.1007/s40279-014-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawley JA, Gibala MJ. What’s new since Hippocrates? Preventing type 2 diabetes by physical exercise and diet. Diabetologia. 2012;55(3):535–9. doi: 10.1007/s00125-012-2460-1. [DOI] [PubMed] [Google Scholar]
- 27.Broadbent S, Rousseau J, Tielemans W, et al. Higher intensity interval training improves aerobic capacity and metabolic profile in men with cardiac disease. J Fit Res. 2013;2:8–16. [Google Scholar]
- 28.Rognmo Ø, Moholdt T, Bakken H, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126(12):1436–40. doi: 10.1161/CIRCULATIONAHA.112.123117. [DOI] [PubMed] [Google Scholar]
- 29.Arena R, Myers J, Forman DE, Lavie CJ, Guazzi M. Should high-intensity-aerobic interval training become the clinical standard in heart failure? Heart Fail Rev. 2013;18(1):95–105. doi: 10.1007/s10741-012-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norton K, Norton L. Pre-Exercise Screening: Guide to the Australian adult pre-exercise screening system. Melbourne, Ausralia: Exercise and Sports Science Australia, Fitness Australia and Sports Medicine Australia; 2012. [Google Scholar]
- 32.Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Chochrane Handbook of Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2008. [Google Scholar]
- 33.Gayda M, Normandin E, Meyer P, Juneau M, Haykowsky M, Nigam A. Central hemodynamic responses during acute high-intensity interval exercise and moderate continuous exercise in patients with heart failure. Appl Physiol Nut Metabol. 2012;37(6):1171–8. doi: 10.1139/h2012-109. [DOI] [PubMed] [Google Scholar]
- 34.Meyer P, Normandin E, Gayda M, et al. High-intensity interval exercise in chronic heart failure: protocol optimization. J Cardiac Fail. 2012;18(2):126–33. doi: 10.1016/j.cardfail.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Guiraud T, Labrunee M, Gaucher-Cazalis K, et al. High-intensity interval exercise improves vagal tone and decreases arrhythmias in CHF. Med Sci Sports Exerc. 2013;45(10):1861–7. doi: 10.1249/MSS.0b013e3182967559. [DOI] [PubMed] [Google Scholar]
- 36.Normandin E, Nigam A, Meyer P, et al. Acute responses to intermittent and continuous exercise in heart failure patients. Can J Cardiol. 2013;29(4):466–71. doi: 10.1016/j.cjca.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Tomczak CR, Thompson RB, Paterson I, et al. Effect of acute high-intensity interval exercise on postexercise biventricular function in mild heart failure. J Appl Physiol. 2011;110(2):398–406. doi: 10.1152/japplphysiol.01114.2010. [DOI] [PubMed] [Google Scholar]
- 38.Guiraud T, Juneau M, Nigam A, et al. Optimization of high intensity interval exercise in coronary heart disease. Eur J Appl Physiol. 2010;108(4):733–40. doi: 10.1007/s00421-009-1287-z. [DOI] [PubMed] [Google Scholar]
- 39.Guiraud T, Nigam A, Juneau M, Meyer P, Gayda M, Bosquet L. Acute Responses to High-Intensity Intermittent Exercise in CHD Patients. Med Sci Sports Exerc. 2011;43(2):211–7. doi: 10.1249/MSS.0b013e3181ebc5de. [DOI] [PubMed] [Google Scholar]
- 40.Currie KD, McKelvie RS, Macdonald MJ. Flow-mediated dilation is acutely improved after high-intensity interval exercise. Med Sci Sports Exerc. 2012;44(11):2057–64. doi: 10.1249/MSS.0b013e318260ff92. [DOI] [PubMed] [Google Scholar]
- 41.Whyte LJ, Ferguson C, Wilson J, Scott RA, Gill JM. Effects of single bout of very high-intensity exercise on metabolic health biomarkers in overweight/obese sedentary men. Metabol. 2013;62(2):212–9. doi: 10.1016/j.metabol.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Gillen JB, Little JP, Punthakee Z, Tarnopolsky MA, Riddell MC, Gibala MJ. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obesity Metabol. 2012;14(6):575–7. doi: 10.1111/j.1463-1326.2012.01564.x. [DOI] [PubMed] [Google Scholar]
- 43.Tjønna AE, Rognmo Ø, Bye A, Stølen TO, Wisløff U. Time course of endothelial adaptation after acute and chronic exercise in patients with metabolic syndrome. J Strength Cond Res. 2011;25(9):2552–8. doi: 10.1519/JSC.0b013e3181fb4809. [DOI] [PubMed] [Google Scholar]
- 44.Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. J Am Med Assoc. 1996;276(3):205–10. [PubMed] [Google Scholar]
- 45.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. J Am Med Assoc. 1995;273(14):1093–8. [PubMed] [Google Scholar]
- 46.Freyssin C, Verkindt C, Prieur F, Benaich P, Maunier S, Blanc P. Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Arch Physic Med Rehabil. 2012;93(8):1359–64. doi: 10.1016/j.apmr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Munk PS, Staal EM, Butt N, et al. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation A randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am Heart J. 2009;158(5):734–41. doi: 10.1016/j.ahj.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Mehta PA, Cowie MR. Gender and heart failure: a population perspective. Heart. 2006;92(suppl 3):iii14–8. doi: 10.1136/hrt.2005.070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57(3):549–56. doi: 10.1161/01.cir.57.3.549. [DOI] [PubMed] [Google Scholar]
- 50.Mittleman MA, Maclure M, Tofler GH, et al. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993;329(23):1677–83. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 51.Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311(14):874–7. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Search strategy for systematic review.
Notes: Medical Subject Heading for Medline (mp) = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier. * = any character.


