Abstract
Objective
To assess the effect of exercise training on insulin sensitivity and plasma ceramides in obesity and type 2 diabetes (T2D).
Methods
Twenty-four adults with obesity and normal glucose tolerance (NGT, n=14), or diabetes (n=10) were studied before and after a 12-week supervised exercise-training program (5 d/wk, 1 hr/d, 80–85% of maximum heart rate). Changes in body composition were assessed using hydrostatic weighing and computed tomography. Peripheral tissue insulin sensitivity was assessed by a 40 mU/m2/min hyperinsulinemic euglycemic clamp. Plasma ceramides (C14:0, C16:0, C18:0, C18:1, C20:0, C24:0 and C24:1) were quantified using electrospray ionization tandem mass spectrometry after separation with HPLC.
Results
Plasma ceramides were similar for the obese NGT and subjects with diabetes, despite differences in glucose tolerance. Exercise significantly reduced body weight and adiposity, and increased peripheral insulin sensitivity in both groups (P<0.05). In addition, plasma C14:0, C16:0, C18:1, and C24:0 ceramide levels were reduced in all subjects following the intervention (P<0.05). Decreases in total (r=-0.51, P=0.02) and C14:0 (r=-0.56, P=0.009) ceramide were negatively correlated with the increase in insulin sensitivity.
Conclusion
Ceramides are linked to exercise training-induced improvements in insulin sensitivity, and plasma C14:0 ceramide may provide a specific target for investigating lipid-related insulin resistance in obesity and T2D.
Keywords: Obesity, Phospholipids, Fatty Acids, Lipids, Insulin Resistance
Introduction
Obesity is the major predisposing factor in the development of insulin resistance. If untreated, the progressive hyperglycemia and subsequent compensatory hyperinsulinemia seen in insulin resistant individuals will lead to beta-cell failure and the onset of type 2 diabetes. Obesity contributes to insulin resistance through increased generation of lipid-related metabolites that inhibit insulin signal transduction. Recently, ceramides were cited as key lipid mediators of insulin resistance (1, 2, 3), and increased ceramide content in muscle, liver and adipose tissue has been demonstrated in insulin resistant models (4, 5, 6). Furthermore, it has now been shown that inhibition of de novo ceramide synthesis in animal models reverses diet-induced insulin resistance (7). Although ceramide levels are increased in the muscle and adipose tissue of subjects with insulin resistance (8, 9), the potential to use tissue ceramide as a biomarker of insulin resistance is limited by access to human tissue. However, using a lipidomic analysis we demonstrated that when compared to lean healthy controls, fasting plasma ceramide concentrations are elevated in those with obesity and type 2 diabetes, and are directly related to rates of insulin-stimulated peripheral tissue glucose uptake (10). Additional studies have since confirmed a role for plasma ceramides in T2D (11, 12). It has also been shown that plasma ceramides are mainly concentrated in VLDL and LDL particles, and the LDL-bound ceramide promotes inflammation and skeletal muscle insulin resistance (13).
Reversal of insulin resistance and hyperglycemia in obesity is critical to preserving pancreatic beta-cell function. We, and others, have shown that exercise training reduces body weight, and improves insulin sensitivity via alterations in lipid metabolism in obesity (14, 15, 16), however the precise mechanisms are not understood. Some studies demonstrate that exercise induced improvements in insulin sensitivity are associated with reductions in intramuscular ceramides. For example, it was reported that prolonged exercise decreased the ceramide content in rat skeletal muscle (17). Likewise, endurance training has been shown to reduce total ceramide and saturated ceramide subspecies in human skeletal muscle (18, 19, 20), although these findings have not been universal (21). Collectively however, the effect of exercise on circulating ceramide in relation to insulin resistance has been overlooked.
Further to our prior findings that plasma ceramide concentrations are related to insulin resistance (10), here we have examined the effect of aerobic exercise training on circulating ceramides in the postabsorptive state, again using a focused lipidomic approach to study a range of ceramide subspecies. We hypothesized that plasma ceramides would be elevated in obese subjects exhibiting diabetic glucose tolerance in contrast to subject with obesity and normal glucose tolerance. Further, we expected that following long-term exercise training, the improvement in insulin resistance would be related to the change in plasma ceramides. We also sought to identify whether ceramide chain length or carbon bond saturation would play an additional role.
Methods
Participants
Potential subjects from the local community and endocrine clinics were recruited to participate as part of our ongoing metabolic studies. Each subject underwent medical screening that included a physical examination, blood chemistry profile, and a resting 12-lead electrocardiogram. Individuals were excluded if they had undergone significant weight loss (>2 kg), or were engaged in regular physical activity in the previous 6 months, demonstrated any contraindication to exercise, were smokers, or if they showed evidence of cardiovascular, renal, hepatic, hypothyroid or hematological disease. During the screening visit the Minnesota Leisure Time Questionnaire was administered to assess daily physical activity. Following medical screening, 24 older (age 63 ± 1 years), obese (BMI 34.1 ± 1.3 kg/m2) volunteers were divided into two groups based on their classification following an oral glucose tolerance test (OGTT). Individuals with normal glucose tolerance (NGT; n = 14) or type 2 diabetes (n = 10) were then entered into a twelve-week aerobic exercise training intervention. Diabetics were previously undiagnosed and were therefore insulin naive, and were not taking statins or oral hypoglycemic drugs. All volunteers gave their informed written consent to partake in the study, and our Institutional Review Board reviewed and approved the protocols.
Dietary Control
For the three days immediately prior to and during the last three days of the intervention, all subjects resided in the Clinical Research Unit. Isocaloric meals based on calorimetry measures of resting metabolic rate were provided during this stay to ensure weight stability prior to metabolic testing. During the study, a registered dietitian counseled the subjects at weekly intervals to ingest a diet isocaloric to their estimated energy expenditure. Participants completed 3-day food records at baseline, and weeks 3, 6, 9, and 12. Food records were analyzed using Nutrition Data System for Research (NDSR, Minnesota) software. During the pre- and post-intervention inpatient stay volunteers underwent assessments of body composition and insulin sensitivity, as described below.
Exercise Training Intervention
Prior to the study an incremental treadmill exercise test was performed to determine each subjects’ maximal aerobic capacity (VO2max). All volunteers then completed a twelve-week period of fully supervised aerobic exercise training: one hour per day, five days per week. Over 80% of the exercise was performed on a treadmill, but in order to provide some variety to the workout, subjects were allowed to alternate between a treadmill and cycle ergometer. Training was initiated at 60% of their maximal heart rate (HRmax; ~50% VO2max), gradually increasing their intensity so that by week 4 they were exercising at 80–85% of HRmax (~70% VO2max). Additionally, to account for training-induced improvements in aerobic fitness, VO2max tests were repeated at weeks 4, 8, and 12. We have previously described these procedures in detail (22, 23).
Body Composition
Body weight and height was assessed by standard techniques. Hydrostatic weighing was used to estimate body density and whole body fat percentage (23). In addition, computed tomography (CT) scanning was used to measure cross-sectional subcutaneous and visceral abdominal adiposity using a Picker PQ6000 Scanner (Marconi/Picker, Highland Heights, OH). Volunteers lay in a supine position and cross-sectional 5 mm slices were obtained at the 4th lumbar vertebral body (L4) using a sampling time of 1 s at 120 kV and 150 mÅ. The location for the post-intervention scan was standardized using distances from bony landmarks identified on the pre-intervention scan. Digitized images were analyzed in a blinded fashion using NIH Image software (http://rsb.info.nih.gov/nih-image/download.html). These images were analyzed in gray scale, and regions of interest (subcutaneous adipose tissue, and visceral adipose tissue [VAT]) were quantified by identifying threshold-highlighted pixels at attenuation values of −250 to −40 Hounsfield units.
Insulin Sensitivity
A 2-hour hyperinsulinemic euglycemic clamp was used to measure subjects’ peripheral insulin sensitivity, prior to and following the study (14). In brief, a primed 40 mU/m2/min infusion of insulin was administered through an antecubital vein accompanied with a variable rate glucose infusion so as to maintain euglycemic conditions (~90 mg/dl). Plasma glucose concentrations were measured every 5 min on blood obtained from a retrograde dorsal hand vein, with the hand kept in a thermostatic heating box to facilitate arterialization. Space-corrected glucose infusion rates (GIR) were calculated during steady state conditions in the final 30 min of the procedure. The GIR is widely considered to represent insulin-mediated glucose uptake in skeletal muscle.
Analytical Measurements
Fasting blood samples were drawn prior to the clamp, upon which the following metabolites were measured. Plasma ceramide subspecies were measured on fasting blood samples, using our published procedure (24). Briefly, plasma samples were spiked with 10 ng of C17:0 and 20 ng of C25:0 ceramides and lipids were extracted according to the protocol of Bligh and Dryer (25). Ceramides were semi-purified by the silica gel column chromatography and analyzed by HPLC coupled electrospray ionization tandem mass spectrometry. In addition, plasma insulin (Diagnostic Products, Los Angeles, CA) and leptin (Linco Research, St. Charles, MO) were determined by radioimmunoassay. Plasma triglycerides and total cholesterol were assayed by enzymatic analysis on an automated platform (Roche Modular Diagnostics, Indianapolis, IN). Plasma TNF-α was determined by Ultrasensitive ELISA (Biosource International, Camarillo, CA).
Statistics
Statistical analysis was performed using Statview (SAS Institute, NC, USA) and all data are expressed as mean ± S.E.M. A two-way repeated measures analysis of variance (ANOVA) was used to identify main effects of time and group. In the event of a significant time-by-group interaction, Bonferroni post hoc procedures were employed to identify specific differences between group means. Simple regression was used to identify relationships between ceramide subspecies and body composition and insulin sensitivity. To further explore potential predictors of change in insulin sensitivity, backward stepwise multiple regression was employed in two separate analyses. The first model examined the relationship between insulin sensitivity and previously described predictors; the second model examined the relationship of GIR with plasma ceramide subspecies. Statistical significance was accepted if P<0.05.
Results
Subject Characteristics (Table 1)
Table 1.
Subject Characteristics
| Subject Characteristics | NGT | T2D | Baseline T-test | Two-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Pre-Study | Post-Study | Pre-Study | Post-Study | time | timexgroup | ||
|
| |||||||
| Sex | 6F, 8M (n=14) | 5F, 5M (n=10) | - | - | - | ||
| Age, y | 62 ± 2 | 65 ± 2 | 0.25 | - | - | ||
| Weight, kg | 98.1 ± 3.3 | 92.2 ± 2.9 | 102.7 ± 6.4 | 98.0 ± 5.7 | 0.48 | <0.0001 | 0.59 |
| BMI, kg/m2 | 32.7 ± 1.2 | 30.8 ± 1.3 | 36.4 ± 2.6 | 34.7 ± 2.2 | 0.16 | <0.0001 | 0.86 |
| FM, kg | 38.8 ± 2.0 | 34.7 ± 2.7 | 41.7 ± 4.4 | 37.1 ± 4.7 | 0.52 | 0.003 | 0.87 |
| FFM, kg | 59.2 ± 2.6 | 57.5 ± 2.8 | 61.1 ± 2.8 | 60.9 ± 3.3 | 0.65 | 0.35 | 0.42 |
| VO2max, l/min | 2.29 ± 0.14 | 2.57 ± 0.20 | 2.08 ± 0.14 | 2.40 ± 0.17 | 0.15 | 0.0001 | 0.90 |
| TAT, cm2 | 573.3 ± 34.6 | 473.2 ± 38.1 | 571.0 ± 80.6 | 445.4 ± 65.3 | 0.98 | 0.0003 | 0.60 |
| VAT, cm2 | 183.6 ± 13.9 | 132.8 ± 16.7 | 191.7 ± 40.3 | 144.6 ± 33.6 | 0.82 | <0.0001 | 0.79 |
| SAT, cm2 | 389.7 ± 30.8 | 340.5 ± 30.9 | 379.3 ± 69.9 | 300.7 ± 55.9 | 0.88 | 0.007 | 0.48 |
| TG, mg/dl | 183.2 ± 21.1 | 126.4 ± 16.9 | 192.3 ± 35.4 | 161.2 ± 30.1 | 0.82 | 0.0009 | 0.40 |
| Chol, mg/dl | 195.3 ± 9.5 | 165.7 ± 7.7 | 177.8 ± 12.5 | 174.9 ± 11.5 | 0.16 | 0.02 | 0.17 |
| VLDL, mg/dl | 29.5 ± 4.1 | 21.4 ± 2.9 | 33.6 ± 5.2 | 23.9 ± 2.9 | 0.55 | <0.05 | 0.83 |
| LDL, mg/dl | 125.2 ± 7.2 | 107.6 ± 5.6 | 110.0 ± 10.3 | 116.6 ± 11.5 | 0.23 | 0.52 | 0.16 |
| HDL, mg/dl | 40.6 ± 4.2 | 37.4 ± 3.3 | 32.9 ± 2.7 | 29.3 ± 1.9 | 0.22 | 0.35 | 0.95 |
| FSI, μU/ml | 15.5 ± 1.0 | 12.0 ± 0.9 | 25.8 ± 3.4 | 17.6 ± 1.9 | <0.01 | <0.001 | 0.22 |
| Leptin, ng/ml | 23.7 ± 5.7 | 17.5 ± 5.1 | 16.1 ± 3.4 | 13.0 ± 3.4 | 0.33 | 0.02 | 0.41 |
| TNF-α, pg/ml | 2.64 ± 0.28 | 2.98 ± 0.41 | 2.30 ± 0.37 | 2.53 ± 0.60 | 0.48 | 0.42 | 0.87 |
Data represent mean ± S.E.M. NGT, normal glucose tolerant subjects; T2D, type 2 diabetic subjects; BMI, Body mass index; FM, whole body fat mass; FFM, fat-free mass; VO2max, maximal oxygen consumption during maximal exercise; TAT, total abdominal adipose tissue; VAT, abdominal visceral adipose tissue; SAT, abdominal subcutaneous adipose tissue; TG, fasting triglycerides; Chol, fasting cholesterol; VLDL, very low density lipoprotein cholesterol; LDL, low density lipoprotein; HDL, high density lipoprotein, FSI, fasting serum insulin.
At baseline, the groups were matched for age, body composition, aerobic fitness, fasting plasma lipids, leptin and TNF-α (baseline t-test: P>0.05). Exercise compliance was 92±3 and 89±4%, for the NGT and T2D groups, respectively. Following the intervention there were significant decreases in body weight, BMI, whole body fat mass, measures of abdominal adiposity (total, subcutaneous, and visceral), leptin, fasting triglycerides, and fasting cholesterol in all subjects (P<0.05). VO2max was significantly increased following the study (P<0.001). These improvements were identical between subjects with NGT or diabetes (group x time interaction; all P>0.05). Whole body fat-free mass and plasma TNF-α was not influenced by the exercise training intervention (both P>0.05).
Dietary Intake
At baseline, caloric intake in those with NGT or diabetes was 2183±322 and 1753±248 kcal/d with a macronutrient composition of 42±6 and 46±2% carbohydrate, 36±3 and 35±3% fat, and 19±2 and 20±2% protein. During the study, mean caloric intake was 1988±197 and 1660±210 kcal/d for individuals with NGT or diabetes, with a macronutrient composition of 47±2 and 48±5% carbohydrate, 35±3 and 33±4% fat, and 18±2 and 19±2% protein. There were no significant differences in dietary intake between groups (P>0.05).
Insulin Sensitivity
At baseline, subjects with diabetes had elevated fasting plasma glucose concentrations compared to individuals with NGT (120.9±4.5 vs. 96.5±1.4 mg/dl, P<0.0001). Fasting plasma glucose concentrations were significantly reduced after exercise training in those with diabetes (120.9±4.5 vs. 110.2±4.9 mg/dl, P<0.0001) but not in those with NGT (96.5±1.4 vs. 94.1±2.0 mg/dl, P>0.05). Figure 1 displays the changes in insulin sensitivity during the clamps performed before and after the intervention. At baseline, peripheral insulin sensitivity in the group with diabetes tended to be lower than in those with NGT but the difference did not reach statistical significance (P=0.08). Following the intervention, insulin sensitivity increased by 70.8±20.8% in NGT and by 48.0±13.4% in those with diabetes (Figure 1; pre- vs. post-study, P<0.001). The improvements in peripheral insulin sensitivity between the groups were not significantly different (P>0.05), although there was a trend toward less of an increase in the group with T2D.
Fig. 1. Exercise training increases insulin-stimulated glucose disposal.
Twenty four older obese volunteers underwent twelve weeks of aerobic exercise training 1 h/day, 5 d/wk at 80% HRmax. Individuals were classified as either NGT (n=14) or T2D (n=10) following OGTT screening. White bars indicate pre-study (PRE) data; black bars indicate post-study (POST) data. Insulin-stimulated glucose disposal rates (GDR) during 40 mU/m2/min hyperinsulinemic euglycemic clamps were increased in both groups following exercise (***, P<0.001). Error bars represent mean ± S.E.M.
Plasma Ceramide Concentrations
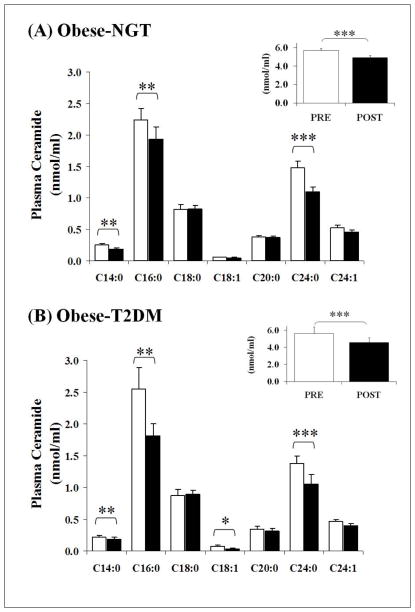
Figure 2, Panel A and B, and Table 2 represent the changes in plasma concentrations of different ceramide subspecies following the twelve-week aerobic exercise training intervention. Total ceramides were close to zero in one of the subjects with NGT and two of the patients with T2D; hence we did not include these data in the final analysis. Our assay could not detect C14:0 and C18:1 in plasma in one additional patient with T2D, hence mean data for these species is representative of 7 subjects. Baseline ceramide concentrations were not different between the groups (Table 2: baseline t-tests, all P>0.05). Total plasma ceramide was reduced by exercise training in both NGT and T2D groups (Figure 2, inset graphs; pre- vs. post-study, P<0.0001). Specifically, exercise training resulted in reductions in C14:0 (P=0.007), C16:0 (P=0.005), C24:0 (P=0.0001), and C24:1 (P=0.08) ceramide subspecies in both groups. In addition, C18:1 ceramide was reduced in the group with diabetes (P=0.02).
Fig. 2. Exercise training lowers fasting plasma ceramide concentrations.
Plasma ceramide concentrations were measured before (white bars) and after (black bars) twelve weeks of aerobic exercise training. Panel (A) illustrates subjects with NGT (N=13); Panel (B) illustrates subjects with T2D (N=8 for total ceramide, and N=7 for the C14:0 and C18:1 subspecies). Total plasma ceramide concentrations were significantly attenuated in both groups (inset graphs; ***, P<0.001). In addition, C14:0, C16:0, and C24:0 ceramide sub-species demonstrated significant decreases in both groups following exercise (**, P<0.01; *, P<0.05). C18:1 ceramide was also suppressed, but only in T2D subjects (*, P<0.05; 2way ANOVA timexgroup interaction, P=0.02). Error bars represent mean ± S.E.M.
Table 2.
Plasma Ceramides
| Plasma Ceramides | NGT | T2DM | Baseline T-test | Two-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Pre-Study | Post-Study | Pre-Study | Post-Study | time | timexgroup | ||
|
| |||||||
| Total, nmol/ml | 5.692 ± 0.199 | 4.892 ± 0.246 | 5.602 ± 0.793 | 4.574 ± 0.579 | 0.45 | <0.05 | 0.79 |
| C14:0, nmol/ml | 0.252 ± 0.023 | 0.180 ± 0.023 | 0.213 ± 0.032 | 0.185 ± 0.027 | 0.33 | 0.007 | 0.20 |
| C16:0, nmol/ml | 2.240 ± 0.186 | 1.940 ± 0.196 | 2.550 ± 0.329 | 1.806 ± 0.193 | 0.39 | 0.005 | 0.19 |
| C18:0, nmol/ml | 0.818 ± 0.078 | 0.831 ± 0.047 | 0.869 ± 0.095 | 0.897 ± 0.064 | 0.69 | 0.70 | 0.89 |
| C18:1, nmol/ml | 0.049 ± 0.005 | 0.046 ± 0.006 | 0.079 ± 0.022 | 0.032 ± 0.012 | 0.09 | 0.02 | 0.04 |
| C20:0, nmol/ml | 0.378 ± 0.029 | 0.373 ± 0.021 | 0.340 ± 0.044 | 0.317 ± 0.042 | 0.46 | 0.35 | 0.52 |
| C24:0, nmol/ml | 1.483 ± 0.109 | 1.102 ± 0.072 | 1.375 ± 0.118 | 1.056 ± 0.148 | 0.64 | 0.0001 | 0.51 |
| C24:1, nmol/ml | 0.526 ± 0.038 | 0.456 ± 0.030 | 0.465 ± 0.033 | 0.399 ± 0.036 | 0.20 | 0.08 | 0.50 |
Data represent mean ± S.E.M. Total and ceramide subspecies were measured in 13 subjects who were obese with NGT and 8 patients who were obese with T2D, with the exception of C14:0 and C18:1, which were quantified for 7 patients with diabetes. NGT, subjects with normal glucose tolerance; T2D, type 2 diabetes.
Regression Analyses
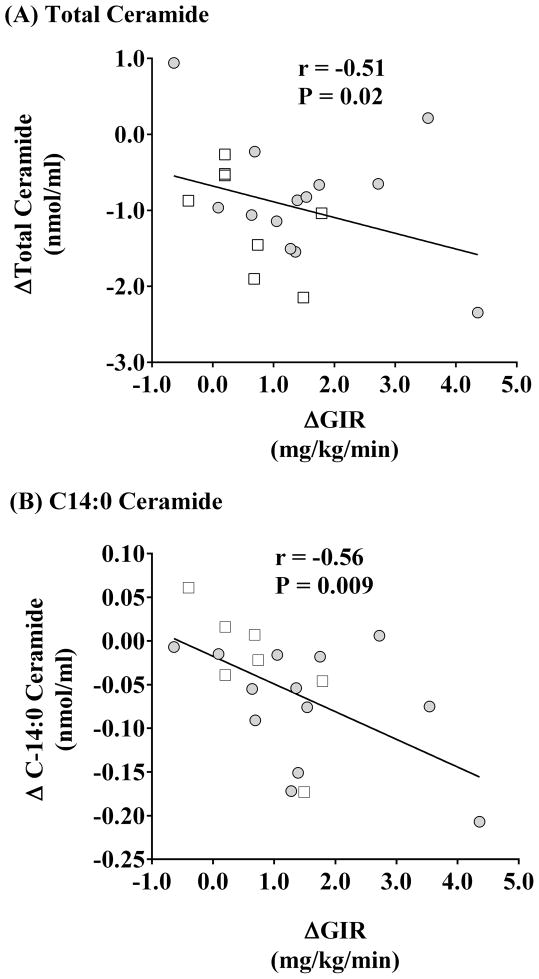
Table 3 shows the relationships between the intervention-induced changes in plasma ceramide concentrations and other metabolic and physiological variables. Reductions in body weight and BMI were significantly related to the decrease in total ceramide (r=0.51, P=0.02) and C14:0 (r=0.58, P=0.006) and C16:0 (r=0.43, P=0.05) ceramide subspecies. Change in whole body adiposity (FM) was also significantly related to total ceramide (r=0.50, P=0.02). Exercise-induced decreases in total abdominal adiposity (TAT) were related to total plasma ceramide (r=0.53, P=0.03) and C14:0 ceramide subspecies (r=0.57, P=0.03). The relationship with the C14:0 subspecies appeared to be largely driven by reductions in visceral abdominal adiposity (VAT; r=0.67, P=0.005). No relationship between VO2max and plasma ceramide was identified, while significant relationships were found between the elevations in rates of insulin-stimulated GIR and the decrease in total and C14:0 ceramide (Figure 3, Panel A and B). Additionally, previously known predictors of insulin resistance (body weight, BMI, FM, VO2max, triglycerides, cholesterol, VAT) and plasma total ceramide were entered into a stepwise multiple regression model. It was demonstrated that decreases in VAT and total ceramide equally contributed to the change in insulin-stimulated GIR, explaining 84% of the variance (P<0.05). An additional analysis to examine the effects of the individual ceramide subspecies on changes in insulin resistance, revealed that change in C14:0 ceramide was the strongest predictor of change in insulin sensitivity, explaining 56% of the variance (P<0.05).
Table 3.
Correlation Matrix between Ceramide Sub-Species and Physiological Variables
| Variable | Total | C14:0 | C16:0 | C18:0 | C18:1 | C20:0 | C24:0 | C24:1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | r | P | r | P | |
| Weight | 0.51 | 0.02 | 0.58 | 0.006 | 0.43 | 0.05 | −0.04 | 0.86 | 0.29 | 0.23 | 0.26 | 0.28 | 0.17 | 0.46 | −0.10 | 0.68 |
| BMI | 0.51 | 0.02 | 0.58 | 0.006 | 0.45 | 0.05 | −0.05 | 0.82 | 0.35 | 0.14 | 0.23 | 0.34 | 0.16 | 0.48 | −0.10 | 0.66 |
| FM | 0.50 | 0.02 | 0.43 | 0.06 | 0.38 | 0.10 | −0.16 | 0.50 | 0.22 | 0.36 | 0.02 | 0.93 | 0.18 | 0.45 | 0.39 | 0.08 |
| TAT | 0.53 | 0.03 | 0.57 | 0.03 | 0.00 | 0.98 | −0.12 | 0.68 | 0.16 | 0.60 | 0.01 | 0.98 | 0.44 | 0.09 | −0.19 | 0.48 |
| SAT | 0.45 | 0.08 | 0.40 | 0.14 | −0.05 | 0.86 | −0.06 | 0.83 | 0.01 | 0.98 | −0.18 | 0.52 | 0.38 | 0.15 | −0.24 | 0.38 |
| VAT | 0.46 | 0.07 | 0.67 | 0.005 | 0.08 | 0.77 | −0.07 | 0.80 | 0.09 | 0.77 | 0.15 | 0.60 | 0.34 | 0.21 | −0.09 | 0.73 |
| VO2max | −0.17 | 0.47 | 0.04 | 0.87 | −0.28 | 0.24 | −0.11 | 0.66 | −0.12 | 0.62 | 0.02 | 0.95 | 0.26 | 0.26 | 0.17 | 0.48 |
| GIR | −0.51 | 0.02 | −0.56 | 0.009 | −0.21 | 0.37 | −0.15 | 0.52 | 0.09 | 0.72 | −0.28 | 0.22 | −0.19 | 0.42 | 0.02 | 0.93 |
Columns represent total fasting plasma ceramide and the specific ceramide subspecies C14:0 through C24:1. BMI = body mass index; FM = whole body fat mass; TAT = total abdominal adiposity; SAT = subcutaneous abdominal adipose; VAT = visceral abdominal adipose; VO2max = maximal aerobic capacity; GIR = mean glucose infusion rate during 40 mU/m2/min hyperinsulinemic clamp. Pearson’s product moment correlation coefficients (r) were calculated, and deemed significant if P<0.05.
Fig. 3. The change in insulin sensitivity is related to the change in fasting plasma ceramides.
Simple regression was used to identify the relationship between the exercise-induced changes (Δ) in insulin-stimulated glucose disposal (GDR) and fasting plasma ceramide concentrations. Analysis revealed that increases in GDR were significantly related to decreases in total (Panel A: r = −0.51, P=0.02) and C14:0 (Panel B: r = −0.56, P=0.009) ceramide. NGT (N=13) and T2D (N=8 for total ceramide, and N=7 for the C14:0 subspecies) subjects are presented by open and black squares, respectively.
Discussion
This study demonstrates that following twelve weeks of aerobic exercise training plasma ceramide concentrations are equally reduced in older obese adults with normal glucose tolerance or diabetes. Further, the exercise-induced decrease in total plasma ceramide levels was related to the improvement in peripheral insulin sensitivity and reductions in whole body and abdominal adiposity. More specifically, the decrease in plasma C14:0 saturated ceramide subspecies following exercise training was related to reductions in body weight and visceral abdominal adiposity, and we have shown that the C14:0 ceramide subspecies is the strongest predictor of change in peripheral insulin sensitivity.
Our previous study demonstrated that obese adults with type 2 diabetes had elevated plasma ceramides when compared to lean healthy controls (10), and that plasma ceramides were inversely related to insulin sensitivity. Our current data show that plasma ceramide levels in older, BMI-matched subjects exhibiting impaired insulin sensitivity are similar to those seen in diabetes. Thus, while plasma ceramides appear to be a good biomarker of insulin resistance in distinctly different sub-groups, they are not sufficiently sensitive to distinguish between groups matched by BMI and age. Nevertheless, plasma ceramides appear to be highly responsive to an exercise-training intervention, and correlate closely to the reversal of insulin resistance. These findings highlight that, despite the clear mechanistic complexity of insulin resistance, ceramides may play a role. This is in agreement with previous findings that, after endurance training in obese humans, both total ceramide content and saturated ceramide species in skeletal muscle are reduced (18, 19, 20, 26). In addition, intramyocellular ceramides have previously been implicated with the impairment of insulin signaling, highlighting the link between ceramide and insulin resistance (5).
A further novel aspect of this study is that following analysis of seven abundant ceramide subspecies, we found that specific reductions in plasma C14:0 ceramide levels were most strongly related to the exercise-induced improvements in insulin sensitivity. This relationship was surprising, because while C16:0 and C24:0 are the most abundant plasma ceramides, C14:0 ceramide levels are lower both in human plasma and tissue. However, it is important to note that dihydroceramide desaturase, a key enzyme in ceramide synthesis, has maximal activity toward C14-dihydroceramide, an immediate precursor for C14:0 ceramide (27). Dihydroceramide desaturase introduces a 4 double bond in the carbon chain of dihydroceramides, which is critical for the biological activity of ceramides. Furthermore, it has been shown that a 14-carbon fatty acid, myristic acid, itself increases the activity of dihydroceramide desaturase 1 isoform in mice through its N-terminal myristoylation (28). Protein myristoylation is an important modification that mediates protein subcellular localization, and the protein-protein and protein-membrane interactions required for the biological interactions of concerned proteins (29). Conditions associated with obesity are characterized by increased availability of palmitate and myristate (30), and analysis of plasma free fatty acids in Japanese patients with diabetes has revealed that, when compared to all other plasma fatty acids, palmitate and myristate (precursors for C14:0 ceramide synthesis) have the strongest correlations with insulin resistance (31). This is also consistent with the findings of a recent study that explored the role of elevated fatty acids and plasma ceramides. Acute lipid infusion in lean human subjects and rats increased plasma ceramide levels (32). Likewise, fatty acid oversupply to HepGe2 cells increased ceramide secretion in dose-dependent manner. Further, lipidomic analysis in subjects with nonalcoholic fatty liver disease (NAFLD), a disease concomitant with insulin resistance, has shown that myristate is one of the most abundant free fatty acids in the liver (30). Therefore, it is possible that increased hepatic palmitate and myristate availability in obesity may stimulate increased synthesis of C14:0 ceramide. Although there is no information about the specific physiological role of C14:0, it is known that ceramides containing different fatty acids play different roles in cell function, growth and survival (33).
The exact source of plasma ceramides in these individuals is not clear. De novo synthesis of ceramides in ER and hydrolysis of sphingomyelin in the Golgi apparatus and the cell membrane appear to be the two major sources of intracellular ceramides. Diet, in the form or a high fat diet has been shown to increase de novo synthesis of ceramides in non-human primates (12). Several lines of evidence also suggest that the liver is the major source of plasma ceramides in animals and humans (34). In a hamster model, de novo synthesis of ceramides in the liver is induced in response to stress and inflammation, and this is paralleled by the increased appearance of ceramides in circulating lipoproteins (35). Further, Wiesner and colleagues have performed very detailed lipid species analysis of lipoprotein fractions where they found that LDL and VLDL are the main ceramide carriers in plasma (36). More recently Boon and colleagues reported that ceramides are packaged with VLDL in the liver and released into the circulation where they target skeletal muscle through internalization in the plasma membrane and downregulation of Akt signaling and insulin-mediated glucose uptake (13). In addition, an LDL-ceramide complex has been shown to activate nuclear factor-κB and initiate increased cytokine production. These cytokines can then target insulin signaling and impair glucose uptake, further exacerbating hyperglycemia and the likelihood of developing diabetes (13, 37). It is conceivable that data in our current study may reflect alterations in ceramide metabolism in the liver.
Lifestyle intervention using exercise training has previously been shown to reduce whole body and abdominal adiposity in line with improvements in insulin sensitivity in obesity (14, 15, 23). Exercise training specifically elevates whole body lipid metabolism (26), via augmentation of skeletal muscle and hepatic lipid oxidation (38). Lipid partitioning plays a key role in the onset of insulin resistance, and exercise training has been shown to improve the lipid profile within skeletal muscle (18, 26) and the liver (38) by reducing lipid moieties known to inhibit insulin signaling events, such as diacylglycerol and ceramide. It is likely that increased lipid utilization following exercise training reduces the availability of substrates required for ceramide synthesis (palmitate, myristate). This, in addition to the reduction in adiposity brought about by an exercise-induced negative energy balance, is a potential mechanism for the suppressed plasma ceramides following our intervention.
The physiological role of plasma ceramides is not well defined. The plasma membrane is not permeable to ceramides containing long-chain fatty acids (39), and no ceramide-binding receptors on the plasma membrane have yet been identified. Therefore, it is unlikely that plasma ceramides exert a direct effect on peripheral insulin sensitivity. However, it has been shown that short-chain ceramides can increase the intracellular content of long-chain ceramides through hydrolysis in extracellular space to sphingosine, and its reacylation with long-chain acyl-CoAs inside the cell (40). Therefore, plasma ceramides may indirectly increase ceramide content in tissues and augment insulin resistance. It is clear that further work is required to understand the mechanisms of plasma ceramide involvement in insulin resistance.
We extend our previous observation that plasma ceramides are elevated in obese adults with type 2 diabetes (10), and now show a direct association between exercise-induced lowering of plasma ceramides and improved peripheral insulin sensitivity in older patients with obesity and type 2 diabetes, and adults who are older, obese but have normal glucose tolerance. The exercise-induced improvement in insulin sensitivity was specifically linked to changes in the C14:0 ceramide subspecies. These data provide support for the idea that ceramides play an important role in insulin resistance and provide novel data showing a link between C14:0 ceramide and improved peripheral insulin sensitivity in obesity and type 2 diabetes.
What is already known about this subject?
Ceramides are implicated as mediators of insulin resistance in skeletal muscle, adipose tissue, and the liver.
Plasma ceramides correlate with the severity of insulin resistance in type 2 diabetes.
Exercise training reduces insulin resistance in obesity and type 2 diabetes.
What does this study add?
Demonstrates that exercise training reduces plasma ceramides in obesity and type 2 diabetes.
Establishes an association between the reduction in plasma ceramides and improved insulin sensitivity.
Highlights a possible role for C14:0 ceramide in insulin resistance.
Acknowledgments
The authors wish to thank the research volunteers for their dedication to the exercise intervention. We also wish to thank the nursing staff of the Clinical Research Unit for their contributions to this work. This study was funded by NIH grants RO1 AG12834 and Diabetes Association of Greater Cleveland 467-R-01 (JPK), GCRC grants MO1 RR00080, and RR018390, and was supported in part by the National Institutes of Health, National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, Ohio.
References
- 1.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell metabolism. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Zierath JR. The path to insulin resistance: paved with ceramides? Cell metabolism. 2007;5:161–163. doi: 10.1016/j.cmet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Consitt LA, Bell JA, Houmard JA. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB life. 2009;61:47–55. doi: 10.1002/iub.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 5.Pickersgill L, Litherland GJ, Greenberg AS, Walker M, Yeaman SJ. Key role for ceramides in mediating insulin resistance in human muscle cells. J Biol Chem. 2007;282:12583–12589. doi: 10.1074/jbc.M611157200. [DOI] [PubMed] [Google Scholar]
- 6.Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59:2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coen PM, Hames KC, Leachman EM, DeLany JP, Ritov VB, Menshikova EV, et al. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring) 2013;21:2362–2371. doi: 10.1002/oby.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blachnio-Zabielska AU, Koutsari C, Tchkonia T, Jensen MD. Sphingolipid content of human adipose tissue: relationship to adiponectin and insulin resistance. Obesity (Silver Spring) 2012;20:2341–2347. doi: 10.1038/oby.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab. 2013;26:995–998. doi: 10.1515/jpem-2012-0407. [DOI] [PubMed] [Google Scholar]
- 12.Brozinick JT, Hawkins E, Hoang Bui H, Kuo MS, Tan B, Kievit P, et al. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. International journal of obesity (2005) 2013;37:1064–1070. doi: 10.1038/ijo.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62:401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O’Carroll SM, et al. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol. 2008;104:1313–1319. doi: 10.1152/japplphysiol.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 16.Houmard JA, Tanner CJ, Yu C, Cunningham PG, Pories WJ, MacDonald KG, et al. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes. 2002;51:2959–2963. doi: 10.2337/diabetes.51.10.2959. [DOI] [PubMed] [Google Scholar]
- 17.Blachnio-Zabielska A, Baranowski M, Zabielski P, Gorski J. Effect of exercise duration on the key pathways of ceramide metabolism in rat skeletal muscles. J Cell Biochem. 2008;105:776–784. doi: 10.1002/jcb.21877. [DOI] [PubMed] [Google Scholar]
- 18.Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJ, et al. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291:E99–E107. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- 19.Helge JW, Dobrzyn A, Saltin B, Gorski J. Exercise and training effects on ceramide metabolism in human skeletal muscle. Experimental physiology. 2004;89:119–127. doi: 10.1113/expphysiol.2003.002605. [DOI] [PubMed] [Google Scholar]
- 20.Amati F, Dube JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devries MC, Samjoo IA, Hamadeh MJ, McCready C, Raha S, Watt MJ, et al. Endurance training modulates intramyocellular lipid compartmentalization and morphology in skeletal muscle of lean and obese women. J Clin Endocrinol Metab. 2013;98:4852–4862. doi: 10.1210/jc.2013-2044. [DOI] [PubMed] [Google Scholar]
- 22.Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151–156. doi: 10.1152/ajpendo.00210.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100:1584–1589. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasumov T, Huang H, Chung YM, Zhang R, McCullough AJ, Kirwan JP. Quantification of ceramide species in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem. 2010;401:154–161. doi: 10.1016/j.ab.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bligh EA, Dyer WJ. A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikami T, Kashiwagi M, Tsuchihashi K, Akino T, Gasa S. Substrate specificity and some other enzymatic properties of dihydroceramide desaturase (ceramide synthase) in fetal rat skin. J Biochem. 1998;123:906–911. doi: 10.1093/oxfordjournals.jbchem.a022023. [DOI] [PubMed] [Google Scholar]
- 28.Beauchamp E, Goenaga D, Le Bloc’h J, Catheline D, Legrand P, Rioux V. Myristic acid increases the activity of dihydroceramide Delta4-desaturase 1 through its N-terminal myristoylation. Biochimie. 2007;89:1553–1561. doi: 10.1016/j.biochi.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Farazi TA, Waksman G, Gordon JI. The biology and enzymology of protein N-myristoylation. J Biol Chem. 2001;276:39501–39504. doi: 10.1074/jbc.R100042200. [DOI] [PubMed] [Google Scholar]
- 30.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology (Baltimore, Md. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 31.Kusunoki M, Tsutsumi K, Nakayama M, Kurokawa T, Nakamura T, Ogawa H, et al. Relationship between serum concentrations of saturated fatty acids and unsaturated fatty acids and the homeostasis model insulin resistance index in Japanese patients with type 2 diabetes mellitus. Journal of Medical Investigation. 2007;54:243–247. doi: 10.2152/jmi.54.243. [DOI] [PubMed] [Google Scholar]
- 32.Watt MJ, Barnett AC, Bruce CR, Schenk S, Horowitz JF, Hoy AJ. Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia. 2012;55:2741–2746. doi: 10.1007/s00125-012-2649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 34.Lightle S, Tosheva R, Lee A, Queen-Baker J, Boyanovsky B, Shedlofsky S, et al. Elevation of ceramide in serum lipoproteins during acute phase response in humans and mice: role of serine-palmitoyl transferase. Arch Biochem Biophys. 2003;419:120–128. doi: 10.1016/j.abb.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Memon RA, Holleran WM, Moser AH, Seki T, Uchida Y, Fuller J, et al. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler Thromb Vasc Biol. 1998;18:1257–1265. doi: 10.1161/01.atv.18.8.1257. [DOI] [PubMed] [Google Scholar]
- 36.Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res. 2009;50:574–585. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Kirwan JP. Plasma ceramides target skeletal muscle in type 2 diabetes. Diabetes. 2013;62:352–354. doi: 10.2337/db12-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 39.Hanada K, Kumagai K, Tomishige N, Kawano M. CERT and intracellular trafficking of ceramide. Biochim Biophys Acta. 2007;1771:644–653. doi: 10.1016/j.bbalip.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, et al. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem. 2002;277:12960–12969. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]