Abstract
Objective
To pool reliable evidences for the optimum anterior transposition technique in the treatment of cubital tunnel syndrome by comparing the clinical efficacy of subcutaneous and submuscular anterior ulnar nerve transposition.
Methods
A comprehensive search was conducted in PubMed MEDLINE, Cochrane Library, EMBASE, Web of Science, OVID AMED, EBSCO and potentially relevant surgical archives. Risk of bias of each included studies was evaluated according to Cochrane Handbook for Systematic Reviews of Interventions. The risk ratio (RR) and 95% confidence intervals (CI) were calculated for the clinical improvement in function compared to baseline. Heterogeneity was assessed across studies, and subgroup analysis was also performed based on the study type and follow-up duration.
Results
Three studies with a total of 352 participants were identified, and the clinically relevant improvement was used as the primary outcomes. Our meta-analysis revealed that no significant difference was observed between two comparison groups in terms of postoperative clinical improvement in those studies (RR 1.04, 95% CI 0.86 to 1.25, P = 0.72). Meanwhile, subgroup analyses by study type and follow-up duration revealed the consistent results with the overall estimate. Additionally, the pre- and postoperative motor nerve conduction velocities were reported in two studies with a total of 326 patients, but we could not perform a meta-analysis because of the lack of concrete numerical value in one study. The quality of evidence for clinical improvement was ‘low’ or ‘moderate’ on the basis of GRADE approach.
Conclusions
Based on small numbers of studies with relatively poor methodological quality, the limited evidence is insufficient to identify the optimum anterior transposition technique in the treatment of cubital tunnel syndrome. The results of the present study suggest that anterior subcutaneous and submuscular transposition might be equally effective in patients with ulnar neuropathy at the elbow. Therefore, more high-quality randomized controlled trials with standardized clinical improvement metrics are required to further clarify this topic and to provide reproducible pre- and postoperative objective outcomes.
Introduction
Cubital tunnel syndrome, also called ulnar neuropathy at the elbow, is referred as the second most common entrapment neuropathy of the peripheral nerves after carpal tunnel syndrome [1, 2]. It predominantly affects the region innervated by ulnar nerve, which is characterized by pain, paraesthesias or anaesthesia, and weakness or atrophy of ulnar nerve innervated muscles. Men have about twice the mean annual incidence of morbidity of women, with estimates of both being affected almost twenty-five cases per 100,000 person-years [3].
Transpositional surgical treatments of cubital tunnel syndrome, including subcutaneous, intramuscular and submuscular [4], remain controversial, which comes from the diverging results for each of the therapeutic modality. Those who prefer anterior subcutaneous transposition claim that it produces less postoperative pain with earlier mobilization and the reduction of tension on the nerve [5, 6]. Those who advocate for anterior submuscular transposition are concerned with the new location of ulnar nerve that has a healthy vascular bed and is well protected by soft tissue [7–9]. Moreover, submuscular transposition, based on the histological study using the rat model, displayed less perineural scar tissue and healthier axons when compared to subcutaneous transposition [10].
Therefore, it is uncertain whether submusclar when compared to subcutaneous produces better clinical improvement. The reliable evidence in favor of one of two surgical treatments remains lack. Controversy exists among hand surgeons when concerning the optimum anterior transposition technique in the treatment of cubital tunnel syndrome. The objective of this systematic and meta-analysis was to pool the reliable evidences to determine which anterior transposition technique is optimum for cubital tunnel syndrome. The present study examines the evidence from randomized controlled trials (RCTs) or quasi-RCTs, includes the estimated zones of representation of approximate clinically equivalent effect sizes, and incorporates the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach [11] to evaluate the overall quality of the evidences for the eligibility studies.
Methods
Protocol registration
We developed a protocol for review in advance, which registered in the PROSPERO database (protocol registration no. CRD42014015653), and followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (S1 Table).
Data source and search strategy
Six public databases (PubMed MEDLINE, Cochrane Library, EMBASE, Web of Science, OVID AMED and EBSCO) were searched by CHL and XBK from the inception of the databases to December 2014 without linguistic restriction. Additionally, the archives of abstracts or grey literatures were searched from the Journal of Hand Surgery, the American Society for Surgery of the Hand (ASSH), the American Association of Hand Surgeons (AAHS) and International Clinical Trials Registry Platform.
Search terms included “cubital /elbow tunnel syndrome,” “ulnar neuropathy,” “ulnar nerve compression /entrapment,” “ulnar nerve compression syndrome,” “ulnar neuropathy,” “ulnar nerve,” “subcutaneous” and “submuscular” combined with “randomized, controlled trial.” Two investigators (CHL and XBK) independently reviewed all title, abstracts, and full text of articles which might meet the inclusion criteria. Meanwhile, a comprehensive search of references from retrieved articles and relevant reviews.
Study eligibility criteria
Studies based on all of the following criteria were selected: (1) RCTs using a truly randomized or quasi-randomized allocation of treatment were included. (2) The target participants consisted of patients who presented with primary cubital tunnel syndrome or primary ulnar neuropathy at the elbow. (3) The intervention group was anterior subcutaneous ulnar nerve transposition; (4) The comparison group was anterior submuscular ulnar nerve transposition (whether original or modified); (5) The outcomes were postoperative clinical and/ or electrodiagnostic variable defined as “improved” versus “not improved.” (6) The study described a follow-up duration of at least 12 months.
Studies were excluded if they described 1 of these conditions: (1) patient population was mixed with compressive neuropathy of ulnar nerve at another site; (2) patients diagnosed with polyneuropathy, brachial plexus injury or a general systemic disease capable of causing a non-compressive ulnar neuropathy; and (3) study was review, case report, letters or conferences.
Assessment of risk of bias
The risk of bias of each included studies was independently evaluated by two investigators in order to assess the methodological quality of each study according to Cochrane Handbook for Systematic Reviews of Interventions. Seven domains were evaluated in each included studies: random sequence generation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective outcome reporting, other risk of bias. We judged the risk of bias as ‘‘low risk”, ‘‘unclear risk” or ‘‘high risk”.
Data extraction
According to the standard protocol, data were independently extracted by two investigators (CHL and XBK) based on the following items: (1) General information of studies included author, year of publication, country, study type. (2) Baseline characteristics of participants such as sample size, age, gender, intervention and follow-up data. (3) Primary outcomes, which were regarded as clinical improvement in function compared to baseline. (4) Secondary outcomes, consisting of adverse events, change from baseline of the cross-sectional area (CSA), motor conduction velocity (MCV), sensory conduction velocity (SCV) and neural action potential (NAP). Disagreements and differences between the investigators were resolved by consensus with all co-authors to come to an agreement. If necessary, authors of each eligible study were also contacted by e-mail to provide further information.
Data analysis
A meta-analysis was performed using the software Review Manager 5.3.5 (Cochrane Collaboration, http://tech.cochrane.org/revman/download). For binary outcomes, the risk ratio (RR) and 95% confidence intervals (CI) were calculated, while mean difference (MD) and associated 95%CI were calculated for continuous outcomes. If outcome measurements in included studies were not conducted on the same scale, we used standardized mean difference (SMD) and 95% CI for continuous outcomes. The level of statistically significance was set at P-value<0.05. Heterogeneity among the included studies was assessed using Cochrane Handbook's Q test and I2 statistics [12, 13]. A P<0.05 or I2>50% was considered significant heterogeneity. The meta-analysis was applied by using the fixed-effect model if there was no significant heterogeneity(p≥0.05, I2≤50%). Otherwise, the random effect model was used or the possible reasons were explored for the significant heterogeneity (P<0.05, I2>50%). When data could not be collected for performing a meta-analysis, the data from these studies were evaluated as descriptive data and still considered in the results of the review.
Subgroup analysis based on study of type and duration of follow-up was then performed comparing RCT to quasi-RCT and 1 year to 2 years. The sensitivity analysis was also conducted by sequential omission of each study in turn to test the stability and strength of pooled results.
GRADE quality assessment
GRADE quality assessment which has been increasingly adopted by many health research organizations was performed using the software GRADEprofiler 3.6 (Cochrane Collaboration, http://tech.cochrane.org/revman/other-resources/gradepro/download). Since data from RCTs were considered high-quality evidence, two investigators rated down the quality of evidence only by one for each following item: risk of bias, inconsistency, imprecision, indirectness and publication bias. Disagreements and discrepancies between the investigators were resolved by consensus with all co-authors to come to an agreement.
Results
Search results
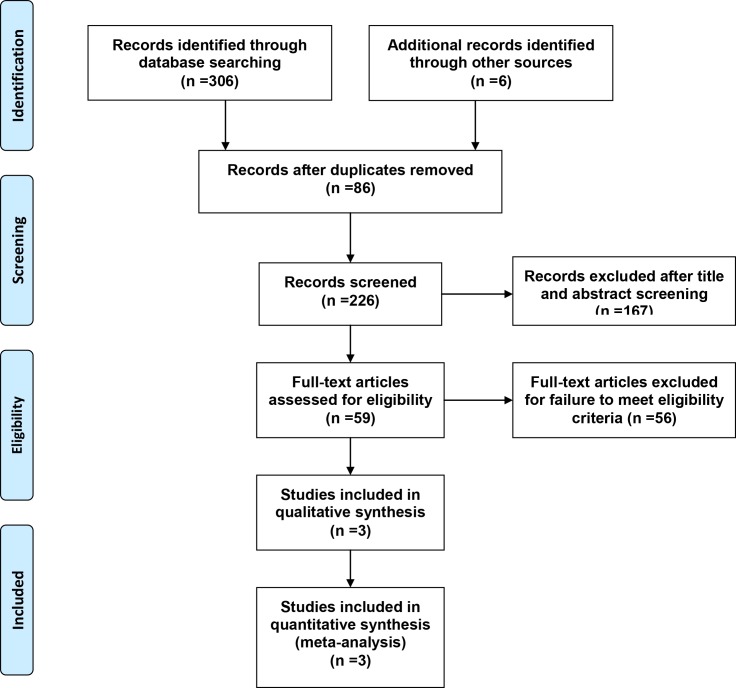
A flow diagram that described the details of literature search was presented in Fig 1. A total of 312 potentially relevant literatures (192 from PubMed MEDLINE, 21 from Cochrane Library, 33 from EMBASE, 41 from Web of Science, 12 from OVID AMED, 7 from EBSCO and 6 from other) were identified in our initial electronic search. After removal of duplicated records, 226 literatures were remained. Then we excluded 167 inappropriate literatures by scanning the titles and abstracts. After this, the full text of remaining 59 articles were obtained and assessed for eligibility. 56 of them were further excluded for failure to meet the predefined standard protocol (S2 Table). Finally, two RCTs and one quasi-RCT were selected and analyzed in our study.
Fig 1. Review flow diagram.
Study characteristics
The summarized characteristics of the three studies included in the present study were presented in Table 1. These studies including 2 RCTs [14, 15] and 1 quasi-RCT [16] were published from 2009 to 2012. Among them, in one studies [16] submuscular technique of ulnar nerve was done in the original operative procedure (13 participants), whereas in the other two study [14, 15] the modified submuscular transposition was used (163 participants). The studies were conducted in Sweden [16], China [14] and Iran [15]. Data from a total of 352 patients (ranging from 26 to 378, only 1 study enrolled≥100 participants) were collected of whom 176 received subcutaneous transposition and 176 received submuscular transposition. The clinical improvement in function compared to baseline was evaluated based on different criteria in included studies. The average follow-up duration of the trails ranged from 12 to 24 months.
Table 1. Characteristics of the included studies.
| Author | Year | Country | Study type | Subcutaneous | Submuscular | Evaluation of Procedure | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n, M/F | Mean Age (y) | Follow-up (y) | n, M/F | Mean Age (y) | Follow-up (y) | |||||
| Jaddue | 2009 | Sweden | quasi-RCT | 13,10/3 | 34 | 1 | 13,10/3 | 34 | 1 | Improvement or not improvement |
| Zarezadeh | 2012 | Iran | RCT | 24,13/11 | 47.58±12.1 | 1 | 24,14/10 | 47.41±12.2 | 1 | Improvement or not improvement |
| Electrophysiological test | ||||||||||
| Zhong | 2011 | China | RCT | 139 | NA(32–66) | 2 | 139 | NA(32–66) | 2 | Improvement or not improvement |
| Electrophysiological test | ||||||||||
| Ultrasound test |
RCTs: randomized controlled trials; NA: not available; M/F: man/female; y: year.
Risk of bias in included studies
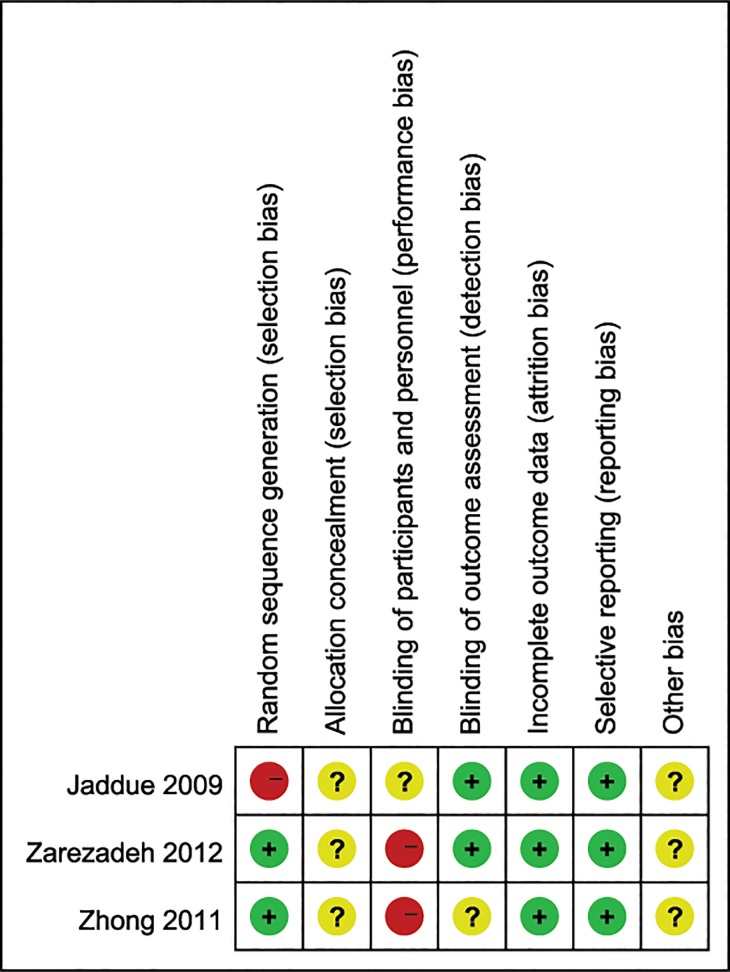
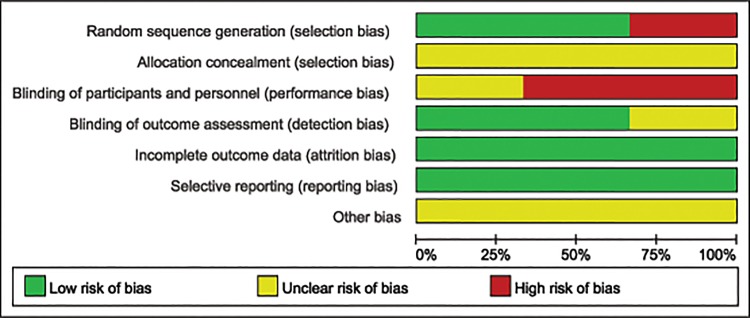
The methodological quality of each included study has been described in Fig 2 and the judgment of “Risk of bias graph” regarding each risk of bias assessment was presented as percentages across all the three included studies in Fig 3. Among the three included studies, two RCTs [14, 15] described adequate methods of randomization, which a computer-generated list was used to randomize the participants in Zhong’s study [14] while Zarezadeh’s study [15] was based on a random table numbers. The participants of the quasi-RCT [16] were randomly assigned by age (2 years margin) and gender. Since no concrete allocation concealment method was described in three included studies [14–16], we described these studies as unclear of allocation concealment. It was not clear whether participants were blinded to the operation in Jaddue’s studies [16], and they were not blinded in two [14, 15] of the three included studies. In two studies [15, 16] all participants were evaluated by the same independent assessors while the other one [14] did not. In these studies no participant was lost to follow-up, so we regarded the included studies as low risk of incomplete outcome data. We also considered all of these studies as low risk of selective outcome reporting for they described complete outcomes in detail. Other potential sources of bias were unclear in 3 included studies since none of the studies mentioned whether or not they had raised funding in support of their research.
Fig 2. Risk of bias summary: This risk of bias tool incorporates the assessment of randomization (sequence generation and allocation concealment), blinding (participants and outcome assessors), incomplete outcome data, selective outcome reporting and other risk of bias.
The items were judged as “low risk”, “unclear risk” or “high risk”.
Fig 3. Risk of bias graph: Each risk of bias assessment was presented as the percentage across all the included studies, which indicates the proportion of different levels of risk of bias for each item.
Effects of interventions
Clinical improvement assessment
All of included studies reported proportion of participants with a clinically relevant improvement in function compared to baseline.
The clinical improvement measures differed in the three studies. Jaddue [16] used the Bishop rating system [17] after operation, which assessed subjective and objective parameters: subjective satisfaction, severity of residual symptoms (evaluated by pain, parasthesia, weakness, clumsiness), work status, leisure activity, grip strength and sensibility (static two point discrimination). Zhong [14] evaluated the clinical improvement postoperatively by including a combination of clinical presentation and physical findings (measurement of sensorimotor function). Zarezadeh [15] used Visual Analogue Scale [18], the Yale sensory scale [19], the Medical Research Council [20] and author-generated clinical scales as a way to evaluate pain, sensation, muscle strength and muscle atrophy respectively. To limit the potential source of bias when using the different assessment of clinical improvement, we reviewed the individual studies for the number of patients who improved or did not improve with each surgical treatment, and converted it into the binary categories of improvement or not improvement for this meta-analysis.
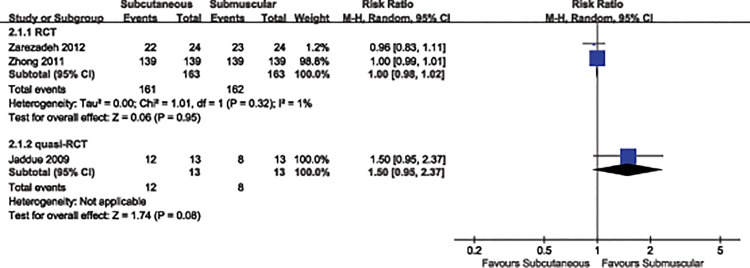
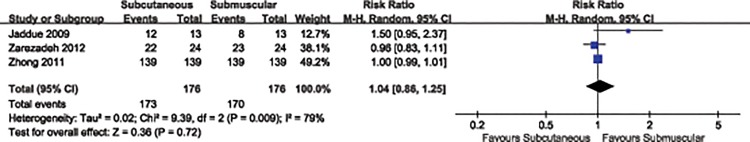
We found clinical improvement compared to baseline in 98.3% of the patients treated with subcutaneous transposition and in 96.7% of those treated with submuscular transposition. No significant difference was observed in postoperative clinical improvement between two treatment groups (RR 1.04, 95% CI 0.86 to 1.25, P = 0.72). (Analysis 1.1, Fig 4). A random-effects model was applied because statistical evidence of heterogeneity was found (P = 0.009, I2 = 79%). Sensitivity analysis revealed that heterogeneity may be attributed to the inclusion of the study reported by Jaddue [16] et al, in which participant populations compared to remaining studies were relatively small. Eliminating this study from the analysis showed a substantially reduced heterogeneity (P = 0.95, I2 = 1%). But we did not drop this study because only three included studies provided the clinically relevant improvement information and moderate methodological quality of the evidence.
Fig 4. Forest plot of comparison: 1 Clinical effect of subcutaneous versus submuscular, outcome: 1.1 Proportion of patients with clinical improvement in function compared to baseline.
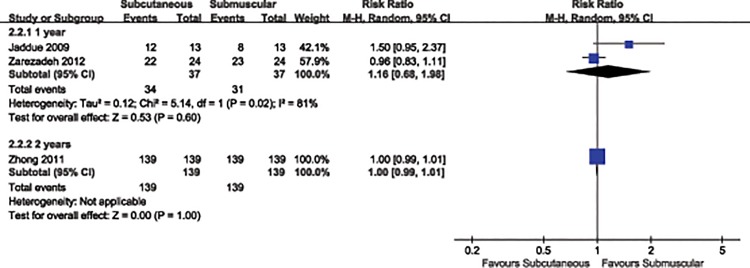
We then conducted two subgroup analyses for the clinical efficacy of anterior transposition of the ulnar nerve by type of study (RCT vs. quasi-RCT) and duration of follow-up (1 year vs. 2 years). In the subgroup analyses by study of type (RCT: RR 1.00, 95% CI 0.98 to 1.02, P = 0.95; quasi-RCT: RR 1.50, 95% CI 0.95to 2.37, P = 0.08) (Analysis 2.1, Fig 5) and duration of follow-up (1 year: RR 1.16, 95% CI 0.68 to 1.98, P = 0.6; 2 years: RR 1.00, 95% CI 0.99 to 1.01, P = 1.00) (Analysis 2.2, Fig 6), the results were in accordance with the overall estimate (Table 2).
Fig 5. Forest plot of comparison: 2 study of subgroup, outcome: 2.1 study of type.
Fig 6. Forest plot of comparison: 2 study of subgroup, outcome: 2.2 duration of follow-up.
Table 2. Results of subgroup analysis.
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 2.1 Study type | 3 | Risk Ratio (M-H, Random, 95% CI) | ||
| 2.1.1 RCT | 2 | 326 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.98, 1.02] |
| 2.1.2 quasi-RCT | 1 | 26 | Risk Ratio (M-H, Random, 95% CI) | 1.50 [0.95, 2.37] |
| 2.2 Follow-up duration | 3 | Risk Ratio (M-H, Random, 95% CI) | ||
| 2.2.1 1 year | 2 | 74 | Risk Ratio (M-H, Random, 95% CI) | 1.16 [0.68, 1.98] |
| 2.2.2 2 years | 1 | 278 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.99, 1.01] |
RCTs: randomized controlled trials.
Electrodiagnostic assessment
The pre- and postoperative motor nerve conduction velocities were reported in two studies [14, 15] with a total of 326 patients. In order to assess the clinical efficacy derived from operation in Zhong’s study [14], all patients were divided into three grades of ulnar neuropathy according to the severity of the neurological signs [21] at the time of operation: patients with mild lesions, but without detectable motor weakness, were McGowan grades I; patients with moderate lesions were McGowan grades II; patients with severe lesions that occurred marked paralysis of the ulnar intrinsic muscles were McGowan grades III.
Zhong reported measurements of postoperative MCV, SCV and NAP of the ulnar nerve at the elbow [14]. In this study, postoperative MCV, SCV and NAP were significantly better than before the operation (P < 0.05). What’s more, it was found that patients with McGowan grade II and III showed significantly greater improvements in MCV, SCV and NAP, in which the changes from baseline in submuscular group were better than in subcutaneous group, where statistically significant differences were demonstrated between MCV (r = –0.832, P<0.01), SCV (r = –0.825, P<0.01), and NAP (r = –0.862, P<0.01), while those with McGowan grades I showed no significant differences between two groups. Therefore, it may show the potential to detect a treatment effect in favor of MCV, SCV and NAP in patients with McGowan grade II and III.
To prevent bias, all participants in Zarezadeh’s study [15] underwent double-blind nerve conduction studies, conducted by the same neurophysiologists according to a standard protocol, without concrete numerical value. We sent an e-mail to the author for the original raw data, but no responses were received.
Ultrasound assessment
Ultrasound test was reported in only one study [14]. In this study, High-resolution ultrasound detection of postoperative CSA of the ulnar nerve was performed using the envelopment method [22–24], within one day of the electrophysiological tests.
Postoperative CSA demonstrated significantly greater improvements than before the operation (P<0.05). For McGowan grades I patients, there was no significant difference in CSA between two groups. For McGowan grades II and III, submuscular group showed significantly greater improvements in CSA than subcutaneous group.
Adverse events
Postoperative wound infection was reported in only one study [16]. In this study, submuscular transposition of ulnar nerve was associated with a higher number of wound infections (1/13, 7%) (one wound infection in the submuscular transposition group, zero wound infection in the subcutaneous transposition group). However, the evidence of wound infection regarding whether subcutaneous group is superior to submuscular group remains insufficient, owning to relatively small sample size of this study.
Discussions
Summary of main results
This systematic review and meta-analysis summarizes the results of 3 RCTs about the clinical efficacy of subcutaneous versus submuscular anterior ulnar nerve transposition for the treatment of cubital tunnel syndrome. Studies [14, 15] investigating the comparison of anterior subcutaneous transposition and modified submuscular transposition were included for this review. The available evidence in the present study suggests that anterior subcutaneous and submuscular transposition might be equally effective in the treatment of ulnar neuropathy at the elbow, because we found no statistically significant difference between two comparison groups in terms of postoperative clinical improvement compared to baseline (RR 1.04, 95% CI 0.86 to 1.25). This is true when identifying only three eligible studies or when combining two real RCTs and one relative under-representation of RCTs. Furthermore, we were not adequately powered to identify whether the detectable difference in proportion of patients with clinical improvement in subcutaneous group (98.3%) versus in submusclar group (96.7%) was actually statistically significant. With these in mind, small numbers of eligible studies and the low or moderate quality of these studies don’t allow us to reach reliable conclusions.
Overall completeness and applicability of evidence
We identified only three studies, enrolling 352 participants, that clinical practice on the basis of varied outcomes definitely differed from population to population, and from centre to centre.
Although the preoperative status of participants among the included studies may have varied, we specified the clinical outcomes as improved or not improved regardless of whatever tool was used, the intention of which was to reduce the potential source of bias according to the predefined standard protocol. Similarly, there is controversy whether the status of participants before surgery affects the eventual postoperative outcome. however, because of inconsistency in reporting of preoperative status among studies, it was not possible to stratify for this variable that would provide useful information from the inclusion of representative studies [25]. In addition, the only three studies included, with similar interventions, small numbers of participants, limited objective information about clinical improvement in function, provide inadequate evidence that is relevant to the areas of clinical application.
Quality of the evidence
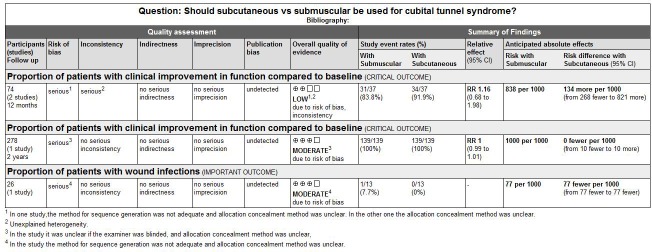
The quality of the evidences for the primary outcomes assessed by GRADE approach was low or moderate in the present study, as shown in Fig 7. All the included studies were RCTs using a truly randomized or quasi-randomized allocation which were substantially less prone to selective bias. The method for sequence generation was adequate in two of the three RCTs included in our meta-analysis. All degrees of severity of symptoms with clinical and electrodiagnostic evidence of ulnar nerve impairment were considered. All participants were followed up for at least 12 months after operation, which showed a low risk of attrition bias.
Fig 7. Grade profile for subcutaneous vs. submuscular for cubital tunnel syndrome.
However, some limitations in the present study should also be noted: Firstly, there was no a clear attempt in methods of randomization and allocation concealment in one study (in other two studies no concrete allocation concealment). Blinding of participants in two studies was inadequate (in one study it was unclear whether the participants were blinded). Secondly, there were only three RCTs with relatively poor methodological quality included in our meta-analysis and the efficacy of our result was relatively low considering that the quality of evidence for the primary outcomes was ‘low’ or ‘moderate’ based on GRADE approach. Thirdly, the assessment of clinical improvement in function compared to baseline was different in the three included studies, resulting in a low reliability of the results in our meta-analysis. In addition, some unpublished studies might not be included, which would lead to nonpublication bias; in the meantime, the lack of high quality of evidence limits us to further investigate the heterogeneity of the studies.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the first systematic review and meta-analysis to evaluate the clinical efficacy of subcutaneous versus submuscular anterior ulnar nerve transposition for the treatment of cubital tunnel syndrome. In some previous meta-analyses [26–29] on the therapeutic management of ulnar neuropathy at the elbow, the author investigated the comparison of simple decompression and decompression with anterior transposition (subcutaneous or submuscular). And the author concluded that no significantly statistical difference in clinical outcomes between two surgical treatments was observed, but rather a trend toward less complication with simple decompression of the ulnar nerve as opposed to anterior transposition in Chen’s study [29]. A similar comparison was used by Bartels [30] and Mowlavi [31] but in these studies the authors introduced therapeutic modalities including simple decompression, anterior transposition and medial epicondylectomy. The majority of these reports analyzed the clinical outcomes as binary outcomes, but Zlowodzki [26] defined the clinical scores as continuous outcomes and used standardized mean difference (SMD). Whereas we converted the clinical outcomes into the binary categories of improved or not improved according to the registered protocol, regardless of whatever tool was used among studies. In review [27, 29], retrospective studies were also considered together in the meta-analysis, which raised the possibility of selection bias, while our study was limited to RCT or quasi-RCT.
Authors’ Conclusions
The quality of available evidence for the primary outcomes varied from ‘low’ to ‘moderate’, and our main findings largely rely on the outcomes data from the few studies with low patient numbers. The limited evidence is insufficient to identify the optimum anterior transposition technique in the treatment of cubital tunnel syndrome. The results of the present study suggest that anterior subcutaneous and submuscular transposition might be equally effective in patients with ulnar neuropathy at the elbow. Thus, it is urgent to conduct RCT level I on the therapeutic management of cubital tunnel syndrome. Future investigation in this area should include high level of scientific evidence RCTs with standardized clinical improvement metrics to evaluate the effectiveness of two surgical options. These RCTs should be sufficiently powered to further clarify this topic and to provide reproducible pre- and postoperative objective outcomes.
Supporting Information
(DOC)
(DOC)
Acknowledgments
We thank academic editor (Nader N. Pouratian, MD PhD) and all the anonymous reviewers for their helpful suggestions on the quality improvement of our paper.
Data Availability
All relevant data are within our paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Zimmerman RM, Jupiter JB, Gonzalez del Pino J. Minimum 6-year follow-up after ulnar nerve decompression and submuscular transposition for primary entrapment. The Journal of hand surgery. 2013;38(12):2398–404. Epub 2013/11/05. 10.1016/j.jhsa.2013.09.017 . [DOI] [PubMed] [Google Scholar]
- 2. Kroonen LT. Cubital tunnel syndrome. The Orthopedic clinics of North America. 2012;43(4):475–86. Epub 2012/10/03. 10.1016/j.ocl.2012.07.017 . [DOI] [PubMed] [Google Scholar]
- 3. Mondelli M, Giannini F, Ballerini M, Ginanneschi F, Martorelli E. Incidence of ulnar neuropathy at the elbow in the province of Siena (Italy). Journal of the neurological sciences. 2005;234(1–2):5–10. Epub 2005/07/05. 10.1016/j.jns.2005.02.010 . [DOI] [PubMed] [Google Scholar]
- 4. Henry M. Modified intramuscular transposition of the ulnar nerve. The Journal of hand surgery. 2006;31(9):1535–42. Epub 2006/11/11. 10.1016/j.jhsa.2006.04.016 . [DOI] [PubMed] [Google Scholar]
- 5. Tada H, Hirayama T, Katsuki M, Habaguchi T. Long term results using a modified King's method for cubital tunnel syndrome. Clinical orthopaedics and related research. 1997;(336):107–10. Epub 1997/03/01. . [DOI] [PubMed] [Google Scholar]
- 6. Artico M, Pastore FS, Nucci F, Giuffre R. 290 surgical procedures for ulnar nerve entrapment at the elbow: physiopathology, clinical experience and results. Acta neurochirurgica. 2000;142(3):303–8. Epub 2000/05/20. . [DOI] [PubMed] [Google Scholar]
- 7. Nouhan R, Kleinert JM. Ulnar nerve decompression by transposing the nerve and Z-lengthening the flexor-pronator mass: clinical outcome. The Journal of hand surgery. 1997;22(1):127–31. Epub 1997/01/01. 10.1016/s0363-5023(05)80192-3 . [DOI] [PubMed] [Google Scholar]
- 8. Brauer CA, Graham B. The surgical treatment of cubital tunnel syndrome: a decision analysis. The Journal of hand surgery, European volume. 2007;32(6):654–62. Epub 2007/11/13. 10.1016/j.jhse.2007.07.001 . [DOI] [PubMed] [Google Scholar]
- 9. Assmus H, Antoniadis G, Bischoff C, Hoffmann R, Martini AK, Preissler P, et al. Cubital tunnel syndrome—a review and management guidelines. Central European neurosurgery. 2011;72(2):90–8. Epub 2011/05/07. 10.1055/s-0031-1271800 . [DOI] [PubMed] [Google Scholar]
- 10. Lee SK, Sharma S, Silver BA, Kleinman G, Hausman MR. Submuscular versus subcutaneous anterior ulnar nerve transposition: a rat histologic study. The Journal of hand surgery. 2009;34(10):1811–4. Epub 2009/11/10. 10.1016/j.jhsa.2009.08.007 . [DOI] [PubMed] [Google Scholar]
- 11.Dijkers M. Introducing GRADE: a systematic approach to rating evidence in systematic reviews and to guideline development Available: http://www.ktdrr.org/products/update/v1n52013.
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557 ; PubMed Central PMCID: PMCPmc192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) Available: http://handbook.cochrane.org/2011 [cited 2010 2004]. Available from: http://handbook.cochrane.org/.
- 14. Zhong W, Zhang W, Zheng X, Li S, Shi J. Comparative study of different surgical transposition methods for ulnar nerve entrapment at the elbow. Journal of International Medical Research. 2011;39(5):1766–72. [DOI] [PubMed] [Google Scholar]
- 15. Zarezadeh A, Shemshaki H, Nourbakhsh M, Etemadifar MR, Moeini M, Mazoochian F. Comparison of anterior subcutaneous and submuscular transposition of ulnar nerve in treatment of cubital tunnel syndrome: A prospective randomized trial. Journal of Research in Medical Sciences. 2012;17(8):745–9. [PMC free article] [PubMed] [Google Scholar]
- 16. Jaddue DA, Saloo SA, Sayed-Noor AS. Subcutaneous vs Submuscular Ulnar Nerve Transposition in Moderate Cubital Tunnel Syndrome. The open orthopaedics journal. 2009;3:78–82. Epub 2009/09/12. 10.2174/1874325000903010078 ; PubMed Central PMCID: PMCPmc2738827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. The Journal of hand surgery. 1989;14(6):972–9. 10.1016/S0363-5023(89)80046-2 [DOI] [PubMed] [Google Scholar]
- 18. Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, Mehta G. Studies with different types of visual analog scales for measurement of pain. Clinical pharmacology and therapeutics. 1983;34(2):234–9. Epub 1983/08/01. . [DOI] [PubMed] [Google Scholar]
- 19. Nabhan A, Ahlhelm F, Kelm J, Reith W, Schwerdtfeger K, Steudel WI. Simple decompression or subcutaneous anterior transposition of the ulnar nerve for cubital tunnel syndrome. Journal of hand surgery (Edinburgh, Scotland). 2005;30(5):521–4. Epub 2005/08/03. 10.1016/j.jhsb.2005.05.011 . [DOI] [PubMed] [Google Scholar]
- 20. Van Allen MW. Aids to the examination of the peripheral nervous system. Archives of Neurology. 1977;34(1):61–. [Google Scholar]
- 21. Mc GA. The results of transposition of the ulnar nerve for traumatic ulnar neuritis. The Journal of bone and joint surgery British volume. 1950;32-b(3):293–301. Epub 1950/08/01. . [DOI] [PubMed] [Google Scholar]
- 22. Beekman R, Schoemaker MC, Van Der Plas JP, Van Den Berg LH, Franssen H, Wokke JH, et al. Diagnostic value of high-resolution sonography in ulnar neuropathy at the elbow. Neurology. 2004;62(5):767–73. Epub 2004/03/10. . [DOI] [PubMed] [Google Scholar]
- 23. Wiesler ER, Chloros GD, Cartwright MS, Shin HW, Walker FO. Ultrasound in the diagnosis of ulnar neuropathy at the cubital tunnel. The Journal of hand surgery. 2006;31(7):1088–93. Epub 2006/09/02. 10.1016/j.jhsa.2006.06.007 . [DOI] [PubMed] [Google Scholar]
- 24. Volpe A, Rossato G, Bottanelli M, Marchetta A, Caramaschi P, Bambara LM, et al. Ultrasound evaluation of ulnar neuropathy at the elbow: correlation with electrophysiological studies. Rheumatology (Oxford, England). 2009;48(9):1098–101. Epub 2009/07/02. 10.1093/rheumatology/kep167 . [DOI] [PubMed] [Google Scholar]
- 25. Foster RJ, Edshage S. Factors related to the outcome of surgically managed compressive ulnar neuropathy at the elbow level. The Journal of hand surgery. 1981;6(2):181–92. Epub 1981/03/01. . [DOI] [PubMed] [Google Scholar]
- 26. Zlowodzki M, Chan S, Bhandari M, Kalliainen L, Schubert W. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome. A meta-analysis of randomized, controlled trials. The Journal of bone and joint surgery American volume. 2007;89(12):2591–8. Epub 2007/12/07. 10.2106/jbjs.g.00183 . [DOI] [PubMed] [Google Scholar]
- 27. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. The Journal of hand surgery. 2008;33(8):1314.e1–12. Epub 2008/10/22. 10.1016/j.jhsa.2008.03.006 . [DOI] [PubMed] [Google Scholar]
- 28. Caliandro P, La Torre G, Padua R, Giannini F, Padua L. Treatment for ulnar neuropathy at the elbow. The Cochrane database of systematic reviews. 2012;7:Cd006839. Epub 2012/07/13. 10.1002/14651858.CD006839.pub3 . [DOI] [PubMed] [Google Scholar]
- 29. Chen HW, Ou S, Liu GD, Fei J, Zhao GS, Wu LJ, et al. Clinical efficacy of simple decompression versus anterior transposition of the ulnar nerve for the treatment of cubital tunnel syndrome: A meta-analysis. Clinical neurology and neurosurgery. 2014;126:150–5. Epub 2014/09/26. 10.1016/j.clineuro.2014.08.005 . [DOI] [PubMed] [Google Scholar]
- 30. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. Journal of neurosurgery. 1998;89(5):722–7. Epub 1998/11/17. 10.3171/jns.1998.89.5.0722 . [DOI] [PubMed] [Google Scholar]
- 31. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: a meta-analysis of clinical studies. Plastic and reconstructive surgery. 2000;106(2):327–34. Epub 2000/08/18. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within our paper.