Abstract
Pathogenesis-related proteins (PR proteins) play crucial roles in the plant defense system. A novel PRP gene was isolated from highly resistant soybean infected with Phytophthora sojae (P. sojae) and was named GmPRP (GenBank accession number: KM506762). The amino acid sequences of GmPRP showed identities of 74%, 73%, 72% and 69% with PRP proteins from Vitis vinifera, Populus trichocarpa, Citrus sinensis and Theobroma cacao, respectively. Quantitative real-time reverse transcription PCR (qRT-PCR) data showed that the expression of GmPRP was highest in roots, followed by the stems and leaves. GmPRP expression was upregulated in soybean leaves infected with P. sojae. Similarly, GmPRP expression also responded to defense/stress signaling molecules, including salicylic acid (SA), ethylene (ET), abscisic acid (ABA) and jasmonic acid (JA). GmPRP was localized in the cell plasma membrane and cytoplasm. Recombinant GmPRP protein exhibited ribonuclease activity and significant inhibition of hyphal growth of P. sojae 1 in vitro. Overexpression of the GmPRP gene in T2 transgenic tobacco and T2 soybean plants resulted in enhanced resistance to Phytophthora nicotianae (P. nicotianae) and P. sojae race 1, respectively. These results indicated that the GmPRP protein played an important role in the defense of soybean against P. sojae infection.
Introduction
Plants, being sessile, are under constant challenge by threats from an array of biological, chemical, and environmental agents. Every plant is thus forced to evolve its own structural and chemical inducible defense mechanisms for survival through various levels of challenges. These challenges activate a defense system that involves an array of induced mechanisms such as the hypersensitive response [1, 2] and the induction of PR proteins [3]. PR proteins are strongly induced in response to wounding or infection by pathogens, accumulate abundantly at the site of infection, and contribute to systemic acquired resistance (SAR) [4, 5].
The production and accumulation of PR proteins in plants in response to invading pathogens or related abiotic stresses are among the crucial steps in the inducible portion of a plant’s self-defense mechanism [6]. Many PR proteins have been characterized in recent years, and they are classified into 17 families [7, 8]. Some PR proteins have been characterized as chitinases, β-1,3-glucanases [9], ribonucleases [10–13], thaumatin-like proteins (TLPs) [14–16], proteinase inhibitors (PIs) [17, 18], plant defensins (PDFs) [19], and lipid transfer proteins (LTPs) [20].
PR proteins not only accumulate in various parts of normal tissues, but are induced by pathogen infection and improve the defensive capacity of plants [21]. The induction of PR10 gene expression has been demonstrated in various plant species following infection by pathogens [22–24]. PR2 and PR3 are strongly induced when plants respond to wounding or infection by fungal, bacterial, or viral pathogens [25, 26]. The PR6 family can be induced upon inoculation with Phytophthora infestans and Pseudomonas syringae pv. Tomato [27, 28]. The PR5 proteins appear to be mainly involved in plant defensive systems that counteract infection by pathogens [15, 29]. PR4 and PR1 proteins have been reported to have antifungal activity and resistance-related properties in many plant species [10, 30].
To further understand the function of PR genes in plant defense reactions, the expression patterns of PR genes was analyzed by various stimulus. PR expression is known to be regulated by signaling compounds such as ABA, ET, JA, and SA [31–35]. PR genes have been shown to be induced by various abiotic stresses, such as treatments with NaCl, heat, cold, PEG [31], UV irradiation [36], and ozone [37]. Some PR proteins have also been reported to accumulate under specific physiological conditions, such as pollen development [38], leaf senescence, fruit development and ripening [39–41].
Plants activate the expression of different PR genes in response to pathogens to improve the defensive capacity of plants. There are also several reports on overexpressing PR genes, resulting in enhanced tolerance to pathogen infection [42–44]. For example, overexpression of PR5 genes has been shown to enhance resistance to Rhizoctonia solani and Phytophthora infestans [45, 46], and overexpression of CABPR1 in tobacco plants enhances tolerance not only to heavy metal stresses but also to pathogen attack [47].
Although the biological and biochemical functions of PR proteins have been studied for several decades, the molecular mechanisms of many PR proteins remain unknown [8, 48, 49]. Thus far, PR proteins have been identified in numerous plants, including hot pepper [12], corn [13], potato [50], wheat [51], lily [52], rice [53], and soybean [54]. However, some PR proteins were not grouped into the 17 PR protein families and were simply named pathogenesis-related proteins (PRPs). Phytophthora root and stem rot of soybean (Glycine max (L.) Merr.), caused by Phytophthora sojae Kaufmann and Gerdemann, is a destructive disease throughout the soybean-growing regions worldwide [55]. We previously reported, using SSH and cDNA microarrays, that a highly upregulated PRP gene (termed GmPRP) was induced by P. sojae in the highly resistant soybean cultivar ‘Suinong 10’ [56]. However, no further studies have been conducted to examine the expression, localization and biochemical activity of this PRP. The objective of the present study was to conduct a functional analysis of GmPRP in the defense against P. sojae for its possible use as a new tool for the management of Phytophthora root and stem rot in soybean.
Materials and Methods
Plant materials and pathogen inoculation
This study used ‘Suinong 10’, a popular soybean cultivar with high genetic resistance against the predominant P. sojae race 1 in Heilongjiang, China [57]. Seeds of ‘Suinong 10’ were planted in pots filled with sterile vermiculite under a 14-h photoperiod at a light intensity of 350 molm-2 s-1 at 25°C and 10-h darkness at 18°C in a growth chamber. Ten days after planting, seedlings at the first-node stage (V1) [58] were used for various treatments.
For P. sojae treatment, the soybean plants were inoculated with P. sojae zoospores following the methods described by Ward et al. (1979) [59] and Morris et al. (1991) [60] with minor modifications. Zoospores were developed using the procedure of Ward et al. (1979) [59], and the concentration was estimated using a hemocytometer to approximately 1 × 105 spores mL-1. All of the seedlings were incubated in a mist chamber at 25°C, with 100% relative humidity and a 14-h photoperiod at a light intensity of 350 μmolm-2 s-1. The unifoliolate leaves of inoculated ‘Suinong 10’ were harvested at 0, 3, 6, 12, 24, 36, and 72 h after the treatment, immediately frozen in liquid nitrogen, and kept at -80°C until quantitative RT-PCR analysis.
RNA extraction and cDNA analysis
Total RNA was extracted from the leaves of the P. sojae-inoculated soybean plants using a Trizol kit (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. The quality and concentration of the RNA samples were examined by agarose gel electrophoresis and analyzed using a Lambda 35 UV/vis Spectrometer (Perkin Elmer, Massachusetts, USA). Total RNAs were converted into cDNAs using a random oligo dT primer and M-MLV reverse transcriptase according to the manufacturer’s instructions.
Cloning of a novel GmPRP gene
The first-strand reaction product from the obtained cDNA was used to clone the full-length GmPRP. Rapid amplification of cDNA ends (RACE) was conducted from soybean mRNA using the CLONTECH SMART RACE cDNA Amplification Kit (Clontech, USA). The gene-specific primers, GmPRP (F: TGATGACGCCATCTTTAGTACC; R: CGGCTAGAGCAGCACAAGTTCAA), were used to amplify GmPRP gene. The PCRs were performed under the following conditions: 94°C for 3 min, then 30 cycles at 94°C for 30 s, 57°C for 30 s, and 72°C for 30 s, with a final extension at 72°C for 10 min. The PCR products were ligated into the pGM-T Easy vector. The clones containing approximately 600-bp and 250-bp fragments were identified by DNA sequencing. Sequence alignment was conducted using DNAMAN software. A clone containing a fragment of approximately 925 bp was obtained and sequenced.
Protein sequence similarity analysis was performed using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/blast). The programs ClustalW (http://www.ebi.ac.uk/clustalw/) and Multiple Alignment ShowB (http://www.bio-soft.net/sms/index.html) were used for multiple sequence alignment. The potential phosphorylation sites of GmPRP protein were analyzed using NetPhos software (http://www.cbs.dtu.dk/services/NetPhos). The phylogenetic relationships of GmPRP homologs in plants were constructed using the neighbor-joining method with the program MEGA 5.1. The structural features of GmPRP protein were analyzed using the Expert Protein Analysis System (http://www.expasy.org/). The promoter sequences of GmPRP gene upstream of ATG were analyzed using Plantcare software (http://bioinformatics.psb.ugent.be/webtools/Plantc-are/html/).
Quantitative Real-time PCR analysis
Quantitative Real-time PCR (qRT-PCR) was conducted to examine the expression of GmPRP under abiotic (phytohormone and chemical) and biotic (P. sojae) stresses in ‘Suinong 10’ soybean. For phytohormone and chemical treatments, ‘Suinong 10’ soybean leaves were sprayed with 0.5 mM SA, 50 mM ABA, or 100 mM JA and harvested for RNA isolation at 1, 3, 6, 9, 12, and 24 h after the treatments and were subjected to qRT-PCR analysis. For treatment with ET, ‘Suinong 10’ soybean plants were kept in a chamber equilibrated with 5% (v/v) gaseous ethylene. Control experiments were carried out in an identical chamber without ethylene.
The leaves of ‘Suinong 10’ soybean seedlings were inoculated with zoospores of P. sojae race 1 following the methods described by Ward et al. (1979) [59] and Morris et al. (1991) [60] with minor modifications and harvested at 6, 12, 24, 36, 48, and 72 h after the treatment for qRT-PCR analysis. Two specific primers, GmPRP F: TTCAGCCTAAACGGAAGGAAGCCT and GmPRP R: TTGTCGTGAAGGCCTTATGGGATG), were used to determine the expression level of GmPRP. qRT-PCR amplifications were performed using a real-time RT-PCR kit according to the manufacturer’s instructions (Takara, Japan) on the CFX96 TouchTM Real-Time PCR Detection System (BioRad, USA). One microgram of total RNA was used for each reverse transcription. Each amplification reaction was performed with 1 μL of the resultant first-strand cDNA solution, 0.2 μM of each primer and 2×SYBR Green PCR Master Mix, in a total reaction volume of 20 μL. The PCR cycling conditions were as follows: 95°C for 5 s, 60°C for 20 s, 72°C for 20 s for 40 cycles and 60°C for 1 min. The relative levels of GmPRP mRNA were evaluated against the soybean housekeeping gene GmActin 4 (GenBank accession number: AF049106) amplified with specific primer pairs (F: CTTGGAGGATCATGTTCGGTT; R: GCATCACAGT-GCAATCTAGCT). For tissue distribution analysis, the tissue with the lowest expression level was used as calibrator. The relative expression of target gene in different tissues of soybean or in different transgenic lines of tobacco and soybean was calculated using the 2−∆∆CT method, and each assay was conducted with three technological replications and statistically analyzed using Student’s t-test (*P < 0.05, **P < 0.01). Bars indicate the standard error of the mean (SE).
Subcellular localization of the GmPRP protein in onion epidermal cells
To analyzed the subcellular localization of the GmPRP protein, the coding sequences of GmPRP were first amplified using primer pairs for GmPRP-GFP (F: CCCATGGCGTCATCAAGTGT; R: GGACTAGTGCCGGTGTTCCTGAGTAC; the underlined bases are for the restriction sites NcoI and SpeI, respectively). After digestion with NcoI and SpeI, the PCR products was ligated into pCAMBIA1302 vector to produce the recombinant plasmid CaMV35S-GmPRP-GFP. The recombinant plasmid was transformed into the Arabidopsis protoplast cells using the method as described by Yoo et al. (2007) [61]. The vector CaMV35S-GFP was used as a control. After 20 h, GFP fluorescence was observed with a Leica TCSSP2 fluorescent stereomicroscope (Leica Microsystems, Wetzlar, Germany).
Expression, purification and renaturation of the GmPRP protein
The coding region of the GmPRP gene were amplified using specific primers, GmPRP (F: GGAAGATCTTATGGCGTCATCAAGTGT, with the BglII site underlined; R: CCGCTCGAGG-CCGGTGTTCCTGAGTAC-3, with the XhoI site underlined). The PCR products were digested with BglII and XhoI and was ligated into the pET-29b vector. The recombinant plasmid pET29b-GmPRP was transformed into BL21 (DE3) cells for protein expression. BL21 (DE3) strains transformed with the pET29b-GmPRP plasmid were grown in LB medium containing 50 mg/mL kanamycin at 37°C to an absorbance of 0.5 at 600 nm. The cultures were induced by 0.5 mM IPTG. After 4 h of induction, the cells were harvested by centrifugation at 4000 rpm for 10 min at 4°C. The expression and purification of the recombinant protein were performed as previously described by Xu et al. (2014) [50].
Antimicrobial activity assays for recombinant GmPRP protein
The inhibition of the growth of P. sojae by the GmPRP protein was assayed using the method described by Schlumbaum et al. (1986) [62]. P. sojae race 1 was grown on carrot agar plates at 28°C. The sterile filter paper discs were treated with 15 μg, 25 μg, or 35 μg of the renatured recombinant GmPRP protein, and treatment with 35 μg of boiled recombinant GmPRP protein served as the control. The growth zones of the pathogen were observed and photographed using a Canon IXUS 860IS camera after incubation at 28°C for 24 h.
Ribonuclease activity assays with recombinant GmPRP protein
To elucidate the ribonuclease activity of the GmPRP protein, RNase activity assays were performed according to the method described by Bantignies et al. (2000) [63] with minor modifications. Briefly, 2 μg, 4 μg, or 6 μg of purified recombinant protein and 10 μg of total RNA extracted from ‘Suinong 10’ soybean leaves were mixed in separate 20 μL reaction mixtures and incubated at 37°C for 2 h. The amount of solubilized RNA in the supernatant was determined by UV absorbance at 260 nm (OD260) with a NanoDrop ND-1000 spectrophotometer (Thermo). The water containing only dissolved RNA was used as a negative control.
Vector construction and tobacco, soybean transformation
GmPRP was amplified using the pGEM-T easy vector template and gene-specific primers. Two specific primers for GmPRP (F: GGGGGATCCAAAGATGGCGTCATC, with the BamHⅠ site underlined; R: ACAAGCCAGAGCTCCAACAACTGCAAT, with the SacⅠ site underlined) were used to amplify the coding region of the GmPRP gene. The PCR products were cloned into the pGM-T easy vector followed by transformation into E. coli DH5α cells (Shanghai Biotech Inc., Shanghai, China) and sequenced. The PCR products and pCAMBIA3301 (www.camia.org), using the bar gene as the selective marker and GUS as the reporter gene, were digested with BamHⅠ and SacⅠ. The recombinant plasmid pCAMBIA3301-GmPRP was produced by ligating the digested PCR products with the digested pCAMBIA3301 plasmid. E. coli DH5α cells were transformed using the pCAMBIA3301-GmPRP plasmid. The plant expression vector was introduced into Agrobacterium tumefaciens LBA4404 by the freezing and thawing method. ‘Dongnong 50’ soybean, which is susceptible to P. sojae race 1, and ‘Havana 425’ tobacco, which is susceptible to Phytophthora nicotianae Breda, were used for gene transformation experiments. Professor WX Shan of the College of Plant Protection, Northwest Agriculture and Forestry University, China kindly provided the P. nicotianae isolate. The tobacco leaf discs were transformed according to the methods described by Horsch et al. (1985) [64], and the soybean cotyledonary nodes were used as explants transformed with the Agrobacterium-mediated method described by Wang et al. (2008) [65]. The T1 seeds were collected, dried at 25°C, and grown in soil to test the transgenic tobacco and soybean plants. T2 seeds were set the same way as T1 seeds.
PCR analysis of transgenic plants
To confirm transgene insertion into the transgenic tobacco (T2) and soybean plants (T2), genomic DNA was extracted by the CTAB method, and PCR analysis was conducted. Two primers (F: ATATCCGAGCGCCTCGTGCAT, R: GGTCTGCACCATCGTCAACCACT) were designed to target the regions of the bar reporter gene. Using genomic DNA as the template, PCR was performed with a pre-denaturing condition of 94°C for 3 min, followed by 30 cycles at 94°C for 30 s, 68°C for 30 s, 72°C for 30 s, and finally 72°C for 8 min.
Southern hybridization analysis
Southern hybridization analysis of PCR-positive soybean plants (T2) was performed using the DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Cat., Germany) according to the manufacturer’s instructions. Specifically, 20μg of genomic DNA was digested with HindIII and electrophoresed on a 1.0% agarose gel. The DNA was transferred to nylon membranes (Schleicher and Schuell, Keene, NH) using the alkaline transfer protocol and UV cross-linking [66]. The PCR products of the bar genes and the target gene from the plasmid were used as the probes, which were labeled using digoxigenin (DIG)-11-dUTP with DIG High Prime DNA Labeling reagents (Roche, Mannheim, Germany). Hybridization was conducted at 42–45°C, and washing, blocking, and detection were performed according to the manufacturer’s instructions. The Southern hybridization was then exposed to X-ray film (Kodak, Japan) using two intensifying screens at room temperature for 20 min and subsequently developed.
Resistance identification of transgenic tobacco and soybean plants
To investigate whether the GmPRP gene could enhance resistance, inoculum preparation and artificial inoculation procedures were modified from those described by Dou et al. (2003) [67]. The leaves of transgenic tobacco plants that were tested through qRT-PCR were inoculated with a P. nicotianae Breda inoculum, and those of the soybean plants that were tested through qRT-PCR and Southern analysis were inoculated with a P. sojae race 1 inoculum. The leaves were incubated for 72 h in a mist chamber at 25°C and 90% relative humidity under a 14-h photoperiod with light intensity of 350 molm-2 s-1. Non-transgenic leaves were used as controls. The disease symptoms on each leaf were observed and photographed using a Canon IXUS 860IS camera at 24, 48 and 72h after the inoculation.
Results
Cloning and sequencing of GmPRP cDNA
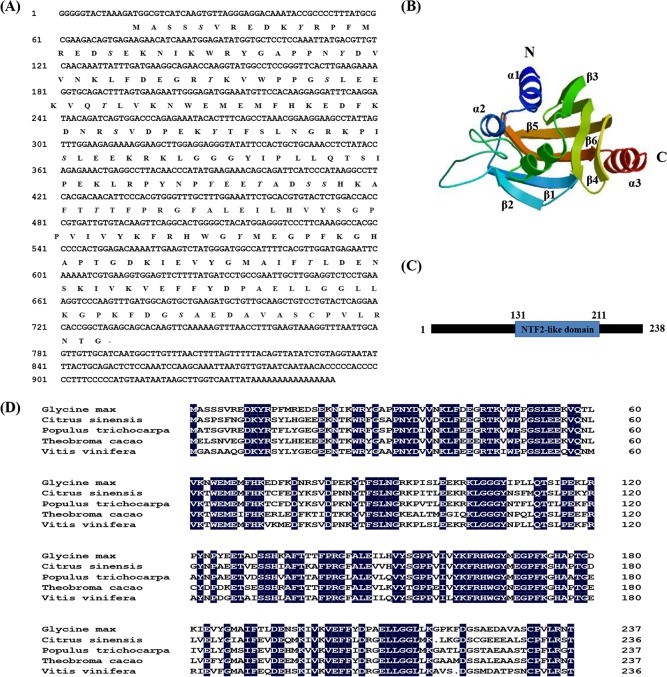
The full-length cDNA sequence was obtained from ‘Suinong 10’ soybean using RACE. The clone, designated GmPRP (GenBank accession no. KM506762), was chosen for further functional analysis. It comprised 952 bp with a single open reading frame (ORF) of 717 nucleotides, encoding a polypeptide of 238 amino acids with a calculated molecular mass of 27.225 kDa and a theoretical PI of 7.07. The nucleotide sequence also showed a 5’ untranslated region (5’ UTR) of 13 nucleotides and a 3’ UTR of 222 nucleotides along with a poly-A signal (AAAAAAAAAAAAAAAA) at 936–952 bp. A database search (http://www.cbs.dtu.dk/services/signalp/) indicated that GmPRP contained no signal peptide. Searches of the NCBI and Phytozome databases (http://www.n.nihcbi.nlm.gov/; http://www.phytozome.net/soybean) revealed that the GmPRP gene had a 694-bp intron and was located on chromosome 6 with two copies. The software NetPhos (http://www.cbs.dtu.dk/services/NetPhos) predicted that GmPRP had eight serines (Ser 5, 19, 52, 79, 96, 131, 132, 223, in bold italics), five tyrosines (Tyr 11, 33, 85, 125, 167, in bold italics), and five lysines (Lys 45, 59, 128, 138, 191, in bold italics) as potential phosphorylation sites (Fig 1A). The 3D structure of the GmPRP protein consisted of a 10-amino-acid C-terminal α-helix (α3) surrounded by a six-stranded antiparallel β-sheet (from β1 to β6) and three N-terminal α-helices (α1, α2 and α3), which are two short α-helices and a long α-helix between the β2 and β3 sheets. The connection sequences between the α-helix and β-strand were short loop structures (Fig 1B). The predicted GmPRP protein contained a conserved motif at residues 131–211 aa that belonged to the NTF2-like superfamily (Fig 1C).
Fig 1. Nucleotide and amino acid sequences of GmPRP cDNA.
(A) Nucleotide and amino acid sequences of GmPRP cDNA. Putative phosphorylation sites are marked in bold italics. (B) Analysis of the 3D structure and conserved motifs of GmPRP. The 3D structure of GmPRP. The N-terminal, C-terminal, α-helix, and β-strand are marked in bold italics. (C) The conserved motif of the GmPRP protein. The predicted GmPRP protein contained a conserved motif at residues 131–211 aa that belonged to the NTF2-like superfamily. (D) Alignments of the amino acid sequences of the GmPRP with other plant PRP proteins. Comparison of the predicted amino acid sequence of GmPRP with other plant PRPs from Vitis vinifera (XP002262980), Populus trichocarpa (XP002306682), Citrus sinensis (XP006472936) and Theobroma cacao (XP007019416). Conserved residues are shaded in black.
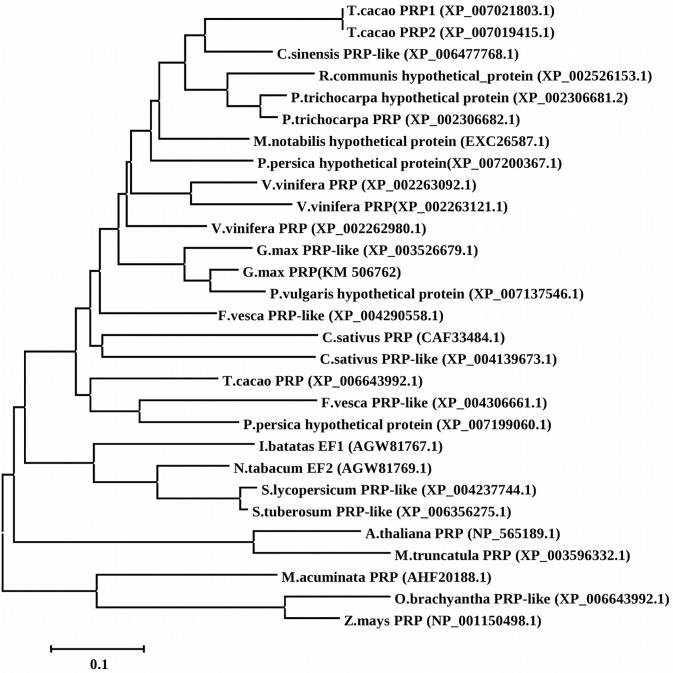
Sequence comparison showed that the putative GmPRP protein was homologous to PRPs from other plants. Its deduced amino acid sequence showed 74%, 73%, 72% and 69% similarity to Vitis vinifera (XP002262980), Populus trichocarpa (XP002306682), Citrus sinensis (XP006472936) and Theobroma cacao (XP007019416), respectively (Fig 1D). The phylogenetic relationships of GmPRP homologs in plants were constructed using the neighbor-joining method with the program MEGA 5.1. A neighbor-joining (NJ) phylogram was used to construct a phylogenetic tree based on the deduced sequence of GmPRP with other members of the PRP family, indicating that they may share a common ancestor and perform similar functions (Fig 2).
Fig 2. Phylogenetic relationships of GmPRP homologs in plants, constructed using the program MEGA 5.1.
Differential transcript levels of GmPRP in response to abiotic stresses
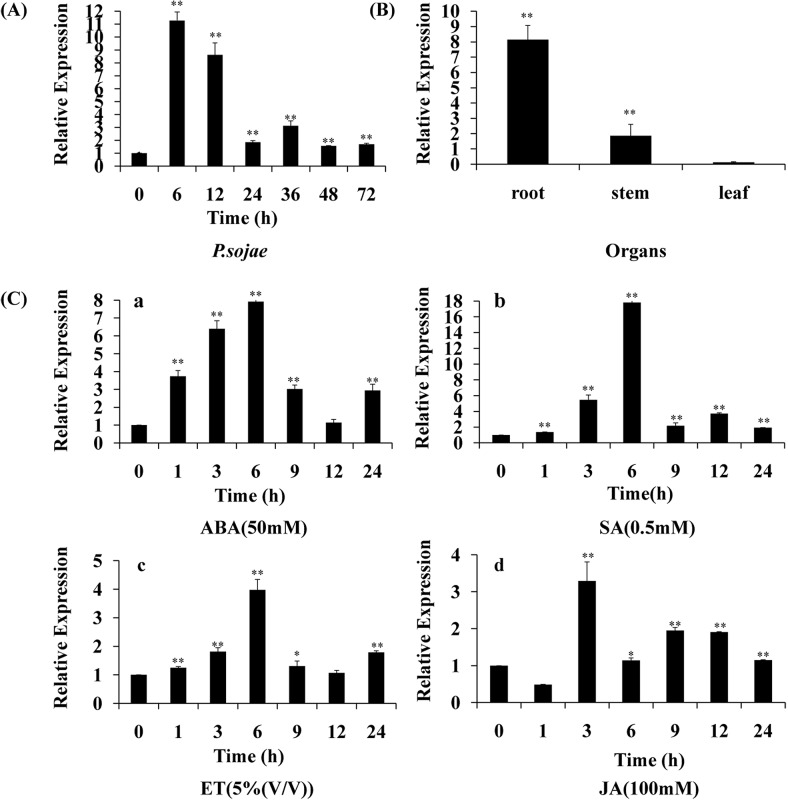
The responsiveness of a gene to certain stresses may indicate its defensive roles through several mechanisms. Therefore, the expression patterns of the GmPRP gene under various abiotic stresses and stress-related chemicals, including SA (0.5 mM), ABA (50μM), JA (100μM), ET (5% (v/v)) and P. sojae race 1, were investigated using qRT-PCR. The time-course qRT-PCR data were analyzed to examine the effects of abiotic stress conditions on GmPRP gene expression.
The transcripts of the GmPRP rapidly increased in leaf under P. sojae infection, reaching a maximum level at 6 h after the treatments, followed by a rapid decline (Fig 3A). GmPRP was constitutively expressed, with the highest expression in roots, followed by the stems and leaves (Fig 3B). Under ABA, SA and ET treatments, GmPRP mRNA transcripts accumulated in leaf and reached maximum levels at 6 h, followed by a decline (Fig 3C-a–3C-c). JA treatment induced the downregulation of GmPRP transcripts at the beginning of the treatment, and the transcripts remained at a low level at 1 h. However, GmPRP transcripts increased and reached a maximum level at 3 h after the treatments, followed by a decline (Fig 3C and 3D).
Fig 3. Relative expression of GmPRP mRNA in leaves of soybean plants after abiotic or biotic stress.
(A) Infection study. The leaves of ‘Suinong 10’ soybean seedlings inoculated with zoospores of P. sojae race 1 were also harvested at 6, 12, 24, 36, 48, and 72 h. (B) Tissue-specific expression study. GmPRP expression in the organs of sterile seedlings. (C) Hormone study. The leaves were collected from soybean plants at 1, 3, 6, 9, 12, and 24 h after treatment with ABA (50 mM) (a), SA (0.5 mM) (b), ET (5%(v/v)) (c), or JA (100 mM) (d). The experiments were performed at least three times for each treatment. Data shown are representative results. Statistical analyses were performed using Student’s t-test (*P < 0.05, **P < 0.01). Bars indicate the standard error of the mean (SE).
Subcellular localization of the GmPRP protein
To investigate the subcellular localization of the GmPRP protein, the GmPRP-GFP fusion gene was transformed into the Arabidopsis protoplast cells. As depicted in Fig 4, the GFP signal of the control was present in both cytoplasm and nuclear, the GFP signal of the GmPRP-GFP fusion gene was present in the cell plasma membrane and cytoplasm.
Fig 4. Subcellular localization of the GmPRP-GFP fusion protein.
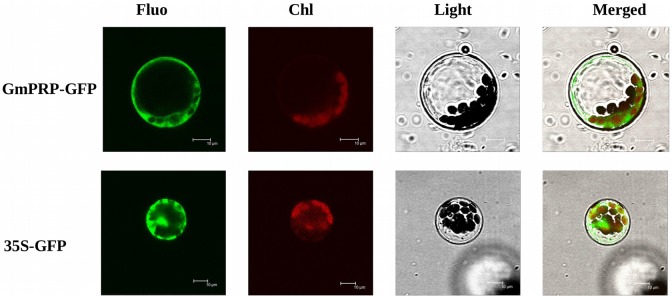
Arabidopsis protoplast cells expressing either GmPRP-GFP fusion protein (top) or GFP (bottom) were observed by fluorescence stereomicroscopy. Scale bar = 100 μm.
Expression of GmPRP in E. coli and properties of the GmPRP protein
Without the induction by 0.5 mM IPTG, all extracts of E. coli with or without the pET-29b vector produced negative results. However, the protein expression was remarkably enhanced by IPTG and increased from 2 to 6 h. Maximum expression of the protein was achieved at 4 h (S1A Fig). The purified recombinant protein migrated at 30 kDa in SDS-PAGE (S1B Fig). That value was consistent with the predicted molecular weight calculated from the amino acid sequence.
Antimicrobial activity assays of recombinant GmPRP protein
To examine the antimicrobial activity effect of the recombinant GmPRP protein on the growth of P. sojae 1, filter-paper discs containing recombinant GmPR10 protein (15, 25, 35 μg) were placed near the front of the growing hyphae of P. sojae 1. After incubation, a 2- to 3-mm zone with inhibited hyphal growth was detected when 25 μg of recombinant protein was applied to the filter-paper discs, and the bacteriostatic effect was enhanced by the application of 35 μg of the protein (Fig 5A). A control filter-paper disc containing boiled recombinant protein did not show an inhibitory effect.
Fig 5. Antimicrobial activity ribonuclease activity and assays for the recombinant GmPRP protein.
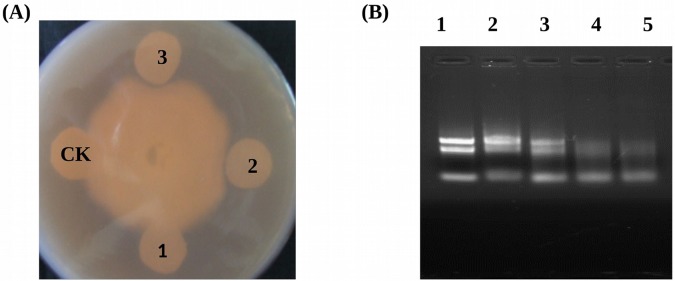
(A) Inhibition of P. sojae race 1 growth by purified recombinant GmPRP. 1, 15 μg renatured recombinant GmPRP protein; 2, 25 μg renatured recombinant GmPRP protein; 3, 35μg renatured recombinant GmPRP protein; CK, 35 μg boiled renatured recombinant GmPRP protein. (B) Ribonuclease activities of recombinant GmPRP proteins on soybean RNA. Gel electrophoresis using 1.0% agarose gel was performed to separate hydrolyzed RNAs. Each reaction mixture containing total RNA from soybean was incubated for 2 h at 37°C. Lane 1, 5 μg RNA+ Elution buffer; Lane 2, 5 μg RNA+ 6 μg of boiled dialytically renatured GmPRP protein. Lane 3, 5 μg RNA+ 2 μg dialytically renatured GmPRP protein; Lane 4, 5 μg RNA+ 4 μg dialytically renatured GmPRP protein; Lane 5, 5 μg RNA+ 6 μg dialytically renatured GmPRP protein.
Ribonuclease activity assays of recombinant GmPRP proteins
The total RNA isolated from the leaves of ‘Suinong 10’ soybean was incubated with or without recombinant GmPRP protein (Fig 5B). The elution buffer did not degrade the total RNA (Fig 5B, lane 1). The control sample, which was incubated with the boiled dialytically renatured GmPRP protein, did not show significant RNase activity against soybean RNA (Fig 5B, lane 2). However, when incubated with the non-boiled dialytically renatured GmPRP protein, RNase activity was clearly visible through the migration of the degradation products in the agarose gel (Fig 5B, lane 3, 4, 5).
Detection of transgenic tobacco and soybean
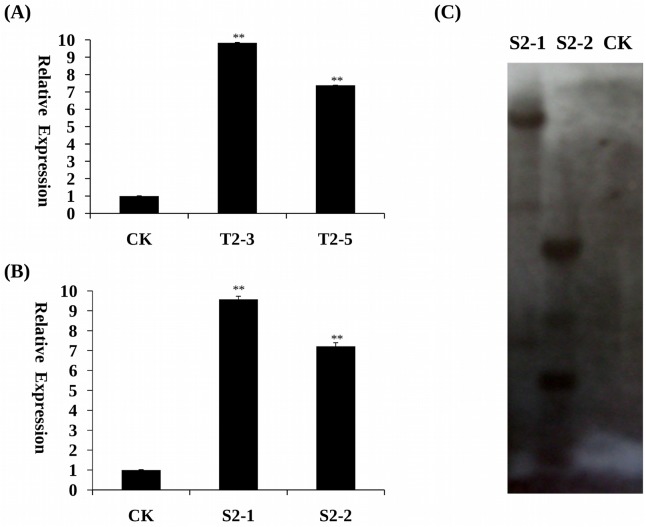
To confirm transgene insertion in transgenic tobacco (T2) and soybean plants (T2), the CTAB method was used to extract genomic DNA from young, fully expanded leaves. Finally, 10 independently transformed T2 transgenic tobacco plants (numbered from T2-1 to T2-10) and 6 independently transformed T2 transgenic soybean plants (numbered from S2-1 to S2-6) were identified by PCR. qRT-PCR of two tobacco transgenic lines (T2-3, T2-5) containing GmPRP and two soybean transgenic lines (S2-1, S2-2) containing GmPRP showed that GmPRP expression was significantly higher than non-transgenic tobacco and soybean plants (Fig 6A and 6B). To determine the copy number of GmPRP in the genome of T2 transgenic soybean, Southern hybridization analysis was performed. Two independently transformed T2 transgenic soybean plants (S2-1 and S2-2) were detected to have three and four copies, respectively (Fig 6C). These results suggest that the GmPRP gene was transformed successfully into soybean plants.
Fig 6. Expression of GmPRP gene in the leaves of transgenic tobacco and soybean plants.
(A) qRT-PCR determining the relative bundance of GmPRP (line T2-3 and T2-5) in the transgenic tobacco plants. The non-transgenic tobacco plants only were used as a control. (B) qRT-PCR determining the relative bundance of GmPRP (line T2-3 and T2-5) in the transgenic soybean plants. The non-transgenic soybean plants only were used as a control. (C) Genomic Southern hybridization analysis confirming stable integration and expression of GmPRP in young fully expanded leaves of transgenic soybean plants (CK, wild-type untransformed soybean control, line S2-1 and S2-2 independently transformed T2 transgenic events). All data represent the mean values of three replications.
Enhanced resistance to P. nicotianae and P. sojae in transgenic plants
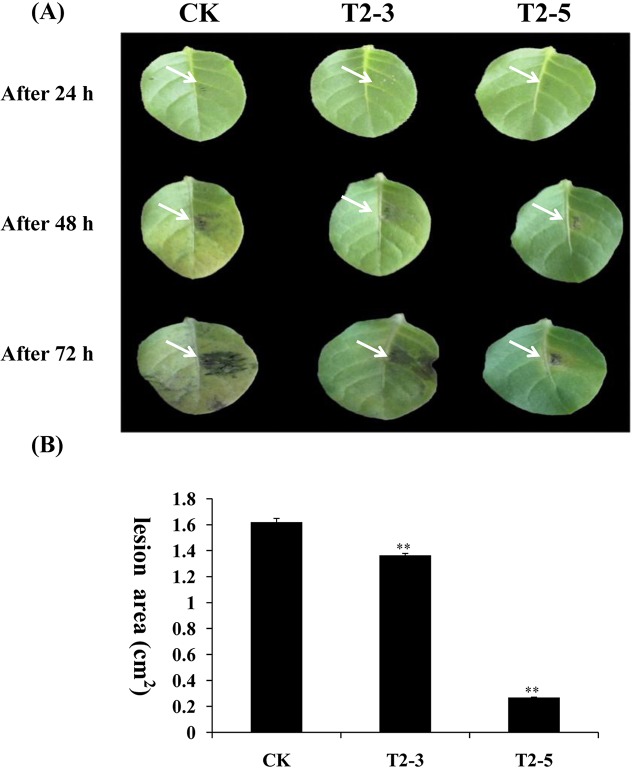
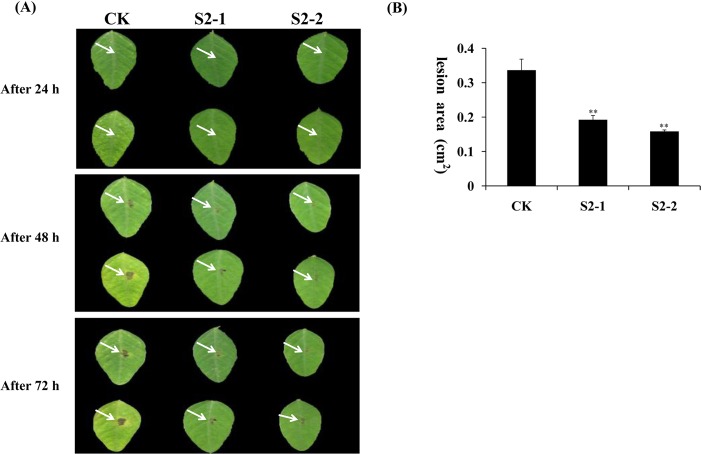
GmPRP was overexpressed in tobacco and soybean to evaluate the antimicrobial activity of this protein in vivo. Two transgenic tobacco plants (T2-3 and T2-5) and two transgenic soybean plants (numbered S2-1 and S2-2) were selected to investigate the susceptibility or resistance to P. nicotianae and P. sojae race 1, respectively. After 72 h of incubation with P. nicotianae or P. sojae race 1, remarkable differences in the development of disease symptoms were observed between the transgenic and non-transgenic tobacco and soybean plants. After 72 h of incubation with P. nicotianae, severe symptoms (necrosis and chlorosis) around the infection areas were observed in non-transgenic tobacco plants (Fig 7A, Lane CK), but the transgenic tobacco plants showed only slight lesions (Fig 7A, Lane T2-3, T2-5). The lesion area of the inoculated CK (1.62 cm2) is significantly different from the lesion area of transgenic lines T2-3 and T2-5 (only 1.36 and 0.27 cm2, respectively) (Fig 7B). After 72 h of incubation with P. sojae race 1, the leaves of the non-transgenic soybean plants exhibited clear and large water-soaked lesions compared with those of the transgenic plants (Fig 8A). The lesion area of the inoculated CK (0.34 cm2) is significantly different from the lesion area of transgenic lines T2-3 and T2-5 (only 0.19 and 0.16 cm2, respectively) (Fig 8B)These results indicate that the overexpression of the GmPRP gene in tobacco and soybean plants improved the resistance to P. nicotianae and P. sojae, respectively.
Fig 7. Overexpression of GmPRP in tobacco leaves enhanced the resistance to P. nicotianae.
(A) Disease symptoms after infection with P. nicotianae. Row a, tobacco leaves 24 h after inoculation. Row b, tobacco leaves 48 h after inoculation. Row c, tobacco leaves 96 h after inoculation. Column CK, leaves of non-transgenic tobacco. Columns T2-3 and T2-5, leaves of transgenic tobacco. (B) Lesion size of transgenic tobacco leaves infection with P. nicotianae after 96 h. All data represent the mean values of three replications.
Fig 8. Overexpression of the GmPRP gene in soybean leaves enhanced the resistance to P. sojae.
(A) Disease symptoms after infection with P. sojae. (a) Soybean leaves 24 h after inoculation. (b) Soybean leaves 48 h after inoculation. (c) Soybean leaves 96 h after inoculation. Column CK, leaves of non-transgenic soybean. Columns S2-1 and S2-2, leaves of transgenic soybean. (B) Lesion size of transgenic soybean leaves infection with P. sojae after 96 h. All data represent the mean values of three replications.
Discussion and Conclusions
In the interaction between soybean and P. sojae, certain plant PR proteins are accumulated upon P. sojae infection, and these proteins may be associated with P. sojae resistance. Moy et al. (2004) [68] reported that PRa in a sensitive soybean was found to be upregulated at 3 h after infection with P. sojae and to maintain active expression until the last sampling time (48 h). Narayanan et al. (2009) [69] found two defense-related PR genes that were upregulated in resistant soybean upon infection by P. sojae. Xu et al. (2012) [56] reported microarray analysis showing four PR protein genes that were upregulated during infection by P. sojae and then confirmed that result using real-time PCR. Among these PR proteins, GmPR10 protein was demonstrated to play an important role in the host defense against P. sojae infection [50].
In a previous study, a novel upregulated cDNA encoding a PRP was screened in the highly resistant soybean cultivar ‘Suinong 10’ [56]. Here, the novel PRP gene (termed GmPRP) and corresponding gene products from soybean (Glycine max) were isolated and characterized to better understand the function of this protein in the defense against P. sojae. To the best of our knowledge, this study is the first report on the biological activity of GmPRP protein from soybean in the defense against a pathogen. Sequence analysis indicating that GmPRP contained no signal peptide suggested that it may be an intracellular protein located in the cell membrane and cytoplasm, and this was verified with subcellular localization of the GmPRP protein in Arabidopsis protoplast cells (Fig 4). Subcellular localization of the GmPRP protein was similar to other intracellular PR proteins, it may be secreted into cell plasma membrane to resist pathogen after being made in the cytoplasm [70]. Most of the intracellular PR genes possess introns and exons, and GmPRP also contained introns and exons. The prediction of the three-dimensional (3D) structure of GmPRP (Fig 1B) was very similar to those of certain other PR proteins, including GmPR10 [50]. However, the predicted GmPRP structure contained a particularly conserved NTF2-like motif that belongs to the Nuclear transport factor 2 (NTF2) superfamily (Fig 1C). This family includes members of the NTF2 family, Delta-5-3-ketosteroid isomerases, scytalone dehydratases, and the beta subunit of Ring hydroxylating dioxygenases [71]. Some reports have provided direct evidence that NTF2 is required for the nuclear import of RanGDP, which is associated with cell proliferation and gene regulation [72, 73]. Hence, how GmPRP protein mediate NTF2 domain against P. sojae still need further study in the plant defense.
To further understand the function of PR genes in plant defense reactions, the expression patterns of PR genes was analyzed by various stimulus [74]. The data presented in this paper demonstrate that the expression of the GmPRP gene could be strongly induced by JA, SA, ABA and ET. Similar results have previously been reported in other plants in which PR proteins were induced by various treatments [31–35]. These results suggest that the expression of GmPRP may depend on the SA, JA, ABA and ET signal transduction pathways. Some reports have shown that the PR genes could be induced by certain factors in response to ET treatment, such as ethylene-response factors (ERFs) [75], which contain a conserved AP2/ERF domain that binds to the GCC box elements present in the promoters of PR genes [76]. However, no GCC box was found in the promoter of GmPRP (S2 Fig), suggesting that it may not be the direct target of ERFs.
P. sojae is a soil-borne pathogen that can survive for many years in soil and depends on sporangia and zoospore formation, as well as the direct penetration of hyphae between the cell walls of the epidermis, to infect soybean [55, 77]. The inhibition of sporangia and zoospore formation or hyphal development will be useful for the host’s resistance to P. sojae. In the present study, recombinant GmPRP protein significantly inhibited the hyphae growth of P. sojae in vitro (Fig 5A), but whether this protein inhibits hyphal development in vivo requires further research.
In the RNA degradation assay, the recombinant GmPRP protein showed ribonucleolytic activity, where part of the RNA was degraded within 2 h of incubation (Fig 5B), indicating that ribonucleolytic activity may be one of the important roles of this protein in the plant defense response to pathogen attack. Furthermore, the increased expression of GmPRP in transgenic tobacco and soybean plants may contribute to enhanced resistance against pathogens. In further experiments, GmPRP was successfully transformed into tobacco and soybean plants, and the antimicrobial activities of GmPRP were evaluated through the inoculation of transgenic tobacco and soybean plants overexpressing GmPRP. These transgenic tobacco and soybean plants showed enhanced levels of resistance to P. nicotianae and P. sojae, respectively. These results suggest that the enhanced resistance to pathogens in tobacco and soybean plants may be associated with the overexpression of GmPRP.
In conclusion, we characterized a novel soybean PRP gene from ‘Suinong 10’ soybean after inoculation with P. sojae. To the best of our knowledge, this is the first report of a PRP gene from soybean to describe its functional accreditation in imparting defense against a pathogen. Further characterization of GmPRP and its regulation under ambient and stress environments will enhance our understanding of the molecular cross-talk among various signaling pathways mediating plant defense responses.
Supporting Information
(A) Lanes 1–7, E. coli BL21 containing the pET-29b(+) vector harboring the GmPRP gene induced by IPTG for 2, 4, and 6 h, respectively; Lane 1, precipitate from E. coli BL21 transformed without pET-29b(+) upon induction by 0.5 mM IPTG; Lane 2, precipitate from E. coli BL21 transformed with pET-29b(+) without induction by 0.5 mM IPTG; Lane 3, precipitate from E. coli BL21 transformed with pET-29b(+) upon induction by 0.5 mM IPTG; Lane 4, precipitate from E. coli BL21 transformed with the recombinant GmPRP and pET-29b(+) without induction by 0.5 mM IPTG; Lanes 5–7, precipitates from E. coli BL21 transformed with the recombinant GmPRP and pET-29b(+) with induction by 0.5 mM IPTG for 2, 4, and 6 h, respectively. (B) Purification of recombinant GmPRP protein from E. coli BL21 transformed with the pET-29b(+) vector containing the GmPRP. Lane 8, purified recombinant GmPRP protein. Lane M, protein marker.
(TIF)
The nucleotide position of the ATG translation initiation codon was assigned as position 1 in the nucleotide sequence, and the nucleotide positions upstream of position 1 were shown as minus numbers. The putative cis-acting elements were upperlined with a gray background, and the names were shown above the elements. Light responsive element: ACE, Box 4, Bow I, G-Box, G-box, Gap-box, Sp1, ATCT-motif, MRE. cis-acting regulatory element essential for the anaerobic induction: ARE; fungal elicitor responsive element: Box-W1; cis-acting element to heat stress responsiveness: HSE; cis-acting element involved in defense and stress the responsiveness: TC-rich repeats; cis-acting element in salicylic acid: TCA-element; auxin-responsive element: TGA-element; binding site of AT-rich DNA bingding protein (ATBP-1): AT-rich element; cis-acting regulatory element involved in MeJA-responsiveness: CGTCA-motif; gibberellins-responsive element: GARE-motif; cis-acting regulatory element required for endosperm expression: Skn-1 motif.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research was supported through funding from the Heilongjiang Province outstanding youth fund (JC201308), National Natural Science Foundation of China Projects (31071439, 31171577, 13 31101167), the Specialized Research Fund for the Doctoral Program of Higher Education (20112325120005), the Science and Technology Innovation Project in Harbin (2012RFQXN011, 2012RFXXN019), and the Research Fund for Young Teachers in Northeast Agricultural University (2012 RCB 08).
References
- 1. Durner J, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Trends Plant Sci. 1997; 2: 266–274. [Google Scholar]
- 2. Montillet JL, Chamnongpol S, Rustérucci C, Dat JF, Van de Cotte B, Agnel JP, et al. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 2005; 138: 1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sels J, Mathys J, De Coninck BM, Cammue BP, De Bolle MF. Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol. Bioch. 2008; 46: 941–950. 10.1016/j.plaphy.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 4. Pozo M J, Cordier C, Dumas-Gaudot E, Gianinazzi S, Barea J M, Azcón‐Aguilar C. Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 2002; 53: 525–534. [DOI] [PubMed] [Google Scholar]
- 5. Ryals JA, Neuensch wander UH, Willits MG, Molina A, Steiner HY, et al. Systemic acquired resistance. Plant Cell 1996; 8: 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jwa NS, Kumar Agrawal G, Rakwal R, Park CH, Prasad Agrawal V. Molecular cloning and characterization of a novel Jasmonate inducible pathogenesis-related class 10 protein gene, JIOsPR10, from rice (Oryza sativa L.) seedling leaves. Biochem. Bioph. Res. Co. 2001; 286: 973–983. [DOI] [PubMed] [Google Scholar]
- 7. Christensen AB, Cho BH, Naesby M, Gregersen PL, Brandt J, Madriz-Ordeñana K, et al. The molecular characterisation of the two barley proteins establishes the novel PR-17 family of pathogenesis-related protein. Mol. Plant Pathol. 2002; 3: 134–144. [DOI] [PubMed] [Google Scholar]
- 8. Van Loon LC, Pierpoint WS, Boller T, Conejero V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 1994; 12: 245–264,. [Google Scholar]
- 9. Van Loon LC, Van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 proteins. Physiol. Mol Plant P. 1999; 55: 85–97. 10927342 [Google Scholar]
- 10. Bai S H, Dong C H, Li B H, Dai H Y. A PR-4 gene identified from Malus domestica is involved in the defense responses against Botryosphaeria dothidea . Plant Physiol. Bioch. 2013; 62: 23–32. 10.1016/j.plaphy.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 11. Liu X J, Huang B B, Lin J, Fei J, Chen Z H, Pang Y, et al. A novel pathogenesis related protein (SsPR10) from Solanum surattense with ribonucleolytic and antimicrobial activity is stress- and pathogen inducible. J. Plant Physiol. 2006; 163: 546–555. [DOI] [PubMed] [Google Scholar]
- 12. Park C J, Kim K J, Shin R, Park J M, Shin Y C, Paek K H. Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant J. 2004; 37: 186–198. [DOI] [PubMed] [Google Scholar]
- 13. Xie Y R, Chen Z Y, Brown R L, Bhatnagar D. Expression and functional characterization of two pathogenesis-related protein 10 genes from Zea mays . J. Plant Physiol. 2010; 67: 121–130. [DOI] [PubMed] [Google Scholar]
- 14. Asselbergh B, Curver K, Franca S C, Audenaert K, Vuylsteke M, Van Breusegem F, et al. Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 2007; 144: 1863–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jayasankar S, Li Z, Gray D J. Constitutive expression of Vitis vinifera thaumatin-like protein after in vitro selection and its role in anthracnose resistance. Funct. Plant Biol. 2003; 30: 1105–1115. [DOI] [PubMed] [Google Scholar]
- 16. Prasath D, El-Sharkawy I, Sherif S, Tiwary K S, Jayasankar S. Cloning and characterization of PR5 gene from Curcuma amada and Zingiber officinale in response to Ralstonia solanacearum infection. Plant Cell Rep. 2011; 30: 1799–1809. 10.1007/s00299-011-1087-x [DOI] [PubMed] [Google Scholar]
- 17. Lorito M, Peterbauer C, Hayes C K, Harman G E. Synergistic interaction between fungal cell wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiology 1994; 140: 623–629. [DOI] [PubMed] [Google Scholar]
- 18. Machida S, Saito M. Purification and characterization of membrane-bound chitin synthase. J. Biol. Chem. 1993; 268: 1702–1707. [PubMed] [Google Scholar]
- 19. Terras F R, Eggermont K, Kovaleva V, Raikhel N V, Osborn R W, Kester A, et al. Small cysteine-richantifungal proteins from radish: their role in host defense. Plant Cell 1995; 7: 573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang S Y, Wu J H, Ng T B, Ye X Y, Rao P F. A non-specific lipid transfer protein with antifungal and antibacterial activities from the mung bean. Peptides 2004; 25:1235–1242. [DOI] [PubMed] [Google Scholar]
- 21. Van Loon L C. Occurrence and properties of plant pathogenesis-related proteins In: Datta S K, Muthukrishnan S, editors. Pathogenesis-Related Proteins in Plants. CRC Press: Boca Raton, FL; 1999. pp. 1–19. [Google Scholar]
- 22. Chadha P, Das R H. A pathogenesis related protein, AhPR10 from peanut: an insight of its mode of antifungal activity. Planta 2006; 225: 213–222. [DOI] [PubMed] [Google Scholar]
- 23. McGee J D, Hamer J E, Hodges T K. Characterization of a PR-10 pathogenesis-related gene family induced in rice during infection with Magnaporthe grisea . Mol.Plant Microbe In. 2001; 14: 877–886. [DOI] [PubMed] [Google Scholar]
- 24. Robert N, Ferran J, Breda C, Coutos-Thevenot P, Boulay M, Buffard D, et al. Molecular characterization of the incompatible interaction of Vitis vinifera leaves with Pseudomonas syringae pv pisi, expression of genes coding for stilbene synthase and class 10 PR protein. Eur. J. Plant Pathol. 2001; 107: 249–261. [Google Scholar]
- 25. Saravanakumar D, Lavanya N, Muthumeena K, Raguchander T, Samiyappan R. Fluorescent pseudomonad mixtures mediate disease resistance in rice plants against sheath rot (Sarocladium oryzae) disease. Biocontrol 2009; 54: 273–286. [Google Scholar]
- 26. Senthilraja G, Anand T, Kennedy J S, Raguchander T, Samiyappan R. Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leafminer insect and collar rot pathogen. Physiol. Mol. Plant Pathol. 2013; 83: 10–19. [Google Scholar]
- 27. Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005; 43: 205–227. [DOI] [PubMed] [Google Scholar]
- 28. Pautot V, Holzer F M, Walling L L. Differential expression of tomato proteinase inhibitor I and II genes during bacterial pathogen invasion and wounding. Mol. Plant Microbe In. 1991; 4: 284–292. [DOI] [PubMed] [Google Scholar]
- 29. González G, Fuentes L, Moya-León M A, Sandoval C, Herrera R. Characterization of two PR genes from Fragaria chiloensis in response to Botrytis cinerea infection: A comparison with Fragaria x ananassa . Physiol. Mol. Plant P. 2013; 82: 73–80. [Google Scholar]
- 30. Mitsuhara I, Iwai T, Seo S, Yanagawa Y, Kawahigasi H, Hirose S, et al. Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180). Mol. Genet. Genomics 2008; 279: 415–427. 10.1007/s00438-008-0322-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borsics T, Lados M. Dodder infection induces the expression of a pathogenesis-related gene of the family PR-10 in alfalfa. J. Exp. Bot. 2002; 53: 1831–1832. [DOI] [PubMed] [Google Scholar]
- 32. Loake G, Grant M. Salicylic acid in plant defence-the players and protagonists. Curr. Opin. Plant Biol. 2007; 10: 466–472. [DOI] [PubMed] [Google Scholar]
- 33. Poupard P, Strull D-G, Simoneau P. Two members of the Bet v 1 gene family encoding birch pathogenesis-related proteins display different patterns of root expression and wound-inducibility. Aust. J. Plant Physiol. 1998; 25: 459–464. [Google Scholar]
- 34. Van Loon L C, Rep M, Pieterse C M J. Significance of inducible defenser elated proteins in infected plants. Annu. Rev. Phytopathol. 2006; 44: 135–162. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y, Shih D S. Isolation of an osmotin-like protein gene from strawberry and analysis of the response of this gene to abiotic stresses. J. Plant Physiol. 2007; 164: 68–77. [DOI] [PubMed] [Google Scholar]
- 36. Rakwal R, Agrawal G K, Yonekura M. Separation of proteins from stressed rice (Oryza sativa L.) leaf tissues by two-dimensional polyacrylamide gel electrophoresis: Induction of pathogenesis-related and cellular protectant proteins by jasmonic acid, UV irradiation and copper chloride. Electrophoresis 1999; 20: 3472–3478. [DOI] [PubMed] [Google Scholar]
- 37. Agrawal G K, Rakwal R, Tamogami S, Yonekura M, Kubo A, Saji H. Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiol.Bioch. 2002; 40: 1061–1069. [Google Scholar]
- 38. Worrall D, Hird D L, Hodge R, Paul W, Draper J, Scott R. Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 1992; 4: 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, et al. The molecular analysis of leaf senescence-a genomics approach. Plant Biotechnol. J. 2003; 1: 3–22. [DOI] [PubMed] [Google Scholar]
- 40. Monteiro S, Picarra-Pereira M A, Loureiro V B, Teixeira A R, Ferreira R B. The diversity of pathogenesis-related proteins decreases during grape maturation. Phytochemistry 2007; 68: 416–425. [DOI] [PubMed] [Google Scholar]
- 41. Quirino B F, Noh Y S, Himelblau E, Amasino R M. Molecular aspects of leaf senescence. Trends Plant Sci. 2000; 5: 278–282. [DOI] [PubMed] [Google Scholar]
- 42. Chassot C, Nawrath C, Metraux J P. Cuticular defects lead to full immunity to a major plant pathogen. Plant J. 2007; 49: 972–980. [DOI] [PubMed] [Google Scholar]
- 43. Iwai T, Kaku H, Honkura R, Nakamura S, Ochiai H, Sasaki T, et al. Enhanced resistance to seed-transmitted bacterial diseases in transgenic rice plants overproducing an oat cell-wall-bound thionin. Mol. Plant Microbe In. 2002; 15: 515–521. [DOI] [PubMed] [Google Scholar]
- 44. Jung H W, Kim K D, Hwang B K. Identification of pathogen-responsive regions in the promoter of a pepper lipid transfer protein gene (CALTPI) and the enhanced resistance of the CALTPI transgenic Arabidopsis against pathogen and environmental stresse. Planta 2005; 221: 361–373. [DOI] [PubMed] [Google Scholar]
- 45. Datta K, Velazhahan R, Oliva N, Ona I, Mew T, Khush G S, et al. Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor. Appl. Genet. 1999, 98: 1138–1145. [Google Scholar]
- 46. Liu D, Raghothama K G, Hasegawa P M, Bressan R A. Osmotin overexpression in potato delays development of disease symptoms. Proc. Natl. Acad. Sci. U.S.A. 1994; 91: 1888–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarowar S, Kim Y J, Kim E N, Kim K D, Hwang B K, Islam R, et al. Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep. 2005; 24: 216–224. [DOI] [PubMed] [Google Scholar]
- 48. Wei Y D, Zhang Z G, Andersen C H, Schmelzer E, Gregersen P L, Collinge D B, et al. An epidermis/papilla-specific oxidase-like protein in the defence response of barley attacked by the powdery mildew fungus. Plant Mol. Biol. 1998; 36: 101–112. [DOI] [PubMed] [Google Scholar]
- 49. Okushima Y, Koizumi N, Kusano T, Sano H. Secreted proteins of tobacco cultured BY2 cells: identification of a new member of pathogenesis-related proteins. Plant Mole. Biol. 2000; 42: 479–488. [DOI] [PubMed] [Google Scholar]
- 50. Zhu B L, Chen T H H, Li P H. Expression of three osmotin-like protein genes in response to osmotic stress and fungal infection in potato. Plant Mol. Biol. 1995; 28: 17–26. [DOI] [PubMed] [Google Scholar]
- 51. Jacobs A S, Pretorius Z A, Kloppers F J, Cox T S. Mechanisms associated with wheat leaf rust resistance derived from Triticum monococcum . Phytopathology 1996; 86: 588–595. [Google Scholar]
- 52. Wang C S, Huang J C, Hu J H. Characterization of two subclasses of PR-10 transcripts in lily anthers and induction of their genes through separate signal transduction pathways. Plant Mol. Biol. 1999; 40: 807–814. [DOI] [PubMed] [Google Scholar]
- 53. Hou M M, Xu W J, Bai H, Liu Y M, Li L Y. Characteristic expression of rice pathogenesis-related proteins in rice leaves during interactions with Xanthomonas oryzae pv. Oryzae . Plant Cell Rep. 2012; 31: 895–904. 10.1007/s00299-011-1210-z [DOI] [PubMed] [Google Scholar]
- 54. Xu P F, Jiang L Y, Wu J J, Li W B, Fan S J, Zhang S Z. Isolation and characterization of a pathogenesis-related protein 10 gene (GmPR10) with induced expression in soybean (Glycine max) during infection with Phytophthora sojae . Mol. Biol. Rep. 2014; 41: 4899–4909. 10.1007/s11033-014-3356-6 [DOI] [PubMed] [Google Scholar]
- 55. Anderson T R, Tenuta A. Phytophthora rot In: Bailey K L, Gossen B D, Gossen B D, Gugel R K, Morrall R A A, editors Diseases of field crops in Canada. The Canadian Phytopathological Society, Saskatoon; 2003; pp. 155–156. [Google Scholar]
- 56. Xu P F, Wu J J, Xue A G, Li W B, Chen W Y, Wei L, et al. Differentially Expressed Genes of Soybean During Infection by Phytophthora sojae . J. Integr. Agr. 2012; 11: 368–377. [Google Scholar]
- 57. Zhang S Z, Xu P F, Wu J J, Xue A G, Zhang J X, Li W B, et al. Races of Phytophthora sojae and their virulences on commonly grown soybean varieties in Heilongjiang, China. Plant Disease 2010; 94: 87–91. [DOI] [PubMed] [Google Scholar]
- 58. Fehr W R, Caviness C E, Burmood D T, Pennington J S. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci. 1971; 11: 929–931. [Google Scholar]
- 59. Ward E W B, Lazarovits G, Unwin C H, Buzzell R I. Hypocotyl reactions and glyceollin in soybeans inoculated with zoospores of Phytophthora megaspuma var. sojae . Phytopathology 1979; 69: 951–955. [Google Scholar]
- 60. Morris P F, Savard M E, Ward E W B. Identification and accumulation of isoflavonoids and isoflavone glucosides in soybean leaves and hypocotyls in resistance responses to Phytophthora megasperma f. sp. Glycinea . Physiol. Mol. Plant P. 1991; 39: 229–244. [Google Scholar]
- 61. Yoo S D, Cho Y H, Sheen J . Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2007; 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 62. Schlumbaum A, Mauch F, Vögeli U, Bolleret T. Plant chitinases are potent inhibitors of fungal growth. Nature 1986; 324: 365–367. [Google Scholar]
- 63. Bantignies B, Seguin J, Muzac I, Dedaldechamp F, Gulick P, Ibrahim R. Direct evidence for rbonucleolytic activity of a PR-10-like protein from white lupin roots. Plant Mol. Biol. 2000; 42: 871–881. [DOI] [PubMed] [Google Scholar]
- 64. Horsch R B, Fry J E, Rogers S G, Sanders P R, Lloyd A. A simple and general method for transferring genes into plant. Science 1985; 227: 1229–1231. [DOI] [PubMed] [Google Scholar]
- 65. Wang H Z, Hu B, Chen G P, Shi N N, Zhao Y, Yin Q C, et al. Application of Arabidopsis AGAMOUS second intron for the engineered ablation of flower development in transgenic tobacco. Plant Cell Rep. 2008; 27: 251–259. [DOI] [PubMed] [Google Scholar]
- 66. Sambrook J, Fritsch E F, Maniatis T. In molecular cloning: a laboratory manual, 2nd edn, New York, Cold Spring Harbor Laboratory, Cold Spring Harbor, 9–62. [Google Scholar]
- 67. Dou D L, Wang B S, Zhu S W, Tang Y X, Wang Z X, Sun J S, et al. Transgenic tobacco with NDR1 gene improved its resistance to two fungal diseases. Scientia. Agricultura Sinica. 2003; 36: 1120–1124 (in Chinese with English abstract). [Google Scholar]
- 68. Moy P, Qutob D, Chapman B P, Atkinson L, Gijzen M. Patterns of gene expression upon infection of soybean plants by Phytophthora sojae . Mol. Plant Microbe In. 2004; 17: 1051–1062. [DOI] [PubMed] [Google Scholar]
- 69. Narayanan N N, Grosic S, Tasma I M, Grant D, Shoemaker R, Bhattacharyya M K. Identification of candidate signaling genes including regulators of chromosome condensation 1 protein family differentially expressed in the soybean-Phytophthora sojae interaction. Theor. Appl. Genet. 2009; 118: 399–412. 10.1007/s00122-008-0895-z [DOI] [PubMed] [Google Scholar]
- 70. Park C J, An J M, Shin Y C, Kim K J, Lee B J, Paek K H. Molecular characterization of pepper germin-like protein as the novel PR-16 family of pathogenesis-related proteins isolated during the resistance response to viral and bacterial infection. Planta 2004; 219: 797–806. [DOI] [PubMed] [Google Scholar]
- 71. Clarkson W D, Corbett A H, Paschal B M, Kent H M, Mccoy A J, Gerace L, et al. Nuclear protein import is decreased by engineered mutants of nuclear transport factor 2 (NTF2) that do not bind GDP-Ran. J. Mol. Biol. 1997; 272: 716–730. [DOI] [PubMed] [Google Scholar]
- 72. Bayliss R, Ribbeck K, Akin D, Kent H M, Feldherr C M, Görlich D, et al. Interaction between NTF2 and xFxFG-containing nucleoporins is required to mediate nuclear import of RanGDP. J. Mol. Biol. 1999; 293: 579–593. [DOI] [PubMed] [Google Scholar]
- 73. Park S H, Park T J, Lim I K. Reduction of exportin 6 activity leads to actin accumulation via failure of RanGTP restoration and NTF2 sequestration in the nuclei of senescent cells. Exp. Cell Res. 2011; 317: 941–954. 10.1016/j.yexcr.2010.12.023 [DOI] [PubMed] [Google Scholar]
- 74. Jones J D G, Dangl I J L. The plant immune system. Nature 2006; 444: 323–329. [DOI] [PubMed] [Google Scholar]
- 75. Knight H, Knight M R. Abiotic stress signaling Pathways: specificity and cross-talk. Trends Plant Sci. 2011; 6: 262–267. [DOI] [PubMed] [Google Scholar]
- 76. Ohme-Takagi M, Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 1995; 7: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Beagle-Ristaino J E, Rissler J F. Histopathology of susceptible and resistant soybean roots inoculated with zoospores of Phytophthora megasperma f sp glycinea . Phytopathology 1983; 73: 590–595. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Lanes 1–7, E. coli BL21 containing the pET-29b(+) vector harboring the GmPRP gene induced by IPTG for 2, 4, and 6 h, respectively; Lane 1, precipitate from E. coli BL21 transformed without pET-29b(+) upon induction by 0.5 mM IPTG; Lane 2, precipitate from E. coli BL21 transformed with pET-29b(+) without induction by 0.5 mM IPTG; Lane 3, precipitate from E. coli BL21 transformed with pET-29b(+) upon induction by 0.5 mM IPTG; Lane 4, precipitate from E. coli BL21 transformed with the recombinant GmPRP and pET-29b(+) without induction by 0.5 mM IPTG; Lanes 5–7, precipitates from E. coli BL21 transformed with the recombinant GmPRP and pET-29b(+) with induction by 0.5 mM IPTG for 2, 4, and 6 h, respectively. (B) Purification of recombinant GmPRP protein from E. coli BL21 transformed with the pET-29b(+) vector containing the GmPRP. Lane 8, purified recombinant GmPRP protein. Lane M, protein marker.
(TIF)
The nucleotide position of the ATG translation initiation codon was assigned as position 1 in the nucleotide sequence, and the nucleotide positions upstream of position 1 were shown as minus numbers. The putative cis-acting elements were upperlined with a gray background, and the names were shown above the elements. Light responsive element: ACE, Box 4, Bow I, G-Box, G-box, Gap-box, Sp1, ATCT-motif, MRE. cis-acting regulatory element essential for the anaerobic induction: ARE; fungal elicitor responsive element: Box-W1; cis-acting element to heat stress responsiveness: HSE; cis-acting element involved in defense and stress the responsiveness: TC-rich repeats; cis-acting element in salicylic acid: TCA-element; auxin-responsive element: TGA-element; binding site of AT-rich DNA bingding protein (ATBP-1): AT-rich element; cis-acting regulatory element involved in MeJA-responsiveness: CGTCA-motif; gibberellins-responsive element: GARE-motif; cis-acting regulatory element required for endosperm expression: Skn-1 motif.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.