Abstract
Background
Repeat blood cultures are frequently obtained in children with persistent fever and neutropenia (FN), but their clinical impact is uncertain.
Methods
We identified children with persistent FN in the context of hematologic malignancy or hematopoietic stem cell transplantation from July 2006 to June 2012. For each episode, we reviewed blood cultures to determine the yield of true positive and false positive results. We then examined episode-level and culture-level predictors to determine factors associated with new bloodstream infections (BSI).
Results
Among 135 children who met inclusion criteria, there were 184 persistent FN episodes, during which 17 new BSI were diagnosed after the first 24 hours of fever (9.2%; 95% CI 5.4–15.3%). After the first 24 hours, the incidence of new BSI was 1.5% (95% CI 1.0–2.4%) per day and the incidence of blood culture contamination was 1.1% (95% CI 0.6–2.1%) per day. Of 17 new BSI identified, 14 (82%) required changes in therapy, while all 12 contaminant blood cultures were followed by additional antibiotic therapy. Increased odds of new BSI were associated with a history of BSI within 30 days of the episode (OR 5.18; 95% CI 1.29–20.8) and increasing time between recurrent fevers (OR 1.29; 95% CI 1.06–1.57).
Conclusions
Repeat blood cultures have an important role in diagnosing new BSI and directing therapy in children with persistent FN. The current strategy could be improved by reducing the frequency of blood cultures after the first 24 hours, and targeting repeat cultures by risk.
Keywords: blood culture, bloodstream infection, bacteremia, fever and neutropenia
Fever and neutropenia (FN) is one of the most common, life-threatening, and costly complications of treatment for childhood cancer [1–3]. Although initial blood cultures identify bloodstream infections (BSI) in 10–20% of children with FN, the majority of episodes do not have an identified infectious etiology, and fever persists despite antibiotic therapy in up to 40% of cases [4–7]. Although blood cultures are frequently repeated in children with persistent FN, it is unclear how often this strategy identifies additional BSI, and how management is altered based on subsequent blood cultures. Potential harms of excessive blood culture collection include iatrogenic blood loss, which may increase transfusion requirements, and false positive results, which lead to additional laboratory testing, unnecessary antibiotic therapy, and increased costs [8–10]. The optimal approach to repeating blood cultures during persistent FN was identified as an important research gap in recent pediatric FN guidelines [11].
Prior studies examining the yield of subsequent blood cultures in patients with FN have demonstrated varying rates of new BSI. In an adult hematopoietic stem cell transplant (HSCT) cohort, 1 (0.9%) of 109 patients who had blood cultures collected after day 1 of FN had a new pathogen identified by follow-up culture [12]. In a study of children with FN whose initial cultures were negative, new BSI were identified via subsequent cultures in 24 (10%) of 220 episodes [13]. Because secondary infections may occur during treatment for the initial infection, it is also important to evaluate the yield of subsequent blood cultures in children whose initial cultures are positive [6, 14]. Subsequent blood culture yield should also be evaluated in pediatric HSCT recipients, a group with high incidence of persistent FN and high risk for BSI [4, 15].
At our center, blood cultures are repeated every 24 hours in the setting of ongoing FN. We evaluated the diagnostic yield of subsequent cultures in order to better characterize the utility of this approach in children with persistent FN, including those with previous positive cultures and HSCT recipients.
Methods
Design
This retrospective cohort study was conducted in patients on the Pediatric Oncology and Pediatric Blood and Marrow Transplant Services at the University of California-San Francisco (UCSF) Benioff Children’s Hospital. The study was approved by the UCSF Committee on Human Research; the requirement for informed consent was waived.
Study Population
Patients were identified via hospital discharge and microbiology records. Patients less than 21 years old with a hematologic malignancy or undergoing HSCT were included if they had any episodes of persistent FN between July 1, 2006 and June 30, 2012. Persistent FN was defined as fever (temperature ≥ 38°C) in the setting of neutropenia (absolute neutrophil count, ANC < 500 cells/µL), that lasted for at least 96 hours without more than 96 hours of interruption [6, 13]. The beginning of an episode was defined as the first recorded temperature ≥ 38°C; the end was defined as the time of the last recorded fever beyond which the patient was afebrile for 96 hours, or the last recorded fever on the day before the patient’s ANC rose to 500 cells/µL.
Patient Management
Per protocol, patients with FN underwent blood cultures every 24 hours in the setting of ongoing fever. The standard empiric therapy for FN was piperacillin-tazobactam with either tobramycin or ciprofloxacin. Vancomycin was added for patients receiving cytarabine or those deemed clinically unstable. Meropenem was substituted for piperacillin-tazobactam in patients with septic shock. Therapy was modified at the clinician’s discretion, with discontinuation of tobramycin, ciprofloxacin and vancomycin recommended if no infection was identified after 48–72 hours. Antifungal therapy with liposomal amphotericin B, voriconazole or caspofungin was started if fever lasted more than 72 hours. Patients did not receive antibacterial prophylaxis for neutropenia in the absence of fever. Antifungal prophylaxis with fluconazole was standard for patients undergoing HSCT and was individualized on the Oncology service. Although we believe that most patients were treated according to these institutional guidelines, due to the observational and retrospective nature of the study, there may have been variation in management of individual patients.
Measurements
The primary outcome was the diagnosis of a new (not previously identified during that episode) BSI via a blood culture collected more than 24 hours from the start of an episode (subsequent blood culture). A patient with a positive blood culture was considered to have a BSI if either a recognized pathogen was isolated, or if two blood cultures collected on the same day or consecutive days were positive for the same commensal organism [16]. The organism, antimicrobial susceptibility, presentation, management and outcome were recorded for each BSI. If a positive culture for a commensal organism did not meet the criteria for BSI, it was considered a false positive result. Changes in therapy and diagnostic evaluation prompted by false positive blood cultures were recorded.
Predictor variables were chosen a priori, based on demonstrated or hypothesized association with BSI. Episode-level predictors were specific to the participant or the persistent FN episode, and did not vary by day. These included the clinical context, categorized as hematologic malignancy with intensive chemotherapy if the episode occurred during induction, consolidation, or intensification chemotherapy, or at the time of diagnosis or relapse; hematologic malignancy with maintenance chemotherapy, allogeneic HSCT, or autologous or syngeneic HSCT. History of prior BSI, type of central venous catheter, whether that catheter was in place at the time of the prior BSI, and presence of mucositis were obtained from the medical record. If multiple central catheters were in place during the episode, the catheter type was categorized by the highest risk catheter that was in place, i.e. from highest to lowest risk – temporary (not surgically implanted) catheters, tunneled catheters, and implanted vascular ports. The duration of neutropenia prior to episode onset was calculated from the first date of documented ANC < 500 cells/µL to the episode onset date.
Culture-level predictors varied daily but were specific to the time interval preceding a blood culture. For each predictor, the vital signs (VS) during the 24 hour period preceding the blood culture collection time were examined; the highest documented body temperature during that interval was recorded. The afebrile interval was the time in hours between the first fever during the pre-culture interval, and the most recent preceding fever. Abnormal heart rate or respiratory rate were coded if any value documented during the interval exceeded the 95th percentile for age [17]. Abnormal systolic or diastolic blood pressure were coded if any value documented during the interval was below the 5th percentile for age and height [18, 19]. If any VS were abnormal during the interval, the interval was coded as having abnormal VS, and if no VS abnormalities were present during the 24 hours immediately preceding the pre-culture interval, the interval was coded as having newly abnormal VS.
Statistical Analysis
Relationships between episode-level and culture-level predictors and new BSI diagnosis via subsequent blood culture were evaluated with Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Results were confirmed with single predictor logistic regression models adjusted for clustering of multiple cultures per episode and multiple episodes per patient. Episode-level models used robust standard errors adjusted for clustering by patient, and culture-level models used generalized estimating equations with episode as the panel variable and adjustment of robust standard errors for clustering by patient. Models were evaluated for validity by comparison to simple logistic regression models and checking continuous predictors for a linear relationship to the logit of risk.
The estimated cost of testing was based on blood cultures alone and was derived using the number of blood cultures collected, multiplied by the estimated cost per culture. The cost per blood culture was estimated at $74.73 by multiplying the blood culture charge of $318.00, obtained from the 2013 Charge Description Master for UCSF Medical Center by the 2013 hospital cost-to-charge ratio of 0.235, obtained from the Centers for Medicare and Medicaid Services. All analysis was performed in Stata version 13 (StataCorp, College Station, TX).
Results
Patient and Episode Characteristics
One-hundred eighty-four episodes of persistent FN were identified in 135 patients. Of 135 patients, 101 (75%) had 1 episode, 23 (17%) had 2 episodes, and 11 (8%) had ≥3 episodes. Fifty-six (41%) patients underwent HSCT for hematologic malignancy, 51 (38%) underwent HSCT for other indications, and 28 (21%) had a hematologic malignancy treated without HSCT (Table I). Of 184 episodes, 120 (65%) occurred in the context of HSCT and 64 (35%) occurred during chemotherapy for hematologic malignancy. Episodes began after a median of 5.7 days (interquartile range [IQR] 1.8–12.8 days) of neutropenia and lasted for a median of 6.7 days (IQR 5.2–9.3 days). Episodes occurring in the context of HSCT started at a median of 4.5 days (IQR 1–9 days) post-transplantation.
Table I.
Characteristics of Patients with Persistent Fever and Neutropenia (n = 135)
| Characteristic | |
|---|---|
| Age at first episode (years, median, IQRa) | 8.2 (3.2–15.0) |
| Male (n, %) | 89 (66) |
| Hematologic malignancy (n, %) | 84 (62) |
| Acute lymphoblastic leukemia | 39 (29) |
| Acute myelogenous leukemia | 26 (19) |
| Otherb | 19 (14) |
| Stem cell transplant recipient (n, %)c | 107 (79) |
| Transplant indication (n, %) | |
| Hematologic malignancy | 56 (42) |
| Solid tumor malignancy | 26 (19) |
| Primary immunodeficiency | 10 (7) |
| Otherd | 15 (11) |
Interquartile range.
Includes 8 patients with myelodysplastic syndrome, 6 with lymphoma, 3 with juvenile myelomonocytic leukemia and 2 with biphenotypic leukemia.
Eight patients received multiple stem cell transplants.
Includes 6 patients with aplastic anemia/bone marrow failure syndromes, 4 with genetic/metabolic disorders, 3 with hemoglobinopathies and 2 with hemophagocytic lymphohistiocytosis.
Blood Culture Diagnostic Yield
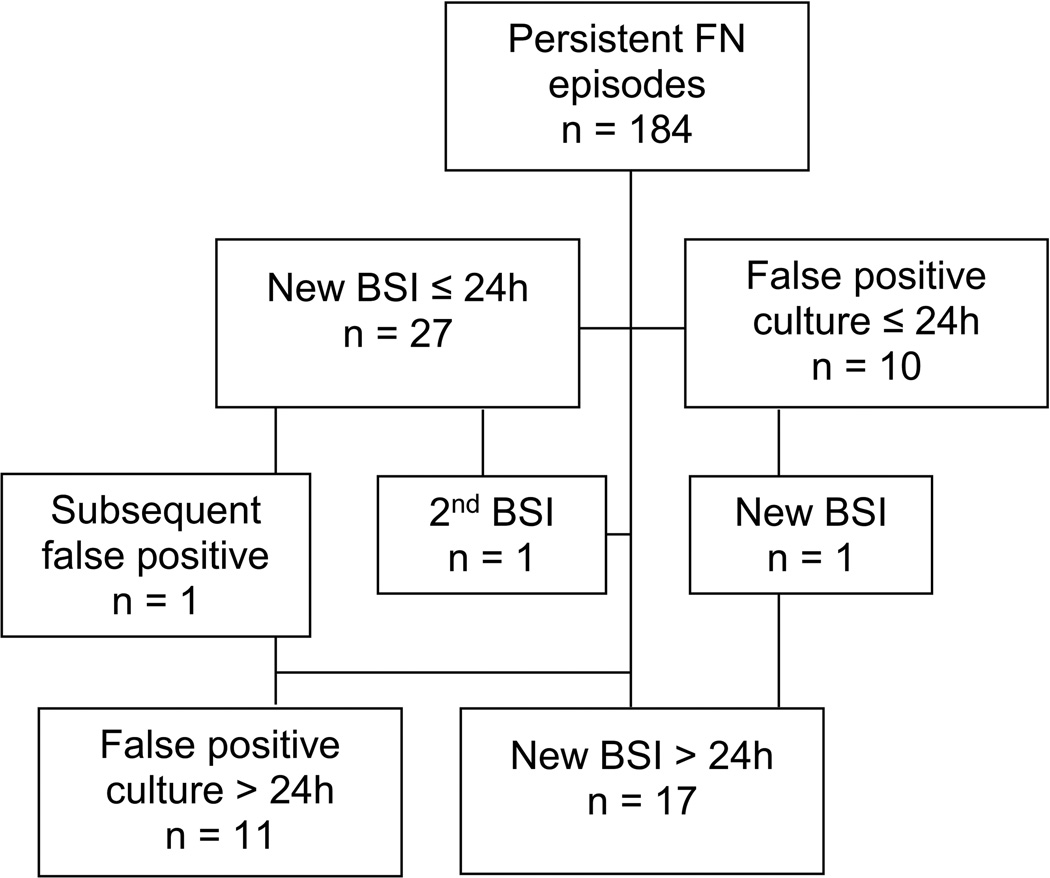
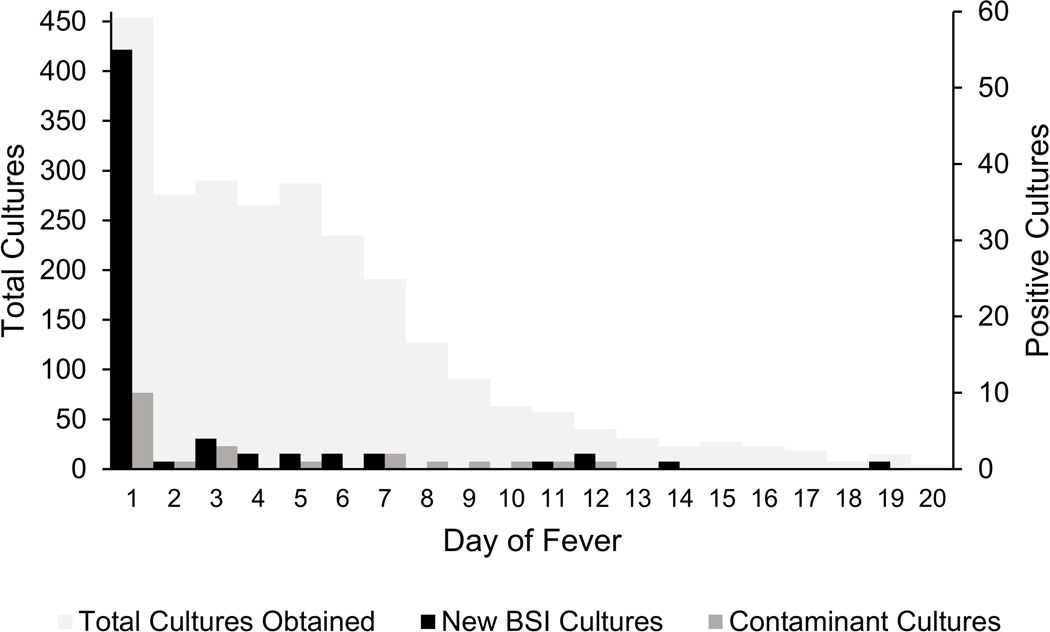
New BSI were diagnosed by cultures collected during the first 24 hours of fever in 27 (14.7%; 95% confidence interval [CI] 10.1–20.7%) episodes and by cultures collected > 24 hours from onset of fever in 17 (9.2%; 95% CI 5.4–15.3%) episodes (Figure 1). The incidence of new BSI diagnosis was 1.5% (95% CI 1.0–2.4%) per day after 24 hours from fever onset, compared to 11.5% (95% CI 8.1–16.1%) per day during the first 24 hours of fever. The incidence of false positive blood cultures was 1.1% (95% CI 0.6–2.1%) per day after 24 hours from fever onset and 4.3% (95% CI 2.3–7.7%) per day during the first 24 hours of fever. Figure 2 shows blood culture utilization and yield of true positive and false positive results by day of fever.
Figure 1.
Flowchart showing total persistent FN episodes evaluated, new bloodstream infections (BSI) diagnosed at ≤24 hours and >24 hours, and false positive blood cultures at ≤24 hours and >24 hours.
Figure 2.
New positive blood cultures leading to diagnosis of bloodstream infections (new BSI cultures) and positive blood cultures determined to be contaminants by day of fever. Total blood cultures obtained by day of fever are plotted on a different axis scale. Cultures collected after day 20 of fever are not shown; 25 cultures were obtained after day 20 of fever and none were positive.
During 184 episodes, 2642 separate blood cultures were performed; 454 (17%) during the first 24 hours, 1020 (39%) during the first 72 hours, and 1622 (61%) after 72 hours from fever onset. The estimated cost of testing for each new BSI diagnosed was $1,257 per BSI during the first 24 hours, $2,459 per BSI during the first 72 hours, and $9,324 per BSI after 72 hours of fever.
Predictors of New BSI Diagnosis
Of the episode-level predictors evaluated, history of prior BSI was the only one found to have a significant relationship to the outcome (Table II). New BSI were diagnosed in 5 (26%) of 19 episodes in which the patient had a history of BSI within the preceding 30 days, compared to 8 (7%) of 124 episodes in which there was no preceding history of BSI and 4 (10%) of 41 episodes in which there was a history of BSI more than 30 days from the episode (p = 0.027). In logistic regression adjusted for clustering of multiple episodes by patient, a history of BSI within 30 days prior to episode onset was associated with increased odds (OR 5.18; 95% CI 1.29–20.8) of having a new BSI diagnosed by subsequent culture, compared to episodes with no preceding BSI.
Table II.
Characteristics of Persistent Fever and Neutropenia Episodes (n = 184) and Their Association with New Bloodstream Infection Diagnosis > 24 Hours from Start of Episode
| Characteristic | No New BSIa (n = 167) |
New BSI (n = 17) |
Pb |
|---|---|---|---|
| Clinical context (n, %) | 0.344 | ||
| Hematologic malignancy – maintenance therapy | 6 (100) | 0 (0) | |
| Hematologic malignancy – intensive therapyc | 54 (93) | 4 (7) | |
| Allogeneic stem cell transplant | 78 (87) | 12 (13) | |
| Autologous or syngeneic stem cell transplantd | 29 (97) | 1 (3) | |
| History of BSI prior to episode (n, %) | 0.027 | ||
| None | 116 (94) | 8 (7) | |
| ≤ 30 days prior to episode start | 14 (74) | 5 (26) | |
| > 30 days prior to episode start | 37 (90) | 4 (10) | |
| History of BSI with same catheter (n, %) | 31 (86) | 5 (14) | 0.333 |
| Central catheter type (n, %) | 0.490 | ||
| None | 3 (100) | 0 (0) | |
| Implanted port | 15 (100) | 0 (0) | |
| Tunneled catheter | 120 (91) | 12 (9) | |
| Temporary cathetere | 29 (85) | 5 (15) | |
| Documented mucositis during episode | 107 (91) | 11 (9) | 1.000 |
| Neutropenic ≥ 1 week prior to episode start | 76 (93) | 6 (7) | 0.455 |
| BSI diagnosed by initial blood culture | 25 (93) | 2 (7) | 1.000 |
Bloodstream infection.
P-values are based on Fisher’s exact test.
Includes patients undergoing induction, consolidation, intensification or salvage chemotherapy regimens.
Only 2 patients received syngeneic transplants.
Temporary catheters included peripherally inserted central catheters and other catheters not surgically implanted and intended for short term use. When multiple central catheters were present, episodes were categorized according to the highest risk catheter in place at the time i.e. temporary catheter if any temporary catheter was in place.
Of the culture-level predictors evaluated, the interval between recorded fevers was found to have a significant relationship to the outcome (Table III). Blood cultures that identified new BSI were associated with fevers occurring at longer intervals from the most recent prior fever, with a median interval of 21 hours (IQR 9–47 hours), compared to a median interval of 9 hours (IQR 4–23 hours) for cultures that did not identify new BSI. In logistic regression adjusted for clustering by episode and by patient, every 12 hour increase in time between documented fevers was associated with a nearly 30% increase in the odds of new BSI (OR 1.29; 95% CI 1.06–1.57).
Table III.
Culture-Level Predictors for New Bloodstream Infection Diagnosis > 24 Hours from Start of Episode
| Predictor | No New BSIa (n = 1086)b |
New BSI (n = 17)b |
Pc |
|---|---|---|---|
| Afebrile interval (hours)d (median, IQRe) | 9 (4–23) | 21 (9–47) | 0.030 |
| Maximum temperature (°C) (median, IQR) | 38.6 (38.3–39.4) | 39.0 (38.4–39.5) | 0.251 |
| Any abnormal VSf (n, %) | 813 (98.3) | 14 (1.7) | 0.585 |
| Heart rate > 95th percentile | 697 (98.2) | 13 (1.8) | 0.444 |
| Respiratory rate > 95th percentile | 387 (98.2) | 7 (1.8) | 0.620 |
| Systolic blood pressure < 5th percentile | 124 (98.4) | 2 (1.6) | 1.000 |
| Diastolic blood pressure < 5th percentile | 99 (98.0) | 2 (2.0) | 0.664 |
| VS newly abnormalg (n, %) | 144 (97.3) | 4 (2.7) | 0.269 |
Bloodstream infection.
Each predictor applies to the 24 hour period preceding the blood culture set; numbers represent blood culture sets rather than individual cultures.
Determined by Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables.
Interval between the first temperature ≥ 38°C within the 24 hours preceding blood culture collection, and the most recent preceding temperature ≥ 38°C.
Interquartile range.
Vital signs.
VS were considered newly abnormal if the subject did not meet any criteria for abnormal VS during the 24 hour period immediately before the 24 hour period preceding blood culture collection.
Combining the two predictors did not identify all cases of new BSI diagnosed via subsequent blood culture, but facilitated risk stratification. In patients with no recent history of BSI, the incidence of new BSI diagnosis was 0.9% (95% CI 0.5–2.0%) per day if the interval between fevers was less than 24 hours, and 1.8% (95% CI 0.7–4.7%) per day if the interval between fevers was greater than 24 hours. In patients with a recent history of BSI, the incidence of new BSI was 4.8% (95% CI 2.2–10.3%) per day.
Review of New BSI and Contaminant Cases
The most commonly identified causative agents of new BSI diagnosed via subsequent culture were coagulase-negative Staphylococcus species in 7 (41%) of 17 cases (Table IV). In most cases (n = 14, 82%), the causative agent was not susceptible to the antibiotics the patient was receiving at the time of blood culture collection. Central lines were removed in 4 cases for persistently positive blood cultures. Two cases of fungal BSI were identified and prompted additional diagnostic evaluation. Two new BSI were associated with focal physical findings present at the time of blood culture collection; one patient had symptoms and imaging consistent with appendicitis, and one had perirectal cellulitis.
Table IV.
Causative Agents of Bloodstream Infections Diagnosed by Initial and Subsequent Blood Cultures During Persistent Fever and Neutropenia Episodes
| Organism | Initial Culture (≤ 24 hours) (n=27) |
Subsequent Culture (>24 hours) (n=17) |
|---|---|---|
| Gram positive | 23 | 10 |
| Viridans group streptococci | 14 | 0 |
| Coagulase-negative staphylococci | 3 | 7 |
| Staphylococcus aureus | 3 | 0 |
| Enterococcus faecium (vancomycin-resistant) | 2 | 2 |
| Other Gram positive organisma | 1 | 1 |
| Gram negativeb | 2 | 5 |
| Fungal | 2 | 2 |
| Candida species | 2 | 1 |
| Bipolaris species | 0 | 1 |
Includes Streptomyces thermocarboxydus (n = 1) detected by initial culture andClostridium difficile (n = 1) detected by subsequent culture.
Gram negative bloodstream infections (BSI) diagnosed by initial culture were caused by Escherichia coli (n = 1) and Klebsiella pneumoniae (n = 1). Gram negative BSI diagnosed by subsequent culture were caused by Escherichia coli (n = 1), Enterobacter cloacae (n = 1), Klebsiella oxytoca (n = 1), and Pseudomonas aeruginosa (n = 1). One patient had a polymicrobial BSI with Escherichia coli and Pseudomonas aeruginosa detected by subsequent culture.
Only one patient had documented signs of severe sepsis aside from VS abnormalities (such as rigors or mental status changes) at the time of repeat blood culture collection. The patient presented with rigors and dyspnea due to a new BSI caused by Escherichia coli. The patient was not receiving antibiotics with Gram negative activity at the time of blood culture collection because of a systemic drug reaction attributed to multiple antibiotics. This patient was transferred to the intensive care unit at the time of presentation and ultimately died. The other patients diagnosed with new BSI after 24 hours of fever recovered with complete resolution in 14 (82%) cases; relapse of BSI with the same organism occurred in 2 (12%) cases.
Twelve patients had false positive results from cultures collected after 24 hours from episode onset. All received empiric therapy with vancomycin and 5 (42%) cases were treated as true BSI with 63 days of therapy with vancomycin and 3 days of therapy with nafcillin attributable to the false positive results. One patient had an implanted vascular port removed due to a false positive result.
Discussion
In this retrospective cohort study of children with persistent FN, repeat blood cultures contributed to the diagnosis of new BSI in 17 (9.2%) of 184 episodes. This diagnostic yield is clinically important, especially since many newly identified pathogens would not have responded to the standard empiric therapy for FN. Our results support the practice of repeating blood cultures in patients with persistent FN, but should prompt re-examination of the frequency of repetition. Although the yield of repeat cultures over the course of the episodes was appreciable, the yield of blood cultures collected on any single day after the first 24 hours of fever was only 1.5%, far lower than the yield of blood cultures obtained within 24 hours from fever onset. After 24 hours of fever, the 95% confidence intervals for the incidence of new BSI diagnosis and the incidence of false positive blood culture results overlap with one another, indicating similar rates of true positive and false positive results. When blood cultures are obtained every 24 hours in the setting of ongoing fevers, the majority of blood culture utilization occurs during the time of lowest diagnostic yield, and patients are subject to additional risks, including excessive phlebotomy, opportunities for introduction of new infection via blood sampling from central lines, and overtreatment for false positive results.
The per episode rate of new BSI diagnosed via subsequent blood culture in our study is similar to that found in a prior pediatric study, in which new BSI were identified via subsequent blood cultures in 24 (10.9%) of 220 episodes [13]. Unlike our study, the previous pediatric study did not include HSCT recipients, and excluded episodes in which BSI were diagnosed by initial blood culture. We did not find HSCT status or the initial blood culture result to be significant modifiers of risk for subsequent BSI. A similar study conducted in adult HSCT recipients found a lower yield of subsequent blood cultures, with only 1 (0.9%) of 109 patients having a new pathogen identified via subsequent blood culture [12]. Potential reasons for the discrepancy between adult and pediatric studies include differences in the epidemiology of BSI between children and adults with FN, lower yield of initial blood cultures in children, use of fluoroquinolone prophylaxis during neutropenia in adults, and differences in empiric therapy for FN between different study populations [12, 20]. Blood culture yield increases with the volume of the inoculum and the number of cultures obtained in a set; since children tend to have lower blood volumes inoculated and fewer cultures obtained, the false negative rate of initial blood cultures may be higher in children, thereby leading to higher rates of apparently new BSI identified by subsequent cultures [21, 22]. The majority of patients in the adult study received vancomycin as empiric therapy; this was not standard at our institution or in the other pediatric cohort study [12, 13]. In both pediatric studies, many of the BSI identified via subsequent cultures were caused by Gram positive organisms that may have been suppressed by vancomycin in the adult study [13].
Identification of specific predictors of new BSI diagnosis via subsequent blood cultures may enable the diagnostic strategy to be tailored. As in a previous pediatric study, we found prior history of BSI to be associated with increased risk of BSI identified by subsequent culture, however we found this to be a stronger risk factor when the prior BSI was diagnosed within 30 days of the current episode [13]. We found that new BSI are more likely to be detected with increasing time between recurrent fevers. Although ours and previous studies have used fever resolution for 96 hours to define the end of a fever episode, this definition may not be appropriate for clinicians who wish to determine whether a recurrent fever represents a new infection [6, 13]. Closer examination of this predictor did not reveal a threshold effect at any specific time point, but rather a steady increase in risk with increasing time between fevers. We did not find VS abnormalities helpful in predicting new BSI diagnosis by subsequent culture. VS abnormalities were common even in patients without BSI, likely reflecting the high prevalence of other serious conditions besides BSI in children with persistent FN, and limited specificity of existing VS cutoffs in children with cancer or undergoing HSCT [4–6].
Our study has limitations, the most important being the small number of new BSI identified by subsequent blood cultures. This limits the precision with which the yield of subsequent cultures is estimated and limits power to identify predictors associated with this event. Inclusion of multiple cultures per episode and multiple episodes per patient introduces clustering effects, which may bias results, however we used regression methods to account for clustering. Finally, the definition used to distinguish true BSI from false positive cultures is meant for surveillance of hospital-acquired infections, and may result in contaminant blood cultures or central line colonization being inappropriately classified as BSI [16, 23]. Several of the BSI identified in this study were caused by coagulase-negative Staphylococcus species and it is possible that some of these were misclassified; if this is the case, it would result in overestimation of the true yield of subsequent blood cultures.
The majority of persistent FN episodes in this study occurred in the setting of HSCT or during intensive phases of chemotherapy for hematologic malignancy, representing times of very high infectious risk [15]. It is possible that evaluating more episodes occurring during maintenance therapy for hematologic malignancy, or chemotherapy for solid tumor malignancies would have led to lower estimate of the yield of subsequent blood cultures, because of the overall lower risk for BSI in these settings [4]. However, our study population represents of the group of patients who experience extended durations of neutropenia and therefore are most likely to undergo repeat blood cultures for persistent FN [24]. Because the yield of subsequent cultures in our study was similar to the yield of subsequent cultures in a more general pediatric oncology population, our results may also be generalizable to other pediatric oncology settings [13].
In summary, repeat blood cultures have a role in diagnosis of new BSI over the course of persistent FN episodes, but the yield of cultures obtained per day of persistent FN is low enough to justify reducing the frequency of repetition. We suggest further evaluating a strategy to reduce the frequency of repeat cultures, such as to every 48 hours instead of every 24 hours. Clinical predictors may identify patients who require more frequent surveillance cultures, such as patients with recent history of BSI, or those with longer intervals between recurrent fevers. This would likely reduce the cost and adverse consequences of frequent testing, but the strategy should be evaluated prospectively to ensure that it is safe and avoids clinically significant delays in therapy for new BSI. A multicenter study would be ideal to ensure generalizability of results across different centers, and could enable further development of risk stratification tools to optimize the approach.
Acknowledgements
We thank Ashley A. Diaz for assistance with data collection, Robert E. Goldsby for assistance with study planning, W. John Boscardin for statistical consultation, and the UCSF Academic Research Systems Unit for data abstraction services. This research was supported by a Caring Wisely Pilot Award from the UCSF Center for Healthcare Value and by the Thrasher Early Career Award (TRF11939) to Dr. Wattier. Dr. Wattier receives training funding through NIGMS for the Training in Clinical Pharmacology, Drug Action and Pharmacogenetics Program (T32 GM007546). Dr. Auerbach was supported by NHLBI K24 K24HL098372. This project benefitted from consultation services of the UCSF Clinical and Translational Science Institute, supported by the National Center for Advancing Translational Sciences though UCSF-CTSI UL1 TR000004. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- FN
Fever and neutropenia
- BSI
Bloodstream infection
- HSCT
Hematopoietic stem cell transplant
- UCSF
University of California-San Francisco
- ANC
Absolute neutrophil count
- VS
Vital signs
- IQR
Interquartile range
- CI
Confidence interval
- OR
Odds ratio
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- 1.Auletta JJ, O’Riordan MA, Nieder ML. Infections in children with cancer: a continued need for the comprehensive physical examination. J Pediatr Hematol Oncol. 1999;21:501–508. [PubMed] [Google Scholar]
- 2.Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103:1916–1924. doi: 10.1002/cncr.20983. [DOI] [PubMed] [Google Scholar]
- 3.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, Martin PJ, Sandmaier BJ, Marr KA, Appelbaum FR, Storb R, McDonald GB. Reduced mortality after allogeneic hematopoietic-cell transplant. New Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castagnola E, Fontana V, Caviglia I, Caruso S, Faraci M, Fioredda F, Garre ML, Moroni C, Conte M, Losurdo G, Scuderi F, Bandettini R, Toma P, Viscoli C, Haupt R. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hematopoietic stem cell transplantation. Clin Infect Dis. 2007;45:1296–1304. doi: 10.1086/522533. [DOI] [PubMed] [Google Scholar]
- 5.Hakim H, Flynn PM, Knapp KM, Srivastava DK, Gaur AH. Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2009;31:623–629. doi: 10.1097/MPH.0b013e3181b1edc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akova M, Paesmans M, Calandra T, Viscoli C. International Antimicrobial Therapy Group of the European Organization for Research and Treatment of Cancer. A European Organization for Research and Treatment of Cancer-International Antimicrobial Therapy Group study of secondary infections in febrile, neutropenic patients with cancer. Clin Infect Dis. 2005;40:239–245. doi: 10.1086/426815. [DOI] [PubMed] [Google Scholar]
- 7.Villarroel M, Aviles CL, Silva P, Guzman AM, Becker A, O'Ryan M, Salgado C, Topelberg S, Tordecilla J, Varas M, Viviani T, Zubieta M, Santolaya ME. Risk factors associated with invasive fungal disease in children with cancer and febrile neutropenia: a prospective multicenter evaluation. Pediatr Infect J. 2010;29:816–821. doi: 10.1097/INF.0b013e3181e7db7f. [DOI] [PubMed] [Google Scholar]
- 8.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. J Am Med Assoc. 1991;265:365–369. [PubMed] [Google Scholar]
- 9.Thuler LC, Jenicek M, Turgeon JP, Rivard M, Lebel P, Lebel MH. Impact of a false positive blood culture result on the management of febrile children. Pediatr Infect Dis J. 1997;16:846–851. doi: 10.1097/00006454-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Alahmadi YM, Aldeyab MA, McElnay JC, Scott MG, Darwish Elhajii FW, Magee FA, Dowds M, Edwards C, Fullerton L, Tate A, Kearney MP. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect. 2011;77:233–236. doi: 10.1016/j.jhin.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Lehrnbecher T, Phillips R, Alexander S, Alvaro F, Fisher B, Hakim H, Santolaya M, Castagnola E, Davis BL, Dupuis LL, Gibson F, Groll AH, Gaur A, Gupta A, Kebudi R, Petrilli S, Steinbach WJ, Villarroel M, Zaoutis T, Sung L. Guidelines for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol. 2012;30:4427–4438. doi: 10.1200/JCO.2012.42.7161. [DOI] [PubMed] [Google Scholar]
- 12.Serody JS, Berrey MM, Albritton K, O'Brien SM, Capel EP, Bigelow SH, Weber DJ, Gabriel DA, Wiley JM, Schell MJ, Gilligan PH, Shea TC. Utility of obtaining blood cultures in febrile neutropenic patients undergoing bone marrow transplantation. Bone Marrow Transplant. 2000;26:533–538. doi: 10.1038/sj.bmt.1702535. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer. 2013;60:923–927. doi: 10.1002/pbc.24358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran TH, Yanofsky R, Johnston DL, Dix D, Gillmeister B, Ethier MC, Portwine C, Price V, Mitchell D, Cellot S, Lewis V, Zelcer S, Silva M, Michon B, Bowers L, Stobart K, Brossard J, Beyene J, Sung L. Second bacteremia during antibiotic treatment in children with acute myeloid leukemia: a report from the Canadian Infections in Acute Myeloid Leukemia Research Group. J Pediatr Infect Soc. 2014;3:228–233. doi: 10.1093/jpids/pit086. [DOI] [PubMed] [Google Scholar]
- 15.Orasch C, Weisser M, Mertz D, Conen A, Heim D, Christen S, Gratwohl A, Battegay M, Widmer A, Fluckiger U. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:521–526. doi: 10.1038/bmt.2009.187. [DOI] [PubMed] [Google Scholar]
- 16.Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Bonafide CP, Brady PW, Keren R, Conway PH, Marsolo K, Daymont C. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131:e1150–e1157. doi: 10.1542/peds.2012-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 19.Haque IU, Zaritsky AL. Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr Crit Care Med. 2007;8:138–144. doi: 10.1097/01.PCC.0000257039.32593.DC. [DOI] [PubMed] [Google Scholar]
- 20.Hann I, Viscoli C, Paesmans M, Gaya H, Glauser M. A comparison of outcome from febrile neutropenic episodes in children compared with adults: results from four EORTC studies. Br J Haematol. 1997;99:580–588. doi: 10.1046/j.1365-2141.1997.4453255.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaditis AG, O’Marcaigh AS, Rhodes KH, Weaver AI, Henry NK. Yield of positive blood cultures in pediatric oncology patients by a new method of blood culture collection. Pediatr Infect J. 1996;15:615–620. doi: 10.1097/00006454-199607000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Scheinemann K, Ethier MC, Dupuis LL, Richardson SE, Doyle J, Allen U, Sung L. Utility of peripheral blood cultures in bacteremic pediatric cancer patients with a central line. Support Care Cancer. 2010;18:913–919. doi: 10.1007/s00520-009-0725-0. [DOI] [PubMed] [Google Scholar]
- 23.Exline MC, Ali NA, Zikri N, Mangino JE, Torrence K, Vermillion B, St. Clair J, Lustberg ME, Pancholi P, Sopirala MM. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17:R41. doi: 10.1186/cc12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander SW, Wade KC, Hibberd PL, Parsons SK. Evaluation of risk prediction criteria for episodes of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2002;24:38–42. doi: 10.1097/00043426-200201000-00011. [DOI] [PubMed] [Google Scholar]