Abstract
Introduction
Visible para-aortic lymph nodes of ≥2 mm in size are common metastatic patterns of colorectal cancer (CRC) seen on imaging. Their prognostic value, however, remains inconclusive. We aimed to assess the prognostic role of visible para-aortic lymph nodes (PALNs).
Methods
Patients with confirmed pathologic diagnosis of CRC were enrolled. Correlations among clinicopathologic variables were analyzed using the χ2 test. The Cox proportional hazards model was applied for univariate and multivariate analyses. Survival was estimated using the Kaplan-Meier method and log-rank test. A prognostic model for visible PALNs in CRC patients was established.
Results
In total, 4527 newly diagnosed CRC patients were enrolled. Patients with visible PALNs had inferior overall survival compared to those without visible PALNs (5-year overall survival, 67% vs. 76%, P = 0.015). Lymphovascular invasion (LVI) (hazard ratio = 1.865, P = 0.015); nodal disease (pN+) status (hazard ratio = 2.099, P = 0.006); elevated preoperative serum carcinoembryonic antigen (CEA) levels (hazard ratio = 2.263, P < 0.001); and visible PALNs ≥10 mm (hazard ratio = 1.638, P = 0.031) were independent prognostic factors for patients with visible PALNs. If each prognostic factor scored one point, 5-year overall survival of lower- (prognostic score 0–1), intermediate- (prognostic score 2), and high- (prognostic score 3–4) risk groups were, 78%. 54%, and 25% respectively (P < 0.001).
Conclusions
The prognostic model, which included LVI, pN+ status, preoperative serum CEA level, and the size of visible PALNs, could effectively distinguish the outcome of patients with visible PALNs.
Introduction
Globally, colorectal cancer (CRC) is the fourth and third most common cancer in men and women respectively [1]. The survival and treatment strategies for patients with CRC correlate with disease-stage status. Adequate treatments would lead to long-term survival, even for advanced-stage patients [2, 3].
With the improvement of imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI), enlarged para-aortic lymph nodes (PALNs) (so-called visible PALNs) are a more commonly observed metastatic pattern in CRC [4]. However, according to the American Joint Committee on Cancer (AJCC) staging system, visible PALN metastases are categorized as clinical stage M1 because they are considered to be non-regional lymph nodes [5]. There have been no original reports addressing the impact of visible PALNs on the clinical behavior of CRC and the survival of patients. Moreover, although some articles have mentioned the poor prognostic value of visible PALNs in recurrence [6], the prognostic role of visible PALNs at initial diagnosis by modern imaging studies remains unclear.
In contrast, extensive surgical dissection and radiation therapy reportedly increases the survival of selected patients with visible PALN metastases. These patients were treated following curative resection or loco-regional recurrence [7–9]. However, dissection of PALNs is difficult, and the incidence of postoperative complications after extensive lymph node dissection is relatively high [4, 10, 11]. Therefore, it remains unclear to clinicians whether visible PALNs in CRC patients represent regional or distant disease; and consequently, whether aggressive treatments such as surgical LN dissection or chemoradiotherapy should be arranged for patients with visible PALN enlargement on initial imaging diagnosis. Thus, the association between visible PALNs and clinic-pathological parameters requires clarification. There is currently, not enough data to stratify patients for aggressive treatment.
In this study, we aimed to assess the prognostic role of visible PALNs in patients with CRC, and attempted to establish a prognostic model for visible PALNs. Although this model could not predict pathologic metastasis of PALNs, it could help us to predict outcomes for CRC patients in combination with the observation of visible PALNs on imaging studies. With this model, we can modify our clinical practice and select some patients with visible PALNs for management.
Materials and Methods
Study design, setting, and patient selection
The study was a single institute, retrospective, cohort study. In this study, all data were collected according to routine clinical care in our hospital. There was no direct contact with patients for any data collection and analysis; as such, the need for written consent from study subjects was waived by the institutional review board. The study was reviewed and approved by the Institutional Review Board of Taipei Veterans General Hospital (No. 2012-11-004BC).
Between January 2001 and December 2011, patients with clinically suspected CRC were selected for advanced survey. Patients with pathologically confirmed CRC at Taipei Veterans General Hospital were enrolled in our database. Patients with no pathologically proven CRC, carcinoma in situ, malignancies other than adenocarcinoma, and secondary primary malignancies were excluded from our database. In order to describe the prognostic role of visible PALNs in CRC, we selected patients without distal metastases and divided them into patient groups with, or without, visible PALNs. Patients with visible PALNs, but no distal metastases (such as lung and liver metastases), were categorized in the visible PALNs group. The remaining patients were categorized as without visible PALNs, and were staged according to the AJCC staging system, 6th edition, and National Comprehensive Cancer Network guidelines [5].
Definition of visible para-aortic lymph nodes
All patients received radiologic (such as CT and MRI) examinations at initial diagnosis. The identification of visible PALNs was retrospectively reviewed from imaging records. All images were read independently by two experienced abdominal radiologists, to determine the short axis diameter of lymph nodes and reach a consensus via discussion. A third radiologist evaluated the lesions if there were inconsistencies in visible PALN identification. Visible PALNs were defined as lymph nodes surrounding the abdominal aorta and inferior vena cava, which were located in the area from the uppermost part of the origin of the celiac trunk to the lower margin of the aortic bifurcation [12]. Visible PALNs measured more than 2 mm in the short axis and were detectable on radiologic examinations.
Imaging Technique
CT images were obtained using a standardized acquisition protocol covering the abdomen and pelvis on multiple row-detector CT systems in all selected cases. Reconstructions were performed in 5.0 mm slice thickness. For lymph nodes measuring less than 5.0mm on reconstructed CT images, additional thin-section CT with less than 1.5mm slice thickness was obtained from the data stored in the server of the PACS system and reviewed at the site of target lesions. MRI imaging was performed on 1.5T MR unit using a pelvic phased-array body coil at a slice thickness of 5 mm and 1mm gap. All images were read independently by experienced abdominal radiologists who determined short axis diameter of lymph nodes and reach the consensus via discussion.
Data collection
Basic clinicopathologic parameters were recorded including: age, gender, stage, tumor location, pathologic features (e.g., histological type, lymphovascular invasion, perineural invasion, and grade), and preoperative serum carcinoembryonic antigen (CEA) levels. The descriptions of visible PALNs (e.g., the largest short axis diameter, number, side) were also recorded according to the image reports. The best supportive care was defined as patients who receive treatment administered with the intent to maximize quality of life without a specific antineoplastic regimen. This included antibiotics, analgesics, antiemetics, thoracentesis, pleurodesis, blood transfusions, nutritional support, and focal external-beam radiation for control of pain, cough, dyspnea, or hemoptysis [13]. Overall survival (OS) was calculated from the date of disease diagnosis to the date of death or the date on which the patient was last evaluated. The final follow-up date was December 31, 2012.
Statistical analysis
The correlations among clinicopathologic variables were analyzed using the χ2 test or Fisher exact test. The Cox proportional hazards model was applied for univariate and multivariate analyses. Survival was estimated using the Kaplan-Meier method, and the log-rank test was used for the comparison of survival curves. Variables with P values <0.05 in univariate analyses were entered into multivariate analysis models. A two-sided P value <0.05 was regarded as statistically significant. SPSS statistical software (version 18.0, SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Baseline characteristics of patients with or without visible PALNs
Between January 1, 2001 and December 31, 2011, there were 4527 newly diagnosed CRC patients in our institution, including 1139 stage IV CRC with distal metastases. The presence of distal metastases is significantly associated with poor patient prognoses for CRC; therefore, only 3388 patients without distal metastases were selected and divided into the groups with or without visible PALNs. Four hundred and nine patients were identified as having visible PALNs without distal metastases. There were 106 patients with visible PALNs who died during follow up. The flow chart of patient enrollment and exclusion is shown in Fig 1. The basic characteristics of patients with and without visible PALNs are presented in Table 1 and S1 Table. Patients with visible PALNs were significantly younger (P = 0.014), predominantly male (P = 0.014), and had more lymphovascular invasion (P < 0.001), advanced pathologic staging (pT, P < 0.001; pN, P < 0.001), and elevated preoperative serum CEA levels (P = 0.021) (Table 1). The OS of patients with visible PALNs compared to patients without visible PALNs was significantly shorter (5-year OS, 67% vs. 76%, P = 0.015) (Fig 2A).
Fig 1. Flow chart of patient enrollment and exclusion.
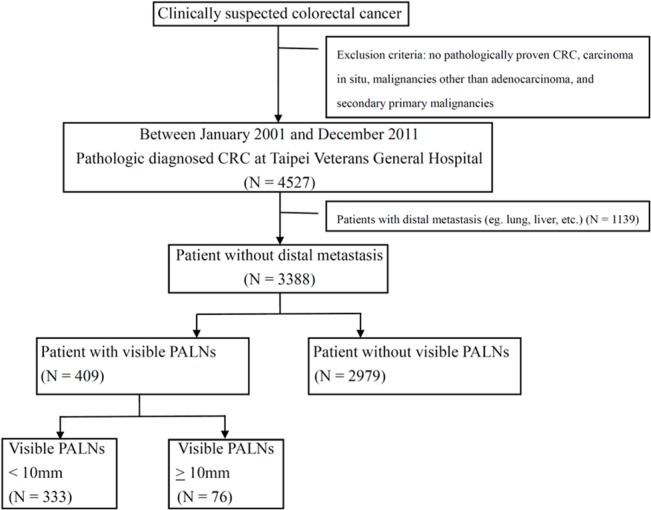
There were 4527 newly diagnosed CRC patients in our institution, including 1139 stage IV CRC with distal metastases. Only 3388 patients without distal metastases were selected and divided into the groups with or without visible PALNs. Four hundred and nine patients were identified as having visible PALNs without distal metastases.
Table 1. Characteristics of patients with or without visible PALNs a .
| Characteristics (N = 3388) | Patients without visible PALNs | Patients with visible PALNs | P value | ||
|---|---|---|---|---|---|
| N = 2979 (%) | N = 409 (%) | ||||
| Age (years) | < 65 | 1075 (36.1) | 171 (41.8) | 0.014 | |
| ≥ 65 | 1904 (63.9) | 238 (58.2) | |||
| Gender | Male | 1878 (63.0) | 281 (68.7) | 0.014 | |
| Female | 1101 (37.0) | 128 (31.3) | |||
| Tumor Location | Colon | 2192 (73.6) | 301 (73.6) | 0.525 | |
| Rectum | 787 (26.4) | 108 (26.4) | |||
| Histological type | Adenocarcinoma | 2817 (94.6) | 286 (94.4) | 0.704 | |
| Mucinous adenocarcinoma | 115 (3.9) | 14 (3.4) | |||
| Signet ring cell adenocarcinoma | 40 (1.3) | 7 (1.7) | |||
| Carcinoma, NOS | 7 (0.2) | 2 (0.5) | |||
| Primary tumor | Lymphovascular invasion | Negative | 2645 (88.8) | 338 (82.6) | <0.001 |
| Positive | 334 (11.2) | 71 (17.4) | |||
| Perineural invasion | Negative | 2886 (96.9) | 393 (96.1) | 0.236 | |
| Positive | 93 (3.1) | 16 (3.9) | |||
| Grade b | Lower | 2734 (91.8) | 369 (90.2) | 0.166 | |
| High | 245 (8.2) | 40 (9.8) | |||
| Pathologic staging | pT | 1 | 435(14.6) | 28(6.8) | <0.001 |
| 2 | 462(15.5) | 50(12.2) | |||
| 3 | 1905(63.9) | 288(70.4) | |||
| 4 | 177(5.9) | 43(10.5) | |||
| pN | N0 | 1974 (66.3) | 220 (53.8) | <0.001 | |
| N+ | 1005 (33.7) | 189 (46.2) | |||
| Preoperative serum CEA level (ng/mL) | <10 | 2424 (85.3) | 322 (81.1) | 0.021 | |
| ≥10 | 419 (14.7) | 75 (18.9) | |||
| Data available | 2843 | 397 | |||
| 5-year overall survival | 76% | 67% | 0.015 | ||
aAll patients were diagnosed as having no distant metastases
bLower grade represents well or moderately differentiated histology and high grade represents poorly differentiated histology or mucinous carcinoma.
PALNs, para-aortic lymph nodes; CRC, colorectal cancer; NOS, not otherwise specified; CEA, carcinoembryonic antigen
Fig 2. Overall survival (OS) of CRC patients with or without visible PALNs.
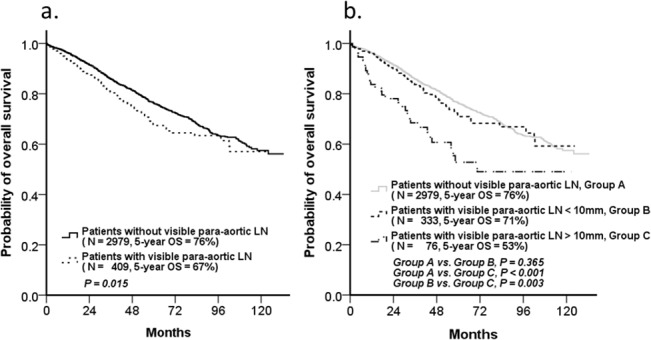
(a) Patients with visible PALNs showed significantly poorer overall survival than those without visible PALNs (5-year OS, 67% vs. 76%P = 0.015). (b) But the survivals between patients with visible PALNs < 10 mm and those without visible PALNs were the same (5-year OS, 71% vs. 76%, P = 0.365).
The impact of visible PALNs
Univariate and multivariate Cox proportional hazards regression models were analyzed to identify the impact of visible PALNs. We classified patients >65 years of age as elderly. The management of elderly CRC patients is an important issue because of more comorbidities and poor performance status [14]; the dosage of treatment for these patients should be modified [15, 16]. Although many factors influence the CEA levels [17], rare conditions result in elevated CEA levels exceeding 10ng/mL due to non-malignancy disease, including inflammation [18]. Therefore, the cut-off CEA level was set at 10 ng/mL. This analysis demonstrated that the presence of visible PALNs was only significant in univariate (P = 0.015) analysis, but was not an independent prognostic factor in multivariate Cox regression analysis for OS (hazard ratio [HR] = 1.198, 95% confidence interval [CI] = 0.970–1.480, P = 0.094) (Table 2).
Table 2. Univariate and multivariate Cox regression analysis of prognostic factors for overall survival in patients without distal metastases.
| Characteristics (N = 3388) | Univariate | Multivariate | ||
|---|---|---|---|---|
| P value | P value | HR (95% CI) | ||
| Age (years) ≥65 | <0.001 | <0.001 | 2.283 (1.912–2.725) | |
| Male | <0.001 | <0.001 | 1.407 (1.196–1.656) | |
| Rectal cancer | 0.044 | 0.001 | 1.318 (1.128–1.541) | |
| Primary tumor | Lymphovascular invasion | <0.001 | 0.006 | 1.334 (1.084–1.641) |
| Perineural invasion | <0.001 | 0.006 | 1.620 (1.149–2.286) | |
| High grade a | <0.001 | 0.005 | 1.368 (1.100–1.700) | |
| Pathologic staging | pT >2 | <0.001 | 0.011 | 1.292 (1.062–1.572) |
| pN+ | <0.001 | <0.001 | 1.661 (1.417–1.946) | |
| Preoperative serum CEA (ng/mL) level ≥10 | <0.001 | <0.001 | 1.568 (1.316–1.868) | |
| Visible PALNs (≥2 mm) | 0.015 | 0.094 | 1.198 (0.970–1.480) | |
aHigh grade represents poorly differentiated histology or mucinous carcinoma.
PALNs, para-aortic lymph nodes; CRC, colorectal cancer; HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen
Analysis of prognostic factors in patients with visible PALNs
Because the presence of visible PALNs was not an independent prognostic factor for OS and the survivals between patients with visible PALNs < 10 mm and those without visible PALNs were the same (5-year OS, 71% vs. 76%, P = 0.365) (Fig 2B), it was important to identify the true poor prognostic factors in patients with visible PALNs to provide the most appropriate treatment. In the past, visible PALNs measuring ≥10 mm in the short axis on radiologic examinations were considered a significant metastatic finding [19]. Therefore we compared the characteristics of patients with, or without, visible PALNs measuring >10 mm in the short axis. Our analysis showed that patients with larger PALNs had more lymphovascular invasion (P = 0.004), higher grade disease (P = 0.019), and more regional lymph node metastasis (P = 0.001). There were no significant differences between the two patient groups in the percentage of patients who received neoadjuvant chemotherapy (P = 0.123), and neoadjuvant concurrent chemoradiotherapy (CCRT) (P = 0.218). Although a significantly higher proportion of patients in the visible PALNs >10 mm group received postoperative chemotherapy (P < 0.001), the role of postoperative chemotherapy (either adjuvant chemotherapy or palliative chemotherapy) was still unclear because the prognostic role of visible PALNs was not evident (Table 3).
Table 3. Characteristics of patients with visible PALNs.
| Characteristics (N = 409) | Diameter of visible PALNs <10 mm | Diameter of visible PALNs ≥10 mm | P value | ||
|---|---|---|---|---|---|
| N = 333 (%) | N = 76 (%) | ||||
| Age (years) | <65 | 141 (42.3) | 30 (39.5) | 0.373 | |
| ≥65 | 192 (57.7) | 46 (60.5) | |||
| Gender | Male | 233 (70.0) | 48 (63.2) | 0.154 | |
| Female | 100 (30.0) | 28 (36.8) | |||
| Tumor Location | Colon | 241 (72.4) | 60 (78.9) | 0.151 | |
| Rectum | 92 (27.6) | 16 (21.1) | |||
| Histological type | Adenocarcinoma | 319 (95.8) | 67 (88.2) | 0.068 | |
| Mucinous adenocarcinoma | 9 (2.7) | 5 (6.6) | |||
| Signet ring cell adenocarcinoma | 4 (1.2) | 3 (3.9) | |||
| Carcinoma, NOS | 1 (0.3) | 1 (1.3) | |||
| Primary tumor | Lymphovascular invasion | Negative | 284 (85.3) | 54 (71.1) | 0.004 |
| Positive | 49 (14.7) | 22 (28.9) | |||
| Perineural invasion | Negative | 321 (96.4) | 72 (94.7) | 0.344 | |
| Positive | 12 (3.6) | 4 (5.3) | |||
| Grade a | Lower | 306 (91.9) | 63 (82.9) | 0.019 | |
| High | 27 (8.1) | 13 (17.1) | |||
| Pathologic staging | pT | 1 | 24(7.2) | 4(5.3) | 0.084 |
| 2 | 40(12.0) | 10(13.2) | |||
| 3 | 240(72.1) | 48(63.2) | |||
| 4 | 20(8.7) | 14(18.4) | |||
| pN | N0 | 193 (58.0) | 28 (36.8) | 0.001 | |
| N+ | 140 (42.0) | 48 (63.2) | |||
| Preoperative serum CEA level (ng/mL) | <10 | 268 (82.7) | 54 (74.0) | 0.063 | |
| ≥10 | 56 (17.3) | 19 (26.0) | |||
| Data available | 324 | 73 | |||
| Radiologic finding of PALNs | Number | Single | 47 (14.1) | 7 (9.2) | 0.171 |
| Multiple | 286 (85.9) | 69 (90.8) | |||
| Side | Unilateral | 98 (29.4%) | 27 (35.5) | 0.183 | |
| Bilateral | 235 (70.6) | 49 (64.5) | |||
| Treatment | Best supportive care | 204 (61.3) | 27 (35.5) | <0.001 | |
| Neoadjuvant chemotherapy | 4 (1.2) | 3 (3.9) | 0.123 | ||
| Neoadjuvant CCRT | 20 (6.0) | 7 (9.2) | 0.218 | ||
| Postoperative chemotherapy | 120 (36.0) | 45 (59.2) | <0.001 | ||
aLower grade represents well or moderately differentiated histology and high grade represents poorly differentiated histology or mucinous carcinoma.
PALNs, para-aortic lymph nodes; CRC, colorectal cancer; NOS, not otherwise specified; CEA, carcinoembryonic antigen; CCRT, concurrent chemoradiotherapy.
To identify independent prognostic factors, the variables affecting survival were examined by univariate and multivariate Cox proportional hazards regression analyses (Table 4). In the univariate analyses, factors associated with poor survival were pathologic features of the primary tumor (lymphovascular invasion, P < 0.001; perineural invasion, P = 0.001; higher grade, P = 0.033), pathologic staging (pN+, P < 0.001), elevated preoperative serum CEA level (P ≥ 0.001), and visible PALNs ≥10 mm (P = 0.003). Although postoperative chemotherapy was a significant prognostic factor for OS in patients with visible PALNs in univariate analysis (P = 0.039), it was insignificant in multivariate analysis (P = 0.284). Only lymphovascular invasion (HR = 1.865; 95% CI = 1.112–3.082; P = 0.015), pN+ status (HR = 2.099; 95% CI = 1.231–3.578; P = 0.006), elevated preoperative serum CEA level (HR = 2.263; 95% CI = 1.470–3.484; P < 0.001), and visible PALNs ≥10 mm (HR = 1.638; 95% CI = 1.047–2.563; P = 0.031) were independent prognostic factors for OS in multivariate regression analysis (Table 4 and Fig 3).
Table 4. Univariate and multivariate Cox regression analysis of prognostic factors for overall survival in patients with visible PALNs.
| Characteristics (N = 409) | Univariate | Multivariate | ||
|---|---|---|---|---|
| P value | P value | HR (95% CI) | ||
| Age (years) ≥ 65 | 0.067 | |||
| Male | 0.352 | |||
| Rectal cancer | 0.814 | |||
| Primary tumor | Lymphovascular invasion | <0.001 | 0.015 | 1.865 (1.112–3.082) |
| Perineural invasion | 0.001 | 0.066 | 2.151 (0.951–4.862) | |
| High grade a | 0.033 | 0.374 | 1.293 (0.734–2.277) | |
| Pathologic staging | pT >2 | 0.259 | ||
| pN + | <0.001 | 0.006 | 2.099 (1.231–3.578) | |
| Preoperative serum CEA level ≥10 (ng/mL) | <0.001 | <0.001 | 2.263 (1.470–3.484) | |
| Radiologic finding of PALNs | Short axis ≥10 mm | 0.003 | 0.031 | 1.638 (1.047–2.563) |
| Multiple | 0.642 | |||
| Bilateral | 0.092 | |||
| Treatment | Neoadjuvant chemotherapy | 0.891 | ||
| Neoadjuvant CCRT | 0.469 | |||
| Postoperative chemotherapy | 0.039 | 0.284 | 0.759 (0.458–1.258) | |
aHigh grade represents poorly differentiated histology or mucinous carcinoma.
PALNs, para-aortic lymph nodes; HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; CCRT, concurrent chemoradiotherapy.
Fig 3. Prognostic factors for overall survival (OS) in patients with visible PALNs.
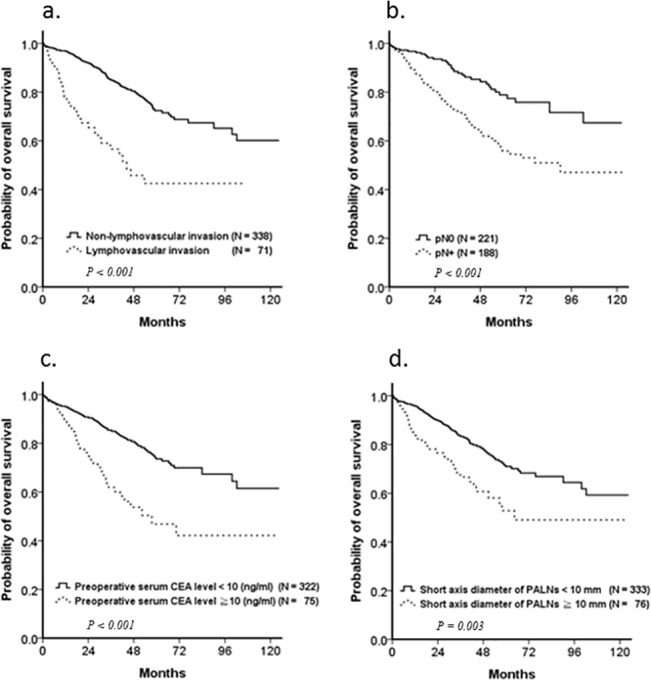
Kaplan–Meier plots revealed that (a) lymphovascular invasion (P < 0.001), (b) pN stage (P < 0.001), (c) preoperative serum CEA level (P < 0.001), and (d) short axis diameter of PALNs (P = 0.003) were independent prognostic factor in patients with visible PALNs.
The prognostic model for patients with visible PALNs
Having identified independent prognostic factors, we established a prognostic scoring system. Each prognostic factor scored one point, and a prognostic model total score ranging from 0–4 was established. Patients with visible PALNs were divided into three groups: lower-risk (prognostic score of 0–1), intermediate-risk (prognostic score of 2), and high-risk (prognostic score of 3–4). 5-year OS of lower-, intermediate-, and high- risk groups were 78%, 54%, and 25%, respectively (P < 0.001).
Compared to patients without visible PALNs, the estimated OS of the lower-risk and high-risk groups were the same as the estimated OS of patients with early stage CRC and stage IV disease respectively. The estimated OS of the intermediate-risk group was between stage III and IVa CRC patients (Fig 4).
Fig 4. Prognostic model for patients with visible PALNs.
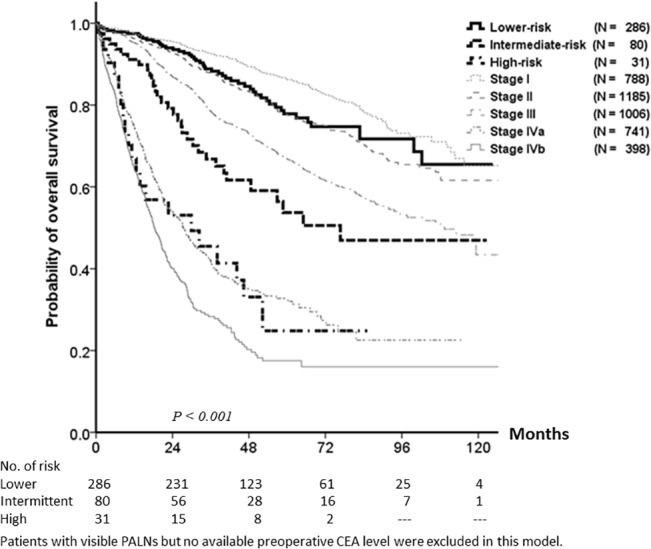
The prognostic scoring system scored one point for the presence of each of the following risk factors: lymphovascular invasion, pN+ status, serum carcinoembryonic antigen (CEA) level ≥10 ng/mL, and short axis diameter of PALNs ≥10 mm. According to the total prognostic score, patients with visible PALNs were divided into three groups: lower-risk (prognostic score of 0–1), intermediate-risk (prognostic score of 2), and high-risk (prognostic score of 3–4). 5-year OS of the lower-, intermediate-, and high-risk groups were, 78%, 54%, and 25% respectively (P <0.001).
Discussion
According to the AJCC staging system and previous studies, the clinical prognostic role and management of visible PALN metastases were equivocal [5, 7], but with the improvement of imaging modalities, these were not rare metastatic patterns in CRC. In our study, visible PALNs were not an independent prognostic factor for OS in patients without distal metastases. To address the prognostic role for these patients, we established a prognostic model to predict the outcome for patients with visible PALNs. Lymphovascular invasion, pN+ status, elevated preoperative serum CEA levels, and visible PALNs >10 mm were identified as independent prognostic factors. After assigning the presence of each prognostic factor one point, patients with visible PALNs were divided into low- (prognostic score of 0–1), intermediate- (prognostic score of 2), and high-risk (prognostic score of 3–4) groups. Only the estimated survival of the intermediate- and high-risk groups was similar to that of patients with stage IV CRC.
Actually, clinicians are uncertain as to whether visible PALNs should be treated as distant metastatic lesions or as regional lymph nodes. The optimal treatment strategies remain inconclusive [4, 10], and there are some issues that lead to undefined treatment strategies. Our findings partially answer these questions.
The cut-off level for the short-axis diameter of clinical PALN metastases has not been well addressed. The detectable sizes of lymph nodes with CT/MRI are dissimilar in different institutions [20, 21]. In our institution, visible PALNs >2 mm would be detectable and mentioned in the image records, but the clinical significance of these visible PALNs should be evaluated. Generally, it is proposed that 10 mm is the acceptable cut-off level for the maximal short-axis diameter of visible PALNs [22]. However, some visible PALNs of less than 10 mm might contain malignant cells, and the sensitivity/specificity of radiologic examination alone for lymph node metastasis is low [23, 24]. Due to the lower sensitivity/specificity of CT/MRI for visible PALNs [22–24], it was difficult to separate benign from malignant nodes with imaging studies; because a low size threshold provides higher sensitivity with low specificity, and a higher size threshold lowers the sensitivity but improves specificity, the evaluation for visible PALNs should depend on other biomarkers. In addition, due to the difficulties of surgical dissection [11], it is hard to prove pathologic metastases of PALNs. To date, a correlation between the size of PALNs and pathological status has not been reported. By survival analysis, our findings showed that 10 mm could be an acceptable cut-off level for PALNs in patients with CRC.
Additionally, the impact of visible PALNs on survival had not been clarified prior to the present study [4, 10]. Although some scattered case reports discuss the prognoses of patients with PALNs, most of them focus on the recurrence and treatment of PALNs [7–9] but not the initial presentation. According to the AJCC staging system [5], PALNs were classified as non-regional lymph nodes, and patients with PALNs were considered clinical stage IV; nevertheless, the characteristics of PALNs were not mentioned in the AJCC staging system. Although regional lymph node size has been used as a strong prognostic factor [25], from past experience, it remains equivocal whether the lymph node size alone could be a good predictor of regional nodal metastases [26, 27]. Concurrent evaluation of biological factors, such as clinicopathologic characteristics, is advised for the prediction of regional nodal metastases [28]. In our study, we point out that although the presence of visible PALNs ≥10 mm is indeed an independent prognostic factor, other biological features such as lymphovascular invasion, pN+ status, and elevated preoperative serum CEA level are also important, and should be considered concurrently.
How to manage visible PALNs is unclear in clinical practice. Previously, the presence of visible PALNs >10mm was an important cut-off level [22]. Visible PALNs <10 mm were considered benign lesions. The treatment for visible PALNs >10mm was inconclusive or controversial, and depended on the discussions of multidisciplinary teams. The prognostic value of visible PALNs needs to be evaluated first. Because the dissection of PALNs is difficult, and the incidence of postoperative complications after extensive lymph node dissection is relatively high, the optimal treatment strategy remains inconclusive [4, 10]. We therefore established a prognostic model to guide the treatment for these patients. According to the prognostic model in our study, patients with lower-risk visible PALNs had similar OS to patients diagnosed with stage I/II CRC, and should be managed as early CRC. The outcome of the intermediate-risk group, is similar to OS of patients diagnosed with CRC between stages III and IV. The treatment is therefore controversial, and more intensive treatments (such as radiotherapy) could be considered. Small cohort studies revealed concurrent chemo-radiotherapy might be useful in treating patients with visible PALNs [7, 29]. However, the development of optimal treatments for intermediate- and high-risk groups requires further study, since there is currently not enough clinical evidence to help guide appropriate treatments for these two groups. In our model,it has been suggested that the following clinicopathologic features, which have also been shown to be predictive factors for nodal metastases, be determined first: lymphovascular invasion, pN+ status, and preoperative CEA levels [30–32]. And we suggest that neo-debulking surgery +/- PALN dissection, followed by involved-field radiation therapy should be considered for patients in intermediate- and high-risk groups with visible PALNs. Patient management determined by AJCC staging system alone might be inadequate.
There were some limitations in this study. The first was the selection bias of visible PALNs; this was a retrospective study, and the limitations of detection of different radiologic machines varied. And example images are shown in S1 Fig. In addition, due to the small number of high-risk group patients, it was difficult to validate the prognostic model [33]. Our findings will require further validation through additional studies. Finally, in our hospital, dissection of PALNs is not a routine technique. The presence of additional risk factors for PALNs did not indicate truly pathologic PALN metastases. The sensitivity and specificity of this prognostic model should help accumulate more pathologic findings in patients with visible PALNs, and detailed studies should be performed to analyze these issues.
In conclusion, lymphovascular involvement, pN+ status, preoperative CEA levels ≥ 10 ng/ml, and the size of the visible PALNs ≥ 10mm were independent prognostic factors for patients with visible PALNs in CRC. These patients should not be routinely classified as AJCC stage IV. A prognostic model could effectively determine the outcome of patients with visible PALNs, and aggressive treatments could be considered for intermediate- and high-risk patients.
Supporting Information
(a,b) Images from a 72-year-old man diagnosed as having pT2N0 ascending colon adenocarcinoma with no lymphovascular invasion (LVI) and a preoperative CEA level of 1.1 ng/mL. The patient survived until the last follow-up, and overall survival (OS) was 43.4 months. Contrast enhanced CT images showed: (a) a clip was placed by endoscopy as a marker for tumor localization; there was focal wall thickening near the clip. (b) A low-risk PALN about 5.0 mm in short-axis diameter at the left para-aortic region (white arrow). (c,d) Images from a 31-year-old man diagnosed as having pT4N2 sigmoid colon adenocarcinoma with LVI and a preoperative CEA level of 2264.0 ng/mL. The images showed: (c) circumferential wall thickening of the sigmoid colon with extension through the colonic wall and invasion of the left psoas muscle; (d) a round-shaped high-risk enlarged lymph node, about 20.0 mm in size, was found at the left para-aortic region(black arrow). Left hydronephrosis due to the tumor compressing the left lower third ureter was observed. The patient died, with the overall survival being only 13.8 months.
(TIF)
(DOC)
Acknowledgments
This study was supported by the Taiwan Clinical Oncology Research Foundation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Taiwan Clinical Oncology Research Foundation.
References
- 1. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA: a cancer journal for clinicians. 2009;59(6):366–78. 10.3322/caac.20038 . [DOI] [PubMed] [Google Scholar]
- 2. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Annals of surgery. 2008;247(1):125–35. 10.1097/SLA.0b013e31815aa2c2 . [DOI] [PubMed] [Google Scholar]
- 3. Iizasa T, Suzuki M, Yoshida S, Motohashi S, Yasufuku K, Iyoda A, et al. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. The Annals of thoracic surgery. 2006;82(1):254–60. 10.1016/j.athoracsur.2006.02.027 . [DOI] [PubMed] [Google Scholar]
- 4. Choi PW, Kim HC, Kim AY, Jung SH, Yu CS, Kim JC. Extensive lymphadenectomy in colorectal cancer with isolated para-aortic lymph node metastasis below the level of renal vessels. Journal of surgical oncology. 2010;101(1):66–71. 10.1002/jso.21421 . [DOI] [PubMed] [Google Scholar]
- 5. Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bulletin of the American College of Surgeons. 2002;87(7):13–5. . [PubMed] [Google Scholar]
- 6. Bowne WB, Lee B, Wong WD, Ben-Porat L, Shia J, Cohen AM, et al. Operative salvage for locoregional recurrent colon cancer after curative resection: an analysis of 100 cases. Diseases of the colon and rectum. 2005;48(5):897–909. 10.1007/s10350-004-0881-8 . [DOI] [PubMed] [Google Scholar]
- 7. Min BS, Kim NK, Sohn SK, Cho CH, Lee KY, Baik SH. Isolated paraaortic lymph-node recurrence after the curative resection of colorectal carcinoma. Journal of surgical oncology. 2008;97(2):136–40. 10.1002/jso.20926 . [DOI] [PubMed] [Google Scholar]
- 8. Ho TW, Mack LA, Temple WJ. Operative salvage for retroperitoneal nodal recurrence in colorectal cancer: a systematic review. Annals of surgical oncology. 2011;18(3):697–703. 10.1245/s10434-010-1322-7 . [DOI] [PubMed] [Google Scholar]
- 9. Yeo SG, Kim DY, Kim TH, Jung KH, Hong YS, Kim SY, et al. Curative chemoradiotherapy for isolated retroperitoneal lymph node recurrence of colorectal cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2010;97(2):307–11. 10.1016/j.radonc.2010.05.021 . [DOI] [PubMed] [Google Scholar]
- 10. Min BS, Kim JS, Kim NK, Lim JS, Lee KY, Cho CH, et al. Extended lymph node dissection for rectal cancer with radiologically diagnosed extramesenteric lymph node metastasis. Annals of surgical oncology. 2009;16(12):3271–8. 10.1245/s10434-009-0692-1 . [DOI] [PubMed] [Google Scholar]
- 11. Liang JT, Huang KC, Lai HS, Lee PH, Sun CT. Oncologic results of laparoscopic D3 lymphadenectomy for male sigmoid and upper rectal cancer with clinically positive lymph nodes. Annals of surgical oncology. 2007;14(7):1980–90. 10.1245/s10434-007-9368-x . [DOI] [PubMed] [Google Scholar]
- 12. Committee on Classification of Regional Lymph Nodes of Japan Society of Clinical Oncology. Classification of regional lymph nodes in Japan. Int J Clin Oncol. 2003;8(4):248–75. Epub 2003/09/05. 10.1007/s10147-003-0343-7 . [DOI] [PubMed] [Google Scholar]
- 13. Jassem J, Ramlau R, Santoro A, Schuette W, Chemaissani A, Hong S, et al. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(10):1698–704. 10.1200/JCO.2006.09.9887 . [DOI] [PubMed] [Google Scholar]
- 14. Lan YT, Chang SC, Yang SH, Lin CC, Wang HS, Jiang JK, et al. Comparison of clinicopathological characteristics and prognosis between early and late recurrence after curative surgery for colorectal cancer. American journal of surgery. 2014;207(6):922–30. 10.1016/j.amjsurg.2013.08.035 . [DOI] [PubMed] [Google Scholar]
- 15. Yang L, Ma Q, Yu YY, Wang C, Meng WJ, Adell G, et al. Efficacy of surgery and adjuvant therapy in older patients with colorectal cancer: a STROBE-compliant article. Medicine. 2014;93(28):e266 10.1097/MD.0000000000000266 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reese ES, Onukwugha E, Hanna N, Seal BS, Mullins CD. Clinical and demographic characteristics associated with the receipt of chemotherapy treatment among 7951 elderly metastatic colon cancer patients. Cancer medicine. 2013;2(6):907–15. 10.1002/cam4.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clinical chemistry. 2001;47(4):624–30. . [PubMed] [Google Scholar]
- 18. Fakih MG, Padmanabhan A. CEA monitoring in colorectal cancer. What you should know. Oncology. 2006;20(6):579–87; discussion 88, 94, 96 passim. . [PubMed] [Google Scholar]
- 19. Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180(2):319–22. 10.1148/radiology.180.2.2068292 . [DOI] [PubMed] [Google Scholar]
- 20. Saokar A, Islam T, Jantsch M, Saksena MA, Hahn PF, Harisinghani MG. Detection of lymph nodes in pelvic malignancies with Computed Tomography and Magnetic Resonance Imaging. Clinical imaging. 2010;34(5):361–6. 10.1016/j.clinimag.2009.07.004 . [DOI] [PubMed] [Google Scholar]
- 21. Choi J, Oh SN, Yeo DM, Kang WK, Jung CK, Kim SW, et al. Computed tomography and magnetic resonance imaging evaluation of lymph node metastasis in early colorectal cancer. World journal of gastroenterology: WJG. 2015;21(2):556–62. 10.3748/wjg.v21.i2.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2004;45(9):1509–18. . [PubMed] [Google Scholar]
- 23. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773–83. 10.1148/radiol.2323031368 . [DOI] [PubMed] [Google Scholar]
- 24. Lee MJ, Yun MJ, Park MS, Cha SH, Kim MJ, Lee JD, et al. Paraaortic lymph node metastasis in patients with intra-abdominal malignancies: CT vs PET. World journal of gastroenterology: WJG. 2009;15(35):4434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dhar DK, Yoshimura H, Kinukawa N, Maruyama R, Tachibana M, Kohno H, et al. Metastatic lymph node size and colorectal cancer prognosis. Journal of the American College of Surgeons. 2005;200(1):20–8. 10.1016/j.jamcollsurg.2004.09.037 . [DOI] [PubMed] [Google Scholar]
- 26. Perez RO, Pereira DD, Proscurshim I, Gama-Rodrigues J, Rawet V, Sao Juliao GP, et al. Lymph node size in rectal cancer following neoadjuvant chemoradiation—can we rely on radiologic nodal staging after chemoradiation? Diseases of the colon and rectum. 2009;52(7):1278–84. 10.1007/DCR.0b013e3181a0af4b . [DOI] [PubMed] [Google Scholar]
- 27. Pomerri F, Maretto I, Pucciarelli S, Rugge M, Burzi S, Zandona M, et al. Prediction of rectal lymph node metastasis by pelvic computed tomography measurement. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35(2):168–73. 10.1016/j.ejso.2008.02.006 . [DOI] [PubMed] [Google Scholar]
- 28. Markl B, Rossle J, Arnholdt HM, Schaller T, Krammer I, Cacchi C, et al. The clinical significance of lymph node size in colon cancer. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25(10):1413–22. 10.1038/modpathol.2012.92 . [DOI] [PubMed] [Google Scholar]
- 29. Fujii S, Ota M, Ichikawa Y, Yamagishi S, Osada S, Suwa H, et al. Paraaortic lymph node metastasis showed CR to UFT/LV therapy in elderly rectal cancer. Hepato-gastroenterology. 2010;57(99–100):472–6. . [PubMed] [Google Scholar]
- 30. Chok KS, Law WL. Prognostic factors affecting survival and recurrence of patients with pT1 and pT2 colorectal cancer. World journal of surgery. 2007;31(7):1485–90. 10.1007/s00268-007-9089-0 . [DOI] [PubMed] [Google Scholar]
- 31. Huh JW, Kim HR, Kim YJ. Lymphovascular or perineural invasion may predict lymph node metastasis in patients with T1 and T2 colorectal cancer. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2010;14(7):1074–80. 10.1007/s11605-010-1206-y . [DOI] [PubMed] [Google Scholar]
- 32. Lou Z, Meng RG, Zhang W, Yu ED, Fu CG. Preoperative carcinoembryonic antibody is predictive of distant metastasis in pathologically T1 colorectal cancer after radical surgery. World journal of gastroenterology: WJG. 2013;19(3):389–93. 10.3748/wjg.v19.i3.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. Bmj. 2009;338:b605 10.1136/bmj.b605 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a,b) Images from a 72-year-old man diagnosed as having pT2N0 ascending colon adenocarcinoma with no lymphovascular invasion (LVI) and a preoperative CEA level of 1.1 ng/mL. The patient survived until the last follow-up, and overall survival (OS) was 43.4 months. Contrast enhanced CT images showed: (a) a clip was placed by endoscopy as a marker for tumor localization; there was focal wall thickening near the clip. (b) A low-risk PALN about 5.0 mm in short-axis diameter at the left para-aortic region (white arrow). (c,d) Images from a 31-year-old man diagnosed as having pT4N2 sigmoid colon adenocarcinoma with LVI and a preoperative CEA level of 2264.0 ng/mL. The images showed: (c) circumferential wall thickening of the sigmoid colon with extension through the colonic wall and invasion of the left psoas muscle; (d) a round-shaped high-risk enlarged lymph node, about 20.0 mm in size, was found at the left para-aortic region(black arrow). Left hydronephrosis due to the tumor compressing the left lower third ureter was observed. The patient died, with the overall survival being only 13.8 months.
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


