Summary
Extracellular adenosine regulates inflammatory responses via A2A adenosine receptor (A2AR). A2AR-deficiency results in much exaggerated acute hepatitis, indicating non-redundancy of adenosine-A2AR pathway in inhibitory mechanisms of immune activation. To identify a critical target of immunoregulatory effect of extracellular adenosine, we focused on NKT cells, which play an indispensable role in hepatitis. A2AR agonist abolished NKT cell-dependent induction of acute hepatitis by Con A or α-galactosylceramide (α-GalCer), corresponding to down-regulation of activation markers and cytokines in NKT cells and of NK cell co-activation. These results show that A2AR signaling can down-regulate NKT cell activation and suppress NKT cell-triggered inflammatory responses. Next, we hypothesized that NKT cells might be under physiological control of the adenosine-A2AR pathway. Indeed, both Con A and α-GalCer induced more severe hepatitis in A2AR−/− mice than in wild-type controls. Transfer of A2AR−/− NKT cells into A2AR-expressing recipients resulted in exaggeration of Con A-induced liver damage, suggesting that NKT cell activation is controlled by endogenous adenosine via A2AR, and this physiological regulatory mechanism of NKT cells is critical in the control of tissue-damaging inflammation. The current study suggests the possibility to manipulate NKT cell activity in inflammatory disorders through intervention to the adenosine-A2AR pathway.
Keywords: NKT cell, adenosine, A2A adenosine receptor, hepatitis, immunoregulation
Introduction
Adenosine in the intracellular pool is at the center of energy and nucleic acid metabolism. However, extracellular adenosine plays a distinctive role in intercellular signaling through cell surface receptors. Four known adenosine receptors, A1, A2A, A2B and A3 receptors, are expressed in many cell types at various levels and regulate physiological functions in cardiovascular, respiratory and central nervous systems [1, 2]. Most immune cells express A2AR at high levels, and adenosine-A2AR interaction suppresses immune functions of granulocytes, macrophages, dendritic cells, NK cells and T cells [2, 3]. Therefore, effect of extracellular adenosine is generally anti-inflammatory and tissue-protective as it prevents inflammatory tissue injury in various experimental models.
Increase of extracellular adenosine levels has been observed in asthma, endotoxic/septic shock, and pulmonary and hepatic injury [4–6]. The increase of adenosine is presumably subsequent to inflammatory tissue damage. Damaged cells may release their intracellular adenosine and adenosine phosphates to extracellular space [7, 8]. Extracellular adenosine phosphates are catabolized to adenosine by ecto-nucleotidases, CD39 metabolizing ATP to AMP and CD73 further metabolizing AMP to adenosine [2, 3]. Extracellular production of adenosine by these cell surface enzymes is responsible for the elevation of adenosine concentration [9, 10]. Upregulation of this anti-inflammatory factor during inflammation implied that adenosine might be produced in inflamed tissue to stop exaggeration of inflammatory activities and prevent excessive damage to vital tissue. Indeed, adenosine’s involvement in this negative feedback mechanism was demonstrated by much exaggerated inflammation in mice lacking A2AR [11–13]. Thus, the danger signal representing potential excessive tissue damage picked up by A2AR downregulates proinflamamtory activities and accelerates resolution of inflammation.
Inflammatory response in the liver is also under control of adenosine. Strong anti-inflammatory effect of A2AR agonists has been shown in acute hepatitis induction by Con A [11], D-galactosamine plus LPS [14] and ischemia-reperfusion [15, 16]. Conversely, antagonists of A2AR exacerbated acute hepatitis [11, 17]. This effect is consistent with exaggerated hepatitis in A2AR-deficient mice, demonstrating that endogenously formed adenosine via A2AR counteracts proinflammatory activities and decreases intensity of hepatic inflammation [11]. Therefore, spontaneous adenosine production plays a crucial role in pathophysiology of hepatitis; however, specific target of adenosine-mediated down-regulation of hepatitis is not clear.
NKT cells are one of the early responders to inflammatory stimuli. NKT cell activation involving robust cytokine production within hours after stimulation influences type and intensity of overall immune response [18–20]. NKT cells, a relatively more frequent population in the liver than in other tissues, play a pivotal role in the induction of hepatitis. To study pathogenesis of hepatitis, Con A-induced liver injury has been widely used for its resemblance to viral and autoimmune hepatitis. NKT cells play a key role in this hepatitis model by producing cytokines such as IL-4, IFN-γ and TNF-α that mediate the liver injury [21–24]. The lack of NKT cells completely abolishes Con A-induced liver injury [23, 24]. More recently, NKT cells were also shown to play an important role in the induction of hepatic ischemia-reperfusion injury [16, 25, 26]. Hepatitis induction by the injection of α-GalCer, an antigenic ligand of NKT cells, indicated that NKT cell activation could be sufficient to trigger inflammatory response resulting in acute hepatitis [27, 28].
Importance of NKT cells in the pathogenesis of hepatitis suggests a possibility that NKT cells represent a critical target of adenosine-dependent regulation of hepatic inflammation. Indeed, A2AR agonist was shown to inhibit activation of NKT cells [16, 29], and suppresses NKT cell-dependent induction of ischemia-reperfusion injury [16, 25, 26]. In this study, we questioned whether NKT cell activation is under control of endogenous adenosine. To test the involvement of A2AR-mediated NKT cell inhibition in adenosinergic regulation of inflammation, we utilized NKT cell-dependent acute hepatitis models: Con A-induced and α-GalCer-induced liver injury. Results of the current study suggest that endogenous adenosine regulates NKT cell activation and thereby suppresses subsequent induction of hepatitis.
Results
A2AR-mediated inhibition of NKT cell activation
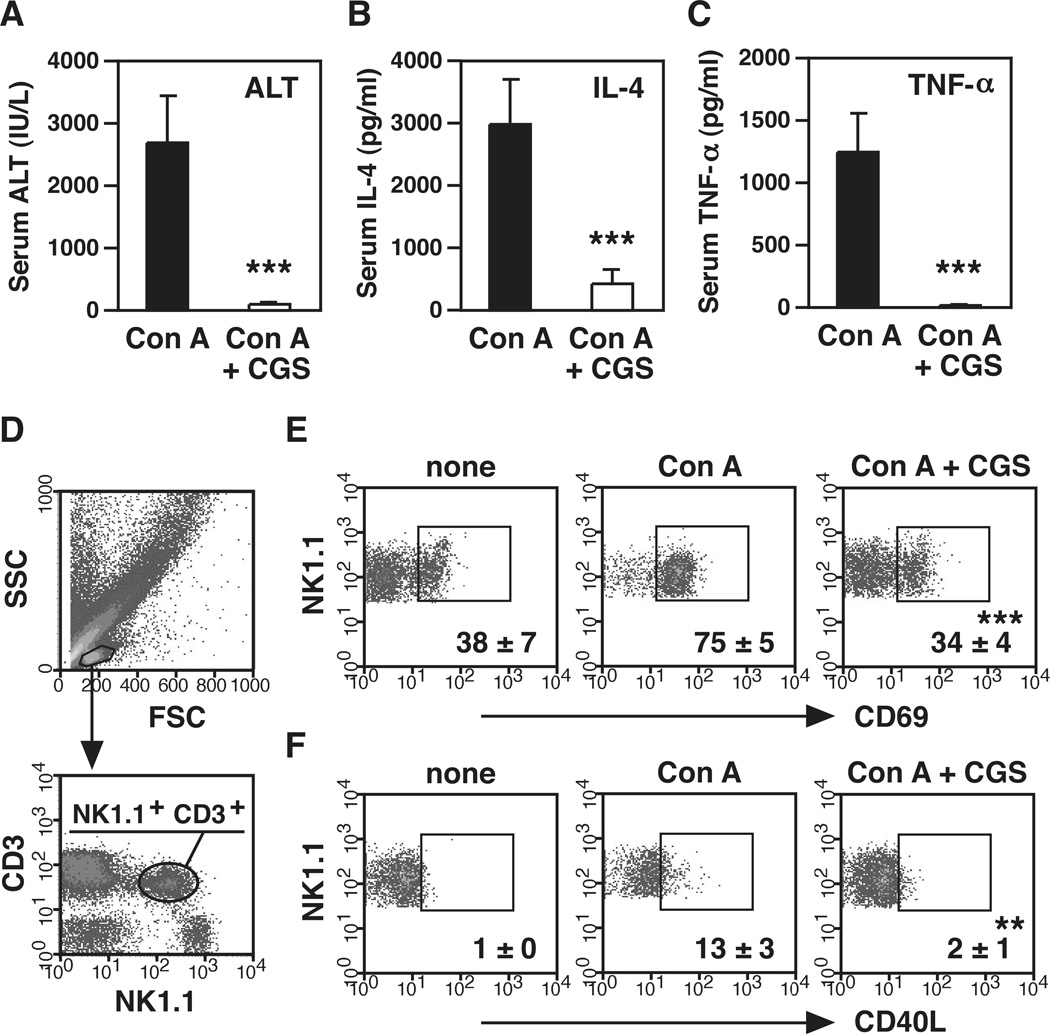
As previously reported in a few different models of acute hepatitis [11, 14–16], administration of A2AR agonist, CGS21680 (CGS), abolished Con A-induced liver injury (Fig. 1A). Suppression of liver injury accompanied remarkable decrease of early production of IL-4 and TNF-α (Fig. 1B, C), which are essential to the induction of hepatitis after Con A injection [21, 23, 24]. NKT cells play an indispensable role in Con A-induced liver injury through early IL-4 production [23, 24]. Extent of NKT cell activation was evaluated by flow cytometry. Consistent with previous reports [30], Con A treatment reduced the proportion of NKT cells to approximately 75 % of control level after 1.5 h. Co-injection of CGS down-regulated Con A-induced CD69 and CD40 ligand (CD40L) on NKT cells (Fig. 1D–F), suggesting that A2AR stimulation suppressed NKT cell activation after Con A injection.
Figure 1.
A2AR stimulation inhibits induction of acute hepatitis and activation of NKT cells. (A–C) Effect of A2AR agonist CGS21680 (CGS) on Con A-induced liver injury. C57BL/6 mice received i.p. injection of CGS (0.5 mg/kg) 10 min prior to Con A administration (12 mg/kg i.v.). Serum ALT levels (A) were determined after 8 h. Serum IL-4 (B) and TNF-α (C) levels were determined after 1.5 h. (D) Gating strategy for flow cytometric analysis of NKT cells in liver mononuclear cells. (E, F) Representative density plots of CD69 (E) and CD40L (F) expression on NK1.1+ CD3+ cells in mice liver 1.5 h after Con A injection. “None” means untreated control. Numbers represent percentages of gated cells. ** P < 0.01, *** P < 0.001 vs Con A alone; Student’s t-test. Data represent average ± SD of 5 mice (A–C) or 3 mice (E, F) and are representative of 3 (A–C) and 2 (E, F) independent experiments.
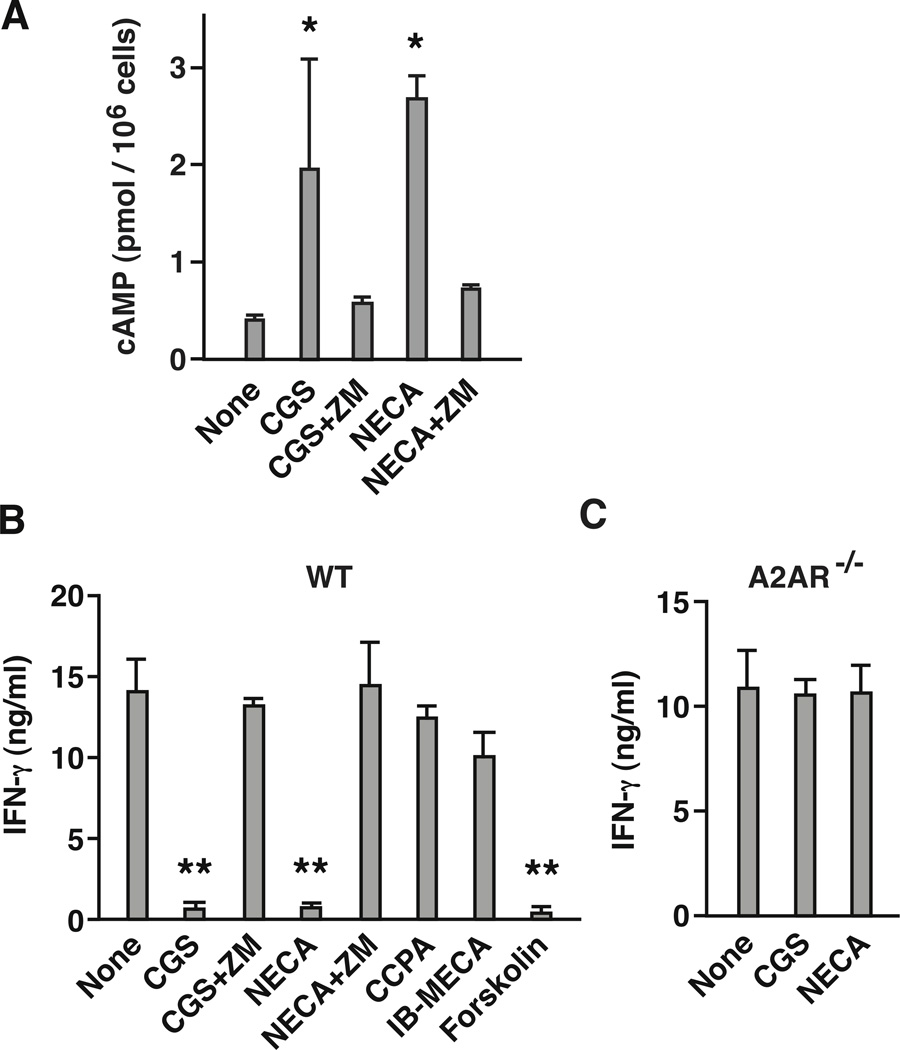
Expression of functional A2AR on NKT cells was confirmed by cAMP induction in response to adenosine receptor agonists. Nonspecific adenosine receptor agonist NECA and A2AR-selective agonist CGS induced cAMP, and A2AR antagonist ZM241385 (ZM) blocked the increase (Fig. 2A). The addition of α-GalCer to cell culture stimulated NKT cells to produce IFN-γ, but NECA and CGS strongly suppressed the IFN-γ production (Fig. 2B). Agonists of other adenosine receptors, CCPA (A1 receptor agonist) and Cl-IB-MECA (A3 receptor agonist), did not significantly reduce IFN-γ levels. A2AR antagonist ZM reversed the inhibitory effect of CGS and NECA. Along with this result, CGS and NECA failed to inhibit IFN-γ production from NKT cells in A2AR-deficient (A2AR−/−) mouse (Fig. 2C), confirming that stimulation of A2AR is strongly immunosuppressive to NKT cells.
Figure 2.
NKT cells express functional A2AR, and stimulation of A2AR suppresses NKT cell activation. (A) cAMP upregulation in NKT cells in response to A2AR stimulation. Purified NKT cells (1 × 105 cells) were incubated with 10 µM CGS (A2AR agonist) and 10 µM NECA (non-specific adenosine receptor agonist) in the presence or absence of A2AR antagonist (ZM; 1 µM). After 15 min of incubation at 37 °C, cAMP levels were determined by ELISA. (B, C) IFN-γ production from wild-type (B) and A2AR−/− (C) NKT cells. Purified NKT cells (5 × 104 cells) were stimulated with α-GalCer (100 ng/ml) in the presence of syngenic dendritic cells (2 × 105 cells). Effects of adenosine receptor stimulation were examined in the presence of various agonists and antagonist (100 nM): CGS (A2AR agonist), NECA (non-specific adenosine agonist), CCPA (A1 receptor agonist), IB-MECA (A3 receptor agonist), and ZM (A2AR antagonist). Forskolin (10 µM) was used to induce adenosine receptor-independent cAMP upregulation. IFN-γ levels in the culture supernatant were determined after 24 h by ELISA. * P < 0.05, ** P < 0.01 vs control; Student’s t-test. Data represent average ± SD of triplicate samples and are representative of 2 (A) and 3 (B, C) independent experiments.
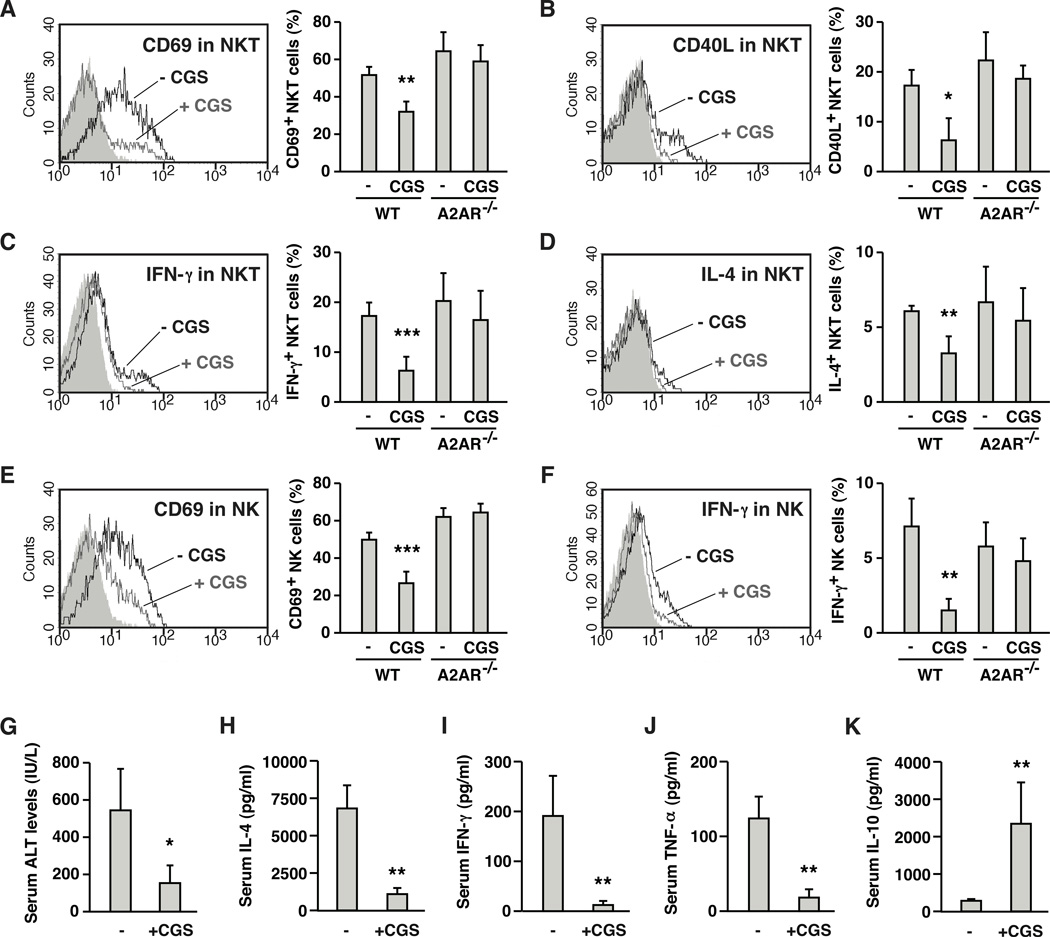
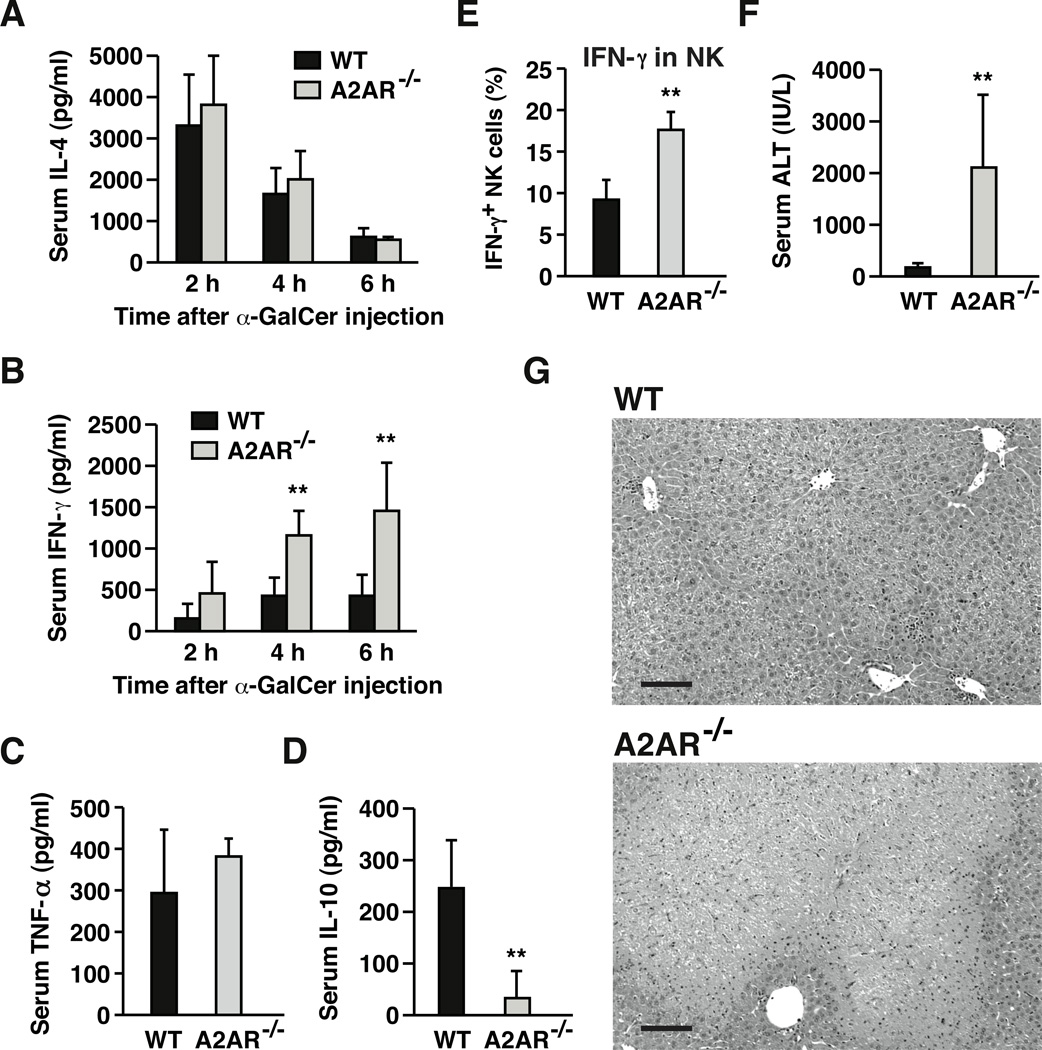
Susceptibility of NKT cells to A2AR agonist was examined in vivo by direct stimulation of NKT cells with α-GalCer. Again, α-GalCer treatment downregulated NKT cell proportion as reported previously [31]. Number of NKT cells were reduced to approximately 75 % of control level after 2 h. α-GalCer induced activation of NKT cells within 2 h as evidenced by upregulation of activation markers, CD69 and CD40L (Fig. 3A, B). At the same time, these NKT cells were producing IFN-γ and IL-4 (Fig. 3C, D). Co-treatment with CGS significantly inhibited upregulation of activation markers and cytokines production (Fig. 3A–D). Early activation of NKT cells is known to induce extensive co-activation of other immune cells [20, 32, 33]. In the current experiment, α-GalCer injection resulted in increases of CD69 and IFN-γ in NK cells; however, CGS also down-regulated such activation of NK cells (Fig. 3E, F). In A2AR−/− mice, CGS did not affect any of activation markers and cytokines in NKT and NK cells, confirming A2AR-dependence of the suppressive effect (Fig. 3A–F). In vivo activation of NKT cells by α-GalCer injection eventually results in hepatic injury. CGS blocked the induction of liver damage along with the increase of serum IL-4, IFN-γ and TNF-α levels after α-GalCer injection (Fig. 3G–J). In addition to the decrease of proinflammatory cytokines, CGS rather induced IL-10, an anti-inflammatory cytokine (Fig. 3K). Thus, A2AR stimulation suppressed NKT cell activation in vivo and consequently blocked propagation of inflammatory response that could cause acute hepatitis.
Figure 3.
A2AR agonist suppresses NKT cell-dependent immune responses in vivo and blocks inflammatory damage in the liver. Wild-type (WT) and A2AR−/− mice received intravenous injection of α-galactosylceramide (α-GalCer; 1 µg/mouse) with or without CGS (0.5 mg/kg i.p.). After 2 h, NKT (NK1.1+ CD3+) cells were analyzed for the expression of CD69 (A), CD40L (B), IFN-γ (C) and IL-4 (D) by flow cytometry. Black and grey lines represent α-GalCer alone and α-GalCer + CGS, respectively. Shaded peaks are background levels. Positive/negative signal threshold is the fluorescence intensity where >98% of background events are included. Fluorescence exceeding this level was defined as positive. To monitor co-activation of NK cells, CD69 (E) and IFN-γ (F) levels in NK1.1+ CD3− cells were also analyzed after 2 h. The intensity of α-GalCer-induced liver damage was determined by serum ALT levels 18 h after α-GalCer injection (G). Serum levels of IFN-γ (H), IL-4 (I), TNF-α (J) and IL-10 (K) were determined 2 h after α-GalCer injection. * P < 0.05, ** P < 0.01, *** P < 0.001 vs control; Student’s t-test. Data represent average ± SD of 4 mice (A-F, H-K) or 3 mice (G) and are representative of 3 independent experiments.
Regulation of NKT cell activation by endogenous adenosine
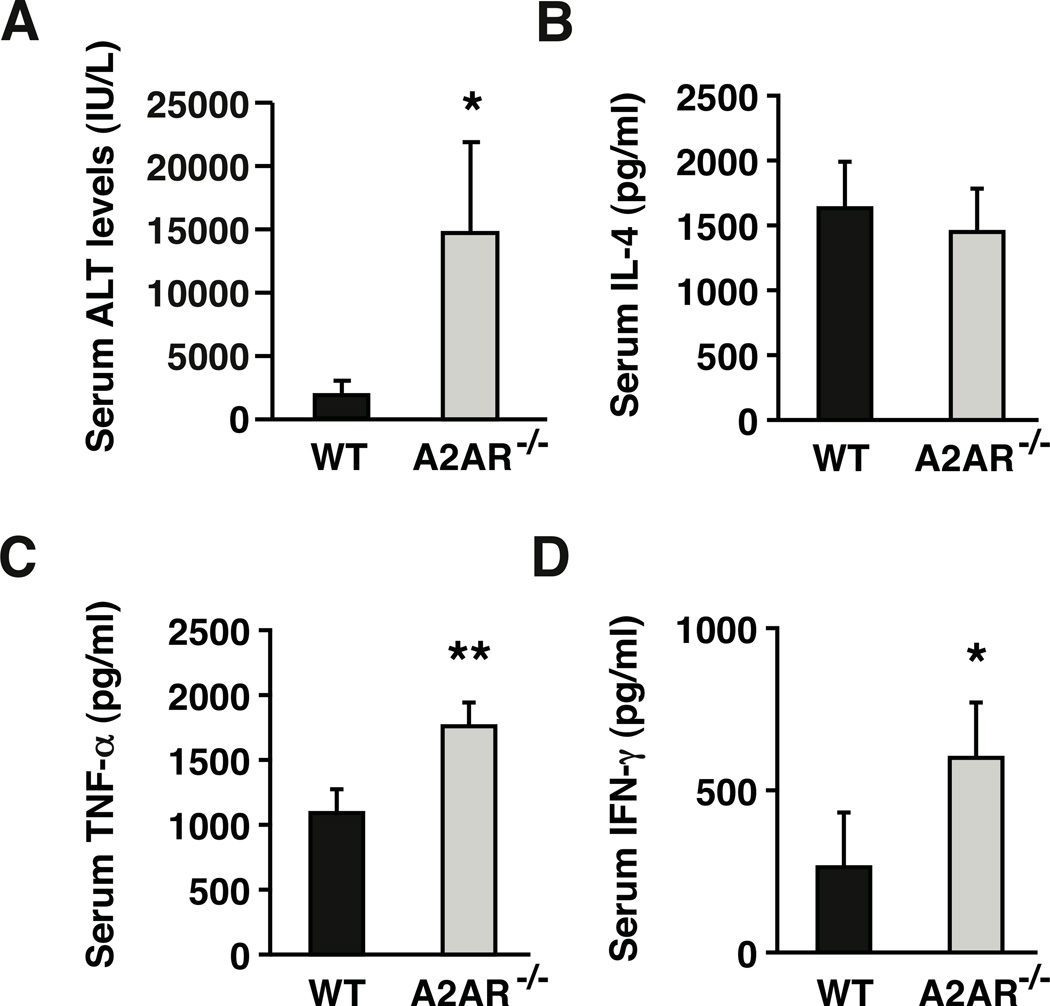
Experiments above showed that the adenosine-A2AR signaling pathway could block hepatitis induction by suppressing NKT cell activation. Next, we questioned whether endogenously produced adenosine is physiologically important in controlling NKT cell activation. To test this issue, we compared outcome of NKT cell activation in A2AR−/− mice with that of wild-type mice. As we previously reported [11], A2AR−/− mice were very susceptible to Con A-induced liver injury along with pronounced early increase of TNF-α and IFN-γ, but not IL-4 (Fig. 4). Since pathogenesis of Con A-induced hepatitis is NKT cell-dependent, we speculated a possible involvement of A2AR in the regulation of NKT cell activation.
Figure 4.
Exacerbation of Con A-induced liver injury in A2AR−/− mice. WT and A2AR−/− mice received intravenous injection of Con A (10 mg/kg). (A) Serum ALT levels 8 h after Con A injection. (B–D) Serum cytokine levels after Con A injection were determined by ELISA. Data shown here are IL-4 (B) and TNF-α (C) levels after 1.5 h and IFN-γ (D) levels 8 h after Con A injection. * P < 0.05, ** P < 0.01 vs WT mice; Student’s t-test. Data represent average ± SD of 5 mice and are representative of 3 independent experiments.
If endogenous adenosine is vitally regulating NKT cells, stronger immune response will be expected in α-GalCer-injected A2AR−/− mice. Two hours after α-GalCer injection, upregulation of CD69 and CD40L in A2AR−/− NKT cells tended to be slightly higher than wild-type NKT cells, though the differences were not statistically significant (Fig. 3A, B). Such trend was the same for cytokine upregulation in NKT cells (Fig. 3C, D). Although exaggerated activation of A2AR−/− NKT cells was not evident from analyses after 2 h, significantly higher levels of IFN-γ production, but not IL-4, were observed in A2AR−/− mice thereafter (Fig. 5A, B). More than 2-fold increase of α-GalCer-induced serum IFN-γ levels after 4 and 6 hours suggests stronger NKT cell-mediated response in A2AR−/− mice. Although TNF-α levels were unchanged (Fig. 5C), serum IL-10 levels were largely decreased in A2AR−/− mice (Fig. 5D), suggesting attenuation of anti-inflammatory response in the absence of A2AR. Consistent with the changes in serum cytokine levels, NKT cell stimulation in A2AR−/− mice enhanced subsequent immune responses. Although extent of NK cell activation was at similar levels after 2 h (Fig. 3E, F), IFN-γ from NK cells became significantly enhanced in A2AR−/− mice after 4 h (Fig. 5E). NKT cell activation in A2AR−/− mice led to exacerbation of α-GalCer-induced acute hepatitis. More extensive hepatocellular damage was reflected in an increase of serum ALT levels (Fig. 5F) and tissue histology (Fig. 5G).
Figure 5.
Exaggerated inflammation and liver damage in A2AR−/− mice after direct activation of NKT cells. WT and A2AR−/− mice received intravenous injection of α-GalCer (1 µg/mouse), and serum IL-4 (A) and IFN-γ (B) levels were monitored between 2–6 h by ELISA. Serum TNF-α (C) and IL-10 levels (D) were also determined after 2 h by ELISA. (E) IFN-γ production from NK (NK1.1+ CD3−) cells was determined 4 h after α-GalCer injection by flow cytometry. (F, G) Intensities of α-GalCer-induced liver damage were evaluated by serum ALT levels (F) and H-E staining of liver tissue (G) after 24 h. Bars represent 100 µm. ** P < 0.01 vs wild-type mice; Student’s t-test. Data represent average ± SD of 4 (A–E) or 5 (F) mice and are representative of 3 independent experiments.
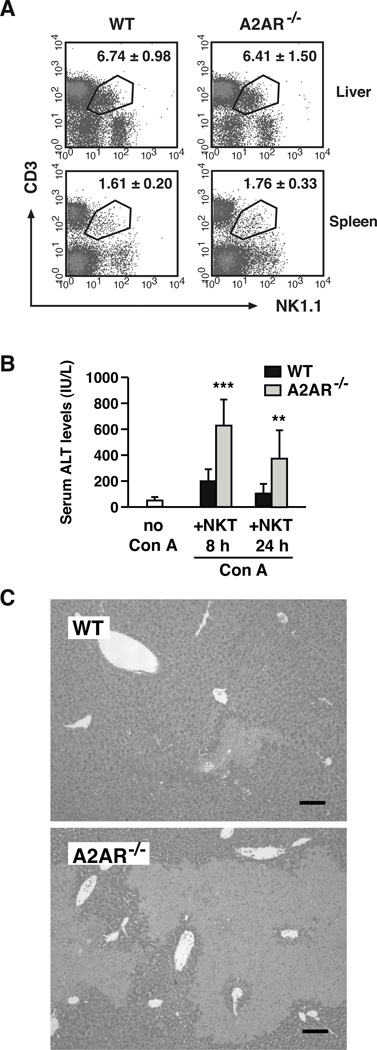
The different outcomes of direct NKT cell stimulation suggested an augmented NKT cell activity in A2AR−/− mice. Since NKT cells were proportional in wild-type and A2AR−/− mice (Fig. 6A), the difference was likely to be qualitative, not quantitative. To examine a difference in NKT cell activity, we injected wild-type or A2AR−/− NKT cells into RAG-1−/− mice and induced Con A hepatitis. Without NKT cell transfer, Con A induced no notable liver damage in RAG-1−/− mice, which lack T, B and NKT cells. Transfer of NKT cells made RAG-1−/− mice vulnerable to Con A-induced liver injury. However, transfer of A2AR−/− NKT cells induced much stronger liver damage (Fig. 6B, C). The lack of A2AR only in NKT cells sufficiently exaggerated acute hepatitis, indicating enhanced inflammatory activity of A2AR−/− NKT cells. This result suggests that activity of NKT cells is under regulation by endogenous adenosine through A2AR, and disruption of this physiological negative regulation can cause massive inflammatory hepatic damage.
Figure 6.
Endogenous adenosine targets A2AR on NKT cells to down-regulate overall inflammatory tissue injury in the liver. (A) Representative density plots of NK1.1+ CD3+ cells in untreated WT and A2AR−/− mice. Numbers represent percentages of NK1.1+ CD3+ cells in the liver mononuclear cells and spleen cells. Data shown are average ± SD of 7 mice. (B, C) Induction of Con A hepatitis after the transfer of NKT cells. RAG-1−/− mice received intrahepatic transfer of NKT cells (1 × 106 cells) purified from WT or A2AR−/− mice. One hour after the cell transfer, Con A (20 mg/kg i.v.) was injected to induce hepatitis, and serum ALT levels (B) were evaluated after 8 and 24 h. ALT levels in RAG-1−/− mice without NKT cell transfer did not increase by Con A injection (ALT 56 ± 4 IU/L after 8 h). Extent of tissue damage was also evaluated in H-E stained liver tissue 24 h after the injection of Con A (C). The images are representative of the liver in Con A-injected RAG-1−/− mice that received transfer of NKT cells from WT or A2AR−/− mice. Bars represent 100 µm. ** P < 0.01, *** P < 0.001 vs control; Student’s t-test. Data represent average ± SD of 7 mice and are pooled from 3 experiments.
Discussion
Divergent roles of extracellular adenosine have been observed in inflammation-related diseases in the liver. As evidenced by much exacerbated acute hepatitis in A2AR−/− mice, formation of extracellular adenosine is an indispensable physiological stop signal to prevent excess inflammation [11–13]. In the resolution of hepatitis, however, adenosine-A2AR interaction may promote induction of cirrhosis. Adenosine was found to facilitate collagen synthesis, and blockade of A2AR signaling prevented hepatic fibrosis [34, 35]. Furthermore, adenosine’s involvement in steatosis was also suggested [36]. Thus, adenosine is a notable regulator of hepatic proinflammatory responses, remodeling of damaged tissue and energy metabolism in the liver, the imbalance of which will lead to disease state.
A2AR stimulation and subsequent cAMP induction strongly impair immune cell activation. Effector function of T cells, especially IFN-γ-producing activity, is very susceptible to A2AR agonist [37, 38]. NKT cells, as well as conventional T cells, were sensitive to A2AR stimulation. In vivo administration of A2AR agonist blocked CD69 and CD40L upregulation and cytokine production in NKT cells (Fig. 3), showing A2AR-mediated inhibitory pathway of NKT cell activation and their functions. Our experiment confirmed that NKT cells express functional A2AR and produce cAMP in response to agonists (Fig. 2) [16, 29]. NKT cells are susceptible to cAMP induction as shown by the impairment of NKT cell activation with cAMP inducers such as forskolin, phosphodiesterase inhibitor and dibutyryl cAMP [39].
NKT cells have been shown to play a major role in liver diseases. Induction of hepatic tissue damage after direct activation of NKT cells indicated their pathogenic role in hepatitis [27, 28]. NKT cells are essential to the induction of Con A hepatitis [23, 24] and ischemia-reperfusion injury [16]. To attenuate ischemia-reperfusion damage in the liver and in sickle cell disease, NKT cells were targeted to block their activity [16, 25, 26]. Indeed, to suppress NKT cell activity, these ischemia-reperfusion studies employed pharmacological stimulation of A2AR.
In the current study, A2AR-mediated suppression of NKT cell activation in vivo corresponded well with the inhibition of acute hepatitis by CGS (Fig. 1, 3). NKT cell activation in a very early phase of immune response further activates diverse types of immune cells, orchestrating later expansion of inflammation [20, 32, 33]. A2AR agonist attenuated NKT cell activation as well as co-activation of NK cells (Fig. 3). These results show that the cascade of inflammatory responses triggered by NKT cells can be inhibited when exogenous A2AR agonist is introduced.
Spontaneous formation of extracellular adenosine in vivo has been shown to down-regulate inflammation and consequently protect tissues from excessive inflammatory damage [11]. This A2AR-mediated function of endogenous adenosine is non-redundant and may represent one of the most important immune-regulatory negative feedback mechanisms [2, 3, 12]. Then, we hypothesized that endogenous adenosine might be a physiological modulator of NKT cell activation. In vitro experiments using adenosine may underestimate adenosine concentration needed for NKT cell inhibition because adenosine deaminase activity present in cell culture media causes rapid degradation of adenosine [40]. Therefore, evaluation of pathophysiological significance of A2AR-mediated NKT cell regulation relies on in vivo assay, which reflects possible effects of local concentration of adenosine. Failure in A2AR−/− mice to receive immunoregulatory signal of endogenous adenosine exaggerated NKT cell-dependent induction of hepatitis. The results from A2AR−/− mice suggested A2AR-dependent negative regulation of NKT cells by physiological adenosine.
De-inhibition of NKT cells from A2AR-mediated suppression might be responsible for the exaggeration of acute hepatitis in A2AR−/− mice, though other immune effector cells could be also de-inhibited at the same time. To examine if the lack of A2AR only in NKT cells causes exacerbation of hepatitis, we injected purified NKT cells to A2AR-expressing recipients. Significant augmentation of hepatitis in mice received A2AR−/− NKT cells indicates that de-inhibition of NKT cells from A2AR-dependent regulation can sufficiently enhance acute hepatitis (Fig. 6). For endogenous immuno-regulators, molecules such as LECT2, CCR5 and BTLA are known to be capable of regulating NKT cells [41–43]. The current study suggests the pathophysiological significance of extracellular adenosine-dependent NKT cell regulation in liver inflammation.
Direct NKT cell activation by α-GalCer injection induced significantly higher levels of IFN-γ, but not IL-4, in A2AR−/− mice (Fig. 5). IL-10 levels were reduced in α-GalCer-injected A2AR−/− mice (Fig. 5), while CGS induced IL-10 in wild-type mice (Fig. 3). These changes correspond to a shift to Th2-type cytokine pattern in NKT cells exposed to adenosine [29]. Early and robust cytokine production from NKT cells is believed to change the course of inflammation. IFN-γ production from NKT cells elicits Th1-type immune response in the activation of anti-tumor immunity [44] and in the prevention of allergic disorders [45]. Enhanced IFN-γ production and NK cell activation after NKT cell stimulation in A2AR−/− mice (Fig. 5) implies promotion of cellular immune response, which may explain the exaggeration of inflammatory liver damage in these mice.
In the exacerbation of Con A-induced liver injury in A2AR−/− mice, levels of IFN-γ and TNF-α, proinflammatory cytokines essential to optimal liver damage [21, 22], were found to increase in the absence of A2AR (Fig. 4) [11]. TNF-α is also essential to hepatitis induction by α-GalCer [28], but TNF-α levels in A2AR−/− mice were comparable to wild-type mice (Fig. 5). Although IFN-γ is not necessary to the induction of liver damage after α-GalCer injection, this cytokine is a strong inducer of IRF-1, which is indispensable to α-GalCer hepatitis [46]. Since IRF-1 increases dependent on IFN-γ concentration, augmented IFN-γ production in A2AR−/− mice may have contributed to the enhancement of liver damage.
In conclusion, we provide evidence for not only A2AR-mediated suppression of NKT cell activation but also augmented NKT cell activity in A2AR-deficient mice. These results strongly suggest physiological immunoregulatory mechanism controlling activation of NKT cells via adenosine-A2AR signaling pathway. Extracellular adenosine is known to spontaneously increase in inflamed tissues [4–6] and tumors [47, 48]. Tissue hypoxia in these environments is conductive to the increase of extracellular adenosine levels. Hypoxia-triggered induction of 5’-nucleotidases, CD39 and CD73, results in promotion of extracellular adenosine formation by increasing degradation of ATP [49, 50]. Hypoxia also suppresses adenosine kinase-dependent conversion of adenosine to AMP [51–53]. Since an increase of extracellular adenosine can directly affect NKT cell activities, attenuation of NKT cell activities are likely in tumors and tissues under inflammation. NKT cells play an important role in inflammatory disorders, infectious diseases, tumor immunity and vaccination [18–20, 54–56]. It may be possible to manipulate NKT cell activity in these immune responses through intervention to the adenosine-A2AR pathway by means of A2AR agonist/antagonist [11, 14–17], CD73 inhibitor [57–59] or atmosphere containing altered levels of oxygen [60, 61].
Materials and methods
Mice
C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA). A2AR−/− mice were backcrossed for 12 times to C57BL/6 mice [17]. C57BL/6-background RAG1−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The mice were housed in the animal facility of Northeastern University and were used at 8–12 weeks of age in accordance with the institutional animal care guidelines.
Concanavalin A (Con A)-induced hepatitis
Con A (Sigma, St. Louis, MO; 12 mg/kg) was injected intravenously to induce acute liver injury. Some mice received intraperitoneal injection of A2AR agonist CGS21680 (0.5 mg/kg) 10 min before Con A. Blood samples were collected at 8 h after Con A injection and liver damage was evaluated by serum ALT levels. ALT levels were determined by a kit from Teco Diagnostics (Anaheim, CA). Serum levels of TNF-α and IL-4 after 1.5 h were determined using ELISA kits from R&D Systems (Minneapolis, MN). CD69 and CD40L upregulation in NKT cells was analyzed 1.5 h after Con A injection by flow cytometry.
Preparation of liver mononuclear cells
LMNC were prepared by density centrifugation using Percoll (GE Healthcare, Upsalla, Sweden). The liver was pressed through stainless steel mesh (#200) and washed by centrifugation. The pellet was resuspended in 40 % Percoll and centrifuged at 500 × g for 15 min at room temperature to isolate liver mononuclear cells from parenchymal hepatocytes and cell debris. The pellet was collected, treated with ACK lysing buffer (Life Technologies, Grand Island, NY), and washed using RPMI1640 medium containing 10 % FCS.
Purification of NKT cells
Mice were pretreated by intraperitoneal injection of anti-asialo GM1 Ab (50 µg/mouse; Wako Chemicals, Richmond, VA) to deplete NK cells. Splenocytes isolated 2 days after the injection of anti-asialo GM1 Ab were labeled with FITC-conjugated anti-NK1.1 mAb (BD Biosciences, San Jose, CA) and with anti-FITC microbeads (Miltenyi Biotec, Auburn, CA). NKT cells were purified by positive selection of NK1.1+ cells using AutoMACS separator (Miltenyi Biotec). Purity of NK1.1+ CD3+ cells was higher than 90 %.
cAMP assay
Induction of cAMP in response to adenosine receptor agonists was measured as described previously [62]. Purified NKT cells (1 × 105) were incubated with NECA or CGS21680 (10 µM) for 15 min at 37 °C in a total volume of 0.2 ml. Adenosine receptor antagonist, ZM241385 was used at 1 µM. After the incubation, 25 µl of 1N hydrochloric acid was added and the samples were stored at –80 °C. cAMP levels were determined by ELISA (GE Healthcare, Buckinghamshire, UK).
Bone marrow-derived dendritic cells
Dendritic cells were induced from bone marrow cells as described [63]. Bone marrow cells from C57BL/6 mouse femora were suspended in RPMI1640 medium containing 10 % FCS and cultured with GM-CSF (10 ng/ml) and IL-4 (10 ng/ml) in a plastic cell culture plate. Non-adherent cells were removed on the next day. After 5–7 days, adherent cells were used to stimulate NKT cells.
In vitro stimulation of NKT cells
Purified NKT cells (1 × 105 cells) were stimulated by α-GalCer (100 ng/ml; Biomol International, Plymouth Meeting, PA) in the presence of bone marrow-derived dendritic cells (1 × 105 cells). Following adenosine receptor agonists and antagonists were added in the culture at 100 nM: 2-chloro-N6-cyclopentyladenosine (CCPA, A1 adenosine receptor-selective agonist), CGS21680 (CGS, A2AR-selective agonist), 5’-N-ethylcarboxamidoadenosine (NECA, non-specific adenosine receptor agonist), 1-[2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-D-ribofuranuronamide (Cl-IB-MECA, A3 adenosine receptor-selective agonist) and ZM241385 (ZM, A2AR antagonist). Forskolin was also used to induce receptor-independent cAMP increase. CCPA, CGS, Cl-IB-MECA and ZM were obtained from Tocris (Ellisville, MO). NECA and forskolin are from Sigma (St. Louis, MO). The cells were cultured for 24 h, and cytokine levels in the supernatant were determined by ELISA.
In vivo NKT cell activation by α-GalCer
α-GalCer (1 µg/mouse) was injected intravenously and serum IL-4, IFN-γ, TNF-α and IL-10 levels were determined after 2–6 h. Spleen cells were analyzed for the expression of CD69, CD40L, IFN-γ and IL-4. Liver damage was assessed 18–24 h after the injection by serum ALT levels and histochemistry. For histochemistry, liver tissue was fixed in 10% formalin-PBS, and the paraffin-embedded tissue slice was stained with hematoxyline-eosin (Mass Histology Service, Worcester, MA).
Flowcytometric analysis of cytokine production from NKT cells
IFN-γ and IL-4 production from NKT cells were analyzed 2 h after Con A injection by intracellular staining [62]. After surface staining, cells were fixed with 4 % paraformaldehyde-PBS for 15 min. After washing with PBS, cells were treated with permeabilizing buffer (50 mM sodium chloride, 5 mM EDTA, 0.02 % sodium azide, 0.5 % Triton X-100, 10 mM Tris-HCl, pH 7.5) for 15 min. Intracellular cytokines were stained with anti-IFN-γ and anti-IL-4 mAbs, and cytokine expression in NK1.1+ CD3+ cells was analyzed by FACSCalibur (BD Biosciences). All antibodies were obtained from BD Biosciences.
Adoptive transfer of NKT cells
Immediately after purification, NKT cells (1 × 106 cells) were suspended in 50 µl saline and injected into RAG1−/− mice at the lateral left lobe of liver [24]. One hour after the cell transfer, the recipient mice received intravenous injection of Con A (20 mg/kg). The liver damage was evaluated after 8 and 24 h.
Statistics
Data represent mean ± SD. Statistical calculations were performed using Student’s t-test. Statistical significance was accepted for p values less than 0.05.
Acknowledgements
This work was supported in part by NIH grants R01 CA112561 (MS) and R01 CA111985 (MS).
Abbreviation
- A2AR
A2A adenosine receptor
- α-GalCer
α-galactosylceramide
- CD40L
CD40 ligand
- CGS
- FasL
Fas ligand
- ZM
ZM241385
Footnotes
Conflict of interest
The authors declare no financial and commercial conflict of interest.
References
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Sitkovsky M, Robson SC. Purinergic signaling during inflammation. New Engl. J. Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am. Rev. Respir. Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 5.Nishiyama A, Miura K, Miyatake A, Fujisawa Y, Yue W, Fukui T, Kimura S, et al. Renal interstitial concentration of adenosine during endotoxin shock. Eur. J. Pharmacol. 1999;385:209–216. doi: 10.1016/s0014-2999(99)00716-5. [DOI] [PubMed] [Google Scholar]
- 6.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizumoto N, Kumamoto T, Robson SC, Sévigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat. Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 8.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J. Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volmer JB, Thompson LF, Blackburn MR. Ecto-5’-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J. Immunol. 2006;176:4449–4458. doi: 10.4049/jimmunol.176.7.4449. [DOI] [PubMed] [Google Scholar]
- 10.Grenz A, Zhang H, Weingart J, von Wietersheim S, Eckle T, Schnermann J, Köhle C, et al. Lack of effect of extracellular adenosine generation and signaling on renal erythropoietin secretion during hypoxia. Am. J. Physiol. Renal Physiol. 2007;293:F1501–F1511. doi: 10.1152/ajprenal.00243.2007. [DOI] [PubMed] [Google Scholar]
- 11.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 12.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 13.Sitkovsky MV, Ohta A. The 'danger' sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Odashima M, Otaka M, Jin M, Komatsu K, Wada I, Matsuhashi T, Horikawa Y, et al. Selective A2A adenosine agonist ATL-146e attenuates acute lethal liver injury in mice. J. Gastroenterol. 2005;40:526–529. doi: 10.1007/s00535-005-1609-9. [DOI] [PubMed] [Google Scholar]
- 15.Harada N, Okajima K, Murakami K, Usune S, Sato C, Ohshima K, Katsuragi T. Adenosine and selective A(2A) receptor agonists reduce ischemia/reperfusion injury of rat liver mainly by inhibiting leukocyte activation. J. Pharmacol. Exp. Ther. 2000;294:1034–1042. [PubMed] [Google Scholar]
- 16.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d–dependent NKT cell activation. J. Exp. Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohta A, Lukashev D, Jackson EK, Fredholm BB, Sitkovsky M. 1,3,7-trimethylxanthine (caffeine) may exacerbate acute inflammatory liver injury by weakening the physiological immunosuppressive mechanism. J. Immunol. 2007;179:7431–7438. doi: 10.4049/jimmunol.179.11.7431. [DOI] [PubMed] [Google Scholar]
- 18.Swain MG. Hepatic NKT cells: friend or foe? Clin. Sci. (Lond.) 2008;114:457–466. doi: 10.1042/CS20070328. [DOI] [PubMed] [Google Scholar]
- 19.Godfrey DI, Rossjohn J. New ways to turn on NKT cells. J. Exp. Med. 2011;208:1121–1125. doi: 10.1084/jem.20110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 21.Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995;21:190–198. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 22.Küsters S, Gantner F, Künstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 23.Toyabe S, Seki S, Iiai T, Takeda K, Shirai K, Watanabe H, Hiraide H, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J. Immunol. 1997;159:1537–1542. [PubMed] [Google Scholar]
- 24.Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, et al. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J. Exp. Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–5020. doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, et al. Dendritic cells tolerized with adenosine A AR agonist attenuate acute kidney injury. J. Clin. Invest. 2012;122:3931–3942. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur. J. Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Biburger M, Tiegs G. Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of Kupffer cells. J. Immunol. 2005;175:1540–1550. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- 29.Nowak M, Lynch L, Yue S, Ohta A, Sitkovsky M, Balk SP, Exley MA. The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur. J. Immunol. 2010;40:682–687. doi: 10.1002/eji.200939897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa R, Nagafune I, Tazunoki Y, Ehara H, Tomura H, Iijima R, Motoki K, et al. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J. Immunol. 2001;166:6578–6584. doi: 10.4049/jimmunol.166.11.6578. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura T, Kitamura H, Iwakabe K, Yahata T, Ohta A, Sato M, Takeda K, et al. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d–expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 2000;12:987–994. doi: 10.1093/intimm/12.7.987. [DOI] [PubMed] [Google Scholar]
- 34.Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng Z, Fernandez P, Wilder T, Yee H, Chiriboga L, Chan ES, Cronstein BN. Ecto-5’-nucleotidase (CD73) -mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB J. 2008;22:2263–2272. doi: 10.1096/fj.07-100685. [DOI] [PubMed] [Google Scholar]
- 36.Peng Z, Borea PA, Varani K, Wilder T, Yee H, Chiriboga L, Blackburn MR, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J. Clin. Invest. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 38.Ohta A, Ohta A, Madasu M, Kini R, Subramanian M, Goel N, Sitkovsky M. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J. Immunol. 2009;183:5487–5493. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 39.Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Inoue H, Murafuji H, Hayashi Y, et al. Phosphodiesterase 7A inhibitor ASB16165 suppresses proliferation and cytokine production of NKT cells. Cell. Immunol. 2009;258:147–151. doi: 10.1016/j.cellimm.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Bynum JW. Characterization of adenosine-induced cytostasis in melanoma cells. Cancer Res. 1980;40:2147–2152. [PubMed] [Google Scholar]
- 41.Saito T, Okumura A, Watanabe H, Asano M, Ishida-Okawara A, Sakagami J, Sudo K, et al. Increase in hepatic NKT cells in leukocyte cell-derived chemotaxin 2-deficient mice contributes to severe concanavalin A-induced hepatitis. J. Immunol. 2004;173:579–585. doi: 10.4049/jimmunol.173.1.579. [DOI] [PubMed] [Google Scholar]
- 42.Ajuebor MN, Aspinall AI, Zhou F, Le T, Yang Y, Urbanski SJ, Sidobre S, et al. Lack of chemokine receptor CCR5 promotes murine fulminant liver failure by preventing the apoptosis of activated CD1d–restricted NKT cells. J. Immunol. 2005;174:8027–8037. doi: 10.4049/jimmunol.174.12.8027. [DOI] [PubMed] [Google Scholar]
- 43.Miller ML, Sun Y, Fu YX. Cutting edge: B and T lymphocyte attenuator signaling on NKT cells inhibits cytokine release and tissue injury in early immune responses. J. Immunol. 2009;183:32–36. doi: 10.4049/jimmunol.0900690. [DOI] [PubMed] [Google Scholar]
- 44.Seino K, Motohashi S, Fujisawa T, Nakayama T, Taniguchi M. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97:807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda H, Takeda K, Koya T, Okamoto M, Shiraishi Y, Miyahara N, Dakhama A, et al. Plasticity of invariant NKT cell regulation of allergic airway disease is dependent on IFN-gamma production. J. Immunol. 2010;185:253–262. doi: 10.4049/jimmunol.0902301. [DOI] [PubMed] [Google Scholar]
- 46.Cao Z, Dhupar R, Cai C, Li P, Billiar TR, Geller DA. A critical role for IFN regulatory factor 1 in NKT cell-mediated liver injury induced by alpha-galactosylceramide. J. Immunol. 2010;185:2536–2543. doi: 10.4049/jimmunol.1000092. [DOI] [PubMed] [Google Scholar]
- 47.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- 48.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, et al. Ecto-5’-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv. Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decking UK, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ. Res. 1997;81:154–164. doi: 10.1161/01.res.81.2.154. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi S, Zimmermann H, Millhorn DE. Chronic hypoxia enhances adenosine release in rat PC12 cells by altering adenosine metabolism and membrane transport. J. Neurochem. 2000;74:621–632. doi: 10.1046/j.1471-4159.2000.740621.x. [DOI] [PubMed] [Google Scholar]
- 53.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 54.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat. Rev. Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 55.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juno JA, Keynan Y, Fowke KR. Invariant NKT cells: regulation and function during viral infection. PLoS Pathog. 2012;8:e1002838. doi: 10.1371/journal.ppat.1002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Choukèr A, Thiel M, Lukashev D, Ward JM, Kaufmann I, Apasov S, Sitkovsky MV, Ohta A. Critical role of hypoxia and A2A adenosine receptors in liver tissue-protecting physiological anti-inflammatory pathway. Mol. Med. 2008;14:116–123. doi: 10.2119/2007-00075.Chouker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohta A, Diwanji R, Kini R, Subramanian M, Ohta A, Sitkovsky M. In vivo T cell activation in lymphoid tissues is inhibited in the oxygen-poor microenvironment. Front. Immun. 2011;2:27. doi: 10.3389/fimmu.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohta A, Kjaergaard J, Sharma S, Mohsin M, Goel N, Madasu M, Fradkov E, et al. In vitro induction of T cells that are resistant to A2 adenosine receptor-mediated immunosuppression. Br. J. Pharmacol. 2009;156:297–306. doi: 10.1111/j.1476-5381.2008.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato M, Iwakabe K, Ohta A, Sekimoto M, Nakui M, Koda T, Kimura S, et al. Functional heterogeneity among bone marrow-derived dendritic cells conditioned by T(h)1- and T(h)2-biasing cytokines for the generation of allogeneic cytotoxic T lymphocytes. Int. Immunol. 2000;12:335–342. doi: 10.1093/intimm/12.3.335. [DOI] [PubMed] [Google Scholar]