Abstract
Understanding the signals used by plants to attract seed disperses is a pervasive quest in evolutionary and sensory biology. Fruit size, colour, and odour variation have long been discussed in the controversial context of dispersal syndromes targeting olfactory-oriented versus visually-oriented foragers. Trade-offs in signal investment could impose important physiological constraints on plants, yet have been largely ignored. Here, we measure the reflectance and volatile organic compounds of a community of Malagasy plants and our results indicate that extant plant signals may represent a trade-off between olfactory and chromatic signals. Blue pigments are the most visually-effective – blue is a colour that is visually salient to all known seed dispersing animals within the study system. Additionally, plants with blue-reflecting fruits are less odiferous than plants that reflect primarily in other regions of the colour spectrum.
Introduction
The physical properties of fruits can act as signals of edibility and nutrition to the seed dispersing animals that play a crucial role in moving seeds away from the parent tree and, thus, increasing plant fitness [1,2,3,4,5]. Numerous studies have demonstrated that plant signals and cues are critical to fruit selection by animals [6,7,8,9]. While ripe fruit signals refer to traits such as colour and odour that are maintained by natural selection because of their ability to reliably convey information to other organisms [10], ripe fruit cues refer to traits that evolved in a context unrelated to animal signalling that may nonetheless convey reliable information to dispersers [11]. Plant signals and cues take a multitude of forms, including fruit chromaticity, odour, and size [7,12,13,14], and have been shown to reliably advertise fruit nutrient content to dispersers [15]. Given variation in disperser sensory abilities, including colour vision and olfactory ability, fruit signals and cues may result in trade-offs between fruit colour and odour signals.
The fruit syndrome hypothesis posits that suites of fruit signals should be directed at the frugivorous guilds that provide the highest quality seed dispersal service, according to their capacity to receive signals [14]. The fruit traits of a given species are, at least in part, predicted to be the subset of the fruit phenotype spectrum reflecting the selective pressures exerted by beneficial seed dispersers [16,17]. One of the most compelling dichotomies in such selective pressures is the conflict between olfactory and chromatic signals. Plants can signal fruit presence and ripeness through visual and olfactory channels [18]. In systems where beneficial dispersers rely variously on visual and olfactory signals, there may be a selective tension imposed on plants. Plants may produce olfactory signals to increase detection by olfactory-driven foragers (e.g., nocturnal and dichromatic mammals), or visual signals to attract visually-oriented foragers (e.g., diurnal avian frugivores). In systems with mixed animal disperser assemblages that include both olfactory and visually oriented frugivores, plant signals may thus represent a tradeoff between fruit colour and odour.
The fruit syndrome hypothesis has intuitive appeal and support for the colour-odour trade-off has been shown to exist for bird- versus bat-dispersed species [14] and in multiple taxon-specific studies [19,20,21]. However, this topic is heavily debated because most fruits are dispersed by multiple taxa possessing diverse sensory phenotypes [16,22,23]. As such, specialized fruit traits targeting a restricted set of seed dispersers may be selected against [4] or result in limited fruit diversification, and therefore be limited to relatively few plant taxa [24,25]. Finally, convergence of fruit traits among different phylogenetically diverse plant species dispersed by different frugivorous guilds has been argued to refute the hypothesis that specific frugivore species are driving the evolution of fruit morphology and generating syndromes [16,26]. Alternately, fruit trait convergence may result from phylogenetic constraint [27].
Our study aims to help clarify this debate by approaching the fruit syndrome hypothesis using a quantitative and multivariate approach. For the fruit syndrome hypothesis to be supported, there should be a negative relationship between chromatically conspicuous fruits and odiferous fruits, i.e., fruits that invest in colour should not invest in odour, due to the predicted trade-off between attracting olfactory-driven versus visually oriented foragers.
Here, we evaluate the fruit syndrome hypothesis by testing whether fruits invest in specific signalling strategies at the expense of others. To evaluate potential phylogenetic constraint on these strategies, we first test for the presence of a phylogenetic structure among traits using a species-level phylogeny. We examine chromatic (visual) and odour (olfactory) signals in an analysis of 56 endemic wild fruit species in a tropical dry forest in Madagascar. We predict that fruits that invest in pigment production in non-photosynthetically active regions of the chromatic spectrum will not invest heavily in odour production. Additionally, because plants with a higher surface-area-to-weight ratio (i.e., smaller fruits) produce more signals relative to overall fruit production (i.e. while the magnitude of signal production may be similar to that produced by larger fruits, the amount of signal relative to the amount of fruit produced will be greater) we predict that the relationship between fruit colour and odour will be altered by surface-area-to-weight ratio of fruits.
Materials and Methods
Colour and odour quantification
Ethics statement—N/A. Permission for fieldwork granted by Madagascar National Parks and the Government of Madagascar (permit number: 092N_EA07/MG12). Between January and December, 2012, between 5–10 ripe fruits of each species were opportunistically collected in the Ampijoroa region of Ankarafantsika National Park, Madagascar (15' '59' -16°22S, 47''56'-47''12E). Ripe fruits of each species were collected directly from trees and all analyses were performed within ~2 hours of fruit collection. Fruit ripeness was determined based on colour, odour, and hardness and confirmed after analysis based on the presence of viable seeds. Seeds were considered viable if they were fully formed, had the approximate mass of seeds known to germinate, and had no evidence of damage. Plants were identified to genus and species using a published tree flora [28] and an unpublished photographic database of the plants of the national park (Sato, pers comm). All known genera were assigned to family using a published tree flora [28] and assigned an order based on the APG III classification [29]. In total, the analysis included species belonging to 19 families and 10 orders (Table 1). In cases where it was not possible to identify plants to the genus level, they were identified either by their local Malagasy name, or categorized as unknown, each of which was given a unique number. Ripe fruits were weighed using a digital scale and measured in three dimensions using calipers.
Table 1. Fruit traits of sampled species including surface-area-scaled VOC sum, percent reflectances per band normalized by brightness, wavelength of peak reflectance in nm (λmax; peak hue), peak reflectance value (brightness), surface area:weight ratios, and number of fruits sampled.
| Species | Family | Order | VOC | UV Reflectance | Blue Reflectance | Green Reflectance | Red Reflectance | λmax | Peak Brightness | Area to Weight | N Fruits |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Androstachys spp | Euphorbiaceae | Malpighiales | 1.16 | 4.14 | 24.12 | 37.66 | 38.21 | 655 | 0.32 | 318.48 | 10 |
| Antidesma petiolare | Euphorbiaceae | Malpighiales | 34.34 | 1.99 | 13.01 | 46.27 | 40.72 | 700 | 0.34 | 849.49 | 5 |
| Asterotrichilia asterotricha | Meliaceae | Sapindales | 1.92 | 12.39 | 17.99 | 51.87 | 30.15 | 557 | 0.18 | 229.67 | 5 |
| Asterotrichilia spp | Meliaceae | Sapindales | 3.97 | 18.81 | 18.05 | 57.11 | 24.83 | 565 | 0.23 | 173.84 | 5 |
| Asterotrichilia marina | Meliaceae | Sapindales | 1.78 | 29.71 | 25.09 | 47.06 | 27.85 | 553 | 0.33 | 279.42 | 5 |
| Badouinia fluggeiformis | Fabaceae | Fabales | 5.22 | 8.7 | 11.63 | 59.83 | 28.54 | 700 | 0.4 | 373.06 | 5 |
| Baudouinia spp | Fabaceae | Fabales | 5.26 | 8.7 | 11.63 | 59.83 | 28.54 | 655 | 0.33 | 548.61 | 5 |
| Berchemia discolor | Rhamnaceae | Rosales | 4.51 | 0.19 | 0 | 95.86 | 4.14 | 562 | 0.24 | 350.79 | 5 |
| Bridellia pervilleana | Euphorbiaceae | Malpighiales | 5.86 | 2.75 | 1.95 | 59.98 | 38.07 | 691 | 0.09 | 662.4 | 10 |
| Croton spp | Euphorbiaceae | Malpighiales | 7.58 | 11.76 | 6.34 | 62.38 | 31.27 | 668 | 0.06 | 1291.5 | 10 |
| Croton spp 2 | Euphorbiaceae | Malpighiales | 4.98 | 7.59 | 11.85 | 35.71 | 52.44 | 684 | 0.43 | 1340.7 | 10 |
| Elaeocarpus subserratus | Elaeocarpaceae | Malvales | 38.8 | 20.1 | 18.36 | 48.64 | 33 | 698 | 0.41 | 532.14 | 5 |
| Empogona ovalifolia | Rubiaceae | Gentianales | 10.3 | 15.58 | 11.02 | 60.39 | 28.59 | 700 | 0.21 | 720.51 | 5 |
| Gaertnera spp | Rubiaceae | Gentianales | 2.75 | 3.98 | 2.13 | 64.58 | 33.28 | 561 | 0.12 | 630.8 | 10 |
| Garcinia arenicola | Clusiaceae | Malpighiales | 1.28 | 7.89 | 8.37 | 56.41 | 35.23 | 698 | 0.12 | 255 | 5 |
| Gardenia rutenbergiana | Rubiaceae | Gentianales | 5.34 | 10.52 | 11.11 | 59.58 | 29.31 | 700 | 0.18 | 181.83 | 5 |
| Grangeria spp | Chyrsobalanaceae | Rosales | 9 | 3.79 | 9.41 | 45.44 | 45.15 | 699 | 0.34 | 429.01 | 5 |
| Grewia madagascariensis | Malvaceae | Malvales | 13.74 | 1.66 | 8.98 | 37.81 | 53.2 | 700 | 0.62 | 490.3 | 5 |
| Grewia triflora | Malvaceae | Malvales | 24.18 | 7.55 | 7.42 | 50.27 | 42.32 | 564 | 0.14 | 638.07 | 10 |
| Landolphia myrtifolia | Apocynaceae | Gentianales | 0.22 | 5.4 | 10.6 | 60.31 | 29.09 | 700 | 0.34 | 113.22 | 5 |
| Mapouria boinensis | Rubiaceae | Gentianales | 10.95 | 7.62 | 5.35 | 47.46 | 47.2 | 699 | 0.32 | 478.6 | 8 |
| Mapouria spp | Rubiaceae | Gentianales | 15.86 | 5.96 | 7.63 | 54.72 | 37.65 | 700 | 0.51 | 469.4 | 10 |
| Monanthotaxis valida | Annonaceae | Magnoliales | 10.93 | 5.5 | 9.08 | 56.44 | 34.48 | 700 | 0.6 | 410.89 | 5 |
| Noronhia spp | Oleaceae | Lamiales | 38.91 | 6.51 | 12.96 | 57.33 | 29.72 | 700 | 0.36 | 466.56 | 5 |
| Petchia spp | Apocynaceae | Gentianales | 1.79 | 20.67 | 26.28 | 35.2 | 38.52 | 669 | 0.84 | 1964.4 | 5 |
| Rothmania renniformis | Rubiaceae | Gentianales | 2.56 | 15.49 | 13.02 | 57.05 | 29.93 | 646 | 0.11 | 242.75 | 5 |
| Rourea orientalis | Connaraceae | Rosales | 4.22 | 15.46 | 17.66 | 52.64 | 29.7 | 692 | 0.49 | 506.1 | 5 |
| Salvadora augustifolia | Salvadoraceae | Brassicales | 1.98 | 13.44 | 15.15 | 44.4 | 40.44 | 563 | 0.31 | 758.21 | 5 |
| Sorindeia madagascariensis | Anacardiaceae | Sapindales | 1.39 | 11.59 | 19.83 | 37.5 | 42.67 | 631 | 0.21 | 244.17 | 6 |
| Strychnos decussata | Loganiaceae | Gentianales | 3.08 | 8.55 | 11.62 | 56.03 | 32.35 | 700 | 0.44 | 247.57 | 5 |
| Strychnos madagascariensis | Loganiaceae | Gentianales | 3.84 | 7.25 | 9.85 | 64.61 | 25.54 | 700 | 0.46 | 153.01 | 6 |
| Strychnos spp. | Loganiaceae | Gentianales | 4.21 | 2.66 | 6.04 | 65.22 | 28.75 | 697 | 0.62 | 240.62 | 5 |
| Strychnos myrtoides | Loganiaceae | Gentianales | 4.16 | 7.09 | 13.82 | 57.22 | 28.96 | 661 | 0.19 | 493.08 | 5 |
| Strychnos spinosa | Loganiaceae | Gentianales | 0.25 | 4.93 | 10.59 | 46.77 | 42.65 | 699 | 0.48 | 66.61 | 5 |
| Tabernaemontana coffeeoides | Apocynaceae | Gentianales | 2.52 | 5.43 | 27.41 | 35.49 | 37.1 | 571 | 0.43 | 325.88 | 5 |
| Terminalia trophophylla | Combretaceae | Myrtales | 23.3 | 7.44 | 8.65 | 59.68 | 31.67 | 551 | 0.17 | 1047.7 | 5 |
| Tina isaloensis | Sapindaceae | Sapindales | 2.26 | 58.39 | 32.85 | 28.59 | 38.56 | 692 | 0.3 | 1200.6 | 5 |
| Tricalysia perrieri | Rubiaceae | Gentianales | 15.75 | 12.92 | 13.03 | 59 | 27.96 | 697 | 0.21 | 614.93 | 10 |
| UK Species 1 | UK | UK | 4.59 | 14.74 | 15.7 | 56.12 | 28.19 | 678 | 0.1 | 547.35 | 10 |
| UK Species 2 | UK | UK | 13.95 | 16.85 | 13.47 | 51.04 | 35.49 | 638 | 0.21 | 157.38 | 5 |
| UK Species 3 | UK | UK | 6.45 | 31.96 | 24.97 | 34.21 | 40.82 | 679 | 0.5 | 470.76 | 5 |
| UK Species 4 | UK | UK | 0.5 | 12.02 | 11.76 | 58.25 | 29.99 | 697 | 0.39 | 123.83 | 5 |
| UK Species 5 | UK | UK | 9.73 | 5.96 | 1.11 | 66.82 | 32.08 | 568 | 0.29 | 884.36 | 10 |
| UK Species 6 | UK | UK | 23.25 | 23.04 | 4.85 | 71.76 | 23.39 | 567 | 0.17 | 674.54 | 10 |
| UK Species 7 | UK | UK | 5.76 | 18.87 | 15.16 | 51.35 | 33.49 | 679 | 0.51 | 567.53 | 5 |
| UK Species 8 | UK | UK | 45.91 | 3.42 | 8.11 | 59.15 | 32.75 | 580 | 0.19 | 428.23 | 5 |
| UK Liana 1 | UK | UK | 9.35 | 2.9 | 3.93 | 56.83 | 39.24 | 578 | 0.12 | 530.81 | 5 |
| UK Liana 2 | UK | UK | 1.94 | 5.19 | 4.47 | 58.64 | 36.89 | 562 | 0.24 | 316.26 | 5 |
| UK Liana 3 | UK | UK | 2.81 | 15.61 | 26.17 | 38.72 | 35.11 | 699 | 0.18 | 582.08 | 10 |
| UK Liana 4 | UK | UK | 17.14 | 22.94 | 4.03 | 45.37 | 50.6 | 700 | 0.21 | 447.56 | 6 |
| UK Liana 5 | UK | UK | 53.44 | 12.79 | 3.44 | 65.51 | 31.05 | 646 | 0.27 | 513.3 | 5 |
| Vitex beraviensis | Lamiaceae | Lamiales | 1.08 | 11.78 | 11.81 | 57.02 | 31.17 | 684 | 0.1 | 106.74 | 5 |
| Vitex perrieri | Lamiaceae | Lamiales | 4.83 | 6.5 | 13.98 | 45.2 | 40.83 | 646 | 0.27 | 436.45 | 5 |
| Vitex spp | Lamiaceae | Lamiales | 1.28 | 3.89 | 9.06 | 54.64 | 36.3 | 630 | 0.13 | 311.88 | 5 |
| Vitex spp 1 | Lamiaceae | Lamiales | 4.56 | 6.5 | 13.98 | 45.2 | 40.83 | 693 | 0.1 | 426.36 | 5 |
| Ximenia caffra | Oleaceae | Lamiales | 1.54 | 5.4 | 14.54 | 47.23 | 38.23 | 699 | 0.08 | 491.01 | 5 |
Peak reflectance values are the proportion reflectance across wavelengths of light in 1-nm increments (400-700nm). Brightness values have been normalized such that the lowest value in the 400-700nm range is set to zero to account for potential spectrometer drift across measurements.
To quantify fruit odour, we measured volatile organic compound (VOC) emissions of ripe fruits. Ripe fruits were placed in inert ~1.5 L plastic sampling bags and the atmosphere within each bag was sampled using a vacuum pump (Gilian 5000, Sensidyne) that pulled air through the sample bag (1L/min, 240 minutes) and into two odourant-adsorbent filters (Amberlite XAD-2, 400-200mg, Sigma-Aldrich). Contamination of the sampling enclosure with ambient VOCs was minimized by passing incoming air through a container of activated carbon. Additionally, blank samples were collected and analysed to identify contamination from the sampling apparatus. Three peaks representing sampling apparatus contamination were detected in each blank sample and these peaks were subtracted from total VOC sums of fruit samples. Trapped VOCs were analysed using the procedure and instrumentation reported in [19]. All VOC sums were divided by the surface area of sampled fruits to obtain VOCs per unit surface area.
Reflectance spectra of one ripe fruit of each species were measured relative to a Spectralon white reflectance standard (Labsphere) on-site using a Jaz portable spectrometer and a PX-2 pulsed xenon lamp (Ocean Optics Inc.) emitting a D-65 light source, with a range of 250-720nm (Figs 1 and 2). The fruit scanning angle was fixed at 45° and external light was blocked using thick black fabric.
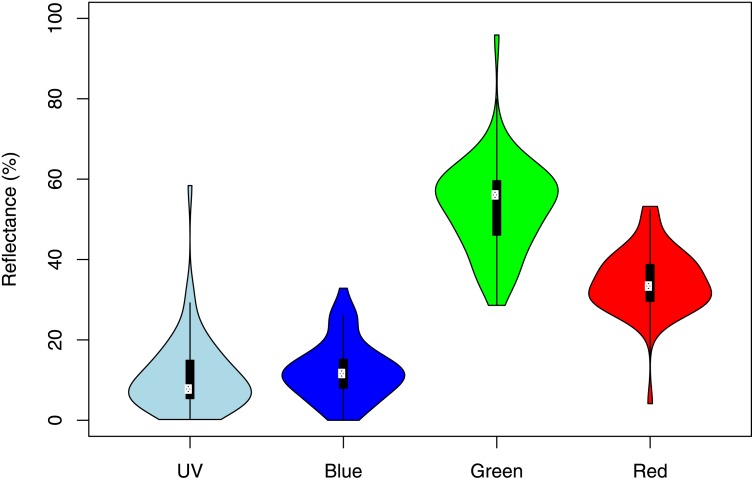
Fig 1. Univariate violin plots showing the reflectance for all fruits in each of the four colour reflectance bands.
Ultraviolet (300-400nm), blue (400-500nm), green (500-600nm), and red (600–700 nm). For each reflectance band, the white dot corresponds to the median, while the lower and upper end of the thick black bars correspond to the 25th and 75th percentiles. The width of the violin plot represents the density of the distribution.
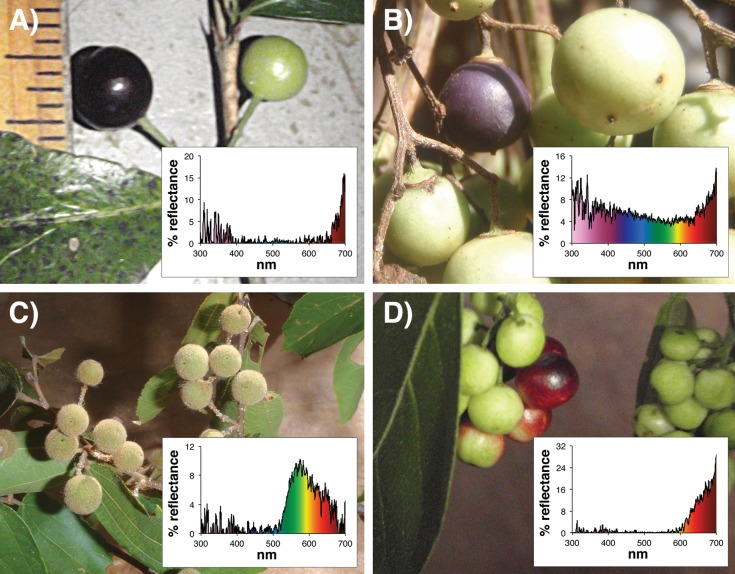
Fig 2. Photographs and associated spectrograms showing four fruits, and their associated reflectance spectra.
A) Tricalysia perrieri, B) UK Liana 3, C) Grewia triflora, D) Antidesma petiolare. Photo credit: KV.
Colour and Odour Measures
We used a VOC index, calculated as log10 surface-area-scaled sum of VOC emissions. We calculated the surface area of each fruit using the following equation for the surface area of an ellipsoid: 4π[((ab)p+(bc)p+(ac)p)/3] 1/p where p = 1.6075 [19]. Four reflectance indices were calculated; ultraviolet (UV, 300-400nm), blue/violet (400-500nm), green/yellow (500-600nm), and orange/red (600-700nm) wavelengths. To control for brightness, the four reflectance indices were calculated as the reflectance in the specified 100nm band divided by the sum of reflectance in the visible range (400-700nm). Brightness was standardized in the visible range because reflectance in the visible range comes at the cost of photosynthetic absorption. Conversely, absorption in the ultraviolet range can result in photoinhibition—absorption of light in this spectrum can be damaging to plants [30]. Reflectance in the ultraviolet range is therefore beneficial in terms of avoiding photoinhibition, and additionally it does not come at a photosynthetic absorption cost. Thus, we chose this method because we wanted to compare relative reflectance within the visible light range (e.g., proportion of blueness, versus redness). This method allows us to compare the relative reflectance of light in the region where it is potentially photosynthetically costly to reflect light (400-700nm). For more detailed multivariate analyses, individual reflectance values (n = 1137) across the 300-700nm range were used directly after normalizing them by the total reflectance in the 400-700nm range, as above.
Phylogenetic Methods
VOC emission, UV, blue, green, and red reflectance were optimized onto a species-level phylogeny as continuous characters, using TNT version 1.1 [31]. The framework phylogeny was adapted from APG III and other classifications [29,32,33,34,35,36] (S1 Fig). The above characters were mapped onto the model phylogeny for a maximally parsimonious arrangement, such that a range of values was optimally assigned to each node. If any of these traits had a higher-level phylogenetic basis we would expect similar values to cluster within a taxon. No such patterns were observed, with optimal character distributions having extensive homoplasy. Assuming maximum parsimony, one would expect a phylogenetically informative trait to exhibit minimal homoplasy, so we calculated consistency and retention indices for each trait (CI and RI, respectively). CI is a direct estimate of homoplasy (i.e., from 0 to 1, CI = 1 if there is none), while RI approximates how well the phylogenetic tree fits a character (i.e., from 0 to 1, RI = 1 if fit is perfect); thus, if any of the measured traits are phylogenetically informative, both values should fall closer to 1 [37]. For a formal test of phylogenetic signal, Blomberg’s K [38] and Pagel’s λ [39] were calculated in R (R Core Development Team, 2014) using the phytools package [40] and compared to a null model using the likelihood-ratio test (S1 File).
Statistical Methods
To assess the association between fruit odour production and fruit chromaticity, we calculated the Pearson correlation of UV, blue, green, and red reflectance with VOC index. To account for correlation across normalized spectrum-reflectance values, we developed a multivariate regression model that included a spline transformation of the spectrum-reflectance values. A series of five predictor variables were calculated as weighted sums of spectrum-reflectance values. The weights for the five predictor variables were determined by a natural-cubic spline that had knots at 350, 410, 470, 530 and 590 nm. Because the summed reflectance values between 400 and 700 nm added to 100%, our spline transformation used the 650nm bandwidth as a fixed referent value to protect against multi-collinearity [41]. Performing the regression using this series of variables rather than the UV, blue, green and red reflectance variables enabled us to model a smooth association between spectrum and VOC. Using the spline-transformed spectrum-reflectance as the principal explanatory variables, we ran a multivariate model that included the log transformed fruit surface-area-to-weight ratios. Because linear regression models handle multiple independent variables but only a single dependent variable, the transformed spectrum-reflectance values were considered as the independent variables in the model. We used this analysis to estimate an adjusted association—an association between variables while holding all other variables constant—and not to imply that fruit colour differences cause changes in VOC production (and not the inverse). For each 100nm colour band, we back-calculated the cumulative coefficient and the variance using the delta method [41]. All analyses were calculated in R (R Core Development Team, 2014), and reported p-values are based on two-tailed hypothesis testing (S2 File).
Results and Discussion
When mapped onto a model phylogeny, none of the measured fruit traits showed any evidence of being phylogenetically informative. Calculated values of CI (maximum = 0.291, UV reflectance) and RI (maximum = 0.386, red reflectance) were much lower than would be sufficient for an informative character [37]. We detected no significant phylogenetic structure for any trait using Blomberg’s K and Pagel’s λ: VOC (K = 0.28, p = 0.59; λ<0.001, p = 1.0), UV (K = 0.36, p = 0.16; λ = 0.22, p = 0.40), blue (K = 0.42, p = 0.23; λ = 0.54, p = 0.24), green (K = 0.36, p = 0.30; λ = 0.28, p = 1.0), red (K = 0.37, p = 0.26; λ = 0.72, p = 0.42) (Table 2). We therefore conclude that traits are not phylogenetically constrained.
Table 2. Results from analyses of fruit traits using a model species-level phylogeny.
| CI | RI | K | p | Λ | p | |
|---|---|---|---|---|---|---|
| VOC | 0.180 | 0.173 | 0.28 | 0.59 | 0.00 | 1.00 |
| UV | 0.291 | 0.275 | 0.36 | 0.16 | 0.22 | 0.40 |
| Blue | 0.193 | 0.270 | 0.42 | 0.23 | 0.54 | 0.24 |
| Green | 0.230 | 0.290 | 0.36 | 0.30 | 0.28 | 1.00 |
| Red | 0.260 | 0.386 | 0.37 | 0.26 | 0.72 | 0.42 |
P-values for Blomberg’s K and Pagel’s λ are from likelihood-ratio tests.
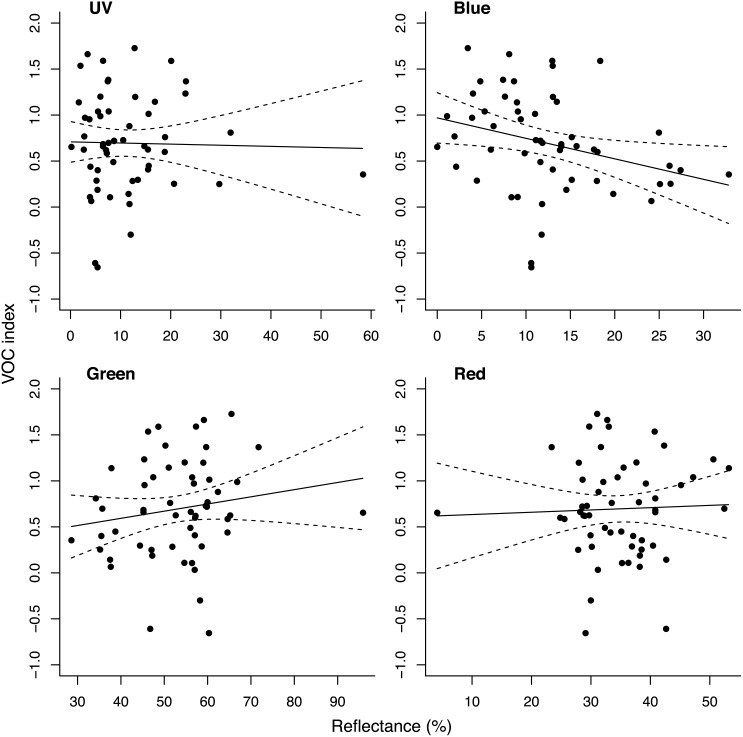
There was a significant negative relationship between blue reflectance and overall VOC index (r = -0.32, p = 0.02). We observed no significant relationship between VOC index and any of the other reflectance ranges: UV (r = -0.01, p = 0.94), green (r = 0.14, p = 0.32), or red reflectance (r = 0.04, p = 0.76; Fig 3). The surface-area-to-weight ratio was a highly significant predictor of the VOC index. Smaller fruits with higher surface-area-to-weight ratios had substantially higher VOC emissions (r = 0.50, p < 0.001) (Table 3).
Fig 3. Bivariate scatter plots showing the relationship between overall odour emission (VOC) and reflectance in each of the four colour reflectance bands.
UV (300-400nm), blue (400-500nm), green (500-600nm), and red (600-700nm). Percent reflectance was calculated as regions of the spectrum reflecting in the specified 100nm band divided by the sum of reflectance in the visible range (400-700nm).
Table 3. Pearson correlation coefficients for fruit traits of sampled species (N = 56) including log transformed surface-area-scaled VOC sum, percent reflectances per band normalized by brightness, surface area:weight ratios.
| VOC | UV Reflectance | Blue Reflectance | Green Reflectance | Red Reflectance | Surface Area to Weight Ratio | |
|---|---|---|---|---|---|---|
| VOC | ||||||
| UV Reflectance | -0.01 | |||||
| Blue Reflectance | -0.32* | 0.43** | ||||
| Green Reflectance | 0.14 | -0.55** | -0.74** | |||
| Red Reflectance | 0.04 | -0.33* | 0.00 | -0.49** | ||
| Surface Area to Weight Ratio | 0.50** | 0.17 | -0.01 | -0.18 | 0.14 |
* p < 0.05
** p < 0.01
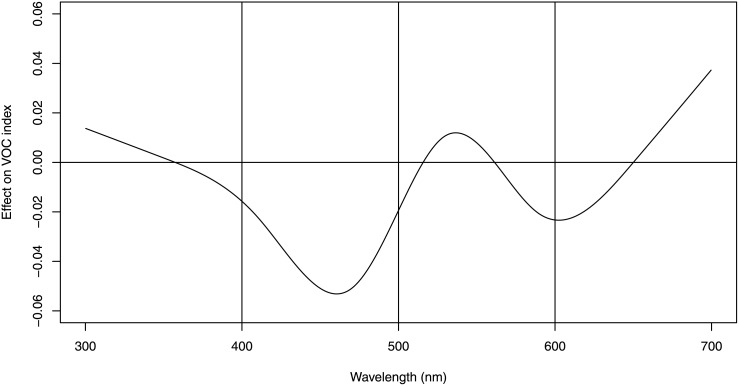
To control for the confounding effect of fruit size and correlated reflectance bands, we ran a multivariate model that included a spline transformation of the normalized reflectance values (Fig 4). The figure demonstrates that higher reflectance in the 400nm to 600nm range was associated with lower VOC in the fruit sample. Overall, reflectance was a significant predictor of VOC emissions (5 d.f., F = 2.8, p = 0.03). As with our bivariate analyses, we found a substantial negative association between VOC and blue reflectance (log10 effect = -0.039, p = 0.009, R2 = 0.41) and a lack of relationship in the other reflectance bands.
Fig 4. The relationship between overall odour emission (log10 VOC) and reflectance across the 300nm to 700nm colour range.
This analysis was based on a natural cubic spline transformation of the spectrum values (300-700nm). Each one-unit increase of reflectance in the blue spectrum (400-500nm) was associated with an 11% decrease (log10 effect = -0.039, p = 0.009) in VOC while reflectance in the UV, green and red spectra were not associated with VOC.
Our prediction that fruit investment in non-photosynthetically active pigments would scale negatively with odour production is partially supported. We find that bluish fruits have significantly lower total VOC emissions than fruits reflecting other hues, which is consistent with the fruit syndrome hypothesis. Specifically our results suggest the existence of a disperser-mediated dichotomy in fruit colour and odour signal production: plants that invest more in blue chromatic signals invest less in odour, yet fruit size is a critical component.
The trade-off between blue chromatic reflectance and VOC emission is particularly compelling because reflectance in the blue range of the spectrum (400-500nm) may be doubly costly to a plant as it occupies critical photosynthetic space—both chlorophyll A and B absorb blue wavelengths of light [42]. Thus plants investing in pigments (e.g., indigoids, anthocyanins) that reflect light at this range of the spectrum may be investing in both pigment production with a concomitant loss of photosynthetic potential [43]. Alternatively, chromatic reflectance and VOC emissions could reflect other constraints, such as chemical constraints of colour producing pigments or exploitation of uncommon colors for a particular environment to increase advertisement [44].
The lack of a relationship between red reflectance (600-700nm) and VOC emission may result from the other benefits to a plant of investment in pigments that reflect at this range of the spectrum (e.g., anthocyanins). Advantages to red plant pigmentation include anti-fungicidal properties, photoprotection against UV damage, prevention of photoinhibition, and chromatic crypsis against dichromatic (red-green colour blind) herbivores [45,46,47]. Thus, while red pigmentation can be available to trichromatic animals as a cue of fruit ripeness, this may result from selective pressures other than disperser signalling.
Our finding that smaller fruits tend to invest heavily in VOC production may reflect the diminutive size of nocturnal, olfactory-driven mammals in Madagascar. Unlike most other tropical systems where the small end of the disperser size spectrum is dominated by avifauna, in Madagascar the smallest seed dispersing animals are mouse and dwarf lemurs of the family Cheirogaleidae [48]. Cheirogaleids are dichromatic (red-green colour blind) and nocturnal, and have been shown to rely heavily on olfaction during fruit selection and detection [19,20]. The fact that these animals are red-green colour blind may explain why red hues did not show a similar pattern to blue hues in our analysis.
Our results provide support for the idea that fruit traits may converge to simultaneously attract multiple dispersers with diverse sensory phenotypes by using both colour and odour signals when possible, or by decreasing olfactory signals when producing colours that all dispersers can see well. An important next step will be to record the behaviour of seed dispersers relative to fruit cues. In this forest there are five known seed dispersing mammals, three nocturnal and two cathemeral, [49,50] and four putative seed dispersing birds [51]. These seed dispersing mammals are cathemeral, nocturnal, and dichromatic, with highly developed olfactory apparatuses, and respond primarily to olfactory cues during fruit selection [20,52]. All frugivorous mammals in this system for which data are available on colour vision capabilities are dichromats, or red-green colour blind [52,53]. While dichromats are not able to distinguish between fruits in the red-green colour channel, they are able to distinguish fruits in the blue-yellow colour channel [54,55] and compelling evidence from studies of primate behaviour, genetics and ambient light measurements suggests that mammalian color vision is useful, even under nocturnal conditions [19,56,57,58]. Avian dispersers, on the other hand, are diurnal, tetrachromatic, visually oriented foragers, with a capacity to distinguish red from green, and into the UV spectrum [59]. Despite the fact that mammalian seed dispersing taxa in this forest are highly olfactorily-driven, [53], the colour that fruits produce at the expense of odour is one that is available to all seed dispersing animals in this system—blue.
Supporting Information
(PDF)
(TXT)
(TXT)
Acknowledgments
We thank MICET and Madagascar National Parks for permission to conduct research in Madagascar. We thank Dr. Scott Mabury for the loan of instrumentation. We thank Dr. Shawn M. Lehman for valuable advice throughout the study. We are grateful to Mr. Paul Tsiveraza, Radoniaina R. Rafaliarison, Francette, Mamy Razafitsalama and Jean de-la-Dieu for contributions in the field, and to four anonymous reviewers for helpful commentary on the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
For funding the authors thank Sigma Xi, GM Women in Science (KV), the University of Toronto and Natural Sciences and Engineering Research Council of Canada (KV). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lomáscolo S, Speranza P, Kimball R (2008) Correlated evolution of fig size and color supports the dispersal syndromes hypothesis. Oecologia 156: 783–796. 10.1007/s00442-008-1023-0 [DOI] [PubMed] [Google Scholar]
- 2. Korine C, Kalko EK, Herre EA (2000) Fruit characteristics and factors affecting fruit removal in a Panamanian community of strangler figs. Oecologia 123: 560–568. [DOI] [PubMed] [Google Scholar]
- 3. Osorio D, Smith AC, Vorobyev M, Buchanan-Smith HM (2004) Detection of fruit and the selection of primate visual pigments for color vision. American Naturalist 164: 696–708. [DOI] [PubMed] [Google Scholar]
- 4. Schaefer H, Schaefer V, Vorobyev M (2007) Are fruit colors adapted to consumer vision and birds equally efficient in detecting colorful signals? The American Naturalist 169: S159–S169. 10.1086/510097 [DOI] [PubMed] [Google Scholar]
- 5. Howe HF (1993) Specialized and generalized dispersal systems: where does ‘the paradigm’stand? Plant Ecology 107: 3–13. [Google Scholar]
- 6. Linn CE Jr., Dambroski HR, Feder JL, Berlocher SH, Nojima S, Rpepf WL, et al. (2004) Postzygotic isolating factor in sympatric speciation in Rhagoletis flies: reduced response of hybrids to parental host-fruit odors. Proceedings of the National Academy of Sciences 101: 17753–17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Korine C, Kalko EKV (2005) Fruit detection and discrimination by small fruit-eating bats (Phyllostomidae): echolocation call design and olfaction. Behavioral Ecology and Sociobiology 59: 12–23. [Google Scholar]
- 8. Hirsch BT (2010) Tradeoff between travel speed and olfactory food detection in ring-tailed coatis (Nasua nasua). Ethology 116: 671–679. [Google Scholar]
- 9. Valido A, Schaefer HM, Jordano P (2011) Colour, design and reward: phenotypic integration of fleshy fruit displays. Journal of Evolutionary Biology 24: 751–760. 10.1111/j.1420-9101.2010.02206.x [DOI] [PubMed] [Google Scholar]
- 10. Schaefer H, Braun J (2009) Reliable cues and signals of fruit quality are contingent on the habitat in black elder (Sambucus nigra). Ecology 90: 1564–1573. [DOI] [PubMed] [Google Scholar]
- 11. Otte D (1974) Effects and functions in the evolution of signaling systems. Annual Review of Ecology and Systematics 5: 385–417. [Google Scholar]
- 12. Janson CH (1983) Adaptation of fruit morphology to dispersal agents in a neotropical forest. Science 219: 187–189. [DOI] [PubMed] [Google Scholar]
- 13. Mazer SJ, Wheelwright NT (1993) Fruit size and shape: allometry at different taxonomic levels in bird-dispersed plants. Evolutionary Ecology 7: 556–575. [Google Scholar]
- 14. Lomáscolo S, Levey D, Kimball R, Bolker B, Alborn H (2010) Dispersers shape fruit diversity in Ficus (Moraceae). Proceedings of the National Academy of Sciences 107: 14668–14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schaefer HM, Schmidt V (2004) Detectability and cntent as opposing signal characteristics in fruits. Proceedings of the Royal Society of London, B S271: S370–S373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer K, Chapman C (1993) Frugivores and fruit syndromes: differences in patterns at the genus and species level. Oikos 66: 472–482. [Google Scholar]
- 17. Lomáscolo S, Schaefer H (2010) Signal convergence in fruits: a result of selection by frugivores? Journal of Evolutionary Biology 23: 614–624. 10.1111/j.1420-9101.2010.01931.x [DOI] [PubMed] [Google Scholar]
- 18. Schaefer HM, Schaefer V, Levey DJ (2004) How plant-animal interactions signal new insights in communication. Trends in Ecology & Evolution 19: 577–584. [Google Scholar]
- 19. Valenta K, Burke RJ, Styler SA, Jackson DA, Melin AD, Lehman S (2013) Colour and odour drive fruit selection and seed dispersal by mouse lemurs. Scientific Reports 3: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siemers BM, Goerlitz HR, Robsomanitrandrasana E, Piep M, Ramanamanjato JB, Rakotodrovony D, et al. (2007) Sensory basis of food detection in wild Microcebus murinus . International Journal of Primatology 28: 291–304. [Google Scholar]
- 21. Luft S, Curio E, Tacud B (2003) The use of olfaction in the foraging behaviour of the golden-mantled flying fox, Pteropus pumilis, and the greater musky fruit bat, Ptenochirus jagori (Megachiroptera: Pteropodidae). Naturwissenschaften 90: 84–87. [DOI] [PubMed] [Google Scholar]
- 22. Herrera C (1987) Vertebrate-dispersed plants of the Iberian Peninsula: a study of fruit characteristics. Ecological Monographs 57: 305–331. [Google Scholar]
- 23. Herrera C (1992) Interspecific variation in fruit shape: allometry, phylogeny, and adaptation to dispersal agents. Ecology 73: 1832–1841. [Google Scholar]
- 24. Whitney KD (2009) Comparative evolution of flower and fruit morphology. Proceedings of the Royal Society of London, B 276: 2941–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wheelwright N, Orians G (1981) Seed dispersal by animals: contrasts with pollen dispersal, problems of terminology and constraints on coevolution. American Naturalist 119: 402–413. [Google Scholar]
- 26. Wheelwright NT, Janson CH (1985) Colors of fruit displays of bird-dispersed plants in two tropical forests. American Naturalist 126: 777–799. [Google Scholar]
- 27. McKitrick MC (1993) Phylogenetic constraint in evolutionary theory: has it any explanatory power? Annual Review of Ecology and Systematics 24: 307–330. [Google Scholar]
- 28. Schatz GE (2001) Generic Tree Flora of Madagascar. St. Louis: Royal Botanic Gardens, Kew and Missouri Gardens. [Google Scholar]
- 29. Chase MW, Reveal JL (2009) A phylogenetic classification of the land plants to accompany APG III. Botanical Journal of the Linnean Society 161: 122–127. [Google Scholar]
- 30. Hakala-Yatkin M, Mantysaari M, Mattila H, Tyystjarvi E (2010) Contributions of visible and ultraviolet parts of sunlight to photoinhibition. Plant Cell Physiology 51: 1745–1753. 10.1093/pcp/pcq133 [DOI] [PubMed] [Google Scholar]
- 31. Goloboff PA, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24: 774–786. [Google Scholar]
- 32. Backlund M, Oxelman B, Bremer B (2000) Phylogenetic relationships within the Gentianales based on ndhF and rbcL sequences, with particular reference to the Loganiaceae. American Journal of Botany 87: 1029–1043. [PubMed] [Google Scholar]
- 33. Gadek PH, Fernando ES, Quinn CJ, Hoot SB, Terrazas T, Sheahan MC, et al. (1996) Sapindales: molecular delimitation and infraordinal groups. American Journal of Botany 83: 802–811. [Google Scholar]
- 34. Tokuoka T, Tobe H (2006) Phylogenetic analyses of Malpighiales using plastid and nuclear DNA sequences, with particular reference to the embryology of Euphorbiaceae sens. str. Journal of Plant Research 119: 599–616. [DOI] [PubMed] [Google Scholar]
- 35. Bremer B, Eriksson T (2009) Time tree of Rubiaceae: phylogeny and dating the family, subfamilies, and tribes. International Journal of Plant Science 170: 766–793. [Google Scholar]
- 36. Nazar N, Goyder DJ, Clarkson JJ, Mahmood T, Chase MW (2012) The taxonomy and systematics of Apocynaceae: where we stand in 2012. Botanical Journal of the Linnean Society 171: 482–490. [Google Scholar]
- 37. Farris JS (1989) The retention index and the resclaed consistency index. Cladistics 5: 417–419. [DOI] [PubMed] [Google Scholar]
- 38. Blomberg SP, Garland T Jr, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- 39. Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- 40. Revell LJ (2012) Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- 41. Gasparrini A, Armstrong B, Kenward MG (2010) Distributed lag non-linear models. Statistics in Medicine 29: 2224–2234. 10.1002/sim.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sims DA, Gamon JA (2002) Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sensing of Environment 81: 337–354. [Google Scholar]
- 43. Willson MF, Whelan CJ (1990) The evolution of fruit color in fleshy-fruited plants. American Naturalist 136: 780–809. [Google Scholar]
- 44. Stournaras KE, Lo E, Bohning-Gaese K, Cazetta E, Dehling DM, Schaleuning M, et al. (2013) How colorful are fruits? Limited color diversity in fleshy fruits on local and global scales. New Phytologist 198: 617–629. 10.1111/nph.12157 [DOI] [PubMed] [Google Scholar]
- 45. Coley PD, Aide TM (1989) Red coloration of tropical leaves: A possible anti-fungal defence? Journal of Tropical Ecology 5: 293–300. [Google Scholar]
- 46. Gould KS, Kuhn DN, Lee DW, Oberbauer SF (1995) Why leaves are sometimes red. Nature 378: 241–242. [Google Scholar]
- 47. Stone BC (1979) Protective coloration of young leaves in certain Malaysian palms. Biotropica 11: 126. [Google Scholar]
- 48. Lahann P (2006) Feeding ecology and seed dispersal of sympatric cheirogaleid lemurs (Microcebus murinus, Cheirogaleus medius, Cheirogaleus major) in the littoral rainforest of south-east Madagascar. Journal of Zoology 271: 88–98. [Google Scholar]
- 49. Sato H (2012) Frugivory and seed dispersal by brown lemurs in a Malagasy tropical dry forest. Biotropica 44: 479–488. [Google Scholar]
- 50. Mittermeier CG, Louis EE, Richardson M, Schwitzer C, Langrand O, Rylands AB, et al. (2010) Lemurs of Madagascar. Bogota: Conservation International. [Google Scholar]
- 51. Langrand O (1990) Guide to the Birds of Madagascar. New Haven: Yale University Press. [Google Scholar]
- 52. Jacobs GH, Deegan JF (1993) Photopigments underlying color vision in ringtail lemurs (Lemur catta) and brown lemurs (Eulemur fulvus). American Journal of Primatology 30: 243–256. [DOI] [PubMed] [Google Scholar]
- 53. Tan Y, Li WH (1999) Trichromatic vision in prosimians. Nature 402: 36 [DOI] [PubMed] [Google Scholar]
- 54. Dominy NJ, Lucas PW (2001) Ecological importance of trichromatic vision to primates. Nature 410: 363–366. [DOI] [PubMed] [Google Scholar]
- 55. Mollon JD (1989) " Tho'she kneel'd in that place where they grew…" The uses and origins of primate colour vision. Journal of Experimental Biology 146: 21–38. [DOI] [PubMed] [Google Scholar]
- 56. Perry GH, Martin RD, Verrelli BC (2007) Signatures of functional constraint at aye-aye opsin genes: The potential of adaptive color vision in a nocturnal primate. Molecular Biology and Evolution 24: 1963–1970. [DOI] [PubMed] [Google Scholar]
- 57. Veilleux CC, Louis EE, Bolnick DA (2013) Nocturnal light environments influence color vision and signatures of selection on the OPN1SW opsin gene in nocturnal lemurs. Molecular Biology and Evolution 30: 1420–1437. 10.1093/molbev/mst058 [DOI] [PubMed] [Google Scholar]
- 58. Melin AD, Moritz GL, Fosbury RA, Kawamura S, Dominy NJ (2012) Why aye-ayes see blue. American journal of Primatology 74: 185–192. [DOI] [PubMed] [Google Scholar]
- 59. Jacobs GH (1981) Comparative color vision: Academic Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(TXT)
(TXT)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.