The inflammatory microenvironment is regulated by epidermal growth factor/epidermal growth factor receptor (EGF-EGFR) signaling and is associated with development of hepatocellular carcinoma (HCC). Precise mechanisms by which EGFR-dependent inflammation causes development of HCC have not been elucidated. In the recent Nature Cell Biology article, Lanaya et al.1 have shown that EGFR has different functions in Kupffer cells (KCs) and in hepatocytes and that the deletion of EGFR in microphages inhibited HCC, whereas deletion of EGFR in hepatocytes promotes HCC. The main results of this article are summarized in Fig. 1.
Figure 1.
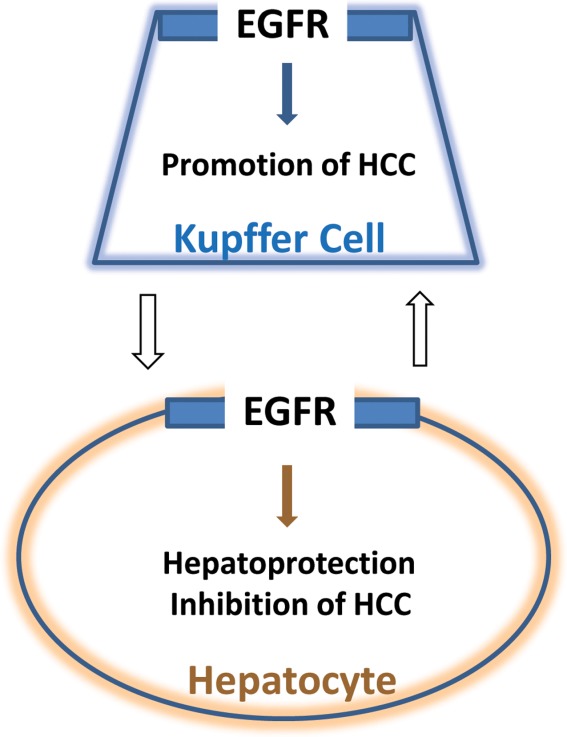
EGFR is elevated in KCs and promotes HCC, whereas EGFR plays different functions in hepatocytes. White arrows show that interaction between KCs and hepatocytes is likely to be involved in liver-specific EGFR functions.
Development of HCC is a multistep process that involves alterations in a number of signaling pathways that synergistically contribute to liver cancer. HCC is usually associated with inflammation and cirrhosis as preneoplastic stages.2 Although the link between inflammation and HCC has been well established, molecular mechanisms are not completely understood. The EGFR is a transmembrane protein receptor that might be activated by EGF and by several additional extracellular ligands. This activation triggers a variety of signaling pathways, including signal transducer and activator of transcription 3, phosphoinositide 3-kinase, Src homology and collagen, Src homology 1, and SH2 domain-containing inositol 5-phosphatase 2 and casitas B-cell lymphoma E3 ubiquitin ligase.3 The growth promotion activities of EGFR have been initially investigated in a partial hepatectomy model of liver proliferation/regeneration. It has been shown that hepatocyte-specific deletion of EGFR1 in mice and rats significantly inhibits liver proliferation after surgical resections.4,5 In agreement with this growth promotion role of EGFR, further studies revealed that expression of EGFR and copy numbers are increased in patients with HCC,6 suggesting that EGFR plays a critical role in development of HCC. These observations prompted clinical trials with inhibitors of EGFR signaling, which, unfortunately, did not show improvements at advanced stages of HCC.7 It is interesting that further studies of effects of an inhibitor of EGFR, erlotinib, on liver cancer in an orthotopic rat model of HCC showed no antitumor effect.8
These unsuccessful trials and negative results in animal models called into question whether our knowledge of the molecular basis for EGFR inflammation in HCC is sufficient for the generation of a strategy for treatments of patients with HCC. To better understand the role of EGFR in liver cancer, Lanaya et al. generated several animal models with a cell-type-specific deletion of EGFR within the liver and examined the development of liver cancer under conditions of diethylnitrosoamine (DEN)-mediated carcinogenesis.1 The response of wild-type (WT) livers to DEN includes DNA damage, apoptosis to remove death hepatocytes, followed by proliferation to replace the dead hepatocytes. The investigators showed that deletion of EGFR in all liver cells (EGFRΔMx, mice) leads to a significant decrease in proliferation and an increase in apoptosis. These initial studies were consistent with previous reports showing the tumor-promoting activities of EGRF. However, subsequent studies of liver tumor development in mice with deletion of EGFR in parenchymal cells (hepatocytes and bile duct cells, EGFRΔhepmice) provided surprising observations that livers of EGFRΔhep mice develop cancer significantly faster and with bigger sizes. The investigators also found that proliferation is significantly increased in livers of EGFRΔhep mice during development of HCC. On the other hand, EGFRΔhep mice were characterized by increased apoptosis, similar to EGFRΔMx mice.
The striking differences in development of liver cancer between EGFRΔhep mice and EGFRΔMx mice prompted the investigators to perform a detailed examination into development of HCC at different additional time points after injection of DEN. It has been found that damaged areas and serum alanine aminotransferase/aspartate aminotransferase levels are significantly increased in these two animal models post-DEN injection and that that necrotic response is also much stronger in EGFRΔhep and EGFRΔMx mice, clearly indicating that expression of EGFR in hepatocytes is required for hepatoprotection. Searching for molecular differences between WT and EGFRΔhep/EGFRΔMx mice, the investigators examined levels of several cytokines and found that expression of interleukin (IL)-1β is significantly increased post-DEN injections in both EGFR mutant mouse models.
The differences in development of HCC between EGFRΔhep and EGFRΔMx mice suggested that EGFR also displays a functional role in non-parenchymal cells (NPCs). Immunochemical examination of NPCs in EGFRΔhep tumors revealed a 4-fold increase of KCs/liver macrophages. To directly test the role of EGFR in KCs, the investigators have generated two additional mouse models that had a deletion of EGFR in both parenchymal cells and KCs and in KCs only. Examination of DEN-mediated liver tumor in these mice demonstrated that deletion of EGFR in KCs inhibits development of HCC. In agreement with these observations, expression of EGFR is increased in KCs of livers of WT mice post-DEN-mediated injury. A quite significant part of the article is a demonstration that EGFR-expressing KCs/liver macrophages are abundant in human HCC with poor prognosis. Examination of two large cohorts of patients with HCC from China and Europe showed that there is no relationship between increased expression of EGFR in hepatocytes to prognosis. However, tumor sections of HCC patients revealed high levels of EGFR in CD68 (macrophage marker)-positive cells, whereas adjacent nontumor tissues had no EGFR in CD68-positive cells. These studies demonstrated that increase of EGFR-positive macrophages in human HCC predicts a poor prognosis.
Identification of EGFR-expressing macrophages as the origin of HCC led to questioning the mechanisms by which EGFR signaling in macrophages promotes liver cancer. KCs produce IL-6 in response to IL-1β, which is derived from damaged hepatocytes. Examination of plasma of DEN-injected mice revealed a significant increase of IL-6 in EGFRΔhep/EGFRΔMx mice, but not in mice with macrophages-specific deletion of EGFR. Consistent with this observation, levels of IL-6 have been found to be increased in plasma of patients with HCC. Further studies showed that IL-1β induces IL-6 production in WT macrophages, but not in EGFR-deleted macrophages. In summary, the investigators showed that the mechanism of IL-1β-mediated activation of EGFR in macrophages includes induction of EGFR ligands and ADAM metallopeptidase domain 17 with subsequent phosphorylation of EGFR by p38 kinase.
The liver contains several cell types that communicate with one another and have the potential to be reprogrammed by specific transcription factors. Although cell-to-cell communications have been previously implicated in regulation of liver biology and development of liver cancer, the precise role of these communications in liver cancer has not been determined. The article by Lanaya et al. presents an excellent example of the studies of the role of EGFR in different cell types of the liver and the significance of these observations for treatments of HCC. Given the tumor-promoting role of EGFR in macrophages, the therapeutic approaches should be designed for a specific inhibition of EGFR only in macrophages and should not affect EGFR in parenchymal cells given that the latter scenario might promote tumorigenesis (see Fig. 1). The data from this article also suggest that inhibition of EGFR could be beneficial at early stages of liver cancer and that patients with advanced HCC will not benefit from EGFR-based therapy.
Author names in bold designate shared co-first authorship.
References
- Lanaya H, Natarajan A, Komposch K, Li L, Amberg N, Chen L, et al. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat Cell Biol. 2014;10:972–981. doi: 10.1038/ncb3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang H. Multiple interactive factors in hepatocarcinogenesis. Cancer Lett. 2014;346:17–23. doi: 10.1016/j.canlet.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Berasian C, Avila MA. The EGFR signaling system in the liver: from hepatoprotection to hepatocarcinogenesis. J Gastrointerol. 2014;49:9–23. doi: 10.1007/s00535-013-0907-x. [DOI] [PubMed] [Google Scholar]
- Natarajian A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;23:17081–17086. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe S, Bowen WC, Tseng GC, Luo JH, Orr A, Michalopoulos GK. RNA interference against hepatic epidermal growth factor receptor has suppressive effect on liver regeneration in rats. Am J Pathol. 2010;25:2669–2681. doi: 10.2353/ajpath.2010.090605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley AF, Burgart LJ, Sahai V, Kakar S. Epidermal growth factor receptor expression and gene copy number in conventional hepatocellular carcinoma. Am J Clin Pathol. 2008;129:245–251. doi: 10.1309/WF10QAAED3PP93BH. [DOI] [PubMed] [Google Scholar]
- Whittaker S, Marias R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Pinter M, Dauser B, Rohr-Udilova N, Piguet AC, Prager G, et al. Erlotinib and sorafenib in an orthotopic rat model of hepatocellular carcinoma. J Hepatol. 2012;57:592–599. doi: 10.1016/j.jhep.2012.04.034. [DOI] [PubMed] [Google Scholar]


