Abstract
α- and β-neurexins are presynaptic cell-adhesion molecules whose general importance for synaptic transmission is well documented. The specific functions of neurexins, however, remain largely unknown because no conditional neurexin knockouts are available, and because targeting all α- and β-neurexins produced by a particular gene is challenging. Using newly-generated constitutive and conditional knockout mice that target all neurexin-3α and -3β isoforms, we here show that neurexin-3 is differentially required for distinct synaptic functions in different brain regions. Specifically, we find that in cultured neurons and acute slices of the hippocampus, presynaptic neurexin-3 mediates trans-synaptic regulation of postsynaptic AMPA-receptors via its extracellular sequences. In cultured neurons and acute slices of the olfactory bulb, however, presynaptic neurexin-3 is selectively required for GABA release by a mechanism involving its intracellular sequences. Thus, our data demonstrate that neurexin-3 performs distinct essential pre- or postsynaptic functions in different brain regions by distinct mechanisms.
Keywords: synaptic cell-adhesion, synapse formation, synaptic transmission, neurexins, AMPA-receptors, olfactory bulb, olfactory circuit
INTRODUCTION
Synapse formation and maintenance require precise coordination between pre- and postsynaptic neurons to produce the properties characteristic of a particular type of synapse. This coordination is thought to be mediated by trans-synaptic cell-adhesion molecules such as neurexins, which are evolutionarily conserved presynaptic proteins that bind to multiple postsynaptic ligands1–4.
Vertebrates express three neurexin genes (Nrxn1, Nrxn2, and Nrxn3 in mice), each of which encodes longer α- and shorter β-neurexins5–7 (Fig. 1a). Human NRXN1 mutations are associated with autism and schizophrenia8–11, and NRXN3 mutations are linked to addiction and obesity12–17. Extensive alternative splicing of neurexins at six canonical sites (SS#1–SS#6) creates thousands of neurexin isoforms18,19 (Fig. 1a). α- and β-neurexins and their various splice variants display a differential regional expression pattern in brain, such that most neurons express multiple α- and β-neurexins in different combinations19,20.
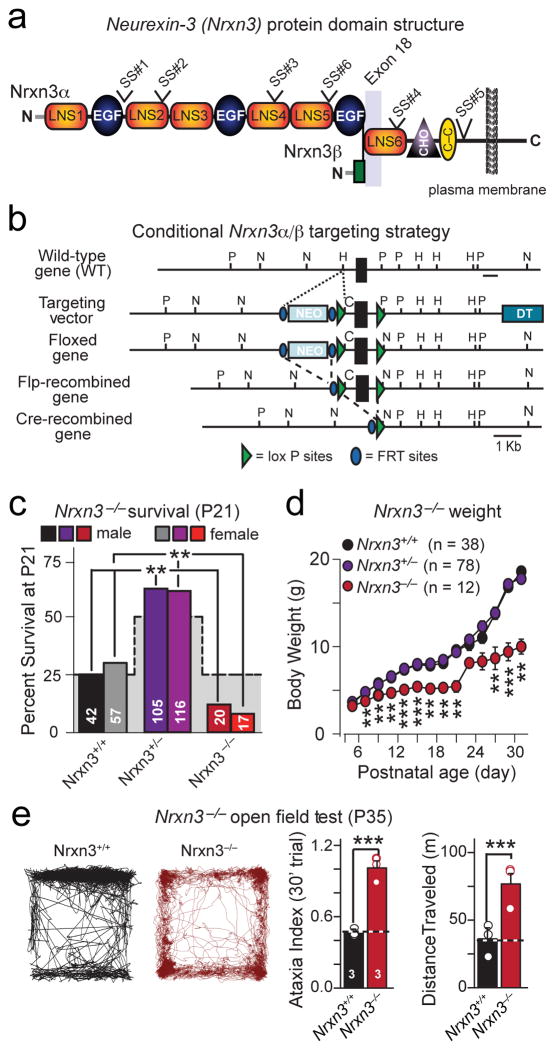
Figure 1. Generation of conditional and constitutive Nrxn3α/β KO mice: Constitutive Nrxn3α/β KO impairs survival.
a, Structure of Nrxn3α and Nrxn3β, with locations of the six canonical sites of alternative splicing (SS#1 to SS#6) and of exon 18. LNS: Laminin G-neurexin-sex hormone binding globulin domain. EGF: Epidermal growth factor-like repeat. CHO: carbohydrate-attachment sequence. C-C: cysteine loop.
b, Targeting strategy for the generation of conditional Nrxn3α/β KO mice in which all Nrxn3α and Nrxn3β forms neurexins are conditionally deleted by cre-recombinase.
c, Survival rates of P21 of male and female offspring from heterozygous constitutive Nrxn3α/β KO mice (stepped background shadow = expected Mendelian ratio; p<0.001).
d, Average body weight of Nrxn3+/+, Nrxn3+/− and Nrxn3−/− mice as a function of age (P5: p = 0.144; P7: p = 0.00375; P9: = 0.003; P11: p = 0.00134; P13: p < 0.0001; P15: p < 0.0001; P17: p = 0.0001; P19: p = 0.000178; P21: p = 0.0151; P23: p = 0.305; P25: p = 0.0807; P27: p = 0.0115; P29: p < 0.0001; P31: p = 0.00999).
e, Distance moved (left) and ataxia indices (right) of P35 Nrxn3α/β KO mice over a 30 min force plate trial (***, p <0.0001).
Data shown in (d and e) are means ± SEMs (numbers in bars = number of mice analyzed. Statistical significance was assessed using the χ2-test comparing the observed to the expected Mendelian distribution (c) or by single-factor ANOVA (d and e). For additional information see supplementary figure 1.
Constitutive KO mice of individual α-neurexins are viable and fertile, whereas double and triple α-neurexin KO mice are lethal, likely due to impaired synaptic function21. Moreover, using knockin (KI) mice expressing Nrxn3 at normal levels but either always with or without an insert in SS#4, we showed that Nrxn3 alternative splicing at SS#4 controls basal and activity-dependent postsynaptic AMPA-receptor (AMPAR) levels in the hippocampus20. However, to date no studies examined the overall functions of all α- and β-neurexins encoded by an individual gene. In this regard it is interesting that invertebrates express only α-neurexin isoforms that also appear to perform essential synaptic functions, although different studies have suggested diverse, and sometimes-contradictory roles22,23.
Here, we generated conditional KO mice ablating all α- and β-forms of Nrxn3, arguably the best studied neurexin isoform20. We examined the Nrxn3α/β KO phenotypes in the hippocampus and olfactory bulb, and directly compared these phenotypes to those of the Nrxn3α/β SS#4 KI and of the Nrxn3α KO mice. Our data reveal that in hippocampus, presynaptic Nrxn3α/β KO phenocopied the Nrxn3α/β SS#4 KI, suggesting that trans-synaptic regulation of postsynaptic AMPARs is the major essential function of Nrxn3 in the hippocampus. In olfactory bulb, by contrast, presynaptic Nrxn3α/β KO revealed an essential role in enabling presynaptic GABA release. Moreover, rescue using Nrxn3 mutants demonstrated that the presynaptic release phenotype in olfactory bulb neurons required intracellular Nrxn3 sequences, whereas the postsynaptic AMPAR-level changes in hippocampal neurons did not. These results suggest that Nrxn3 is differentially essential for distinct functions mediated by diverse mechanisms in different neural circuits; consistent with the notion that differential expression of various neurexin isoforms in different types of neurons shapes the synaptic properties.
RESULTS
Conditional and constitutive Nrxn3α/β KO mice
We used homologous recombination in mouse ES cells to flank the first common exon for Nrxn3α and Nrxn3β (exon 18 [5]) with loxP sites (Fig. 1b). Following the deletion of exon 18 by cre recombinase, the resulting mRNA contains a frame-shift in the sixth LNS-domain. If the mutant mRNAs were sufficiently stable to still produce protein, such protein would be C-terminally misfolded and lack a membrane anchor, rendering it non-functional. Thus, we refer to the floxed Nrxn3 allele as a conditional KO (cKO) allele.
When we converted the conditional Nrxn3α/β KO mice into constitutive Nrxn3α/β KO mice by cre-mediated germline recombination, we found that most homozygous Nrxn3α/β KO mice died at birth (Fig. 1c). Surviving homozygous constitutive Nrxn3α/β KO mice were runted and exhibited ataxia and hyperactivity when examined on a force plate actometer24 (Figs. 1d & 1e). However, no major changes in protein composition were noted (Supplementary Figs. 1a and 1b). Thus, constitutive Nrxn3α/β KO mice exhibit a significant overall phenotype that is more severe than that of Nrxn3α KO and Nrxn3α/β SS#4 KI mice20,21, prompting us to explore the consequences of Nrxn3α/β ablation on synaptic function.
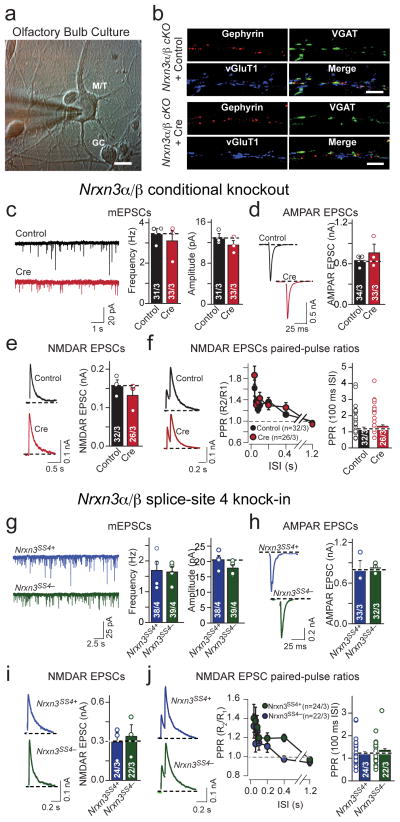
Deletion of Nrxn3α/β but not of Nrxn3α alone in cultured hippocampal neurons selectively impairs AMPAR-mediated synaptic transmission
To analyze Nrxn3α/β function in precisely matching mutant and control neurons while avoiding the lethality of constitutive Nrxn3α/β KO mice, we cultured hippocampal neurons from newborn homozygous Nrxn3α/β cKO mice. We infected the neurons with lentiviruses expressing inactive (control) or active cre-recombinase (cre) fused to EGFP, and examined the neurons by whole-cell patch-clamp recordings two weeks later.
We first measured spontaneous ‘minis’ (miniature excitatory and inhibitory postsynaptic currents [mEPSCs and mIPSCs, respectively]; Figs. 2a–d). We found that the Nrxn3α/β deletion did not change mEPSC and mIPSC mini frequencies, but produced a dramatic decrease in mEPSC amplitude, while the mIPSC amplitude was unaffected. The loss of Nrxn3α/β did not alter passive membrane properties or mEPSC and mIPSC kinetics (Supplementary Figs. 2a–c). The selective decrease in mEPSC amplitude in Nrxn3α/β cKO neurons mirrors the phenotype we previously observed in hippocampal Nrxn3α/β SS#4 KI neurons which express only the Nrxn3α/β SS#4 variant containing an insert in SS#420.
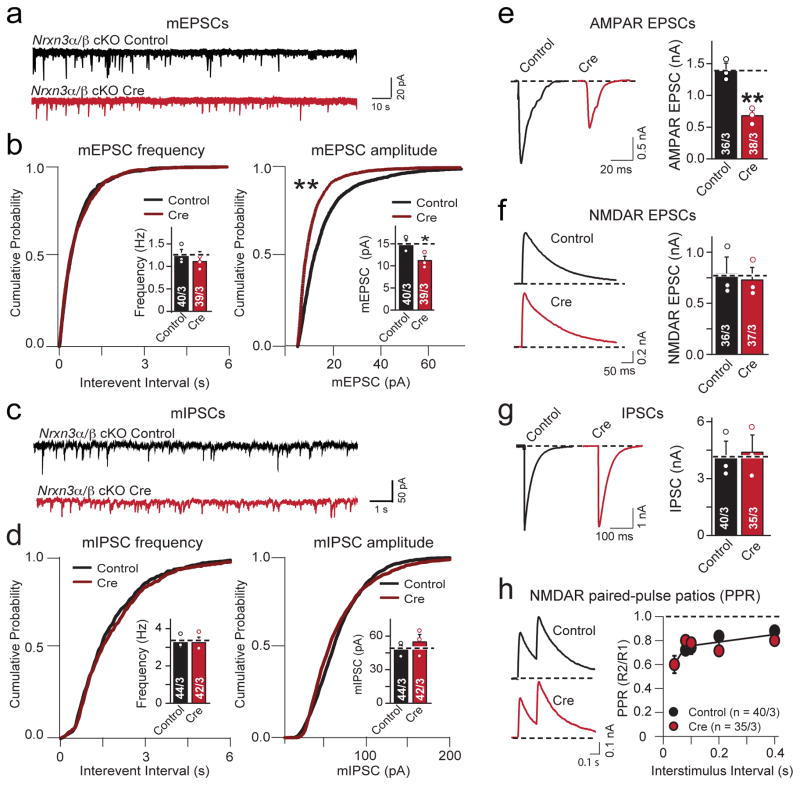
Figure 2. Conditional Nrxn3α/β KO selectively decreases AMPAR-mediated synaptic transmission in cultured hippocampal neurons.
a, Representative traces of mEPSCs recorded from cultured hippocampal pyramidal neurons from homozygous Nrxn3α/β cKO mice in 1 μM tetrodotoxin and 100 μM picrotoxin. Neurons were infected with lentiviruses expressing inactive (control) or active cre-recombinase (cre) at 4–5 days in vitro (DIV), and analyzed at 14–16 DIV.
b, Cumulative distribution plots of the mEPSC frequency and amplitude in hippocampal Nrxn3α/β cKO neurons infected with lentiviruses expressing inactive (control) or active cre-recombinase (cre) (mEPSC frequency cumulative probability: p = 0.972, frequency: p = 0.128; amplitude cumulative probability: p < 0.0001, mEPSC amplitude bar graph: p = 0.0216).
c & d, Same as in a & b, but analyzing mIPSCs (mIPSC frequency cumulative probability: p = 0.481, frequency: p = 0.879; amplitude cumulative probability: p = 0.392, amplitude: p = 0.467).
e–g, Evoked synaptic responses in control and Nrxn3α/β cKO neurons. Panels show representative traces (left) and summary graphs of the response amplitudes (right) for AMPAR- (e; p = 0.0036) and NMDAR-mediated EPSCs (f; p = 0.752) and for GABA-receptor-mediated IPSCs (g; p = 0.821).
h, Paired-pulse ratio measurements of NMDAR-mediated EPSCs demonstrate that there is no change in release probability in Nrxn3α/β deficient neurons (80 ms ISI: p = 0.969)
Data shown are means ± SEM; numbers of cells/independent cultures analyzed are indicated. Statistical significance was assessed by K-S test for cumulative probability plots, (b and d) and single-factor ANOVA. For more information, please supplementary figure 2.
We next examined the effect of the conditional Nrxn3α/β KO on evoked synaptic transmission. We observed a specific and selective ~50% decrease in AMPAR- but not in NMDAR-mediated excitatory responses or in GABAergic inhibitory responses (Figs. 2e–2g). The reduction in mEPSC amplitude and selective decrease in AMPAR- but not NMDAR-mediated synaptic transmission indicates that the Nrxn3α/β cKO impairs postsynaptic AMPAR levels but does not alter presynaptic glutamate release. To test if Nrxn3α/β cKO regulates presynaptic release probability at excitatory synapses, we measured the paired-pulse ratio of NMDAR-mediated EPSCs and the progressive, activity-dependent, blockade of NMDAR-mediated EPSCs induced by the open-channel blocker MK-801 (Fig. 2h, Supplementary Fig. 2d). Both approaches provide sensitive but indirect assessments of the presynaptic release probability25–28, but revealed no changes induced by acute deletion of Nrxn3α/β.
Does Nrxn3α alone reproduce the Nrxn3α/β cKO phenotype? We cultured hippocampal neurons from littermate Nrxn3α+/+ and constitutive Nrxn3α−/− mice. We detected no significant change in passive membrane properties or mEPSC amplitude or frequency (Supplementary Fig. 2e & 2f). Moreover, evoked EPSCs mediated by AMPARs or NMDARs were unaltered by the constitutive Nrxn3α KO, as were paired-pulse ratios of NMDAR-mediated EPSCs (Supplementary Figs. 2g–i). Thus, Nrxn3β must have the capacity to functionally compensate for the loss of Nrxn3α in the hippocampus of Nrxn3α KO mice.
Nrxn3α/β deletion decreases surface AMPAR levels and enhances GluA1 internalization
The unexpected similarity in phenotypes between the Nrxn3α/β KO and the Nrxn3α/β SS#4 KI prompted us to ask whether these electrophysiological phenotypes are mediated by the same biological mechanisms.
To address this question, we live surface labeled Nrxn3α/β cKO neurons infected with inactive or active cre-recombinase using antibodies against the extracellular N-terminus of GluA1. We then fixed and permeabilized the GluA1 labeled neurons, and immunostained them for the excitatory pre- and postsynaptic markers vGluT1 and PSD-95, respectively (Figs. 3a–d, Supplementary Figs. 3a–c). The puncta densities representing GluA1, vGluT1 or PSD95 were unchanged between control and Nrxn3α/β-deficient neurons, consistent with a normal synapse density (Fig. 3b). However, GluA1-immunoreactive surface puncta sizes were reduced ~50% in Nrxn3α/β KO neurons, whereas vGluT1- and PSD95-positive puncta sizes were unchanged. as were the intensities (signal strength per unit area) of GluA1-, vGluT1- or PSD95-positive puncta (Figs. 3c & 3d, Supplementary Figs. 3a & 3b). In parallel experiments, we quantified the surface levels of GluA2, and observed a ~50% reduction in surface puncta size without significant changes in puncta density or intensity (Figs. 3a–3d, Supplementary Figs. 3a–c) Thus, total surface levels of AMPARs are selectively decreased in Nrxn3α/β-deficient neurons compared to control neurons. This specific reduction in GluA1 and GluA2 surface immunoreactivity is likely not due to a reduction in total AMPAR levels because the total protein levels of GluA1 and GluA2 in constitutive Nrxn3α/β KO animals was not detectably changed (Supplementary Figs. 1a & 1b).
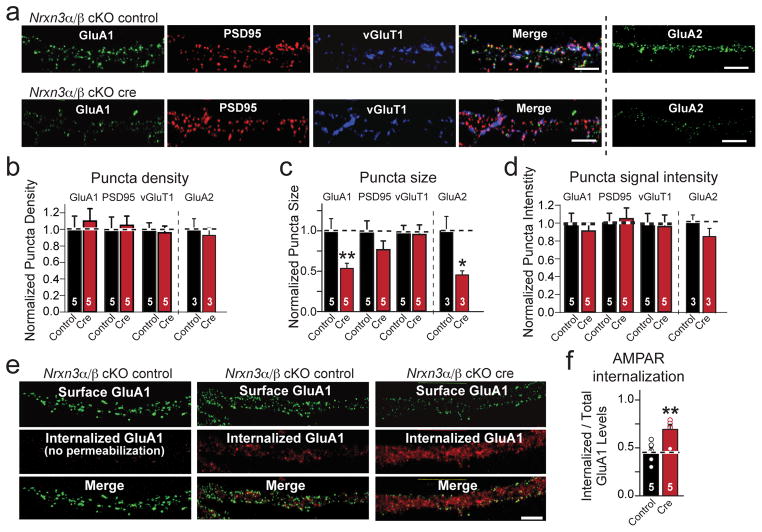
Figure 3. Conditional Nrxn3α/β KO destabilizes postsynaptic AMPAR-levels.
a, Representative immunofluorescence images of dendritic surface-exposed AMPA-receptor subunits GluA1 and GluA2 and of synaptic markers vGluT1 and PSD95 in control and Nrxn3α/β cKO neurons (scale bar = 5 μm).
b–d, Density (b), size (c), and intensity (d) of GluA1-, GluA2-, PSD95-, and vGluT1-containing synapses in control and Nrxn3α/β cKO neurons (Density: GluA1, p = 0.698; GluA2, p = 0.631; PSD95, p = 0.818; vGluT1, p = 0.758. Size: GluA1, p = 0.018; GluA2, p = 0.0387, PSD95, p = 0.186; vGluT1, p = 0.421. Intensity: GluA1, p = 0.579; GluA2, p = 0.267; PSD95, p = 0.821; vGluT1, p = 0.796).
e–f, Representative images (e) and summary graph (f) of GluR1 internalization experiments in which control and Nrxn3α/β KO neurons were incubated with GluA1 antibodies for 5 min, chased for 20 min, and then the surface-exposed and internalized GluR1 was labeled with two different secondary antibodies. Control images on the left are from neurons without permeabilization to ensure that surface-exposed and internalized GluA1 can be cleanly differentiated (scale bar = 5 μm). Internalization is quantified (f) as the ratio of internalized to total GluA1 (**, p = 0.0034).
Data in b–d and f are means ± SEM (n=5 cultures); statistical significance was evaluated by single factor ANOVA. Please see supplementary figure 3 for absolute quantification of puncta parameters.
To test how acute deletion of Nrxn3α/β decreases postsynaptic surface AMPAR levels, we measured basal GluA1 internalization20,39. We incubated Nrxn3α/β cKO neurons expressing inactive or active cre-recombinase with an antibody raised against the extracellular N-terminus of GluA1 for 5 min, followed by a 15–20 min chase to allow partial internalization of surface-labeled GluA1. We fixed the neurons without permeabilization, and labeled them with an Alexa-488 (green) conjugated secondary antibody to detect the uninternalized population of surface GluA1, and subsequently permeabilized the neurons and labeled them with a different Alexa-546 (red) conjugated secondary antibody to detect the internalized fraction of GluA1. As a control, non-permeabilized neurons displayed no red immunofluorescence signal, indicating that the first secondary antibody, AlexaFluor488, completely saturated the surface population of labeled GluA1 (Fig. 3e).
We found that the Nrxn3α/β KO increased the GluA1 internalization (measured as the internalized/total fluorescence) by ~60% relative to control neurons (Figs. 3e & 3f; Supplementary Fig. 3d). Thus, the genetic ablation of Nrxn3α/β destabilizes the surface levels of postsynaptic GluA1-containing AMPARs presumably via a trans-synaptic interaction, independent of excitatory synapse formation.
This phenotype is strikingly similar to that of Nrxn3α/β KI neurons constitutively expressing Nrxn3α/βSS#4+, suggesting that the primary, non-redundant Nrxn3α/β function in hippocampus is to maintain normal postsynaptic AMPAR levels, which is critically dependent on the expression of the Nrxn3α/βSS#4− isoform.
Both α- and β-neurexins rescue hippocampal Nrxn3α/β KO phenotype
We next asked whether the decrease in postsynaptic AMPAR levels in Nrxn3α/β KO neurons reflects a general function of neurexins that depends on SS#4, and whether this activity is specific for Nrxn3α or Nrxn3β. To address these questions, we performed rescue experiments in cultured hippocampal neurons.
Both Nrxn3α and Nrxn3β rescued the Nrxn3α/β KO phenotype, but only when they lacked an insert at SS#4 (Figs. 4a & b). To define the minimal properties of Nrxn3 required to mediate postsynaptic AMPAR function, we attempted to rescue the phenotype with mutant Nrxn3. The extracellular domain of Nrxn3α without the splice-site #4 insert, anchored to the membrane via a GPI anchor was sufficient to restore AMPAR-mediated transmission to wild-type levels (Fig. 4c). Whereas the GPI anchored Nrxn3α SS#4− extracellular domain succeeded in stabilizing AMPAR transmission, a soluble Nrxn3β variant – resulting from a SS#5 insert containing an early stop codon5 – failed to rescue the Nrxn3α/β KO phenotype (Fig. 4d, Supplementary Fig. 4a). We found that Nrxn1β and Nrxn2β without an insert in SS#4 also rescued the Nrxn3α/β KO phenotype, demonstrating the potential for splice-site #4 dependent functional redundancy among neurexins (Fig. 4e). Moreover, when the Nrxn1β transmembrane region and cytoplasmic tail were exchanged for those of the unrelated PDGF receptor was still fully capable of rescue (Fig. 4f, Supplementary Fig. 4b). These experiments define the fundamental neurexin characteristics necessary for controlling postsynaptic AMPARs, demonstrating that neurexins lacking an insert at SS#4 can redundantly rescue the conditional Nrxn3α/β KO phenotype without requiring their intracellular sequences, suggesting that neurexin-mediated trans-synaptic interactions stabilize postsynaptic AMPARs in cultured hippocampal neurons independent of neurexin-mediated intracellular signaling.
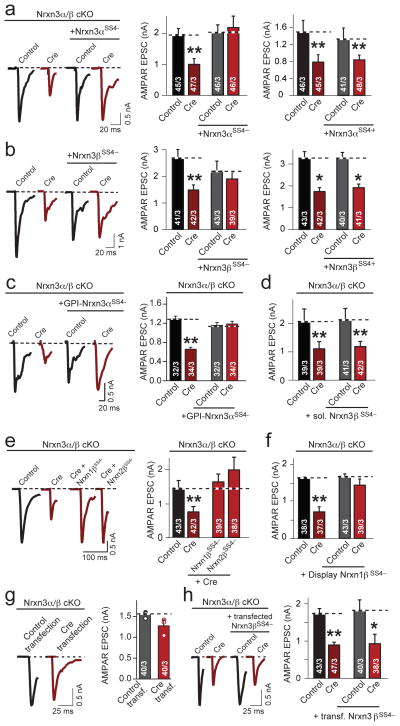
Figure 4. Rescue of AMPAR-mediated EPSCs in cultured hippocampal Nrxn3α/β cKO neurons by pre-synaptic neurexins lacking an insert in SS#4.
a–f, Summary data of AMPAR-mediated EPSC rescue experiments, performed as in Figure 2a and co-infected with lentivirus expressing the following:
a, Nrxn3αSS4− (cre: p = 0.0014; cre + Nrxn3αSS4−: p = 0.962) or Nrxn3αSS4+ (cre: p = 0.00836; cre + Nrxn3αSS4+: p = 0.00450).
b, Nrxn3β SS4− (cre: p = 0.00785; cre + Nrxn3βSS4−: p = 0.647) or Nrxn3βSS4− (cre: p = 0.0138; cre + Nrxn3βSS4+: p = 0.0118).
c, GPI-Nrxn3αSS4− (cre: p = 0.0009; cre + GPI-Nrxn3αSS4−: p = 0.742).
d, Endogenous soluble form of Nrxn3βSS4− (cre: p = 0.003; cre + sNrxn3βSS4−: p = 0.007).
e, Nrxn1βSS4− and Nrxn2βSS4− (cre: p = 0.0025; cre + Nrxn1βSS4−: p = 0.717; cre + Nrxn2βSS4−: p = 0.697).
f, Nrxn1βSS4− fused to the PDGF receptor (cre: p = 0.0073; cre + Nrxn1βSS4−-PDGFR: p = 0.535).
g, Traces and summary graph of AMPAR-mediated EPSCs measured in GFP-positive Nrxn3α/β cKO neurons sparsely co-transfected with inactive (control) or active cre-recombinase (cre) and EGFP (p = 0.100).
h, Traces and summary graph of AMPAR-mediated EPSCs measured in GFP+ Nrxn3α/β cKO neurons, infected with lentivirus (as in (a)), and sparsely co-transfected with EGFP and Nrxn3βSS4−,plasmids (**, p = 0.00112; *, p = 0.0186).
Data shown are means ± SEMs; numbers in bars: cells/independent cultures analyzed. Statistical significance was assessed using single-factor ANOVA. For more information, please see supplementary figure 4.
Presynaptic Nrxn3α/β SS#4− acts trans-synaptically on AMPARs by binding to a postsynaptic neurexin ligand, likely LRRTM220. To assess if the same mechanism applies for the Nrxn3α/β KO phenotype, we took two separate approaches. First, we tested whether postsynaptic deletion of Nrxn3α/β in isolated neurons produced the same phenotype as the Nrxn3α/β deletion in all neurons. We sparsely transfected primary hippocampal cultures from Nrxn3α/β cKO mice with EGFP fused to inactive (control) or active cre-recombinase (cre), which exhibit strict nuclear localization, and performed whole-cell patch-clamp recordings from neurons with EGFP+ nuclei, but found no change in AMPAR-mediated synaptic responses (Fig. 4g). Second, we infected all Nrxn3α/β cKO neurons in a culture dish with lentiviruses expressing inactive or active cre-recombinase, and then asked if sparse postsynaptic co-transfection of Nrxn3β without the SS#4 insert and cytoplasmic EGFP could rescue the Nrxn3α/β KO phenotype. We identified transfected versus untransfected neurons by intense cytoplasmic EGFP fluorescence and found that postsynaptic rescue did not restore AMPAR-mediated synaptic responses (Fig. 4h). Together, these experiments support the notion that presynaptic Nrxn3 is required to stabilize postsynaptic AMPA-receptors and to enable AMPAR-mediated synaptic transmission.
Our current findings, together with previous data on Nrxn3α/β SS#4 KI neurons20, thus suggest that Nrxn3SS4− isoforms perform a dominant and essential active function in retaining postsynaptic AMPARs in hippocampal cultures. This function is lost either when only Nrxn3SS4+ is expressed, or when Nrxn3α/β is deleted entirely. Furthermore, our findings suggest that extracellular Nrxn3 sequences, anchored to the presynaptic plasma membrane and lacking an insert at SS#4, are necessary and sufficient to trans-synaptically stabilize postsynaptic AMPARs. However, all of these observations were made in vitro in developing cultured hippocampal neurons, prompting us to ask whether Nrxn3α/β performs a similar function in adult hippocampal neurons in vivo.
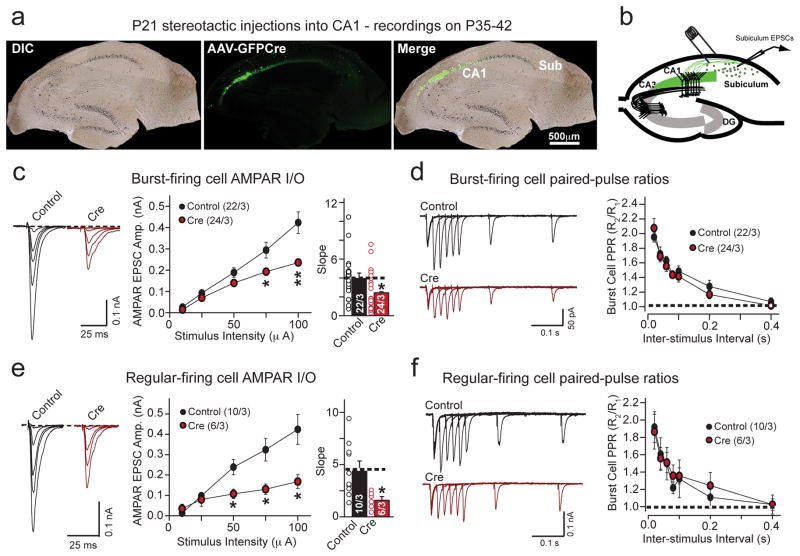
Presynaptic deletion of Nrxn3α/β in vivo at CA1-subiculum synapses impairs postsynaptic AMPAR-mediated responses
We stereotactically injected adeno-associated viruses (AAVs) expressing active or inactive Cre-recombinase fused to EGFP into the CA1 region of Nrxn3α/β cKO mice at P21 and analyzed AMPAR-mediate transmission at CA1-subiculum synapses on P35-42. We first confirmed that our injection coordinates largely confined the AAV infection to the CA1 region with minimal spillover into the adjacent subiculum (Fig. 5a). Using horizontally cut slices (300 μm thickness), we stimulated CA1 afferents with an extracellular stimulating electrode in the stratum oriens/alveus at the CA1-subiculum border, and recorded from uninfected postsynaptic subicular neurons (Fig. 5b). The subiculum is populated by two types of pyramidal neurons, identified by their intrinsic firing properties as burst- and regular-firing neurons (Supplementary Fig. 5a and b). We observed a significant reduction in AMPAR input-output relationship without a change in presynaptic release probability or intrinsic membrane properties in both types of neurons (Figs. 5c–f; Supplementary Figs. 5c & d). These results confirm that the primary role of presynaptic Nrxn3α/β in the hippocampus in vivo is to stabilize postsynaptic AMPARs at mature synapses.
Figure 5. Conditional in vivo ablation of Nrxn3α/β in presynaptic hippocampal CA1-region neurons impairs postsynaptic AMPAR-mediated responses in the subiculum.
a, Hippocampal brain sections of P35 male animals illustrating complete targeted infection of the CA1-region without significant infection of the subiculum, following the stereotactic injection of AAVs expressing EGFP-tagged inactive (control) or active (cre) cre-recombinase at P21 (scale bar: 500 μm).
b, Schematic illustrating the electrophysiological recording configuration to selectively evoke AMPAR-mediated EPSCs at CA1-subiculum synapses.
c & e, Input-output representative traces (left), summary graph (middle) and slope (right) for AMPAR EPSCs measured in burst spiking (c; 10μA: p = 0.0728; 25μA: p = 0.177; 50μA: p = 0.122; 75μA: p = 0.0198; 100μA: p = 0.00748 and slope: p = 0.0362) or regular spiking (e; 10μA: p = 0.132; 25μA: p = 0.188; 50μA: p = 0.0248, 75μA: 0.0349, 100μA: 0.035 and slope: p = 0.0172) neurons from mice prepared as in (a).
d & f, Paired-pulse ratio measurements from burst spiking (d; 60ms ISI: p = 0.422) or regular spiking (f; 60ms ISI: p = 0.954) subicular neurons in mice injected with inactive (control) or active (cre) cre-recombinase in region CA1. Representative traces (left) and summary graph (right) are shown.
Data are means ± SEMs; means were calculated from the total number of cells. Numbers in bars: cell/animal. Statistical significance was assessed using single-factor ANOVA. For more information on subicular neuron identification and passive membrane properties of each cell type, see supplementary figure 5.
Nrxn3 is required for presynaptic GABA release in olfactory bulb neurons independent of SS#4
Our results thus far reveal only one essential function of Nrxn3α/β in hippocampal neurons: to trans-synaptically maintain postsynaptic AMPAR levels. These results do not rule out SS#4-independent Nrxn3α/β functions that are otherwise masked in hippocampal neurons due to redundant expression of other neurexins,. Since neurexins are known to be differentially expressed in brain regions19,20, we reasoned that it may be possible to unmask other Nrxn3α/β functions by analyzing other types of neurons in which compensating neurexin expression may be reduced. We focused on the olfactory bulb because previous studies found that olfactory bulb neurons express Nrxn3αSS4+ at particularly high levels19,20.
We cultured olfactory bulb neurons from homozygous Nrxn3α/β cKO mice and infected the neurons with lentiviruses encoding inactive (control) or active cre-recombinase (cre) (Fig. 6a). Nrxn3α/β-deficient olfactory bulb neurons did not exhibit changes in excitatory or inhibitory synapse morphology (Fig. 6b; Supplementary Fig. 6a – c). We next patched large mitral/tufted cells and measured mEPSC frequency and amplitude. Surprisingly in view of the results in hippocampus (Fig. 2), we detected no change (Fig. 6c, Supplementary Figs. 6d & e). We also examined evoked AMPAR- and NMDAR-mediated EPSCs as well as the excitatory presynaptic release probability, and observed no change in Nrxn3α/β cKO neurons (Fig. 6d – f). Thus, in olfactory bulb synapses Nrxn3α/β is not essential for excitatory synaptic transmission.
Figure 6. Different from the hippocampus Nrxn3α/β is not required for AMPAR-mediated synaptic responses in the olfactory bulb.
a, Representative image of DIV 14 cultured olfactory bulb neurons. Note the difference in soma size that was used to identify neuron subtypes (M/T: mitral/tufted cell; GC: granule cell; scale bar: 10 μm).
b, Representative images of excitatory and inhibitory synapse density immunostained for inhibitory (vGAT and gephyrin) and excitatory (vGluT1) markers in cultured Nrxn3α/β cKO olfactory bulb neurons infected with lentiviruses expressing EGFP-tagged inactive (control) or active (cre) cre-recombinase. n = 3 experiments; scale bar: 5 μm.
c–f, Sample traces and summary graphs of mEPSCs (c; frequency: p = 0.603; amplitude: p = 0.276), AMPAR EPSCs (d; p = 0.469), NMDAR EPSCs (e; p = 0.537) and NMDAR paired-pulse ratios (f; 100ms ISI: p = 0.300) recorded from Nrxn3α/β cKO mitral/tufted olfactory bulb neurons, cultured as in (b).
g–j, Sample traces and summary graphs of mEPSCs (g; frequency: p = 0.883; amplitude: p = 0.181), evoked AMPAR EPSCs (h; p = 0.881), evoked NMDAR EPSCs (i; p = 0.721) and NMDAR-EPSC paired-pulse ratios (j; 100ms ISI: 0.807) recorded from Nrxn3α/β SS#4 KI M/T olfactory bulb neurons infected with lentiviruses expressing EGFP-tagged inactive (Nrxn3α/βSS4+) or active (Nrxn3α/βSS4−) cre-recombinase.
Data are means ± SEM. Bar graphs: cells/ independent culture experiments. Means and SEMs: from three independent experiments except for figures f and j, where the number of cells was. Statistical significance was determined by single-factor ANOVA. For more information, please see supplemental figure 6.
The lack of an effect of the Nrxn3α/β cKO on AMPARs in olfactory bulb neurons prompted us to ask whether the previously observed correlation between Nrxn3α/β function and alternative splicing at SS#4 in Nrxn3α/β also applies to the olfactory bulb. We examined if the experimental manipulation of Nrxn3 SS#4 would result in an excitatory transmission phenotype in olfactory bulb neurons, but detected none (Figs. 6g – j, Supplementary Figs. 6f & g). This result appears to confirm the correlation between the functions of Nrxn3α/β and its alternative splicing at SS#4.
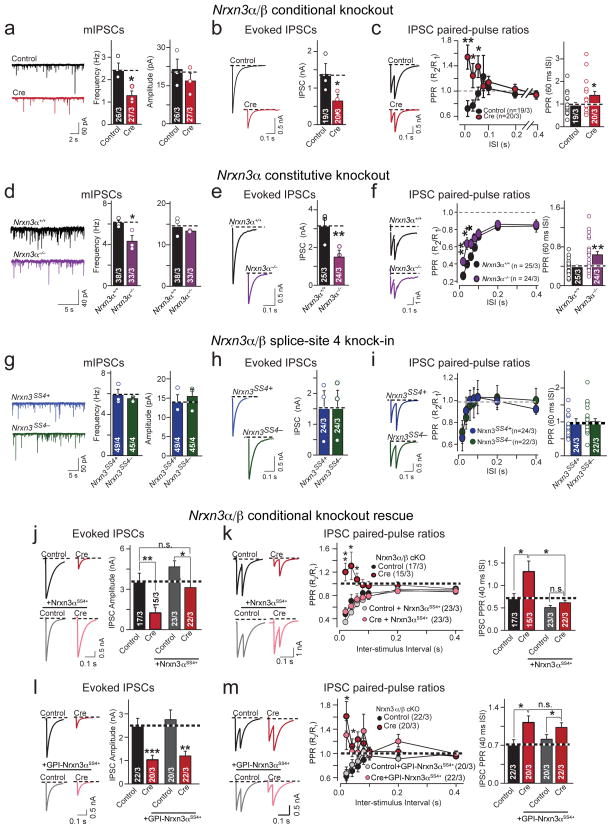
We next tested inhibitory synaptic transmission. In contrast to the hippocampus, we observed a marked reduction in mIPSC frequency (~40%), but not in mIPSC amplitude or mIPSC kinetics in Nrxn3α/β-deficient olfactory bulb neurons (Fig. 7a, Supplementary Figs. 7a & b). We detected a large reduction (~60%) in the amplitude of evoked IPSCs in Nrxn3α/β KO neurons (Fig. 7b). The decreased mIPSC frequency and evoked IPSC amplitude in Nrxn3α/β KO neurons could be explained by a smaller readily-releasable pool (RRP) of GABA-containing synaptic vesicles, or by impaired coupling of an action potential to GABA release. To test these hypotheses, we first measured IPSCs evoked by an application of 0.5 M hypertonic sucrose, which monitors the size of the RRP of synaptic vesicles28, but observed no change in RRP size (Supplementary Fig. 7c). However, the conditional ablation of Nrxn3α/β resulted a large increase in IPSC PPR, consistent with a decrease in release probability (Fig 7c). These data suggest that Nrxn3α/β deletion decreases GABAergic transmission in cultured olfactory bulb neurons – in which granule cells make up the vast majority of GABAergic neurons – by decreasing the efficacy with which an action potential triggers GABA release from granule cells.
Figure 7. Nrxn3 is essential for presynaptic GABA release in olfactory bulb neurons by an SS#4-independent mechanism.
a–c, Sample traces and summary graphs for mIPSCs (a; frequency: p = 0.039; amplitude: 0.419), evoked IPSCs (b; p = 0.024) and IPSC PPRs (c; p = 0.00264 (20ms ISI), 0.0365 (40ms), 0.450 (60ms)), recorded from mitral/tufted cells in cultured Nrxn3α/β cKO olfactory bulb neurons infected with lentiviruses expressing EGFP-tagged inactive (control) or active (cre) cre-recombinase
d–f, Same as (a–c), but measured in cultured Nrxn3α+/+ and Nrxn3α−/− olfactory bulb neurons. (d: frequency: p = 0.0435; amplitude: p = 0.367; e: p = 0.0005; f: p = 0.00805 (20ms ISI), 0.00744 (40ms), 0.0327 (60ms)).
g–i, Same as (a–c), but measured in cultured Nrxn3α/β SS#4 KI olfactory bulb neurons (g: frequency: p = 0.536, amplitude: p = 0.614; h: p = 0.983; i: 60 ms ISI: p = 0.130). Neurons were infected with lentiviruses as in Fig. 6g20.
j & k, Rescue of the GABAergic transmission in Nrxn3α/β cKO neurons by full-length Nrxn3αSS4+. Evoked IPSC (j; cre: p = 0.0034; cre + Nrxn3αSS4+: p = 0.0217) and IPSC PPRs (k; cre: p = 0.000515 (20ms ISI), 0.0363 (40ms), 0.02891 (60ms), cre + Nrxn3αSS4+: p = 0.0334 (20ms), 0.128 (40ms), 0.284 (60ms)) in mitral/tufted cells as in Fig. 7c, with or without full-length Nrxn3αSS4+.
l & m, Same as in (j) and (k) but with a GPI-anchored Nrxn3αSS4+. Evoked IPSCs (l; cre: p < 0.001; cre + GPI-Nrxn3αSS4+: p = 0.00165) and IPSC PPRs (m; cre: p = 0.0006 (20ms ISI), 0.0107 (40ms), 0.0055 (60ms); cre + GPI-Nrxn3αSS4+: p = 0.0231 (20ms), 0.0213 (40ms), ISI: 0.219 (60ms).
Data shown are means ± SEMs; the number of cells/independent cultures analyzed are indicated in the bars. Statistical analyses were calculated from the number of independent cultures (for a, d and g) and from cell number (b, e, h, j–m). Statistical significance was assessed using single-factor ANOVA. For more information, see supplementary figure 7.
Does Nrxn3α/β function in GABA release from olfactory bulb neurons depend on both Nrxn3α and Nrxn3β, and is it regulated by Nrxn3α/β alternative splicing at SS#4 similar to the Nrxn3α/β function in stabilizing postsynaptic AMPARs in hippocampal neurons? To address these questions, we analyzed inhibitory synaptic transmission in olfactory bulb neurons (1) cultured from littermate Nrxn3α+/+ or Nrxn3α−/− mice, and (2) cultured from homozygous Nrxn3α/β SS#4 KI mice, infected with lentiviruses expressing inactive (which retains the SS#4+ genotype) or active cre-recombinase (which generates the SS#4− genotype).
The constitutive ablation of Nrxn3α phenocopied the GABA release phenotype observed in Nrxn3α/β cKO cultured olfactory bulb neurons, suggesting that only Nrxn3α is essential for GABA release in granule cell neurons (Fig. 7d–f, Supplementary Fig. 7d and 7e). Conversely, we observed that SS#4 alternative splicing had no effect on inhibitory synaptic transmission in cultured olfactory bulb neurons (Figs. 7g–i, Supplementary Figs. 7f and 7g). Together, these data suggest that different from the hippocampus, where Nrxn3 is essential for postsynaptic retention of AMPARs via the redundant action of both Nrxn3α and Nrxn3β in a manner regulated by SS#4 alternative splicing, in olfactory bulb Nrxn3 is required for presynaptic GABA release primarily via the action of Nrxn3α in a manner independent of SS#4 alternative splicing. In other words, these results suggest that Nrxn3α/β has brain region-specific pre- and postsynaptically manifesting functions that are mediated by distinct mechanisms.
Nrxn3α function in GABA release from olfactory bulb neurons requires its intracellular sequences
We asked whether the extracellular sequences of Nrxn3 are sufficient for its function in maintaining normal GABA release in olfactory bulb neurons, similar to their role in retaining postsynaptic AMPARs (Fig. 4c). We tested whether GABA release probability in olfactory bulb neurons could be restored by full-length Nrxn3αSS4+ or by truncated Nrxn3αSS4+ that is tethered to the presynaptic plasma membrane via a GPI-anchor (Supplementary Figs. 7h and 7i). We performed these rescue experiments using Nrxn3α with an insert in SS#4 to match the endogenous isoform expression found in the olfactory bulb20.
Full-length Nrxn3αSS4+ fully rescued evoked IPSCs, demonstrating that the direct genetic manipulation of Nrxn3 directly affects GABAergic release probabilities (Fig. 7j & k). Strikingly, GPI-anchored Nrxn3α failed to rescue the GABA release phenotype (Fig. 7l & m), despite its ability to localize to the synapse and restore AMPAR-mediated synaptic transmission in hippocampal neurons (Fig. 4c). Thus, in contrast to the hippocampus where the SS#4-dependent precise identity of trans-synaptic interactions is sufficient and necessary to stabilize excitatory transmission, these data establish that in olfactory bulb, the initiation of Nrxn3-dependent trans-synaptic interactions alone is not sufficient to regulate presynaptic release but also requires the intracellular sequences of Nrxn3.
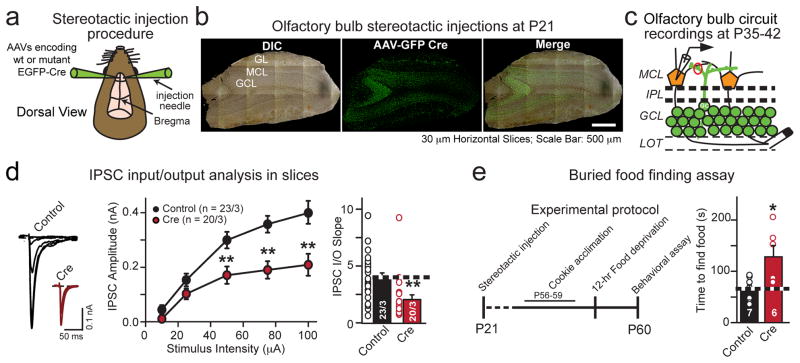
In vivo olfactory bulb ablation of Nrxn3 impairs GABAergic synaptic transmission and olfaction
To examine whether Nrxn3α/β in adult olfactory bulb neurons functions in vivo in a similar capacity as in developing cultured olfactory bulb neurons, we bilaterally stereotactically injected AAVs expressing inactive (control) or active cre-recombinase (cre) fused to EGFP into the granule cell layer of Nrxn3α/β cKO olfactory bulbs at P21 (Fig. 8a–c). 14–21 days after injections, we cut 300 μm horizontal olfactory bulb slices,,placed an extracellular stimulating electrode in the lateral olfactory tract (LOT), and evoked granule cell-mediated IPSCs by antidromic stimulation of mitral cell axons, while recording in whole-cell voltage-clamp mode from uninfected postsynaptic mitral cells in the mitral cell layer (Fig. 8c and Supplemental Fig. 8a). Consistent with our findings in culture, we observed a striking reduction in GABAergic synapse strength measured by a large impairment in the IPSC input-output relationship following the loss of Nrxn3α/β in granule cells (Fig. 8d).
Figure 8. Conditional in vivo deletion of Nrxn3α/β in the olfactory bulb decreases GABAergic synaptic transmission at granule cell-mitral/tufted cell synapses and impairs an olfactory behavior.
a, Schematic of bilateral stereotactic injections in vivo into the olfactory bulb.
b, Representative images of horizontal (30 μm) sections showing. complete infection of the granule cell layer. Nrxn3α/β cKO mice were bilaterally injected with AAV expressing EGFP fused to inactive (control) or active (cre) cre-recombibase at P21, and analyzed at P35-42. (scale bar: 500 μm).
c, Schematic of recording configuration from uninfected postsynaptic mitral/tufted cells in the mitral cell layer (MCL). Granule cell IPSCs were evoked by antidromic extracellular stimulation in the lateral olfactory tract (LOT).
d, IPSC input-output representative traces (left), plotted data (middle) and slope (right) from olfactory bulb slices from Nrxn3α/β cKO mice that had been infected as in (b). 10 μA: p = 0.0749; 25μA: p = 0.111; 50 μA: p = 0.00578; 75 μA: p = 0.00052; 100 μA: p = 0.0026; slope: p = 0.00370.
e, Schematic of the experimental protocol (left) and summary graph (right) of the buried food finding assay. Bilateral genetic ablation of Nrxn3α/β in olfactory bulbs (as in (b)) significantly increased the latency of food finding in behaving mice (p = 0.0162).
Data are means ± SEMs; the number of cells/independent animals analyzed are indicated in the bars (d) or number of animals (e). Statistical significance was assessed using single-factor ANOVA. For more information, see supplementary figure 8.
To determine if the reduction in local inhibition in the olfactory bulb circuit following conditional ablation of Nrxn3α/β in adult mice resulted in a behavioral change, we subjected the mice to a buried food-finding assay. Previous reports have demonstrated that the disruption of granule cell-dependent inhibitory transmission alters olfactory discrimination and impairs food-finding behavior29. We found that the deletion of Nrxn3α/β from the majority of inhibitory granule cells increased the latency to find buried food relative to animals infected with control AAVs, confirming the functional importance of the role of Nrxn3α/β in inhibitory synaptic transmission in the olfactory bulb (Fig. 8e, Supplementary Fig. 8b).
DISCUSSION
Here, we generated conditional Nrxn3α/β KO mice using a strategy that ablates all α- and β-Nrxn3 forms, analyzed the phenotypes induced by constitutive or conditional KO of all Nrxn3 forms at multiple levels, and compared these phenotypes to those produced by the constitutive Nrxn3α KO21, and the conditional Nrxn3 SS#4 KI that allows control of SS#4 alternative splicing with normal Nrxn3α/β expression levels20. We examined two brain regions, the hippocampus and the olfactory bulb, by electrophysiology and immunocytochemistry using cultured neurons that were infected with cre-recombinase expressing lentiviruses in vitro, or acute slices/tissue sections that were obtained from mice after stereotactic infections with cre-recombinase expressing AAVs in vivo. Our results identified two major, non-redundant synaptic functions of Nrxn3α/β: Nrxn3α/β was selectively required for maintaining normal postsynaptic AMPAR levels in the hippocampus, whereas, it was selectively essential for controlling the presynaptic release probability at inhibitory synapses in the olfactory bulb (Supplementary Table 1).
The Nrxn3α/β cKO AMPAR phenotype observed in the hippocampus is very similar to that of Nrxn3α/β SS#4 KI mice that express Nrxn3α/β at normal levels but with a constitutively included insert in SS#420. In the Nrxn3α/β SS#4 KI mice, the phenotype can be rescued simply by excising the SS#4 insert; accordingly, we found that the Nrxn3α/β cKO phenotype could be rescued only by neurexins without an insert in SS#4, but not with an insert in SS#4 (Fig. 3 and Supplementary Table 1). Moreover, both in Nrxn3α/β KO and in Nrxn3α/β SS#4 KI neurons the AMPAR impairment was rescued by a C-terminally truncated version of Nrxn3α or Nrxn3β in which its extracellular sequences (without an insert at SS#4) were tethered to the presynaptic plasma membrane by an unrelated sequence (Fig. 3)20. Thus, the hippocampal phenotypes of both the Nrxn3α/β cKO and the constitutive Nrxn3α/β SS#4 KI are likely a direct result of disrupting trans-synaptic Nrxn3-ligand interactions (likely with LRRTMs) which serve to anchor AMPARs at the synapse and suggest that the predominant essential function of Nrxn3α/β in excitatory hippocampal synapses is to mediate presynaptic, SS#4-dependent regulation of postsynaptic AMPARs, which is surprising considering the many ligands and functions that are generally associated with neurexins. Moreover, these results raise the fundamental question whether alternative splicing of other neurexins at SS#4 also plays similarly critical functions, and whether the lack of the AMPAR-related phenotype in Nrxn3α/β-deficient olfactory bulb neurons may be due to the compensating functions of other, correctly alternatively spliced neurexins in these neurons.
In contrast to the postsynaptic phenotype of Nrxn3α/β-deficient hippocampal neurons, the presynaptic release phenotype we observed in olfactory bulb neurons is consistent with previous notions of neurexin functions as organizers of the presynaptic release machinery21. Specifically, our results suggest that in inhibitory olfactory bulb neurons (likely granule cells where Nrxn3α is predominantly expressed19), Nrxn3α/β functions to maintain evoked GABA release. The interpretation that the decrease in inhibitory synaptic strength in olfactory bulb neurons is due to a decrease in the Ca2+-dependent release is based on the decrease in mIPSC frequency and the severe change in PPR observed in the Nrxn3α/β KO neurons, without a change in the readily-releasable pool size. Since the GABA release phenotype was similarly observed in olfactory bulb neurons lacking only Nrxn3α, Nrxn3β clearly does not functionally compensate in inhibitory olfactory bulb synapses likely due to the undetectable/low levels of Nrxn3β expression in granule cells19. Moreover, since the GABA release phenotype in Nrxn3α/β-deficient neurons could not be rescued by a membrane-tethered Nrxn3α construct lacking the intracellular Nrxn3α sequences, Nrxn3α is likely essential for normal GABA release via an action mediated by its cytoplasmic tail. Intriguingly, we failed to observe excitatory postsynaptic changes in Nrxn3α/β-deficient olfactory bulb neurons, and we detected no effect of alternative splicing of Nrxn3α/β at SS#4 on the Nrxn3α release function. Together, these findings indicate that Nrxn3 cell-autonomously governs presynaptic release probability in inhibitory olfactory bulb neurons. It remains to be established whether this Nrxn3 function also requires its extracellular sequences and involves trans-synaptic interactions as well as intracellular signaling, and if so, what postsynaptic neurexin ligands that bind to both SS#4 isoforms may be involved.
How is it possible that the same gene (Nrxn3α/β) performs two distinct, non-overlapping functions in two different brain regions and at two different types of synapses? This very observation illustrates the intriguing yet confounding layers of molecular and functional redundancy and complexity of neurexins. This observation can most plausibly be explained by the finding that different types of neurons and different brain regions express distinct patterns of neurexins (both in terms of principal isoforms and splice variants) and of neurexin ligands (of which there are many, ranging from postsynaptic cell-adhesion molecules to secreted proteins). Since neurexins exhibit a high degree of sequence homology, they are likely redundant for their central functions, but may additionally perform isoform-specific functions. Moreover, α- and β-neurexins share many sequences including the LNS6 domain which is the minimal extracellular domain required for binding to most known neurexin ligands as well as the entire intracellular sequences, and might provide an explanation for some but not all functions. Thus, different patterns of expression of neurexin variants and of neurexin ligands will result in distinct patterns of essential vs. redundant functions for a given neurexin gene. Genetic manipulations can only unmask functions that are not redundant with other genes, and the two very different functions of Nrxn3α/β we here describe could therefore be the simple consequence of differential patterns of expression of redundant other neurexins and of postsynaptic ligands. For example, if hippocampal neurons expressed lower levels of Nrxn1SS#4− and Nrxn2SS#4− than Nrxn3SS#4− but similar absolute levels of all three neurexins, a release function mediated by all three neurexins may not be apparent in these neurons even when the AMPAR-maintenance function becomes manifest. Conversely, if olfactory bulb neurons expressed significant levels of Nrxn1βSS#4− or Nrxn2βSS#4− but these β-neurexins were unable to sustain GABA release, loss of Nrxn3α/β would cause a selective GABA release phenotype, but not an AMPAR-related phenotype.
Our current findings provide systematic and comprehensive insight into Nrxn3α/β function at excitatory and inhibitory synapses in two distinct brain regions. To our knowledge, this is the first study to systematically analyze the effects of different mutations in the same gene (Nrxn3) in different brain regions using conditional manipulations. Our findings illustrate the multifarious fundamental functions of neurexins, and strengthen the notion that the circuit-level consequences associated with disease-relevant genetic abnormalities in neurexins must be addressed in a cell type-specific context.
METHODS
Mouse generation and husbandry
Nrxn3αβ cKO mutant mice were generated by homologous recombination in R1 embryonic stem cells using the strategy outlined in Fig. 1b. The Nrxn3αβ cKO mouse line was obtained after flp-recombinase-mediated germ line deletion of the neo cassette. The Nrxn3α βconstitutive KO mouse line was produced by cre-recombinase mediated deletion of exon 18 in the germ line of Nrxn3α βcKO mutant mice by breeding to a CMV-cre line34. The Nrxn3α KO and Nrxn3SS4+ mouse lines were previously described20,21. All animal are maintained in a hybrid genetic background consisting of C57/B6 × Sv/129 and housed on a 12hr light/dark cycle. All animals studies were approved by the Stanford animal use committees, and all original mouse lines were deposited in Jackson Labs for distribution.
mRNA measurements
Validation of genetic manipulation of exon excision was performed on mRNA isolated from DIV14 primary hippocampal cultures infected with inactive (control) or active cre-recombinase (cre). qRT-PCR reactions were performed in triplicates for each condition (150ng total RNA) with 2× VeriQuest master-mix (Affymetrix) and gene-specific qRT-PCR probes (IDT DNA). Reactions were run on a 7900HT Fast RT-PCR instrument (Applied Biosystems). mRNA from single Mitral/Tufted or inhibitory granule cells was isolated from primary olfactory bulb cultures and neurexin expression profiles were generated using Fluidigm BioMark (Fluidigm). Briefly, cytoplasm from single neurons was aspirated into patch electrodes and immediately snap-frozen in 2× cells-direct buffer (Invitrogen). Single-cell RNA was then pre-amplified for 18 cycles using gene specific primers to the target genes of interest. For internal controls (not shown), total RNA was also isolated from P21 olfactory bulbs was purified using the TriZol method (Invitrogen), and pre-amplified together with the single cell samples. Critical threshold cycles (Ct) values were then determined by using custom designed Taqman assays and BioMark48×48 Dynamic Array integrated fluidic circuit (Fluidigm), and the resulting data were analyzed by custom made scripts in Mathematica 9 (Wolfram Research). The data are plotted as normalized expression relative to β-actin.
DNA constructs
Inactive and active cre-recombinase viral constructs for lentivirus and adeno-associated virus production were previously described20. Briefly, inactive and active cre recombinase constructs are engineered to express EGFP fusion proteins that are localized to the nucleus. Full-length Nrxn1, Nrxn2, and Nrxn3 SS#4+/− rescue constructs have been described previously20. All constructs were cloned from whole brain cDNA isolated from C57/B6 mice and primers were designed against the appropriate sequences5 and subcloned into the lentiviral plasmid, FSW.
Electrophysiology in primary culture
Electrophysiological measurements were performed in DIV14-16 primary neurons cultured from the hippocampus or olfactory bulbs of newborn Nrxn3αβ cKO, Nrxn3α KO or Nrxn3SS4+ mice. Nrxn3α/β cKO and Nrxn3SS4+ neuronal cultures were infected with lentivirus on DIV3-4 that expressed either inactive (control; Nrxn3SS4+) or active (cre; Nrxn3SS4−) cre-recombinase. Rescue experiments in Nrxn3α/β cKO neurons were performed by concurrent superinfection with rescue viruses. Whole-cell patch-clamp recordings were performed in cultured neurons at DIV13-16 as described29,34. Cultures were continuously superfused with extracellular solution (ACSF; in mM): 126 NaCl, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 2.5 CaCl2, 1.3 MgSO4-7H2O, 11 D-Glucose, ~290 mOsm. Excitatory and inhibitory currents (mEPSCs/EPSCs and mIPSCs/IPSCs) were recorded from excitatory pyramidal neurons (hippocampal culture) and larger mitral/tufted cells (olfactory bulb culture) with 3–4 MΩ pipettes (WPI) filled with an internal solution containing the following (in mM): 119 Cesium-Methanesulfonate, 15 CsCl, 8 NaCl, 10 TEA-Cl, 0.2 EGTA, 4 Na2-ATP, 0.3 Na2-GTP, 10 HEPES, 10 Phosphocreatine at pH 7.3 with CsOH, ~300 mOsm. A constant hyperpolarizing current was used to maintain a resting potential of –60 to –70 mV. mEPSCs and mIPSCs were recorded in the presence of 0.5 μM tetrodotoxin (TTX) plus either 100 μM picrotoxin (mEPSCs) or 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50 μM D-(−)-2-amino-5-phosphonopentanoic acid (D-APV) (mIPSCs), respectively. Miniature events were visually inspected, individually selected and analyzed in Clampfit 10 (Molecular Devices) using template matching and an amplitude threshold of 5 pA. Evoked synaptic currents were examined as previously described35–37. Briefly, a bipolar stimulating electrode, made with nichrome wire (A-M Systems, Carlsborg, WA), placed at a position 100–150 μm from the soma of recorded neurons triggered evoked synaptic currents. After the formation of the whole-cell configuration and equilibrium of the intracellular pipette solution, the series resistance was compensated to 5–8 MΩ. To isolate individual glutamatergic and GABAergic currents, evoked responses were recorded in the presence of 100 μM picrotoxin and 50 μM D-APV (AMPAR), and 10 μM CNQX (NMDAR), and 10 μM CNQX and 50 μM D-APV (IPSCs). Presynaptic release probability was monitored by measurements of the paired-pulse ratios at inter-stimulus intervals ranging from 40 ms – 400 ms. For evoked release experiments, 10 μM QX-314 (final concentration) was added to the internal solutions and dialyzed into postsynaptic neurons to prevent firing.
Electrophysiology in acute slices
Electrophysiological recordings from acute hippocampal slices from Nrxn3α/β cKO animals injected with AAVs expressing GFP fused to inactive (control) or active (cre) cre-recombinase on P21 were performed as previously described20. Briefly, on P35-42, 300 μm horizontal brain slices were cut in a high sucrose cutting solution (in mM): 85 NaCl, 75 sucrose, 2.5 KCl, 1.3 NaH2PO4, 24 NaHCO3, 0.5 CaCl2, 4 MgCl2 and 25 D-Glucose and allowed to recover in ACSF at 31°C for 30 min followed by a hour at room temperature. To record AMPAR input-output current, a nichrome stimulating electrode was placed at the most distal portion of CA1 in the Stratum Oriens/alveus. Postsynaptic AMPAR currents were measured in burst and regular spiking neurons in the subiculum. The identity of the postsynaptic neuron was determined in whole-cell current clamp mode using an internal that consisted of (in mM): 140 K-gluconate, 5 KCl, 10 HEPES, 4 ATP-Mg2, 0.5 GTP-Na2, 10 phosphocreatine and 0.2 EGTA. After identification of subicular neuron cell-type, the AMPAR input-output relationship was measured in whole-cell voltage clamp mode. AMPAR paired-pulse ratios were performed at ISIs ranging from 20 ms – 400 ms. All recordings in the hippocampus were performed in the presence of 100 μM D-APV and 100 μM picrotoxin.
Slice electrophysiology from olfactory bulb preparations were performed as previously described29. Briefly, olfactory bulbs were stereotactically injected on P21 and prepared for recording on P35-42. 300mm horizontal slices were made from the olfactory bulb in the same sucrose-based cutting solution described above and allowed to recover as described. To evoke granule cell-mediated IPSC current, a nichrome stimulating electrode was placed in the lateral olfactory tract. Uninfected (GFP negative) mitral/tufted cells, located in the mitral cell layer of the olfactory bulb, were subjected to whole cell voltage clamp recordings. The internal solution for these experiments consisted of (in mM): 119 Cesium-Methanesulfonate, 15 CsCl, 8 NaCl, 10 TEA-Cl, 0.2 EGTA, 4 Na2-ATP, 0.3 Na2-GTP, 10 HEPES, 10 Phosphocreatine at pH 7.3 with CsOH, ~300 mOsm.
Data was collected at 10 kHz and filtered with a lowpass filter at 2 kHz. The frequency, duration, and magnitude of the extracellular stimulus was controlled by a Model 2100 Isolated Pulse Stimulator (A-M Systems, Inc.) synchronized with the Clampex 10 data acquisition software (Molecular Devices). For all electrophysiological experiments, the experimenter was blind to the recording condition.
Production of recombinant lentivirus for primary culture
Lentiviruses were produced as previously described34. Lentiviral transfer vectors were co-transfected with third-generation helper plasmids (VSVG, pRSV-REV, and pMDLg/pRE) into HEK293T cells by calcium phosphate transfection. Viral particles were harvested 48 hours after transfection, spun at 500 × g for 5 minutes to pellet cellular debris, and added to cultured neurons.
Production of recombinant Adeno-Associated Viruses for in vivo injection
AAV-DJ expressing GFP fused to inactive or active cre-recombinase was generated as previously described20. Briefly, HEK293T cells were co-transfected with the AAV vector along with helper plasmids (pDJ and pHelper) by the calcium phosphate method. 72 hours post transfection, cells were harvested, nuclei lysed and virus purified over an iodixanol gradient column (2 hrs at 80K RPM in an S80AT3 rotor). Virus was dialyzed to remove excess iodixanol and aliquoted for injection.
Stereotactic injection of AAV
Stereotactic injection of P21 animals was previously described20. The localization and AAV expression were confirmed by the presence of GFP. The coordinates for P21 CA1 (hippocampus) injections are (A-P/M-L/D-V from Bregma): −3.2, ±3.5, −2.6. The coordinates for P21 granule cell (olfactory bulb) injections are (A-P/M-L/D-V from Bregma): +4.4, ±1.0, −1.5.
Buried food-finding assay
Animal behavior was performed as previously described29. Nrxn3α/β cKO male and female mice were stereotactically injected on P21 with AAV expressing GFP fused to inactive (control) or active (cre) cre-recombinase. On P60, the animals were singly housed for one week prior to the experiment. 18 hours before the start of the experiment, the animals were deprived of food to increase food-finding motivation. On the day of the experiment, the animals were acclimated to the testing room for 1hr. The mice were removed from their home cage and placed into a holding cage while a single food pellet was buried randomly in one of the corners. The mouse was returned to the home cage and the latency to food finding was measured. All trials were performed blind to the injection conditions and video recorded for post hoc analysis.
AMPAR surface labeling
GluA1 (1:4; Calbiochem, PC246) and GluA2 (1:100; Millipore, MAB397) surface labeling was performed as previously described20,38. Briefly, cultures were washed with PBS containing 0.5 mM CaCl2 and 1 mM MgCl2 (PBSMC) with 4% sucrose. Neurons were preincubated at 37° C for 5 min with primary antibodies against GluA1 to allow labeling of surface AMPA receptors, washed with ice-cold PBSMC, fixed with 4% PFA + 4% sucrose for 15 minutes, then blocked in a detergent-free blocking solution (PBS with 2% normal goat serum (Sigma, St. Louis, MO), and 0.02% sodium azide) for 1 hr, followed by secondary antibody incubation at room temp for 1 hour. To immunolabel for excitatory synaptic markers, cultures were then postfixed with –20°C methanol for 1 min to permeabilize the neurons. Cells were blocked in blocking solution for 1 hr, followed by incubation with primary antibodies for PSD95 (1:200; Pierce, 6G6-1C9) and VGluT1 (1:500; Millipore AB5905). Neurons were washed, then incubated with a secondary antibody at room temp for 1hr.
AMPAR internalization assays
The GluA1 internalization assay was essentially performed as previously described20, 39. Live hippocampal neurons at 14–16 days in vitro were labeled at 37°C for 5 min with a primary antibody against an extracellular epitope of GluA1 to allow labeling of surface AMPA receptors. Cells were washed in PBSMC then incubated at 37°C with neuron growth media for 15–20 minutes to allow for basal internalization. Cells were then washed and fixed with 4% PFA/4% sucrose, blocked in a detergent-free blocking solution for 1 hr, followed by an AlexaFluor488 secondary antibody incubation at room temp for 1hr to label the surface population. Neurons were then postfixed with 100% methanol at –20°C and stained with an AlexaFluor546 secondary antibody to immunolabel the pre-labeled internalized fraction. To calculate the percent internalization, the integrated red fluorescence intensity, identifying internalized GluA1, was divided by the total (red + green) intensity.
Olfactory bulb immunocytochemistry
Olfactory bulb cultures (DIV14-15) were fixed with 4% PFA + 4% sucrose, permeabilized with 0.02% Triton-X-100 in PBS (3×5′) and blocked for 1 hr in blocking solution. Fixed and permeabilized neurons were incubated with primary antibodies to detect Gephyrin (1:500; Synaptic Systems 147 003), vGAT (1:500; Synaptic Systems 131 011) and vGluT1. Cells were washed and stained with fluorescently conjugated secondary antibodies.
Image acquisition and quantifications
Image acquisition was performed previously20,38,40. Cells were chosen at random from three or more independent cultures. Images were taken from at least three coverslips per experiment. Fluorescent images were acquired at room temperature with an Olympus (Tokyo, Japan) FV1000 BX61WI laser-scanning confocal microscope using an Olympus Plan Apochromat 60× oil objective (NA: 1.42; WD: 0.15) set to 3× zoom with sequential acquisition, averaging of 3 frames, and set to 1024×1024 pixel resolution. Laser power and photomutiplier settings were set so bleed-through was negligible between channels. Within the same experiment, the same settings for laser power, PMT gain, and offset were used. These settings provided images where the brightest pixels were just under saturation. Digital images were taken using the Olympus Fluoview Imaging software (Olympus). 7–12 0.5 μm optical sections were used and maximum pixel intensity projections were created. Quantification of images was performed as previously described20,38,40. In brief, images were thresholded by intensity to exclude background signals and puncta were quantified by counting the number of puncta whose areas ranged from 0.1–4.0 μm2. For each experiment, at least 15 cells per condition were analyzed and the mean and SEM were calculated. Data shown represent the average of the mean values from at least 3 independent experiments with each experiment consisting of the analysis of 12–20 cells per experimental condition. An experimenter blind to the conditions performed image-capture and quantification.
Quantitative immunoblotting was performed using 125I secondary antibodies an imaged using a Typhoon Phosphoimager (GE Healthcare). Protein samples were either from adult littermate wild-type or constitutive Nrxn3α/β KO mice.
Statistics
The experimenter was blinded to all conditions during data collection and analysis. All values from culture electrophysiology experiments are represented by the average of independent experiments ± SEM with the exceptions being the paired-pulse ratio measurements in figures: 6e &f, 6i & 6j, 7e & 7f, 7h & i, 7j – m, in these experiments, the numbers represent the number of total cells recorded ± SEM. Acute slice experiments (Fig. 5c–f and 8d) are represented by the total number of cells per condition ± SEM. For the buried food-finding behavior (Fig. 8e), the total number of animals was used to determine the average latency ± SEM. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those generally employed in the field. Data distribution was assumed to be normal but this was not formally tested, thus we have displayed individual data points for all 2-bar bar graphs. Statistical significance for electrophysiological and behavioral experiments was determined by single-factor ANOVA from a two-tailed test. Significance of weight distribution was determined by 2-way ANOVA. Statistical analysis of cumulative probabilities was tested by a Kolmogorov-Smirnov test (K-S test) from: http://www.physics.csbsju.edu/stats/KS-test.n.plot_form.html. A supplementary methods checklist is available.
Supplementary Material
Acknowledgments
We thank Drs. D. Martinelli, P. Rothwell, L. Chen and R.C. Malenka for advice and technical help. This study was supported by grants from the NIMH (R37MH52804 to T.C.S.), the NIDA (K99DA034029 to C.F.) and the A.H.A. (11POST7360078 to J.A.) and NIMH (NIMH: 1K99MH103531to J.A.).
Footnotes
J.A., K.T. and T.C.S. conceived the project. K.T. generated the Nrxn3α/β cKO mice and performed biochemical analyses of protein expression. J.A. designed and performed all immunocytochemistry, culture and acute hippocampal slice electrophysiology and animal behavior experiments. J.A. and C.F. performed olfactory bulb acute slice analyses. J.A. and T.C.S. wrote and edited the manuscript.
References
- 1.Bang ML, Owczarek S. A matter of balance: role of neurexin and neuroligin at the synapse. Neurochem Res. 2013;38:1174–1189. doi: 10.1007/s11064-013-1029-9. [DOI] [PubMed] [Google Scholar]
- 2.Knight D, Xie W, Boulianne GL. Neurexins and neuroligins: recent insights from invertebrates. Mol Neurobiol. 2011;44:426–440. doi: 10.1007/s12035-011-8213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krueger DD, Tuffy LP, Papadopoulos T, Brose N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol. 2012;22:412–422. doi: 10.1016/j.conb.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabuchi K, Südhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- 6.Ushkaryov YA, et al. Conserved domain structure of beta-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. J Biol Chem. 1994;269:11987–11992. [PubMed] [Google Scholar]
- 7.Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- 8.Reichelt AC, Rodgers RJ, Clapcote SJ. The role of neurexins in schizophrenia and autistic spectrum disorder. Neuropharmacology. 2012;62:1519–1526. doi: 10.1016/j.neuropharm.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Kim HG, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirov G, et al. Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophr Bull. 2009;35:851–854. doi: 10.1093/schbul/sbp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato N, et al. Association between neurexin 1 (NRXN1) polymorphisms and the smoking behavior of elderly Japanese. Psychiatr Genet. 2010;20:135–136. doi: 10.1097/YPG.0b013e32833a21f9. [DOI] [PubMed] [Google Scholar]
- 12.Docampo E, et al. Association of neurexin 3 polymorphisms with smoking behavior. Genes Brain Behav. 2012;11:704–711. doi: 10.1111/j.1601-183X.2012.00815.x. [DOI] [PubMed] [Google Scholar]
- 13.Hishimoto A, et al. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum Mol Genet. 2007;16:2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- 14.Kelai S, et al. Nrxn3 upregulation in the globus pallidus of mice developing cocaine addiction. Neuroreport. 2008;19:751–755. doi: 10.1097/WNR.0b013e3282fda231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lachman HM, et al. Genomewide suggestive linkage of opioid dependence to chromosome 14q. Hum Mol Genet. 2007;16:1327–1334. doi: 10.1093/hmg/ddm081. [DOI] [PubMed] [Google Scholar]
- 16.Novak G, Boukhadra J, Shaikh SA, Kennedy JL, Le Foll B. Association of a polymorphism in the NRXN3 gene with the degree of smoking in schizophrenia: a preliminary study. World J Biol Psychiatry. 2009;10:929–935. doi: 10.1080/15622970903079499. [DOI] [PubMed] [Google Scholar]
- 17.Prats-Puig A, et al. Variations in the obesity genes FTO, TMEM18 and NRXN3 influence the vulnerability of children to weight gain induced by short sleep duration. Int J Obes (Lond) 2013;37:182–187. doi: 10.1038/ijo.2012.27. [DOI] [PubMed] [Google Scholar]
- 18.Treutlein B, Gokce O, Quake SR, Südhof TC. Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc Natl Acad Sci U S A. 2014;111:E1291–1299. doi: 10.1073/pnas.1403244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullrich B, Ushkaryov YA, Südhof TC. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 20.Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Südhof TC. Presynaptic Nrxn3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell. 2013;154:75–88. doi: 10.1016/j.cell.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missler M, et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 22.Chen YC, et al. Drosophila neuroligin 2 is required presynaptically and postsynaptically for proper synaptic differentiation and synaptic transmission. J Neurosci. 2012;32:16018–16030. doi: 10.1523/JNEUROSCI.1685-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Z, et al. Neurexin and neuroligin mediate retrograde synaptic inhibition in C. elegans. Science. 2012;337:980–984. doi: 10.1126/science.1224896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler SC, et al. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods. 2001;107:107–124. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- 25.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 26.Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 27.Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 28.Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 29.Cao P, Maximov A, Südhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell. 2011;145:300–311. doi: 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Citri A, Pang ZP, Südhof TC, Wernig M, Malenka RC. Comprehensive qPCR profiling of gene expression in single neuronal cells. Nat Protoc. 2012;7:118–127. doi: 10.1038/nprot.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Földy C, Malenka RC, Südhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron. 2013;78:498–509. doi: 10.1016/j.neuron.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iijima T, Iijima Y, Witte H, Scheiffele P. Neuronal cell type-specific alternative splicing is regulated by the KH domain protein SLM1. J Cell Biol. 2014;204:331–342. doi: 10.1083/jcb.201310136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iijima T, et al. SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell. 2011;147:1601–1614. doi: 10.1016/j.cell.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaeser PS, et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maximov A, Pang ZP, Tervo DG, Südhof TC. Monitoring synaptic transmission in primary neuronal cultures using local extracellular stimulation. J Neurosci Methods. 2007;161:75–87. doi: 10.1016/j.jneumeth.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Pang ZP, et al. Doc2 supports spontaneous synaptic transmission by a Ca(2+)-independent mechanism. Neuron. 2011;70:244–251. doi: 10.1016/j.neuron.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, et al. Neurexins physically and functionally interact with GABA(A) receptors. Neuron. 2010;66:403–416. doi: 10.1016/j.neuron.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin JW, et al. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 40.Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.