Abstract
Invasive species can affect the function and structure of natural ecological communities, hence understanding and predicting their potential for spreading is a major ecological challenge. Once established in a new region, the spread of invasive species is largely controlled by their dispersal capacity, local environmental conditions and species interactions. The mussel Mytilus galloprovincialis is native to the Mediterranean and is the most successful marine invader in southern Africa. Its distribution there has expanded rapidly and extensively since the 1970s, however, over the last decade its spread has ceased. In this study, we coupled broad scale field surveys, Ecological Niche Modelling (ENM) and Lagrangian Particle Simulations (LPS) to assess the current invaded distribution of M. galloprovincialis in southern Africa and to evaluate what prevents further spread of this species. Results showed that all environmentally suitable habitats in southern Africa have been occupied by the species. This includes rocky shores between Rocky Point in Namibia and East London in South Africa (approx. 2800 km) and these limits coincide with the steep transitions between cool-temperate and subtropical-warmer climates, on both west and southeast African coasts. On the west coast, simulations of drifting larvae almost entirely followed the northward and offshore direction of the Benguela current, creating a clear dispersal barrier by advecting larvae away from the coast. On the southeast coast, nearshore currents give larvae the potential to move eastwards, against the prevalent Agulhas current and beyond the present distributional limit, however environmental conditions prevent the establishment of the species. The transition between the cooler and warmer water regimes is therefore the main factor limiting the northern spread on the southeast coast; however, biotic interactions with native fauna may also play an important role.
Introduction
Invasive species represent one of the main threats to global biodiversity, affecting the function and structure of natural ecological communities and causing annual economic losses amounting to billions of dollars worldwide [1–4]. Since the publication of Charles Elton’s book “The ecology of invasions by animals and plants” in 1958 [5], biological invasions have gained exponentially growing attention [6]. The realization that most successful invasions are irreversible [7] has motivated ecologists to attempt to identify the environmental factors influencing the spread of invaders in new ecosystems and to quantify and forecast the success of extant invasive processes (e.g., [8–14]). While most of these studies assume that the spread of new colonizers is primarily controlled by the potential for invasive species to disperse and the availability of suitable habitats in the new environments, the use of fragmented approaches focusing on only one of these possibilities may have precluded important conclusions regarding invasive processes.
Many marine species have a planktonic dispersal phase and the distance that propagules can travel is often positively correlated to their Pelagic Larval Duration (PLD; but see [15]). As a result, many studies have adapted Lagrangian Particle Simulations (LPS; see [16–17] for a review) to assess the potential for invasive species to disperse from neighbouring regions where establishment has already taken place [18–21]. These simulations allow the tracking of virtual particles advected by ocean current fields (e.g., [22]), and their realism can be increased by including important biological parameters such as larval behaviour or the PLD of drifting particles (e.g., [23–24]). However, the potential dispersal distance, as measured by LPS, and the realized one, often differ when behavioural strategies, local hydrodynamic conditions and most importantly, habitat suitability are not taken into account [25]. When propagules arrive at new environments, the establishment success and abundance of invasive species are dependent on the availability of suitable habitats, which is largely determined by local abiotic conditions such as climate [9,11] and biotic interaction with the indigenous biota [26,27]. Ecological Niche Models (ENM; see [28] for a review) can be used alongside LPS to predict suitable habitats for putative invasions in non-native regions. However, a major challenge arises in using ENM in the invasive context because the fundamental niche of a species may diverge between native and invasive ranges due to local selection in the face of novel environmental conditions [29–30]. This results in the violation of one of the fundamental assumptions of ENM due to an asymmetric transference of model fitting between environmental spaces [31]. Thus, a crucial step in applying ENM for invasive species is to test the agreement between the environmental spaces used by both the native and the invasive populations [32].
Among marine animals, one of the most successful invasive species is the mussel Mytilus galloprovincialis. This species is native to the Mediterranean and has an antitropical distribution [33], being recorded to date on the coasts of all continents except Antarctica [34]. It possesses many features that make it a successful invasive species, including high fecundity [26], fast growth, competitive abilities [34–36] and ease of transport of larvae and adults in ballast waters [37]. M. galloprovincialis arrived in South Africa in the 1970’s, probably with shipping from a Mediterranean source population [38]. It rapidly colonized the west and south coastlines of southern Africa, but its spread appears to have ceased over the last decade [39–40]. The arrival of this invader had significant ecological impacts (reviewed by [34]). On the west coast of South Africa, it has replaced the indigenous mussels Aulacomya ater and Choromytilus meridionalis as the dominant species. On sheltered shores, the limpets Scutellastra granularis and S. argenvillei have also been negatively affected. Despite several negative effects, the presence of M. galloprovincialis in the South African intertidal has moved the centre of distribution of intertidal mussel beds higher on the shore thus increasing the overall mussel biomass. This has important positive effects for intertidal predators such as the African black oystercatcher Haematopus moquini. On the west coast, this near-threatened species has shifted its diet and now feeds primarily on the invasive mussel [41]. Critically, the economic impact of M. galloprovincialis has also been significant as the South African mussel culture industry is mostly based on this invasive species [42].
In this study, we combine extensive field surveys, ENM using climatic variables and LPS approaches to assess the current invasive distribution of M. galloprovincialis in southern Africa and evaluate whether dispersal and/or environmental conditions limit the further spread of this marine invader in southern Africa. Specifically, we: (1) assessed the potential and realized niche of M. galloprovincialis in the invaded range, (2) inferred whether there might have been niche adaptation (divergence) between native and recent establishments and (3) tested the null hypothesis of no potential for larval dispersal beyond the already established non-native ranges due to major oceanographic discontinuities promoted by ocean currents.
Materials and Methods
Ethics Statement
All field surveys did not involve disruptive sampling and thus no specific permits were required with the exception of the South African surveys that were performed under permit number RES2014/12 issued by the Department of Agriculture Forestry and Fisheries to the Department of Zoology and Entomology at Rhodes University.
Data on species records and climate
To have georeferenced occurrences of Mytilus galloprovincialis in southern Africa, we combined two types of datasets: wide-ranging field surveys and existing literature. The field surveys were carried out during low spring tides between 2010 and 2013 on rocky intertidal shores along the southern African coastline from Mowe (9.37°S, 12.70°E), in Namibia, to Ponta do Ouro (26.85°S, 32.89°E), in Mozambique. Most of the locations were visited twice, covering winter and summer months; two observers performed searches lasting approximately 60 min across all microhabitats present. Because invasive ENMs benefit from records that include species native ranges [43], we further expanded the field surveys to Northern Africa (Western Sahara, Morocco and Tunisia) and southern Europe. These were performed between 2008 and 2013 following the same procedure as for the surveys in the invasive range. The literature search covering native records also included the GBif database. Since M. galloprovincialis can hybridise with the congeneric M. edulis [44] and M. trossulus [44–45] in northern Europe, these regions were excluded. While the modelling approach used (Boosted Regression Trees; see Ecological Niche Modelling section) can account for some degree of spatial autocorrelation [46], completely neglecting the effect of spatial dependence among occurrence records may lead to poorly estimated regression coefficients and to the selection of unimportant environmental predictors [47]. To reduce spatial autocorrelation in our models, all occurrence records were gridded (0.08° x 0.08° resolution) and duplicate entries were considered only once (e.g., [48]).
Environmental predictors commonly used for intertidal species (e.g., [49–52]) were produced by determining the long-term averages (2000–2010) summarizing the annual extremes in sea surface temperature, air temperature, sea surface salinity and cloud cover. The raw environmental information was gathered from the Operational Sea Surface Temperature and Sea Ice Analysis (OSTIA) [53], the World Ocean Database 2013 [54] and the European Centre for Medium-Range Weather Forecasts (ECMWF) [55]. All predictors were gridded with bilinear interpolation to match the resolution of the distribution data.
Ecological Niche Modelling
The niche modelling implemented in our study was performed following previous studies with high predictive performance when modelling intertidal species [52,56]. In the framework used, we identified a set of models with higher potential for spatial transferability (i.e., the ability to predict distributions in regions outside the domain of known records of occurrence; [57]) among numerous candidate models. We adopted the machine-learning method Boosted Regression Trees (BRT; [58]) because of its ability to model non-linear relationships and detect complex interactions among predictors.
Because M. galloprovincialis may be absent from some regions of southern Africa due to factors other than simple niche availability (e.g., dispersal constraints), the models were performed with pseudo-absences rather than the absences collated from surveys. The selection of this information was performed following the recommendations of Barbet-Massin et al. [59] for Boosted Regression Trees, by using the same number of pseudo-absences as the presence records, randomly selected from regions outside the species’ ecological domain (throughout both native and non-native ranges). In this step, we computed a habitat suitability map using Mahalanobis distance, relating all presence records with the normalized environmental predictors (see [60] for details). However, the threshold allowing one to extract pseudo-absences from the suitability gradient has a direct effect on both predicted areas and accuracy scores, with low thresholds for instance (e.g., 0.1), elevating accuracy scores while over-predicting distributions [61]. We adopted a threshold of 0.2, which allows a good balance between accuracy scores and predicted distributions close to realised ranges (see [61] for details).
A cross-validation framework was implemented by randomly dividing both presence and pseudo-absence records into two independent datasets, one set with 70% of the records for model training and another with 30% to test the model’s accuracy. Numerous models were trained using all possible combinations of environmental predictors with no signs of strong correlation (Spearman’s R < |0.7|). Fitting each BRT model was a process dependent on the learning rate, tree complexity, number of trees and bag fraction (see [58,62] for details). The optimal values for these parameters were determined by 10-fold cross-validation performed in the training dataset, using deviance reduction as an estimate of success (e.g., [62]). Each model was fitted using a range of learning rates (0.1, 0.05, 0.01, 0.005, 0.001) and tree complexities (from 1 up to the number of predictors in a given subset). The optimal number of trees was identified by varying values of trees from 100 to 10000 (step 50) and ensuring that at least 1000 trees were fitted [58,62]. A default value of 0.5 for bag fraction was used because it consistently results in optimal binomial responses [62]. Careful identification of the optimal parameters allowed an increased level of generality, while avoiding the problems of over fitting when predicting distributions. The accuracy of each combination of predictors was then verified using True Skill Statistics (TSS) [63], by comparing the test dataset with a reclassified predictive map that maximizes the ability to detect true absences and presences (specificity and sensitivity, respectively). Using these statistics, accuracy scores higher than 0.8 were interpreted as an excellent model, from 0.8 to 0.6 a good model, from 0.6 to 0.2 a fair to poor model, and values lower than 0.2 a model with no predictive ability.
The cross-validation framework was conducted 30 times and, for each one, the pseudo-absences were randomized along with the training and testing datasets. The contribution of the environmental predictors to the responses of models was determined by the mean accuracy when a predictor was modelled alone (univariate effect) and the mean gain in accuracy when added to a model with other predictors. To identify the combination of predictors with the highest potential for transferability we ran independent tests with the null hypothesis of no differences between the mean TSS. This was performed by sorting the combinations of predictors by decreasing accuracy scores and successively adding them to non-parametric Kruskal-Wallis rank tests until the level of significance was reached (alpha = 0.05).
The final predictive map was produced with the full set of distribution records. However, because several models could be equally transferrable (no differences in their mean accuracy), we merged (with a median function) the predictive surfaces resulting from those models trained with the highest transferrable combinations of predictors (i.e. ensemble modelling), an approach that reduces the inherent uncertainty of predictions [52,64]. The ensemble was then reclassified with a threshold maximizing the sum of sensitivity and sensibility, so the output would reflect predicted presences and absences rather than the probability of occurrence. The standard deviation of the ensemble was also computed as a measure of uncertainty. Finally, to compare the available niche with the current realized distribution of M. galloprovincialis, we determined the sensitivity and specificity of the ensemble against our field observations (both presences and absences). This approach allowed verification of the proportion of surveyed sites with suitable environmental conditions already invaded by the species.
Niche divergence between ranges
Principal Component Analysis (PCA) was used to infer niche divergence between native and non-native ranges (e.g., [65]). All presence records were used to extract the values from the environmental predictors included in the ensemble modelling (i.e. those making the greatest contribution to the niche of this species). In this step, the predictors were scaled to have a mean of zero and a standard deviation of one. The first two PCA components were plotted and their raw values used to test the significance of niche evolution (i.e., divergence) by permutational multivariate ANOVA (PERMANOVA; [66]) performed on the Euclidian similarity matrix under 9999 randomizations, using ranges as independent variables. In this analysis, the rejection of the null hypothesis suggests niche evolution due to differences in the relative position of environmental data in the multivariate space, in their degree of dispersion, or both [67]. To disentangle the possible causes for niche divergence, we performed a permutational analysis of multivariate dispersion (PERMDISP; [67]).
Lagrangian Particle Simulations
At large spatial scales, the larvae of M. galloprovincialis are dispersed as passive particles and their dispersal patterns and range of dispersal can be linked closely to hydrogeographic data [68]. Thus, the probability of M. galloprovincialis dispersing beyond the already established range in southern Africa was inferred by individual-based Lagrangian Particle Simulations (LPS). Data for currents were derived from the Hybrid Coordinate Ocean Model (HYCOM), a daily high-resolution product forced by wind speed, wind stress, heat flux and precipitation [69]. This model can resolve oceanic eddies, meandering currents, filaments and fronts [69], important mesoscale processes required to simulate accurately dispersing larvae (e.g., [70]). The LPS covered two separate coasts where the northern range limits are presently defined: (1) the west coast from Capulo (Angola; 8.00° S) to Walvis Bay (Namibia; 23.00° S) and (2) the southeast coast, from St Francis Bay (South Africa; 34.00° S) to Beira (Moçambique; 20.00° S). Both coastlines were gridded so the cells would match the spatial resolution of HYCOM (0.08° x 0.08°). Passive particles simulating drifting larvae were released from each coastal cell on a daily basis throughout the spawning season of M. galloprovincialis (from May to July and from October to January; [71]). The particles were allowed to drift for 30 and 90 days, which are the average and upper end (when metamorphic delay occurs) Pelagic Larval Duration (PLD) known for Mytilus spp. [68,72–75]. In these experiments, the geographical position of each particle was determined every two hours using the local bilinear interpolation of HYCOM’s velocity fields. The aggregated trajectories allowed the production of a connectivity matrix between every pair of cells, by determining the number of temporal steps that a particle released from cell i crossed cell j, divided by the number of steps simulated per particle (30 days PLD * 12 steps per day). To account for inter-annual variability, simulations were run individually for each year for a 5-year period (2008 to 2012), and the mean connectivity matrix was calculated by averaging the annual matrices. The null hypotheses of no correlation between the connectivity matrices performed with contrasting PLDs (30 and 90 days) for both west and southeast coasts were tested using Mantel non-parametric test based on 9999 permutations.
Niche modeling analysis, niche divergence tests and dispersal simulations were performed in R [76] using the packages: adehabitat, dismo, gbm, gstat, mda, parallel, raster, SDMTools and vegan.
Results
The field surveys provided 104 georeferenced records for M. galloprovincialis in southern Africa. For modelling purposes, this dataset was enhanced with 170 additional records from native ranges derived from field surveys in North Africa and Southern Europe, the available literature and an electronic database (Fig 1A; S1 Text).
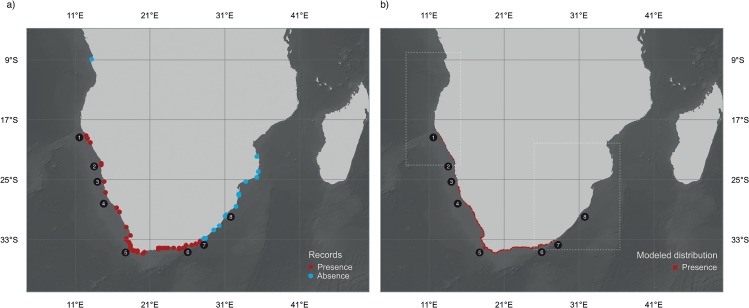
Fig 1.
(a) Records of occurrence for M. galloprovincialis throughout the southern African coast (Red dots for presence and blue dots for absence). (b) Reclassified ensemble showing the occurrence of M. galloprovincialis for the present (2000–2010). Numbers show Regions Of Interest: ROI 1. Rocky Point, ROI 2. Walvis Bay, ROI 3. Sossusvlei, ROI 4. Elizabeth Bay, ROI 5. Cape Town, ROI 6. Port Elizabeth, ROI 7. East London, ROI 8. Durban. The dashed squares indicate those regions where the Lagrangian Particle Simulations where performed: the west coast, from Capulo to Walvis Bay, and the southeast coast, from St Francis Bay to Beira. Credits for the background of both maps: General Bathymetric Chart of the Oceans (GEBCO).
Ecological Niche modelling of M. galloprovincialis
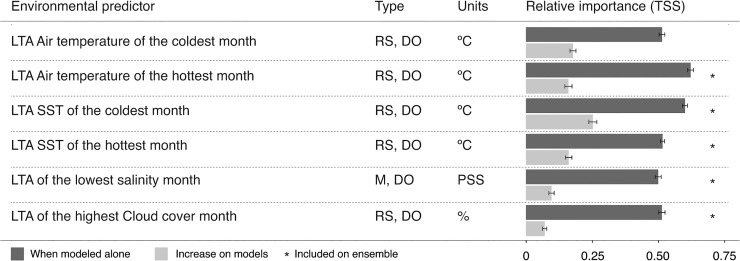
Measuring the relative importance of each environmental predictor to the response of the models showed that the distribution of M. galloprovincialis is best explained by the extremes of high air and low sea temperatures (Fig 2). These two predictors retrieved good accuracy scores when modelled alone (TSS > 0.6), while when combined with others, they added gains in TSS from 0.16±0.01 to 0.25±0.01. All other predictors produced null models when used alone, retrieving accuracy scores ranging from 0.48±0.01 (low salinity) to 0.51±0.01 (hottest sea temperatures). Nevertheless, they showed a fair contribution to the models when combined with other predictors, adding accuracy gains from 0.09±0.01 (high cloud cover) up to 0.17±0.01 (coldest air temperatures).
Fig 2. Environmental predictors used in niche modelling as Long Term Averages (LTA), type of data (M—Modelled; RS—Remote sensing; DO—Direct observation), units and relative importance on cross-validation (TSS—True Skill Statistics).
Asterisks show the predictors included in the final ensemble.
The ensemble produced with the best transferrable models resulted in an excellent description of M. galloprovincialis distribution (TSS: 0.95), while showing low levels of uncertainty (S1 Fig.; SD ranging from 0 to 0.15). This prediction indicated that this species’ niche is available from Rocky Point in Namibia (ROI 1, Fig 1B) southwards to East London in South Africa (ROI 7). Within this wide region (~2800 km), the predicted niche was not continuous though, showing low suitability scores in parts of Namibia, specifically south of Walvis Bay (ROI 2), in Sossusvlei (ROI 3) and Elizabeth Bay (ROI 4, Fig 1B). Our field surveys substantially corroborated the niche modelling (Fig 1A and 1B), resulting in high specificity and sensitivity scores (1 and 0.97, respectively). While all modelled presences were validated in the field, only one false absence was predicted (at Sylvia Hill, Namibia, near ROI 3).
Niche divergence between ranges
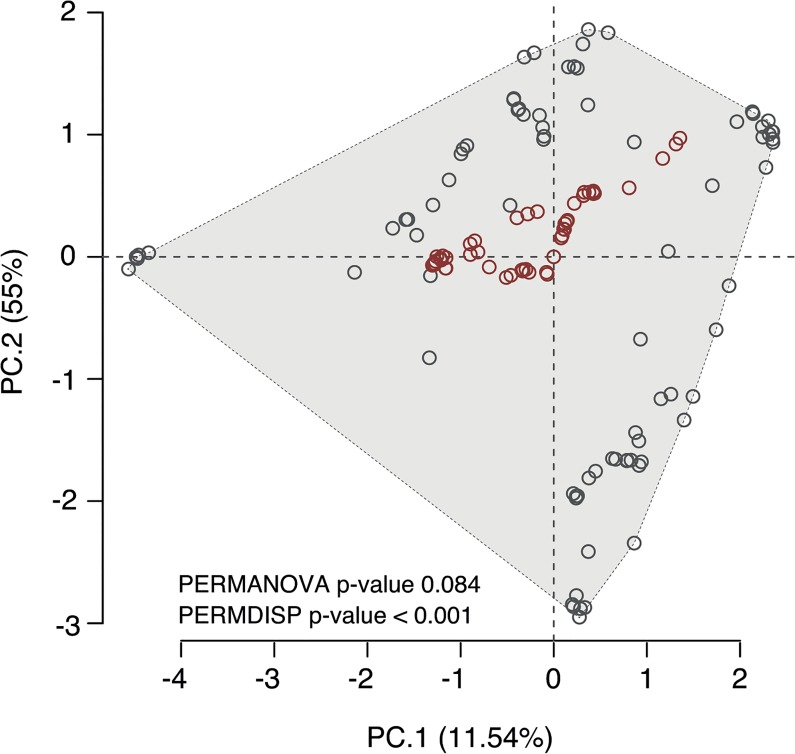
The first two PCA components performed to infer niche divergence explained 66.54% of the variability found in the environmental data. This analysis showed that the invasive occurrences use part of the native environmental space (Fig 3). PERMANOVA and PERMDISP further confirmed this, indicating that a niche shift by M. galloprovincialis during its spread through southern Africa was unlikely (PERMANOVA Pseudo-F: 2.801; p: 0.084) and that the native ecological niche is wider (i.e., more dispersed) than the invaded one (PERMDISP F: 138.1; p: < 0.001).
Fig 3. Principal Component Analysis (PCA) of the environmental space used by native (grey circles) and non-native (red circles) occurrences of M. galloprovincialis.
The grey polygon indicates the overall environmental space of the species. The significance levels of the permutational multivariate ANOVA (PERMANOVA) and permutational analysis of multivariate dispersion (PERMDISP) to test for niche divergence between native and non-native ranges are shown.
M. galloprovincialis dispersal potential
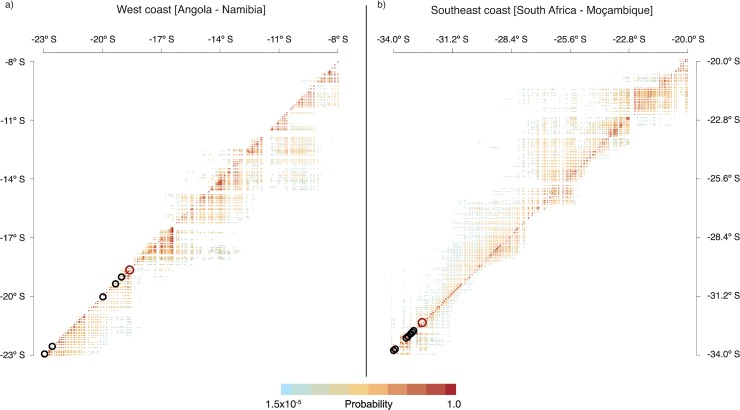
The particle simulations fed by HYCOM velocity fields over the entire 5-year period allowed the release of 5040 particles per site (3,512,880 tracked particles in total). The aggregated trajectories resulted in different connectivity matrices corresponding to how long the passive particles were allowed to drift (30 or 90 days of PLD) and to where the simulations took place (west or southeast coasts). Regarding the experiments using contrasting PLDs, we verified high resemblances (Fig 4; S2 Fig), no statistical differences and strong correlations between connectivity matrices (west coast: Mantel r = 0.944, p-value < 0.001; southeast coast: Mantel r = 0.927, p-value < 0.001). Therefore, we centre further analysis and assumptions on the matrices produced for the west and southeast coasts using 30 days PLD, which is the more likely scenario for pelagic states of M. galloprovincialis. These matrices showed a high probability for larval retention (matrix’ diagonals), although their general trends were found to be contrasting. The particles tracked on the west coast (Fig 4A) displayed a marked northward trend, with an exception from Benguela (12.50° S) to Tombua (16.50° S), where particles also moved southward, though for shorter distances. On the southeast coast of Africa (Fig 4B) the particles moved mostly along southward trajectories. In this region, with the exception of a few oceanographic discontinuities, particles were also found to move northward, though for shorter distances when compared to the general trends for this region.
Fig 4. Potential connectivity matrices for the Lagrangian Particle Simulations performed on the (a) west and (b) southeast coasts of southern Africa using a PLD of 30 days.
Black circles show locations where M. galloprovincialis occurs, while the open red circles show where the niche models predicted northern edges.
The inferred trajectories indicate that particles released from Rocky Point and Maui Bay (northern locations where M. galloprovincialis occurs on the west coast) have little probability of moving northward (probabilities < 0.05) due to a strong oceanographic discontinuity found at Cape Frio (18.50° S; close to ROI 1, Fig 1). On the southeast coast, particles released from Kidd’s Beach and Kayser’s Beach (close to the most easterly locations for M. galloprovincialis on the southeast coast) may move further to the northeast, up to the shores located at 32.60° S (probabilities > 0.05, Fig 4). In a year-to-year stepping-stone scenario, particles released from these two locations could move further to the northeast, although they never crossed the oceanographic discontinuities found at Durban (29.80° S) or near Kosi Bay (27.00° S).
Discussion
The distribution of M. galloprovincialis in southern Africa
The contribution of each environmental predictor to the models was corroborated by previous studies focused on the physiology of M. galloprovincialis, which showed that extreme air and sea temperatures greatly influence its growth and survival [26,49–51]. The strong effects of temperature were further substantiated by the distributional limits inferred by the models, which coincide with steep transitions between cool temperate and warmer subtropical climates. The first is located in the proximity of the Angola-Benguela Front (16oS; [77]). This region lies at the convergence of the south-flowing Angola Current and the north-flowing cold water of the Benguela upwelling system and corresponds to the northern boundary of coastal upwelling off the Namibian coast [78–79]. The second distributional limit for M. galloprovincialis occurs on the South African southeast coast (32oS). There, the continental shelf gradually widens, deflecting the warm Agulhas current, which flows to the south-west following the continental break, away from the coast, thus reducing sea surface temperatures significantly [80]. Because warm waters tend to have less nutrients than cold waters [81], the distributional limits of M. galloprovincialis may also be affected by unfavourable trophic conditions beyond these boundaries, in addition to prohibitive thermal conditions. This effect could be particularly evident during the austral summer, when upwelling conditions there are minimal [82], reducing the availability of plankton throughout southern Africa [83].
The additional environmental predictors used in our framework also produced fair contributions to the accuracy of models. Several authors have shown that distribution patterns of several Mytilus spp. and their hybrids correlate with salinity gradients (e.g., [84–85]). The influence of salinity is further supported by laboratory experimental evidence showing that it affects the physiology (i.e. heart rate) and behaviour (i.e. valve closure) of Mytilidae [49]. Furthermore, low cloud cover may interact significantly with other environmental variables affecting the thermal stress experienced by intertidal organisms [86] by reducing the importance of solar radiation and therefore lowering mussels’ body temperatures significantly [87]. In fact, this is a prominent feature in the study region, particularly throughout the Namibian shores, where the interaction between cold Benguela water and air temperature saturates the low altitude climate with moisture from the sea.
The total agreement found between modelled occurrences and the realized invasive distribution (sensitivity = 1) allowed us to deduce that M. galloprovincialis has already occupied the entire ~2800 km of suitable habitat in southern Africa since its arrival in the late 1970s. Soon after its arrival in South Africa, M. galloprovincialis spread rapidly to the north at an average speed of 115 km yr–1 and to the south at about 25 km yr–1 [26]. In the last 20 years, its expansion on the south coast has essentially ceased, and it has previously been suggested that it might have now reached stability at its eastern limit [88]. Our study is the first to explicitly assess the distribution of this invader and to evaluate environmental determinants of its distributional limits. Furthermore, our results suggest that such rapid spread was not based on adaptation to novel environmental conditions, as niche adaptation was not demonstrated; the invasive populations inhabiting southern Africa shores currently use a narrower part of the native environmental space. Rather the spread of M. galloprovincialis must have relied on very efficient dispersal of planktotrophic larvae and the better physiological performance of the species compared to native mussels (e.g., [37]).
While our distribution models only considered climate predictors, biotic conditions may also play an important role in the success of a potential invasion. An invasive species must interact with the native species and such interactions can be fundamental to invasion dynamics. On the west coast, the spread of M. galloprovincialis was likely facilitated by its superior competitive characteristics compared to the native mussels Aulcaomya ater and Choromytilus meridionalis, and it rapidly displaced them to become the dominant intertidal mussel [27]. In contrast, on the south coast, M. galloprovincialis interacts with a different native species, Perna perna, and the two show partial habitat segregation [26], largely based on their differing competitive abilities across the steep environmental gradient of the intertidal [89].
Dispersal of larvae beyond present limits
Our dispersal simulations are in agreement with the directionality of two main oceanographic features characterizing the western and southeastern coasts, suggesting that the methodology implemented here can provide a reasonable estimate of particle transport across main current flows (as previously shown by [17]). On the west coast, particles followed the predominant northward flow of the Benguela current and, to a lesser degree, the flow of the Angola Current (from 12.50° to 16.50° S; [68]). Both currents deflect westwards, bringing intense upwelling and transporting particles offshore [77,90]. This effect was particularly evident at Cape Frio, at the northern boundary of the Benguela front, where we inferred a permanent dispersal barrier that limits the transport of M. galloprovincialis larvae to latitudes further north [90–91], even when considering extreme pelagic durations of up to 90 days (S2 Fig).
All suitable habitat has been occupied by M. galloprovincialis along the west coastline as far as the northern distributional limit. This is probably a result of two main factors: a) the species’ high reproductive output and capacity to colonize new areas [26,92–93] and b) the strong northward flow of the Benguela Current, which would have favoured an initial, strongly unidirectional spread of M. galloprovincialis along this coast [34]. Although these factors would promote rapid spread of the species, environmental conditions are unsuitable beyond the present northern limit; probably inhibiting any settlement of larvae passing through the physical barrier at Cape Frio.
On the southeast coast, the main trend observed in the simulations was caused by the prevailing fast-flowing Agulhas Current, which pushed most particles southwestwards. This is in agreement with previous studies that employed nearshore drogues. Drifters released off the south coast were caught by the Agulhas Current and moved primarily offshore and towards the southwest [94]. However, in contrast to our findings, there was remarkably little overlap between the trajectories of drifters released off the south coast and those released on the east coast, suggesting that ocean dynamics may limit larval dispersal between the two regions. Instead, our simulations supported some potential eastward movement of drifting larvae, beyond the present limits of M. galloprovincialis along this coast (East London, South Africa, Fig 1B). This may be associated with wind-driven surface currents as they have been previously demonstrated to be an important element of mussel larvae transport along the southeast coast [68]. Inshore counter-currents that lie closer to the coast (< 2–3 km) than the release points of drogues may also allow larvae to disperse to the northeast against the general trend imposed by the strong Agulhas Current. In fact, larvae of M. galloprovincialis (identified following [26]) have been recently collected using a plankton pump near Mazeppa Bay (~85 km east of East London, 32° 29'S 28° 39'E) at very low densities (1 M. galloprovincialis larvae per ~900 L of seawater) [95]. Thus, occasional dispersal as much as 150 km beyond the present distributional limit does occur, although our simulations indicate that larvae are unlikely to cross strong environmental discontinuities further to the northeast at Durban and Kosi Bay.
As larvae can pass beyond the present established range, the complete absence of adults/recruits beyond established ranges (as inferred by field surveys and niche models) shows that environmental conditions are major drivers of local selection. Both northern ranges of M. galloprovincialis currently coincide with a strong environmental transition between the warm sea temperate (15–22°C) and subtropical bioregions (22–27°C) [96]. These boundaries characterizing the species’ one-dimensional distribution along the coastline presumably indicate a permanent physiological barrier, beyond which environmental conditions fall outside this species’ tolerances. Such steep transitions defined by climate are also phylogenetic breaks for two other coastal species, the native mussel P. perna and the estuarine mudprawn Upogebia africana [97]. It has been suggested that as climate change raises temperatures in the near future, the niche of M. galloprovincialis may become more common throughout the globe, thereby favouring the further spread of this species [51]. While this may be true for other warm-temperate regions, our results suggest that the distribution of M. galloprovincialis may become more constrained in southern Africa due to the advance of the subtropical isotherms to higher latitudes [98], where this species is already present. Furthermore, because upwelling systems of the world are predicted to become more intense in the face of climatic changes [99], the current oceanographic discontinuities inferred in our study may become stronger and more permanent, further limiting the distribution of this species.
We draw our main conclusions that the limiting of M. galloprovincialis post-invasion spread is strictly through ocean currents. While our simulations are based on the actual oceanographic patterns of southern Africa, which are presumably the main drivers of the spread of this species after its earlier colonisation stages, we cannot rule out dispersal thorough subsequent, human-mediated introductions. It is known that the maritime network of cargo ships plays a crucial role, with seawater discharges from ballast tanks and hull fouling seen as major vectors for biological invasions between ports worldwide [100–102]. These processes may have assisted the spread of M. galloprovincialis in our study region, particularly on the southern shores, where major ports structure a local network of trading that has intensified in the last decades between Cape Town, Port Elizabeth, East London and Durban (ROI 5–8, Fig 1) [102–103]. Nowadays, since all suitable habitats are fully occupied (as shown by our results), these nodes may be contributing to a mere homogenization effect among populations of M. galloprovincialis (e.g., [104]) and not to promote the further spread of the species throughout southern African shores.
Our main findings broaden our knowledge about the processes driving the spread of M. galloprovincialis, but may be extended to the global invasion dynamics of marine species relying on pelagic larvae as the primary dispersal vector. A recent assessment of invasive marine species in our study region revealed 86 introduced and 39 cryptogenic species, of which 13 are bivalves [105]. Among these, Semimytilus algosus has recently spread along the west coast of South Africa [106] using an identical dispersal strategy as M. galloprovincialis ([107] and references therein). The distributional range of S. algosus now extends for approximately 500km and it occupies lower portions of the mussel zone below M. galloprovincialis [106]. Given the high pelagic larvae duration and strong competitive abilities of both species, it is likely that environmental conditions alone play the crucial role limiting their spread from the initial sources of introduction. If ocean currents favour dispersal of other bivalve species with the same potential for spreading and competition, invasions may cover broad regions (in the orders of 1000s km) in just a few decades, reshaping the population structure of native intertidal communities, like those already impacted in southern Africa by M. galloprovincialis and S. algosus [105].
Supporting Information
Credits for the background of both maps: General Bathymetric Chart of the Oceans (GEBCO).
(TIF)
Black circles show locations where M. galloprovincialis occurs, while the open red circles show where the niche models predicted northern edges.
(TIF)
(CSV)
Acknowledgments
Financial support information was stated during the submission process (on-line form).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Portuguese National Science Foundation (FCT, project EXCL/AAG-GLO/0661/2012, EXPL/BIA-BIC/1471/2012 and fellowship CCMAR/BPD/0045/2013 to JA), and partially supported by a bilateral project (Acordo Portugal-Tunisia: 2010/11, to GIZ), the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation.
References
- 1. Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, O’Connell C, et al. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric Ecosyst Environ. 2001;84: 1–20. 10.1016/S0167-8809(00)00178-X [DOI] [Google Scholar]
- 2. Bax N, Williamson A, Aguero M, Gonzalez E, Geeves W. Marine invasive alien species: A threat to global biodiversity. Mar Policy. 2003;27: 313–323. 10.1016/S0308-597X(03)00041-1 [DOI] [Google Scholar]
- 3. Molnar JL, Gamboa RL, Revenga C, Spalding MD. Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ. 2008;6: 485–492. 10.1890/070064 [DOI] [Google Scholar]
- 4. Gallardo B, Aldridge DC. The “dirty dozen”: Socio-economic factors amplify the invasion potential of 12 high-risk aquatic invasive species in Great Britain and Ireland. J Appl Ecol. 2013;50: 757–766. 10.1111/1365-2664.12079 [DOI] [Google Scholar]
- 5. Elton CS, The Ecology of Invasions by Animals and Plants London: University of Chicago Press; 2000. [Google Scholar]
- 6. Richardson DM, Pyšek P. Fifty years of invasion ecology—The legacy of Charles Elton. Divers Distrib. 2008;14: 161–168. 10.1111/j.1472-4642.2007.00464.x [DOI] [Google Scholar]
- 7. Thresher RE, Kuris AM. Options for managing invasive marine species. Biol Invasions. 2004;6: 295–300. 10.1023/B:BINV.0000034598.28718.2e [DOI] [Google Scholar]
- 8. Quinn A, Gallardo B, Aldridge DC. Quantifying the ecological niche overlap between two interacting invasive species: The zebra mussel (Dreissena polymorpha) and the quagga mussel (Dreissena rostriformis bugensis). Aquat Conserv Mar Freshw Ecosyst. 2014;24: 324–337. 10.1002/aqc.2414 [DOI] [Google Scholar]
- 9. Schneider KR, Helmuth B. Spatial variability in habitat temperature may drive patterns of selection between an invasive and native mussel species. Mar Ecol Prog Ser. 2007;339: 157–167. 10.3354/meps339157 [DOI] [Google Scholar]
- 10. Hastwell GT, Daniel AJ, Vivian-Smith G. Predicting invasiveness in exotic species: Do subtropical native and invasive exotic aquatic plants differ in their growth responses to macronutrients? Divers Distrib. 2008;14: 243–251. 10.1111/j.1472-4642.2007.00367.x [DOI] [Google Scholar]
- 11. Sarà G, Palmeri V, Rinaldi A, Montalto V, Helmuth B. Predicting biological invasions in marine habitats through eco-physiological mechanistic models: A case study with the bivalve Brachidontes pharaonis . Divers Distrib. 2013;19: 1235–1247. 10.1111/ddi.12074 [DOI] [Google Scholar]
- 12. Soberón J, Nakamura M. Niches and distributional areas: concepts, methods, and assumptions. Proc Natl Acad Sci U S A. 2009;106 Suppl 2: 19644–19650. 10.1073/pnas.0901637106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tonn W, Kornis MS, Vander Zanden MJ. Forecasting the distribution of the invasive round goby (Neogobius melanostomus) in Wisconsin tributaries to Lake Michigan. Can J Fish Aquat Sci. 2010;87: 553–562. 10.1139/F10-002 [DOI] [Google Scholar]
- 14. Viard F, Ellien C, Dupont L. Dispersal ability and invasion success of Crepidula fornicata in a single gulf: Insights from genetic markers and larval-dispersal model. Helgol Mar Res. 2006;60: 144–152. 10.1007/s10152-006-0033-8 [DOI] [Google Scholar]
- 15. Mora C, Treml EA, Roberts J, Crosby K, Roy D, Tittensor DP. High connectivity among habitats precludes the relationship between dispersal and range size in tropical reef fishes. Ecography. 2012;35: 89–96. 10.1111/j.1600-0587.2011.06874.x [DOI] [Google Scholar]
- 16. Cowen RK, Sponaugle S. Larval dispersal and marine population connectivity. Ann Rev Mar Sci. 2009;1: 443–466. 10.1146/annurev.marine.010908.163757 [DOI] [PubMed] [Google Scholar]
- 17. Fossette S, Putman NF, Lohmann KJ, Marsh R, Hays GC. A biologist’s guide to assessing ocean currents: A review. Mar Ecol Prog Ser. 2012;457: 285–301. 10.3354/meps09581 [DOI] [Google Scholar]
- 18. Keller RP, Frang K, Lodge DM. Preventing the spread of invasive species: Economic benefits of intervention guided by ecological predictions. Conserv Biol. 2008;22: 80–88. 10.1111/j.1523-1739.2007.00811.x [DOI] [PubMed] [Google Scholar]
- 19. Mariani P, MacKenzie BR, Iudicone D, Bozec A. Modelling retention and dispersion mechanisms of bluefin tuna eggs and larvae in the northwest Mediterranean Sea. Prog Oceanogr. 2010;86: 45–58. 10.1016/j.pocean.2010.04.027 [DOI] [Google Scholar]
- 20. Hamann M, Grech A, Wolanski E, Lambrechts J. Modelling the fate of marine turtle hatchlings. Ecol Modell. 2011;222: 1515–1521. 10.1016/j.ecolmodel.2011.02.003 [DOI] [Google Scholar]
- 21. Ospina-álvarez A, Palomera I, Parada C. Changes in egg buoyancy during development and its effects on the vertical distribution of anchovy eggs. Fish Res. 2012;117–118: 86–95. 10.1016/j.fishres.2011.01.030 [DOI] [Google Scholar]
- 22. Chassignet EP, Hurlburt HE, Smedstad OM, Halliwell GR, Hogan PJ, Wallcraft AJ, et al. The HYCOM (HYbrid Coordinate Ocean Model) data assimilative system. J Mar Syst. 2007;65: 60–83. 10.1016/j.jmarsys.2005.09.016 [DOI] [Google Scholar]
- 23. Butler IV MJ, Paris CB, Goldstein JS, Matsuda H, Cowen RK. Behavior constrains the dispersal of long-lived spiny lobster larvae. Mar Ecol Prog Ser. 2011;422: 223–237. 10.3354/meps08878 [DOI] [Google Scholar]
- 24. Fiksen Ø, Jørgensen C, Kristiansen T, Vikebø F, Huse G. Linking behavioural ecology and oceanography: Larval behaviour determines growth, mortality and dispersal. Mar Ecol Prog Ser. 2007;347: 195–205. 10.3354/meps06978 [DOI] [Google Scholar]
- 25. Jones GP, Jones GP, Srinivasan M, Srinivasan M, Almany GR, Almany GR. Population connectivity and conservation of marine biodiversity. Oceanography. 2007;20: 100–111. 10.5670/oceanog.2007.33 [DOI] [Google Scholar]
- 26. Bownes SJ, McQuaid CD. Will the invasive mussel Mytilus galloprovincialis Lamarck replace the indigenous Perna perna L. on the south coast of South Africa? J Exp Mar Bio Ecol. 2006;338: 140–151. 10.1016/j.jembe.2006.07.006 [DOI] [Google Scholar]
- 27.Griffiths CL, Hockey PAR, Van Erkom Schurink C, Le Roux PJ. Marine invasive aliens on South African shores: implications for community structure and tropillc functioning. Afr J Mar Sci. 1992;713–722. 10.2989/02577619209504736 [DOI]
- 28. Elith J, Leathwick JR. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu Rev Ecol Evol Syst. 2009;40: 677–697. 10.1146/annurev.ecolsys.110308.120159 [DOI] [Google Scholar]
- 29. Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson AT, Guisan A. Evidence of climatic niche shift during biological invasion. Ecol Lett. 2007;10: 701–709. 10.1111/j.1461-0248.2007.01060.x [DOI] [PubMed] [Google Scholar]
- 30. Fitzpatrick MC, Weltzin JF, Sanders NJ, Dunn RR. The biogeography of prediction error: Why does the introduced range of the fire ant over-predict its native range? Glob Ecol Biogeogr. 2007;16: 24–33. 10.1111/j.1466-8238.2006.00258.x [DOI] [Google Scholar]
- 31. Randin CF, Dirnböck T, Dullinger S, Zimmermann NE, Zappa M, Guisan A. Are niche-based species distribution models transferable in space? J Biogeogr. 2006;33: 1689–1703. 10.1111/j.1365-2699.2006.01466.x [DOI] [Google Scholar]
- 32. Beaumont LJ, Gallagher R V., Thuiller W, Downey PO, Leishman MR, Hughes L. Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib. 2009;15: 409–420. 10.1111/j.1472-4642.2008.00547.x [DOI] [Google Scholar]
- 33. Hilbish TJ, Mullinax A, Dolven SI, Meyer A, Koehn RK, Rawson PD. Origin of the antitropical distribution pattern in marine mussels (Mytilus spp.): routes and timing of transequatorial migration. Marine Biology. 2000;136: 69–77. 10.1007/s002270050010 [DOI] [Google Scholar]
- 34.McQuaid CD, Porri F, Nicastro KR, Zardi GI. Simple, scale-dependent patterns emerge from very complex effects: an example from the intertidal mussels Mytilus galloprovincialis and Perna perna. In: Hughes RN, Hughes DJ, Smith IP, Dale AC (Eds). Oceanogr Mar Biol. 2015;53: 127–156 (in press)
- 35. Zardi GI, Nicastro KR, McQuaid CD, Rius M, Porri F. Hydrodynamic stress and habitat partitioning between indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels: Constraints of an evolutionary strategy. Mar Biol. 2006;150: 79–88. 10.1007/s00227-006-0328-y [DOI] [Google Scholar]
- 36. Nicastro KR, Zardi GI, McQuaid CD, Stephens L, Radloff S, Blatch GL. The role of gaping behaviour in habitat partitioning between coexisting intertidal mussels. BMC Ecol. 2010;10: 17 10.1186/1472-6785-10-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee JE, Chown SL. Mytilus on the move: Transport of an invasive bivalve to the Antarctic. Mar Ecol Prog Ser. 2007;339: 307–310. 10.3354/meps339307 [DOI] [Google Scholar]
- 38. Grant WS, Cherry MI. Mytilus galloprovincialis Lmk. in Southern Africa. J Exp Mar Bio Ecol. 1985;90: 179–191. 10.1016/0022-0981(85)90119-4 [DOI] [Google Scholar]
- 39. Robinson T, Griffiths C, McQuaid C, Rius M. Marine alien species of South Africa—status and impacts. Afr J Mar Sci. 2005;27: 297–306. 10.2989/18142320509504088 [DOI] [Google Scholar]
- 40. Bownes SJ, McQuaid CD. Mechanisms of habitat segregation between an invasive and an indigenous mussel: Settlement, post-settlement mortality and recruitment. Mar Biol. 2009;156: 991–1006. 10.1007/s00227-009-1143-z [DOI] [Google Scholar]
- 41. Kohler SA, Connan M, Hill JM, Mablouké C, Bonnevie B, Ludynia K, et al. Geographic variation in the trophic ecology of an avian rocky shore predator, the African black oystercatcher, along the southern African coastline. Mar Ecol Prog Ser. 2011;435: 235–249. 10.3354/meps09215 [DOI] [Google Scholar]
- 42.DAFF. Department of Agriculture, Forestry and Fisheries Aquaculture Annual Report 2011. South Africa. Department of Agriculture, Forestry and Fisheries Aquaculture; 2012.
- 43. Verbruggen H, Tyberghein L, Belton GS, Mineur F, Jueterbock A, Hoarau G, et al. Improving Transferability of Introduced Species’ Distribution Models: New Tools to Forecast the Spread of a Highly Invasive Seaweed. PLoS One. 2013;8 10.1371/journal.pone.0068337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bierne N, Borsa P, Daguin C, Jollivet D, Viard F, Bonhomme F, et al. Introgression patterns in the mosaic hybrid zone between Mytilus edulis and M. galloprovincialis . Mol Ecol. 2003;12: 447–461. 10.1046/j.1365-294X.2003.01730.x [DOI] [PubMed] [Google Scholar]
- 45. Rawson PD, Agrawal V, Hilbish TJ. Hybridization between the blue mussels Mytilus galloprovincialis and M. trossulus along the Pacific coast of North America: Evidence for limited introgression. Mar Biol. 1999;134: 201–211. 10.1007/s002270050538 [DOI] [Google Scholar]
- 46. Crase B, Liedloff AC, Wintle B A. A new method for dealing with residual spatial autocorrelation in species distribution models. Ecography (Cop). 2012;35: 879–888. 10.1111/j.1600-0587.2011.07138.x [DOI] [Google Scholar]
- 47. Dormann CF. Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Glob Ecol Biogeogr. 2007;16: 129–138. 10.1111/j.1466-8238.2006.00279.x [DOI] [Google Scholar]
- 48. Waltari E, Hijmans RJ, Peterson AT, Nyári ÁS, Perkins SL, Guralnick RP. Locating pleistocene refugia: Comparing phylogeographic and ecological niche model predictions. PLoS One. 2007;2: e563 10.1371/journal.pone.0000563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Braby CE, Somero GN. Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). J Exp Biol. 2006;209: 2554–2566. 10.1242/jeb.02259 [DOI] [PubMed] [Google Scholar]
- 50. Braby CE, Somero GN. Ecological gradients and relative abundance of native (Mytilus trossulus) and invasive (Mytilus galloprovincialis) blue mussels in the California hybrid zone. Mar Biol. 2006;148: 1249–1262. 10.1007/s00227-005-0177-0 [DOI] [Google Scholar]
- 51. Schneider KR. Heat stress in the intertidal: comparing survival and growth of an invasive and native mussel under a variety of thermal conditions. Biol Bull. 2008; 215: 253–264. Available: http://www.ncbi.nlm.nih.gov/pubmed/19098146 [DOI] [PubMed] [Google Scholar]
- 52. Assis J, Serrão EA, Claro B, Perrin C, Pearson GA. Climate-driven range shifts explain the distribution of extant gene pools and predict future loss of unique lineages in a marine brown alga. Mol Ecol. 2014;23: 2797–2810. 10.1111/mec.12772 [DOI] [PubMed] [Google Scholar]
- 53. Donlon CJ, Martin M, Stark J, Roberts-Jones J, Fiedler E, Wimmer W. The Operational Sea Surface Temperature and Sea Ice Analysis (OSTIA) system. Remote Sens Environ. 2012;116: 140–158. 10.1016/j.rse.2010.10.017 [DOI] [Google Scholar]
- 54. Levitus S, Antonov JI, Baranova OK, Boyer TP, Coleman CL, Garcia HE, et al. The World Ocean Database. Data Sci J. 2013;12: WDS229–WDS234. 10.2481/dsj.WDS-041 [DOI] [Google Scholar]
- 55. Dee DP, Uppala SM, Simmons AJ, Berrisford P, Poli P, Kobayashi S, et al. The ERA-Interim reanalysis: Configuration and performance of the data assimilation system. Q J R Meteorol Soc. 2011;137: 553–597. 10.1002/qj.828 [DOI] [Google Scholar]
- 56. Neiva J, Assis J, Fernandes F, Pearson GA, Serrão EA. Species distribution models and mitochondrial DNA phylogeography suggest an extensive biogeographical shift in the high-intertidal seaweed Pelvetia canaliculata. J Biogeogr. 2014;41: 1137–1148. 10.1111/jbi.12278 [DOI] [Google Scholar]
- 57. Elith J, Leathwick JR. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu Rev Ecol Evol Syst. 2009;40: 677–697. 10.1146/annurev.ecolsys.110308.120159 [DOI] [Google Scholar]
- 58. De’ath G. Boosted trees for ecological modeling and prediction. Ecology. 2007;88: 243–251. 10.1890/0012-9658(2007)88[243:BTFEMA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 59. Barbet-Massin M, Jiguet F, Albert CH, Thuiller W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol Evol. 2012;3: 327–338. 10.1111/j.2041-210X.2011.00172.x [DOI] [Google Scholar]
- 60. Calenge C, Darmon G, Basille M, Loison A, Jullien JM. The factorial decomposition of the Mahalanobis distances in habitat selection studies. Ecology. 2008;89: 555–566. 10.1890/06-1750.1 [DOI] [PubMed] [Google Scholar]
- 61. Chefaoui RM, Lobo JM. Assessing the effects of pseudo-absences on predictive distribution model performance. Ecol Modell. 2008;210: 478–486. 10.1016/j.ecolmodel.2007.08.010 [DOI] [Google Scholar]
- 62. Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. Journal of Animal Ecology. 2008;77: 802–813. 10.1111/j.1365-2656.2008.01390.x [DOI] [PubMed] [Google Scholar]
- 63. Allouche O, Tsoar A, Kadmon R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J Appl Ecol. 2006;43: 1223–1232. 10.1111/j.1365-2664.2006.01214.x [DOI] [Google Scholar]
- 64. Araújo MB, Araújo MB, New M, New M. Ensemble forecasting of species distributions. Trends Ecol Evol. 2007;22: 42–7. 10.1016/j.tree.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 65. Moreno-Letelier A, Ortíz-Medrano A, Piñero D. Niche divergence versus neutral processes: combined environmental and genetic analyses identify contrasting patterns of differentiation in recently diverged pine species. PLoS One. 2013;8: e78228 10.1371/journal.pone.0078228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26: 32–46. 10.1046/j.1442-9993.2001.01070.x 11469182 [DOI] [Google Scholar]
- 67. Anderson MJ. PERMDISP: A FORTRAN computer program for permutational analysis of multivariate dispersions (for any two-factor ANOVA design) using permutation tests Department of Statistics, University of Auckland, New Zealand: 2004. [Google Scholar]
- 68. McQuaid CD, Phillips TE. Limited wind-driven dispersal of intertidal mussel larvae: In situ evidence from the plankton and the spread of the invasive species Mytilus galloprovincialis in South Africa. Mar Ecol Prog Ser. 2000;201: 211–220. 10.3354/meps201211 [DOI] [Google Scholar]
- 69. Chassignet EP, Hurlburt HE, Smedstad OM, Halliwell GR, Hogan PJ, Wallcraft AJ, et al. The HYCOM (HYbrid Coordinate Ocean Model) data assimilative system. J Mar Syst. 2007;65: 60–83. 10.1016/j.jmarsys.2005.09.016 [DOI] [Google Scholar]
- 70. Lett C, Verley P, Mullon C, Parada C, Brochier T, Penven P, et al. A Lagrangian tool for modelling ichthyoplankton dynamics. Environ Model Softw. 2008;23: 1210–1214. 10.1016/j.envsoft.2008.02.005 [DOI] [Google Scholar]
- 71. Nicastro KR, Zardi GI, McQuaid CD. Differential reproductive investment, attachment strength and mortality of invasive and indigenous mussels across heterogeneous environments. Biol Invasions. 2010;12: 2165–2177. 10.1007/s10530-009-9619-9 [DOI] [Google Scholar]
- 72. Suchanek TH. The ecology of Mytilus edulis L. in exposed rocky intertidal communities. J Exp Mar Bio Ecol. 1978;31: 105–120. 10.1016/0022-0981(78)90139-9 [DOI] [Google Scholar]
- 73. Jørgensen CB. Mortality, growth, and grazing impact of a cohort of bivalve larvae, Mytilus edulis L. Ophelia. 1981;20: 185–192. 10.1080/00785236.1981.10426570 [DOI] [Google Scholar]
- 74. Bayne BL. Growth and the delay of metamorphosis of the larvae of Mytilus edulis (L.). Ophelia. Taylor & Francis; 1965;2: 1–47. 10.1080/00785326.1965.10409596 [DOI] [Google Scholar]
- 75. Gilg MR, Hilbish TJ. The geography of marine larval dispersal: Coupling genetics with fine-scale physical oceanography. Ecology. 2003;84: 2989–2998. 10.1890/02-0498 [DOI] [Google Scholar]
- 76. R Developement Core Team. R: A Language and Environment for Statistical Computing [Internet] R Foundation for Statistical Computing; 2013. 10.1007/978-3-540-74686-7 [DOI] [Google Scholar]
- 77. Gordon AL, Bosley KT, Aikman F. Tropical Atlantic water within the Benguela upwelling system at 27°S. Deep Sea Res Part 1 Oceanogr Res Pap. 1995;42: 1–12. 10.1016/0967-0637(94)00032-N [DOI] [Google Scholar]
- 78. Branch SF, Road B, Point S, Town C, Africa S. Upwelling in the Southern Benguela Current. Prog Oceanogr. 1980;9: 1–81. [Google Scholar]
- 79. Teske PR, Von der Heyden S, McQuaid CD, Barker NP. A review of marine phylogeography in southern Africa. Afr J Mar Sci. 2011;107: 1–11. 10.4102/sajs.v107i5/6.514 [DOI] [Google Scholar]
- 80. Jackson JM, Rainville L, Roberts MJ, McQuaid CD, Lutjeharms JRE. Mesoscale bio-physical interactions between the Agulhas Current and the Agulhas Bank, South Africa. Cont Shelf Res. 2012;49: 10–24. 10.1016/j.csr.2012.09.005 [DOI] [Google Scholar]
- 81. Kamykowski D, Zentara SJ. Predicting plant nutrient concentrations from temperature and sigma-t in the upper kilometer of the world ocean. Deep Sea Res Part A Oceanogr Res Pap. 1986;33: 89–105. 10.1016/0198-0149(86)90109-3 [DOI] [Google Scholar]
- 82. Hagen E, Feistel R, Agenbag JJ, Ohde T. Seasonal and interannual changes in intense Benguela upwelling (1982–1999). Oceanol Acta. 2001;24: 557–568. 10.1016/S0399-1784(01)01173-2 [DOI] [Google Scholar]
- 83. Huenerlage K, Buchholz F. Krill of the northern Benguela Current and the Angola-Benguela frontal zone compared: Physiological performance and short-term starvation in Euphausia hanseni . J Plankton Res. 2013;35: 337–351. 10.1093/plankt/fbs086 [DOI] [Google Scholar]
- 84. Sarver SK, Foltz DW. Genetic population structure of a species’ complex of blue mussels (Mytilus spp.). Mar Biol 1993;117: 105–112. 10.1007/BF00346431 [DOI] [Google Scholar]
- 85. Bierne N, David P, Langlade A, Bonhomme F. Can habitat specialisation maintain a mosaic hybrid zone in marine bivalves? Mar Ecol Prog Ser. 2002;245: 157–170. 10.3354/meps245157 [DOI] [Google Scholar]
- 86. Helmuth B. Thermal biology of rocky intertidal mussels: Quantifying body temperatures using climatological data. Ecology. 1999;80: 15–34. 10.1890/0012-9658(1999)080[0015:TBORIM]2.0.CO;2 [DOI] [Google Scholar]
- 87. Gilman SE, Wethey DS, Helmuth B. Variation in the sensitivity of organismal body temperature to climate change over local and geographic scales. Proc Natl Acad Sci U S A. 2006;103: 9560–9565. 10.1073/pnas.0510992103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Branch GM, Steffani NC. Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamarck). J Exp Mar Bio Ecol 2004;300: 189–215. 10.1016/j.jembe.2003.12.007 [DOI] [Google Scholar]
- 89. Rius M, McQuaid CD. Wave action and competitive interaction between the invasive mussel Mytilus galloprovincialis and the indigenous Perna perna in South Africa. Mar Biol. 2006;150: 69–78. 10.1007/s00227-006-0322-4 [DOI] [Google Scholar]
- 90. Agenbag JJ, Shannon JV. A suggested physical explanation for the existence of a biological boundary at 24°30′S in the Benguela system. South African J Mar Sci. 1988;6: 119–132. [Google Scholar]
- 91. Zardi GI, McQuaid CD, Teske PR, Barker NP. Unexpected genetic structure of mussel populations in South Africa: Indigenous Perna perna and invasive Mytilus galloprovincialis . Mar Ecol Prog Ser. 2007;337: 135–144. 10.3354/meps337135 [DOI] [Google Scholar]
- 92. Van Erkom Schurink C, Griffiths CL. A comparison of reproductive cycles and reproductive output in four southern African mussel species. Mar Ecol Prog Ser. 1991;76: 123–134. 10.3354/meps076123 [DOI] [Google Scholar]
- 93. Erlandsson J, Pal P, McQuaid CD. Re-colonisation rate differs between co-existing indigenous and invasive intertidal mussels following major disturbance. Mar Ecol Prog Ser. 2006;320: 169–176. 10.3354/meps320169 [DOI] [Google Scholar]
- 94. Zardi GI, Nicastro KR, McQuaid CD, Hancke L, Helmuth B. The combination of selection and dispersal helps explain genetic structure in intertidal mussels. Oecologia. 2011;165: 947–958. 10.1007/s00442-010-1788-9 [DOI] [PubMed] [Google Scholar]
- 95.Hall J. What limits an invasive? Biotic and abiotic effects on the distribution of the invasive mussel Mytilus galloprovincialis on the South African coastline. M.Sc. Thesis. Rhodes University. 2015.
- 96.Harrison TD. Preliminary assessment of the biogeography of fishes in South African estuaries. Mar Freshw Res. 2002;479–490. 10.1071/MF01121 [DOI]
- 97. Teske PR, Papadopoulos I, Newman BK, Dworschak PC, McQuaid CD, Barker NP. Oceanic dispersal barriers, adaptation and larval retention: an interdisciplinary assessment of potential factors maintaining a phylogeographic break between sister lineages of an African prawn. BMC Evol Biol. 2008;8: 341 10.1186/1471-2148-8-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Teske PR, Zardi GI, McQuaid CD, Nicastro KR. Two sides of the same coin: extinctions and originations across the Atlantic/Indian Ocean boundary as consequences of the same climate oscillation Journal Front Biogeogrpahy. 2013;5: 48–59. Available: http://escholarship.org/uc/item/0vb3b7mp [Google Scholar]
- 99. McGregor HV, Dima M, Fischer HW, Mulitza S. Rapid 20th-century increase in coastal upwelling off northwest Africa. Science. 2007;315: 637–639. 10.1126/science.1134839 [DOI] [PubMed] [Google Scholar]
- 100. Ruiz GM, Rawlings TK, Dobbs FC, Drake LA, Mullady T, Huq A, et al. Global spread of microorganisms by ships. Nature. 2000;408: 49–50. 10.1038/35040695 [DOI] [PubMed] [Google Scholar]
- 101. Drake JM, Lodge DM. Hull fouling is a risk factor for intercontinental species exchange in aquatic ecosystems. Aquat Invasions. 2007;2: 121–131. 10.3391/ai.2007.2.2.7 [DOI] [Google Scholar]
- 102. Kaluza P, Kölzsch A, Gastner MT, Blasius B. The complex network of global cargo ship movements. J R Soc Interface. 2010;7: 1093–1103. 10.1098/rsif.2009.0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Foster V, Briceno-Garmendia C. Ports and Shipping: Landlords Needed. In: Foster V, Briceno-Garmendia C, editors. Africa’s Infrastructure: A Time for Transformation. Washington, DC: The World Bank; 2010. pp. 249–258. ISBN: 978–0821380413
- 104.Zardi GI, Nicastro KR, McQuaid CD, Castilho R, Costa J, Serrão EA, Pearson GA. Intraspecific genetic lineages of a marine mussel show behavioural divergence and spatial segregation over a tropical/subtropical biogeographic transition. BMC Evol Biol (Accepted) [DOI] [PMC free article] [PubMed]
- 105. Mead A, Carlton JT, Griffiths CL, Rius M. Revealing the scale of marine bioinvasions in developing regions: A South African re-assessment. Biol Invasions. 2011;13: 1991–2008. 10.1007/s10530-011-0016-9 [DOI] [Google Scholar]
- 106. de Greef K, Griffiths CL, Zeeman Z. Deja vu? A second mytilid mussel, Semimytilus algosus, invades South Africa’s west coast. African J Mar Sci. 2013;35: 37–41. 10.2989/1814232X.2013.829789 [DOI] [Google Scholar]
- 107.Bigatti G, Signorelli JH, Schwindt E. Potential invasion of the Atlantic coast of South America by Semimytilus. 2014;3: 241–246.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Credits for the background of both maps: General Bathymetric Chart of the Oceans (GEBCO).
(TIF)
Black circles show locations where M. galloprovincialis occurs, while the open red circles show where the niche models predicted northern edges.
(TIF)
(CSV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.