FE may be a safe and feasible rehabilitation approach to augment recovery of motor and nonmotor function while improving aerobic fitness in people with chronic stroke.
MeSH TERMS: exercise therapy, motor skills, recovery of function, rehabilitation, stroke
Abstract
OBJECTIVE. Previously, we demonstrated that forced aerobic exercise (FE) increases the pattern of neural activation in Parkinson’s disease. We sought to evaluate whether FE, when coupled with repetitive task practice, could promote motor recovery poststroke.
METHOD. A 46-yr-old man with ischemic stroke exhibited chronic residual upper-extremity deficits, scoring 35/66 on the Fugl-Meyer Assessment (FMA) at baseline. He completed 24 training sessions comprising 45 min of FE on a motorized stationary bicycle followed by 45 min of upper-extremity repetitive task practice.
RESULTS. From baseline to end of treatment, the FMA score improved by 20 points, perceived level of recovery on the Stroke Impact Scale increased by 20 percentage points, and cardiovascular function measured by peak oxygen uptake improved 30%. These improvements persisted 4 wk after the intervention ceased.
CONCLUSION. FE may be a safe and feasible rehabilitation approach to augment recovery of motor and nonmotor function while improving aerobic fitness in people with chronic stroke.
Of the estimated 795,000 people in the United States who have a stroke each year, approximately 50% will continue to experience limitations in functional activities, activities of daily living (ADLs), and community reintegration for as many as 6 mo after the stroke, thus highlighting the need for continued innovation in effective stroke rehabilitation approaches (Go et al., 2014; Mayo, Wood-Dauphinee, Côté, Durcan, & Carlton, 2002). Although aerobic exercise has been shown to improve cardiovascular fitness in people with stroke (Stoller, de Bruin, Knols, & Hunt, 2012), its potential role in enhancing neuroplasticity and motor recovery after stroke has not been examined.
It is well accepted that motor recovery after stroke is achieved through cortical reorganization, in which the brain and central nervous system (CNS) adapt in response to environmental and behavioral change to acquire novel information by modifying neural connectivity and function (Knaepen, Goekint, Heyman, & Meeusen, 2010; Mang, Campbell, Ross, & Boyd, 2013). Although the exact mechanism for cortical reorganization is not known, neurotrophins are thought to play a major role by enabling neuronal survival, potentiation, and differentiation; promoting dendritic growth and remodeling; and promoting synaptic plasticity (Lin & Kuo, 2013; Voss, Nagamatsu, Liu-Ambrose, & Kramer, 2011).
Brain-derived neurotrophic factor (BDNF) is of particular interest in basic science and rehabilitation research because of its responsiveness to physical activity and exercise (Knaepen et al., 2010). The activity-dependent changes in the CNS and elevation of BDNF levels after acute bouts of aerobic exercise provide a theoretical rationale for exploiting the potential neurophysiologic effects of aerobic exercise as a means to promote motor recovery after stroke (Mang et al., 2013). Therefore, we developed a model to integrate aerobic exercise with task practice in an attempt to optimize motor recovery.
The American Heart Association recommends that people who have experienced a stroke participate in moderate to vigorous aerobic exercise 3–4 times per week for an average of 40 min (Kernan et al., 2014). However, residual neurological impairments have been shown to prevent people from exercising at their full aerobic potential (Billinger et al., 2014). We have overcome this barrier in people with Parkinson’s disease (PD) through the implementation of forced exercise (FE), an approach in which the voluntary efforts of people exercising on a stationary bicycle are augmented by a motor, allowing them to achieve and sustain an active pedaling rate greater than their voluntary rate (Alberts, Linder, Penko, Lowe, & Phillips, 2011; Ridgel, Vitek, & Alberts, 2009).
Although the motor is augmenting rate, the person is still actively contributing to exercise within a prescribed aerobic heart rate (HR) zone. When this mode of exercise was implemented in people with PD, significant improvements were noted in motor functioning, including rigidity, tremor, and bradykinesia as well as interlimb coordination and grasping force production (Alberts et al., 2011; Ridgel et al., 2009). These changes in global motor function, and the cortical and subcortical changes seen in functional connectivity magnetic resonance imaging, suggest that FE alters CNS processing (Alberts et al., 2011; Beall et al., 2013; Ridgel et al., 2009).
The successful implementation of FE in people with PD, coupled with a body of literature linking acute bouts of aerobic exercise training with activity-dependent changes in the CNS, warrants the investigation of the potential role of aerobic exercise in the recovery of motor function after stroke. In people with stroke, aerobic exercise may serve to prime the CNS and, if paired with well-selected motor training, has the potential to facilitate neuroplastic changes (Mang et al., 2013). Motor training tasks must be appropriately selected to be meaningful, intense, and task specific to create changes in the CNS (Bayona, Bitensky, Salter, & Teasell, 2005; Wolf, Blanton, Baer, Breshears, & Butler, 2002).
Repetitive task practice (RTP) is a well-studied rehabilitation intervention that implements these principles of motor learning and promotes cortical reorganization (Matsuo et al., 2013). The clinical rationale behind RTP is to maximize the intensity and dosage of task practice to facilitate motor learning. The role of the occupational or physical therapy practitioner who is administering RTP is to select and grade appropriately challenging functional tasks to meet each person’s abilities and goals. The aim of this case study is to describe the implementation of a FE paradigm coupled with RTP in a person with chronic stroke to improve motor and nonmotor function.
Method
Participant
The participant was an African-American man age 46 yr who incurred a left anterior cerebral artery stroke 10.5 mo before enrollment in the study. Important past medical history included hypertension, hyperlipidemia, obesity, and Type 2 diabetes mellitus controlled with oral medication. At the time of enrollment, he exhibited right upper-extremity (UE) and lower-extremity (LE) strength and coordination deficits, expressive aphasia, and deficits in executive functioning and memory. His right UE lacked end-range active range of motion (ROM) in the shoulder, elbow, wrist, and fingers with 3/5 strength throughout using manual muscle strength testing (Fan et al., 2010) His right LE exhibited full ROM and 4/5 strength, which resulted in a Trendelenburg gait deviation during right midstance, decreased step length, and diminished push off at preswing. He displayed no sensory deficits, and visual fields were intact.
Upon enrollment, the participant was residing in his own home and had part-time assistance from his brother. He was able to ambulate short distances in the community with a cane and reported activity limitations in household chores such as cooking and cleaning and difficulty with fine motor tasks. He acknowledged deficits in executive functioning and memory but had compensated with a well-designed reminder program on his smartphone.
The participant was selected for the FE program because of his potential for continued motor recovery, exhibition of motor control that would allow him to actively participate in a program of cycling and RTP, and motivation to improve. He had voluntary movement in his wrist and finger extensors, making him an excellent candidate for RTP (Wolf et al., 2006). In addition, he had no medical contraindications to cardiovascular exercise, and his primary care physician deemed him safe to undergo a cardiopulmonary exercise (CPX) test to determine his cardiovascular response to intensive aerobic exercise activity.
This study was part of a larger clinical trial approved by the institutional review board at the Cleveland Clinic (Cleveland, OH), and the participant completed the informed consent process.
Measures
The participant completed a battery of clinical testing to obtain his baseline cardiopulmonary function, motor function, mood, and quality of life. Outcomes used to measure UE motor function were the Wolf Motor Function Test (Morris, Uswatte, Crago, Cook, & Taub, 2001), including the Functional Ability and Performance Time scales; the Fugl-Meyer Assessment (FMA; Gladstone, Danells, & Black, 2002); and the Nine-Hole Peg Test(Chen, Chen, Hsueh, Huang, & Hsieh, 2009). Nonmotor function was assessed using the Center for Epidemiologic Studies Depression Scale (Shinar et al., 1986), the Stroke Impact Scale (SIS; Duncan et al., 1999), and the Trail Making Test Parts A and B (Tamez et al., 2011). Outcomes for cardiopulmonary function were a CPX test and the Six-Minute Walk Test (6MWT; Fulk, Echternach, Nof, & O’Sullivan, 2008). Motor and nonmotor outcomes are described in detail in the online supplemental materials (available at http://ajot.aotapress.net; navigate to this article, and click on “Supplemental Materials”). An occupational therapist who was blind to the intervention and was standardized in the administration of all outcome measures conducted all testing at baseline, end of treatment (EOT), and 4 wk after EOT (EOT+4).
The participant underwent CPX testing to determine baseline cardiorespiratory function and to evaluate his ability to safely participate in aerobic exercise training. A continuous incremental metabolic stress test protocol was used with a stationary bike beginning with a workload of 20 W and increasing by 20 W every 2 min. A 12-lead electrocardiogram and gas analysis to determine peak oxygen uptake (VO2peak) were continuously monitored. At baseline, the participant’s VO2peak was 13.7 mL/kg/min, or 33% of predicted value based on his age, weight, and gender. A cardiologist interpreted the participant’s CPX test and determined he was safe to start the exercise program.
Intervention
The participant attended training sessions 3 times/wk for 8 wk for a total of 24 sessions. Each intervention session began with 45 min of cycling that consisted of a 5-min warm-up, a 35-min main exercise set, and a 5-min cool down. A target HR zone of 105–120 beats per minute was calculated on the basis of his CPX results using the Karvonen formula (Swain, 2014). Cycling was performed on a stationary motor-driven bicycle that augmented the participant’s voluntary rate. The FE rate was 30% greater than the participant’s self-selected pedaling rate (in revolutions per minute [RPM]) during CPX testing. The 30% increase was selected on the basis of our experience with people with PD (Ridgel et al., 2009). The participant began at a forced rate of 70 RPM. In Session 4, he progressed to 75 RPM, and by Session 12, his cadence was 80 RPM, where he remained for the duration of the program.
An exercise physiologist, physical therapist, or occupational therapist supervised each cycling session and monitored the participant’s hemodynamic response. Blood pressure and rating of perceived exertion using the 10-point Borg Scale (Borg & Kaijser, 2006) were recorded at baseline, every 10 min during exercise, and immediately after exercise cessation. HR, power, and cadence were recorded every 5 min. Because of the participant’s history of Type 2 diabetes mellitus, blood glucose levels were monitored before and after the cycling session.
Within 15 min of cycling cessation, a 45-min session of UE RTP was administered under the guidance of a neurologically trained occupational therapist or physical therapist. Tasks were selected on the basis of the participant’s impairments and goals and were graded to increase difficulty as the therapist deemed appropriate. For example, the participant reported difficulty putting his dishes onto high shelves in his kitchen as a result of ROM, strength, and motor-planning deficits. He was given the RTP task of grasping the handle of a coffee cup on the counter, lifting the cup, reaching to an overhead shelf, placing the cup on the shelf, and then bringing the cup down to the counter. This task was graded by adding a 1-lb weight to the cup or by raising the height of the shelf. The therapist monitored movement quality, and the participant was cued to avoid compensatory strategies such as recruitment of the upper trapezius during shoulder elevation or lateral flexion of the trunk to counteract deltoid weakness. The therapist selected a variety of activities on the basis of the participant’s impairments and functional limitations. Several examples of tasks are provided in Table 1.
Table 1.
Examples of Repetitive Task Practice Tasks
| Impairment or Functional Limitation | Task | Grading of Task |
| Impaired wrist and intrinsic hand coordination limiting writing legibility | Writing on a lined whiteboard | Changing distance between lines to vary letter size |
| Decreased shoulder external rotation resulting in difficulty washing hair | Overhead throwing to a target, with an emphasis on external rotation during wind-up | Varying the distance of the target |
| Limitations in pronation and supination, making cooking tasks difficult | Transferring water from cup to cup | Varying amount of water in cup or varying distance between cups, requiring the participant to shift his weight to challenge limits of stability outside of his center of mass |
On average, each RTP session involved three to four different tasks with a focus on maximizing movement quality and repetitions during the 45-min session. Each task was repeated approximately 75–100 times, often divided into sets (i.e., 10 sets of 10 repetitions) that typically took 45–75 s to complete. Because this participant also had LE strength deficits that impaired his balance and gait, the therapist elected to have him perform the majority of the tasks while standing. Predominately blocked practice was used to facilitate learning of novel tasks and to maximize repetitions (Tanaka, Honda, Hanakawa, & Cohen, 2010).
Both explicit and implicit learning techniques were used to establish a balance between extrinsic guidance from the therapist and the participant’s intrinsic observations of task completion and movement quality (Halsband & Lange, 2006). Both strategies have been shown to have an important role in motor learning, because the brain’s presupplemental motor area is involved in explicit learning, whereas the supplemental motor area and parietal cortex are involved in implicit learning (Halsband & Lange, 2006).
When presenting a novel task to the participant, the therapist initially used extrinsic feedback based on knowledge of performance. As tasks advanced and sessions progressed, the therapist transitioned to a motor learning strategy focused on knowledge of results using the implicit learning approach (Subramanian, Massie, Malcolm, & Levin, 2010). An example of the progression of feedback is as follows: During a 60-s period, the participant was asked to grasp a standard spoon, use the spoon to pick up a round marble from a bowl, and transfer the marble to another bowl to simulate a cooking task. At the beginning of the intervention, the therapist provided feedback on the quality of movement by stating, “Turn the palm of your hand all the way down toward the bowl” to facilitate full pronation when transferring the marble. Once the participant understood the task and movement characteristics desired, extrinsic feedback was eliminated to allow for the intrinsic awareness of knowledge of results with the goal of facilitating self-awareness of task completion and movement quality.
Results
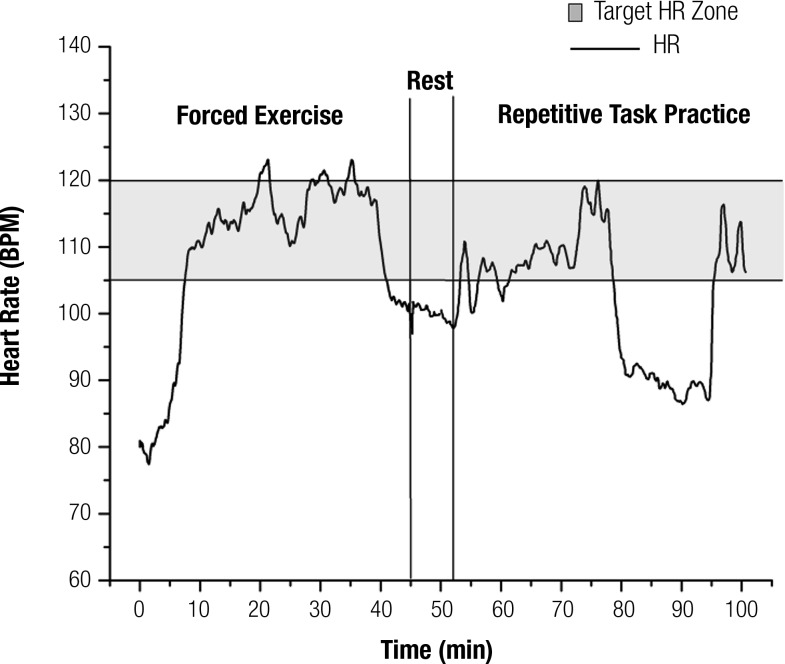
The participant completed all 24 intervention sessions without adverse event. During the cycling sessions, he was at or above his target HR range for 83% of the main 35-min session (5-min warm-up and 5-min cool-down excluded). Although not a primary goal of RTP, the participant’s cardiovascular exertion was observed to be elevated during RTP sessions; therefore, during Sessions 8–24, HR was continuously recorded using a Garmin™ (Olathe, KS) Edge® 800. The participant was in his target HR zone 46% of the time during RTP sessions and completed an average of 281 UE repetitions during each 45-min RTP session. An example of this participant’s HR response during a 45-min cycling session and a 45-min RTP session is displayed in Figure 1.
Figure 1.
Heart rate (HR) response during a representative 45-min session of forced exercise followed by a 45-min session of repetitive task practice (RTP). The participant was within or above his target HR zone (105–120) during 94% of this forced exercise session and 51% of this RTP session. Variable HR response during the RTP session is evident and was dependent on the mode of RTP administration (standing or sitting) and task difficulty.
Note. BPM = beats per minute.
The participant demonstrated improvement on all motor outcomes and all but one nonmotor outcome at EOT and maintained or improved at EOT+4 (Tables 2 and 3). Tables 2 and 3 also display minimal clinically important difference (MCID) and minimal detectable change (MDC; Fulk et al., 2008; Lang, Edwards, Birkenmeier, & Dromerick, 2008; Lin et al., 2009; Page, Fulk, & Boyne, 2012). The participant demonstrated a 20-point improvement on the FMA at EOT, achieving the MCID and MDC at EOT and EOT+4. In terms of cardiopulmonary measures, VO2peak improved by more than 4 mL/kg/min and the 6MWT improved by more than 43 m at EOT. For nonmotor outcomes, in four subsets of the SIS that may be more likely to change as a result of a motor intervention (i.e., ADLs, strength, mobility, UE use), the participant achieved MCID values in two subsets at EOT and in all four subsets at EOT+4 (Lin et al., 2010). His self-reported recovery from stroke improved from 70% at baseline to 90% at EOT and 95% at EOT+4.
Table 2.
Results of Motor Outcomes
| Measure | Baseline | EOT | EOT+4 |
| WMFT PTS, average s | 1.84 | 1.69 | 1.55 |
| WMFT FAS score | 4.53 | 4.93a,b | 4.93a,b |
| FMA score | 35 | 55a,b | 52a,b |
| NHPT, s | 35.6 | 32.3 | 30.2 |
| 6MWT, m | 367 | 410 | 408 |
| VO2peak, mL/kg/min | 13.7 | 17.8 | — |
Note. — = not tested; 6MWT = Six-Minute Walk Test; EOT = end of treatment; EOT+4 = 4 wk after end of treatment; FAS = Functional Ability scale; FMA = Fugl-Meyer Assessment; NHPT = Nine-Hole Peg Test; PTS = Performance Time scale; VO2peak = peak oxygen uptake; WMFT = Wolf Motor Function Test.
Change ≥ the minimal clinically important difference compared with baseline.
Change ≥ the minimal detectible change compared with baseline.
Table 3.
Results of Nonmotor Outcomes
| Measure | Baseline | EOT | EOT+4 |
| CES–D score | 5 | 6 | 3 |
| SIS score | |||
| ADLs | 85 | 85 | 100a |
| Strength | 56.3 | 89.3a,b | 87.5a,b |
| Mobility | 83.8 | 94.4a | 100a,b |
| UE use | 80 | 90 | 100a |
| Recovery from stroke, % | 70 | 90 | 95 |
| TMT, s | |||
| Part A | 53.12 | 50.87 | 51.67 |
| Part B | 168.52 | 81.51 | 91.97 |
Note. ADLs = activities of daily living; CES–D = Center for Epidemiologic Studies Depression Scale; EOT = end of treatment; EOT+4 = 4 wk after end of treatment; SIS = Stroke Impact Scale; TMT = Trail Making Test; UE = upper extremity.
Change ≥ the minimal clinically important difference compared with baseline.
Change ≥ the minimal detectible change compared with baseline.
Discussion
This participant with chronic stroke completed a total of 24 sessions of forced rate, high-intensity cycling coupled with RTP without adverse event. He improved in all motor outcomes and all but one nonmotor outcome from baseline to EOT, many of which met MCID and MDC values, and maintained or improved on most outcome measures at EOT+4.
Because intensity is an important variable in neurotrophin release (Knaepen et al., 2010), achieving and maintaining target HR was a key focus during cycling. This participant remained at or above his HR range 83% of the time. A challenge for people with neurologic impairment is difficulty exercising within an aerobic HR zone. Bateman and colleagues (2001) found that in a population of 78 people with brain injury, 9 were unable to achieve 60%–80% of their predicted HR zone. The remaining 69 participants spent an average of 56% of their exercise session in their target HR range. Billinger et al. (2012) found comparable results in a subacute stroke population with a target HR between 50% and 70% of peak HR, observing that these participants achieved their prescribed HR range 66% of the time.
We hypothesize that the participant in this case study successfully exercised for a substantial amount of time within his target HR zone because of the forced nature of the intervention. It is possible that the motor-driven stationary bike provided increased intrinsic and extrinsic feedback to overcome his decreased cortical and motor output, allowing him to achieve and maintain exercise intensity greater than what has previously been reported in patients with stroke (Bateman et al., 2001; Billinger et al., 2012).
Interestingly, in addition to sustaining his HR range during the cycling intervention, the participant also was within or above his target HR zone for approximately 46% of his 45-min RTP sessions. To the best of our knowledge, HR during an RTP session has not been formally monitored and reported previously in clinical research. However, as part of an observational study performed in a clinical setting, it was noted that patients achieved 40%–85% of their predicted maximum HR for approximately 5% of their physical therapy session (mean [M] = 2.8 min, standard deviation [SD] = 0.9 min) and 2% of their occupational therapy session (M = 0.7 min, SD = 0.2 min; MacKay-Lyons & Makrides, 2002). These observations indicate that little physical exertion occurs during traditional therapy. During this participant’s RTP sessions, an average of 281 repetitions were completed over a 45-min period, predominately while standing and with minimal rest between sets and activities, which may have contributed to his elevated cardiovascular response.
It has been reported that only 25% of people with stroke return to a level of participation and physical functioning comparable to that of community-matched peers without stroke (Dobkin, 2005). Decreased physical activity level poststroke causes a decline in VO2peak as low as 50%–70% of the age- and gender-matched value in sedentary people (Dobkin, 2008; Eng, Dawson, & Chu, 2004; MacKay-Lyons & Makrides, 2004). The participant in this study improved his VO2peak by 30% during the 8-wk intervention. This improvement is on the high end of what has been reported in the literature, which ranges from 9.0% to 34.8% in improvement in VO2peak after aerobic training (Pang, Eng, Dawson, & Gylfadóttir, 2006).
Notably, even with a 30% improvement in VO2peak, the participant’s exercise capacity was less than the 20.1 mL/kg/min reported as a threshold for functional performance in older adults (Cress & Meyer, 2003). In a recent article examining clinical outcomes in people with heart failure, a 6% increase in VO2peak was associated with a 5% decrease in mortality and hospitalization (Swank et al., 2012). Because cardiovascular risk factors and stroke are closely linked (Gordon et al., 2004), a 30% improvement may be meaningful in reducing risk of recurrent stroke.
Implications for Occupational Therapy Practice
The results of this case study have the following implications for occupational therapy practice:
Aerobic exercise is an important component of rehabilitation after stroke for cardiorespiratory fitness; however, its effect on brain health after stroke has not been established.
A FE cycling intervention was able to overcome physical barriers to achieve sustained, high-intensity aerobic exercise in the study participant after stroke.
Forty-five minutes of FE cycling followed by 45 min of RTP was sufficient to produce clinically meaningful results on motor and nonmotor outcomes in the study participant.
Conclusion
The development of a rehabilitative intervention for people with stroke that facilitates motor recovery while simultaneously decreasing cardiovascular risk factors would be a valuable adjunct to current stroke rehabilitation approaches. The dosage of 45 min of cycling followed within 15 min by 45 min of RTP is feasible in a clinical environment and was sufficient to facilitate improvements for the participant in this case study. However, this was a single-participant case study, and further research with more participants and a control group is currently under way to gauge the generalizability of this intervention. Moreover, although we provide a theoretical framework for the neurophysiological mechanism associated with the neuroplastic effects of aerobic exercise, only clinical outcomes were obtained in this case study. Future research, including neuroimaging to complement clinical outcomes and more precisely determine the mechanisms associated with motor recovery is necessary. Nevertheless, the motor and nonmotor improvements achieved by this participant suggest that the intervention may be safe, feasible, and efficacious in a chronic stroke population.
Acknowledgments
The authors thank the following for their support of the study: Cindy Clark for participant recruitment and evaluation, Amanda Penko for exercise monitoring, and Andrew Bazyk for support with data sorting and analysis. The Research Electronic Data Capture© database was used to record and store data for this study. This study was supported by National Institutes of Health (NIH) Award No. R03HD073566 from the National Institute of Neurological Disorders and Stroke (NINDS). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or NINDS. This trial is listed at ClinicalTrials.gov registration number NCT02076776.
Contributor Information
Susan M. Linder, Susan M. Linder, PT, DPT, NCS, is Research Scientist, Department of Biomedical Engineering, Cleveland Clinic, Cleveland, OH; linders@ccf.org
Anson B. Rosenfeldt, Anson B. Rosenfeldt, PT, DPT, MBA, is Senior Physical Therapist, Department of Biomedical Engineering, Cleveland Clinic, Cleveland, OH
Matthew Rasanow, Matthew Rasanow is Research Assistant, Department of Biomedical Engineering, Cleveland Clinic, Cleveland, OH.
Jay L. Alberts, Jay L. Alberts, PhD, is Staff, Department of Biomedical Engineering, Cleveland Clinic, and Investigator, Cleveland FES Center, L. Stokes Cleveland VA Medical Center, Cleveland, OH
References
- Alberts J. L., Linder S. M., Penko A. L., Lowe M. J., & Phillips M. (2011). It is not about the bike, it is about the pedaling: Forced exercise and Parkinson’s disease. Exercise and Sport Sciences Reviews, 39, 177–186. http://dx.doi.org/10.1097/JES.0b013e31822cc71a [DOI] [PubMed] [Google Scholar]
- Bateman A., Culpan F. J., Pickering A. D., Powell J. H., Scott O. M., & Greenwood R. J. (2001). The effect of aerobic training on rehabilitation outcomes after recent severe brain injury: A randomized controlled evaluation. Archives of Physical Medicine and Rehabilitation, 82, 174–182. http://dx.doi.org/10.1053/apmr.2001.19744 [DOI] [PubMed] [Google Scholar]
- Bayona N. A., Bitensky J., Salter K., & Teasell R. (2005). The role of task-specific training in rehabilitation therapies. Topics in Stroke Rehabilitation, 12, 58–65. http://dx.doi.org/10.1310/BQM5-6YGB-MVJ5-WVCR [DOI] [PubMed] [Google Scholar]
- Beall E. B., Lowe M. J., Alberts J. L., Frankemolle A. M., Thota A. K., Shah C., & Phillips M. D. (2013). The effect of forced-exercise therapy for Parkinson’s disease on motor cortex functional connectivity. Brain Connect, 3, 190–198. http://dx.doi.org/10.1089/brain.2012.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billinger S. A., Arena R., Bernhardt J., Eng J. J., Franklin B. A., Johnson C. M., . . . Tang A.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Clinical Cardiology. (2014). Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 45, 2532–2553. http://dx.doi.org/10.1161/STR.0000000000000022 [DOI] [PubMed] [Google Scholar]
- Billinger S. A., Mattlage A. E., Ashenden A. L., Lentz A. A., Harter G., & Rippee M. A. (2012). Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. Journal of Neurologic Physical Therapy, 36, 159–165. http://dx.doi.org/10.1097/NPT.0b013e318274d082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg E., & Kaijser L. (2006). A comparison between three rating scales for perceived exertion and two different work tests. Scandinavian Journal of Medicine and Science in Sports, 16, 57–69. http://dx.doi.org/10.1111/j.1600-0838.2005.00448.x [DOI] [PubMed] [Google Scholar]
- Chen H. M., Chen C. C., Hsueh I. P., Huang S. L., & Hsieh C. L. (2009). Test–retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabilitation and Neural Repair, 23, 435-440. http://dx.doi.org/10.1177/1545968308331146 [DOI] [PubMed] [Google Scholar]
- Cress M. E., & Meyer M. (2003). Maximal voluntary and functional performance levels needed for independence in adults aged 65 to 97 years. Physical Therapy, 83, 37–48. [PubMed] [Google Scholar]
- Dobkin B. H. (2005). Clinical practice: Rehabilitation after stroke. New England Journal of Medicine, 352, 1677–1684. http://dx.doi.org/10.1056/NEJMcp043511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin B. H. (2008). Training and exercise to drive poststroke recovery. Neurology, 4, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan P. W., Wallace D., Lai S. M., Johnson D., Embretson S., & Laster L. J. (1999). The Stroke Impact Scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke, 30, 2131–2140. http://dx.doi.org/10.1161/01.STR.30.10.2131 [DOI] [PubMed] [Google Scholar]
- Eng J. J., Dawson A. S., & Chu K. S. (2004). Submaximal exercise in persons with stroke: Test–retest reliability and concurrent validity with maximal oxygen consumption. Archives of Physical Medicine and Rehabilitation, 85, 113–118. http://dx.doi.org/10.1016/S0003-9993(03)00436-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan E., Ciesla N. D., Truong A. D., Bhoopathi V., Zeger S. L., & Needham D. M. (2010). Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Medicine, 36, 1038–1043. http://dx.doi.org/10.1007/s00134-010-1796-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulk G. D., Echternach J. L., Nof L., & O’Sullivan S. (2008). Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke. Physiotherapy Theory and Practice, 24, 195–204. http://dx.doi.org/10.1080/09593980701588284 [DOI] [PubMed] [Google Scholar]
- Gladstone D. J., Danells C. J., & Black S. E. (2002). The Fugl-Meyer Assessment of Motor Recovery after stroke: A critical review of its measurement properties. Neurorehabilitation and Neural Repair, 16, 232–240. http://dx.doi.org/10.1177/154596802401105171 [DOI] [PubMed] [Google Scholar]
- Go A. S., Mozaffarian D., Roger V. L., Benjamin E. J., Berry J. D., Blaha M. J., . . . Turner M. B.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. (2014). Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation, 129, e28–e292. http://dx.doi.org/10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N. F., Gulanick M., Costa F., Fletcher G., Franklin B. A., Roth E. J., & Shephard T.; American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; Council on Cardiovascular Nursing; Council on Nutrition, Physical Activity, and Metabolism; Stroke Council. (2004). Physical activity and exercise recommendations for stroke survivors: An American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation, 109, 2031–2041. http://dx.doi.org/10.1161/01.CIR.0000126280.65777.A4 [DOI] [PubMed] [Google Scholar]
- Halsband U., & Lange R. K. (2006). Motor learning in man: A review of functional and clinical studies. Journal of Physiology, 99, 414–424. http://dx.doi.org/10.1016/j.jphysparis.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Kernan W. N., Ovbiagele B., Black H. R., Bravata D. M., Chimowitz M. I., Ezekowitz M. D., . . . Wilson J. A.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Peripheral Vascular Disease. (2014). Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 45, 2160–2236. http://dx.doi.org/10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- Knaepen K., Goekint M., Heyman E. M., & Meeusen R. (2010). Neuroplasticity—exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Medicine, 40, 765–801. http://dx.doi.org/10.2165/11534530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Lang C. E., Edwards D. F., Birkenmeier R. L., & Dromerick A. W. (2008). Estimating minimal clinically important differences of upper-extremity measures early after stroke. Archives of Physical Medicine and Rehabilitation, 89, 1693–1700. http://dx.doi.org/10.1016/j.apmr.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K. C., Fu T., Wu C. Y., Wang Y. H., Liu J. S., Hsieh C. J., & Lin S. F. (2010). Minimal detectable change and clinically important difference of the Stroke Impact Scale in stroke patients. Neurorehabilitation and Neural Repair, 24, 486–492. http://dx.doi.org/10.1177/1545968309356295 [DOI] [PubMed] [Google Scholar]
- Lin K. C., Hsieh Y. W., Wu C. Y., Chen C. L., Jang Y., & Liu J. S. (2009). Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Neurorehabilitation and Neural Repair, 23, 429–434. http://dx.doi.org/10.1177/1545968308331144 [DOI] [PubMed] [Google Scholar]
- Lin T. W., & Kuo Y. M. (2013). Exercise benefits brain function: The monoamine connection. Brain Science, 3, 39–53. http://dx.doi.org/10.3390/brainsci3010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay-Lyons M. J., & Makrides L. (2002). Cardiovascular stress during a contemporary stroke rehabilitation program: Is the intensity adequate to induce a training effect? Archives of Physical Medicine and Rehabilitation, 83, 1378–1383. http://dx.doi.org/10.1053/apmr.2002.35089 [DOI] [PubMed] [Google Scholar]
- MacKay-Lyons M. J., & Makrides L. (2004). Longitudinal changes in exercise capacity after stroke. Archives of Physical Medicine and Rehabilitation, 85, 1608–1612. http://dx.doi.org/10.1016/j.apmr.2004.01.027 [DOI] [PubMed] [Google Scholar]
- Mang C. S., Campbell K. L., Ross C. J., & Boyd L. A. (2013). Promoting neuroplasticity for motor rehabilitation after stroke: Considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Physical Therapy, 93, 1707–1716. http://dx.doi.org/10.2522/ptj.20130053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo J., Nakamura S., Ito A., Yamazaki T., Ishida K., Hayashi Y., . . . Yamaguchi H. (2013). Protochlamydia induces apoptosis of human HEp-2 cells through mitochondrial dysfunction mediated by chlamydial protease-like activity factor. PLoS ONE, 8, e5600 http://dx.doi.org/10.1371/journal.pone.0056005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo N. E., Wood-Dauphinee S., Côté R., Durcan L., & Carlton J. (2002). Activity, participation, and quality of life 6 months poststroke. Archives of Physical Medicine and Rehabilitation, 83, 1035–1042. http://dx.doi.org/10.1053/apmr.2002.33984 [DOI] [PubMed] [Google Scholar]
- Morris D. M., Uswatte G., Crago J. E., Cook E. W. 3rd, & Taub E. (2001). The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Archives of Physical Medicine and Rehabilitation, 82, 750–755. http://dx.doi.org/10.1053/apmr.2001.23183 [DOI] [PubMed] [Google Scholar]
- Page S. J., Fulk G. D., & Boyne P. (2012). Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Physical Therapy, 92, 791–798. http://dx.doi.org/10.2522/ptj.20110009 [DOI] [PubMed] [Google Scholar]
- Pang M. Y., Eng J. J., Dawson A. S., & Gylfadóttir S. (2006). The use of aerobic exercise training in improving aerobic capacity in individuals with stroke: A meta-analysis. Clinical Rehabilitation, 20, 97–111. http://dx.doi.org/10.1191/0269215506cr926oa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgel A. L., Vitek J. L., & Alberts J. L. (2009). Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabilitation and Neural Repair, 23, 600–608. http://dx.doi.org/10.1177/1545968308328726 [DOI] [PubMed] [Google Scholar]
- Shinar D., Gross C. R., Price T. R., Banko M., Bolduc P. L., & Robinson R. G. (1986). Screening for depression in stroke patients: The reliability and validity of the Center for Epidemiologic Studies Depression Scale. Stroke, 17, 241–245. [DOI] [PubMed] [Google Scholar]
- Stoller O., de Bruin E. D., Knols R. H., & Hunt K. J. (2012). Effects of cardiovascular exercise early after stroke: Systematic review and meta-analysis. BMC Neurology, 12, 45 http://dx.doi.org/10.1186/1471-2377-12-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S. K., Massie C. L., Malcolm M. P., & Levin M. F. (2010). Does provision of extrinsic feedback result in improved motor learning in the upper limb poststroke? A systematic review of the evidence. Neurorehabilitation and Neural Repair, 24, 113–124. http://dx.doi.org/10.1177/1545968309349941 [DOI] [PubMed] [Google Scholar]
- Swain D. P. (Ed.). (2014). ACSM’s resource manual for guidelines for exercise testing and prescription (7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.
- Swank A. M., Horton J., Fleg J. L., Fonarow G. C., Keteyian S., Goldberg L., . . . Kraus W. E.; HF-ACTION Investigators. (2012). Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: Results from heart failure and a controlled trial to investigate outcomes of exercise training. Circulation: Heart Failure, 5, 579–585. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.111.965186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamez E., Myerson J., Morris L., White D. A., Baum C., & Connor L. T. (2011). Assessing executive abilities following acute stroke with the Trail Making Test and Digit Span. Behavioral Neurology, 24, 177–185. http://dx.doi.org/10.3233/BEN-2011-0328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Honda M., Hanakawa T., & Cohen L. G. (2010). Differential contribution of the supplementary motor area to stabilization of a procedural motor skill acquired through different practice schedules. Cerebral Cortex, 20, 2114–2121. 10.1093/cercor/bhp276. http://dx.doi.org/10.1093/cercor/bhp276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M. W., Nagamatsu L. S., Liu-Ambrose T., & Kramer A. F. (2011). Exercise, brain, and cognition across the life span. Journal of Applied Physiology, 111, 1505–1513. http://dx.doi.org/10.1152/japplphysiol.00210.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. L., Blanton S., Baer H., Breshears J., & Butler A. J. (2002). Repetitive task practice: A critical review of constraint-induced movement therapy in stroke. Neurologist, 8, 325–338. http://dx.doi.org/10.1097/01.nrl.0000031014.85777.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. L., Winstein C. J., Miller J. P., Taub E., Uswatte G., Morris D., . . . Nichols-Larsen D.; EXCITE Investigators. (2006). Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA, 296, 2095–2104. http://dx.doi.org/10.1001/jama.296.17.2095 [DOI] [PubMed] [Google Scholar]