Abstract
Production of pro-inflammatory cytokines by innate immune cells at the early stages of bacterial infection is important for host protection against the pathogen. Many intracellular bacteria, including Francisella tularensis, the agent of tularemia, utilize the anti-inflammatory cytokine IL-10, to evade the host immune response. It is well established that IL-10 has the ability to inhibit robust antigen presentation by dendritic cells and macrophages, thus suppressing the generation of protective immunity. The pathogenesis of F. tularensis is not fully understood, and research has failed to develop an effective vaccine to this date. In the current study, we hypothesized that F. tularensis polarizes antigen presenting cells during the early stages of infection towards an anti-inflammatory status characterized by increased synthesis of IL-10 and decreased production of IL-12p70 and TNF-α in an IFN-ɣ-dependent fashion. In addition, F. tularensis drives an alternative activation of alveolar macrophages within the first 48 hours post-infection, thus allowing the bacterium to avoid protective immunity. Furthermore, we demonstrate that targeting inactivated F. tularensis (iFt) to Fcγ receptors (FcɣRs) via intranasal immunization with mAb-iFt complexes, a proven vaccine strategy in our laboratories, reverses the anti-inflammatory effects of the bacterium on macrophages by down-regulating production of IL-10. More specifically, we observed that targeting of iFt to FcγRs enhances the classical activation of macrophages not only within the respiratory mucosa, but also systemically, at the early stages of infection. These results provide important insight for further understanding the protective immune mechanisms generated when targeting immunogens to Fc receptors.
Introduction
For many intracellular bacteria the induction of a robust innate immune response is a critical factor in host protection and bacterial clearance [1–3]. The induction of innate immunity is triggered upon recognition of bacterial components such as lipopolysaccharides, peptidoglycans, or bacterial DNA by cellular receptors and regulated by a number of cytokines including IL-12, TNF-α and IFN-γ [4, 5].
Interleukin-10, an anti-inflammatory cytokine secreted by many different cell populations including T cells, B cells, macrophages, dendritic cells and keratinocytes [6, 7] inhibits pro-inflammatory cytokine synthesis and the antigen presenting ability of monocytes/macrophages and dendritic cells [8]. A number of studies have demonstrated that many intracellular pathogens, such as F. tularensis, a Gram-negative intracellular bacterium that causes tularemia, use IL-10 to evade the host immune defense especially in the initial stages of infection [9–12]. F. tularensis can be transmitted through insect bites, infected carcasses, contaminated water, and inhalation of contaminated air, although inhalation of as little as 1–2 bacteria can lead to respiratory failure and death if left untreated [13]. For this reason, the Centers for Disease Control and Prevention has designated F. tularensis as a Category A biological agent [14]. Since no licensed vaccine for tularemia is currently available in the United States, there is a need for development of an effective vaccine.
Several studies reported that early innate immune responses, in particular secretion of inflammatory cytokines such as TNF-α, IFN-γ and IL-12, provide immediate control over F. tularensis replication [15–17]. F. tularensis infection, however, is characterized by the absence of early immune responses throughout the first 2–3 days after infection [18–20]. This is believed to occur partly because of the low endotoxicity of F. tularensis lipopolysaccharide (LPS), which is structurally different from other Gram-negative bacterial LPS [21–24]. In addition, despite the absence of an early protective inflammatory response against F. tularensis infection, the potential role of anti-inflammatory cytokines, such as IL-10, in the progression of infection has not been clearly elucidated.
We have previously demonstrated that targeting inactivated F. tularensis (iFt) bacteria to the Fcγ receptors (FcγRs) in mice, via immunization with mAb-iFt immune complexes, resulted in: (1) enhanced uptake and presentation of the immunogen (iFt) by professional antigen presenting cells, (2) increased recruitment and activation of dendritic cells in the lungs of immunized mice, (3) enhanced F. tularensis-specific cytokine and antibody responses, (4) generation of effector memory CD4+ T cells, and (5) increased protection against F. tularensis infection [19, 25, 26]. In the current study we hypothesized that F. tularensis polarizes antigen presenting cells (APCs) during the first 48 hours post-infection towards an anti-inflammatory status, characterized by IL-10 production, thus allowing the pathogen to avoid protective anti-bacterial innate immune responses. Furthermore, we seek to determine whether targeting of mAb-iFt immune complexes to FcγRs reverses the potential detrimental role of IL-10 during the early stages of Francisella infection. Using our vaccine platform we demonstrate that targeting of inactivated F. tularensis (iFt) bacteria to FcγRs leads to systemic macrophage activation, shifts the cytokine profile from anti-inflammatory to pro-inflammatory, and alters the alveolar macrophage activation state from alternatively to classically activated macrophages in the lungs of mAb-iFt immunized mice during the early stages of F. tularensis infection. In summary, this study identifies a critical link between the ability of F. tularensis to suppress the immune response and the ability of FcγR-targeted immunogen to alter that response and thereby enhance protection against infection.
Materials and Methods
Mice and bacteria
C57BL/6 and IL-10 genetically deficient mice were purchased from Jackson Laboratories (Bar Harbor, Maine). All mice were housed at the Animal Research Facility at Seton Hall University. The mice were used at 6–10 weeks of age. All protocols were reviewed and approved by the Seton Hall University Ethics Committee utilizing NIH standards.
F. tularensis LVS (ATCC 29684; American Type Culture Collection) was provided by K. Elkins (U.S. Food and Drug Administration, Bethesda, MD).
Ethics Statement
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the Seton Hall University Animal Care and Use Committee (Approval # CB1401). Guidelines provided by the NIH were followed in all experimentation. Briefly, mouse anesthesia was performed via i.p. injections of a ketamine / xylazine cocktail, while mouse euthanasia was achieved via CO2 administration followed by cervical dislocation.
Antibodies
Mouse IgG2a anti-Ft. LPS mAb used to generate mAb-iFt immune complexes was purchased from Fitzgerald (Cat# 10-F02, clone#M023621, Acton, MA). The following flow cytometry antibodies were purchased from BD Biosciences (San Jose, California): F4/80 (PE), CD11b (FITC), CCR7 (PE-Cy5.5), MHC class II (APC), B7.1 and B7.2 (PercP), CD11c (APC)
Inactivation and Labeling of F. tularensis
Inactivated F. tularensis LVS (iFt) was generated by growing F. tularensis LVS in Mueller Hinton broth (MHB) media (BD Biosciences) up to a density of 1x109 CFU/mL. The culture was then spun down at 22,000g for 20 min. at 4°C, and washed 3 times with PBS, resuspended in 2% Paraformaldehyde (Sigma) and incubated for 2 hours at room temperature on a rocker. Bacteria were then washed 3 more times with PBS and 1x109 organisms were plated on a chocolate agar plate (BD Biosciences) and incubated for 7 days at 37°C to confirm inactivation. The final concentration of iFt organisms was determined by OD at 610 nm.
mAb-iFt immune complex (IC) Generation
To generate ICs, 1x109 iFt organisms were incubated at 4°C overnight on a rocker with 0 μg/mL or 1μg/mL of anti-Ft mAb in PBS. Following the incubation, iFt or mAb-iFt preparations were administered to mice intranasally. Generation of ICs has been previously confirmed by ELISA and SDS-PAGE [19, 26].
Immunization and Challenge Studies
C57BL/6 and IL-10 deficient mice were divided into three groups consisting of 5–6 mice/group, 6–10 weeks of age. Each mouse was immunized on days 0 and 21 with 2x107 iFt organisms alone or in the form of mAb plus iFt ICs. On day 35 the mice were challenged with 10,000 CFU of live F. tularensis LVS. Following challenge survival was monitored twice-daily for 21–25 days. Death due to the infection was considered as the experimental end-point although in the occasions were animals were deemed to suffer (completely immobile, hunched backs, eyes shut), mice were sacrificed via CO2 administration followed by cervical dislocation, per our approved animal protocol (Approval # CB1401), and that was considered as the experimental end-point for the particular animals. Exact CFU administered were also verified by culturing and counting the inoculum subsequent to challenge on chocolate agar plate.
Lung leukocyte isolation
Lungs of immunized mice were harvested 24, 48 and 96 hours post-infection, perfused with cold 1x PBS containing a protease inhibitor cocktail, shredded into small pieces, and placed in digestion buffer containing RPMI (Life technologies), 0.2mg/ml DNaseI (Sigma), 0.4mg/ml Collagenase D (Sigma), and 1M MgCl2. After a 30 minute incubation at 37°C the digested tissue samples were forced through a cell strainer and the cell suspension obtained was washed and resuspended in RPMI containing 2% FBS. The cell suspension was then carefully layered on 5 mLs of Lympholyte M (Cedarlane Laboratories- Burlington, NC), and spun down at 15,000g for 30 minutes at room temperature. Following centrifugation, the interface containing the majority of immune cells was obtained and added in RPMI with 2% FBS, prior to enumeration. Identification and enumeration of alveolar macrophages cells was based on the expression of surface antigens F4/80 and CD11b.
Peritoneal exudate cell (PEC) isolation
PECs were harvested 48 hours post-infection, centrifuged in a refrigerated centrifuge 4,000g for 10 minutes. Following centrifugation, the interface containing PECs was resuspended in RPMI with 10% FBS, prior to enumeration. Identification and enumeration of PECs was based on the expression of surface antigens F4/80.
Flow cytometry
Peritoneal exudate cells or lung cells were obtained from immunized mice at different time-points post-LVS infection as described above. For cell surface marker staining, cells were washed with PBS-BSA-azide, resuspended in blocking buffer [PBS-BSA-azide plus 30 μg/ml of normal mouse IgG (Sigma)] and incubated on ice for 30 minutes. Cells were then washed with PBS-BSA-azide and fluorescently labeled antibodies to CD11b, F4/80, MHC class II, B7.1, B7.2, CCR7, or their corresponding isotype controls were added. The cells were then incubated on ice for 30 minutes, washed, and then fixed with 2% paraformaldehyde. Cells were then analyzed by flow cytometry on an LSRII flow cytometer (BD Biosciences).
Cytokine measurements
C57BL/6 mice were immunized intranasally (i.n) with PBS, iFt, or mAb-iFt, boosted on day 21 and challenged on day 35 with 10,000 CFUs of live Ft LVS. After 48 hours post-infection peritoneal cells were obtained from all groups and cultured for 24 hours with either LVS (1:10 and 1:100 MOI), Ft-LPS (kindly provided by Dr. Timothy Sellati-Albany Medical College, Albany, NY) or E. coli-LPS (Sigma) at 1 ng/mL, 10 ng/mL and 20 ng/ml, or recombinant IFN-γ (Invitrogen) at 100 U/ml. Supernatants were collected at designated time points and the levels of IL-12p70, TNF-α and IL-10 cytokines were measured using BD Biosciences Cytometric Bead Array (CBA) following vendor instructions.
In a separate experiment, lung tissue (left lobe) was harvested from immunized mice and homogenized (Omni Homogenizer, Omni International). Homogenates were then spun down at 15,000g for 30 minutes at room temperature to remove tissue debris and cytokine levels were detected by using the IL-6, IL-10, TNF-α and IFN-γ ELISA kits by following vendor instructions (Biolegend).
Statistical Analysis
Statistical differences among the groups were analyzed using a one-way analysis of variances (ANOVA) or the unpaired, one-tailed student t-test. GraphPad Prism 4 provided the software for the statistical analysis (San Diego, CA).
Results
Administration of mAb-iFt immune complexes (ICs) reverses the anti-inflammatory properties of LVS ex vivo and increases the activation of mouse peritoneal exudate cells (PECs)
One of the critical immune responses to bacterial infection is the synthesis and release of pro-inflammatory cytokines by innate immune cells during the early stages of infection [1–3]. Given the ability of F. tularensis to evade the immune system by favoring the short-term secretion of anti-inflammatory cytokines [12, 27], it was of interest to investigate the cytokine levels produced by PECs at the early stages of infection. Therefore, we analyzed the production of inflammatory cytokines IL-12p70 and TNF-α as well as the anti-inflammatory cytokine IL-10 using PECs from immunized and subsequently challenged mice. On day 35 post-immunization mice were challenged with 10,000 CFU of live F. tularensis LVS and cells were isolated two days post-infection. PECs were further stimulated with LVS ex vivo (at 1:10 and 1:100 MOI) for 24 hours and the cytokine levels in the supernatant were measured by the BD Biosciences Cytometric Bead Array (CBA). We observed that the levels of IL-12p70 (Fig 1A) and TNF-α (Fig 1B) in supernatants from PECs of mAb-iFt immunized mice were significantly higher (two to three fold) in comparison to mice immunized with iFt alone. By contrast, the levels of IL-10 production were 2-fold lower in the mAb-iFt compared to mice immunized with iFt alone (Fig 1C) independent of the MOI tested.
Fig 1. Administration of mAb-iFt immune complexes reverses the anti-inflammatory properties of LVS in mouse PECs ex vivo.
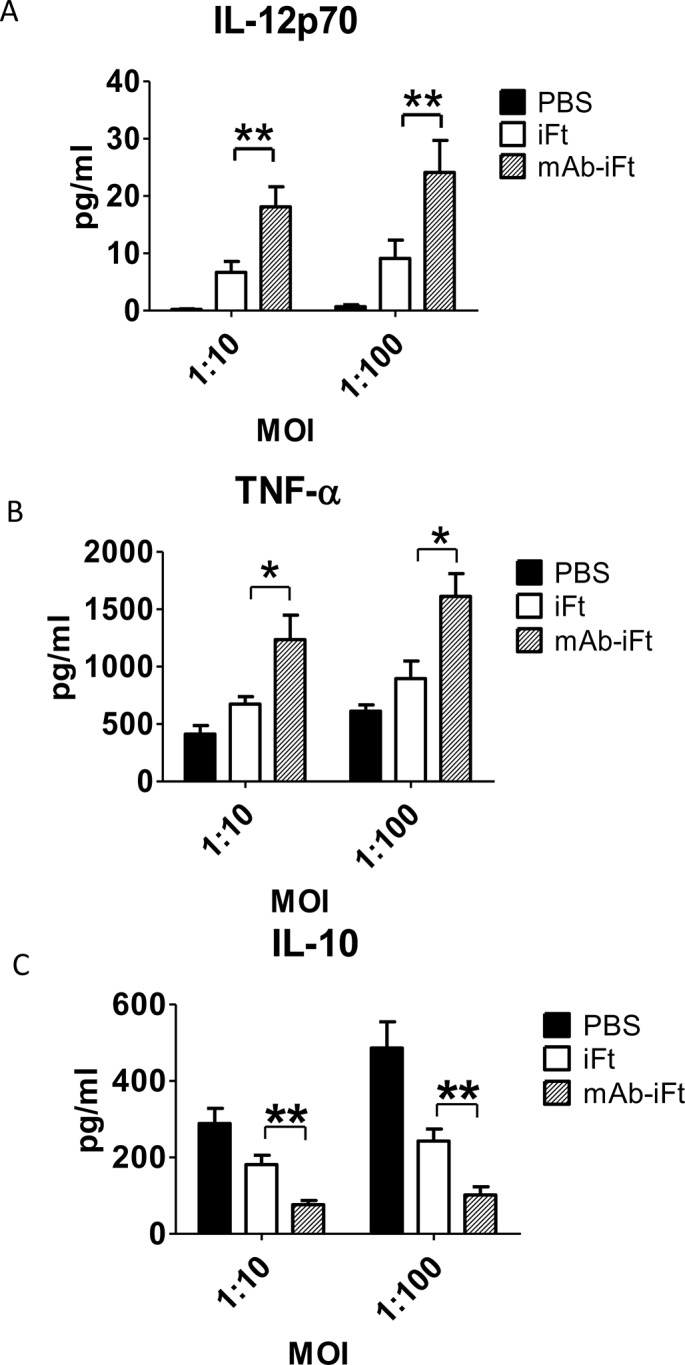
C57BL/6 mice were immunized i.n. with PBS, iFt (2x107 CFUs), or mAb-iFt, boosted on day 21 and challenged on day 35 with 10,000 CFUs of Ft LVS. After 48 hours post—LVS challenge, the PECs of immunized mice were harvested and cultured in the presence or absence of Ft. LVS at 1:10 and 1:100 MOI for 24 hrs. The cytokine production was measured as previously described. Results are representative of three independent experiments. (*) P-value < 0.1; (**) P-value < 0.05; bars represent the SD.
IL-10 is known to decrease the cell surface expression of MHC class II and co-stimulatory molecules CD80 and CD86 on murine macrophages [28]. However, one of the mechanisms by which immune complexes trigger immune responses is via T cell activation that requires robust antigen presentation by activated antigen presenting cells and increased expression of co-stimulatory molecules. Previous experiments have shown that co-culturing of Ft- specific T cell hybridoma with mouse PECs in the presence of mAb-iFt noticeably increased Ft-specific T cell responses compared to using the iFt immunogen alone [26]. In addition, in vivo administration of mAb-iFt ICs intranasally increased the activation status of mucosal dendritic cells in the lungs of immunized mice [25]. Therefore, we hypothesized that targeting of immunogen to Fcγ receptors (FcγRs) increases systemically the activation status of antigen presenting cells following LVS challenge. Thus, we examined the expression of MHC class II and co-stimulatory molecules CD80 and CD86 on PECs from immunized mice post-infection.
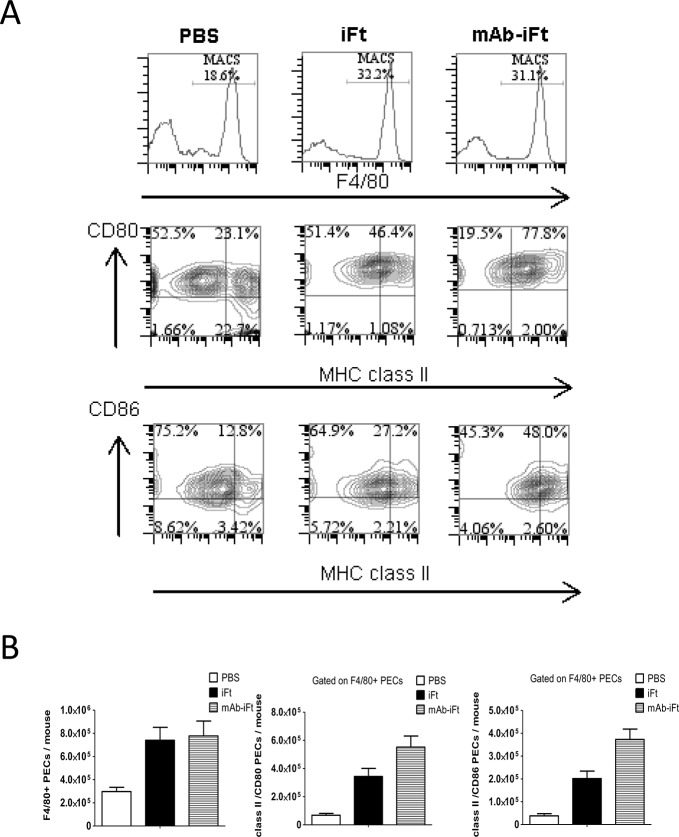
Peritoneal cells were obtained from immunized mice two days post-LVS challenge as described in Materials and Methods, and the expression of the murine macrophage cell-surface marker F4/80, the co-stimulatory molecules CD80 and CD86, as well as MHC class II was determined by flow cytometry. Although the number of cells expressing the F4/80 cell surface marker was similar between the iFt and mAb-iFt immunized mice, the number and frequency of cells expressing both MHC class II and CD80/CD86 molecules was significantly increased in the mAb-iFt group (Fig 2). This enhancement in surface marker expression upon immunization with mAb-iFt correlates with the increased presentation of iFt in the presence of mAb-iFt in vitro [26].
Fig 2. Immunization with mAb-iFt immune complexes increases the activation of PECs following LVS challenge.
C57BL/6 mice were immunized i.n. with PBS, iFt (2x107 CFUs), or mAb-iFt, boosted on day 21 and challenged on day 35 with 10,000 CFUs of Ft LVS. On day 2 post-infection the peritoneal exudate cells of immunized mice were harvested and the expression of F4/80, MHC class II, B7.1 (CD80), and B7.2 (CD86) were analyzed by flow cytometry. Results are representative of three independent experiments. (*) P-value < 0.1; (**) P-value < 0.05; bars represent the SD.
These results demonstrate for the first time that intranasal immunization with mAb-iFt favors a pro-inflammatory cytokine profile secreted by murine macrophages as depicted by an increase of IL-12p70 and TNF-α production and inhibition of IL-10. Moreover, it triggers activation of peritoneal macrophages during F. tularensis infection, indicating the induction of a systemic response in vivo.
F. tularensis LPS contributes to the anti-inflammatory properties of F. tularensis LVS in an IFN-γ and IL-10 dependent manner
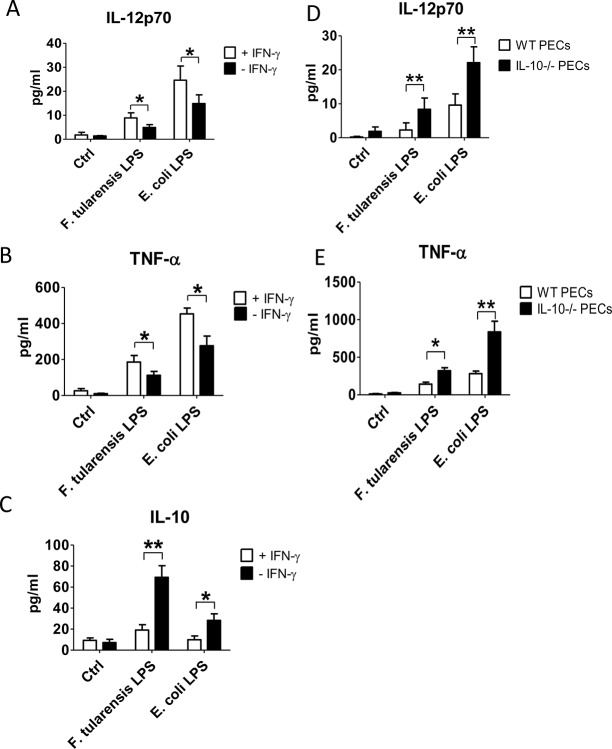
It is well established that F. tularensis LPS is structurally different from other intracellular bacteria and elicits a subdued inflammatory response in the initial stages of infection, which otherwise are critical in controlling bacterial burden [21–24]. To investigate the effect F. tularensis LPS on the cytokine profile produced by PECs, we measured the levels of IL-12p70, TNF-α and IL-10 in the supernatants of PECs from naïve C57BL/6 mice cultured with various concentrations of F. tularensis LPS. As expected, incubation with F. tularensis LPS at different concentrations triggered significantly lower levels of IL-12p70 (Fig 3A) and TNF-α (Fig 3B) secretion compared to E. coli LPS (positive control). In contrast, IL-10 production was elevated in the presence of F.tularensis LPS (Fig 3C). This observation not only confirms the low endotoxic activity of F. tularensis LPS but also indicates that it down-regulates Th1 cell mediated inflammatory responses via up-regulation of IL-10 and down-regulation of IL-12p70 and TNF-α.
Fig 3. tularensis LPS contributes to the anti-inflammatory properties of F. tularensis LVS during the early stages of infection.
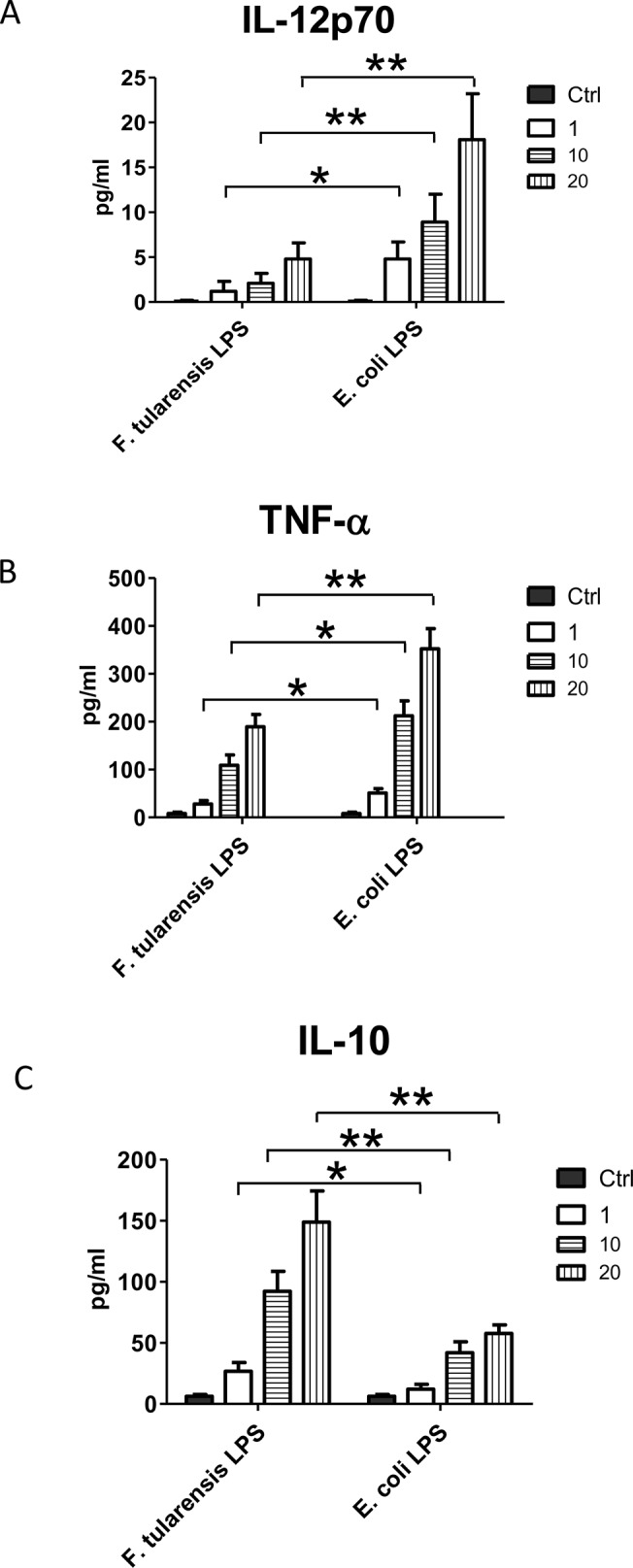
F. PECs from C57BL/6 mice were obtained and cultured in a 96-well plate at 2 x 105 cells/well in the presence or absence of either Ft-LPS or E. coli-LPS at 1 ng/mL, 10 ng/mL and 20 ng/ml. Cells incubated with PBS were used as a control. Levels of IL-12p70, TNF-α and IL-10 were measured using BD Biosciences Cytometric Bead Array (CBA) following vendor instructions. Results are representative of three independent experiments. (*) P-value < 0.1; (**) P-value < 0.05; bars represent SD.
Several studies have shown that IL-10 negatively regulates synthesis of IFN-γ as well as synthesis of other pro-inflammatory cytokines in monocytes and macrophages [29–31]. Due to the increased levels of IL-10 synthesis after incubation of PECs with F. tularensis LPS it was of interest to examine whether this up-regulation correlates with the decrease in TNF-α and IL-12p70 secretion. To investigate this, PECs from C57BL/6 wild-type and IL-10 genetically deficient mice were incubated with F. tularensis LPS or E. coli LPS. As previously observed, in the absence of endogenous IFN-γ, F. tularensis LPS portrayed anti-inflammatory properties via up-regulation of IL-10 production and decrease in the synthesis of IL-12p70 and TNF-α by the PECs. Interestingly, these results were reversed by the addition of exogenous IFN-γ (Fig 4A–4C). Lack of endogenous IL-10, in turn, resulted in an elevated synthesis of IL-12p70 (Fig 4D) and TNF-α (Fig 4E). These results indicate that increased levels of IL-10 synthesis is one of the mechanisms responsible for suppressing the protective pro-inflammatory cytokines such as TNF-α and IL-12p70 leading to an overall suppression of the Th1 response and reduced IFN-γ levels in the early stages of F. tularensis infection. In fact, the importance of IFN-γ during F. tularensis infection was reported by Rawool and colleagues [19], who observed a significant drop in survival rate in the mAb-iFt immunized IFN-γ-/- mice compared to the wild type control.
Fig 4. Anti-inflammatory effect of F. tularensis LPS on mouse PECs is IFN-γ and IL-10 dependent.
PECs from naïve and IL-10 genetically deficient C57BL/6 mice were obtained and resuspended in cell culture media. PECs were cultured in a 96-well plate at 2 x 105 cells/well with either Ft-LPS or E. coli-LPS at 1 ng/mL in the presence or absence of recombinant IFN-γ at 100 U/ml. Cells cultured with PBS were used as a control. The cytokine production was measured using BD Biosciences Cytometric Bead Array (CBA) following vendor instructions. Results are representative of three independent experiments. (*) P-value < 0.1, (**) P-value < 0.05, bars represent SD.
Administration of mAb-iFt immune complexes reverses the anti-inflammatory properties of LVS in the lungs of immunized mice
The balance and kinetics of pro- and anti-inflammatory cytokine secretion during F. tularensis challenge are key players in controlling the outcome of infection [18, 32, 33]. Consequently, having shown that immunization of mice with mAb-iFt favors a pro-inflammatory cytokine profile secreted by PECs in vitro, we attempted to determine the effect of our FcγR targeting approach on the levels of inflammatory cytokines in the lungs of immunized mice during the early stages of LVS infection. To accomplish this, C57BL/6 mice were immunized with PBS, or iFt, or mAb-iFt, boosted on day 21 and infected with a lethal dose of LVS on day 35 post-immunization. The lungs of euthanized mice were harvested after 24, 48 and 96 hours post-challenge, homogenized, and the IL-6, IL-10, TNF-α and IFN-γ cytokine levels were measured by direct sandwich ELISAs. Our results indicate a faster pro-inflammatory response in the lungs of mAb-iFt immunized mice compared to mice immunized with iFt alone, as assessed by the IL-6 and TNF-α production kinetics. Levels of IL-6 and TNF-α peaked at 48 hours post-infection, while they were significantly decreased by day 4 post-challenge (Fig 5). This observation was accompanied by a drop of the bacterial load (data not shown). On the other hand, the pro-inflammatory cytokine levels tested continued to increase at 96 hours post infection in both the PBS and iFt immunized mice accompanied by an increase in the bacterial burden in the lungs (Fig 5 and data not shown). Similar results among the three groups of mice were noted with IFN-γ (Fig 5). On the contrary, the levels of IL-10 were significantly higher in mice immunized with either iFt or PBS versus mAb-iFt within the first 24 of infection, indicating the early anti-inflammatory properties of F. tularensis LVS. Importantly, the decrease of IL-10 in the mAb-iFt immunized mice observed at 96 hours post-infection was consistent with our previous observation, indicating that FcγR targeting shifts towards a pro-inflammatory cytokine profile at the early stages of F. tularensis infection (Figs 2 and 5).
Fig 5. Administration of mAb-iFt immune complexes reverses the anti-inflammatory properties of LVS in the lungs of immunized mice.
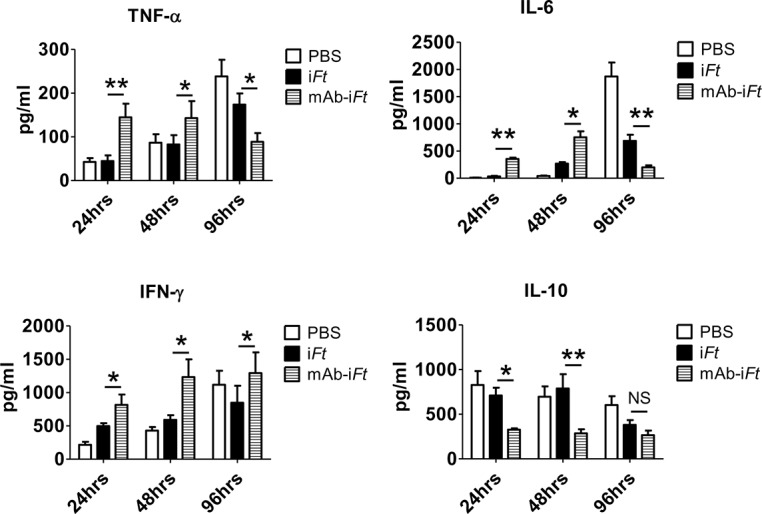
C57BL/6 mice were immunized i.n. with PBS, iFt (2x107 CFUs), or mAb-iFt, boosted on day 21 and challenged on day 35 with 10,000 CFUs of Ft LVS. Lung tissue homogenates were obtained from immunized mice 24, 48 and 96 hours post-infection as indicated above and spun down at 15,000g for 30 minutes at room temperature to remove tissue debris. Cytokine levels were detected by using the IL-6, IL-10, TNF-α and IFN-γ ELISA kits and following vendor instructions (Biolegend). Results are representative of three independent experiments. (*) P-value < 0.1; (**) P-value < 0.05; bars represent the SD.
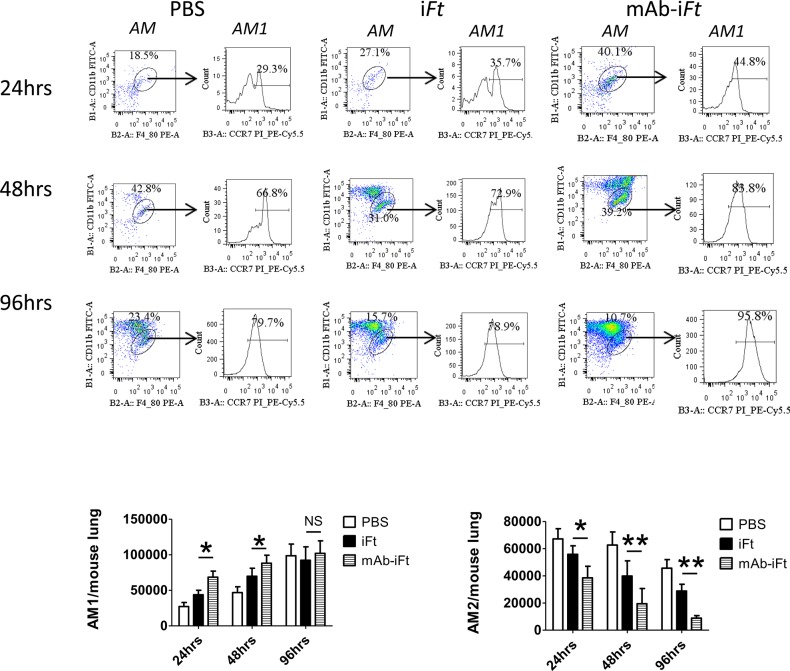
FcγR targeting drives polarization of mouse macrophages towards the AM1 phenotype at the early stages of LVS infection
Our results have suggested that one of the immune evasion mechanisms exploited by F. tularensis is the reduction of pro-inflammatory cytokines during the early stages of infection. To further investigate possible mechanisms responsible for this shift in the innate immune response, we analyzed the effect of FcγR targeting on macrophage activation and phenotype in the lungs of immunized mice. Previous research showed that one of the ways of F. tularensis survival and replication within the host cell is its ability to alter the macrophage activation from classically activated alveolar macrophages (AM1) to alternatively activated alveolar macrophages (AM2) [34]. The AM1 macrophages are characterized by high levels of pro-inflammatory cytokines and thus, play an essential role in anti-bacterial innate immune response. In contrast, AM2 macrophages are associated with high levels of anti-inflammatory cytokines, in particular IL-10 [35, 36]. Therefore, we performed flow cytometric analysis to assess the number of AM1 and AM2 present upon immunization with mAb-iFt. C57BL/6 mice were immunized with PBS, iFt, or mAb-iFt, boosted on day 21 and infected with a lethal dose of LVS on day 35 post-immunization. The lungs of euthanized mice were harvested 24, 48 and 96 hours post-challenge and the levels of F4/80, CD11b, CCR7, MHC class II and B7.2 marker expression on white blood cells was analyzed by flow cytometry. Classically activated AM1 cells are characterized as F4/80+/CD11bint/CCR7+/MHC class II+/B7.2+ cells, while AM2 cells were identified as F4/80+/CD11bint/CCR7-/ MHC class II-/B7.2- [35, 37]. Our results indicate that the frequency and number of AM1 cells was significantly higher in mAb-iFt immunized group of mice compared to mice immunized with iFt alone at 24, 48, and 96 hours post challenge (Fig 6). AM that were CCR7+ were also positive for MHC class II and B7.2 and thus considered classically activated macrophages (AM1) while AM that were CCR7- were also negative for the MHC class II and B7.2 markers, indicative of an alternative activated macrophage (AM2) (data not shown). Interestingly, the levels of AM1 cells were comparable among the different immunized groups at 96 hours of post challenge. In addition, our data showed a significantly lower number of AM2 cells in the lungs of mAb-iFt immunized mice relative to mice immunized with iFt alone at all three time points post-infection. It is also of interest that mice immunized with PBS had the highest frequency of AM2 cells, proposing that AM2 polarization in vivo may be an additional immune evasion strategy of F. tularensis, especially at the early stages of infection.
Fig 6. FcγR targeting drives polarization of mouse macrophages towards the AM1 phenotype at the early stages of LVS infection.
Lungs of immunized mice were harvested 24, 48 and 96 hours post-infection. For cell surface marker staining, cells were fluorescently labeled with antibodies against CD11b, F4/80, MHC class II, B7.1, B7.2, CCR7, or their corresponding isotype controls were added. Cells were then analyzed by flow cytometry on an LSRII flow cytometer (BD Biosciences). Results are representative of three independent experiments. (*) P-value < 0.1; (**) P-value < 0.05; bars represent the SD.
Discussion
In the current study, we demonstrated that targeting of inactivated F. tularensis (iFt) bacteria to Fcγ receptors via formation of immune complexes reverses the potential detrimental effect of IL-10 during the early stages of F. tularensis infection. Early control of bacterial infection depends on the secretion of pro-inflammatory cytokines that regulate the activation of antigen presenting cells and the generation of a protective Th1-like response [1–3]. One of the characteristics of F. tularensis infection is the lack of such responses during early progression of infection. While some studies depict that this effect is due to the low endotoxicity of F. tularensis lipopolysaccharide, which is structurally different from other Gram-negative bacteria [21–24], other studies show that the potential up-regulation of anti-inflammatory cytokines is an important factor in the progression of F. tularensis infection [12, 38]. The latter could possibly be due to suppression of the early activation of inflammasome by F. tularensis, one of the mechanisms that the bacteria utilizes to evade the early immune response [39–41].
The anti-inflammatory cytokine IL-10 plays a major role in suppression of host immune system by inhibiting the antigen presentation ability of macrophages and dendritic cells mainly by down-regulating the expression of CD80, CD86 and MHC class II molecules, as well as the production of pro-inflammatory cytokines [7]. The ability of IL-10 to suppress innate, inflammatory responses against intracellular pathogens has been previously reported. For instance, lack of endogenous IL-10 production led to a decrease in number of Trypanosoma cruzi parasites in the blood of infected mice [9]. Similarly, lack of IL-10 resulted in higher resistance of mice to Listeria monocytogenes [42]. In addition, viruses have also evolved mechanisms to escape the immune response by altering the Th1/Th2 balance. Thus, it has been shown that elevated levels of IL-10 correlated with higher viral load in HIV infected individuals [43].
IL-10 has also been identified as a key regulator of immune response to F. tularensis infection [12]. In particular, IL-10 mediates suppression of IL-17, a cytokine that is essential in the initiation of a protective immune response to F. tularensis infection [15–17, 44]
It is well established that targeting of antigen to FcγRs using a receptor subclass-specific monoclonal antibodies increases the binding, internalization and processing of an antigen by antigen presenting cells (APCs) in the absence of an adjuvant [45]. Furthermore, targeting of inactivated F. tularensis bacteria to FcγRs through intranasal immunization of mice with mAb-iFt enhances immune response and protection against F. tularensis infection possibly via FcγR dependent enhanced uptake of the antigen by APCs and further presentation to T cells leading to enhanced T cell activation [19, 26]. In the current study, we showed that a potential mechanism of protection in our F. tularensis model, following FcγR targeting of fixed bacteria, entails the generation of a Th1 response during the early phases of F. tularensis infection, by inhibiting the synthesis of the anti-inflammatory cytokine IL-10.
Numerous studies showed that during F. tularensis infection the early pro-inflammatory responses are significantly suppressed [18, 46], followed by overwhelming up-regulation after 48 hours of infection leading to a severe sepsis [32, 33]. Our results show that iFt targeting to FcγRs up-regulates pro-inflammatory responses at early stages of F. tularensis infection both ex vivo and in vivo. Particularly, immunization of C57BL/6 mice with mAb-iFt shifted the cytokine profile towards a pro-inflammatory Th1 type in the lungs and peritoneum of immunized mice during LVS infection, which was associated with reduction in bacterial load. Conversely, immunization with PBS and the inactivated immunogen alone resulted in considerably lower amounts of Th1 cytokines and higher amounts of IL-10 synthesis accompanied by an increase in bacterial burden in the lungs of infected mice. These observations indicate that immunization of animals with mAb-iFt elicits a robust, pro-inflammatory immune response in the early phases of F. tularensis infection by reversing the inhibitory effect of IL-10 both locally and systemically.
The components of F. tularensis that contribute to the inhibition of pro-inflammatory responses are not completely understood yet. However, it is well established that F.tularensis LPS shows low endotoxic activity due to its structural differences from other intracellular pathogens [21–24]. Our findings that F. tularensis LPS elicits inefficient pro-inflammatory response and up-regulates IL-10 synthesis indicates that LPS is partly responsible for the anti-inflammatory activities of F. tularensis LVS. The importance of pro-inflammatory cytokines, in particular IFN-γ, in controlling the intracellular infection has been described in previous reports. IFN-γ deficiency in mice during Listeria monocytogenes infection compromised activation of macrophages allowing bacteria to escape from phagolysome and further replicate within cytoplasm [42]. Similarly, lack of IFN-γ resulted in decreased survival of mice and increase in bacterial burden during Legionella pneumophila infection [47]. In addition, IFN-γ dependent activation of human monocyte-derived macrophages inhibited escape of F. tularensis into the cytoplasm, thus preventing bacterial replication [48]. In our study we observed that the increased IL-10 synthesis in the early stages of F. tularensis infection coincided with a decrease of the pro-inflammatory cytokines IL-12p70 and TNF-α in the absence of endogenous IFN-γ. Addition of exogenous IFN-γ to cultures of LPS-treated PECs, however, was able to reverse this suppression. Likewise, an increase in the synthesis of IL-12p70 and TNF-α was observed in IL-10 deficient mice after stimulation with F. tularensis LPS. Therefore, these data demonstrate that early up-regulation of IL-10 is one of the means of Th1 immune response suppression during early stages of F. tularensis infection, enabling the bacteria to avoid classical anti-bacterial mechanisms.
The source of pro-inflammatory cytokines during infection is primarily the professional antigen presenting cells, such as dendritic cells and macrophages. Proper activation and differentiation of macrophages is important for the generation of robust, innate immune response against bacterial pathogens. Depending on the extracellular cytokine background, the activated macrophages are divided into two distinct groups, classically activated macrophages (AM1) associated with high levels of pro-inflammatory cytokines and alternatively activated macrophages (AM2) characterized by increased levels of anti-inflammatory cytokines, in particular IL-10 [49]. In fact, IL-10 and CCL17, both produced by AM2, are key players in suppressing induction of AM1 cells [36]. Shirey et al. (2009) demonstrated that F. tularensis LVS skews macrophage activation from anti-bacterial AM1 phenotype towards AM2, which allows its survival and uncontrolled replication within host cells [34]. Based on these findings, we hypothesized that targeting of mAb-iFt may alter the macrophage phenotype from anti-inflammatory AM2 to pro-inflammatory AM1. Our results showed that immunization of mice with mAb-iFt resulted in higher frequency and number of AM1 cells compared to mice immunized with iFt alone. In addition, our data showed a significantly lower number of AM2 cells in the lungs of mAb-iFt immunized mice relative to mice immunized with iFt alone. Interestingly, immunization with PBS resulted in the highest frequency of AM2 cells, suggesting that AM2 polarization in vivo may be an additional immune evasion strategy of F. tularensis, especially at the early stages of infection.
In summary, we have demonstrated for the first time that targeting of inactivated F. tularensis bacteria to FcγRs reverses the potential detrimental effect of IL-10 during the early stages of F. tularensis infection. The anti-inflammatory cytokine IL-10 secreted by F. tularensis infected murine macrophages promotes the bacterial survival and replication by suppressing the synthesis of pro-inflammatory cytokines TNF-α, IL-12, IL-6 and IFN-γ. The immunization of mice with mAb-iFt triggers activation of macrophages, shifts the cytokine profile from anti-inflammatory towards pro-inflammatory and alters macrophage activation state from alternatively activated to classically activated macrophages. These findings support our hypothesis that targeting bacterial immunogens to FcγRs on antigen presenting cells is an effective way to enhance innate immunity against intracellular pathogens during the early stages of infection. Additional studies using the virulent type A F. tularensis SchuS4 strain will enhance our understanding of the pathogenesis and evasion mechanisms of F. tularensis. Further investigation of the IL-10 pathway, as well as identification of additional survival mechanisms utilized by F. tularensis bacteria will aid the development of new vaccine approaches against F. tularensis infection.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was financially supported by Seton Hall University (www.shu.edu) Start Up Funds and the National Institutes of Health (www.nih.gov; R01 AI076408, P01 AI05632). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Medzhitov R, Janeway CA Jr (1997) Innate immunity: the virtues of a nonclonal system of recognition. Cell 91: 295–298. [DOI] [PubMed] [Google Scholar]
- 2. Pashine A, Valiante NM, Ulmer JB (2005) Targeting the innate immune response with improved vaccine adjuvants. Nat Med 11: S63–68. [DOI] [PubMed] [Google Scholar]
- 3. Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188: 21–28. 10.4049/jimmunol.1101029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alfano M, Poli G (2005) Role of cytokines and chemokines in the regulation of innate immunity and HIV infection. Mol Immunol 42: 161–182. [DOI] [PubMed] [Google Scholar]
- 5. Lacy P, Stow JL (2011) Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood 118: 9–18. 10.1182/blood-2010-08-265892 [DOI] [PubMed] [Google Scholar]
- 6. Shibata Y, Foster LA, Kurimoto M, Okamura H, Nakamura RM, et al. (1998) Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-gamma-inducing factors but enhances NK cell production of IFN-gamma. J Immunol 161: 4283–4288. [PubMed] [Google Scholar]
- 7. Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, et al. (2004) Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 22: 929–979. [DOI] [PubMed] [Google Scholar]
- 8. Asadullah K, Sterry W, Volk HD (2003) Interleukin-10 therapy—review of a new approach. Pharmacol Rev 55: 241–269. [DOI] [PubMed] [Google Scholar]
- 9. Abrahamsohn IA, Coffman RL (1996) Trypanosoma cruzi: IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol 84: 231–244. [DOI] [PubMed] [Google Scholar]
- 10. Sing A, Roggenkamp A, Geiger AM, Heesemann J (2002) Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J Immunol 168: 1315–1321. [DOI] [PubMed] [Google Scholar]
- 11. Salek-Ardakani S, Arrand JR, Mackett M (2002) Epstein-Barr virus encoded interleukin-10 inhibits HLA-class I, ICAM-1, and B7 expression on human monocytes: implications for immune evasion by EBV. Virology 304: 342–351. [DOI] [PubMed] [Google Scholar]
- 12. Metzger DW, Salmon SL, Kirimanjeswara G (2013) Differing effects of interleukin-10 on cutaneous and pulmonary Francisella tularensis live vaccine strain infection. Infect Immun 81: 2022–2027. 10.1128/IAI.00024-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Metzger DW, Bakshi CS, Kirimanjeswara G (2007) Mucosal immunopathogenesis of Francisella tularensis. Ann N Y Acad Sci 1105: 266–283. [DOI] [PubMed] [Google Scholar]
- 14. Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM (2002) Public health assessment of potential biological terrorism agents. Emerg Infect Dis 8: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elkins KL, Cowley SC, Bosio CM (2003) Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect 5: 135–142. [DOI] [PubMed] [Google Scholar]
- 16. Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA (1992) In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun 60: 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duckett NS, Olmos S, Durrant DM, Metzger DW (2005) Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun 73: 2306–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bosio CM, Bielefeldt-Ohmann H, Belisle JT (2007) Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J Immunol 178: 4538–4547. [DOI] [PubMed] [Google Scholar]
- 19. Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, et al. (2008) Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol 180: 5548–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh A, Rahman T, Malik M, Hickey AJ, Leifer CA, et al. , Discordant results obtained with Francisella tularensis during in vitro and in vivo immunological studies are attributable to compromised bacterial structural integrity. PLOS ONE 8: e58513 10.1371/journal.pone.0058513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, et al. (2006) Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun 74: 6730–6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duenas AI, Aceves M, Orduna A, Diaz R, Sanchez Crespo M, et al. (2006) Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than E. coli LPS. Int Immunol 18: 785–795. [DOI] [PubMed] [Google Scholar]
- 23. Ancuta P, Pedron T, Girard R, Sandstrom G, Chaby R (1996) Inability of the Francisella tularensis lipopolysaccharide to mimic or to antagonize the induction of cell activation by endotoxins. Infect Immun 64: 2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barker JH, Weiss J, Apicella MA, Nauseef WM (2006) Basis for the failure of Francisella tularensis lipopolysaccharide to prime human polymorphonuclear leukocytes. Infect Immun 74: 3277–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bitsaktsis C, Babadjanova Z, Gosselin EJ (2015) In Vivo Mechanisms Involved in Enhanced Protection Utilizing an FcR-Targeted Mucosal Vaccine Platform in a Bacterial Vaccine and Challenge Model. Infect Immun 83(1): 77–89. 10.1128/IAI.02289-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iglesias BV, Bitsaktsis C, Pham G, Drake JR, Hazlett KR, et al. (2013) Multiple mechanisms mediate enhanced immunity generated by mAb-inactivated F. tularensis immunogen. Immunol Cell Biol 91: 139–148. 10.1038/icb.2012.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bosio CM (2011) The subversion of the immune system by francisella tularensis. Front Microbiol 2: 9 10.3389/fmicb.2011.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM (1993) IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol 151: 1224–1234. [PubMed] [Google Scholar]
- 29. Donnelly RP, Freeman SL, Hayes MP (1995) Inhibition of IL-10 expression by IFN-gamma up-regulates transcription of TNF-alpha in human monocytes. J Immunol 155: 1420–1427. [PubMed] [Google Scholar]
- 30. Brustoski K, Moller U, Kramer M, Petelski A, Brenner S, et al. (2005) IFN-gamma and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. J Immunol 174: 1738–1745. [DOI] [PubMed] [Google Scholar]
- 31. Cope A, Le Friec G, Cardone J, Kemper C (2011) The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol 32: 278–286. 10.1016/j.it.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 32. Sharma J, Li Q, Mishra BB, Georges MJ, Teale JM (2009) Vaccination with an attenuated strain of Francisella novicida prevents T-cell depletion and protects mice infected with the wild-type strain from severe sepsis. Infect Immun 77: 4314–4326. 10.1128/IAI.00654-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mares CA, Ojeda SS, Morris EG, Li Q, Teale JM (2008) Initial delay in the immune response to Francisella tularensis is followed by hypercytokinemia characteristic of severe sepsis and correlating with upregulation and release of damage-associated molecular patterns. Infect Immun 76: 3001–3010. 10.1128/IAI.00215-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shirey KA, Cole LE, Keegan AD, Vogel SN (2008) Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J Immunol 181: 4159–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tomioka H, Tatano Y, Maw WW, Sano C, Kanehiro Y, et al. (2012) Characteristics of suppressor macrophages induced by mycobacterial and protozoal infections in relation to alternatively activated M2 macrophages. Clin Dev Immunol 2012: 635451 10.1155/2012/635451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Katakura T, Miyazaki M, Kobayashi M, Herndon DN, Suzuki F (2004) CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol 172: 1407–1413. [DOI] [PubMed] [Google Scholar]
- 37. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, et al. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686. [DOI] [PubMed] [Google Scholar]
- 38. Woolard MD, Wilson JE, Hensley LL, Jania LA, Kawula TH, et al. (2007) Francisella tularensis-infected macrophages release prostaglandin E2 that blocks T cell proliferation and promotes a Th2-like response. J Immunol 178: 2065–2074. [DOI] [PubMed] [Google Scholar]
- 39. Huang MT, Mortensen BL, Taxman DJ, Craven RR, Taft-Benz S, et al. (2010) Deletion of ripA alleviates suppression of the inflammasome and MAPK by Francisella tularensis. J Immunol 185: 5476–5485. 10.4049/jimmunol.1002154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ulland TK, Buchan BW, Ketterer MR, Fernandes-Alnemri T, Meyerholz DK, et al. (2010) Cutting edge: mutation of Francisella tularensis mviN leads to increased macrophage absent in melanoma 2 inflammasome activation and a loss of virulence. J Immunol 185: 2670–2674. 10.4049/jimmunol.1001610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dotson RJ, Rabadi SM, Westcott EL, Bradley S, Catlett SV, et al. (2013) Repression of inflammasome by Francisella tularensis during early stages of infection. J Biol Chem 288: 23844–23857. 10.1074/jbc.M113.490086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dai WJ, Kohler G, Brombacher F (1997) Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol 158: 2259–2267. [PubMed] [Google Scholar]
- 43. Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, et al. (2009) IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114: 346–356. 10.1182/blood-2008-12-191296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin Y, Ritchea S, Logar A, Slight S, Messmer M, et al. (2009) Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31: 799–810. 10.1016/j.immuni.2009.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gosselin EJ, Wardwell K, Gosselin DR, Alter N, Fisher JL, et al. (1992) Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J Immunol 149: 3477–3481. [PubMed] [Google Scholar]
- 46. Walters KA, Olsufka R, Kuestner RE, Cho JH, Li H, et al. (2013) Francisella tularensis subsp. tularensis induces a unique pulmonary inflammatory response: role of bacterial gene expression in temporal regulation of host defense responses. PLOS ONE 8: e62412 10.1371/journal.pone.0062412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shinozawa Y, Matsumoto T, Uchida K, Tsujimoto S, Iwakura Y, et al. (2002) Role of interferon-gamma in inflammatory responses in murine respiratory infection with Legionella pneumophila. J Med Microbiol 51: 225–230. [DOI] [PubMed] [Google Scholar]
- 48. Santic M, Molmeret M, Abu Kwaik Y (2005) Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-gamma. Cell Microbiol 7: 957–967. [DOI] [PubMed] [Google Scholar]
- 49. Benoit M, Desnues B, Mege JL (2008) Macrophage polarization in bacterial infections. J Immunol 181: 3733–3739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.