Abstract
Background
Survivors of pediatric acute lymphoblastic leukemia (ALL) have a significantly higher body mass index (BMI) than their peers. Understanding the critical time periods in which patients with pediatric ALL are vulnerable to unhealthy weight gain will lay the groundwork for developing effectively timed interventions.
Procedure
We determined the growth patterns of patients with pediatric ALL during and after treatment through the conduct of a systematic review and meta-analysis. A search of MEDLINE, Scopus, and Web of Science was performed from its inception through May 2014. Studies met the inclusion criteria if they included at least 10 patients of pediatric ALL, and longitudinally assessed BMI at diagnosis and at least one time point after diagnosis
Results
Twenty-one studies met the inclusion criteria for the systematic review and 16 were included in meta-analysis. The mean increase in BMI z-score during treatment in 1,514 patients with pediatric ALL was 0.81 (95% CI: 0.25–1.38). Specifically, patients experienced substantial weight gain in early treatment (Δ=0.41, 95% CI: −0.34, 1.17) and again during maintenance (Δ=0.34, 95% CI: −0.22, 0.90). The mean increase in BMI z-score ranged between 0.52 and 0.89 beyond treatment completion. Subgroup analyses found unhealthy weight gain occurred regardless of patients’ receipt of cranial radiation therapy, sex, and weight status at diagnosis.
Conclusions
Patients with pediatric ALL experience unhealthy weight gain early in treatment, and increases in weight are maintained beyond treatment completion. Preventing early onset of obesity is a priority for improving the care and outcomes for patients with pediatric ALL.
Keywords: Obesity, Growth, Acute Lymphoblastic Leukemia, Pediatric, Survivors
INTRODUCTION
Advances in treatment have resulted in significant improvements in survival rates of childhood cancer. Approximately 90% of children diagnosed with acute lymphoblastic leukemia (ALL), the most common childhood cancer, can expect to be cured (1). This success translates into a growing population of long-term survivors (2). At the same time, cancer treatment at a young age also produces late-effects, including obesity (3, 4). Our meta-analysis in 1,742 survivors of pediatric ALL, identified a mean body mass index (BMI) z-score of 0.83, corresponding to the 80th BMI percentile (5). This indicates that pediatric ALL survivors have a significantly higher BMI than their peers.
Obesity adds additional risks to the increased chronic health conditions already experienced by pediatric ALL survivors. Lifestyle interventions promoting healthy eating and physical activity can potentially prevent unhealthy weight gain in pediatric ALL survivors, but a critical question that needs to be addressed is when to intervene. In a retrospective cohort of 83 patients with pediatric ALL diagnosed between 1985–2010, we found a rapid weight gain during induction and the first 6 months of maintenance therapy (6). There is now accumulating evidence suggesting that pediatric ALL survivors experience excessive weight gain early in treatment (7, 8), and increases in weight tend to be maintained throughout the treatment and beyond.
This systematic review aims to identify growth patterns of patients with pediatric ALL by summarizing existing longitudinal evidence on changes in BMI z-score during treatment and years post treatment completion. Understanding the critical time periods in which patients with pediatric ALL are vulnerable to unhealthy weight gain will lay the groundwork for developing effective interventions to reduce premature mortality and serious morbidity in this population.
METHODS
We followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement for reporting our results (9) (Supplemental Table I). A protocol was developed prior to the conduct of the systematic review and submitted to PROSPERO (#: CRD42014010315), an international register of systematic review protocols (10).
Literature Search
We searched MEDLINE, Scopus, and Web of Sciences from inception through May 27, 2014 to identify studies that investigated obesity in patients or survivors of pediatric ALL. We used medical subject heading (MeSH) and text words related to obesity (obesity, weight, body composition, growth, etc.) in combination with survivors (survivors, remission, disease free survival, etc.) and acute lymphoblastic leukemia (acute lymphoblastic leukemia, leukemia, precursor cell lymphoblastic leukemia-lymphoma, etc.) (10). We consulted a research librarian in specifying the search and searched reference lists of eligible studies and relevant narrative reviews to identify additional studies that met inclusion criteria. While we did not set any language restrictions for the search, we did not screen studies without abstracts available in English.
Eligibility Criteria and Study Selection
Two authors (FFZ and SL) independently screened the titles and abstracts to identify potentially eligible studies using the software Abstrackr (http://www.cebm.brown.edu/software). Discrepancies were jointly reviewed to reach consensus. These two authors then independently screened the full texts of the identified studies to determine which were eligible for review. Studies were eligible if they met the following criteria: (1) were research articles published in peer-reviewed journals; (2) included at least 10 patients who were diagnosed with ALL prior to age 21 years; (3) assessed BMI z-score or percentile according to a reference population (e.g., the 2000 Centers for Disease Control and Prevention (CDC) growth reference (11)); and (4) followed BMI z-score or percentile longitudinally at diagnosis and at least one time point after diagnosis. We excluded review articles, case reports and studies that assessed only weight or only height as the outcome. We also excluded studies that assessed survivors who received hematopoietic stem-cell transplantation (HSCT) based on the rationale that HSCT survivors might experience a different pattern of growth compared to other ALL survivors.
Data collection and extraction
Two authors (FFZ and SL) extracted data and each verified the other’s extracted information. Discrepancies were resolved by consensus. For each eligible study, we extracted the following information (1) author, year and country of publication; (2) characteristics of the study population (sample size, years at diagnosis, mean age at diagnosis, treatment protocol, percentage of receiving cranial radiation therapy (CRT), percentage of males, and mean BMI z-score at diagnosis); (3) number of time points assessed; (4) length of follow-up from diagnosis to each of the time points; (5) primary outcomes (BMI z-score or percentile) reported at each time point; and (6) findings by receipt of CRT, sex, and weight status at diagnosis when available. BMI z-score or percentile calculated based on age- and sex-specific BMI cut-offs of a reference population was used to evaluate growth patterns because for individuals < 20 years of age BMI normally changes with age and varies by sex (12). The BMI z-score indicates the number of standard deviations the measurement is away from the age- and sex-specific mean value in the reference population. A BMI z-score >0 or BMI >50th percentile indicates a higher-than-average BMI.
Assessment of Study Validity/Quality Assessment
There is no standard scale to assess quality in observational studies (13, 14). We modified the Newcastle-Ottawa Scale (15) to create a checklist including the following quality items: whether the study adequately described the patients characteristics including age at diagnosis, sex distribution, and treatment protocols and/or years at diagnosis, whether the study clearly described the length of follow-up and the sample size at each follow-up, and whether the study clearly descried the methods of assessing the outcome, and provided the standard deviation, standard error, confidence interval or p-value for the outcome assessed at each follow-up (Supplemental Table II).
Statistical Analysis
In light of clinical heterogeneity in study characteristics, we performed random-effect meta-analysis to combine studies that reported the mean and standard deviation/error of BMI z-score at diagnosis and at least one time point after diagnosis. Studies were grouped and evaluated at seven time periods including (1) diagnosis; (2) end of induction (approximately 1 month post diagnosis); (3) start of maintenance (approximately 6–9 months post diagnosis); (4) end of treatment (approximately 2–3 years post diagnosis); (5) short-term follow-up (<2 years post-treatment); (6) median-term follow-up (2 – 4.9 years post-treatment); and (7) long-term follow-up (≥5 years post-treatment). If multiple BMI z-scores were reported within one time period, we chose the one with the most complete data (i.e. the largest sample size).
Among the 16 included studies, eight (6, 16–22) explicitly reported the mean BMI z-score for the overall cohort, one (23) reported the mean BMI percentile, which was converted to BMI z-score based on the standard normal curve, and we obtained the mean BMI z-score directly from authors in one study (24). For the remaining six studies, the mean BMI z-score was calculated as a weighted average based on subgroup values (using sample size as weights) in five studies (7, 25–28), or was estimated based on median BMI z-score in one study (29). For standard deviation (SD) of the BMI z-score, nine studies (6, 16–23) explicitly reported the standard deviation/error or confidence interval of the BMI z-score or percentile, and we obtained SD directly from authors in one study (24). For the remaining six studies, the SD was either calculated as a pooled standard deviation based on subgroup values assuming equal variance in five studies (7, 25–28), or estimated from median and range in one study (29) using methods described by Hozo et al (30). Among all 16 included studies, the mean and SD of the BMI z-score were estimated based on graphs using DigitizeIt (http://www.digitizeit.de/) in three studies (22, 25, 29).
Changes in BMI z-score during and after treatment were calculated for the following time periods using methods described in the Cochrane Handbook (31) from diagnosis to: (1) end of induction; (2) start of maintenance; (3) end of treatment; (4) <2 years post treatment; (5) 2 – 4.9 years post treatment; and (6) ≥5 years post treatment. Briefly, the changes in BMI z-score were calculated as the difference between the two time points. Only two studies explicitly reported changes in BMI z-score and SDs of the changes (21, 25). We estimated the SDs of the mean changes (SDchange) using the SDs for each periods (SDfollow-up=SD of mean BMI z-score at the longer follow-up time period; SDC=SD of mean BMI z-score at diagnosis or the shorter follow-up time period) and an imputed correlation coefficient (Corr) for the pre-post measurements using this formula SDchange = √ (SDfollow-up2 + SDC2 − 2×Corr×SDfollow-up× SDC). We used the correlation coefficients between time periods in our previous study as Corr (6) (Supplemental Table III) to impute SDs of the changes. To evaluate whether the growth pattern differed by key study characteristics, we performed subgroup meta-analysis in patients of pediatric ALL treated with CRT and those treated without CRT separately, in female and male patients separately, and in patients with BMI z-score ≥0 and <0 at diagnosis separately.
We performed sensitivity analyses to assess the robustness of our findings. First, we performed the analysis after excluding six studies for which the mean BMI z-score or its standard deviation was not explicitly reported. We also repeated the analysis after excluding three studies for which the mean and SD of BMI z-score was estimated based on graphs using DigitizeIt. In addition, we performed a “leave-one-out meta-analysis” to evaluate the impact of individual studies on the summary estimates (32).
We assessed between-study heterogeneity using Cochran’s Q statistic (33) and the I2 index.(34) The Cochran’s Q was considered statistically significant at PQ < 0.1. The I2 index, ranging between 0 and 100%, quantified the extent of heterogeneity beyond chance, with higher values indicating greater inconsistency across studies. All analyses were conducted using Stata version IC/12.1 (Stata Corp., College Station, TX, 2012). Statistical significance was defined as a two-sided p-value <0.05 for all tests except those for heterogeneity.
RESULTS
Included Studies
Our initial search identified 1,265 studies from MEDLINE, 522 studies of Web of Science, and 292 studies from Scopus, for a total of 2,079 studies. After screening titles and abstracts, 40 non-overlapping studies were considered potentially eligible and were retrieved for full text review. Of these, 19 studies were excluded, and 21 were included in this systematic review that reported results on 1,791 pediatric ALL survivors (Figure 1). Tables 1 summarizes the characteristics of the 21 studies.
Figure 1.
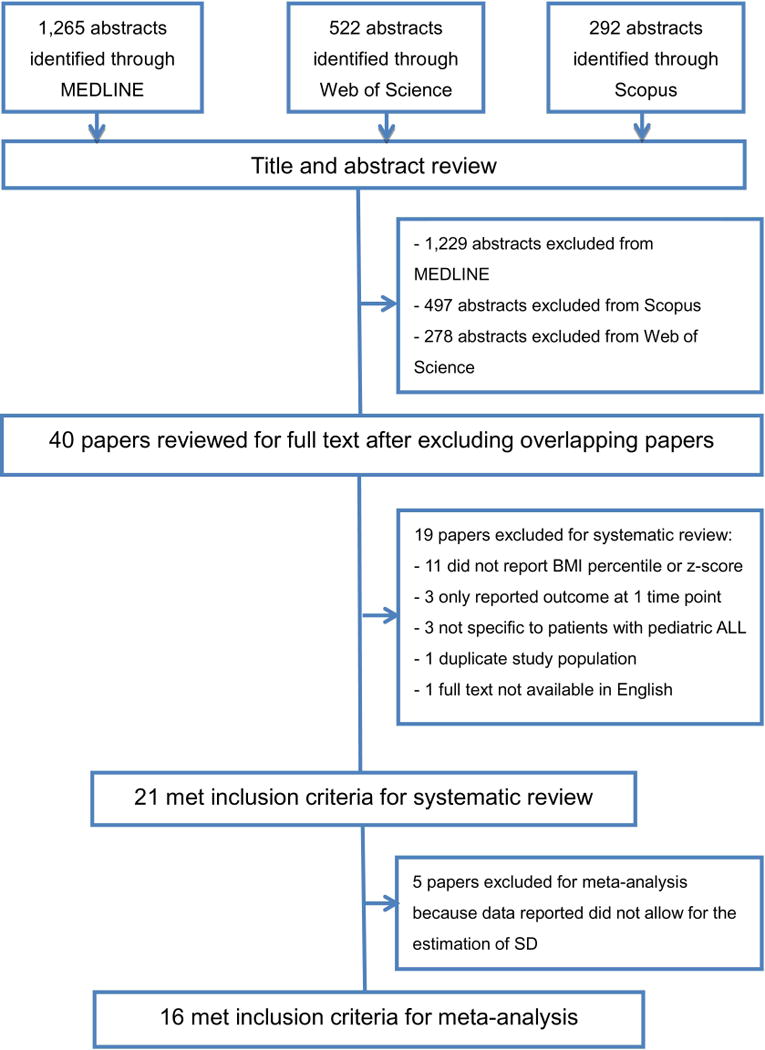
Study Flow Chart
Table I.
Characteristics of studies that assessed changes in BMI z-score during and after treatment in patients with pediatric ALL
| Author, Year (Location) |
N of Patients | Years at Diagnosis | Mean Age at Diagnosis (year) |
Treatment Protocol | % CRT | % Male | Mean BMI Z-score (SD) at Diagnosis |
|---|---|---|---|---|---|---|---|
| Odame et al, 1994 (United Kingdom) | 40 | 1980–1990 | 3.5 | MRC UKALL VIII and X | 100 | 47.5 | −0.006 (1.11) |
| Van Dogen-Melman et al, 1995 (Netherlands) | 113 | 1978–1990 | 3.8 | Dutch HR76, IIIB, V, VI, VI, VII | 46.0 | 51.3 | −0.50 (1.24) |
| Birkebaek et al, 1998 (a) (Denmark) | 33 | 1973–1984 | 4.3 | N/A | 66.7 | 42.4 | 0.35 (N/A) |
| Birkebaek et al, 1998 (b) (Denmark) | 30 | 1973–1984 | 4.3 | N/A | 60.0 | 50.0 | −0.64 (N/A) |
| Nysom et al, 1999 (Denmark) | 95 | 1970–1990 | 4.0 | N/A | 41.1 | N/A | −0.17 (0.85) |
| Reilly et al, 2000 (United Kingdom) | 98 | 1990–1997 | 5.2 | MRC UKALL-XI | N/A | 54.1 | −0.10 (1.06) |
| Sklar et al, 2000 (United States) | 126 | 1969–1982 | 6.4 | N/A | 69.8 | 46.8 | −0.18 (0.90) |
| Van der Sluis et al, 2002 (Netherland) | 61 | N/A | 7.1 | DCLSG-ALL9 | 0 | 61.0 | −0.29 (1.39) |
| Dalton et al, 2003 (United States) | 618 | 1987–1995 | 6.3 | DFCI ALL Consortium protocol 87-01 or 91-01 | N/A | 51.3 | −0.02 (1.30) |
| Davies et al, 2004 (United Kingdom) | 14 | N/A | 7.5 | MRC ALL 97 or the MRC ALL 97/99 | 0 | 50.0 | −0.33 (0.30) |
| Marinovic et al, 2005 (France) | 37 | 1995–1999 | 3.3 | EORTC 58881 | 0 | 54.1 | N/A |
| Baillargeon et al, 2007 (United States) | 307 | 1990–2002 | N/A | Multiple POG protocols | 0 | 60.6 | 0.22 (1.40) |
| Chow et al, 2007 (United States) | 165 | 1993–2003 | 4.8 | N/A | 19.4 | 57.0 | 0.24 (1.24) |
| Collins et al, 2010 (Canada) | 161 | 1985–2004 | 6.4 | DFCI protocols 85-01, 87-01, 91-01, 95-01, and 2000-01 | 60.0 | 61.0 | −0.20 (1.20) |
| Esbenshade et al, 2011 (United States) | 183 | 2000–2008 | 5.7 | COG | 12.6 | 61.7 | 0.64 (1.11) |
| Love et al, 2011 (Canada) | 102 | N/A | 3.3 | N/A | 7.8 | 46.1 | 0.12 (0.83) |
| Bang et al, 2012 (Korea) | 107 | 2005–2008 | 5.2 | Institutional protocol, CMCPL-2005 | 0 | 51.4 | 0.11 (1.03) |
| Karakurt et al, 2012 (Turkey) | 44 | 2000–2007 | 6.0 | BFM-95 | 59.0 | 57.0 | −0.25 (1.24) |
| Esbenshade et al, 2013 (USA) | 34 | 2008–2010 | N/A | COG | 26.5 | 64.7 | 0.52 (N/A) |
| Harper et al, 2013 (United Kingdom) | 77 | 1997–2003 | 4.6 | MRC/UKALL97 | 0 | 48.1 | 0.24 (1.16) |
| Zhang et al, 2014 (United States) | 83 | 1985–2010 | 4.0 | POG/DFCI/COG/others | 15.7 | 48.2 | 0.20 (1.20) |
Meta-Analysis of Changes in BMI Z-Score During and After Treatment
Sixteen studies provided (6, 7, 16–21, 23–29, 35) data for mean and SD of BMI z-score or percentile for at least two time points during and after treatment and were included in the meta-analysis. Baseline BMI z-scores ranged from −0.64 to 0.64 across studies. In 14 studies (6, 7, 16, 17, 19–28) that examined changes in BMI z-score from diagnosis to end of treatment, a significant increase in pooled BMI z-score was observed in 1,514 patients with pediatric ALL (Figure 2) (Δ=0.81, 95% CI: 0.25, 1.38). When different treatment phases were considered, a rapid weight gain occurred during the early treatment (i.e. from diagnosis to start of maintenance) (Δ=0.41, 95% CI: −0.34, 1.17) in 990 patients with pediatric ALL. Specifically, there was a substantial increase in pooled BMI z-score during induction (Δ=0.67, 95% CI: −0.72, 2.06) (i.e. from diagnosis to end of induction), followed by a decrease in pooled BMI z-score during consolidation (Δ=−0.50, 95% CI: −1.78, 0.77) (i.e. from diagnosis to start of maintenance). Weight gain occurred again from start of maintenance to end of treatment (Δ=0.34, 95% CI: −0.22, 0.90) in 966 patients with pediatric ALL (6, 7, 16, 17, 21, 22, 24, 25) (Supplemental Figure 1), although the increase in pooled BMI z-score during early treatment and during maintenance did not reach statistical significance.
Figure 2. Changes in BMI z-score during and after treatment in patients with pediatric ALL.
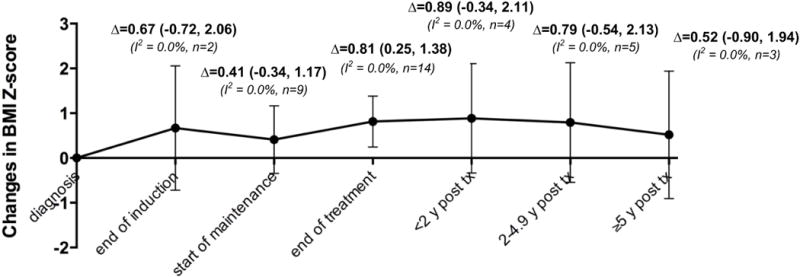
Δ (95% confidence interval) represents pooled random-effects meta-analysis results for the mean change in BMI z-score since diagnosis at each time period during and after treatment. I2 quantifies the extent of heterogeneity across studies. n represents the number of studies included in each meta-analysis.
Weight gain that occurred during treatment persisted beyond completion of treatment. Eleven studies evaluated growth patterns after treatment completion. The increase in pooled BMI z-score was 0.89 (95 CI: −0.34, 2.11) from diagnosis to <2 years post-treatment in 208 patients with pediatric ALL, was 0.79 (95 CI: −0.54, 2.13) from diagnosis to 2 – 4.9 years post-treatment in 370 patients with pediatric ALL, and was 0.52 (95 CI: −0.90, 1.94) from diagnosis to ≥5 years post-treatment in 378 patients with pediatric ALL (Figure 2, Supplemental Figure 2). Little between-study statistical heterogeneity was observed for changes in BMI z-score during and after treatment with I2 all approaching 0 (Supplemental Figures 1 and 2).
Sensitivity Analysis
Sensitivity analysis demonstrated similar growth patterns during and after treatment in patients with pediatric ALL after excluding studies that did not explicitly report the mean or SD of the BMI z-score/percentile or after excluding studies that obtained the mean and standard deviation of the BMI z-score based on graphs (Supplemental Figure 3). Leave-one-out meta-analysis did not find any specific study significantly impacted the meta-analysis results.
Assessment of Quality and Reporting
All 21 studies clearly defined the outcome and described the methods used to calculate BMI z-score/percentile. Most studies clearly stated patients’ age at study evaluation (95%), sex distribution (95%), and treatment protocols or years at diagnosis (71%). Fifteen studies (71%) described the length of follow-up, and 19 (90%) provided the sample size at the follow-up. Thirteen studies (62%) provided SD, standard error, confidence interval, or presented sufficient data for the estimation of SD (Supplemental Table II). Because all studies met at least five of the seven quality assessment criteria, we did not perform subgroup meta-analysis by study quality.
Growth Patterns in Patients with pediatric ALL by Key Study Characteristics
CRT
Four studies (6, 27, 28, 36) reported changes in BMI z-score separately for patients treated with CRT and those treated without CRT; one study (26) exclusively included patients treated with CRT; and five studies (16, 17, 19, 22, 24) exclusively included patients treated without CRT. Among the 10 studies, two (27, 36) did not provide SD for BMI z-score at diagnosis and were excluded in meta-analysis. Pooled changes in BMI z-score from diagnosis to end of treatment was 0.95 (95% CI: −0.06, 1.96) in the 127 survivors treated with CRT based on four studies, and was 0.97 (95% CI: 0.41, 1.54) in the 668 survivors treated without CRT based on eight studies. Growth patterns were also similar in patients treated with and without CRT beyond treatment completion (Figure 3A).
Figure 3. Changes in BMI z-score during and after treatment in patients with pediatric ALL by patient and treatment characteristics.
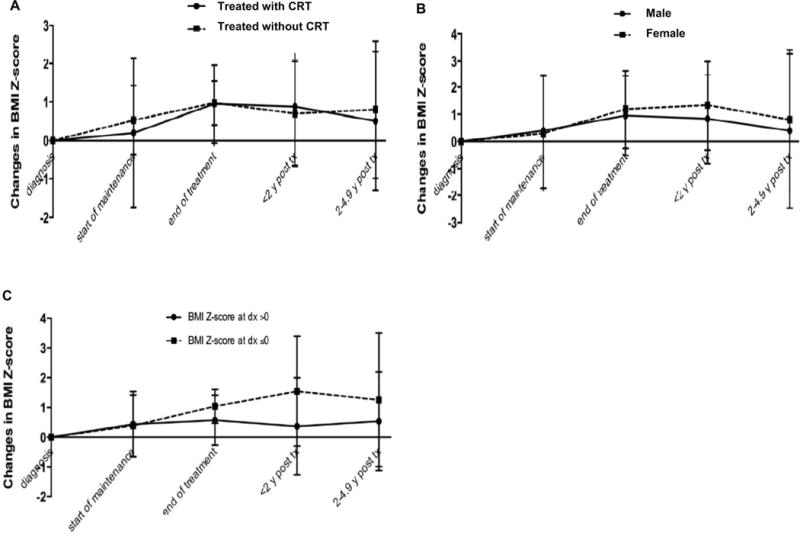
A. Changes in BMI z-score by receipt of cranial radiation therapy (CRT). B. Changes in BMI z-score by sex. C. Changes in BMI z-score by weight status at diagnosis.
Sex
Two studies (6, 26) reported changes in BMI z-score separately in male and female survivors. Pooled changes in BMI z-score from diagnosis to end of treatment were 1.19 (95% CI: −0.26, 2.64) in the 64 female survivors of pediatric ALL and was 0.95 (95% CI: −0.52, 2.42) in the 59 male survivors. Increase in BMI z-score was observed beyond treatment completion in both male and female survivors although it was slightly higher in female survivors (Figure 3B). Both male and female survivors experienced weight gain post treatment completion.
Weight status at diagnosis
Eight studies (20–22, 25–29) assessed changes in BMI z-score in patients with a mean BMI z-score <0 (i.e. below-average BMI) at diagnosis, and eight (6, 7, 16–19, 23, 24) in patients with a mean BMI z-score ≥0 (i.e. average or above-average BMI) at diagnosis. During treatment, weight gain was observed regardless of weight status at diagnosis, although meta-analysis combining cohorts of 667 patients with below-average BMI showed a greater increase in pooled BMI z-score (Δ=1.55, 95% CI: −0.30, 3.39) than that combining cohorts of 862 patients with average/above-average BMI (Δ=0.57, 95% CI: −0.27, 1.42). The same pattern persisted beyond treatment completion (Figure 3C).
DISCUSSION
This systematic review synthesized the longitudinal evidence for the growth patterns in patients with pediatric ALL during and after treatment. The meta-analysis demonstrated a significant increase in mean BMI z-score during treatment in 1,514 patients with pediatric ALL, and increases in weight persist beyond treatment completion. The meta-analysis also found unhealthy weight gain is prevalent in patients with pediatric ALL regardless of receipt of CRT, sex, and patients’ weights status.
Survivors of pediatric ALL have significantly high risk for obesity (5) but we did not know at which time period they experienced excessive weight gain. This meta-analysis found that patients with pediatric ALL gained substantial weight during early treatment (i.e. from diagnosis to start of maintenance), and again during the maintenance phase of the treatment. Overall, the mean increase in BMI z-score during treatment was 0.8 in patients with pediatric ALL. For a 5-year old male with an average BMI (i.e. BMI z-score = 0 or BMI = 50th percentile) at diagnosis who becomes 7-year old after treatment completion, this increase corresponds to 4 lbs. extra weight gain compared to his peers assuming normal growth in height. These findings support the notion that cancer treatment period is a sensitive window for unhealthy weight gain in patients with pediatric ALL.
Importantly, the meta-analysis suggested that patients with pediatric ALL did not return to their pre-treatment weight and were consistently more overweight or obese than their peers beyond treatment completion. From diagnosis to several years post treatment completion, the mean increase in BMI z-score remained high, ranging from 0.5 to 0.9. These findings, taken together, suggest that excessive weight gain occurred early in treatment is unlikely to be reversed after children complete cancer treatment.
Cancer and its treatment can affect energy intake and expenditure through complex pathways. CRT has been associated with obesity in pediatric ALL survivors but CRT has been gradually replaced by intrathecal and systemic chemotherapy. The meta-analysis supports the notion that patients treated under modern protocols that do not involve CRT still experience substantial weight gain during and after treatment. Corticosteroids are known to be critically involved in regulating energy intake, storage, and mobilization. Prolonged use of corticosteroids has demonstrated effects on increasing energy intake and percentage of body fat in patients with pediatric ALL (37, 38). Strategies to reduce the use of steroids during maintenance may ameliorate some of the unhealthy weight gain seen during this treatment period. Some of the international pediatric oncology groups have omitted steroids completely during maintenance (39) while others are investigating a reduction in steroids during maintenance (40). Other chemotherapeutic agents such as anthracyclines and vincristine may also contribute to weight gain through impairing cardiovascular fitness and muscle strength and subsequently reducing levels of physical activity (41–43). Although these changes were originally thought to be acute responses to cancer treatment, recent evidence indicates that children had difficulty reversing unhealthy eating habits and sedentary behaviors after treatment completion (41, 44, 45). Cancer treatment may have long-lasting impacts on survivors’ dietary intake and physical activity, contributing to unhealthy weight gain in this population.
Patients with below-average BMI at diagnosis seemed to experience a larger degree of weight gain during and after treatment compared to those with average or above-average BMI at diagnosis, which may reflect a greater impact of cancer treatment on “catch-up growth” for those who were leaner at diagnosis. Nevertheless, we did not find that the overall trajectory of changes in BMI z-score differed significantly by receipt of CRT, sex, and weight status during and after treatment, although our meta-analysis was limited by the number of longitudinal studies with available data for longer periods of follow-up to be included.
It is important to note that cancer treatment only attributes partially, less than 50%, to the elevated obesity risk in childhood cancer survivors (3). It is critical to further improve our understanding of modifiable behaviors such as nutritional intake and physical activity in association with obesity risk in childhood cancer survivors, and in particular, to elucidate the immediate and long-term effect of cancer treatment on hypothalamic-pituitary function and subsequent energy balance profiles by different patient and treatment characteristics.
Limitations should be considered when interpreting our findings. Our systematic review comprised heterogeneous studies that included patients from different countries, treated under different protocols, and with different weight status at diagnosis. Studies were also conducted over a period of time over which the treatment protocols have changed. We explored this clinical heterogeneity with subgroup analyses and accounted for unexplained variability through random effects models. Our meta-analysis did not find substantial differences in changes in BMI z-scores when comparing subgroups based on patient- and treatment- related characteristics. However, it is possible that true findings between these subgroups exist, but that our subgroup analyses were under-powered to detect them. Although BMI is widely used to measure obesity, it may not correspond well to the degree of body fatness in children.(46) A recent study suggested that the use of BMI led to underestimation of body fatness in adult survivors of childhood cancer. (47)
In conclusion, our meta-analysis suggests that patients with pediatric ALL are at risk becoming overweight or obese early in treatment, and increases in weight are likely to be maintained throughout treatment and beyond. Excessive weight gain occurs in all patients with pediatric ALL across treatment received, sex, and weight status at diagnosis. Given that approximately 90% of children and adolescents treated for ALL will be cured, our finding have important implications for pediatric oncologists, general pediatricians, and internal medicine/family medicine physicians, all of whom will provide long-term care to the growing cohort of childhood cancer survivors. Our results strongly suggest the needs for incorporating weight management early in treatment to prevent the early onset of obesity-related morbidities in patients with pediatric ALL.
Supplementary Material
Supplemental Figure 1. Changes in BMI z-score from diagnosis to end of treatment in patients with pediatric ALL.
Supplemental Figure 2. Changes in BMI z-score from diagnosis to years post treatment completion in patients with pediatric ALL.
Supplemental Figure 3. Sensitivity analyses for changes in BMI z-score during and after treatment in patients with pediatric ALL.
Sensitivity analysis 1 excluded six studies that did not explicitly report the mean and/or standard deviation of the BMI z-score. Sensitivity analysis 2 excluded three studies for which the mean and standard deviation of the BMI z-score were estimated based on reported graphs.
Supplemental Table 1. PRISMA 2009 Checklist.
Supplemental Table 2. Quality Assessment of Studies Evaluating Growth Patterns During and After Treatment in Patients with Pediatric ALL.
Supplemental Table 3. Pearson Correlation Coefficients for BMI Z-Score During and After Treatment in Patients with Pediatric ALL.
Table II.
Longitudinal changes in BMI z-score during and after treatment in patients with pediatric ALL
| Author, Year (Location) | Diagnosis | End of induction | Start of Maintenance | End of Treatment | ≤;2 years Post-Treatment | 2–5 years Post-Treatment | >5 years Post-Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Odame et al, 1994 (United Kingdom) | 40 | −0.01 (1.11) | 40 | 1.63 (1.10) | 40 | 1.78 (1.22) | ||||||||
| Van Dogen-Melman et al, 1995 (Netherlands) | 113 | −0.50 (1.24) | 113 | 0.70 (1.42) | 96 | 0.85 (1.43) | ||||||||
| Birkebaek et al (a), 1998 (Denmark)a | 33 | 0.35 (N/A) | 33 | 0.18 (N/A) | 33 | 0.61 (N/A) | ||||||||
| Birkebaek et al (b), 1998 (Denmark)a,c | 29 | −0.64 (N/A) | 29 | 0.18 (N/A) | 29 | 0.69 (N/A) | ||||||||
| Nysom et al, 1999 (Denmark) | 95 | −0.17 (0.85) | 95 | 0.66 (0.97) | 95 | 0.48 (1.04) | ||||||||
| Reilly et al, 2000 (United Kingdom) | 98 | −0.10 (1.06) | 98 | 0.56 (1.42) | 98 | 0.77 (1.27) | ||||||||
| Sklar et al, 2000 (United States) | 126 | −0.18 (0.90) | 126 | 0.41 (1.01) | 126 | 0.50 (1.23) | ||||||||
| van der Sluis et al, 2002 (Netherland)b | 61 | −0.29 (1.39) | 50 | 0.69 (1.37) | 30 | 1.08 (1.15) | 15 | 1.05 (0.54) | ||||||
| Dalton et al, 2003 (United States)a | 618 | −0.02 (1.30) | N/A | N/A | ||||||||||
| Davies et al, 2004 (United Kingdom) | 14 | −0.33 (0.30) | 8 | 0.004 (0.83) | 13 | 0.78 (0.46) | ||||||||
| Marinovic et al, 2005 (France)a,c | N/A | N/A | 37 | 0.90 (N/A) | 37 | 0.70 (N/A) | ||||||||
| Baillargeon et al, 2007 (United States) | 307 | 0.22 (1.40) | 307 | 0.64 (1.20) | 307 | 0.79 (1.00) | ||||||||
| Chow et al, 2007 (United States) | 165 | 0.24 (1.24) | 165 | 0.76 (0.94) | ||||||||||
| Collins et al, 2010 (Canada)b | 161 | −0.20 (1.20) | 158 | −0.63 (1.63) | 152 | 0.51 (1.33) | ||||||||
| Esbenshade et al, 2011 (United States)c | 183 | 0.64 (1.11) | 183 | 1.45 (1.09) | 183 | 0.67 (1.09) | 183 | 1.02 (1.20) | ||||||
| Love et al, 2011 (Canada) | 157 | 0.12 (0.83) | 157 | 0.65 (0.80) | 157 | 0.43 (0.87) | ||||||||
| Bang et al, 2012 (Korea) | 107 | 0.11 (1.03) | 107 | 1.09 (1.14) | 107 | 0.72 (1.24) | 92 | 0.35 (1.15) | ||||||
| Karakurt et al, 2012 (Turkey)b,c | 44 | −0.25 (1.24) | 44 | 0.94 (1.00) | ||||||||||
| Esbenshade et al, 2013 (United States)a,c | 34 | 0.52 (N/A) | 34 | 1.61 (N/A) | 34 | 0.29 (N/A) | ||||||||
| Harper et al, 2013 (United Kingdom) | 37 | 0.24 (1.16) | 32 | 1.04 (1.19) | 23 | 0.84 (1.16) | 10 | 0.03 (1.28) | ||||||
| Zhang, et al 2014 (United States) | 83 | 0.20 (1.20) | 78 | 0.70 (1.20) | 79 | 0.50 (1.00) | 76 | 0.80 (0.80) | 61 | 0.70 (1.00) | 42 | 0.70 (0.90) | ||
Studies were excluded for meta-analysis because SD was not reported and could not be estimated based on available data
Mean and SD of the BMI z-score were obtained based on graphs using DigitizeIt
Median of the BMI z-score was presented because mean was not reported.
Acknowledgments
All phases of this study were supported by the Boston Nutrition Obesity Research Center Grant Number P30DK46200, the National Center for Research Resources Grant Number UL1 RR025752, the National Center for Advancing Translational Sciences and the National Institutes of Health Grant Number UL1 TR000073. The funding source had no role in the design, conduct, or analysis of this study or the decision to submit the manuscript for publication. Dr. Chung contributed in analysis plan, statistical analysis, and the interpretations of results without receiving salary support.
Footnotes
The authors have no conflicts of interests to disclose.
References
- 1.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663–9. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariotto AB, Rowland JH, Yabroff KR, Scoppa S, Hachey M, Ries L, Feuer EJ. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1033–40. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, Sklar CA, Srivastava DK, Robison LL. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang FF, Kelly MJ, Saltzman E, Must A, Roberts SB, Parsons SK. Obesity in Pediatric ALL Survivors: A Meta-Analysis. Pediatrics. 2014;133(3):704–15. doi: 10.1542/peds.2013-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang FF, Rodday AM, Kelly MJ, Must A, Macpherson C, Roberts SB, Saltzman E, Parsons SK. Predictors of being overweight or obese in survivors of pediatric acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2014;61(7):1263–9. doi: 10.1002/pbc.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esbenshade AJ, Simmons JH, Koyama T, Koehler E, Whitlock JA, Friedman DL. Body mass index and blood pressure changes over the course of treatment of pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(3):372–8. doi: 10.1002/pbc.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Withycombe JS, Post-White JE, Meza JL, Hawks RG, Smith LM, Sacks N, Seibel NL. Weight patterns in children with higher risk ALL: A report from the Children’s Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53(7):1249–54. doi: 10.1002/pbc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Zhang FF, Liu SS, Chung M, Kelly MJ. Growth Patterns of Pediatric ALL Patients During and After Treatment PROSPERO International prospective register of systematic reviews. 2014 [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2000 CDC growth charts. 2000 [Google Scholar]
- 12.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 13.Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii–x. 1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–76. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2013 [Google Scholar]
- 16.Baillargeon J, Langevin AM, Lewis M, Estrada J, Grady JJ, Mullins J, Pitney A, Pollock BH. Demographic correlates of body size changes in children undergoing treatment for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(6):793–6. doi: 10.1002/pbc.21063. [DOI] [PubMed] [Google Scholar]
- 17.Bang KW, Seo SY, Lee JW, Jang PS, Jung MH, Chung NG, Cho B, Jeong DC, Suh BK, Kim HK. Evaluation of changes in random blood glucose and body mass index during and after completion of chemotherapy in children with acute lymphoblastic leukemia. Korean J Pediatr. 2012;55(4):121–7. doi: 10.3345/kjp.2012.55.4.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110(10):2313–20. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 19.Harper RL, Breene RA, Gattens M, Williams RM, Murray MJ. Non-irradiated female survivors of childhood acute lymphoblastic leukaemia are at risk of long-term increases in weight and body mass index. Br J Haematol. 2013;163(4):510–3. doi: 10.1111/bjh.12552. [DOI] [PubMed] [Google Scholar]
- 20.Nysom K, Holm K, Michaelsen KF, Hertz H, Muller J, Molgaard C. Degree of fatness after treatment for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 1999;84(12):4591–6. doi: 10.1210/jcem.84.12.6205. [DOI] [PubMed] [Google Scholar]
- 21.Reilly JJ, Ventham JC, Newell J, Aitchison T, Wallace WH, Gibson BE. Risk factors for excess weight gain in children treated for acute lymphoblastic leukaemia. Int J Obes Relat Metab Disord. 2000;24(11):1537–41. doi: 10.1038/sj.ijo.0801403. [DOI] [PubMed] [Google Scholar]
- 22.van der Sluis IM, van den Heuvel-Eibrink MM, Hahlen K, Krenning EP, de Muinck Keizer-Schrama SM. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr. 2002;141(2):204–10. doi: 10.1067/mpd.2002.125728. [DOI] [PubMed] [Google Scholar]
- 23.Love E, Schneiderman JE, Stephens D, Lee S, Barron M, Tsangaris E, Urbach S, Staneland P, Greenberg M, Nathan PC. A cross-sectional study of overweight in pediatric survivors of acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2011;57(7):1204–9. doi: 10.1002/pbc.23010. [DOI] [PubMed] [Google Scholar]
- 24.Davies JH, Evans BA, Jones E, Evans WD, Jenney ME, Gregory JW. Osteopenia, excess adiposity and hyperleptinaemia during 2 years of treatment for childhood acute lymphoblastic leukaemia without cranial irradiation. Clin Endocrinol (Oxf) 2004;60(3):358–65. doi: 10.1111/j.1365-2265.2003.01986.x. [DOI] [PubMed] [Google Scholar]
- 25.Collins L, Zarzabal LA, Nayiager T, Pollock BH, Barr RD. Growth in children with acute lymphoblastic leukemia during treatment. J Pediatr Hematol Oncol. 2010;32(8):e304–7. doi: 10.1097/MPH.0b013e3181ece2bb. [DOI] [PubMed] [Google Scholar]
- 26.Odame I, Reilly JJ, Gibson BE, Donaldson MD. Patterns of obesity in boys and girls after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1994;71(2):147–9. doi: 10.1136/adc.71.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sklar CA, Mertens AC, Walter A, Mitchell D, Nesbit ME, O’Leary M, Hutchinson R, Meadows AT, Robison LL. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: role of cranial irradiation. Med Pediatr Oncol. 2000;35(2):91–5. doi: 10.1002/1096-911x(200008)35:2<91::aid-mpo1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Van Dongen-Melman JE, Hokken-Koelega AC, Hahlen K, De Groot A, Tromp CG, Egeler RM. Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res. 1995;38(1):86–90. doi: 10.1203/00006450-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Karakurt H, Sarper N, Kilic SC, Gelen SA, Zengin E. Screening survivors of childhood acute lymphoblastic leukemia for obesity, metabolic syndrome, and insulin resistance. Pediatr Hematol Oncol. 2012;29(6):551–61. doi: 10.3109/08880018.2012.708892. [DOI] [PubMed] [Google Scholar]
- 30.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Green S. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Higgins JP, Green S, editors. The Cochrane Collaboration; 2011. [Google Scholar]
- 32.Olkin I. Diagnostic statistical procedures in medical meta-analyses. Stat Med. 1999;18(17–18):2331–41. doi: 10.1002/(sici)1097-0258(19990915/30)18:17/18<2331::aid-sim259>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 34.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 35.van der Sluis IM, van den Heuvel-Eibrink MM, Hahlen K, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density, body composition, and height in long-term survivors of acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 2000;35(4):415–20. doi: 10.1002/1096-911x(20001001)35:4<415::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Birkebaek NH, Clausen N. Height and weight pattern up to 20 years after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1998;79(2):161–4. doi: 10.1136/adc.79.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansen H, Postma A, Stolk RP, Kamps WA. Acute lymphoblastic leukemia and obesity: increased energy intake or decreased physical activity? Support Care Cancer. 2009;17(1):103–6. doi: 10.1007/s00520-008-0531-0. [DOI] [PubMed] [Google Scholar]
- 38.Reilly JJ, Brougham M, Montgomery C, Richardson F, Kelly A, Gibson BE. Effect of glucocorticoid therapy on energy intake in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2001;86(8):3742–5. doi: 10.1210/jcem.86.8.7764. [DOI] [PubMed] [Google Scholar]
- 39.Stary J, Zimmermann M, Campbell M, Castillo L, Dibar E, Donska S, et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol. 2014;32(3):174–84. doi: 10.1200/JCO.2013.48.6522. [DOI] [PubMed] [Google Scholar]
- 40.Children’s Oncology Group. Risk-Adapted Chemotherapy in Younger Patients With Newly Diagnosed Standard-Risk Acute Lymphoblastic Leukemia. 2014 ClinicalTrials.gov Identifier: NCT01190930.
- 41.Florin TA, Fryer GE, Miyoshi T, Weitzman M, Mertens AC, Hudson MM, Sklar CA, Emmons K, Hinkle A, Whitton J, Stovall M, Robison LL, Oeffinger KC. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1356–63. doi: 10.1158/1055-9965.EPI-07-0048. [DOI] [PubMed] [Google Scholar]
- 42.Ness KK, Leisenring WM, Huang S, Hudson MM, Gurney JG, Whelan K, Hobbie WL, Armstrong GT, Robison LL, Oeffinger KC. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115(9):1984–94. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips-Salimi CR, Lommel K, Andrykowski MA. Physical and mental health status and health behaviors of childhood cancer survivors: findings from the 2009 BRFSS survey. Pediatr Blood Cancer. 2012;58(6):964–70. doi: 10.1002/pbc.23359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stern M, Lamana L, Russell C, Edwin L, Thompson A, Trapp S, Bitsko M, Mazzeo S. Adaptation of an Obesity Intervention Program for Pediatric Cancer Survivors (NOURISH-T) Clinical Practice in Pediatric Psychology. 2013;1(3):264–75. [Google Scholar]
- 45.Arroyave WD, Clipp EC, Miller PE, Jones LW, Ward DS, Bonner MJ, Rosoff PM, Snyder DC, Demark-Wahnefried W. Childhood cancer survivors’ perceived barriers to improving exercise and dietary behaviors. Oncol Nurs Forum. 2008;35(1):121–30. doi: 10.1188/08.ONF.121-130. [DOI] [PubMed] [Google Scholar]
- 46.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124(Suppl 1):S23–34. doi: 10.1542/peds.2008-3586E. [DOI] [PubMed] [Google Scholar]
- 47.Blijdorp K, van den Heuvel-Eibrink MM, Pieters R, Boot AM, Delhanty PJ, van der Lely AJ, Neggers SJ. Obesity is underestimated using body mass index and waist-hip ratio in long-term adult survivors of childhood cancer. PLoS One. 2012;7(8):e43269. doi: 10.1371/journal.pone.0043269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Changes in BMI z-score from diagnosis to end of treatment in patients with pediatric ALL.
Supplemental Figure 2. Changes in BMI z-score from diagnosis to years post treatment completion in patients with pediatric ALL.
Supplemental Figure 3. Sensitivity analyses for changes in BMI z-score during and after treatment in patients with pediatric ALL.
Sensitivity analysis 1 excluded six studies that did not explicitly report the mean and/or standard deviation of the BMI z-score. Sensitivity analysis 2 excluded three studies for which the mean and standard deviation of the BMI z-score were estimated based on reported graphs.
Supplemental Table 1. PRISMA 2009 Checklist.
Supplemental Table 2. Quality Assessment of Studies Evaluating Growth Patterns During and After Treatment in Patients with Pediatric ALL.
Supplemental Table 3. Pearson Correlation Coefficients for BMI Z-Score During and After Treatment in Patients with Pediatric ALL.


