Abstract
Objectives
Congenital heart defects (CHDs) occur in nearly 1% of live births. We sought to assess factors associated with prenatal CHD diagnosis in the National Birth Defects Prevention Study (NBDPS).
Methods
We analyzed data from mothers with CHD-affected pregnancies from 1998–2005. Prenatal CHD diagnosis was defined as affirmative responses to questions about abnormal prenatal ultrasounds and/or fetal echocardiography obtained during a structured telephone interview.
Results
Fifteen percent (1,097/7,299) of women with CHD-affected pregnancies (excluding recognized syndromes and single-gene disorders) reported receiving a prenatal CHD diagnosis. Prenatal CHD diagnosis was positively associated with advanced maternal age, family history of CHD, type 1 or type 2 diabetes, twin or higher order gestation, CHD complexity and presence of extracardiac defects. Prenatal CHD diagnosis was inversely associated with maternal Hispanic race/ethnicity, prepregnancy overweight or obesity, and pre-existing hypertension. Prenatal CHD diagnosis varied by time to NBDPS interview and NBDPS study site.
Conclusions
Further work is warranted to identify reasons for the observed variability in maternal reports of prenatal CHD diagnosis and the extent to which differences in health literacy or health system factors such as access to specialized prenatal care and/or fetal echocardiography may account for such variability.
INTRODUCTION
Congenital heart defects (CHDs) occur in nearly 1% of live births1, 2 and are associated with substantial morbidity and mortality.3, 4 Prenatal diagnosis of heart defects can lead to changes in medical management that may improve clinical outcomes. For example, decisions to deliver at tertiary care centers with ready access to pediatric medical and surgical specialties are associated with decreased neonatal morbidity and mortality.5 Prenatal diagnosis can be particularly important in the case of critical CHDs (those that require surgery or catheterization within the first year of life) that may cause hypoxia and lead to severe organ damage or death in the absence of timely intervention.6-8 Although several risk factors for CHDs have been identified, such as family history, exposure to teratogenic medications, lack of prenatal vitamin and folic acid use, prepregnancy obesity, and pregestational diabetes, the causes of the majority of CHDs remain unexplained.9
In the United States, prenatal diagnosis rates for CHDs vary by type, and ranged from 6% during 1990-1994 to 36% during 2004-2005 and 39% during 1997-2007 in select populations.10-12 The American Institute of Ultrasound in Medicine (AUIM) recommends that pregnant women receive a second or third trimester ultrasound during which sonographers conduct basic cardiac examinations including a four-chamber view of the heart and, when technically feasible, views of the outflow tracts.13 Indications for fetal echocardiography include, but are not limited to: 1) an abnormal routine ultrasound; 2) a family history of CHD; 3) pregestational diabetes; or 4) a pregnancy conceived by in vitro fertilization.14 Reports on the variability of prenatal diagnosis rates for CHD in the U.S. and possible correlates of such variability are limited. The objectives of this study were to use data from mothers of CHD-affected pregnancies enrolled in the National Birth Defects Prevention Study (NBDPS) to estimate the proportion of mothers of infants with non-syndromic CHDs who report receiving a prenatal diagnosis and to investigate maternal and infant characteristics associated with maternal report of prenatal diagnosis.
METHODS
Study Population
The NBDPS is an on-going population-based case-control study of risk factors for selected major birth defects. The NBDPS enrolled pregnant women with dates of delivery on or after October 1, 1997 and we limited this analysis to infants with an estimated date of delivery (EDD) from January 1, 1998 to December 31, 2005. The 10 study sites include: the states of Arkansas (since 1998), Iowa, New Jersey (1998-2002 only), and Utah (since 2003) and select counties in California (Central Valley counties), Georgia (metropolitan Atlanta counties), Massachusetts (eastern counties, including the Boston metropolitan area), North Carolina (northern Piedmont region counties, since 2003), New York (Western New York, Lower Hudson Valley counties), and Texas (Lower Rio Grande Valley counties). The study methodology has been described previously.15 Briefly, NBDPS cases include live-born infants (all sites), stillbirths of ≥20 weeks gestation (all sites except NY before the year 2000 and NJ), and elective terminations ≥20 weeks gestation (all sites except NY before the year 2000, MA, and NJ). Controls are live-born infants without any major birth defects and are randomly selected from vital records or hospital discharge information from the same catchment areas from which cases were selected.
Clinical Review of CHD Cases
Medical records from all fetuses/infants with CHDs were reviewed by trained abstractors as part of the surveillance systems in each contributing study center. Fetuses/infants with documentation of major chromosomal abnormalities, single-gene disorders and birth defects with known etiology are excluded from the NBDPS which means the exclusion of those with an identified 22q11 deletion. Furthermore, for a CHD case to be included in the NBDPS, diagnosis of the CHD must be confirmed by echocardiography, cardiac catheterization, surgery, or autopsy.15 Cases with only a clinical diagnosis recorded in their medical record, such as diagnoses using only physical exam, chest radiography, or electrocardiogram, were not considered to have a definitive diagnosis and were excluded from NBDPS. Prenatally diagnosed and terminated pregnancies at any gestational age are included if there was a postmortem examination to confirm the defect or if the prenatal examination was done by a pediatric cardiologist or at a prenatal diagnosis center with expertise in pediatric cardiology.16 Medical records abstractions for all potential CHD cases were reviewed and their heart defects were categorized by a physician with specialized training in pediatric cardiology. The complexity of each case was determined; cases with either a single heart defect or well-defined constellation of heart defects such as hypoplastic left heart syndrome or tetralogy of Fallot were categorized as having a simple heart defect; cases with more than one distinct heart defect, such as aortic stenosis with coarctation of the aorta, heterotaxy, or single ventricle, were categorized as having an association or complex heart defect.16 Each case was also categorized according to whether extracardiac defects (defect(s) in an organ other than the heart) were present; cases with no extracardiac defects were categorized as isolated.
Reporting of Prenatal Diagnosis
The NBDPS does not specifically ascertain the date of initial diagnosis from medical records; rather, the study systematically ascertains the dates and results from echocardiography, catheterization, surgery or autopsy reports documentation required to confirm the CHD diagnosis for inclusion in the study. We therefore decided not to use these clinical data elements to estimate the frequency of prenatal CHD diagnosis, knowing that prenatal procedures were likely to be under-reported. Rather, we relied on maternal self-report during the standardized computer-assisted telephone interview (CATI) that was administered in English or Spanish by a trained interviewer six weeks to two years after the EDD. In addition to being asked a wide range of questions about lifestyle, medical, nutritional, and occupational exposures, mothers were also asked several questions about their prenatal care. In this analysis, we used two interview questions to define maternal report of a prenatal CHD diagnosis. Diagnoses based on abnormal ultrasounds were derived from the question “Did you have any ultrasounds which showed any abnormalities with the fetus, placenta or fluid?” The description of the abnormality(ies) found, and the date or timing during pregnancy (week, month or trimester) of abnormal ultrasounds were obtained. Prenatal diagnoses based on fetal echocardiography were derived from the question “Did you have any prenatal diagnostic tests such as fetal echocardiography or fetal dye studies?” The type of test performed, the description of the abnormality(ies) found, and the date or timing during pregnancy (week, month or trimester) of the test for each abnormal fetal echocardiography were recorded.
Unique verbatim descriptions of abnormal ultrasounds and fetal echocardiography from case and control women were categorized (3,271 unique descriptions). A study team member with expertise in pediatric cardiology reviewed all prenatal ultrasound and fetal echocardiography descriptions to determine whether the description represented a prenatal diagnosis of a CHD (“strongly indicative of a CHD”, “weakly indicative of a CHD” or “not indicative of a CHD”). Specific descriptions such as “tetralogy of Fallot” or “hypoplastic left heart syndrome” were categorized as “strongly indicative of a CHD” while more vague descriptions that may indicate the presence of a CHD such as “heart problem” were evaluated as “weakly indicative of a CHD”. A woman was categorized as reporting a prenatal CHD diagnosis if she reported having an ultrasound or fetal echocardiography that detected an abnormality assessed as “weakly” or “strongly” indicative of a CHD before the date of birth. Mothers who reported having either test but were missing data on the timing of the test during pregnancy were excluded. Mothers who reported having either test but reported dates after the baby's date of birth were categorized as not having a prenatal diagnosis.
Data Analysis
We excluded mothers of CHD-affected pregnancies who resided in New Jersey (for all years), and those who resided in Texas before June 1998 because during these time periods only a sample of infants from among all clinically-eligible fetuses/infants with CHDs were enrolled in the NBDPS. For all other time periods, there was no sampling of CHD types by study personnel and all interviewed mothers of CHD-affected pregnancies that met the NBDPS case definition were included in the NBDPS. Our final sample included mothers who reported receiving prenatal care and who could recall the timing of their abnormal ultrasound or fetal echocardiography.
We explored factors posited a priori to be associated with the report of a prenatal diagnosis, including the type of CHD, complexity of CHD, presence of extracardiac defects, year of estimated date of delivery, first degree family history of CHD, gestational age, plurality, maternal race/ethnicity, maternal age at delivery, maternal level of education, maternal prepregnancy body mass index (BMI), maternal pregestational diabetes, maternal diagnosis of hypertension, maternal fertility treatments, number of pregnancy losses before this pregnancy, pregnancy intention, trimester of first prenatal care visit, time from EDD to NBDPS interview, and NBDPS study site. We used the following categories for gestational age (very preterm [<32 completed weeks), preterm [32–36 completed weeks), or term [≥ 37 completed weeks)), plurality (singleton or multiple), maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or Other), maternal age at delivery (<30 years or >=30 years), maternal level of education (0-12 years or >12 years of education), prepregnancy BMI (underweight [<18.5 kg/m2), normal weight [18.5–<25.0 kg/m2), overweight [25.0–<30.0 kg/m2), or obese [≥30.0 kg/m2)), pregestational diabetes (none, type 1, or type 2), number of pregnancy losses before this pregnancy (none, one, two, or more than two losses), pregnancy intention (intended, mistimed/ambivalent, or unwanted17), and time to interview (6 weeks-6 months, 7-12 months, 13-18 months, and 19-24 months18). Data on CHD type, CHD complexity, presence of extracardiac defects, gestational age and study site came from previously abstracted medical records. All other variables included in the analyses were based on self-report during structured interviews.
We examined the frequency of maternal report of prenatal diagnosis according to the aforementioned factors. We also examined frequencies by the joint distribution of study site and CHD type. However, because of unstable estimates in smaller strata, we only present the frequencies for CHD types with ≥ 300 simple, isolated cases and >10% maternal report of prenatal diagnosis. We used bivariate and multivariable Poisson regression models with a “sandwich” or robust covariance matrix estimator to calculate crude and adjusted prevalence ratios (cPR, aPR) and corresponding 95% confidence intervals (95% CIs).19 This model was chosen because, unlike logistic regression models, the Poisson regression model with robust error estimator can provide an unbiased estimate of the prevalence ratio when the outcome under study (in this case, the frequency of maternal reported prenatal CHD diagnosis) is not “rare”.20 Although robust error estimators are typically used with clustered data, they are used in this context with uncorrelated data to account for the overestimation of the variance that can occur when modeling binary data with Poisson regression.20 All factors, with the exception of CHD type, were included in the multivariable model. Reference values for variables were determined a priori, with the exception of study site, for which the NBDPS site with the lowest frequency of maternal report of prenatal detection and sufficient sample size was selected as the referent. All significance tests used a two-sided alpha of 0.05 and all analyses were conducted using SAS v.9.2 (SAS Institute Inc., Cary, NC). This study was approved by the Centers for Disease Control and Prevention's and study centers’ Institutional Review Boards.
RESULTS
There were 7,971 mothers with non-syndromic CHD-affected pregnancies with EDDs from January 1998–December 2005. After excluding mothers who resided in New Jersey (n=548), those who resided in Texas and had EDDs before June, 1998 (n=10), those with unknown or no prenatal care (n=90), and those with missing dates of abnormal ultrasounds or fetal echocardiography (n=24), 7,299 mothers (91.6%) were eligible for further analysis. Almost all fetuses/infants (n=7,230; 99.1%) were live-born, 74.4% (n=5,404) had simple heart defects, and 83.6% (n=6,104) had no extracardiac defects.
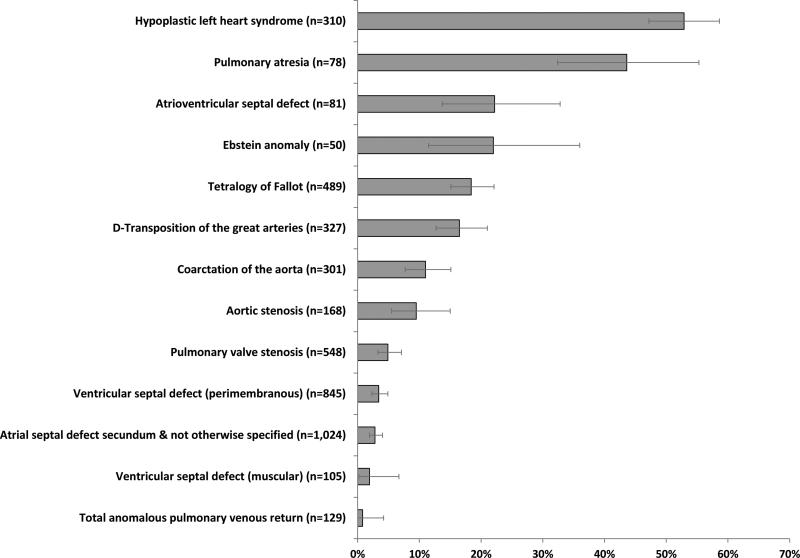
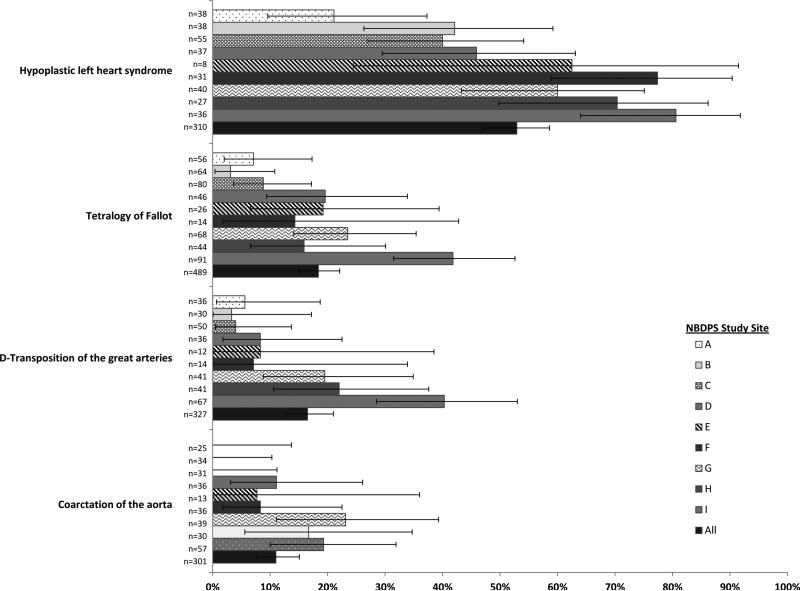
Of the 7,299 mothers with CHD-affected pregnancies, 1,097 (15.0%) reported having an abnormal ultrasound or fetal echocardiography that suggested a CHD, the majority of which (n=1,011; 92.2%) were assessed as being “strongly” indicative of a CHD. Among those with simple, isolated defects, maternal report of prenatal diagnosis varied by CHD type, from 0.8% for total anomalous pulmonary venous return to 52.9% for hypoplastic left heart syndrome (HLHS) (Figure 1). There was also substantial variability by NBDPS study site; maternal report of prenatal CHD diagnosis ranged from 7.1% to 25.6% by site (Table). In addition, there was substantial variability by site within CHD types. Among isolated cases of HLHS, maternal report ranged from 21.1–80.6% and other CHD types exhibited similar variation as well (Figure 2). Maternal report of prenatal CHD diagnosis ranged from 12.7% in 1998 to 17.1% in 2002 though there was no evidence of a temporal trend (Table), even after accounting for changes in study site catchment area over time (data not shown).
Figure 1.
Frequency of maternal report of prenatal congenital heart defect diagnosis by defect type, for simple, isolated defectsa, National Birth Defects Prevention Study, 1998–2005 (n=4,455)
Note: Error bars represent exact 95% confidence intervals
a A case was categorized as having a “simple” heart defect if s/he had one heart defect or a well-defined constellation of defects such as hypoplastic left heart syndrome or tetralogy of Fallot and “isolated” if no extracardiac defects were present. For presentation purposes, we only present the frequencies for CHD types with ≥ 50 simple, isolated cases across all study sites.
Table.
Maternal and infant characteristics associated with maternal report of prenatal CHD diagnosis, National Birth Defects Prevention Study, 1998-2005 (n=7,299)
| Report of prenatal diagnosis | ||||
|---|---|---|---|---|
| Characteristic | N | n(%) | cPR (95% CI) | aPR (95% CI) |
| CHD Complexity | ||||
| Simple (one heart defect) | 5404 | 685 (12.7%) | Reference | Reference |
| Two or more associated or complex heart defects | 1895 | 412 (21.7%) | 1.72 (1.54,1.92) | 1.61 (1.44,1.81) |
| Presence of Extracardiac Defects | ||||
| No (isolated; no extracardiac defects) | 6104 | 812 (13.3%) | Reference | Reference |
| Yes (extracardiac defects present) | 1195 | 285 (23.8%) | 1.79 (1.59,2.02) | 1.65 (1.46,1.87) |
| Year of Due Date | ||||
| 1998 | 659 | 84 (12.7%) | 0.78 (0.61,0.99) | 0.79 (0.62,1.01) |
| 1999 | 782 | 114 (14.6%) | 0.89 (0.72,1.11) | 0.88 (0.71,1.10) |
| 2000 | 851 | 126 (14.8%) | 0.91 (0.74,1.12) | 0.90 (0.73,1.12) |
| 2001 | 880 | 126 (14.3%) | 0.88 (0.71,1.08) | 0.86 (0.70,1.07) |
| 2002 | 813 | 139 (17.1%) | 1.05 (0.86,1.28) | 0.95 (0.77,1.16) |
| 2003 | 959 | 146 (15.2%) | 0.93 (0.76,1.14) | 0.91 (0.74,1.11) |
| 2004 | 1246 | 181 (14.5%) | 0.89 (0.74,1.08) | 0.90 (0.74,1.09) |
| 2005 | 1109 | 181 (16.3%) | Reference | Reference |
| Family History of CHD (first degree relative) | ||||
| No | 7047 | 1038 (14.7%) | Reference | Reference |
| Yes | 252 | 59 (23.4%) | 1.59 (1.26,2.00) | 1.47 (1.16,1.86) |
| Gestational Age | ||||
| Very preterm (<32 completed weeks) | 613 | 84 (13.7%) | 0.93 (0.76,1.15) | 0.97 (0.78,1.20) |
| Preterm (32-36 completed weeks) | 1309 | 224 (17.1%) | 1.17 (1.02,1.34) | 1.09 (0.94,1.25) |
| Term (≥ 37 completed weeks) | 5376 | 789 (14.7%) | Reference | Reference |
| Unknown/missing | 1 | 0 (0%) | NC | NC |
| Plurality | ||||
| Singleton | 6765 | 989 (14.6%) | Reference | Reference |
| Twins or higher order gestation | 525 | 106 (20.2%) | 1.38 (1.15,1.65) | 1.26 (1.03,1.54) |
| Unknown/missing | 9 | 2 (22.2%) | NC | NC |
| Maternal Race/Ethnicity | ||||
| Non-Hispanic white | 4300 | 745 (17.3%) | Reference | Reference |
| Non-Hispanic black | 823 | 109 (13.2%) | 0.76 (0.63,0.92) | 0.83 (0.68,1.02) |
| Hispanic | 1741 | 166 (9.5%) | 0.55 (0.47,0.65) | 0.72 (0.59,0.89) |
| Other | 434 | 76 (17.5%) | 1.01 (0.82,1.25) | 0.97 (0.78,1.20) |
| Unknown/missing | 1 | 1 (100.0%) | NC | NC |
| Maternal Age (at delivery) | ||||
| <30 years | 4336 | 542 (12.5%) | Reference | Reference |
| >=30 years | 2963 | 555 (18.7%) | 1.50 (1.34,1.67) | 1.13 (1.00,1.28) |
| Maternal Level of Education | ||||
| 0-12 years | 3169 | 376 (11.9%) | Reference | Reference |
| >12 years | 4040 | 709 (17.5%) | 1.48 (1.32,1.66) | 1.14 (1.00,1.30) |
| Unknown/missing | 90 | 12 (13.3%) | NC | NC |
| Prepregnancy Maternal Body Mass Index (BMI) | ||||
| Underweight (<18.5 kg/m2) | 384 | 60 (15.6%) | 0.92 (0.72,1.17) | 1.00 (0.79,1.28) |
| Normal weight (18.5-24 kg/m2) | 3492 | 596 (17.1%) | Reference | Reference |
| Overweight (25-29 kg/m2) | 1643 | 231 (14.1%) | 0.82 (0.72,0.95) | 0.87 (0.76,1.00) |
| Obese (>=30 kg/m2) | 1478 | 183 (12.4%) | 0.73 (0.62,0.85) | 0.78 (0.66,0.92) |
| Unknown/missing | 302 | 27 (8.9%) | NC | NC |
| Pregestational Diabetes | ||||
| None | 6994 | 1016 (14.5%) | Reference | Reference |
| Type 1 | 108 | 26 (24.1%) | 1.66 (1.18,2.33) | 1.57 (1.10,2.24) |
| Type 2 | 138 | 41 (29.7%) | 2.05 (1.57,2.66) | 2.46 (1.86,3.24) |
| Unknown/missing | 59 | 14 (23.7%) | NC | NC |
| Hypertension (before or during index pregnancy) | ||||
| No | 6100 | 951 (15.6%) | Reference | Reference |
| Yes | 1193 | 145 (12.2%) | 0.78 (0.66,0.92) | 0.81 (0.68,0.97) |
| Unknown/missing | 6 | 1 (16.7%) | NC | NC |
| Maternal Fertility Treatments | ||||
| No | 6801 | 1002 (14.7%) | Reference | Reference |
| Yes | 426 | 84 (19.7%) | 1.34 (1.10,1.63) | 0.86 (0.68,1.08) |
| Unknown/missing | 72 | 11 (15.3%) | NC | NC |
| Number of Pregnancy Losses before this Pregnancy | ||||
| None | 4681 | 679 (14.5%) | Reference | Reference |
| One | 1670 | 269 (16.1%) | 1.11 (0.98,1.26) | 1.05 (0.92,1.20) |
| Two | 606 | 84 (13.9%) | 0.96 (0.77,1.18) | 0.87 (0.70,1.07) |
| More than two | 338 | 65 (19.2%) | 1.33 (1.05,1.67) | 1.15 (0.90,1.45) |
| Unknown/missing | 4 | 0 (0.0%) | NC | NC |
| Pregnancy Intention | ||||
| Intended | 4191 | 668 (15.9%) | Reference | Reference |
| Mistimed/ambivalent | 1700 | 247 (14.5%) | 0.91 (0.80,1.04) | 1.10 (0.95,1.27) |
| Unwanted | 1379 | 178 (12.9%) | 0.81 (0.69,0.94) | 0.96 (0.82,1.13) |
| Unknown/missing | 29 | 4 (13.8%) | NC | NC |
| Trimester of First Prenatal Care Visit | ||||
| 1st | 6121 | 947 (15.5%) | Reference | Reference |
| 2nd | 895 | 117 (13.1%) | 0.84 (0.71,1.01) | 0.98 (0.82,1.18) |
| 3rd | 66 | 7 (10.6%) | 0.69 (0.34,1.38) | 0.78 (0.41,1.47) |
| Unknown/missing | 217 | 26 (12.0%) | NC | NC |
| Time to Interview | ||||
| 6 weeks-6 months | 1652 | 341 (20.6%) | Reference | Reference |
| 7-12 months | 3132 | 477 (15.2%) | 0.74 (0.65,0.84) | 0.77 (0.67,0.88) |
| 13-18 months | 1663 | 209 (12.6%) | 0.61 (0.52,0.71) | 0.71 (0.60,0.84) |
| 19-24 months | 766 | 62 (8.1%) | 0.39 (0.30,.51) | 0.51 (0.39,0.68) |
| Unknown/missing | 86 | 8 (9.3%) | NC | NC |
| Study Site | ||||
| A | 1188 | 84 (7.1%) | Reference | Reference |
| B | 1199 | 86 (7.2%) | 1.01 (0.76,1.36) | 1.19 (0.86,1.64) |
| C | 855 | 112 (13.1%) | 1.85 (1.42,2.42) | 1.96 (1.46,2.64) |
| D | 741 | 113 (15.2%) | 2.16 (1.65,2.82) | 2.18 (1.65,2.87) |
| E | 285 | 45 (15.8%) | 2.23 (1.59,3.13) | 2.16 (1.51,3.11) |
| F | 483 | 77 (15.9%) | 2.25 (1.69,3.01) | 2.13 (1.56,2.90) |
| G | 944 | 188 (19.9%) | 2.82 (2.21,3.59) | 2.64 (2.05,3.41) |
| H | 548 | 122 (22.3%) | 3.15 (2.43,4.08) | 2.71 (2.07,3.55) |
| I | 1056 | 270 (25.6%) | 3.62 (2.87,4.55) | 3.09 (2.42,3.94) |
Bold indicates significance at < 0.05 level
CHD=congenital heart defect
NBDPS=National Birth Defects Prevention Study
cPR=crude prevalence ratio estimated from a Poisson regression model with robust error variance
aPR=adjusted prevalence ratio estimated from a Poisson regression model with robust error variance
CI=confidence interval
NC=not calculated
a Adjusted for all variables listed; excludes cases with at least one missing response
b A case was categorized as having a “simple” heart defect if s/he had one heart defect or a well-defined constellation of defects such as hypoplastic left heart syndrome or tetralogy of Fallot
c The study catchment area changed over time
Figure 2.
Frequency of maternal report of prenatal congenital heart defect diagnosis by defect type and study site, for select simple, isolated defects,a National Birth Defects Prevention Study, 1998–2005
Note: Error bars represent exact 95% confidence intervals
a A case was categorized as having a “simple” heart defect if s/he had one heart defect or a well-defined constellation of defects such as hypoplastic left heart syndrome or tetralogy of Fallot and “isolated” if no extracardiac defects were present. For presentation purposes, we only present the frequencies for CHD types with > 300 simple, isolated cases and >10% maternal report of prenatal diagnosis across all study sites.
Factors significantly associated with maternal reports of a prenatal CHD diagnosis in multivariable analyses included CHD complexity, presence of extracardiac defects, maternal factors such as advanced age (≥ 30 years), family history of CHD, type 1 diabetes, type 2 diabetes, and twins or higher order gestation, as well as NBDPS study site. Factors such as maternal Hispanic race/ethnicity (compared to non-Hispanic white race/ethnicity), prepregnancy overweight (25.0–<30.0 kg/m2) or obese (≥ 30 kg/m2) BMI, pre-existing hypertension and NBDPS interview at 7-12 months, 13-18 months, and 19-24 months post-EDD (compared to those interviewed at 6 weeks-6 months after their EDD) were each inversely associated with maternal report of prenatal diagnosis (Table).
DISCUSSION
In our study, 15% of mothers with non-syndromic CHD-affected pregnancies reported receiving a prenatal diagnosis. Maternal report of prenatal CHD diagnosis varied by NBDPS study site as well as by maternal characteristics such as plurality, race/ethnicity, age, presence of comorbid conditions (i.e., diabetes and obesity), and family history of CHD, and by the complexity of the CHD, presence of extracardiac defects, and CHD type.
Many of the characteristics associated with maternal report of prenatal CHD diagnosis are plausible given their likelihood to cause a woman's pregnancy to be closely monitored, such as family history of CHD, twin or a higher order gestation, presence of type 1 or type 2 diabetes, and older maternal age.14, 21 In our study, overweight and obese mothers were each significantly less likely to have a prenatal CHD diagnosis than those in the normal prepregnancy weight BMI category. Maternal BMI has been found to be associated with lower prenatal diagnosis rates22, 23 and higher false positive rates23 for CHDs in some studies, but not others.12, 24 Detection might be more difficult in women with higher BMIs because of the increased layer of fat that limits the ability of ultrasound or echocardiography to visualize fetal structures, as has been found with prenatal detection of orofacial clefts.25
The prevalence of maternally-reported prenatal CHD diagnosis in our study varied significantly by CHD type, which is consistent with previous studies.2, 5, 12, 24, 26 Pinto et al.'s (2012) study in Utah from 1997-2007 found the prevalence of prenatal CHD diagnosis ranged from 0% for interrupted aortic arch type B to 100% for single ventricle, not otherwise specified, and three of the four defects most likely to be detected prenatally were considered to exhibit an abnormal four-chamber view (e.g., HLHS and atrioventricular septal defects). However, defects categorized as exhibiting abnormal outflow tracts or other abnormal views (e.g., coarctation of the aorta) tended to have lower rates of prenatal diagnosis,12 similar to what was seen in our study. These findings are not unexpected given the difficulty of detecting outflow tract obstruction defects via ultrasound. We also found increasing maternally-reported prenatal CHD diagnosis with increasing defect complexity (more than one heart defect), and the presence of extracardiac defects; studies of prenatal diagnosis of CHDs12 and other types of birth defects25 have similarly found increased prenatal diagnosis when multiple birth defects were present.
Unlike some previous U.S. studies, we were able to assess geographic differences in maternal report of prenatal CHD diagnosis and found variation by NBDPS study site (and even greater variation within specific CHD types). In multivariable analysis, study site remained one of the strongest predictors of maternal report of prenatal CHD diagnosis and there remained a 3-fold difference between the lowest prenatal diagnosis study site and the highest. A study of the 15 referral centers in the U.S. Pediatric Heart Network from 2005-2009 found that prenatal diagnosis rates for CHDs, even among these specialized centers, ranged from 59% to 82%.27 Referral centers that drew from larger population areas had significantly higher prenatal diagnosis rates. Prenatal diagnosis rates in the United Kingdom also varied significantly across postal codes and areas with higher prenatal diagnosis rates were more likely to detect defect types that exhibited abnormal outflow tracts, as well as abnormal four-chamber views.26 As controls enrolled in the NBDPS have been found to be representative of their respective source populations,28 it is unlikely that the geographic differences observed in our study are attributable to selection bias. Rather, geographic differences in prenatal diagnosis rates might be explained by differences in timely access to prenatal care, access to high-quality imaging technology or variation in proficiency of persons interpreting the ultrasounds and echocardiography. In Utah, the likelihood of prenatal CHD diagnosis was 1.6 times higher when the ultrasound was performed at a hospital and 10-times more likely when it was performed at a high-risk clinic.12 Increasing skill of the ultrasound reader and increasing number of ultrasounds also improved the likelihood of diagnosing a CHD prenatally.
Our prenatal diagnosis rate may be lower than previous studies for a number of reasons, some of which are limitations to NBDPS data in the context of this research question. First, the NBDPS inclusion criteria limit CHD cases to those without a recognized single-gene disorder or chromosomal abnormality, which might be less easily prenatally diagnosed. Previous studies reporting higher prenatal diagnosis rates did not note a similar restriction in the study population.10, 12, 27 Atrioventricular septal defects (AVSD) represent the extreme of the impact of this exclusion criterion. A recent comparison of AVSDs included in the NBDPS to those reported to their respective birth defects surveillance systems in Georgia, Iowa and Massachusetts found that 61% of all AVSD cases included in the surveillance system were excluded from the NBDPS because of chromosomal abnormalities and 1% were excluded because of single-gene disorders.29 However, many of the other CHD types under consideration in this study are not as frequently associated with genetic syndromes,30 so the frequency of maternal report of prenatal detection may be more likely to reflect clinical practice. Second, we had a high proportion (99%) of live-born infants in our study sample, which is likely due to the stringent NBDPS criteria for confirmation of CHDs, as discussed previously. Other studies, both in Europe and in the United States, which have reported higher prenatal CHD diagnosis rates, including those approaching 50% in Paris from 1995-2000, have included more prenatally-diagnosed and terminated cases.2, 10, 26, 31
One might argue that the use of maternal self-report to define prenatal diagnosis could lead to an underestimate because mothers might not recall receiving a prenatal diagnosis. However, the receipt of a CHD diagnosis is a stressful event in a pregnancy.32 A prenatal, as opposed to a postnatal diagnosis, carries with it the weight of having to make decisions about whether to continue the pregnancy and thus the recall of the event is likely to be accurate. Even among women with pregnancies unaffected by birth defects, Githens et al.'s small study found that 89% of 85 mothers accurately recalled receiving a prenatal ultrasound and 97% of 19 mothers accurately recalled receiving amniocentesis.33 Although time to interview was a significant predictor of prenatal diagnosis in our analysis, and we observed a decreasing frequency of maternal report of prenatal CHD diagnosis with increasing time to interview, this does not necessarily mean that mothers interviewed later were poorly recalling a prenatal diagnosis. Rather, it may be that those infants/fetuses that were diagnosed prenatally are captured earlier by their respective birth defects surveillance systems, and these mothers are contacted earlier by the NBDPS than mothers of infants/fetuses diagnosed postnatally.
Still, the strengths of this analysis include the use of a large, multi-center case-control study, as opposed to studies restricted to single states or metropolitan areas; the blinded review of the maternal reports of the abnormalities identified on prenatal ultrasounds and fetal echocardiography; and the clinician verification of CHD diagnosis, as opposed to reliance solely on administrative coding to identified CHD cases.
Overall, we found that a low proportion (15%) of women with pregnancies affected by a non-syndromic CHD reported receiving a prenatal CHD diagnosis but the prevalence of prenatal diagnosis varied by both maternal and infant characteristics. Some of this variability may represent unmeasured differences in the quality of prenatal care received, differences in health literacy, or health system factors such as access to specialized prenatal care and/or fetal echocardiography. Countries which have observed marked increases in prenatal CHD detection rates have attributed such changes to improvements in sonographer training and availability of technology. For instance, in the Czech Republic, prenatal detection rates of a dozen types of CHD increased from 1986-1999 to 2000-2006, including from 31% to 96% for HLHS and 6% to 26% for transposition of the great arteries.34 Ensuring access to high-quality prenatal care and adequate training of providers about prenatal diagnosis of CHDs has been shown to increase CHD prenatal diagnosis rates12,35 and including an assessment of outflow tracts in addition to a basic four chamber cardiac view can improve prenatal detection of CHD.36 However, it is unclear what proportion of obstetric practices are following guidelines put forth by the AUIM.37 Further work is warranted not only to understand the reasons for the observed variability in maternal reports of prenatal diagnosis but also to improve overall rates of prenatal diagnosis for CHD, since early diagnosis might improve survival and clinical outcomes.
What's already known about this topic:
Prenatal diagnosis of congenital heart defects (CHD) can lead to more timely interventions which may improve outcomes
What does this study add:
15% of mothers with a CHD-affected pregnancy reported receiving a prenatal diagnosis
Prenatal diagnosis varied by infant/fetal characteristics such as CHD type, CHD complexity and presence of extracardiac defects; maternal factors including age, race/ethnicity, family history of CHD, plurality, prepregnancy diabetes, hypertension, and body mass index; and NBDPS study site and time to interview
ACKNOWLEDGEMENTS
This work was supported through cooperative agreements under PA 96043, PA 02081 and FOA DD09-001 from the Centers for Disease Control and Prevention to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study.
Footnotes
Conflicts of interest: None declared.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Reller M, Strickland M, Riehle-Colarusso T, et al. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. The Journal of Pediatrics. 2008;153(6):807–13. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoshnood B, Lelong N, Houyel L, et al. Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects: a population-based study. Heart. 2012;98(22):1667–73. doi: 10.1136/heartjnl-2012-302543. [DOI] [PubMed] [Google Scholar]
- 3.Meberg A, Lindberg H, Thaulow E. Congenital heart defects: the patients who die. Acta Pediatrica. 2007;94(8):1060–5. doi: 10.1111/j.1651-2227.2005.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilboa SM, Salemi JL, Nembhard WN, et al. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122(22):2254–63. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelle M, Raio L, Pavlovic M, et al. Prenatal diagnosis and treatment planning of congenital heart defects-possibilities and limits. World J Pediatr. 2009;5(1):18–22. doi: 10.1007/s12519-009-0003-8. [DOI] [PubMed] [Google Scholar]
- 6.Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Archives of disease in childhood Fetal and neonatal edition. 2008;93(1):F33–5. doi: 10.1136/adc.2007.119032. [DOI] [PubMed] [Google Scholar]
- 7.Brown KL, Ridout DA, Hoskote A, et al. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart. 2006;92(9):1298–302. doi: 10.1136/hrt.2005.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahle WT, Newburger JW, Matherne GP, et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the AHA and AAP. Pediatrics. 2009;124(2):823–36. doi: 10.1542/peds.2009-1397. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins KJ, Correa A, Feinstein JA, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal Detection of Congenital Heart Disease. The Journal of Pediatrics. 2009;155(1):26–31. doi: 10.1016/j.jpeds.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 11.Montana E, Khoury M, Cragan J, et al. Trends and outcomes after prenatal diagnosis of congenital cardiac malformations by fetal echocardiography in a well defined birth population, Atlanta, Georgia, 1990-1994. Journal of the American College of Cardiology. 1996;28(7):1805–9. doi: 10.1016/S0735-1097(96)00381-6. [DOI] [PubMed] [Google Scholar]
- 12.Pinto NM, Keenan HT, Minich LL, et al. Barriers to Prenatal Detection of Congenital Heart Disease: A Population Based Study. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2012;40(4):418–25. doi: 10.1002/uog.10116. [DOI] [PubMed] [Google Scholar]
- 13.American Institute of Ultrasound in Medicine AIUM practice guideline for the performance of obstetric ultrasound examinations. Journal of ultrasound in medicine. 2010;29(1):157–66. doi: 10.7863/jum.2010.29.1.157. [DOI] [PubMed] [Google Scholar]
- 14.Fetal Echocardiography Task Force, American Institute of Ultrasound in Medicine Clinical Standards Committee of the American College of Obstetricians Gynecologists Society for Maternal-Fetal Medicine AIUM practice guideline for the performance of fetal echocardiography. Journal of ultrasound in medicine. 2011;30(1):127–36. doi: 10.7863/jum.2011.30.1.127. [DOI] [PubMed] [Google Scholar]
- 15.Yoon PW, Rasmussen SA, Lynberg MC, et al. The National Birth Defects Prevention Study. Public health reports. 2001;116(Suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botto LD, Lin AE, Riehle-Colarusso T, et al. Seeking causes: Classifying and evaluating congenital heart defects in etiologic studies. Birth defects research Part A, Clinical and molecular teratology. 2007;79(10):714–27. doi: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- 17.Dott M, Rasmussen SA, Hogue CJ, et al. Association between pregnancy intention and reproductive-health related behaviors before and after pregnancy recognition, National Birth Defects Prevention Study, 1997-2002. Maternal and child health journal. 2010;14(3):373–81. doi: 10.1007/s10995-009-0458-1. [DOI] [PubMed] [Google Scholar]
- 18.Tinker SC, Gibbs C, Strickland MJ, et al. Impact of time to maternal interview on interview responses in the national birth defects prevention study. American journal of epidemiology. 2013;177(11):1225–35. doi: 10.1093/aje/kws352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Tan CS, Chia KS. A practical guide for multivariate analysis of dichotomous outcomes. Annals of the Academy of Medicine, Singapore. 2009;38(8):714–9. [PubMed] [Google Scholar]
- 21.Johnson JA, Tough S, Society of Obstetricians and Gynaecologists of Canada Delayed child-bearing. Journal of obstetrics and gynaecology Canada. 2012;34(1):80–93. doi: 10.1016/S1701-2163(16)35138-6. [DOI] [PubMed] [Google Scholar]
- 22.Dashe JS, McIntire DD, Twickler DM. Effect of maternal obesity on the ultrasound detection of anomalous fetuses. Obstetrics and gynecology. 2009;113(5):1001–7. doi: 10.1097/AOG.0b013e3181a1d2f5. [DOI] [PubMed] [Google Scholar]
- 23.Aagaard-Tillery KM, Flint Porter T, Malone FD, et al. Influence of maternal BMI on genetic sonography in the FaSTER trial. Prenatal diagnosis. 2010;30(1):14–22. doi: 10.1002/pd.2399. [DOI] [PubMed] [Google Scholar]
- 24.Wong SF, Chan FY, Cincotta RB, et al. Factors influencing the prenatal detection of structural congenital heart diseases. Ultrasound in obstetrics & gynecology. 2003;21(1):19–25. doi: 10.1002/uog.7. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CY, Honein MA, Hobbs CA, et al. Prenatal diagnosis of orofacial clefts, National Birth Defects Prevention Study, 1998-2004. Prenatal diagnosis. 2009;29(9):833–9. doi: 10.1002/pd.2293. [DOI] [PubMed] [Google Scholar]
- 26.Bull C. Current and potential impact of fetal diagnosis on prevalence and spectrum of serious congenital heart disease at term in the UK. British Paediatric Cardiac Association. Lancet. 1999;354(9186):1242–7. doi: 10.1016/s0140-6736(99)01167-8. [DOI] [PubMed] [Google Scholar]
- 27.Atz AM, Travison TG, Williams IA, et al. Prenatal diagnosis and risk factors for preoperative death in neonates with single right ventricle and systemic outflow obstruction: screening data from the Pediatric Heart Network Single Ventricle Reconstruction Trial. The Journal of thoracic and cardiovascular surgery. 2010;140(6):1245–50. doi: 10.1016/j.jtcvs.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cogswell ME, Bitsko RH, Anderka M, et al. Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. American journal of epidemiology. 2009;170(8):975–85. doi: 10.1093/aje/kwp226. [DOI] [PubMed] [Google Scholar]
- 29.Hartman RJ, Riehle-Colarusso T, Lin A, et al. Descriptive study of nonsyndromic atrioventricular septal defects in the National Birth Defects Prevention Study, 1997-2005. American journal of medical genetics Part A. 2011;155A(3):555–64. doi: 10.1002/ajmg.a.33874. [DOI] [PubMed] [Google Scholar]
- 30.Wimalasundera RC, Gardiner HM. Congenital heart disease and aneuploidy. Prenatal diagnosis. 2004;24(13):1116–22. doi: 10.1002/pd.1068. [DOI] [PubMed] [Google Scholar]
- 31.Khoshnood B, De Vigan C, Vodovar V, et al. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983-2000: a population-based evaluation. Pediatrics. 2005;115(1):95. doi: 10.1542/peds.2004-0516. [DOI] [PubMed] [Google Scholar]
- 32.Rychik J, Donaghue DD, Levy S, et al. Maternal Psychological Stress after Prenatal Diagnosis of Congenital Heart Disease. J Pediatr. 2012;162(2):302–7. doi: 10.1016/j.jpeds.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Githens PB, Glass CA, Sloan FA, et al. Maternal recall and medical records: an examination of events during pregnancy, childbirth, and early infancy. Birth. 1993;20(3):136–41. doi: 10.1111/j.1523-536x.1993.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 34.Marek J, Tomek V, Skovranek J, et al. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart. 2011;97(2):124–30. doi: 10.1136/hrt.2010.206623. [DOI] [PubMed] [Google Scholar]
- 35.McBrien A, Sands A, Craig B, et al. Impact of a regional training program in fetal echocardiography for sonographers on the antenatal detection of major congenital heart disease. Ultrasound in obstetrics & gynecology. 2010;36(3):279–84. doi: 10.1002/uog.7616. [DOI] [PubMed] [Google Scholar]
- 36.Sklansky MS, Berman DP, Pruetz JD, et al. Prenatal screening for major congenital heart disease: superiority of outflow tracts over the 4-chamber view. Journal of ultrasound in medicine. 2009;28(7):889–99. doi: 10.7863/jum.2009.28.7.889. [DOI] [PubMed] [Google Scholar]
- 37.Smulian JC, Vintzileos AM, Rodis JF, et al. Community-based obstetrical ultrasound reports: documentation of compliance with suggested minimum standards. Journal of clinical ultrasound. 1996;24(3):123–7. doi: 10.1002/(SICI)1097-0096(199603)24:3<123::AID-JCU3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]