Abstract
A widespread biogeographic pattern in nature is that population abundance is not uniform across the geographic range of species: most occurrence sites have relatively low numbers, whereas a few places contain orders of magnitude more individuals. The Bolson tortoise Gopherus flavomarginatus is endemic to a small region of the Chihuahuan Desert in Mexico, where habitat deterioration threatens this species with extinction. In this study we combined field burrows counts and the approach for modeling species abundance based on calculating the distance to the niche centroid to obtain range-wide abundance estimates. For the Bolson tortoise, we found a robust, negative relationship between observed burrows abundance and distance to the niche centroid, with a predictive capacity of 71%. Based on these results we identified four priority areas for the conservation of this microendemic and threatened tortoise. We conclude that this approach may be a useful approximation for identifying key areas for sampling and conservation efforts in elusive and rare species.
Introduction
Spatial variation in abundance across species’ geographic ranges has been a topic of interest for decades (e.g., Brown [1]; Brown et al. [2]; Gaston et al. [3]; Guo et al. [4]; Pearce & Ferrier [5]). A widespread pattern across taxonomic groups is that in most occurrence sites within species’ ranges population numbers are generally low, whereas a few sites have orders of magnitude more individuals [6]. Abundance does not often follow a spatial radial pattern, in which maximum abundance is held towards the geographic center of species’ range and decreases towards the edges [7,8]. Spatial patterns of abundance in nature are more complex than this simple rule and several factors seem to have an influence [9].
First, the processes driving the distribution-abundance relationship operate at different spatial and temporal scales (e.g., local resource availability, environmental suitability of the landscape, dispersal capacity of the species) [10]. Second, spatial patterns are strongly autocorrelated (i.e., nearby sites tend to have more similar abundances than sites that are far apart [1]); therefore, the stronger the spatial autocorrelation, the smaller the changes in population size from site to site across the species’ range [11]. According to Brown and collaborators [2], population abundance is determined by two main factors: (i) the degree to which local conditions fulfill the niche requirements of species, and (ii) the interactions between abiotic and biotic variables. Therefore, spatial variation in abundance depends on the number and nature of niche variables and the way these vary across space.
Abundance is a key parameter for conservation purposes, since it is frequently used as one of the criteria for deciding whether a species is rare or common: for rare species there are few individuals per sample, and thus low absolute variation among samples [2,12,13]. In spite of its importance, abundance is seldom measured across the geographic range of the species because it is time-consuming and requires a great deal of effort, and resources. Species abundance is usually estimated based on data from a handful of sites [14,15].
Methods for estimating the spatial distribution of abundance include the fractal method [16] and the negative binomial distribution [17]. A recent study [18] has proposed an alternative approach, namely the distance to the niche centroid (DNC) method. This is based on Hutchinson’s [19] theory of the multidimensional ecological niche and a further theoretical development, which suggests that abundance, is determined by the internal structure of the niche. The optimal conditions (i.e., where birth rate is maximal and death rate is minimal and thus abundance is highest) occur toward the geometric centroid of the niche in ecological space, and abundance decreases with distance from the centroid [20]. This ecological centrality principle would thus manifest in different geographic patterns across the landscape, depending on the eco-spatial structure [18]. The DNC method fits a curve for the relationship between known abundance samples across the species’ geographic range and the distance to the ecological niche centroid to make range-wide estimates of the species’ abundance. It has proven robust in different geographic contexts and at different scales [21].
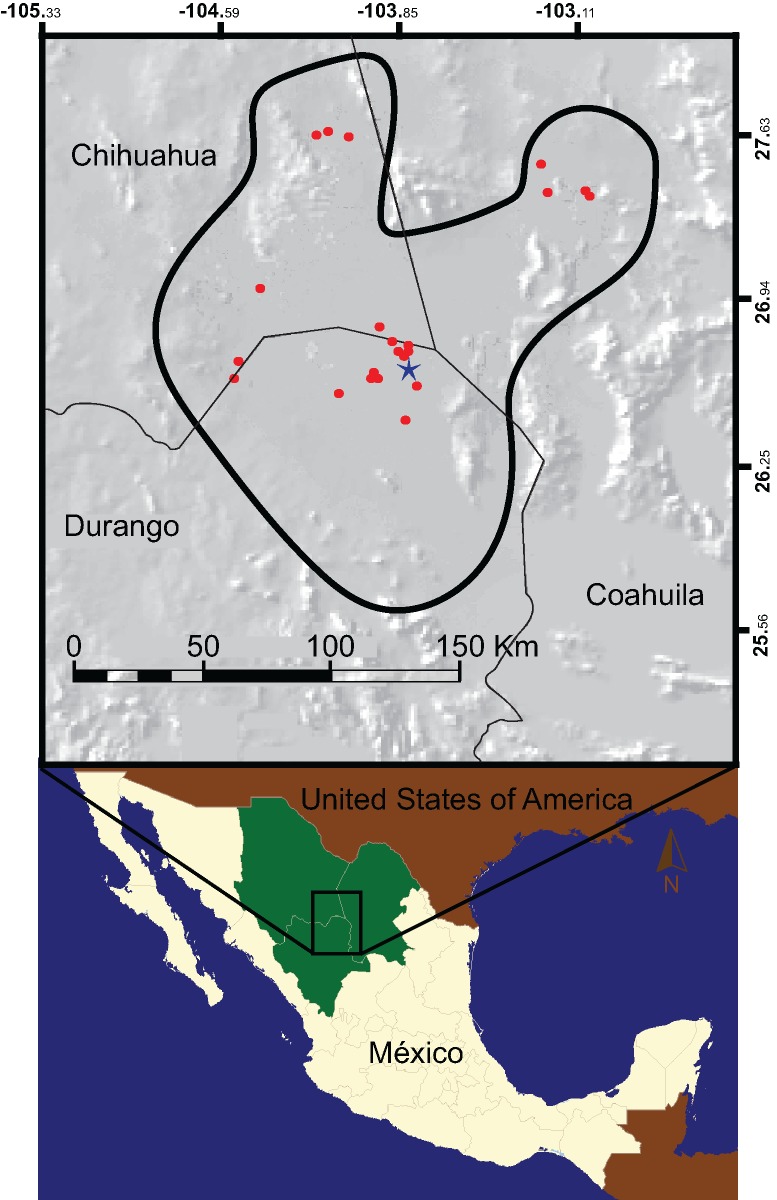
Gopherus flavomarginatus (the Bolson tortoise) is endemic to a portion of the Mapimí Basin, in the Chihuahuan Desert, Mexico (Fig 1). Widespread until the Pleistocene when it ranged from southern USA to central Mexico [22], several factors have brought the Bolson tortoise to the verge of extinction: climate changes in the Pleistocene-Holocene transition, recent anthropogenic activities, including habitat destruction due to overgrazing and agriculture, overexploitation for wildlife trade [23–26], and low genetic variation [27].
Fig 1. Geographic distribution of Gopherus flavomarginatus.
The thick black line delineates the polygon of the Mapimí Biosphere Reserve, whereas the state borders are mark by the thin black lines. Red dots pinpoint the sites where the abundance data for the Bolson tortoises was recorded. The star indicates the location of the Mapimí field station.
Limiting factors in the distribution of the Bolson tortoise include very specific habitat requirements, such as soft slopes and the presence of dry lake beds for nesting [28]. The only previous study in which abundance was estimated reported a mean density of 10 tortoises/Km2, and an area of occupancy covering 6,090 Km2 [29]. Based on field surveys and niche modeling, in this study we estimated the geographic and ecological distribution of the Bolson tortoise, as well as its range-wide abundance with the DNC method. These data helped us to identify critical areas for concentrating future fieldwork and conservation efforts.
Materials and Methods
Ethics statement
The current study was carried out across the whole distribution of Gopherus flavomarginatus, including the Mapimí Biosphere Reserve. Nonetheless, special permits were not required. This study did not involve any sort of tortoise or habitat manipulation. In addition to the Mapimí Biosphere Reserve we obtained access to private properties Rancho Americanos, Rancho La Mena, Rancho Cerros Emilio, Rancho La Parva, Rancho Los Remedios, Rancho San Miguel; and some Ejidos, including Las Flores, Lagunetas, Emiliano Zapata, and Ejido Vicente Guerrero. If needed, the authors can be contacted to provide further details from the respective owners or legal representatives.
Study area
The current known distribution of Gopherus flavomarginatus occupies the central part of the Chihuahuan Desert, in the Bolsón de Mapimí, which covers parts of the states of Chihuahua, Coahuila and Durango (Fig 1). The Bolsón de Mapimí is an endoreic basin composed of a series of small sub-basins intermixed with valleys, but is generally flat with a mean altitude of 1,150 m.a.s.l. Climate is of the type “continental tropical arid” with a mean annual temperature of 20.8°C and an annual precipitation of 264.2 mm [30]. The main vegetation type is desert shrubland [31] and the Bolsón has the richest herpetofauna of the whole Chihuahuan Desert with several endemic species, including the Bolson tortoise [32].
Field surveys
We gathered two types of the tortoise presence information: occurrence and abundance data. Occurrence data were drawn from electronic databases (www.gbif.org and http://www.conabio.gob.mx/remib/doctos/remib_esp.html) and the literature [29,33]. Based on that information we went to the field to record the presence of the species across its distribution range from April 2008 to May 2010. The occurrence data were used to build a niche model for the Bolson tortoise via Mahalanobis distances (see below). Whereas, the abundance data were gathered in 22 independent ~1 km2 plots randomly distributed across the distribution range of the species. In each plot we counted all active and inactive adult burrows, excluding all abandoned or destroyed ones. Active burrows were identified by the presence of tortoise footprints and food leftovers (e.g., grass, leaves), whereas other animals such as owls and snakes usually inhabit the abandoned ones. Abundance data were used to calibrate and validate our abundance model (see below).
Ecological Niche Modeling and distances to the centroid
We assembled a data matrix with the 19 bioclimate variables from the WorldClim database [34], and three topographic variables from the Hydro 1k database [35] (Table 1). All variables were under the geographic coordinate system (WGS84 Datum), and pixel size was 30 arc-seconds (~1 km2).
Table 1. Climate and topographic variables used for inferring the ecological niche and abundance models of the Bolson tortoise.
| Name | Variable |
|---|---|
| BIO 01 | Mean Annual Temperature |
| BIO 02 | Diurnal Temperature Range |
| BIO 03 | Isothermality |
| BIO 04 | Temperature Seasonality |
| BIO 05 | Max Temperature of the Warmest Month |
| BIO 06 | Min Temperature of the Coldest Month |
| BIO 07 | Temperature Annual Range |
| BIO 08 | Mean Temperature of the Wettest Quarter |
| BIO 09 | Mean Temperature of the Driest Quarter |
| BIO 10 | Mean Temperature of the Warmest Quarter |
| BIO 11 | Mean Temperature of the Coldest Quarter |
| BIO 12 | Annual Precipitation |
| BIO 13 | Precipitation of the Wettest Month |
| BIO 14 | Precipitation of the Driest Month |
| BIO 15 | Precipitation Seasonality |
| BIO 16 | Precipitation Wettest |
| BIO 17 | Precipitation of the Driest Quarter |
| BIO 18 | Precipitation of the Warmest Quarter |
| BIO 19 | Precipitation of the Coldest Month |
| CTI | Compound Topographic Index (or Wettest Index) |
| ALT | Altitude |
| SLOPE | Slope |
Currently, there are many algorithms to produce niche-based distribution models, and their performance capacity varies depending on the type, amount and bias of the biological data [36]. Due to the collinearity observed in the environmental variables, for this study we implemented the Mahalanobis Distance method. Fieldwork yielded a total of 241 confirmed presence points. These points were used to infer the species ecological niche by obtaining the particular values for each environmental variable (Table 1). Then, these values were used to calculate a multidimensional environmental mean (i.e., the niche centroid), then, Mahalanobis distances were calculated from the niche centroid to each occurrence point. We considered as the potential distribution of the Bolson tortoise in environmental space the climate envelope generated around the 241 occurrence points with a radius equal to the distance observed from the niche centroid to the farthest of such points. Finally, Mahalanobis distances were calculated for all pixels in the study area where the potential distribution of the species in geographic space thus encompassed all pixels with distance values equal to or lower than the maximum distance to the occurrence points.
Relationship between the distance to the centroid and abundance, and the generation of the abundance map
We performed regression analyses between the distance to the centroid and the observed abundance of the Bolson tortoise to find the best fit using the Statistical Package SPSS Ver. 19 (IBM). Then, using the best-fit model we generated the estimated abundance map of the Bolson tortoise across its entire potential distribution range in ArcGis v.10.0 (ESRI, Redlands, CA, USA).
Abundance Model validation and Uncertainty Map
The predictive capacity of the abundance model (i.e., best-fit model) was assessed applying a regression procedure using a random 70/30 data split for training/validation. We re-estimated the best-fit model using the 70% fraction of the data, and the resulting function was used to predict the abundance of the remaining 30% points. Then, we performed a simple linear regression between the expected and the observed abundance of the 30% data fraction. The R2 of this second regression is proportional to the predictive power of the inferred model. We repeated this procedure ten times to observe the standard deviation of R2.
Alternatively, we applied a bootstrap procedure consisting of generating 1000 estimates of abundance using a random 65/35 data split for training/validation. We calculated the 95% regression confidence intervals for the iterations, and counted the number of points that fell within them. In this way, we obtained a percentage that reflects the predictive capacity of the model. Finally, we generated an uncertainty map using the confidence intervals [21]. The bootstrap was implemented in R-v.2.15.1 software (R Development Core Team).
Results
The potential distribution of the Bolson tortoise, inferred with the Mahalanobis distance method, match well with its known distribution [29] (Fig 2A). Most of the predicted area corresponds to flat lands. The map, however, include a few sites with steep slopes from which the species is known to be absent. Those areas, therefore, were removed from the potential distribution.
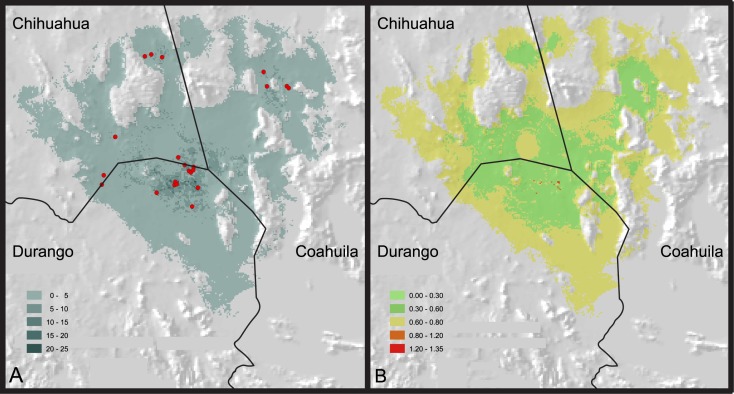
Fig 2. Maps showing the potential distribution and abundance of Gopherus flavomarginatus.
(A) Inferred abundance for the Bolson tortoise (burrows/Km2); red dots pinpoint the sites where the abundance data for the Bolson tortoises was recorded. (B) Standard deviation of abundance.
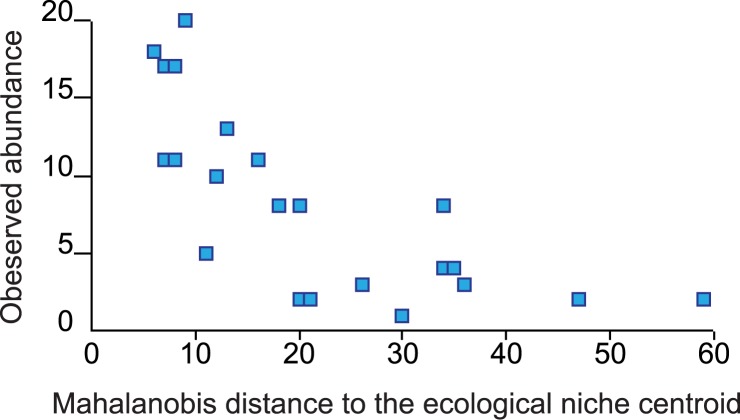
All the regression functions that we explored found a significant relationship between the observed abundance and the distance to the ecological niche centroid (Table 2). The best-fit model, nonetheless, was the Inverse Regression explaining more of the variation in the relationship between the Mahalanobis distance to the centroid and the abundance (adjusted R2 = 0.681, P < 0.001). As expected, the observed abundance drops drastically when the environmental conditions depart from those found nearby the ecological niche centroid. Our field data showed that the abundance value drops nearly two thirds once the Mahalanobis distance to the niche centroid increase beyond 20 units (Fig 3).
Table 2. Goodness-of-fit of the regression models between Mahalanobis distance to the ENC and burrow abundance of Gopherus flavomarginatus.
| Model | regular R2 | adjusted R2 | P-value |
|---|---|---|---|
| Inverse | 0.696 | 0.681 | < 0.001 |
| Logaritmic | 0.677 | 0.660 | < 0.001 |
| Cubic | 0.707 | 0.658 | < 0.001 |
| Quadratic | 0.680 | 0.647 | < 0.001 |
| Power | 0.628 | 0.609 | < 0.001 |
| Growth | 0.545 | 0.522 | < 0.001 |
| Exponential | 0.545 | 0.522 | < 0.001 |
| Logistic | 0.545 | 0.522 | < 0.001 |
| Lineal | 0.540 | 0.517 | < 0.001 |
| SAR | 0.685 | 125.923 1 | < 0.001 |
1 Results inferred from Akaike Information Criterion (AIC)
Fig 3. Relationship between the observed abundance (burrows/Km2) of Gopherus flavomarginatus and the distance to the species´ ecological niche centroid.
The highest estimated abundance of the Bolson tortoise is recovered mainly towards the center of the potential distribution area predicted by the Mahalanobis distance method (Fig 2A). This putative rich section is encompassed within the polygon of the Mapimí Biosphere Reserve. However, several other potentially rich areas are predicted outside of officially protected lands. Two of those areas are located in remote isolated regions. One is north of Sierra del Diablo Mountains, in the state of Chihuahua, whereas the other is east of Sierra Mojada Mountains, in the state of Coahuila. Nonetheless, other unprotected areas with high abundance are close to populated zones and are accessible by dirt roads.
According to the validation procedure, the predictive capacity of the best-fit model averaged an R2 of 0.712 (p = 0.023, standard deviation = 0.127; Table 3). Abundance estimates are within reasonable ranges (1 to 20 burrows/km2), and coincide with our observed field data (Table 4). The uncertainty map, generated with the bootstrap procedure, shows that the inferred abundance has a standard deviation between 0 and 1.35 (Fig 2B).
Table 3. Validation for the Inverse Regression Model between Mahalanobis distance to the ENC and burrow abundance.
| Trial | R | R2 | P-value | |
|---|---|---|---|---|
| 1 | 0.779 | 0.638 | 0.031 | |
| 2 | 0.843 | 0.653 | 0.017 | |
| 3 | 0.812 | 0.660 | 0.026 | |
| 4 | 0.669 | 0.447 | 0.101 | |
| 5 | 0.886 | 0.785 | 0.008 | |
| 6 | 0.887 | 0.787 | 0.008 | |
| 7 | 0.923 | 0.852 | 0.003 | |
| 8 | 0.927 | 0.858 | 0.003 | |
| 9 | 0.805 | 0.648 | 0.029 | |
| 10 | 0.891 | 0.794 | 0.007 | |
| Mean | 0.844 | 0.712 | 0.023 | |
| Standard deviation | 0.078 | 0.127 | 0.029 | |
| Minimum | 0.669 | 0.447 | 0.003 | |
| Maximum | 0.927 | 0.858 | 0.101 |
Table 4. Recorded abundance of Gopherus flavomarginatus expressed as the number of burrows/Km2.
| Sampling Locality | District | Abundance |
|---|---|---|
| Americanos | I: Los Americanos, Coahuila | 8 |
| Las Flores | I: Los Americanos, Coahuila | 2 |
| Lagunetas | I: Los Americanos, Coahuila | 4 |
| La Mena | I: Los Americanos, Coahuila | 3 |
| Cerros Emilio | II: Sierra del Diablo, Chihuahua | 8 |
| Ejido Emiliano Zapata | II: Sierra del Diablo, Chihuahua | 2 |
| La Parva | II: Sierra del Diablo, Chihuahua | 2 |
| Los Remedios | VI: Sierra de los Remedios, Chihuahua | 1 |
| San Miguel | III: Rancho Diana, Chihuahua | 4 |
| Ejido Vicente Guerrero | III: Rancho Diana, Chihuahua | 2 |
| El Pujo | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 11 |
| Tortugas | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 10 |
| La Flor (Brecha) | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 13 |
| Las Lolas | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 18 |
| Cajones | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 20 |
| La Flor (Bebedero) | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 17 |
| La Flor (Joyita) | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 17 |
| San Ignacio Yermo | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 11 |
| Laboratory MBR North | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 8 |
| Laboratory MBR South | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 11 |
| La Soledad | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 3 |
| San Carlos | V: MCR 1 , Durango-Coahuila-Chihuahua 2 | 5 |
Roman numerals before the district name correspond to the designation assigned by Bury et al. [29].
1 MCR = Mapimí Central Region
2 District V was modified and we included the state of Chihuahua because several sites lie within this region.
Discussion
An abundance estimate of the Bolson tortoise is key to establishing the conservation status of the species. This is the first range-wide study in which abundance is estimated at the regional scale; thus, it has potentially important implications for further conservation and management actions.
Ecological niche modeling has been a helpful approach for predicting species distributions with conservation purposes, particularly when data are limited [37–41]. Interestingly, niche model outcome probabilities (or similar results) are frequently interpreted as a measure of habitat suitability, thus it is implicitly thought that they somehow provide information about population potential performance [42]; however, this assumption has seldom been tested, mainly due to a lack of information on performance parameters across the whole species range, but when such suitability has been compared with the abundance of species, the results are inconclusive, but generally indicate that this relationship is weak [43–46].
In this study we implemented the Mahalanobis distance to the niche centroid approach to estimate the abundance of the Bolson tortoise across its geographic range [18]. This method is data demanding, which may be a potential pitfall. Several tens of occurrences are needed to obtain a reliable estimation of the species’ niche.
We found a significant relationship suggesting that the abundance of G. flavomarginatus is strongly determined by the internal structure of the species’ niche throughout its geographic range [20]. Interestingly, we observed that its abundance distribution followed a centralized pattern both in the ecological and geographic spaces, where abundance tends to be highest toward the center of these spaces and decrease toward the boundaries [2,47]. Although irregular and containing unoccupied areas, the shape of the species range is roughly circular, so the centralized pattern found in both ecological and geographic spaces is not surprising [18].
Our results were similar to those reported for the Spotted Turtle (Clemmys guttata), in terms of the form of the function and the explanatory power of abundance by the distance to the niche centroid [18]. Interestingly, these authors found that for 9 out of the 10 species analyzed in their study, the relationship between the distance to the centroid and abundance was not inverse, such as in our study, but rather logarithmic or exponential [18]. This has implications in the demography of species, since the reduction in the population size of the chelonids with increasing distance from the niche centroid is abrupt and not, monotonic as in the other species.
On the other hand, the relationship that we found was not as strong as that reported for the White-tailed Deer (Odocoileus virginianus; R2 = 0.902 and R2 = 0.761) [21]. If the hypothesis that the centriod of the ecological niche encompasses the optimal conditions for the species is correct, differences in the predicting power of the model might be due to environmental or ecological variables that were not taken into account [48]. The Bolson tortoise, for instance, is an herbivore that inhabits “sabaneta” (Pleuraphis mutica) grassland borders and biotopes with soft slopes, fine-texture soils with a mixture of shrubs (Larrea divaricata, Prosopis juliflora, Parthenium incanum, and Fluorensia cernua) and halophilic grasses [26], where cattle also feed. Therefore, the cattle may be interfering with tortoises, directly or indirectly [49]. Studies for other Gopherus species have reported that young individuals are frequently kicked or stepped on by cattle [50,51]. Also, the Bolson tortoise is illegally exploited for meat and sold as a pet [26,52,53]; however, there is no hard data to evaluate the impact of these activities on the demography of the species. In addition, the Bolson tortoise has a very low dispersal rate, which may cause low population numbers in suitable habitats, as might be the lack of field surveys in several areas. Therefore, besides abiotic variables, biotic interactions are important factors that also drive species’ distributions [54–56]; and ultimately might influence the species’ abundance [47,57].
The distance to the centroid method is a static approach that does not capture the spatio-temporal dynamics of populations [21]. The method assumes unimodality and centrality of abundance in relation to all environmental variables (i.e., that optimal conditions are always close to the mean values for all variables). This, may not necessarily hold true, because for some variables optimal conditions might actually be closer to the extreme values. Despite these shortcomings, the distance to the centroid approach represented the abundance distribution patterns of the Bolson tortoise fairly well.
Other approaches have been developed to obtain spatial estimates of abundance, like the fractal model [16] and the negative binomial distribution method [17]. This negative binomial distribution approach is the most popular (e.g., Tosh, Reyers & van Jaarsveld [58]; Figueiredo & Grelle [59]), but is strongly dependent on spatial scale [60]. Unfortunately, these methods cannot be directly compared to our implementation of the distance to the niche centroid because their performance is not measured via a determination coefficient. Our approach (i.e., DNC) is more flexible because the results are inferred from presence-absence data, instead of presence-only data as in other methods. Furthermore, for good-fit models the distance to the centroid alone can be a good approximation of the suitability of the environment to the species.
Previous field studies on the abundance of G. flavomarginatus in some areas of its geographic range reported a marked contrast in tortoise density across its range (5–44 burrows/0.5 km2; [29]). Our results suggest that after three decades the abundance of the Bolson tortoise seems to be declining, in some areas by as much as 91%. For instance, Bury et al. [29] reported the presence of 88 individual of this species in Cerros Emilio, in Chihuahua, whereas we only recorded 8 different individuals (Table 4). The largest colonies of tortoise that we found are in the Mapimí Biosphere Reserve, with up to 20 individuals/Km2. Previous reports suggest that the abundance of the species has decreased by 25% in the sites for which historical data is available. In 1988 a total of 25 individuals were reported at Las Lolas [29]; but during the two years that our field survey lasted we only observed 18 tortoises at the exact same locality (Table 4). Anecdotal information also suggests that populations seem to be smaller today than a couple of decades ago, particularly at the distributional limits of the species (G. Aguirre, pers. comm.). This trend seems to be a consequence of the expansion of the railroad network, cattle ranching and agriculture in the Chihuahuan Desert during the 20th century [25,33].
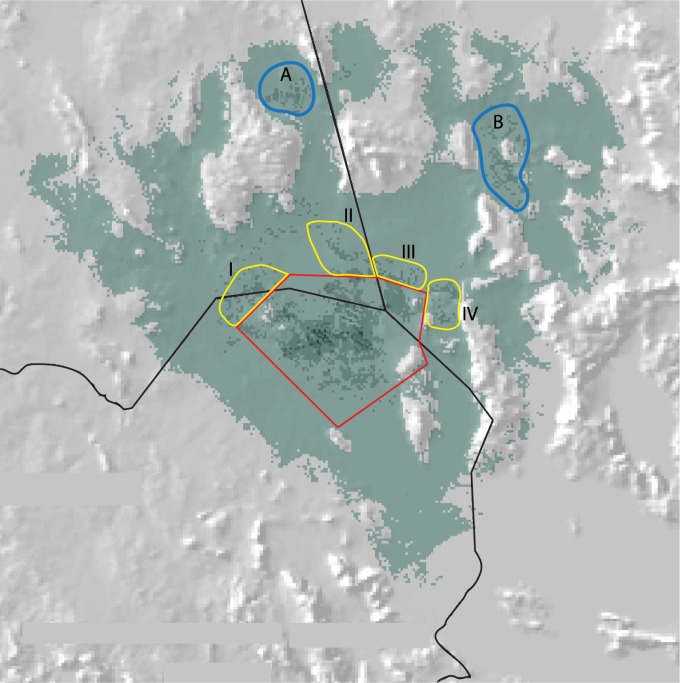
Based on our results we identified four high-abundance areas, contiguous to the Mapimí Biosphere Reserve polygon, worthy of considering for legal protection (Fig 4). According to our results, the most convenient sites for this purpose are Areas III (236 Km2; 26.88°N, -103.59°W) and IV (291 Km2; 26.68°N, -103.44°W) in the state of Coahuila because these two areas hold very low human population and road densities, especially Area III. Area I (528 Km2; 26.79° N, -104.29°W), and II (575 Km2; 27.01° N, -103.98° W) in Chihuahua are the largest, but they are the most populated and intensively used areas for human activities, given its closeness to Lake Palomas. Additionally, Sierra del Diablo (27.63° N, -104.15°W), and Sierra Mojada (27.37° N, -103.11°W), located in the states of Chihuahua and Coahuila respectively, represent important regions in terms of the expected abundance. Currently, these regions are not subjected to any type of protection; however they might be considered of least concern because they are relatively isolated, and human population density is very low (and currently is declining).
Fig 4. Proposed key sites (yellow polygons) for the conservation of the Bolson tortoise, Gopherus flavomarginatus.
The red line delineates the current protected polygon of the Mapimí Biosphere Reserve. Sierra del Diablo (A) and Sierra Mojada (B) represent putative high abundance sites; nonetheless, these are low priority areas due to their natural isolation and low human population density.
Protection of the four areas proposed would add 1,279 Km2 to the 3,423.88 Km2 currently under protection within the Mapimí Biosphere Reserve polygon for the Bolson tortoise. According to previous studies [61,62], a fifth potential area for the tortoise protection is the Sierra del Diablo District (20 Km2 surrounding Cerros Emilio 27.42°N, 103.97°W), in Chihuahua. This area has high levels of abundance according to our model; however, it is located at the northern distribution edge of the species and is similar in area and characteristics to Area IV. Therefore, a carefully thought out strategy would be necessary to create a multi-polygon protected area taking into account the management systems of local land resources. The conservation of the Bolson tortoise is of the highest priority; decisions should be made with the best information available and this study aims to provide relevant information in this regard. The results presented here can be used as a first and simple approach for inferring abundance patterns in space. Such information is important for making management, or conservation decisions for any species.
Conclusions
This study aims to contribute to the conservation of the Bolson tortoise by providing new insights about the abundance distribution of the species at the range-wide scale. First, the aggregated nature of the distribution of the species across the landscape should be taken into account for conservation strategies. Compared to the other areas where the Bolson tortoise is distributed, the Mapimí Biosphere Reserve successfully protects some of its populations [61] because it holds the largest colonies and actively prohibits their exploitation. Even there, however, the population is still declining. Moreover, given the restricted distribution of the species and the lack of protection in other areas, top priority must be given to including most-if not all- of this species’ range under a protection scheme.
Acknowledgments
We are deeply grateful to A. R. Monroy for his help with ArcGis. We also thank G. Aguirre for his support and for sharing his extensive knowledge on the biology of the Bolson tortoise and A. R. Trápaga for his help throughout the fieldwork. We also acknowledge all the valuable support provided by the people in charge and inhabitants of the Mapimí Biosphere Reserve. Comments from S. Varela, M. Siqueira, and one anonymous reviewer greatly improve the manuscript, thanks very much.
Funding Statement
The Instituto de Ecología, A.C. and its Graduate Studies office provided support during the studies of CAUA, who was awarded a Doctorate Scholarship by the Mexican Council of Science and Technology CONACyT (No. 235792/213554). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brown JH. On the relationship between abundance and distribution of species. Am Nat. 1984;124: 255–279. 10.1086/284267 [DOI] [Google Scholar]
- 2. Brown JH, Mehlman DW, Stevens GC. Spatial variation in abundance. Ecology. 1995;76: 2028–2043. 10.2307/1941678 [DOI] [Google Scholar]
- 3. Gaston KJ, Blackburn TM, Greenwood JJD, Gregory RD, Quinn RM, Lawton JH. Abundance—occupancy relationships. J Appl Ecol. 2000;37: 39–59. 10.1046/j.1365-2664.2000.00485.x [DOI] [Google Scholar]
- 4. Guo Q, Brown JH, Valone TJ. Abundance and distribution of desert annuals: are spatial and temporal patterns related? J Ecol. 2000;88: 551–560. 10.1046/j.1365-2745.2000.00466.x [DOI] [Google Scholar]
- 5. Pearce J, Ferrier S. The practical value of modelling relative abundance of species for regional conservation planning: a case study. Biol Conserv. 2000;98: 33–43. 10.1016/S0006-3207(00)00139-7 [DOI] [Google Scholar]
- 6. Brown JH. Macroecology. Chicago, IL: University of Chicago Press; 1995. [Google Scholar]
- 7. Maurer BA, Villard MA. Geographic variation in abundance of North American birds. Res Explor. 1994;10: 306–317. [Google Scholar]
- 8. Maurer BA, Taper ML. Connecting geographical distributions with population processes. Ecol Lett. 2002;5: 223–231. 10.1046/j.1461-0248.2002.00308.x [DOI] [Google Scholar]
- 9. Lawton JH. Range, population abundance and conservation. Trends Ecol Evol. 1993;8: 409–413. 10.1016/0169-5347(93)90043-O [DOI] [PubMed] [Google Scholar]
- 10. Krabbe MB, Rahbek C. Causality of the relationship between geographic distribution and species abundance. Q Rev Biol. 2010;85: 31–53. 10.1086/650265 [DOI] [PubMed] [Google Scholar]
- 11. Maurer BA. Geographical population analysis: tools for the analysis of biodiversity. Oxford: Wiley-Blackwell; 1994. [Google Scholar]
- 12. Gaston KJ. Rarity. London: Springer; 1994. [Google Scholar]
- 13. Yu J, Dobson FS. Seven forms of rarity in mammals. J Biogeogr. 2000;27: 131–139. 10.1046/j.1365-2699.2000.00366.x [DOI] [Google Scholar]
- 14. Gaston KJ, Blackburn TM. Global scale macroecology: interactions between population size, geographic range size and body size in the Anseriformes. J Anim Ecol. 1996;65: 701–714. 10.2307/5669 [DOI] [Google Scholar]
- 15. Murray BR, Fonseca CR, Westoby M. The macroecology of Australian frogs. J Anim Ecol. 1998;67: 567–579. 10.1046/j.1365-2656.1998.00217.x [DOI] [Google Scholar]
- 16. Kunin WE. Extrapolating species abundance across spatial scales. Science. 1998;281: 1513–1515. 10.1126/science.281.5382.1513 [DOI] [PubMed] [Google Scholar]
- 17. He F, Gaston KJ. Estimating species abundance from occurrence. Am Nat. 2000;156: 553–559. 10.1086/303403 [DOI] [PubMed] [Google Scholar]
- 18. Martínez-Meyer E, Díaz-Porras D, Peterson AT, Yáñez-Arenas C. Ecological niche structure and rangewide abundance patterns of species. Biol Lett. 2013;9: 20120637 10.1098/rsbl.2012.0637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hutchinson G. Concluding remarks, Cold Spring Harbor Symposium. Quant Biol. 1957;22: 415–427. 10.1101/SQB.1957.022.01.039 [DOI] [Google Scholar]
- 20. Maguire B Jr. Niche response structure and the analytical potentials of its relationship to the habitat. Am Nat. 1973;107: 213–246. [Google Scholar]
- 21. Yáñez-Arenas C, Martínez-Meyer E, Mandujano S, Rojas-Soto O. Modelling geographic patterns of population density of the white-tailed deer in central Mexico by implementing ecological niche theory. Oikos. 2012;121: 2081–2089. 10.1111/j.1600-0706.2012.20350.x [DOI] [Google Scholar]
- 22. Auffenberg W. Tortoise behavior and survival Biological Sciences Curriculum Study, Patterns of Life Series. Chicago, IL: Rand McNally and Co; 1969. [Google Scholar]
- 23.SEMARNAT. Norma Oficial Mexicana NOM-059-SEMARNAT-2010. Protección ambiental. Especies nativas de México de flora y fauna silvestres—Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio—Lista de especies en riesgo. Diario Oficial de la Federación México. 30 Dec 2010. Available: http://www.profepa.gob.mx/innovaportal/file/435/1/NOM_059_SEMARNAT_2010.pdf. Accessed 24 Apr 2015.
- 24. Adest GA, Aguirre G, Morafka DJ, Jarchow JV. Bolson tortoise (Gopherus flavomarginatus) conservation. I. Life history. Vida Silvestre Neotropical. 1989;2:7–13. [Google Scholar]
- 25. Morafka DJ, Aguirre G, Adest GA. Gopherus flavomarginatus Bolson tortoise In: Swingland IR, Klemens MW, editors. The Conservation Biology of Tortoises. Switzerland: Occasional Paper of the IUCN Species Survival Comission 5; 1989. pp. 10–13. [Google Scholar]
- 26. González TR, Aguirre G. La tortuga del Bolsón, Gopherus flavomarginatus . Reptilia. 2006;62: 26–33. [Google Scholar]
- 27. Ureña-Aranda CA, Espinosa de los Monteros A. The genetic crisis of the Mexican Bolson tortoise (Gopherus flavomarginatus: Testudinidae). Amphibia-Reptilia. 2012;33: 45–53. 10.1163/156853811X621508 [DOI] [Google Scholar]
- 28. Lieberman SS, Morafka DJ. Part II. Ecological distribution of the Bolson tortoise. Ann Carnegie Mus. 1988;57: 31–46. [Google Scholar]
- 29. Bury RB, Morafka DJ, McCoy CJ. Part I. Distribution, Abundance and Status of the Bolson tortoise. Ann Carnegie Mus. 1988;57: 5–30. [Google Scholar]
- 30. Cornet AF, Delhoume JP, Montaña C. Dynamics of striped vegetation patterns and water balance in the Chihuahuan Desert In: During JJ, Werger MJA, Willems JH, editors. Diversity and pattern in land communities. The Hague: SPB Academic Publishing; 1988. pp. 221–231. [Google Scholar]
- 31. Rzedowski J. Vegetación de México. México: Limusa; 1978. [Google Scholar]
- 32. Morafka DJ. A historical biogeography of the Chihuahuan herpetofauna In: Morafka DJ, editor. A Biogeographical Analysis of the Chihuahuan Desert Through its Herpetofauna. Dordrecht, Netherlands: Springer; 1978. pp. 159–215. [Google Scholar]
- 33. Morafka DJ. Part III. Historical biogeography of the Bolson tortoise. Ann Carnegie Mus. 1988;57: 47–72. [Google Scholar]
- 34. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25: 1965–1978. 10.1002/joc.1276 [DOI] [Google Scholar]
- 35.US Geological Survey. HYDRO 1k elevation derivative database; 2001. Available: https://lta.cr.usgs.gov/HYDRO1K.
- 36. Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006;29: 129–151. 10.1111/j.2006.0906-7590.04596.x [DOI] [Google Scholar]
- 37. Lobo JM, Jiménez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecol Biogeog. 2008;17: 145–151. 10.1111/j.1466-8238.2007.00358.x [DOI] [Google Scholar]
- 38. Williams RAJ, Peterson AT. Ecology and geography of avian influenza (HPAI H5N1) transmission in the Middle East and northeastern Africa. Int J Health Geogr. 2009;8: 47–58. 10.1186/1476-072X-8-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barve N. Tool for Partial-ROC (Biodiversity Institute, Lawrence, KS), ver 1.0. 2008. [Google Scholar]
- 40. Rangel TF, Diniz-Fihlo JAF, Bini LM. SAM: a comprehensive application for Spatial Analysis in Macroecology. Ecography. 2010;33: 46–50. 10.1111/j.1600-0587.2009.06299.x [DOI] [Google Scholar]
- 41. Chefaoui RM, Hortal J, Lobo JM. Potential distribution modelling, niche characterization and conservation status assessment using GIS tools: a case study of Iberian Copris species. Biol Conserv. 2005;122: 327–338. 10.1016/j.biocon.2004.08.005 [DOI] [Google Scholar]
- 42. Martínez-Meyer E, Peterson AT, Servín JI, Kiff LF. Ecological niche modelling and prioritizing areas for species reintroductions. Oryx. 2006;40: 411–418. 10.1017/5003060530600 [DOI] [Google Scholar]
- 43. Jiménez-Valverde A, Diniz FA, de Azeved EB, Borges PAV. Species distribution models do not account for abundance: the case of arthropods on Terceira Island. Ann Zool Fennici. 2009;46: 451–464. 10.5735/086.046.0606 [DOI] [Google Scholar]
- 44. van der Wal J, Shoo LP, Johnson CN, Williams SE. Abundance and the environmental niche: environmental suitability estimated from niche models predicts the upper limit of local abundance. Am Nat. 2009;174: 282–291. 10.1086/600087 [DOI] [PubMed] [Google Scholar]
- 45. Oliver TH, Gillings S, Girardello M, Rapacciuolo G, Brereton TM, Siriwardena GM, et al. Population density but not stability can be predicted from species distribution models. J Appl Ecol. 2012;49: 581–590. 10.1111/j.1365-2664.2012.02138.x [DOI] [Google Scholar]
- 46. Tôrres NM, De Marco P, Santos T, Silveira L, de Almeida Jácomo AT, Diniz-Filho JAF. Can species distribution modelling provide estimates of population densities? A case study with jaguars in the Neotropics. Divers Distrib. 2012;18: 615–627. 10.1111/j.1472-4642.2012.00892.x [DOI] [Google Scholar]
- 47. Luoto M, Kuusaari M, Rita H, Salminen J, von Bonsdorff T. Determinants of distribution and abundance in the clouded Apollo butterfly: a landscape ecological approach. Ecography. 2001;24: 601–617. 10.1111/j.1600-0587.2001.tb00494.x [DOI] [Google Scholar]
- 48. Thuiller W, Albert CH, Dubuis A, Randin C, Guisan A. Variation in habitat suitability does not always relate to variation in species' plant functional traits. Biol Lett. 2010;6: 120–123. 10.1098/rsbl.2009.0669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Avery HW, Neibergs A. Effects of cattle grazing on the desert tortoise, (Gopherus agassizii): nutritional and behavioral interactions In: van Abbema J, editor. Conservation, Restoration, and Management of Tortoises and Turtles—An International Conference. New York: New York Turtle and Tortoise Society; 1997. pp. 13–20. [Google Scholar]
- 50. Kazmaier RT, Hellgren EC, Ruthven DC III, Synatzke DR. Effects of grazing on the demography and growth of the Texas tortoise. Conserv Biol. 2001;15: 1091–1101. 10.1046/j.1523-1739.2001.0150041091.x [DOI] [Google Scholar]
- 51. Boarman WI. The desert tortoise In: Boarman WI, Beaman K, editors. Sensitive animals and plants in the western Mojave Desert. Sacramento, CA: U.S. Geological Survey, Western Ecological Research Center; 2002. pp. 1–10. [Google Scholar]
- 52.Morafka DJ. The status and distribution of the Bolson tortoise (Gopherus flavomarginatus). In: Bury RB, editor. North American Tortoises: Conservation and Ecology. USDI, Fish and Wildlife Service. Wildlife Research Report 12; 1982. pp. 71–94.
- 53. Aguirre G. Conservation of the Bolson tortoise, Gopherus flavomarginatus In: Aguirre G, McCoy ED, Mushinsky H, Villagrán-Santa Cruz M, García-Collazo R, Casas-Andreu G, editors. Proceeding of North American Tortoise Conference. Sociedad Herpetológica Mexicana; 1995. pp. 6–9. [Google Scholar]
- 54. Araújo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Global Ecol Biogeogr. 2007;16: 743–753. 10.1111/j.1466-8238.2007.00359.x [DOI] [Google Scholar]
- 55. Heikkinen RK, Luoto M, Virkkala R, Pearson RG, Körber J. Biotic interactions improve prediction of boreal bird distributions at macro-scales. Global Ecol Biogeogr. 2007;16: 754–763. 10.1111/j.1466-8238.2007.00345.x [DOI] [Google Scholar]
- 56. Meier ES, Kienast F, Pearman PB, Svenning JC, Thuiller W, Araújo MB, et al. Biotic and abiotic variables show little redundancy in explaining tree species distributions. Ecography. 2010;33: 1038–1048. 10.1111/j.1600-0587.2010.06229.x [DOI] [Google Scholar]
- 57. Leathwick JR. Intra-generic competition among Nothofagus in New Zealand’s primary indigenous forests. Biodivers Conserv. 2002;11: 2177–2187. 10.1023/A:1021394628607 [DOI] [Google Scholar]
- 58. Tosh CA, Reyers B, van Jaarsveld AS. Estimating the abundances of large herbivores in the Kruger National Park using presence—absence data. Anim Conserv. 2004;7: 55–61. 10.1017/S1367943003001112 [DOI] [Google Scholar]
- 59. Figueiredo MSL, Grelle CEV. Predicting global abundance of a threatened species from its occurrence: implications for conservation planning. Divers Distrib. 2009;15: 117–121. 10.1111/j.1472-4642.2008.00525.x [DOI] [Google Scholar]
- 60. Hwang WH, He F. Estimating abundance from presence/absence maps. Methods Ecol Evol. 2011;2: 550–559. 10.1111/j.2041-210X.2011.00105.x [DOI] [Google Scholar]
- 61. Aguirre G, Morafka DJ, Adest GA . Conservation strategies for the Bolson tortoise, Gopherus flavomarginatus, in the Chihuahuan Desert In: van Abbema J, editor. Conservation, Restoration and Management of Tortoises and Turtles—An International Conference. New York: New York Turtle and Tortoise Society; 1997. pp. 333–338. [Google Scholar]
- 62. Treviño E, Morafka DJ, Aguirre G. A second reserve for the Bolson tortoise, Gopherus flavomarginatus, at Rancho Sombreretillo, Chihuahua, Mexico In: van Abbema J, editor. Conservation, Restoration and Management of Tortoises and Turtles—An International Conference. New York: New York Turtle and Tortoise Society; 1997. pp. 417–420. [Google Scholar]