Abstract
Background
It is unclear whether an intensive program of weight loss combined with exercise prevents the onset of knee pain among those at high risk. We examined whether an intensive lifestyle intervention (ILI) prevents incident knee pain compared with a diabetes support and education (DSE) comparison group among overweight adults with diabetes.
Methods
We conducted a secondary analysis of the Action for Health in Diabetes (Look AHEAD) study, which is a randomized intervention trial of adults who were obese and had type 2 diabetes starting in 2001. We studied a sub-cohort of 2889 who reported no knee pain at baseline, but were at high risk due to obesity. Risk ratios (RR) were calculated to examine the association of ILI vs. DSE with incident knee pain at year 1 and 4. All analyses were adjusted for potential confounders.
Results
Age, sex, and BMI were similar among ILI and DSE participants with no knee pain at baseline. At year 1, ILI participants were 15% less likely to develop knee pain compared with DSE participants (RR = 0.85, 95% CI [0.74, 0.98]). At year 4, this decreased to 5% and was no longer statistically significant.
Conclusions
An intensive lifestyle intervention of diet and exercise may prevent the development of knee pain among those at high risk in the short-term. Health care providers may consider recommending diet and exercise as a means to prevent the development of knee pain among those at high risk.
Introduction
Knee pain is present in about one-fifth of men and one-quarter of women and has increased in prevalence by 65% over the past 40 years in the United States.1 Knee pain in older adults often leads to disability,2 is a frequent reason for medical visits,3 and is most commonly caused by knee osteoarthritis (OA).4, 5 The effective treatment of knee pain remains a major unmet clinical need. This is because pharmacologic treatment reduces knee pain by only a modest amount6–8 and is associated with side effects, particularly in older adults. Diet combined with exercise modestly reduces knee pain.9, 10 However, pain often persists at unsatisfactory levels since these conservative approaches are often prescribed to people with advanced pain.11
A preferable alternative to addressing knee pain may be through prevention among those at high risk, specifically through diet and exercise. Health professionals and public health organizations already promote healthy eating and consistent physical activity to target obesity and physical inactivity. Targeting diet and exercise for treatment of knee pain is a reasonable approach since obesity is a major risk factor for knee pain12 and knee OA,13 and weight loss interventions are effective at reducing knee pain.14 Adding regular exercise increases the effectiveness of a weight loss intervention to reduce knee pain.10, 15 This is possibly because exercise helps with weight loss and strengthens lower extremity skeletal muscles that consequently protect the knee joint. Nevertheless, no evidence to date supports a strategy of weight loss and exercise to prevent the onset of knee pain in those at high risk. In addition, the individual and combined effects of weight loss and regular exercise to potentially prevent knee pain are not known.
The Action for Health in Diabetes (Look AHEAD) study was a large multicenter diet and exercise intervention study of adults, with type 2 diabetes. Since the Look AHEAD study participants were overweight or obese and aged 45 to 76 years, they were, by extension, at high risk of knee OA.13, 16 Study participants were randomized into either an Intensive Lifestyle Intervention (ILI) with the goal to lose ≥7% of body weight and participate in ≥175 minutes/week of moderate to vigorous physical activity or a Diabetes Support and Education (DSE) comparison group. The purpose of our study was to conduct a secondary analysis to evaluate whether ILI participants without knee pain at baseline were less likely to develop knee pain one and four years later compared to the DSE comparison group. We also evaluated the risk of developing knee pain among participants meeting weight loss and/or exercise goals in a sub-cohort with objectively measured physical activity, compared with their counterparts not meeting these goals.
Method
The Look AHEAD study was a multicenter, randomized clinical trial designed to evaluate the long-term health effects of an intensive lifestyle intervention compared with usual care for 5,145 overweight or obese adults between the ages of 45 to 76 years with type 2 diabetes. Study participants were recruited from 16 outpatient centers in the United States beginning in September of 2001. There was no racial or gender bias in the selection of participants. A complete description of the design and methods for the Look AHEAD trial was previously published.17 Study participants were randomized to either an ILI or a DSE comparison group stratified by the clinic sites and blocked with random block sizes between October 2001 and ending in May 2004. Participants in both intervention arms continued to receive medical treatment for diabetes from their personal physicians as well as general medical care. Informed consent was obtained from all participants prior to screening. The Look AHEAD study protocol was approved by the Institutional Review Board from each clinical site and was registered to ClinicalTrials.gov, NCT00017953.
Study subsample
At the baseline study visit participants were asked “Have you had any pain or discomfort in your knees in the past month?” Similarly worded questions to define the presence of knee pain have been previously employed in large epidemiologic studies.18–21 We included study participants who answered “No” at baseline, n=2889. A second analysis was performed examining physical activity adherence and included participants without knee pain who wore an accelerometer, n=989.
Intervention groups
Intensive Lifestyle Intervention (ILI): The overall goal of this intervention was to teach and encourage behavioral change strategies for weight loss and exercise over four years. Study participants in the ILI were given a study-wide goal of ≥ 7% weight loss by the end of the first year and ≥ 175 minutes/week of MVPA physical activity by 6 months. Only physical activity bouts lasting at least 10 continuous minutes were counted towards this goal. During the first year of intervention, months 1 to 6 consisted of 24 sessions (three weekly group sessions followed by one individual session with a lifestyle counselor). Months 7 to 12 consisted of two group sessions and one individual session/month. During years 2 to 4, study participants had a minimum of two contacts/month with one being on-site and the other by phone, mail, or e-mail. A more detailed description of the diet and exercise portions of the ILI has been previously described.22 Briefly, the group and individual sessions of the ILI were modeled after programs developed for the treatment of people who are obese and have type 2 diabetes.23 The ILI utilized strategies shown to be most effective for long-term weight loss and maintenance. These included a portion-controlled diet, behavioral techniques, physical activity, social support, and ongoing regular contact throughout the follow-up period. All study intervention sites utilized the same standardized ILI treatment manual developed by Look AHEAD.
For diet, restriction of caloric intake was the primary method of achieving weight loss. The recommended diet was based on guidelines from the American Diabetes Association limiting the total calories from fat to 30%, and including at least 10% of calories from protein.24 Participants were encouraged to consume a target of 1,200 to 1,500 kcal/day for those ≤ 114kg at baseline and a target of 1,500 to 1,800 kcal/day for those > 114kg using meal replacements for breakfast and lunch. This approach produces more weight loss than self-selected diet of conventional foods.23 Participants chose from two diets. The first used a commercially available liquid meal replacement combined with an evening meal or frozen entrée. The second option for those unable to accept or tolerate the liquid meal prototype involved the consumption of a structured meal plan using foods the participant prepared themselves.25 Individual meetings with a lifestyle counselor on a monthly basis provided an opportunity to tailor these diet options to the participant’s personal preferences.
For exercise, intervention relied heavily on unsupervised exercise (at home) with a gradual progression to ≥ 175 minutes/week of moderate to vigorous physical activity (MVPA). For most participants this consisted of brisk walking and moderate-intensity walking was encouraged as a primary type of physical activity. The intervention did allow for participants to work with a lifestyle counselor to choose different types of moderate intensity activities based on individual preferences and physical ability. While unsupervised exercise was encouraged for most sessions, study sites did provide one supervised exercise session/week to stimulate social support. Exercise was recommended to occur five days/week and occupational activity was not counted towards the physical activity goal.
Cognitive behavioral strategies were employed to promote and maintain changes in dietary intake and physical activity. Specifically, study participants self-monitored their caloric intake by recording calories and fat grams throughout the first six months and periodically thereafter. Physical activity was self-monitored as well. Individual sessions with the lifestyle counselor focused on goal setting, problem solving, and motivational interviewing based on self-monitored diet and physical activity adherence in order to promote behavioral change.
Diabetes Support and Education (DSE): Participants in this comparison condition received general recommendations related to healthy eating and exercise, and strategies for safe and effective implementation of these recommendations from three group sessions during the first year. However, in contrast to the ILI, DSE participants were not given specific strategies or goals to promote weight loss or physical activity and did not receive individual sessions with a lifestyle counselor.
Outcome Measures
Incident knee pain
The primary outcome measure for our sub-study was knee pain, which was recorded at baseline, year 1, and year 4. Those with knee pain at year 1 and/or 4 were classified as having incident knee pain. In particular, we classified the documentation of the presence of pain at year 1 as ‘knee pain by year 1’, and the presence of pain at year 4 (i.e., they answered ‘no’ at baseline and ‘yes’ at year 4) as ‘knee pain by year 4’.
Pain severity
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire was given to study subjects who responded ‘yes’ to knee pain at each study visit. The pain subscale of the WOMAC is calculated from 5 items and the score ranges from 0 to 20 with higher scores representing more pain.
Weight
Weight was measured using a digital scale at baseline, year 1, and year 4. Height was measured with a wall-mounted stadiometer.
Physical Activity
Eight of the 16 Look AHEAD clinical sites measured physical activity objectively by an accelerometer at year 1, and in our study, this subsample comprised 989 subjects. An RT3 triaxial accelerometer (Stayhealthy, Inc. Monrovia, CA) measured physical activity in units of Metabolic Equivalents (METS)/minute. The accelerometer was worn on the hip starting on the assessment day (partial day) and for the next six consecutive days. Wearing the accelerometer for one week was repeated after each clinic visit. The accelerometer was worn during all waking hours and removed for periods of bathing, showering, or other water-based activities. A detailed description of how accelerometer data was processed has been published previously.26 MVPA was defined as ≥3 METS.
Potential confounders
The following factors measured at baseline were considered as potential confounders based on the association with physical activity and pain in previous studies: age,27 sex,28 race, use of non-steroidal anti-inflammatory drugs (NSAID) assessed via self-report, body mass index29 (BMI) calculated from weight and height measures,30 and depressive symptoms21 assessed with the Beck Depressive Index.31
Statistical Analysis
We compared characteristics of participants without knee pain at baseline (n=2889) in the ILI (n=1448) and DSE (n=1441) conditions by performing t-tests for continuous covariates, and chi-square tests for categorical covariates. Next, we examined the proportion of study subjects with incident knee pain within ILI and DSE conditions. We calculated risk ratios and 95% confidence intervals to examine the association of being assigned to the ILI with incident knee pain at year 1 and at year 4, respectively, compared with DSE participants. We also described pain severity only among study subjects with incident knee pain, as these study subjects were administered the WOMAC pain subscale. Next, we investigated the association of meeting the weight loss and/or physical activity treatment goals with incident knee pain in a sub-group that combined both treatment groups (ILI and DSE) and wore an accelerometer (n=989). We first compared characteristics of participants without knee pain who received (n=989) and did not receive an accelerometer (n=1900). Next, we classified participants as meeting neither treatment goal, the weight loss goal only, the physical activity goal only, or both goals at 1 year. We examined the association of meeting weight loss and/or physical activity goals with the risk of incident knee pain by year 1 and by year 4, respectively by calculating risk ratios and 95% confidence intervals. We used participants who did not meet either treatment goal as a reference. All analyses were adjusted for potential confounders. All statistical analyses were conducted with SAS software (Version 9.1, SAS Institute, Cary, NC).
Results
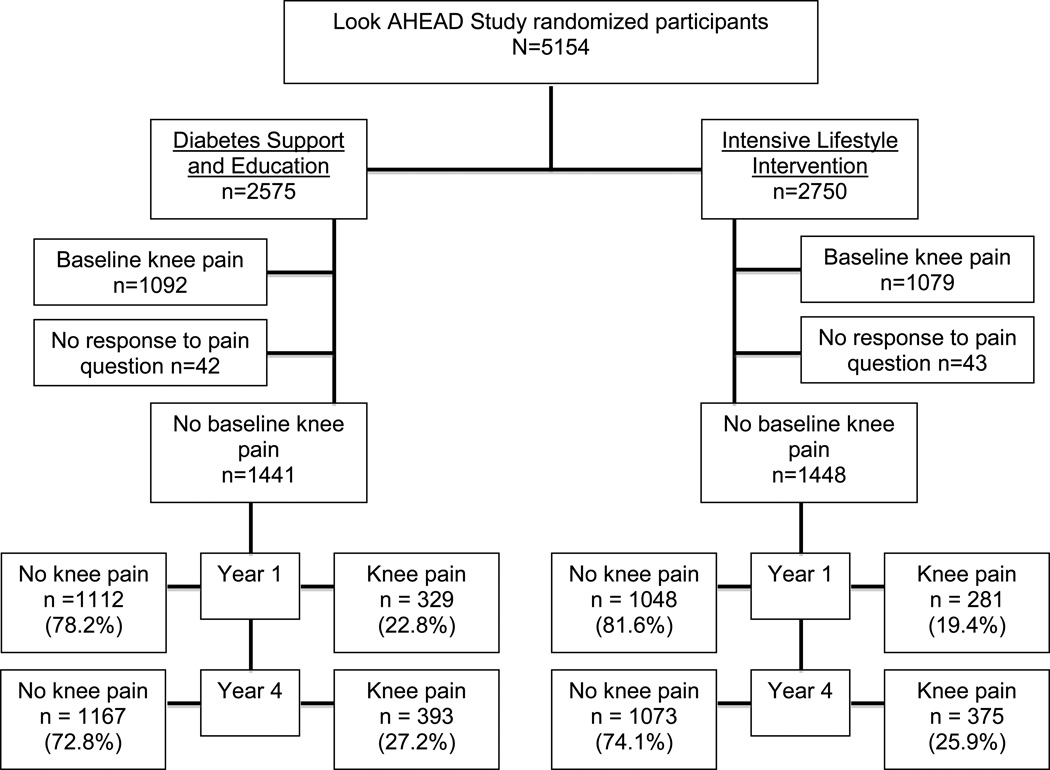
Of the 5145 study participants included in Look AHEAD, there were 2889 who reported no knee pain at baseline and were included in this sub-study. There were 50.1% (1448/2889) in the ILI group and 49.9% (1441/2889) in the DSE comparison group. (Figure 1) Study participants in the ILI and DSE groups were similar with respect to age, sex, BMI, NSAID use, and depressive symptoms. (Table 1) There were slightly fewer Whites (62.7%), and more African Americans (15.3%) and Hispanics (15.7%) in the DSE group than the ILI group (65%, 13.4%, and 14.5%, respectively).
Figure 1.
Flow diagram of knee pain at baseline, 1 year, and 4 years for Look AHEAD study participants
Table 1.
Characteristics of study participants with no knee pain at baseline in the Intensive Lifestyle Intervention (ILI) and Diabetes Support and Education (DSE) groups
| Combined (n=2889) |
ILI (n=1448) | DSE (n=1441) | p-value (ILI vs DSE) |
|
|---|---|---|---|---|
| Age [mean (sd)] | 58.5 (6.7) | 58.4 (6.8) | 58.5 (6.7) | 0.80 |
| Men [n (%)] | 1297 (44.9) | 641 (44.3) | 656 (45.5) | 0.50 |
| Race [n (%)] | ||||
| White | 1845 (63.9) | 941 (65.0) | 904 (62.7) | 0.21 |
| Black | 415 (14.4) | 194 (13.4) | 221 (15.3) | 0.14 |
| Hispanic | 436 (15.1) | 210 (14.5) | 226 (15.7) | 0.38 |
| BMI [mean (sd)] | 35.1 (5.6) | 35.0 (5.8) | 35.1 (5.3) | 0.90 |
| Change in BMI [mean (sd)] | ||||
| Year 1 – baseline | −1.7 (2.6) | −3.1 (2.6) | −0.2 (1.7) | <0.01 |
| Year 4 – baseline | −0.9 (3.4) | −1.5 (3.3) | −0.2 (3.2) | <0.01 |
| NSAID use at baseline [n (%)] | 819 (28.3) | 408 (28.2) | 411 (28.5) | 0.84 |
| Beck score for depression [mean (sd)] | 4.7 (4.6) | 4.7 (4.7) | 4.8 (4.5) | 0.53 |
| Physical Activity* [minutes/day of METS ≥ 3] [mean(sd)] | 58.1 (37.5) | 57.9 (37.7) | 58.2 (37.4) | 0.89 |
| Change in Physical Activity* [minutes/day] [mean (sd)] | ||||
| Year 1 – baseline* | 1.1 (53.6) | 6.4 (65.0) | −4.3 (38.2) | <0.01 |
| Year 4 – baseline* | −8.3 (43.1) | −4.5 (40.5) | −12.2 (41.9) | <0.01 |
Among the subcohort who wore an accelerometer at baseline (n=989)
Knee pain developed in 19.4% ILI participants and in 22.8% of DSE participants at year 1. (Table 2) After adjustment for potential confounders, ILI participants had 15% lower risk of developing knee pain at the one-year follow-up than DSE participants. By year 4, knee pain was newly reported in 25.9% of ILI participants and 27.2% of DSE participants from baseline. Those in the ILI had 5% lower risk of incident knee pain than DSE participants, an adjusted difference that was not statistically significant. The mean (sd) knee pain severity among study participants who developed knee pain was 3.8 (3.2) and 4.3 (3.4) at year 1 and year 4, respectively. (See Supplementary Table 1)
Table 2.
Association of an intensive weight loss and exercise program with the development of knee pain over 4 years.
| Knee pain at year 1 | Knee pain at year 4 | |||
|---|---|---|---|---|
| n (%) | Adjusted* RR [95% CI] |
n (%) | Adjusted* RR [95% CI] |
|
| Diabetes Support and Education (n=1441) | 329 (22.8) | 1.00 [REF] | 393 (27.2) | 1.00 [REF] |
| Intensive Lifestyle Intervention (n=1448) | 281 (19.4) | 0.85 [0.74, 0.98] | 375 (25.9) | 0.95 [0.84, 1.07] |
Adjusted for age, sex, BMI, race, NSAID use, and depressive symptoms
We focused on objectively monitored physical activity in the subset of 989 in either the ILI (n=494) or DSE (n=495) groups who wore an accelerometer. These participants were similar to those not receiving an accelerometer (n=1900) with respect to BMI, NSAID use, and depressive symptoms. Those receiving an accelerometer were older, more likely to be male, and White compared with those who did not. (See Supplementary Table 2)
Among the subset that wore an accelerometer, those meeting the weight loss goal at year 1 were 37% and 38% less likely to develop knee pain at year 1 and 4, respectively, compared with those not meeting either treatment goal. (Table 3) Those meeting the physical activity goal were 31% and 21% less likely to develop knee pain at year 1 and 4, respectively, which was not statistically significant. Lastly, those meeting both the weight loss and physical activity goals were 47% less likely to develop knee pain at year 1, which was statistically significant, and were 24% less likely to develop knee pain at year 4, which was not statistically significant.
Table 3.
Association of meeting treatment goals at one year with the development of knee pain at year 1 and year 4 among all participants who wore an accelerometer (n=989).
| Treatment goal reached at year 1 |
Knee pain at year 1 | Knee pain at year 4 | ||
|---|---|---|---|---|
| n (%) | n (%) | Adjusted* RR [95% CI] |
n (%) | Adjusted* RR [95% CI] |
| Did not meet either treatment goal 549 (55.5) | 137 (25.0) | 1.00 [REF] | 166 (30.2) | 1.00 [REF] |
| Met only the weight loss goal 229 (23.1) | 37 (16.2) | 0.63 [0.46, 0.88] | 44 (19.2) | 0.62 [0.47, 0.83] |
| Met only the physical activity goal 106 (10.7) | 15 (14.2) | 0.69 [0.42, 1.13] | 21 (19.8) | 0.79 [0.53, 1.20] |
| Met both treatment goals 106 (10.7) | 13 (12.3) | 0.53 [0.31, 0.90] | 22 (20.8) | 0.76 [0.51, 1.13] |
Adjusted for age, sex, BMI, race, NSAID use, and depressive symptoms
Discussion
Among middle to older aged adults who were overweight or obese and had diabetes, an intensive lifestyle intervention program of diet and exercise reduced the relative risk of developing knee pain by 15% at one year compared with a diabetes support and education comparison group. At four years, this protective effect was attenuated. These findings provide evidence from a secondary analysis of an intervention trial that an intensive program of diet and exercise may prevent the development of knee pain.
Diet and exercise had a small statistically significant protective against the development of knee pain at year 1. The risk of incident knee pain at year 1 was 3.4% less in those in the ILI (19.4%) compared with those in the DSE (22.8%). This effect is consistent with a broader theme that dietary weight loss, strengthening, and aerobic walking programs decrease knee pain by a small to moderate magnitude (Standardized Mean Difference = 0.4, 95%CI [0.30, 0.50]).32 Our study extends this previous work by adding that diet and exercise may be effective for preventing the development of knee pain in adults at risk.
We found that the protective effect of the ILI to prevent the development of knee pain to diminish from 15% at year 1 to 5% at year 4. A possible explanation for this decline may be the waning ability of study subjects to maintain diet and exercise recommendations over time. Lifestyle interventions achieve maximal weight loss at 1 year, which is followed by a increase in weight33 and the same trend is found for physical activity.34 In our sample, the greatest positive change in weight and physical activity occurred at year 1 for those in the ILI. Hence, it is possible the effect of the ILI to prevent knee pain decreased by year 4 as a consequence of diminishing adherence to diet and exercise recommendations.
Our primary analysis of all those without knee pain does not depend on adherence to the prescribed exercise or dietary regimens. Therefore, the protective effect at one year seen should not be tainted by reverse causality. However, in our findings focused on those who adhered to the regimens, persons developing knee pain during follow-up may not have been able to adhere to the physical activity program, leading to reverse causality. Thus, our per-protocol findings on adherents should be treated with caution. The relative importance of weight loss and exercise for preventing knee pain should ideally be tested in a clinical trial to truly determine whether weight loss alone is more important than exercise to prevent the development of knee pain.
The severity of pain as reported by those with incident knee pain ranged from 3 to 4 on the 5-item WOMAC pain subscale, which represents mild pain on 3 to 4 items or extreme pain on one item. This severity is similar to that reported in cohorts of people with early knee OA35 and is close to exceeding a minimum patient acceptable symptom state, or the severity of pain below which patients consider as satisfactory.36 While the duration and frequency of knee pain was not assessed in the Look AHEAD cohort, the observed pain severity was not inconsequential.
Limitations of our study should be acknowledged. First, knee pain was a secondary end point of the Look AHEAD cohort and we conducted our analysis in a subgroup of people without knee pain. Hence, there is a possibility of confounding by factors that were either not included in analyses or were unmeasured. Nevertheless, we did adjust for common potential confounders such as age, sex, BMI, and depressive symptoms in all analyses. Second, we are unable to confirm the presence of knee OA by radiograph since no knee x-rays were taken in this sample. Hence, there is a chance that knee pain may be emerging from other sources, such as acute injury or referred from low back pain. However, given that this sample is composed of middle- to older-aged adults who were overweight or obese, it is most likely that incident knee pain developed from OA. Third, we were unable to distinguish whether knee pain at each follow-up visit was truly the first occurrence or a recurrence of pain. As with any study of incidence, the outcome could have occurred at any time during the follow-up. We acknowledge that we may have missed some individuals at year 4 who had pain between year 1 and year 4, but did not report any pain at year 4. Nevertheless, the presence of knee pain and not radiographic evidence of knee OA is troublesome for older adults, given that it drives older adults to seek medical attention, and leads to functional limitation and a reduced quality of life.4, 5, 37 Fourth, generalizability of our study findings to people without diabetes should be done with caution given our sample consisted entirely of people with diabetes.
Despite these limitations, our study has several strengths. We took advantage of a large multi-center randomized controlled trial to investigate whether diet and exercise protected against the development of knee pain among those at high risk. This cohort provided adequate sample size to investigate this question. Also, study subjects were followed for four years, which provides a generous longitudinal perspective on the efficacy of weight loss and exercise to prevent the development of knee pain.
In conclusion, in a secondary analysis of the Look AHEAD study we found an intensive intervention program of weight loss and exercise reduced the short-term risk for developing knee pain among overweight adults with type 2 diabetes. These findings suggest that diet and exercise may be effective for preventing the development of knee pain. Health care providers should consider recommending diet and exercise to their patients who are overweight or obese as a potentially effective means to prevent the development of knee pain.
Supplementary Material
Significance and Innovation.
Diet and exercise are commonly recommended by health professionals to people who are overweight and consequently at high risk of developing knee pain. However, it is not known whether following such recommendations prevents the development of knee pain.
We found an intensive program of diet and exercise had a small statistically significant protective effect against the development of knee pain in the short term among overweight adults with diabetes.
Acknowledgments
Funding Drs. White, Neogi, and Felson: R24HD0065688, NIH AR47785, R01AR062506, ACR/RRF Rheumatology Investigator Award, ACR/RRF Bridge Funding Award, Boston Claude D. Pepper Older Americans Independence Center (P30-AG031679), and the Foundation for Physical Therapy.
REFERENCES
- 1.Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155(11):725–732. doi: 10.1059/0003-4819-155-11-201112060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 3.Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey, 2006 summary. Natl Health Stat Report. 2008;(3):1–39. [PubMed] [Google Scholar]
- 4.Hadler NM. Knee pain is the malady--not osteoarthritis. Ann Intern Med. 1992;116(7):598–599. doi: 10.7326/0003-4819-116-7-598. [DOI] [PubMed] [Google Scholar]
- 5.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52(4):258–262. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjordal JM, Ljunggren AE, Klovning A, Slordal L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ. 2004;329(7478):1317. doi: 10.1136/bmj.38273.626655.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15(9):981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18(4):476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 10.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill SD, McBurney H. Does exercise reduce pain and improve physical function before hip or knee replacement surgery? A systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2013;94(1):164–176. doi: 10.1016/j.apmr.2012.08.211. [DOI] [PubMed] [Google Scholar]
- 12.Riddle DL, Stratford PW. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: a cohort study. Arthritis Care Res (Hoboken) 2013;65(1):15–22. doi: 10.1002/acr.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 14.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66(4):433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foy CG, Lewis CE, Hairston KG, Miller GD, Lang W, Jakicic JM, et al. Intensive lifestyle intervention improves physical function among obese adults with knee pain: findings from the Look AHEAD trial. Obesity (Silver Spring) 2011;19(1):83–93. doi: 10.1038/oby.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109(1):18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 17.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 18.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27(6):1513–1517. [PubMed] [Google Scholar]
- 20.Hochberg MC, Lawrence RC, Everett DF, Cornoni-Huntley J. Epidemiologic associations of pain in osteoarthritis of the knee: data from the National Health and Nutrition Examination Survey and the National Health and Nutrition Examination-I Epidemiologic Follow-up Survey. Semin Arthritis Rheum. 1989;18(4) Suppl 2:4–9. doi: 10.1016/0049-0172(89)90008-5. [DOI] [PubMed] [Google Scholar]
- 21.White DK, Keysor JJ, Neogi T, Felson DT, LaValley M, Gross KD, et al. When it hurts, a positive attitude may help: association of positive affect with daily walking in knee osteoarthritis. Results from a multicenter longitudinal cohort study. Arthritis Care Res (Hoboken) 2012;64(9):1312–1319. doi: 10.1002/acr.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med. 1994;97(4):354–362. doi: 10.1016/0002-9343(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 24.Nutrition recommendations and principles for people with diabetes mellitus. Diabetes Care. 2000;23(Suppl 1):S43–S46. [PubMed] [Google Scholar]
- 25.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord. 1996;20(1):56–62. [PubMed] [Google Scholar]
- 26.Jakicic JM, Gregg E, Knowler W, Kelley DE, Lang W, Miller GD, et al. Activity patterns of obese adults with type 2 diabetes in the look AHEAD study. Med Sci Sports Exerc. 2010;42(11):1995–2005. doi: 10.1249/MSS.0b013e3181e054f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tudor-Locke C, Schuna JM, Jr, Barreira TV, Mire EF, Broyles ST, Katzmarzyk PT, et al. Normative steps/day values for older adults: NHANES 2005–2006. J Gerontol A Biol Sci Med Sci. 2013;68(11):1426–1432. doi: 10.1093/gerona/glt116. [DOI] [PubMed] [Google Scholar]
- 28.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 29.Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White DK, Neogi T, Zhang Y, Felson D, Lavalley M, Niu J, et al. The association of obesity with walking independent of knee pain: the multicenter osteoarthritis study. J Obes. 2012;2012:261974. doi: 10.1155/2012/261974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 32.Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. J Rheumatol. 2009;36(6):1109–1117. doi: 10.3899/jrheum.090058. [DOI] [PubMed] [Google Scholar]
- 33.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125(9):1157–1170. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hobbs N, Godfrey A, Lara J, Errington L, Meyer TD, Rochester L, et al. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med. 2013;11:75. doi: 10.1186/1741-7015-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesseling J, Bierma-Zeinstra SM, Kloppenburg M, Meijer R, Bijlsma JW. Worsening of pain and function over 5 years in individuals with 'early' OA is related to structural damage: data from the Osteoarthritis Initiative and CHECK (Cohort Hip & Cohort Knee) study. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203829. [DOI] [PubMed] [Google Scholar]
- 36.Escobar A, Gonzalez M, Quintana JM, Vrotsou K, Bilbao A, Herrera-Espineira C, et al. Patient acceptable symptom state and OMERACT-OARSI set of responder criteria in joint replacement. Identification of cut-off values. Osteoarthritis Cartilage. 2012;20(2):87–92. doi: 10.1016/j.joca.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Ayis S, Ebrahim S, Williams S, Juni P, Dieppe P. Determinants of reduced walking speed in people with musculoskeletal pain. J Rheumatol. 2007;34(9):1905–1912. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.