Abstract
Purpose
To date, there is no effective therapy for triple-negative breast cancer (TNBC), which has dismal clinical outcome. Upregulation of tissue factor (TF) expression leads to increased patient morbidity and mortality in many solid tumor types, including TNBC. Our goal is to employ the Fab fragment of ALT-836, a chimeric anti-human TF mAb, for PET imaging of TNBC, which can be used to guide future TNBC therapy.
Methods
ALT-836-Fab was generated by enzymatic papain digestion. SDS-PAGE and FACS studies were performed to evaluate the integrity and TF binding affinity of ALT-836-Fab before NOTA conjugation and 64Cu-labeling. Serial PET imaging and biodistribution studies were carried out to evaluate the tumor targeting efficacy and pharmacokinetics in MDA-MB-231 TNBC model, which expresses high level of TF on the tumor cells. Blocking studies, histological assessment, as well as RT-PCR was performed to confirm TF specificity of 64Cu-NOTA-ALT-836-Fab.
Results
ALT-836-Fab was produced with high purity, which exhibited superb TF binding affinity and specificity. Serial PET imaging revealed rapid and persistent tumor uptake of 64Cu-NOTA-ALT-836-Fab (5.1 ± 0.5 %ID/g at 24 h post-injection; n = 4) and high tumor/muscle ratio (7.0 ± 1.2 at 24 h post-injection; n = 4), several-fold higher than that of the blocking group and tumor models that do not express significant level of TF, which was confirmed by biodistribution studies. TF specificity of the tracer was also validated by histology and RT-PCR.
Conclusions
64Cu-NOTA-ALT-836-Fab exhibited prominent tissue factor targeting efficiency in MDA-MB-231 TNBC model. The use of a Fab fragment led to fast tumor uptake and good tissue/muscle ratio, which may be translated into same-day immunoPET imaging in the clinical setting to improve TNBC patient management.
Keywords: Triple-Negative Breast Cancer (TNBC), Positron emission tomography (PET), Tissue Factor (TF), Antibody fragment, Fab
Introduction
Triple negative breast cancer (TNBC) is among the most common types of breast cancer, which does not express estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER-2) [1]. Almost 15% of the patient population with invasive breast cancer are diagnosed as TNBC, and there is no effective diagnosis method or treatment regimen due to the lack of targets for effective delivery of imaging probes and therapeutic agents [1, 2]. Most TNBC is aggressive, which is characterized with early dissemination and high risk of relapse [2]. Patients with TNBC tend to have a poor survival rate compared with those from other breast cancer types [3]. Therefore, the seeking of targets to boost diagnosis and treatment efficiency of TNBC has attracted tremendous interest from global researchers.
Tissue factor (TF), also known as platelet tissue factor, factor III, thrombokinase, or CD142, has been confirmed to be abundantly present on TNBC cells (e.g. MDA-MB-231) [4, 5]. TF plays various important roles in cancer cell signaling, including the inhibition of apoptosis and promotion of cell migration [6]. Numerous animal and patient studies have pointed out the impacts of TF on TNBC progression [7-9]. Importantly, tumor TF expression level can serve as a prognostic index, since high TF expression usually contributes to a decreased overall survival rate in TNBC patients [10, 11]. Based on the above-mentioned findings, TF could be adopted as a promising target for effective diagnosis and therapy of TNBC. To the best of our knowledge, TF has not been tested with in vivo targeting and imaging of TNBC to date.
ALT-836, a chimeric antihuman TF monoclonal antibody that binds to the factor X-binding site in TF with subnanomolar affinity [12, 13], has been employed in this study for TF targeting. Recently, a phase 2 clinical trial using ALT-836 in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) has been completed (Clinical Trials.gov Identifier: NCT00879606), and the results indicated that ALT-836 was well tolerated and beneficial to the whole patient population. In addition, a clinical trial in which ALT-836 will be used against solid tumors with elevated TF level has also been initiated (Clinical Trials.gov Identifier: NCT01325558).
Positron emission tomography (PET) imaging was applied in this study to examine the TF targeting of an ALT-836-based imaging agent in a mouse TNBC model. Although antibody-based imaging agents possess optimal tumor accumulation, one of the major obstacles for PET imaging with an intact antibody is the prolonged circulation half-life [14]. The tumor uptake might not reach the peak until a few days after injection [12]. As alternatives, antibody fragments, which maintain the targeting specificity and acquire rapid blood clearance, have been utilized for PET imaging [15-19]. IgG type antibodies are typically composed of Fab and Fc, with Fab containing the antigen-binding sites [19]. The specificity of ALT-836-Fab for TF on TNBC cells was assessed here for the potential application as an imaging agent for TNBC.
In this study, 64Cu was introduced as a radiolabel for ALT-836-Fab via 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA). Various in vitro, in vivo, and ex vivo studies were performed to validate the expression of TF in the TNBC model and correlate the tumor uptake of 64Cu-NOTA-ALT-836-Fab with TF expression. Rapid blood clearance and TF-specific tumor uptake from 64Cu-NOTA-ALT-836-Fab were witnessed.
Materials and methods
Chemicals
ALT-836 was provided by Altor Bioscience Corporation (Miramar, FL). S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) was purchased from Macrocyclics, Inc. (Dallas, TX). Chelex 100 resin (50-100 mesh) and fluorescein isothiocyanate (FITC) were acquired from Sigma-Aldrich (St. Louis, MO). Rat anti-mouse CD31 primary antibody was purchased from BD Biosciences (San Diego, CA). AlexaFluor488- and Cy3-labeled secondary antibodies were purchased from Jackson Immunoresearch Laboratories, Inc. (West Grove, CA). PD-10 desalting columns were purchased from GE Healthcare (Piscataway, NJ). 64Cu was produced by a GE PETrace cyclotron using the 64Ni(p,n)64Cu reaction, which has specific activity of > 5 Ci/μmol at the end of bombardment. Water and all buffers were of Millipore grade and pretreated with Chelex 100 resin to ensure that the aqueous solution was free of heavy metals. All other reaction buffers and chemicals were from Thermo Fisher Scientific.
Generation and characterization of ALT-836-Fab
The production of ALT-836-Fab was carried out by digestion of ALT-836 (5 mg/mL) by immobilized papain (weight ratio: papain/ALT-836 = 1:40) in a reaction buffer (20 mM sodium phosphate dibasic, 10 mM disodium ethylenediaminetetraacetic acid (EDTA), and 80 mM L-Cysteine hydrochloride) for 4 h at 37 °C under constant stirring [15, 19]. Subsequently, the supernatant was collected and purified by a Sephadex G-75 size exclusion column using phosphate-buffered saline (PBS) as the mobile phase. The elution from Sephadex G-75 size exclusion column was examined by UV absorbance at 280 nm [20]. The purity of ALT-836-Fab was evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 5 % stacking gel and 8 % resolving gel), which was run under nonreducing conditions and stained by Coomassie brilliant blue R-250 [21].
Antibody fragment conjugation
ALT-836-Fab was reacted with p-SCN-Bn-NOTA or FITC in a similar buffer condition, at a molar ratio of 1:10 at pH 9.0 for 2 h [12]. The final products (NOTA-ALT-836-Fab or FITC-ALT-836-Fab) were purified by PD-10 size exclusion columns with PBS as the mobile phase.
Cell lines and animal models
MDA-MB-231 (high TF expression) [22-25] and MDA-MB-435 (low TF expression) [23] human breast carcinoma cell lines were both obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured according to the supplier’s instructions. Cells were used for in vitro and in vivo experiments when they reached ~80% confluence. All animal studies were conducted under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. Four to five-week-old female nude mice (Harlan, Indianapolis, IN) were each injected with 2 × 106 MDA-MB-231 or MDA-MB-435 cells subcutaneously in the right flank. The tumor sizes were monitored daily and the mice were used for in vivo experiments when the tumor diameter reached 5-8 mm.
Flow cytometry
MDA-MB-231 and MDA-MB-435 cells were harvested and suspended in cold PBS with 2% bovine serum albumin at a concentration of 5 × 106 cells/mL, incubated with FITC-ALT-836-Fab at different concentrations (1 μg/mL and 5 μg/mL) for 30 min at room temperature, centrifuged at 1,000 rpm for 5 min, and washed three times with cold PBS. Subsequently, the cells were analyzed using a BD FACSCalibur 4-color analysis cytometer (Becton-Dickinson, San Jose, CA) and FlowJo analysis software (Tree Star, Inc., Ashland, OR).
Radiolabeling
64Cu was produced with an onsite cyclotron (GE PETrace). 64CuCl2 (74 MBq) was diluted in 300 μL of 0.1 M sodium acetate buffer (pH 5.5) and mixed with 200 μL of NOTA-ALT-836-Fab (0.3 mg/mL). The reaction was conducted at 37 °C for 30 min with constant shaking. The resulting 64Cu-NOTA-ALT-836-Fab was purified by PD-10 size exclusion column chromatography, using PBS as the mobile phase. The radioactive fraction containing 64Cu-NOTA-ALT-836-Fab was collected for further in vitro and in vivo studies.
PET imaging and biodistribution studies
At different time points post-injection (p.i.) of 5-10 MBq of 64Cu-NOTA-ALT-836-Fab via tail vein, PET scans of MDA-MB-231 and MDA-MB-435 tumor-bearing mice (4 mice per group) were carried out using a microPET/microCT Inveon rodent model scanner (Siemens Medical Solutions USA, Inc.). Data acquisition, image reconstruction, and region-of-interest (ROI) analysis of the PET data were performed as previously described [12, 19]. Briefly, the images were acquired by 10-min static PET scans and reconstructed using maximum a posteriori (MAP) algorithm, without attenuation or scatter correction. ROI analysis of each PET scan was carried out using software (Inveon Research Workplace, IRW) based on decay-corrected whole-body images, calculated with the injected dose measured by a dose calibrator (Capintec, Inc., Ramsey, NJ). Quantitative PET data of the tumor and major organs was presented in the format of percentage injected dose per gram of tissue (%ID/g). After the last scan at 24 h p.i., biodistribution studies were performed to corroborate PET data. Mice were euthanized and tumor, blood and major organs/tissues were collected and wet-weighed. The radioactivity in the tissue was measured using a γ counter (PerkinElmer) and presented as %ID/g (mean ± SD). To confirm the in vivo targeting specificity, blocking test was also performed in MDA-MB-231 tumor-bearing mice after pre-injection of 2 mg ALT-836 intact antibody.
Histology
MDA-MB-231 and MDA-MB-435 tumor-bearing mice were euthanized to collect positive and negative tumor sample respectively. Liver, spleen and muscle were collected from MDA-MB-231 tumor-bearing mice as controls. Collected tissues were frozen and cryo-sectioned for histological analysis. Frozen tissue slices of 7 μm thickness were fixed with cold acetone and stained for endothelial marker CD31 by using rat anti-mouse CD31 antibody as the primary antibody and Cy3-labeled donkey anti-rat IgG as the secondary antibody. To stain TF, the same tissue slices were also incubated with ALT-836 as the primary antibody and AlexaFluor488-labeled goat anti-human IgG as the secondary antibody. All images were acquired by using a Nikon Eclipse Ti microscope.
Quantitative real time RT-PCR
To confirm TF expression in both MDA-MB-231 and MDA-MB-435 tumors, quantitative real time RT-PCR was performed. MDA-MB-231 and MDA-MB-435 tumor tissues were homogenized in an icy environment, and the total RNA was isolated using Qiagen RNeasy Minikit (Qiagen Inc., Valencia, CA), reversely transcribed and amplified using Qiagen OneStep RT-PCR kit (Qiagen Inc., Valencia, CA). Real time PCR was performed with Bio-Rad MyIQ detection system, using TaqMan primers for TF (Hs01076029_ml F3) and GAPDH (Mm99999915_g1 Gapdh) (Applied Biosystems, Carlsbad, CA). The PCR product was run on 0.5% agarose gel under 100 V for 1 h [26].
Results
Generation and characterization of ALT-836-Fab
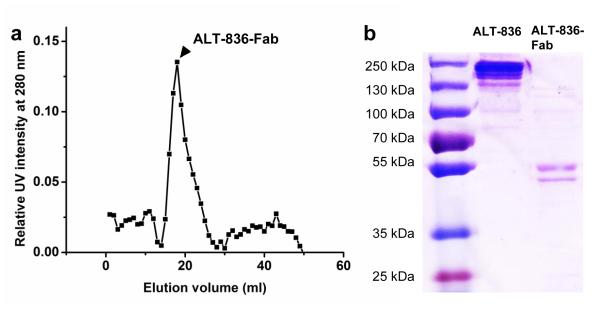
ALT-836 was digested with Papain in a reaction buffer at 37 °C [19]. After papain digestion, ALT-836-Fab was separated from the reaction mixture through a Sephadex G-75 column (fractionation range 3,000-80,000 Da) using PBS as the mobile phase. The elution profile was tested under a 280 nm UV spectrum (Fig. 1a) and elution factions from 17-19 mL (peak ALT-836-Fab concentration) were used in later experiments. The results acquired from SDS-PAGE suggested a molecular weight of 50-55 kDa for ALT-836-Fab with high purity (Fig. 1b).
Fig. 1.
Purification and characterization of ALT-836-Fab. a Elution profile of ALT-836-Fab from a Sephadex G-75 column (arrowhead: the single fraction used for further in vitro and in vivo studies. b SDS-PAGE of ALT-836 (left lane) and ALT-836-Fab (right lane).
In vitro TF targeting
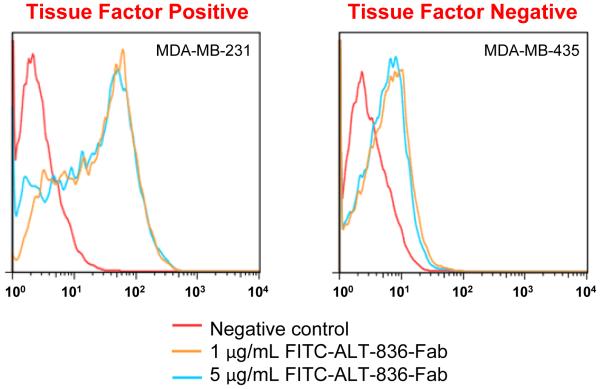
Before in vivo studies, flow cytometry was performed to examine the targeting efficiency and specificity of ALT-836-Fab in vitro. After treatment with FITC-ALT-836-Fab at a low concentration (1 μg/mg), the fluorescence signal from MDA-MB-231 cells (TF positive) exhibited a 25-fold increase over the negative group, whereas that from MDA-MB-435 cells (TF negative) only exhibited 4-fold increase (n = 3; Fig. 2). This result clearly demonstrated the TF specificity of ALT-836-Fab.
Fig. 2.
Flow cytometry analysis of ALT-836-Fab at different concentrations (1 μg/mL and 5 μg/ml) in MDA-MB-231 (TF positive) and MDA-MB-435 (TF negative) cells. All data are repeated 3 times per group.
In vivo TF targeting and ex vivo biodistribution studies
NOTA was conjugated to ALT-836-Fab as a chelator, since NOTA has been confirmed as one of the most potent chelators for 64Cu labeling, and 64Cu-NOTA complex demonstrated excellent stability both in serum and in vivo [27]. After 64Cu labeling, the resulting conjugate (64Cu-NOTA-ALT-836-Fab) was purified with a size exclusion column and then injected to tumor bearing mice intravenously. Same as in vitro studies, MDA-MB-231 tumor served as the positive group, while MDA-MB-435 tumor served as the negative group.
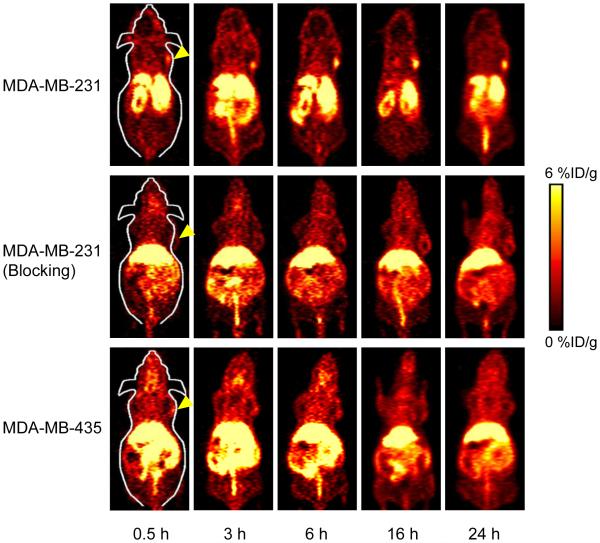
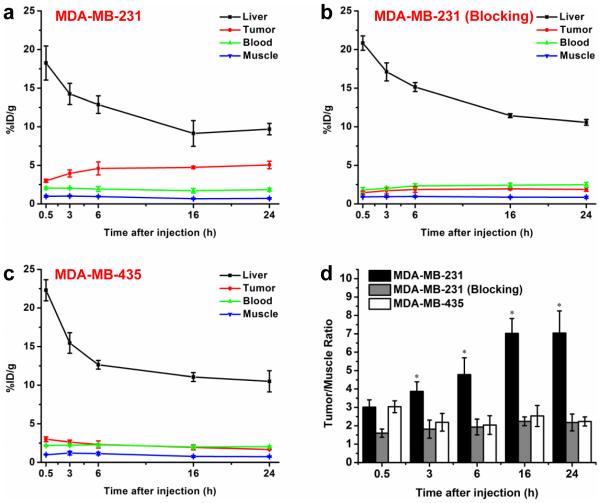
Coronal PET imaging was applied using a microPET/microCT Inveon rodent model scanner at 0.5, 3, 6, 16, 24 h p.i. The PET slice images with tumor uptake in different groups are shown in Fig. 3 and quantitative ROI analysis is shown in Fig. 4. The results show that MDA-MB-231 tumors became readily visible as early as 0.5 h p.i. (3.0 ± 0.2 %ID/g; n = 4) and remained relatively stable until 24 h p.i. (3.9 ± 0.5, 4.6 ± 0.8, 4.7 ± 0.2, 5.1 ± 0.5 %ID/g at 3, 6, 16, and 24 h p.i., respectively; n = 4; Fig. 3 and 4a). A blocking test was also carried out on MDA-MB-231 tumor-bearing mice after pre-injection of 2 mg ALT-836 to validate the TF specificity of 64Cu-NOTA-ALT-836-Fab in vivo. Tumor uptake in the blocking group was very low throughout the scan (1.5 ± 0.2, 1.7 ± 0.4, 1.9 ± 0.4, 2.0 ± 0.1, 1.9 ± 0.2 %ID/g at 0.5, 3, 6, 16, and 24 h p.i., respectively; n = 4; Fig. 3 and 4b), suggesting excellent in vivo targeting specificity and minimal non-specificity binding. On the other hand, the tumor uptake of 64Cu-NOTA-ALT-836-Fab in TF-negative MDA-MB-435 tumor was significantly lower at all time points examined (3.0 ± 0.3%ID/g at 0.5 h p.i., and gradually decreasing with time: 2.6 ± 0.3, 2.3 ± 0.5 1.9 ± 0.3, 1.7 ± 0.1 %ID/g at 3, 6, 16, and 24 h p.i., respectively; n = 4; Fig. 3 and 4c). Taken together, a significant enhancement of tumor uptake has been achieved with ALT-836-Fab (2.7-fold higher than the blocking group and 3.0-fold higher than the negative group at 24 h p.i.), suggesting that ALT-836-Fab could specifically target TF in TNBC.
Fig. 3.
Serial coronal PET images at different time points post-injection of 64Cu-NOTA-ALT-836-Fab were acquired in MDA-MB-231 tumor-bearing mice, MDA-MB-231 tumor-bearing mice pre-injected with 2 mg ALT-836 (blocking), and MDA-MB-435 tumor-bearing mice.
Fig. 4.
Quantitative analysis of the PET data. a Time activity curves of the liver, MDA-MB-231 tumor, blood, and muscle upon intravenous injection of 64Cu-NOTA-ALT-836-Fab (positive group). b Time activity curves of the liver, MDA-MB-231 tumor, blood, and muscle upon intravenous injection of 64Cu-NOTA-ALT-836-Fab after pre-injection of 2 mg ALT-836 (blocking group). c Time activity curves of the liver, MDA-MB-435 tumor, blood, and muscle upon intravenous injection of 64Cu-NOTA-ALT-836-Fab (negative group). d Comparison of tumor/muscle ratio in positive, blocking and negative groups. The differences of the tumor uptake and tumor/muscle ratio in three groups were statistically significant (P < 0.05) at all time points except 0.5 h. All data represent 4 mice per group.
Due to the smaller size of the Fab fragment (50-55 kDa) compared to the intact antibody (~150 kDa), Fab could be cleared through both the hepatobiliary and renal pathways (Fig. 3). The tracer accumulation in the liver and blood was similar in all three groups (MDA-MB-231, MDA-MB-231 with blocking, and MDA-MB-435; Fig. 4a, b and c), confirming that 64Cu-NOTA-ALT-836-Fab maintained the same pharmacokinetics, and the difference of tumor uptake was only caused by specific TF targeting. More importantly, the radioactivity in the blood was low from the first time point examined, validating the rapid blood clearance of 64Cu-NOTA-ALT-836-Fab. Improved tumor contrast was achieved in MDA-MB-231 (tumor/muscle ratio: 3.0 ± 0.4, 3.9 ± 0.5, 4.8 ± 0.9 7.0 ± 0.8, 7.0 ± 1.2 at 0.5, 3, 6, 16, and 24 h p.i., respectively; n = 4; Fig. 4d), in comparison with that in the blocking group (tumor/muscle ratio: 1.6 ± 0.2, 1.8 ± 0.5, 1.9 ± 0.4 2.2 ± 0.2, 2.2 ± 0.5 at 0.5, 3, 6, 16, and 24 h p.i., respectively; n = 4) and MDA-MB-435 (tumor/muscle ratio: 3.0 ± 0.3, 2.2 ± 0.5, 2.0 ± 0.5 2.5 ± 0.6, 2.2 ± 0.2 at 0.5, 3, 6, 16, and 24 h p.i., respectively; n = 4), highlighting the potential of ALT-836-Fab as a good contrast agent for TF delineation in TNBC.
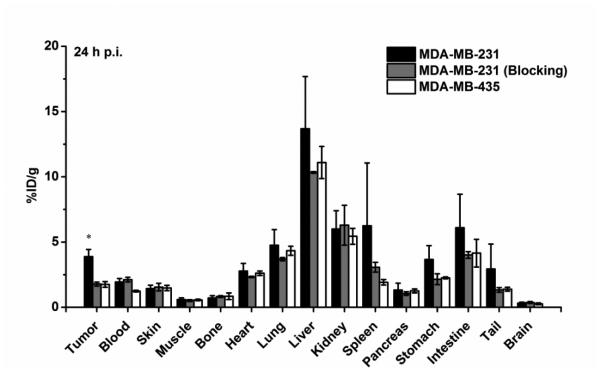
After the last scan at 24 h p.i., mice were sacrificed for biodistribution studies to validate the ROI analysis of PET images (Fig. 5). The quantitative results based on biodistribution studies and ROI analysis matched very well, suggesting that ROI analysis from PET accurately reflected the distribution of 64Cu-NOTA-ALT-836-Fab in tumor-bearing mice.
Fig. 5.
Biodistribution of 64Cu-NOTA-ALT-836-Fab in MDA-MB-231 tumor-bearing mice (positive group), MDA-MB-231 tumor-bearing mice pre-injected with 2 mg ALT-836 (blocking group), and MDA-MB-435 tumor-bearing mice (negative group) at 24 h post-injection (n = 4).
Histology
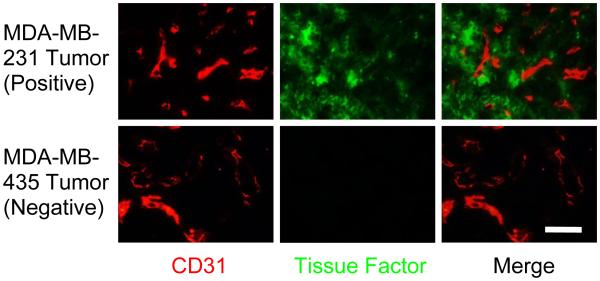
Histological studies were performed to validate the expression level of TF in positive and negative tumors (Fig. 6). Red fluorescence indicated the expression of CD31, which represents the location of vasculature, whereas green fluorescence demonstrated the expression of TF, which is the target of ALT-836-Fab. In MDA-MB-231 tumor, a significantly high TF expression level was achieved, and the TF expression did not overlap with the location of vasculature. In MDA-MB-435 tumor, TF expression was relatively low. Taken together, the histology study further demonstrated the TF specificity of ALT-836-Fab.
Fig. 6.
Immunofluorescence TF/CD31 double-staining of MDA-MB-231 tumor and MDA-MB-435 tumor. Scale bar: 100 μm.
Quantitative real time RT-PCR
Real time RT-PCR was applied to further confirm the TF expression level in both MDA-MB-231 and MDA-MB-435 human breast carcinoma. The TF expression in the MDA-MB-231 tumor (CT = 21.7 ± 0.2 min, where CT denotes critical time; n = 3) was significantly higher than that in the MDA-MB-435 tumor (CT = 29.1 ± 0.2 min; n = 3), while the expression levels of the positive control GAPDH in both tumors are similar (CT ≈ 15.2 ± 0.4 min; n = 3; Fig. 7). DNA gel electrophoresis confirmed real time RT-PCR results (Fig. 7a).
Fig. 7.
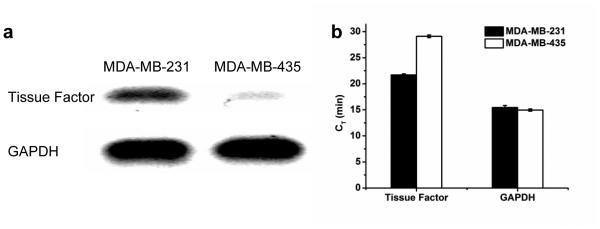
Quantitative real time RT-PCR of TF expression in MDA-MB-231 and MDA-MB-435 tumors. a Agarose gel electrophoresis and b Critical time of RT-PCR products proved that TF has higher expression in the MDA-MB-231 tumor than the MDA-MB-435 tumor.
Discussion
There is no effective management for TNBC, which has dismal clinical outcome [28-30]. To date, most of the studies on PET imaging of TNBC are performed with 18F-fluoro-deoxy-glucose (18F-FDG) [31-33]. Although conventional 18F-FDG PET imaging has been proven to be useful for the detection of TNBC in vivo, poor specificity is a major concern that limits the application of 18F-FDG in clinical setting, since 18F-FDG can only represent the metabolic level and fail to recognize the information from tumors at cellular/molecular level. Therefore, the pursuit of novel targeting agents of TNBC is highly desired.
Antibody/antibody fragment could function as alternative imaging probes for diagnosis and therapy of TNBC with superior specificity. TF has been found to be highly expressed in several human TNBC cell lines, including MDA-MB-231, MDA-MB-468, and HCC-1806 [34]. In this study, we achieved excellent TNBC imaging with 64Cu-NOTA-ALT-836-Fab in MDA-MB-231 TNBC xenografts. Since TF plays a critical role in cancer cell signaling for tumor growth and metastasis [6], the inhibition of TF might open another window for effective treatment of TNBC in the future, which now has limited therapeutic options.
One of the best advantages of Fab fragment is its quick blood clearance. Compared with the full antibody which has a prolonged circulation half-life and high background, 64Cu-ALT-836-Fab exhibited very low background signal as early as 0.5 h p.i. (~ 2 %ID/g in blood and ~ 1 %ID/g in muscle, Fig. 4a). The quick blood clearance endows 64Cu-ALT-836-Fab with superior tumor contrast, making it applicable to other cancer types in organs with abundant blood flow, such as lung cancer. Antibody fragments could be cleared through both the hepatobiliary and renal pathways. The clearance varies with different Fab fragments, possibly dependent on their size, structure, and hydrohilicity. In this study, ALT-836-Fab was cleared predominantly by the liver, whereas other antibody fragments, such as TRC105-Fab and Cetuximab-Fab, were mainly cleared by kidney [19, 35]. Therefore, the types of Fabs should be correctly selected for different cancers. The other advantage of Fab is the prompt tumor uptake, and hence ALT-836-Fab may be translated into same-day immunoPET imaging in the clinical setting to improve TNBC patient management. Alternative short half-life isotopes (e.g. 61Cu, 45 Ti, 44Sc, 68Ga) could also be used as the radiolabel for the PET imaging with Fab. The limitation of Fab is lower binding avidity compared to the full antibody, since Fab has only one antigen-binding site. For example, the uptake in positive tumor (MDA-MB-231) was only 5.1 %ID/g in this study, which is ~3-fold higher than negative tumor (MDA-MB-435) and ~2.5-fold higher than blood. This might restrict the future application of Fabs in tumor imaging. If high absolute tumor uptake rather than high tumor contrast is required, Fab may not be very suitable [12].
Radiolabeling stability is a common concern for PET imaging, since PET only detects the location of the radiolabel. We have demonstrated in previous studies that 64Cu is very stably bound to NOTA, which is one of the best chelators for 64Cu [27, 36]. NOTA-ALT-836-Fab is also easily applicable to 68Ga labeling, which can potentially make the clinical translation much easier. We have also demonstrated that the conjugation of NOTA and radiolabeling did not affect the targeting efficiency and specificity of ALT-836 [12]. Therefore, as-designed imaging probe in this study truly represented the TF expression in TNBC.
Conclusion
Herein, we reported the generation, characterization of ALT-836-Fab and used it for PET imaging of TF expression in triple-negative breast cancer. Rapid, persistent, and TF-specific uptake of 64Cu-NOTA-ALT-836-Fab was achieved in MDA-MB-231 tumors. Because of the quick blood clearance and tumor uptake, 64Cu-NOTA-ALT-836-Fab could be potentially translated into same day immunoPET imaging to improve patient management. Considering the current limited diagnosis and therapy options for TNBC, TF targeting and imaging could pave the way for effective delivery of imaging/therapy agents to TNBC.
Acknowledgements
This work is supported, in part, by the University of Wisconsin-Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365, P30CA014520, and T32CA009206), the Department of Defense (W81XWH-11-1-0644 and W81XWH-11-1-0648), and the American Cancer Society (125246-RSG-13-099-01-CCE).
Footnotes
Conflicts of interest Bai Liu and Hing C. Wong are employees of Altor Bioscience Corporation. The other authors declare that they have no conflict of interest.
Statement of human rights This article does not contain any studies with human participants performed by any of the authors.
Statement on the welfare of animals All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. New Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Terwisscha van Scheltinga AG, Berghuis P, Nienhuis HH, Timmer-Bosscha H, Pot L, Gaykema SB, et al. Visualising dual downregulation of insulin-like growth factor receptor-1 and vascular endothelial growth factor-A by heat shock protein 90 inhibition effect in triple negative breast cancer. Eur J Cancer. 2014;50:2508–16. doi: 10.1016/j.ejca.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–92. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 4.Callander NS, Varki N, Rao LV. Immunohistochemical identification of tissue factor in solid tumors. Cancer. 1992;70:1194–201. doi: 10.1002/1097-0142(19920901)70:5<1194::aid-cncr2820700528>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Vrana JA, Stang MT, Grande JP, Getz MJ. Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: paracrine regulation by carcinoma cell-derived members of the transforming growth factor beta family. Cancer Res. 1996;56:5063–70. [PubMed] [Google Scholar]
- 6.Jiang X, Bailly MA, Panetti TS, Cappello M, Konigsberg WH, Bromberg ME. Formation of tissue factor-factor VIIa-factor Xa complex promotes cellular signaling and migration of human breast cancer cells. J Thromb Haemost. 2004;2:93–101. doi: 10.1111/j.1538-7836.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- 7.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209–15. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 8.Ruf W, Yokota N, Schaffner F. Tissue factor in cancer progression and angiogenesis. Thromb Res. 2010;125(Suppl 2):S36–8. doi: 10.1016/S0049-3848(10)70010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryden L, Grabau D, Schaffner F, Jonsson PE, Ruf W, Belting M. Evidence for tissue factor phosphorylation and its correlation with protease-activated receptor expression and the prognosis of primary breast cancer. Int J Cancer. 2010;126:2330–40. doi: 10.1002/ijc.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 11.Ueno T, Toi M, Koike M, Nakamura S, Tominaga T. Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. Br J Cancer. 2000;83:164–70. doi: 10.1054/bjoc.2000.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong H, Zhang Y, Nayak TR, Engle JW, Wong HC, Liu B, et al. Immuno-PET of tissue factor in pancreatic cancer. J Nucl Med. 2012;53:1748–54. doi: 10.2967/jnumed.112.105460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao JA, Kelly AB, Marzec UM, Nieves E, Acevedo J, Burkhardt M, et al. Inhibition of acute vascular thrombosis in chimpanzees by an anti-human tissue factor antibody targeting the factor X binding site. Thromb Haemost. 2010;103:224–33. doi: 10.1160/TH09-06-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu AM, Olafsen T. Antibodies for molecular imaging of cancer. Cancer J. 2008;14:191–7. doi: 10.1097/PPO.0b013e31817b07ae. [DOI] [PubMed] [Google Scholar]
- 15.Andrew SM, Pimm MV, Perkins AC, Baldwin RW. Comparative imaging and biodistribution studies with an anti-CEA monoclonal antibody and its F(ab)2 and Fab fragments in mice with colon carcinoma xenografts. Eur J Nucl Med. 1986;12:168–75. doi: 10.1007/BF00256915. [DOI] [PubMed] [Google Scholar]
- 16.Hoeben BA, Kaanders JH, Franssen GM, Troost EG, Rijken PF, Oosterwijk E, et al. PET of hypoxia with 89Zr-labeled cG250-F(ab')2 in head and neck tumors. J Nucl Med. 2010;51:1076–83. doi: 10.2967/jnumed.109.073189. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths GL, Goldenberg DM, Roesch F, Hansen HJ. Radiolabeling of an anti-carcinoembryonic antigen antibody Fab' fragment (CEA-Scan) with the positron-emitting radionuclide Tc-94m. Clin Cancer Res. 1999;5:3001s–3s. [PubMed] [Google Scholar]
- 18.Yoshida C, Tsuji AB, Sudo H, Sugyo A, Sogawa C, Inubushi M, et al. Development of positron emission tomography probe of 64Cu-labeled anti-C-kit 12A8 Fab to measure protooncogene C-kit expression. Nucl Med Biol. 2011;38:331–7. doi: 10.1016/j.nucmedbio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Hong H, Orbay H, Valdovinos HF, Nayak TR, Theuer CP, et al. PET imaging of CD105/endoglin expression with a (6)(1)/(6)(4)Cu-labeled Fab antibody fragment. Eur J Nucl Med Mol Imaging. 2013;40:759–67. doi: 10.1007/s00259-012-2334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rousseaux J, Rousseaux-Prevost R, Bazin H. Optimal conditions for the preparation of Fab and F(ab')2 fragments from monoclonal IgG of different rat IgG subclasses. J Immunol Methods. 1983;64:141–6. doi: 10.1016/0022-1759(83)90392-7. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Nayak TR, Goel S, Valdovinos HF, Hong H, Theuer CP, et al. In Vivo Tumor Vasculature Targeted PET/NIRF Imaging with TRC105(Fab)-Conjugated, Dual-Labeled Mesoporous Silica Nanoparticles. Mol Pharm. 2014 doi: 10.1021/mp500306k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewett PW, Murray C. Modulation of human endothelial cell procoagulant activity in tumour models in vitro. Int J Cancer. 1996;66:784–9. doi: 10.1002/(SICI)1097-0215(19960611)66:6<784::AID-IJC13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Hjortoe GM, Petersen LC, Albrektsen T, Sorensen BB, Norby PL, Mandal SK, et al. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood. 2004;103:3029–37. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J, Stenlinder M, Linder S. Tissue factor mRNA expression in breast carcinoma cells. Oncol Rep. 1996;3:443–5. doi: 10.3892/or.3.3.443. [DOI] [PubMed] [Google Scholar]
- 25.Zhou JN, Ljungdahl S, Shoshan MC, Swedenborg J, Linder S. Activation of tissue-factor gene expression in breast carcinoma cells by stimulation of the RAF-ERK signaling pathway. Mol Carcinog. 1998;21:234–43. [PubMed] [Google Scholar]
- 26.Orbay H, Zhang Y, Hong H, Hacker TA, Valdovinos HF, Zagzebski JA, et al. Positron emission tomography imaging of angiogenesis in a murine hindlimb ischemia model with 64Cu-labeled TRC105. Mol Pharm. 2013;10:2749–56. doi: 10.1021/mp400191w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Hong H, Engle JW, Bean J, Yang Y, Leigh BR, et al. Positron emission tomography imaging of CD105 expression with a 64Cu-labeled monoclonal antibody: NOTA is superior to DOTA. PLoS One. 2011;6:e28005. doi: 10.1371/journal.pone.0028005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ademuyiwa FO, Ellis MJ, Ma CX. Neoadjuvant therapy in operable breast cancer: application to triple negative breast cancer. J Oncol. 2013;2013:219869. doi: 10.1155/2013/219869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16(Suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 30.Schmadeka R, Harmon BE, Singh M. Triple-negative breast carcinoma: current and emerging concepts. Am J Clin Pathol. 2014;141:462–77. doi: 10.1309/AJCPQN8GZ8SILKGN. [DOI] [PubMed] [Google Scholar]
- 31.Tchou J, Sonnad SS, Bergey MR, Basu S, Tomaszewski J, Alavi A, et al. Degree of tumor FDG uptake correlates with proliferation index in triple negative breast cancer. Mol Imaging Biol. 2010;12:657–62. doi: 10.1007/s11307-009-0294-0. [DOI] [PubMed] [Google Scholar]
- 32.Hama Y, Nakagawa K. Early distant relapse in early stage triple-negative breast cancer: usefulness of FDG-PET for diagnosis of distant metastases. Breast Cancer. 2013;20:191–3. doi: 10.1007/s12282-009-0195-8. [DOI] [PubMed] [Google Scholar]
- 33.Dogan BE, Turnbull LW. Imaging of triple-negative breast cancer. Ann Oncol. 2012;23(Suppl 6):vi23–9. doi: 10.1093/annonc/mds191. [DOI] [PubMed] [Google Scholar]
- 34.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/"triple-negative" breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–26. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarty R, Goel S, Valdovinos HF, Hernandez R, Hong H, Nickles RJ, et al. Matching the Decay Half-Life with the Biological Half-Life: ImmunoPET Imaging with (44)Sc-Labeled Cetuximab Fab Fragment. Bioconjug Chem. 2014;25:2197–204. doi: 10.1021/bc500415x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi S, Yang K, Hong H, Valdovinos HF, Nayak TR, Zhang Y, et al. Tumor vasculature targeting and imaging in living mice with reduced graphene oxide. Biomaterials. 2013;34:3002–9. doi: 10.1016/j.biomaterials.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]