Abstract
In high risk/inoperable patients with severe symptomatic aortic stenosis, transcatheter aortic valve implantation (TAVI) is a proven alternative to standard (i.e., medical) therapy (ST) or surgical AVR. Concerns have been raised, however, about patients who survive the procedure but have a short subsequent survival. We therefore sought to identify correlates of early out-of-hospital mortality (EOHM) among patients undergoing successful TAVI, rendering TAVI potentially “futile”. Patients who were discharged from the hospital and survived >30 days but <12 months after TAVI were identified (EOHM group). Independent predictors of EOHM were explored, including patient-level factors and procedural non-fatal major complications (NFMC). A sensitivity analysis was also performed excluding patients with NFMC. Among 485 patients who were discharged from the hospital and survived 30 days following TAVI, 101 (21%) were dead within one year. Independent predictors of EOHM included serum creatinine, liver disease, coagulopathy, mental status, body mass index, male gender, and STS score. Although NFMC were strongly associated with EOHM, patient-level risk factors for EOHM were similar among patients who did and did not experience NFMC. Compared to ST, TAVI patients with EOHM had similar 6-month 6-minute walk distance & functional class, with higher rates of repeat hospitalization. In conclusion, among high risk/inoperable patients undergoing TAVI who were discharged and alive at 30 days, EOHM was not infrequent, and was largely determined by presenting characteristics and the occurrence of peri-procedural NFMC. Careful screening and minimization of NFMC may maximize the benefit of TAVI.
Keywords: Valves, Aortic stenosis, TAVI, Futility
Introduction
Among high-risk adults with severe symptomatic aortic stenosis (AS), transcatheter aortic valve implantation improves survival compared with medical therapy in patients not suitable for surgery, and is non-inferior to surgical aortic valve replacement (SAVR) among high-risk surgical candidates. 1-4 Additionally, quality of life improvements are associated with TAVI.5,6 Despite these encouraging results, all-cause mortality following TAVI in high-risk patients remains high (44% at 3 years)7. Much of this mortality is not procedural, but rather a result of existing comorbidities and other contributing patient characteristics, including advanced age, that adversely influence long-term outcomes. Because the TAVI procedure does incur some patient risk as well as societal cost, it is reasonable to seek to maximize the benefit of the procedure through the application of the procedure to patients for whom meaningful clinical benefit is expected. We therefore sought to use data from the Placement of AoRTic TraNscathetER Valve (PARTNER) trial to characterize a cohort of patients for whom the TAVI procedure was successful, but did not provide a long-term mortality benefit. To accomplish this objective, we compared those patients who underwent TAVI and were successfully discharged from the hospital and alive at 30 days after the procedure but yet died within a year of the procedure, with those who survived beyond a year in order to identify predictors of early out-of-hospital mortality (EOHM) after TAVI.
Methods
The PARTNER I trial incorporated two parallel prospective, multicenter, randomized, active-treatment-controlled clinical trials. The study design and patient selection for the randomized, multicenter PARTNER I trial have been previously described. 1,3Briefly, the PARTNER I Trial enrolled patients with severe symptomatic AS (aortic-valve area ≤0.8 cm2 plus a peak velocity ≥4 meters per second or a mean transaortic valve gradient ≥40 mmHg) who were deemed high-risk for conventional surgery (Cohort A) or inoperable (Cohort B). The institutional review board at each participating site approved the study and all patients provided written informed consent.
Clinical outcomes analyzed included all cause and cardiovascular mortality, repeat hospitalization (due to AS or complications of the valve procedure), NYHA functional class, and 6-minute walk test (distance measured in meters walked in 6 minutes in a monitored walk)8. Procedural success was defined as patients who received a valve implant during the index procedure with < moderate aortic insufficiency. Major arrhythmia was defined as the presence of: Atrial fibrillation/flutter, supraventricular tachycardia, ventricular arrhythmia or a high degree atrio-ventricular block. An independent clinical events committee adjudicated all adverse events.
The current analysis pooled all patients from cohorts A and B who underwent TAVI via the TF or TA approach (as treated analysis). In order to minimize the influence of fatal procedural events (e.g. complications) in determining one-year survival, patients who died during their index (procedural) hospitalization or within the first 30 days post-TAVI were then excluded from this analysis. In total, 519 patients underwent TAVI of which 29 patients died within 30 days and 5 suffered in-hospital death (IH) after 30 days. This left a total of 485 patients included in this analysis.
Patients who had undergone TAVI were then stratified into two groups: Patients who were discharged from the index hospitalization and were alive at 30 days post-TAVI but died from any cause within the year following TAVI were characterized as EOHM; patients undergoing TAVI who were alive at 1-year post-TAVI were considered no early mortality (no EOHM). We further analyzed the EOHM group based upon whether a peri-procedural non-fatal major complication (NFMC) had occurred. NFMC included the occurrence (within 7 days of TAVI) of any stroke, major vascular complications, major bleeding complications, and aortic valve re-intervention. In a further comparison, outcomes of EOHM patients who were discharged alive after TAVI were compared with those of 179 inoperable patients who underwent standard therapy without TAVI (medical therapy alone with or without balloon aortic valvuloplasty).
Baseline demographic, clinical, and echocardiographic characteristics were compared between EOHM and no EOHM patients. The Society of Thoracic Surgery predicted risk (STS score) of in-hospital/30-day mortality for an isolated SAVR and logistic EuroSCORE (LES) were computed for all patients. Baseline cognitive function was assessed using the standardized Mini Mental Status Examination (MMSE).9 Differences between patients with EOHM and with no EOHM were tested using chi-squared or Fisher’s exact test for dichotomous variables and Student’s t-test or Wilcoxon rank sum test for continuous variables, as appropriate. The rates of clinical outcomes in EOHM and no EOHM groups were assessed according to the method of Kaplan and Meier and compared using the log-rank test.
Multivariable Cox proportional hazards models were constructed in order to identify baseline variables independently associated with EOHM. A tiered approach was used whereby the initial model included only the baseline variables with univariate associations with EOHM at a level of p≤0.1. A second model included NFMC, added as time-dependent covariates, and the final model included only those variables that remained significant after multivariable adjustment. As a sensitivity analysis, an additional model was constructed restricting the dataset to subjects without NFMC. A second sensitivity analysis was performed by redefining EOHM as those who survived to hospital discharge and 30 days post TAVI, but died within 6 months after TAVI; the results of this analysis was similar to the primary analysis and, thus, is not shown. All statistical analyses were performed with the use of SAS software, (SAS version 9.2 SAS Institute, Cary, North Carolina), and a 2-sided alpha level of 0.05 was used to determine statistical significance.
Results
A total of 485 patients in the PARTNER I randomized trial were discharged from the hospital and survived ≥30 days following TAVI, and were therefore included in the primary analysis. Of these, 101 (21%) were dead within one year (categorized as EOHM), and 384 (79%) survived at least one year (categorized as no EOHM).
Baseline characteristics of the EOHM vs no EOHM groups are shown in Table 1. Also included, for comparison, are the baseline characteristics of the 179 patients randomized to ST. There were similar rates of TA vs. TF access in the EOHM and no EOHM groups. (18% TA in the EOHM group vs. 19% TA in the no EOHM group, p=0.79)
Table 1.
Baseline Clinical and Echocardiographic Characteristics of the early out-of-hospital mortality (EOHM, TAVR survivors <1 year) compared to no early out-of-hospital mortality (no EOHM, TAVR survivors >1 year) and standard therapy (ST) groups
| Variable |
Early Out-of- Hospital Mortality (n=101) |
No Early Out-of- Hospital Mortality (n=384) |
Standard Therapy (n = 179) |
p-value
EOHM vs No EOHM |
p-value
EOHM vs ST |
|---|---|---|---|---|---|
| Age (median, IQR) | 84.68 [78.26, 88.47] | 84.42 [79.56, 88.43] | 84.75 [79.10, 88.55] | 0.92 | 0.97 |
| Age ≥ 80 years | 71 (70%) | 282 (73%) | 128 (72%) | 0.53 | 0.83 |
| Male | 62 (61%) | 199 (52%) | 84 (47%) | 0.09 | 0.02 |
| Body Mass Index (median, IQR) (kg/m2) |
25.05 [21.63, 28.94] | 26.63 [22.86, 30.69] | 25.25 [22.15, 29.44] | 0.007 | 0.23 |
| STS Score (median, IQR) | 12.00 [9.70, 15.80] | 10.95 [9.50, 12.90] | 11.50 [8.80, 15.00] | 0.009 | 0.26 |
| STS > 10 | 72 (71%) | 261 (68%) | 117 (66%) | 0.52 | 0.34 |
| LogEuroSCORE (median, IQR) | 26.61 [16.18, 43.08] | 24.06 [14.64, 37.50] | 26.00 [16.19, 41.48] | 0.17 | 0.73 |
| Diabetes mellitus | 33 (33%) | 159 (41%) | 63 (35%) | 0.11 | 0.67 |
| Hyperlipidemia | 73 (72%) | 309 (81%) | 141 (79%) | 0.07 | 0.22 |
| Smoker | 47 (47%) | 185 (48%) | 86 (48%) | 0.77 | 0.81 |
| Hypertension | 89 (88%) | 342 (89%) | 153 (86%) | 0.74 | 0.54 |
| Heart Failure NYHA class 3/4 | 95 (94%) | 360 (94%) | 168 (94%) | 0.91 | 0.95 |
| Coronary Artery Disease | 77 (76%) | 279 (73%) | 133 (74%) | 0.47 | 0.72 |
| Peripheral Vascular disease | 38 (38%) | 145 (38%) | 45 (25%) | 0.94 | 0.03 |
| Porcelain aorta | 8 (8%) | 26 (7%) | 20 (11%) | 0.69 | 0.38 |
| Prior balloon aortic valvuloplasty | 21 (21%) | 45 (12%) | 39 (22%) | 0.02 | 0.85 |
| Major Arrhythmia | 58 (57%) | 175 (46%) | 90 (50%) | 0.03 | 0.25 |
| Coagulopathy | 6 (6%) | 6 (2%) | 3 (2%) | 0.02 | 0.07 |
| Creatinine ≥ 2 mg/dl | 28 (28%) | 62 (16%) | 35 (20%) | 0.008 | 0.12 |
| Liver disease | 6 (6%) | 7 (2%) | 6 (3%) | 0.03 | 0.36 |
| Chronic obstructive pulmonary disease |
44 (44%) | 158 (41%) | 94 (53%) | 0.66 | 0.15 |
| Oxygen Dependent | 16 (16%) | 46 (12%) | 46 (26%) | 0.30 | 0.06 |
| Chest wall radiation | 4 (4%) | 15 (4%) | 15 (8%) | 1.00 | 0.16 |
| Chest wall deformities | 3 (3%) | 11 (3%) | 9 (5%) | 1.00 | 0.55 |
| MMSE (median, IQR) | 27.0 [24.5, 29.0] | 28.0 [25.0, 29.0] | 28.0 [25.0, 30.0] | 0.02 | 0.03 |
| Anemia | 78 (77%) | 255 (67%) | 129 (73%) | 0.04 | 0.42 |
| Mean AV Gradient (mmHg, median, IQR) |
40.24 [32.22,49.24] | 42.19[33.25, 52.44] | 40.66 [32.64, 49.90] | 0.14 | 0.53 |
| Peak AV Gradient (mmHg, median, IQR) |
66.74 [51.67, 82.32] | 70.09 [56.53, 86.60] | 70.11 [56.46, 86.44] | 0.12 | 0.22 |
| Aortic Valve Area (EOA) (cm2, median (IQR) |
0.65 [0.51, 0.77] | 0.63 [0.53, 0.76] | 0.64 [0.50, 0.74] | 0.68 | 0.98 |
| Baseline LVEF (%, median, IQR) |
55 [43, 60] | 57[45, 62] | 55 [41, 60] | 0.14 | 0.85 |
- Anemia is defined as Hemoglobin (g/dL) < 12 (female) and Hemoglobin (g/dL) < 13 (male).
- EOA: estimated orifice area
NFMC were more frequent among patients experiencing EOHM (28% with EOHM vs. 16% without EOHM, p= 0.007) (Table 2). Among NFMC, major vascular complications (17% vs. 10%, p=0.04) were more frequent among EOHM as compared with no EOHM patients. A total of 54 patients developed major vascular complications of which 52 (96%) were TF and 2 (4%) were TA. There was a trend toward more frequent major bleeding (16% vs. 9%, p=0.06) but no difference in the rates of any stroke, or the need for any aortic valve re-intervention. The rate of new permanent pacemaker implantation was similar in the EOHM and no EOHM groups (6% vs. 12%, p=0.23) and was not found to be a significant univariate predictor of EOHM. The rate of post-procedure moderate or severe paravalvular aortic insufficiency was similar among EOHM as compared with no EOHM patients (12% vs. 9%, p=0.46). Transfemoral or transapical access was not a significant univariate predictor of early out of hospital mortality (transapical vs. transfemoral: Hazard Ratio [95% CI]: 1.02 [0.62, 1.68], p=0.93).
Table 2.
Non-fatal peri-procedural complications among early out-of-hospital mortality (EOHM, TAVR survivors <1 year) compared to no early out-of-hospital mortality (no EOHM, TAVR survivors >1 year)
| Variable | EOHM (n=101) |
No EOHM (n=384) |
p-value | Independent Hazard of EOHM [95% CI] |
|---|---|---|---|---|
| Any NFMC+ | 28 (28%) | 62 (16%) | 0.007 | 1.81 [1.16, 2.83] |
| Stroke (any) | 7 (7%) | 14 (4%) | 0.17 | 3.19 [1.82, 5.62] |
| Aortic valve re-intervention | 5 (5%) | 6 (2%) | 0.057 | 3.74 [1.61, 8.67] |
| Major bleeding | 16 (16%) | 36 (9%) | 0.06 | 2.43 [1.54, 3.83] |
| Major vascular complications |
17 (17%) | 37 (10%) | 0.04 | 1.71 [1.00, 2.90] |
P-value from Chi-square test or Fisher’s exact test, as appropriate, for categorical variables and from Student’s t-test for means and Wilcoxon Rank Sum for medians for continuous variables.
Non-fatal major complications: Complications occurring within 7 days of index TAVR.
In a multivariable model examining the independent correlates of EOHM (Table 3a), baseline and pre-procedural characteristics independently associated with EOHM included: serum creatinine, liver disease, coagulopathy, total Mini Mental State Exam score (MMSE), Body Mass Index (BMI) and STS score. Of note, these correlates (and the strengths and magnitude of their associations with EOHM) remained independently associated with EOHM in a model excluding patients without NFMC (Table 3b).
Table 3.
Baseline Predictors of early out-of-hospital mortality
| Variable | Hazard Ratio | p value |
|---|---|---|
| 3a. All patients | ||
| Body Mass Index (lbs/in2) | 0.95 [0.92, 0.99] | 0.0095 |
| Coagulopathy | 3.46 [1.49, 8.03] | 0.0039 |
| Serum creatinine | 1.08 [1.03, 1.14] | 0.0029 |
| Liver disease | 2.69 [1.15, 6.31] | 0.0225 |
| Total Mini Mental State Exam Score | 0.95 [0.90, 1.00] | 0.0454 |
| STS Risk Score | 1.06 [1.02, 1.11] | 0.0034 |
| 3b. Patients without NFMC | ||
| Body Mass Index (lbs/in2) | 0.94 [0.90, 0.99] | 0.0181 |
| Coagulopathy | 3.47 [1.24, 9.70] | 0.0177 |
| Serum creatinine | 1.09 [1.03, 1.15] | 0.0031 |
| Liver disease | 3.00 [1.07, 8.39] | 0.0365 |
| Male | 2.09 [1.23, 3.54] | 0.0063 |
| Total Mini Mental State Exam Score | 0.93 [0.88, 0.99] | 0.0238 |
| STS Risk Score | 1.08 [1.03, 1.13] | 0.0008 |
Multivariate predictors using stepwise Cox regression with entry/stay criteria of 0.1/0.1
Potential covariates include: Gender, BMI, STS Score, Prior BAV, Liver Disease, MMSE, Major arrhythmia, Anemia, Renal disease, Coagulopathy, Hyperlipidemia
NFMC after TAVI were also strongly associated with EOHM. After adjustment for baseline correlates of EOHM, individual complications that were associated with subsequent EOHM were: any stroke, major bleeding, and the need for any aortic valve re-intervention (Table 2). In the final adjusted model, the independent contributors to EOHM were: coagulopathy, liver disease, NFMC, baseline serum creatinine, baseline STS risk score, BMI, and total MMSE score (Table 4).
Table 4.
Predictors of Early Out of Hospital Mortality (Full model including NFMC modeled as a time-dependent covariate):
| Variable | Hazard Ratio | p value |
|---|---|---|
| Body Mass Index (lbs/in2) | 0.95 [0.92, 0.99] | 0.0098 |
| Coagulopathy | 3.05 [1.29, 7.17] | 0.0108 |
| Serum creatinine | 1.09 [1.03, 1.15] | 0.0017 |
| Liver disease | 2.84 [1.20, 6.73] | 0.0174 |
| Total Mini Mental State Exam | 0.95 [0.90, 1.00] | 0.0458 |
| NFMC | 1.81 [1.16, 2.83] | 0.0087 |
| STS Risk Score | 1.07 [1.02, 1.11] | 0.002 |
Multivariate predictors using stepwise Cox regression with entry/stay criteria of 0.1/0.1
Potential covariates include: Baseline variables as in table 3, NFMC
Among patients in whom 6-minute walk distance could be assessed at 30 days, this distance was non-significantly lower among EOHM compared with no EOHM patients (median, IQR: 139.25 (78.34, 253.65) vs. 180 (109.0, 271.80), p=0.25). More EOHM patients were in NYHA class III/IV (54% vs. 20%, p< 0.0001) and fewer had improved by greater than one NYHA class (30% vs. 53%, p=0.0001). At 1 year of follow- up, the rate of repeat hospitalization was more frequent among EOHM compared with no EOHM (50% vs. 14%, p< 0.0001).
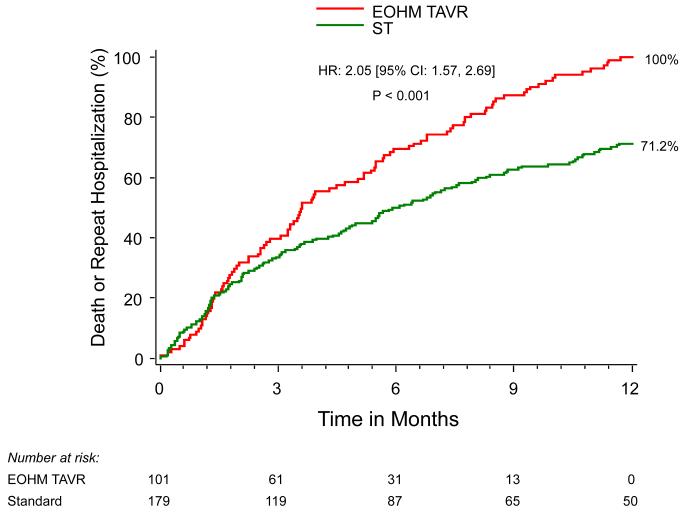
Outcomes were additionally compared between patients who underwent TAVI and had subsequent EOHM and PARTNER I patients randomized to ST alone without TAVI. Among patients in whom 6-minute walk distance could be assessed at 6 months, this distance was similar for EOHM as compared to ST (median [IQR]) (122.00 [106.68, 146.00] vs. 139.35 [9.00, 257.00], p=0.49), as was the proportion of EOHM patients in NYHA class III/IV (50% vs. 66%, p=0.09). At 6 months, the rate of repeat hospitalization was more frequent among EOHM as compared to ST patients (38% vs. 21%, p=0.004) and mortality was additionally > 2-fold higher among EOHM as compared with ST patients (55% vs. 22%, p<0.0001). (Figure 1)
Figure 1.
Kaplan-Meier curve for the combined endpoint of All-Cause Mortality + Rehospitalization (EOHM vs. ST)
Discussion
The principal findings of this analysis of the PARTNER trial are: 1) 1 of 5 patients discharged from the hospital alive at 30 days after TAVI was subsequently dead within one year following the procedure; 2) not unexpectedly, patients with EOHM had higher STS scores, lower MMSE scores, lower BMI and more frequently had other comorbidities such as liver and kidney disease; 3) patients with EOHM had more frequent non-fatal periprocedural major complications, but even among patients with NFMC, baseline covariates contributed to EOHM; and 4) compared with a cohort of patients who underwent standard therapy without TAVI, TAVI patients with EOHM had higher rates of repeat hospitalization and demonstrated no other evidence of functional improvement measured by either 6-minute walk distance or NYHA functional class.
The patients undergoing TAVI enrolled in the PARTNER I trial represented a cohort of some of the highest-risk patients with severe AS, with multiple coexisting comorbidities contributing to this risk. In addition to the demonstration of feasibility (and effectiveness) of TAVI in PARTNER, one of the most striking observations from the trial was the subsequent outcomes of patients enrolled in the trial, with significant late mortality consistent with the overall risk profile of the enrolled patients. When adopting a new treatment for patients who remain at high risk for death, it is important to attempt to identify a population of patients who undergo a successful procedure, but yet may be at risk of deriving minimal longer-term clinical benefit from the procedure. In the context of this analysis, we defined those patients (EOHM) as those who were able to be discharged from the hospital and were alive at 30 days following the procedure, but yet did not survive beyond one year following TAVI. Notably, the overall frequency of EOHM even for patients who were discharged alive at 30 days was 21%.
A prior multivariable analysis from the SOURCE registry identified logistic EuroSCORE, renal disease, liver disease, and smoking as variables with the highest hazard ratios for 1-year mortality.10 Unlike our analysis, patients that died in-hospital or within 30-days of index procedure were included in the analysis from the SOURCE registry. Additionally, events were site reported in the SOURCE registry but adjudicated in the PARTNER trial from which the present analysis is derived. The PARTNER trial utilized prospectively calculated STS scores as an entry criterion but not the Logistic EuroSCORE. With respect to the factors that contribute to EOHM in the present analysis, several but not all measures of baseline comorbidity were similar (baseline liver and kidney disease) but others dissimilar (STS scores, lower MMSE scores, lower BMI) to those in the SOURCE registry analysis as predictors of subsequent 1-year outcomes.
The logistic EuroSCORE and Society of Thoracic Surgeons scores were established as a mortality risk assessment of open cardiac surgery and not for TAVI, and while they have been extensively used to determine eligibility and prognosticate on TAVI outcomes, their utility in TAVI is very much open to question.11-13 It has previously been shown that higher STS scores (indicating greater surgical risk) are associated with significantly higher 2-year mortality and that there may be a lack of survival benefit with TAVI as compared with standard therapy at highest STS scores (STS ≥ 15).2 The STS score accurately predicts mortality in low risk patients undergoing bypass surgery but has not been well-validated in high risk patients undergoing SAVR or TAVI.14 Until a dedicated TAVI risk tool is available, the STS-PROM may aid in identifying patients at risk, but it should be complemented with clinical judgment, assessment of patient’s level of independent function, and other clinical factors identified in TAVI populations to assist in patient selection.
In the present analysis, cognitive impairment as measured by MMSE was shown to predict early death after TAVI – an association previously described in elderly patients with known cardiovascular disease.15 Cognitive impairment may influence discharge location, nourishment, medication compliance, ability for a patient to participate in an organized rehabilitation program; increasing the risk of hospital readmission and death. 15 Other factors associated with EOHM included serum creatinine, liver disease, coagulopathy, and BMI. In another analysis of patients undergoing TAVI, chronic renal failure was found to be the strongest single predictor of mortality at late follow-up.16 It has previously been shown that patients with liver disease who require surgery are at greater risk for surgical and anesthesia related complications than those with a healthy liver. 17 A BMI < 20 kg/m2 has been associated with a higher risk of stroke and death in patients undergoing TAVI in other series.18 This association between low BMI and outcomes may be mediated through patient frailty and vulnerability to procedural complications. Careful screening and emphasis on these comorbidities is critical to tease out patients whose long-term survival will not be modified after valve replacement. Although patients having fatal complications were excluded from this analysis by design, the occurrence of NFMC were independently associated with subsequent EOHM. Pre-operative anemia and consequent blood transfusions after cardiac and non-cardiac surgical procedures may increase future mortality of patients19 and therefore this may be an important and readily remediable risk factor that should be addressed before patients undergo TAVI. Procedural complications have an additive effect on the high-risk patients undergoing TAVI, and, as a result, every attempt should be made to minimize such complications. Improvements in catheter valve prosthesis, the use of embolic protection devices, newer generation devices that require smaller arteriotomies, the availability and widespread experience of non-transfemoral access routes, and improved patient screening will likely decrease TAVI related complications and their consequences.
Defining medical futility is a complicated task, and its definition will differ depending on the population being studied. For the purpose of this analysis, futility was defined as those patients who had EOHM – identifying those patients who failed to derive a long-term benefit for the procedure irrespective of changes in quality of life. This definition has its limitations, as due to patients’ inherent characteristics (age and functional limitations) some will prefer symptom improvement over longevity.
Extrapolating from this example, it has been previously shown that when compared with ST, TAVI decreases mortality and reduces hospital readmission.5 In our analysis, however, EOHM were associated with higher 6 month mortality and increased need for repeat hospitalization, and no improvement in 6-minute walk distance or NYHA functional class when compared to ST patients. Thus, better identification of patients who are at the highest risk of EOHM may aid in limiting procedures that have limited efficacy or may not affect survival.
This manuscript presents a secondary analysis of the data collected as part of the PARTNER randomized controlled trial and therefore should be considered hypothesis generating. The analyzed patient population was part of the initial United States TAVI experience, and the risk profile may not be representative of current high-risk profile patients being screened for TAVI. Futility was defined based on strict mortality cut-off criteria and did not take into account quality of life or patient/family preferences. The relatively small sample size may have introduced a type II error in analyzing the risk factors considered for defining futility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: clinicaltrials.gov #NCT00530894
References
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB. Transcatheter Aortic-Valve Replacement for Inoperable Severe Aortic Stenosis. N Engl J Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 3.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 4.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 5.Georgiadou P, Kontodima P, Sbarouni E, Karavolias GK, Smirli A, Xanthos T, Troupis T, Khouri M, Papadimitriou L, Voudris V. Long-term quality of life improvement after transcatheter aortic valve implantation. Am Heart J. 2011;162:232–237. doi: 10.1016/j.ahj.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 7.Thourani VH. Three-Year Outcomes after Transcatheter or Surgical Aortic Valve Replacement in High-Risk Patients with Severe Aortic Stenosis American College of Cardiology Annual Scientific Sessions. San Francisco, CA: 2013. [Google Scholar]
- 8.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. CMAJ. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H, Eggebrecht H, Rubino P, Colombo A, Lange R, Schwarz RR, Wendler O. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2011;124:425–433. doi: 10.1161/CIRCULATIONAHA.110.001545. [DOI] [PubMed] [Google Scholar]
- 11.Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–187. doi: 10.1016/j.jtcvs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Wendt D, Osswald B, Thielmann M, Kayser K, Tossios P, Massoudy P, Kamler M, Jakob H. The EuroSCORE - still helpful in patients undergoing isolated aortic valve replacement? Interact Cardiovasc Thorac Surg. 2010;10:239–244. doi: 10.1510/icvts.2009.218149. [DOI] [PubMed] [Google Scholar]
- 13.Parolari A, Pesce LL, Trezzi M, Cavallotti L, Kassem S, Loardi C, Pacini D, Tremoli E, Alamanni F. EuroSCORE performance in valve surgery: a meta-analysis. Ann Thorac Surg. 2010;89:787–793. 793, e781–782. doi: 10.1016/j.athoracsur.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Mack MJ. Risk scores for predicting outcomes in valvular heart disease: how useful? Curr Cardiol Rep. 2011;13:107–112. doi: 10.1007/s11886-010-0167-9. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell M, Teo K, Gao P, Anderson C, Sleight P, Dans A, Marzona I, Bosch J, Probstfield J, Yusuf S. Cognitive impairment and risk of cardiovascular events and mortality. Eur Heart J. 2012;33:1777–86. doi: 10.1093/eurheartj/ehs053. [DOI] [PubMed] [Google Scholar]
- 16.Webb JG, Altwegg L, Boone RH, Cheung A, Ye J, Lichtenstein S, Lee M, Masson JB, Thompson C, Moss R, Carere R, Munt B, Nietlispach F, Humphries K. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation. 2009;119:3009–3016. doi: 10.1161/CIRCULATIONAHA.108.837807. [DOI] [PubMed] [Google Scholar]
- 17.Friedman LS. The risk of surgery in patients with liver disease. Hepatology. 1999;29:1617–1623. doi: 10.1002/hep.510290639. [DOI] [PubMed] [Google Scholar]
- 18.Pilgrim T, Kalesan B, Wenaweser P, Huber C, Stortecky S, Buellesfeld L, Khattab AA, Eberle B, Gloekler S, Gsponer T, Meier B, Juni P, Carrel T, Windecker S. Predictors of clinical outcomes in patients with severe aortic stenosis undergoing TAVI: a multistate analysis. Circulation Cardiovasc interv. 2012;5:856–861. doi: 10.1161/CIRCINTERVENTIONS.112.974899. [DOI] [PubMed] [Google Scholar]
- 19.Ranucci M, Di Dedda U, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G. Impact of preoperative anemia on outcome in adult cardiac surgery: a propensity-matched analysis. Ann Thorac Surg. 2012;94:1134–1141. doi: 10.1016/j.athoracsur.2012.04.042. [DOI] [PubMed] [Google Scholar]