Non alcoholic fatty liver disease (NAFLD) is the most common form of liver disease affecting nearly a third of the population and its spectrum ranges from simple fat accumulation in the hepatocytes to non alcoholic steatohepatitis (NASH) to cirrhosis (1). Few diseases have attracted the global scientific attention that NAFLD has from such diverse fields as molecular genetics, endocrinology, sleep medicine and hepatology. From this extensive body of literature a few defining concepts have emerged. The current view on the pathogenesis of NAFLD is that a two or possibly multiple hit process results in the progression of disease (2). Excess caloric intake precedes, accompanies or is followed by insulin resistance in both the adipose tissue and skeletal muscle (3). This results in increased circulating fatty acids and their hepatocyte uptake followed by partitioning of the fatty acids to either beta oxidation or esterification to triglycerides (4). The focus of therapy has therefore been to reverse or prevent both hepatic and peripheral insulin resistance. A critical regulatory mechanism for hepatic fat accumulation has also been the reduced fatty acid oxidation and accumulation of triglycerides in the liver. β-oxidation of fatty acids occurs in both peroxisomes and mitochondria that generate acetyl CoA that needs to be oxidized via the Kreb’s cycle in the mitochondrial matrix. Mitochondrial dysfunction in NAFLD contributes to the shift of fatty acids from oxidation into the esterification and export pathways (5). Since fatty liver is intimately linked to the metabolic syndrome, disordered signaling responses have been identified in the triad of metabolically active organs comprised of the liver, adipose tissue and skeletal muscle. Alteration in insulin signaling, substrate metabolism and mitochondrial function contribute to the development and possibly progression of NAFLD.
Adiponectin, an adipocytokine, is a central regulatory link between insulin resistance, disordered substrate oxidation and mitochondrial dysfunction in multiple organs (6). Adiponectin expression is highly specific to adipose tissue but has been shown in other organs including the liver and skeletal muscle (7). Circulating adiponectin exists in different isoforms: high molecular weight (HMW) and low molecular weight (LMW) multimers that bind to the cell surface receptor, T-cadherin but require additional co-receptors for intracellular signaling (7). Other circulating forms include the full length adiponectin that binds to adiponectin receptor 2 (expressed primarily in the liver) and the globular domain trimer (lacking the N terminal domain) that binds to the adiponectin receptor 1 (expressed primarily in the skeletal muscle). Ligand binding to the adiponectin receptors regulates substrate metabolism by activation of the critical energy sensors, AMPK and Sirtuins, activity of the nuclear receptor, PPARα as well as modulation of inflammatory responses (8, 9). Additional hepatic salutary effects of adiponectin include anti-inflammatory and antifibrotic effects. Despite the increasing understanding of the pathogenesis and progression of NAFLD, a number of questions remain, not the least of which are the mechanisms of progression and identifying potential molecular therapeutic targets.
In the current issue, Handa et al (10) report the results of a series of very elegant in-vivo studies in the liver and adipose tissue of a murine model that replicates the spectrum of NAFLD from steatosis to NASH and complementary in-vitro studies in a murine hepatocyte cell line as well as in primary hepatocytes. They demonstrate that adiponectin depletion is a direct consequence of weight gain and plays a critical regulatory role in the development and progression of NAFLD. Their studies specifically provide answers to 2 specific questions: why does plasma adiponectin decrease with progression of NAFLD and is there a mechanistic relation between reduced adiponectin and progression of NAFLD. Using a standard murine model of insulin resistance, the Leprdb/db mice fed a high fat diet, they demonstrated hypoadiponectemia and reduced activation of AMPK and its target, acyl CoA carboxylase (ACC). Since AMPK activation is a cellular response to activate oxidative phosphorylation, reduced adiponectin acts via blunted cellular energy sensing mechanisms (9). Additionally, the authors demonstrate a novel and potentially paradigm shifting link between adiponectin and mitochondrial biogenesis (10). Interestingly, NASH was induced in mice with a liquid high fat diet with omega-6 polyunsaturated fatty acids.
Relationship between decreased plasma adiponectin and progression of NAFLD
The current study helps identify the potential mechanisms for a number of clinical and molecular observations in patients with NAFLD who have an increased adipose tissue mass and reduced adiponectin (8). Since adiponectin is synthesized primarily by adipocytes, it has been a challenge to explain the low adiponectin despite an expansion of adipose tissue mass. There has been controversy regarding the mechanism of low circulating adiponectin and the hyperinsulinemia of NAFLD since insulin directly stimulates adiponectin biosynthesis and secretion (11). In vitro studies in NIH 3T3 L1 adipocytes showed increased adiponectin in the medium in response to insulin. In contrast, in vivo, hyperinsulinemia consistently is associated with hypoadiponectemia (6). Physiologically, insulin inhibits adipose tissue FOXO1 that in turn represses its downstream target PPARγ, a transcriptional inducer of the adiponectin gene (12). In NAFLD, insulin resistance is accompanied by reduced suppression of FOXO1 activation with downstream consequences resulting in reduced adiponectin transcription. There is however, very limited evidence to support this mechanistic hypothesis. Additional contributors to the reduced adiponectin include inflammation-induced oxidative stress in adipose tissue. The very interesting direct studies by Handa et al on adipose tissue showing an increased inflammatory response suggest that expression of adiponectin expression is inversely related to a number of inflammatory genes such as NFkB, IL6 and TNFα (10).
A possible mechanistic relationship between reduced adiponectin and progression of NAFLD
The critical question of how adiponectin functions as a mediator of the adipose tissue - liver axis was answered by studies in liver tissue as well as direct studies in hepatocytes. NAFLD and specifically NASH in vivo, was associated with reduced liver AMPK activation via impaired adiponectin signaling. Low adiponectin was accompanied by reduced adiponectin receptor 2 as well as reduced phosphorylation of AMPK. Previous studies have suggested that AMPK phosphorylation by adiponectin was mediated via APPL1 (leucine zipper motif) while Handa et al show that LKB1 expression was reduced in NASH suggesting a novel signaling pathway of adiponectin- adiponectin R2- LKB- AMPK. Additional studies with knockout mice are necessary to confirm these in vitro studies on this novel pathway. Whether activation of AMPK by alternate mechanisms including pharmacological interventions using 5 amino-imidazole-4-carboxamide (AICAR) can overcome the hypoadiponectinemia is yet to be resolved but provides an exciting targeted therapeutic option. In addition to demonstrating the biochemical basis of fat accumulation via the increased ACC due to reduced AMPK activation, mitochondrial biogenesis was also affected. Conflicting reports on disordered hepatic mitochondrial function and increased oxidative stress have been reported in NASH but its mechanism is not entirely clear (5, 13). The present study sheds light on this by providing direct evidence of regulation of mitochondrial structure by adiponectin. Even though the authors did not show direct evidence of mitochondrial dysfunction by either oxygen consumption ratio or substrate oxidation, their data are compelling to reiterate that mitochondrial dysfunction is an essential component of NASH and they show that this a direct effect of reduced adiponectin function.
The skeletal muscle is the third component of the metabolically active organ triad and plays a significant role in the development of the metabolic syndrome, insulin resistant states and fatty liver. Since NAFLD is being recognized as a state of sarcopenic obesity, whether the loss of muscle mass or altered signaling responses in the skeletal muscle contributes to development and progression of NAFLD to cirrhosis is an area of intense research interest (14). In the skeletal muscle, adiponectin binds to the adiponectin receptor 1 and regulates peripheral insulin sensitivity as well as fatty acid oxidation (7, 8). Myostatin, a skeletal muscle myokine of the TGFβ superfamily, is a potential therapeutic target in insulin resistant states (15, 16). Evidence that blocking myostatin protects against both diet induced obesity and fatty liver (17) opens an exciting area of whether myostatin and adiponectin cross talk (18) and if so, does it occurs at the transcriptional, posttranslational or receptor level?
Recent data show additional novel adiponectin responses that include ceramidase activity (19) and suppression of gluconeogenic enzymes, independent of AMPK via the APPL1 pathway (7). These and other mechanisms including interaction of adiponectin with the suppressor of glucose from autophagy protein (20) are very exciting areas that may play a contributory role to the progression of NAFLD and need further studies.
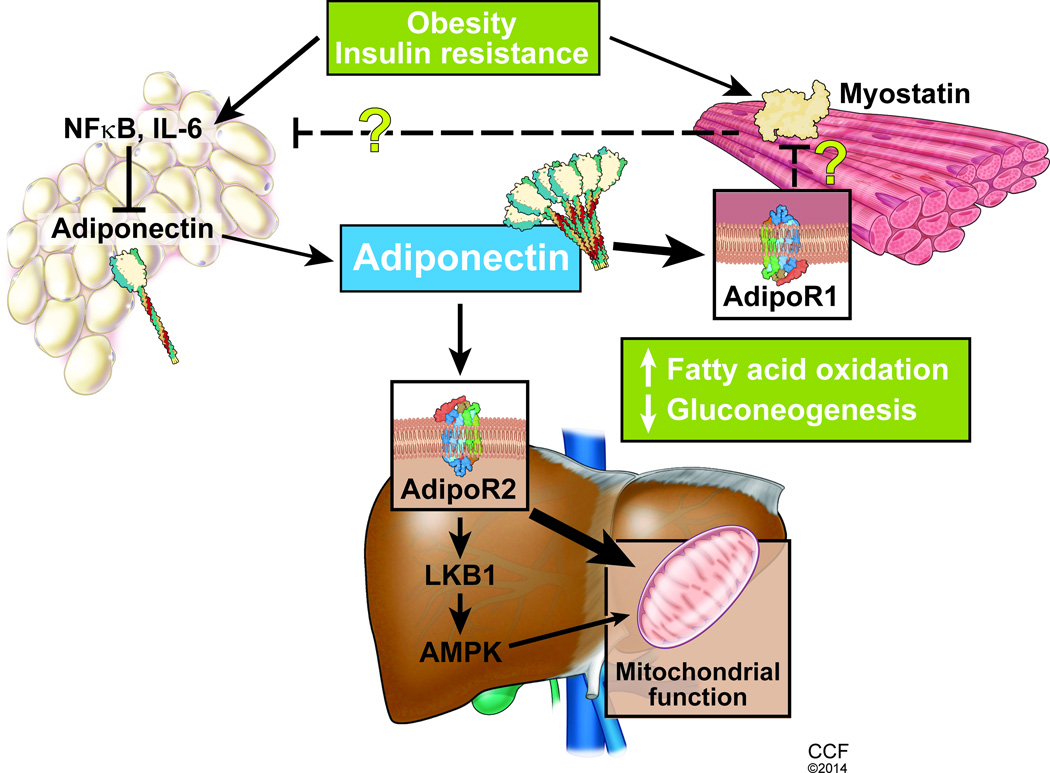
In summary, these data provide compelling evidence for novel targets and pathways in the development and progression of fatty liver and specifically identify adiponectin as a mediator of the liver- skeletal muscle axis (Figure 1). The present study provides compelling and paradigm shifting evidence of the central role of adiponectin mediated AMPK dependent regulation of signaling responses in vivo in NAFLD and specifically, in NASH. Given the increasing recognition of the considerable adverse effects of the thiazolidinedione class of insulin sensitizers that have effects beyond adiponectin, studies focusing on the development of adiponectin analogs or inducers as well as adiponectin sensitizers will provide novel therapeutic options for patients with NAFLD.
Figure 1.
Multiorgan metabolic regulatory role of adiponectin as a central link in NAFLD. Insulin resistance and obesity contribute to the adipose tissue inflammatory response with reduced adiponectin secretion. Hepatic signaling and putative signaling effects on the skeletal muscle via myostatin regulation suggest the systemic metabolic regulatory effects of adiponectin.
Reference List
- 1.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013 Jul 1;178(1):38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH) Int J Mol Sci. 2013;14(10):20704–20728. doi: 10.3390/ijms141020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi K, Clements RH, Saeed F, Abrams GA. Comparative evaluation of whole body and hepatic insulin resistance using indices from oral glucose tolerance test in morbidly obese subjects with nonalcoholic Fatty liver disease. J Obes. 2010 doi: 10.1155/2010/741521. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zammit VA. Mechanisms of regulation of the partition of fatty acids between oxidation and esterification in the liver. Prog Lipid Res. 1984;23(1):39–67. doi: 10.1016/0163-7827(84)90005-5. [DOI] [PubMed] [Google Scholar]
- 5.Grattagliano I, de BO, Bernardo TC, Oliveira PJ, Wang DQ, Portincasa P. Role of mitochondria in nonalcoholic fatty liver disease--from origin to propagation. Clin Biochem. 2012 Jun;45(9):610–618. doi: 10.1016/j.clinbiochem.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Calton EK, Miller VS, Soares MJ. Factors determining the risk of the metabolic syndrome: is there a central role for adiponectin? Eur J Clin Nutr. 2013 May;67(5):485–491. doi: 10.1038/ejcn.2013.1. [DOI] [PubMed] [Google Scholar]
- 7.Combs TP, Marliss EB. Adiponectin signaling in the liver. Rev Endocr Metab Disord. 2013 Dec 3; doi: 10.1007/s11154-013-9280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006 Jul;116(7):1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010 Apr 29;464(7293):1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 10.Handa P, Maliken B, Nelson J, Morgan-Stevenson V, Dhillon B, Klintworth H, et al. Reduced adiponectin signaling due to weight gain results in nonalcoholic steatohepatitis through impaired mitochondrial biogenesis. 2013 doi: 10.1002/hep.26946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajri T, Tao H, Wattacheril J, Marks-Shulman P, Abumrad NN. Regulation of adiponectin production by insulin: interactions with tumor necrosis factor-alpha and interleukin-6. Am J Physiol Endocrinol Metab. 2011 Feb;300(2):E350–E360. doi: 10.1152/ajpendo.00307.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karki S, Chakrabarti P, Huang G, Wang H, Farmer SR, Kandror KV. The multi-level action of fatty acids on adiponectin production by fat cells. PLoS One. 2011;6(11):e28146. doi: 10.1371/journal.pone.0028146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasarathy S, Kasumov T, Edmison JM, Gruca LL, Bennett C, Duenas C, et al. Glycine and urea kinetics in nonalcoholic steatohepatitis in human: effect of intralipid infusion. Am J Physiol Gastrointest Liver Physiol. 2009 Sep;297(3):G567–G575. doi: 10.1152/ajpgi.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasarathy J, Periyalwar P, Allampati S, Bhinder V, Hawkins C, Brandt P, et al. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int. 2013 Sep 5; doi: 10.1111/liv.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009;4(3):e4937. doi: 10.1371/journal.pone.0004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonala S, McFarlane C, Ang J, Lim R, Lee M, Chua H, et al. Pid1 induces insulin resistance in both human and mouse skeletal muscle during obesity. Mol Endocrinol. 2013 Sep;27(9):1518–1535. doi: 10.1210/me.2013-1048. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Burgess K, Xu T, Brown R, Han B, Welle S. Effect of myostatin depletion on weight gain, hyperglycemia, and hepatic steatosis during five months of high-fat feeding in mice. PLoS One. 2011;6(2):e17090. doi: 10.1371/journal.pone.0017090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki ST, Zhao B, Yang J. Enhanced muscle by myostatin propeptide increases adipose tissue adiponectin, PPAR-alpha, and PPAR-gamma expressions. Biochem Biophys Res Commun. 2008 May 2;369(2):767–773. doi: 10.1016/j.bbrc.2008.02.092. [DOI] [PubMed] [Google Scholar]
- 19.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011 Jan;17(1):55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowherd RB, Asmar MM, Alderman JM, Alderman EA, Garland AL, Busby WH, et al. Adiponectin lowers glucose production by increasing SOGA. Am J Pathol. 2010 Oct;177(4):1936–1945. doi: 10.2353/ajpath.2010.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]