Abstract
Rationale
While it is known that tobacco use varies across the 24-hour day, the time-of-day effects are poorly understood. Findings from several previous studies indicate a potential role for melatonin in these time-of-day effects; however the specific underlying mechanisms have not been well characterized. Understanding of these mechanisms may lead to potential novel smoking cessation treatments.
Objective
Examine the role of melatonin and melatonin receptors in nicotine free choice consumption
Methods
A two-bottle oral nicotine choice paradigm was utilized with melatonin supplementation in melatonin deficient mice (C57BL/6J) or without melatonin supplementation in mice proficient at melatonin synthesis (C3H/Ibg) compared to melatonin proficient mice lacking both or one of the high affinity melatonin receptors (MT1 and MT2; double null mutant DM, or MT1 or MT2). Preference for bitter and sweet tastants also was assessed in wild type and MT1 and MT2 DM mice. Finally, home cage locomotor monitoring was performed to determine the effect of melatonin administration on activity patterns.
Results
Supplemental melatonin in drinking water significantly reduced free-choice nicotine consumption in C57BL/6J mice, which do not produce endogenous melatonin, while not altering activity patterns. Independently, genetic deletion of both MT1 and MT2 receptors in a melatonin proficient mouse strain (C3H) resulted in significantly more nicotine consumption than controls. However single genetic deletion of either the MT1 or MT2 receptor alone did not result in increased nicotine consumption. Deletion of MT1 and MT2 did not impact taste preference.
Conclusions
This study demonstrates that nicotine consumption can be affected by exogenous or endogenous melatonin and requires at least one of the high-affinity melatonin receptors. The fact that expression of either the MT1 or MT2 melatonin receptor is sufficient to maintain lower nicotine consumption suggests functional overlap and potential mechanistic explanations. Keywords: Melatonin, Nicotine, Mice, Preference Drinking
Introduction
Strong associations are seen between being a late chronotype or a shift worker with drug abuse (Prat and Adan, 2011). For nicotine in particular, there are time-of-day differences on subjective effects, consumption and smoking intensity (Grainge et al., 2009; Benowitz et al., 1982; Mooney et al., 2006). Interestingly, smoking decreases prior to sleep onset (Mooney et al., 2006) at a time point which coincides with the rise in plasma melatonin (Wright, Jr. et al., 2005; Wright, Jr. et al., 2013). Findings from the rodent literature suggest a potential role for melatonin in modulating drug sensitivity. For example, the psychomotor stimulant and reinforcing properties of cocaine varies with time-of-day; being greater during the light versus dark phase of the light-dark cycle (Akhisaroglu et al., 2004; Uz et al., 2002; Kurtuncu et al., 2004; Abarca et al., 2002). However, in mouse strains that do not synthesize melatonin, this time-of-day effect on cocaine sensitivity is absent (Uz et al. 2002; Akhisaroglu et al., 2004). Furthermore, day-night differences in cocaine-induced conditioned place preference and locomotor sensitization are abolished if the pineal gland (the major source of circulating melatonin) is ablated (Uz et al., 2003; Kurtuncu et al., 2004). Time-of-day differences in sensitivity to the hypothermic effects of several drugs including nicotine, ethanol and apomorphine have also been shown (Morley and Garner, 1990; Russell, 1993; Mexal et al., 2012). Moreover, recent reports demonstrate that the daily variation in sensitivity to the hypothermic effects of nicotine (Mexal et al., 2012) and the sensitizing effect of methamphetamine (Hutchinson et al., 2014) are dependent upon melatonin signaling. In addition, melatonin can modulate the function of neuronal nicotinic receptors (Markus et al., 2003; Lax, 2008). Taken together, these results suggest that melatonin is sufficient to alter sensitivity and response to drugs of abuse, including nicotine. The importance of understanding the potential role of melatonin in modulating the effects of nicotine is highlighted by the finding that melatonin treatment reduces withdrawal-induced craving in abstinent smokers (Zhdanova and Piotrovskaya, 2000).
Findings from the alcohol field have demonstrated that initial drug sensitivity is correlated with future addiction outcomes (Schuckit, 1994). Similar hypotheses have been put forward for initial sensitivity to nicotine and nicotine addiction liability (Pomerleau et al., 1993). Despite these observations, and the findings described above showing that melatonin modulates initial drug sensitivity, little is known regarding the impact of melatonin on chronic measures of drug seeking. Therefore, the goal of this study was to determine the effect of melatonin and the high affinity melatonin receptors MT1 and MT2 on nicotine intake. Specifically, the experiments described in this study examined the ability of melatonin to modulate nicotine consumption in a two-bottle choice task and the impact of MT1 and/or MT2 receptor deletion on nicotine intake in the same task. Finally, several control experiments were performed including 24-hour home cage locomotor activity measurements to determine the effect of melatonin supplementation on the amplitude of the activity rhythm and the timing of peaks and nadirs of locomotor activity. In addition tastant drinking was performed to see if genetic deletion of MT1 and MT2 melatonin receptors altered bitter or sweet taste sensitivity.
Methods
Animals
Male C3H/Ibg (C3H), C3H MT1 and MT2 double null mutant (MT1/MT2 DM), C3H MT1 alone (MT1), C3H MT2 alone (MT2) and C57BL/6J (C57) mice were maintained on a normal 12h/12h light-dark cycle (lights on at 0700 and off at 1900). C57 is a commonly used strain for studies of drug abuse, but is deficient in melatonin production. Therefore, this strain was used to assess the effect of exogenous melatonin on nicotine intake. In contrast, C3H is one of the few commonly used inbred mouse strains able to produce endogenous melatonin (Goto et al., 1989; Vivien-Roels et al., 1998) and is thus well suited for studies of the effects of endogenous melatonin on nicotine intake. For the endogenous melatonin studies, C3H mice or C3H mice lacking one or both G-protein coupled melatonin receptors (MT1 and MT2) were utilized. C3H MT1/MT2 double null mutant (DM) mice were generously provided by Dr. David Weaver (University of Massachusetts) and were maintained as double homozygous breeders. The MT1/MT2 DM was originally bred onto a C3H/He genetic background for 10 generations as described elsewhere (Jin et al. 2003; Liu et al. 1997). These double mutants were used to generate the MT1 and MT2 single receptor null mutant strains by crossing to C3H/Ibg mice. After the initial cross, MT1 and MT2 single mutants were maintained with a heterozygous x heterozygous breeding scheme so that homozygous mutant animals were matched with wild-type littermate controls for testing.
Importantly, C57 mice were only used in exogenous melatonin supplementation studies while C3H background mice were only used to study endogenous melatonin. No comparisons were made between the exogenous and endogenous melatonin experiments due to the different strains utilized for the experiments. Animals had ad libitum access to food (Teklad 22/5 rodent diet, Harlan, Madison, WI) and water. All animals were tested between 60–120 days of age. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Colorado’s Institutional Animal Care and Use Committee. All efforts were made to minimize pain and suffering of animals and to minimize the number of animals used.
Drugs
Nicotine free base was purchased from Sigma (St. Louis, MO, USA) and diluted to the appropriate concentration in tap water for drinking studies. Melatonin was purchased from Tocris (St. Louis, MO, USA) and dissolved in 100% ethanol to a concentration of 400 μg/mL, which was further diluted to 400 ng/mL in tap water (for a final ethanol concentration of 0.1% in drinking solutions). To avoid potential confounds, control groups were also supplemented to 0.1% ethanol in drinking solutions. Quinine and saccharin were obtained from Sigma (St. Louis, MO, USA) and were resuspended in tap water at two different concentrations based on previously published results (Kamens et al., 2010; Kamens et al., 2005). The high concentration for quinine was 0.03 mM, the low was 0.015 mM. For saccharin, the high concentration was 0.066%, and low was 0.033%.
Preference drinking
The 2-bottle choice paradigm has been extensively used by our lab as a measure of drug intake (Stitzel et al., 2000; Wilking et al., 2010; Li et al., 2007; Li et al., 2005). In order to investigate the effect of melatonin on nicotine 2-bottle preference drinking, C57 mice were utilized. Furthermore, to examine the effect of melatonin signaling, MT1/MT2, MT1 and MT2 null mutant animals on a C3H background were used. Briefly, on day 0 animals were weighed, singly housed and provided with 2 water filled tubes fitted with sipper tops. Food was available ad libitum for the entirety of the study. For 4 subsequent days, each tube was weighed to determine consumption and the position was reversed. After 4 days of water, a nicotine solution was substituted for one of the water bottles and the procedure was repeated for 4 more days. This pattern repeated with increasing concentrations of nicotine. Control cages with sipper tubes that did not house an animal, but were otherwise treated identically with experimental cages were included to account for handling-induced spillage and evaporative loss. This correction was extremely consistent across all experiments and averaged between 0.1 – 0.2 mL per bottle per day. The concentrations varied depending on the background strain because of known differences in nicotine intake (Robinson et al., 1996; Li et al., 2005; Li et al., 2007). For C57 animals, the concentrations were 0, 25, 50, 100, 150 and 200 μg/mL and for C3H and melatonin DM animals the concentrations were 0, 10, 20, 35, 50, 75 and 100 μg/mL, while an abbreviated range of 0, 25, 50, 75 and 100 μg/mL was used for single melatonin receptor null mutant studies. In addition, the C57 animals in the melatonin supplement groups had 400 ng/mL melatonin in 0.1% ethanol vehicle added to both the water and nicotine drinking bottles, while melatonin control group had 0.1% ethanol without melatonin added to both drinking solutions. The dependent variables measured in these studies were nicotine consumption both for each nicotine concentration and in total across the entire study, preference ratio (percent of total fluid consumption occurring from the nicotine bottle), and total fluid intake.
Home cage locomotor activity
Locomotion assessments were done in the home cage using infrared sensors mounted on one side of the cage top (Mini Mitter, Bend, OR, USA). To mimic the conditions from the 2-bottle nicotine preference studies, animals were singly housed on day 0 and provided with drinking bottles containing either plain tap water plus vehicle or tap water supplemented with 400 ng/mL melatonin in vehicle. Every 4 days, a new solution was prepared with fresh melatonin. Food was available ad libitum throughout the study. Water bottles were weighed each day between 1300 and 1400, and the amount of fluid consumed was recorded. Bottles were weighed during this restricted time window to control for potential increases in locomotor activity from the slight disturbance of the bottle weighing procedure. Locomotor activity was assessed as number of beam breaks recorded in 10 minute bins across a 24 hour period for 28 days. Non-orthogonal spectral analysis (Czeisler et al., 1999) was used to estimate the activity rhythm amplitude, time of the peak and nadir of activity, along with the standard deviation for each (a measure of consistency of the rhythm). These measures were separately calculated across all 28 days of the experiment, as well as in 4 day bins corresponding to the 4 days blocks in the preference studies.
Tastant drinking
A similar procedure to the 2-bottle nicotine preference paradigm was used to test saccharin and quinine preference of the MT1/MT2 DM animals. Two bottles were provided; one contained pure tap water while the other contained the low concentration of either saccharin or quinine. The order of the tastant presentation was randomized and counterbalanced. Each day the water bottles were weighed to determine consumption and the position on the cage top was switched. At the end of the 4th day, the tastant was switched to the higher concentration. After 8 days with the initial tastant, the procedure was repeated for 8 more days with the alternate tastant. From daily consumption data, the preference for each solution was calculated.
Statistics
Dependent variables in the preference drinking studies (both for nicotine and for tastants) were analyzed with a repeated measures 2-way ANOVA (genotype or melatonin treatment x day) and included nicotine dose consumed (mg/kg/day), preference for nicotine-containing bottle (% preference) and total fluid consumed (mL). Melatonin’s effect on locomotor activity patterns was assessed with a t-test between melatonin and control groups for all 28 days of the study. In addition, to test for any tolerance development, data was analyzed in sets of 4 days to mirror the preference drinking studies. This data was analyzed using a 2-way ANOVA with time and treatment as the factors. Post-hoc tests included analysis by the Tukey method. Significant results were considered to have an α-level of 0.05 or less. All statistical analyses were performed in SPSS 21.0 (IBM Corp. Armonk, NY) and figures were constructed in Prism 5.0 (GraphPad Software Inc, La Jolla, CA).
Results
Melatonin effects on nicotine preference drinking in C57BL6/J mice
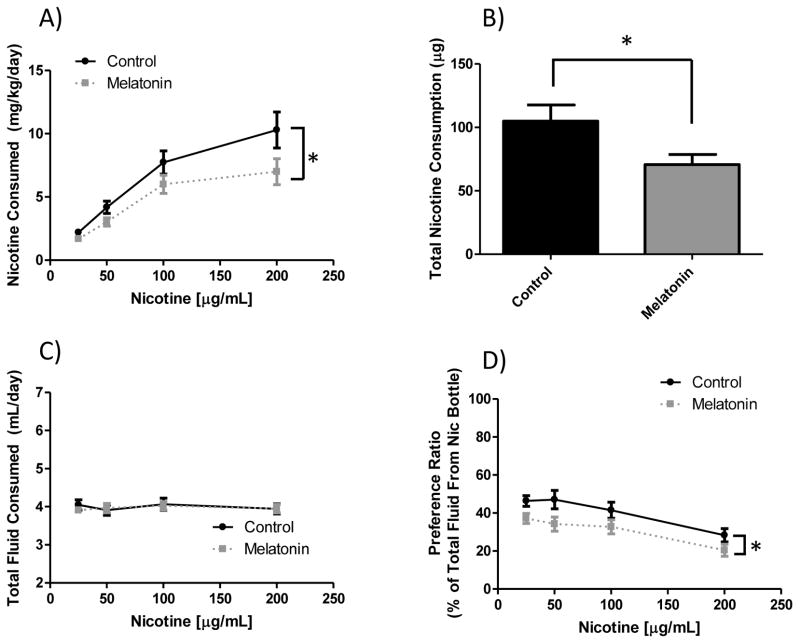
C57 mice were tested in a two-bottle choice nicotine intake paradigm in which the drinking water was either tap water plus vehicle or 400 ng/mL melatonin in vehicle added to tap water. This melatonin dose was arrived at after a dose-response experiment was performed with melatonin concentrations ranging from 100 ng/mL to 2 μg/mL (data not shown) indicated that the 400 ng/mL dose was the most effective at reducing nicotine intake. As seen in figure 1, when C57 animals received supplemental melatonin in their drinking water, they significantly decreased their nicotine intake without altering total fluid consumption. In panel A, main effects of melatonin treatment (p < 0.005) and nicotine concentration (p = 0.0001) were observed for nicotine dose consumed. There was also a significant effect of total dose consumed over the course of the study (p < 0.05, panel B). Importantly, these reductions in nicotine consumption were independent of total fluid consumption, where there was no main effect of melatonin treatment (p = 0.73, panel C). For nicotine preference, seen in panel D, there were again significant main effects of melatonin supplementation (p < 0.0005) and nicotine concentration (p < 0.0001). This measure also shows that melatonin supplementation reduced preference for the nicotine bottle.
Figure 1. Effect of melatonin supplementation on nicotine 2-bottle preference drinking in C57 mice.
When C57BL/6J mice have supplemental melatonin, they drink significantly less nicotine than the controls. Panel (A) shows nicotine dose consumed per day. There was a significant main effect of nicotine concentration (F3, 132 = 32.00, p < 0.0001), and melatonin supplementation (F1, 132 = 9.68, p < 0.005). Panel (B) shows total nicotine intake over the course of the study, where a significant effect of melatonin supplementation (t = 2.335, df = 34, p < 0.05) was observed. Panel (C) shows total fluid intake where there are no differences by melatonin supplementation (F1, 132 = 0.12, p = 0.73) or nicotine concentration (F3, 132 = 0.30, p = 0.82). Panel (D) shows nicotine preference, which demonstrated main effects of melatonin treatment (F1, 132 = 14.45, p < 0.0005) and nicotine concentration (F3, 132 = 9.827, p < 0.0001). Data is displayed as mean ± SEM and n=17 control, 19 melatonin supplemented animals. *denotes main effect differences between melatonin and control conditions.
The effect of melatonin supplementation on circadian parameters
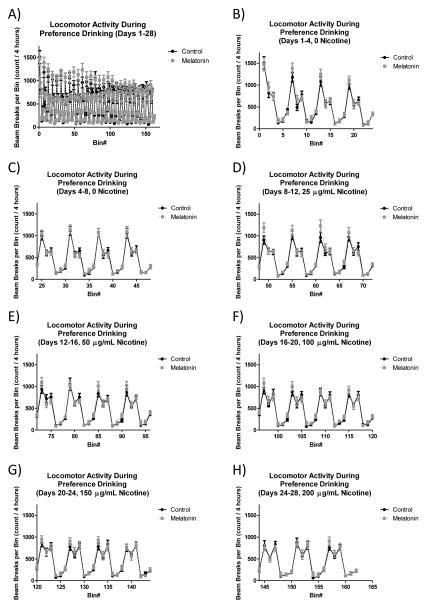
To test if the 400 ug/mL melatonin supplementation that resulted in lowered nicotine preference drinking also affected circadian parameters, home cage locomotor activity was examined. When data were analyzed for all 28 days in aggregate (see figure 2, panel A), there was no significant effect of melatonin supplementation on the rhythm parameters. As shown in table 1, the amplitude of the calculated activity rhythm (p = 0.69) and its standard deviation (p = 0.81) were unchanged between melatonin supplemented groups compared to controls. The timing for the peak (p = 0.75) and nadir (p = 0.32) of the activity rhythm also remained unchanged when animals consumed the 400 ng/mL supplemental melatonin compared to control (table 1). The standard deviation for the nadir of activity (a measure of how stable the timing of the nadir of activity is) showed a trend toward significance (p = 0.06) while the standard deviation for the peak in activity was not significant (p = 0.96). Finally, when data was analyzed in 4 day bins that would correspond to nicotine concentrations in the 2-bottle preference drinking studies described above (see figure 2, panels B–H), no significant effects of melatonin supplementation were observed. Table 2 shows calculated amplitude of the activity rhythm along with standard deviation across the study. There was a significant main effect of 4-day bin (p < 0.005 for amplitude, p < 0.0005 for standard deviation of amplitude), but not of melatonin supplementation (p = 0.72 for amplitude and p = 0.80 for standard deviation of amplitude). Similarly, table 2 also shows that there were significant effects of 4-day bin on measures of the peak and nadir of activity. For the nadir of activity, there was a non-significant trend for a main effect of day (p = 0.07) and there was a significant effect on standard deviation of the nadir (p < 0.01), however there was no effect of melatonin supplementation (p = 0.89 and p = 0.13, respectively). There was also a significant main effect of day on peak activity (p < 0.001) and standard deviation of peak activity (p < 0.05), but no effect of melatonin supplementation on these measures (p = 0.19 and p = 0.78, respectively).
Figure 2. Effect of melatonin supplementation on locomotor activity in C57 mice.
Supplemental melatonin administration in drinking water does not affect circadian patterns of locomotor activity. Panel (A) shows overall locomotor activity across the entire 28-day study period. For clarity, in the remaining panels (B–H) data was collapsed into 2-hour bins and plotted for 4-day periods that mimic the conditions in the presented preference drinking studies. Data was analyzed for time of minimum and maximum activity as well as amplitude of the rhythm, and no significant main effects of melatonin supplementation were seen (see tables 1&2). For this study n=15 control and 16 melatonin supplemented mice.
Table 1. Effect of melatonin supplementation on circadian parameters in C57 mice across the entire 28-day study period.
Values obtained from non-orthogonal spectral analysis of home cage locomotor activity across all 28 days of the study. Values are presented as mean (SEM) and n = 15 control and 16 melatonin supplemented animals.
| 28 Days Combined | ||
|---|---|---|
| Control | Melatonin | |
| Amplitude | 8.4 (0.9) | 7.9 (0.8) |
| SD Amplitude | 2.6 (0.2) | 2.7 (0.1) |
| Time of Nadir | 12.8 (1.9) | 11.8 (1.4) |
| SD Time of Nadir | 2.7 (0.3) | 2.7 (0.4) |
| Time of Peak | 19.7 (1.3) | 21.7 (1.5) |
| SD Time of Peak | 3.1 (0.3) | 3.9 (0.3) |
Table 2. Effect of melatonin supplementation on circadian parameters in C57 mice in 4-day bins.
Values obtained from non-orthogonal spectral analysis of home cage locomotor activity when data was combined into 4-days bins corresponding to the 4-day access to each concentration of nicotine. Values are presented as mean (SEM) and n = 15 control and 16 melatonin supplemented animals.
| Days | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–4 | 5–8 | 9–12 | 13–16 | 17–20 | 21–24 | 25–28 | ||||||||
| Control | Melatonin | Control | Melatonin | Control | Melatonin | Control | Melatonin | Control | Melatonin | Control | Melatonin | Control | Melatonin | |
| Amplitude | 24.5 (4.8) | 26.6 (3.6) | 11.9 (1.6) | 12.7 (2.3) | 13.3 (2.7) | 12.9 (2.2) | 16.5 (3.2) | 16.4 (4.7) | 14.2 (3.5) | 14.2 (3.1) | 18.3 (4.2) | 12.5 (3.4) | 14.0 (2.6) | 13.0 (2.0) |
| SD Amplitude | 7.6 (0.5) | 7.9 (0.5) | 6.2 (0.3) | 6.2 (0.3) | 6.2 (0.4) | 6.2 (0.4) | 6.4 (0.4) | 6.3 (0.4) | 5.8 (0.3) | 6.1 (0.3) | 6.4 (0.5) | 6.4 (0.4) | 6.5 (0.4) | 6.8 (0.3) |
| Time of Nadir | 13.0 (1.3) | 14.9 (2.1) | 120.6 (2.1) | 122.8 (2.0) | 220.6 (1.7) | 221.4 (2.0) | 315.1 (2.3) | 303.5 (7.1) | 409.8 (1.4) | 411.3 (1.9) | 507.8 (1.9) | 504.3 (2. 1) | 605.8 (2.1) | 605.2 (2.0) |
| SD Time of Nadir | 3.4 (0.5) | 3.0 (0.4) | 4.7 (0.4) | 4.4 (0.5) | 4.6 (0.4) | 4.6 (0.5) | 4.6 (0.5) | 4.4 (0.5) | 4.4 (0.5) | 4.2 (0.5) | 4.1 (0.4) | 5.1 (0.5) | 4.1 (0.5) | 4.5 (0.4) |
| Time of Peak | 22.4 (1.3) | 22.0 (1.7) | 121.8 (1.4) | 125.1 (1. 8) | 217.7 (1. 8) | 219.9 (1.7) | 312.6 (2.0) | 312.3 (1.3) | 405.1 (1.9) | 407.2 (2.0) | 503.3 (2.1) | 507.6 (1. 8) | 599.5 (1.3) | 599.9 (1.2) |
| SD Time of Peak | 3.1 (0.5) | 2.8 (0.3) | 4.3 (0.5) | 5.0 (0.4) | 4.4 (0.5) | 4.3 (0.5) | 4.4 (0.6) | 4.8 (0.4) | 4.3 (0.5) | 4.2 (0.5) | 3.5 (0.5) | 5.2 (0.5) | 4.2 (0.4) | 4.5 (0.4) |
Melatonin effects on nicotine preference drinking in C3H inbred mice and MT1/MT2 DM mice
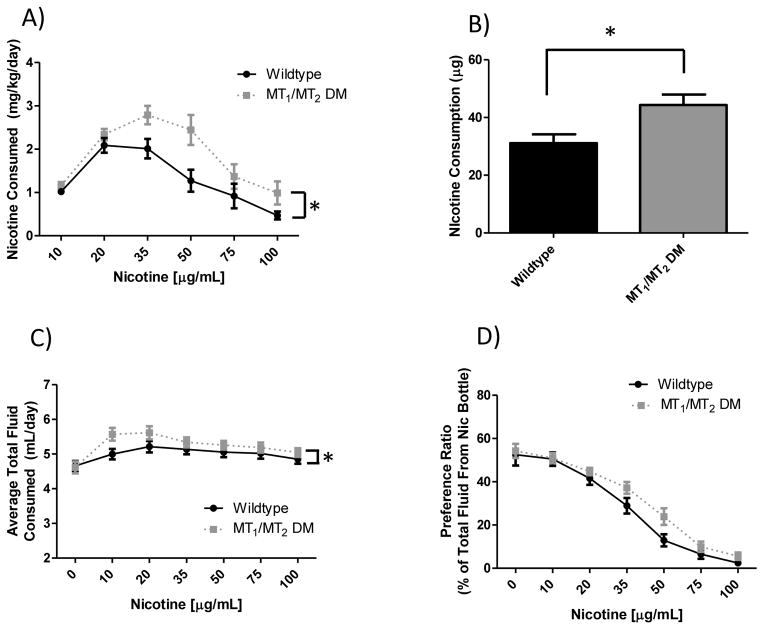
To more specifically examine the effect of melatonin signaling on nicotine preference drinking, animals harboring genetic deletions of both high-affinity melatonin receptors (MT1 and MT2) on a C3H background (animals which do produce endogenous melatonin) were tested in the two-bottle choice paradigm. As seen in figure 3, animals that lack melatonin receptors consumed significantly more nicotine than did controls. A main effect of genotype was observed for nicotine dose consumed (p > 0.0001, panel A), and approached significance for preference for the nicotine bottle (p = 0.063, panel D). There was also a main effect of nicotine concentration for measures of both nicotine dose (p < 0.0001) and preference rating (p < 0.0001), but no interaction of genotype x day. No main effect of genotype on total fluid consumption (p = 0.21) was found, however there was a main effect of nicotine concentration (p < 0.0001), where mutants drank slightly more over the course of the study. This could be confounding, so post-hoc tests were performed, demonstrating no effect of genotype at any of the concentrations measured.
Figure 3. Effect of melatonin signaling on nicotine 2-bottle preference drinking in C3H mice.
Panel (A) shows nicotine dose consumed. There was a significant main effect of nicotine concentration (F5, 180 = 19.18, p > 0.0001), and genotype (F1, 180 = 18.84, p > 0.0001) with MT1/MT2 DM animals drinking more nicotine. Panel (B) shows total nicotine intake over the course of the study where a significant effect of genotype (t = 2.78, df = 32, p < 0.01) was observed. Panel (C) shows total fluid intake which demonstrated a significant main effect of nicotine concentration (F6, 180 = 13.20, p < 0.0001) and genotype (F1, 180 = 3.46, p = 0.21), which resulted in slightly increased fluid intake in null mutant animals. Post-hoc tests revealed no significant difference at any nicotine concentration for genotype, though the 10 and 20 μg/mL concentrations approached significance. Panel (D) shows nicotine preference, which displayed a main effect of nicotine concentration (F6, 180 = 72.10, p < 0.0001) and a trend towards significance for genotype (F1, 180 = 1.02, p = 0.063). Data is represented as mean ± SEM and n=16 wildtype and 16 DM mice. MT1/MT2 DM = MT1 and MT2 double melatonin receptor null mutant animals. *denotes main effect differences between wildtype and MT1/MT2 DM animals.
Tastant drinking C3H inbred mice and MT1/MT2 dKO mice
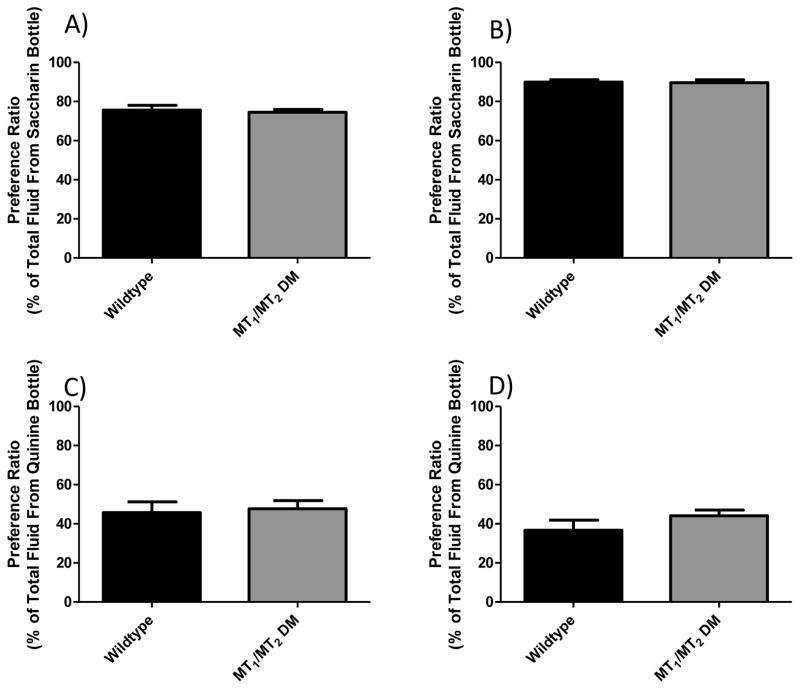
Genetic differences that impact taste perception may impact oral nicotine preference (Gyekis et al., 2012). Because we were unable to find data in the literature for the tastant preference in the MT1/MT2 double-null mutant animals, we tested their preference for the bitter quinine solution and the sweet tastant saccharin. As seen in figure 4, there were no significant differences between genotypes on preference for high or low concentrations of quinine (p = 0.22 and p = 0.77 respectively) or preference for high or low saccharin concentrations (p = 0.86 and p = 0.70).
Figure 4. Effect of MT1/MT2 genetic deletion on saccharine and quinine 2-bottle preference drinking.
Panels (A & B) show preference data for the sweet tasting saccharin solution at low and high concentrations respectively. No significant differences were seen at either saccharin concentration (t = 0.38, df = 30, p = 0.70) and (t = 0.18, df = 30, p = 0.86 for low and high respectively). Panels (C & D) show preference ratios for the bitter quinine consumption at high and low concentrations respectively. There were no significant differences seen in preference for the low concentration of quinine (t = 0.30, df = 30, p = 0.77) or the high concentration (t = 1.24, df = 30, p = 0.22). Data is represented as mean ± SEM and n=16 wildtype and 16 DM mice. MT1/MT2 DM = MT1 and MT2 double melatonin receptor null mutant animals
Dissection of MT1 and/or MT2 receptor effects on preference drinking
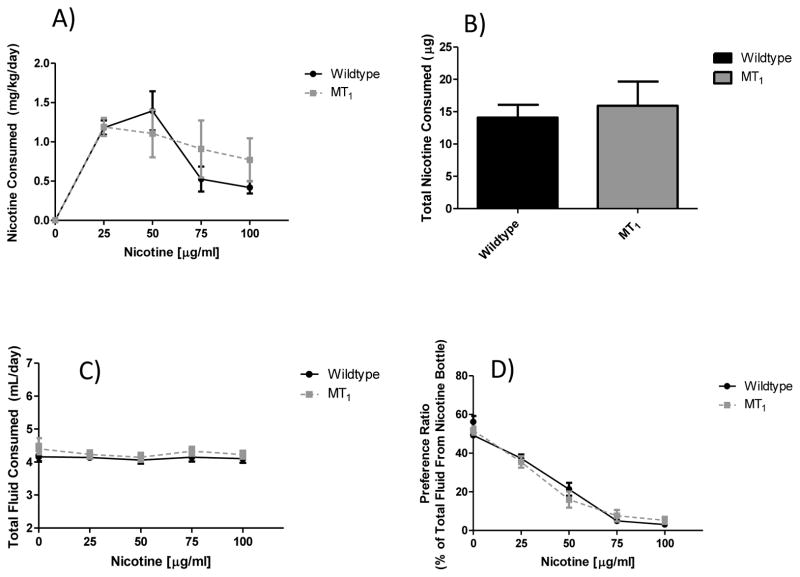
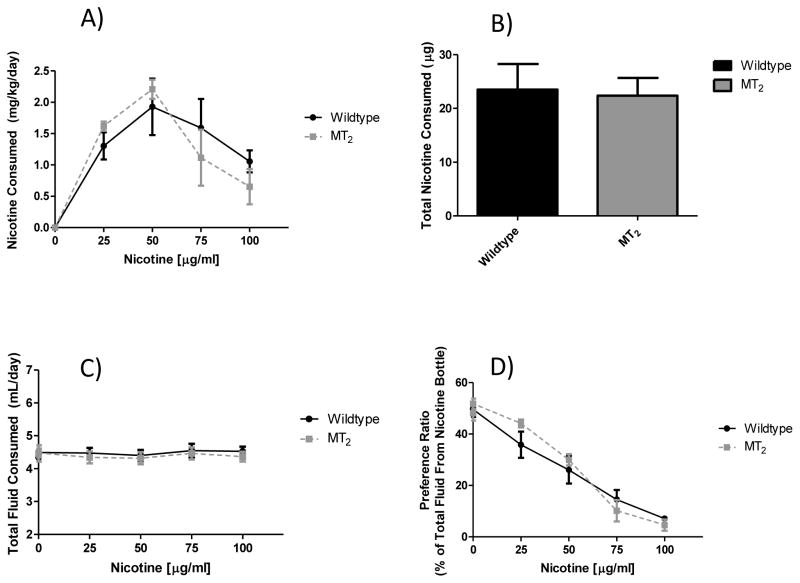
To further examine the contributions of the MT1 and MT2 melatonin receptors for nicotine intake, animals with deletions of either the MT1 or MT2 melatonin receptor genes were tested. As seen in figure 5, when the MT1 null mutant line was tested, a significant main effect of nicotine concentration on nicotine consumption (p < 0.0001) was observed, but not a main effect of MT1 genotype (p = 0.67). We also did not see an effect of MT1 genotype effect on total nicotine consumption throughout the study (p = 0.67). While not significant, there was a trend for a main effect of nicotine concentration on total fluid consumption (p = 0.06), but not MT1 genotype (p = 0.37). Finally, the expected reduction in preference for the nicotine containing bottle as nicotine concentrations increase was observed (p < 0.0001), but there was no main effect of MT1 genotype (p = 1.0). Results were nearly identical for the MT2 line as seen in figure 6. A significant main effect of nicotine concentration on nicotine consumption (p < 0.0001) emerged, but no main effect of MT2 genotype (p = 0.86) was observed. We did not see a MT2 genotype effect on total nicotine consumption throughout the study (p = 0.86). Neither nicotine concentration (p = 0.45) nor MT2 genotype (p = 0.65) resulted in a significant effect on total fluid consumption. Finally, there was again a significant main effect of nicotine concentration on preference for the nicotine containing bottle (p < 0.0001), but not a main effect of the MT2 genotype (p = 0.70).
Figure 5. Effect of genetic deletion of only the MT1 melatonin receptor on nicotine preference drinking.
Panel (A) shows nicotine consumption by nicotine concentration. No significant main effect of genotype was observed (F1, 100 = 0.19, p = 0.67), but there was a main effect of nicotine concentration (F4, 100 = 22.55, p < 0.0001). Panel (B) shows total nicotine consumption across the entire study, where no significant effect of genotype was observed (t = 0.44, df = 25, p = 0.67). As seen in panel (C) there were no significant main effects of nicotine concentration (F4, 100 = 2.36, p = 0.06) or genotype (F1, 100 = 0.82, p = 0.37) on total fluid consumption. Finally, in panel (D) there was no main effect of genotype (F1, 100 = 0.0003, p = 1.0) on preference for the nicotine bottle, however there was a significant main effect of nicotine concentration (F4, 100 = 230.7, p < 0.0001). Values are presented as mean (SEM) and n = 14 control and 13 MT1 null mutant.
Figure 6. Effect of genetic deletion of only the MT2 melatonin receptor on nicotine preference drinking.
Panel (A) shows nicotine consumption by nicotine concentration. No significant main effect of genotype was observed (F1, 64 = 0.03, p = 0.86), but there was a main effect of nicotine concentration (F4, 64 = 23.70, p < 0.0001). Panel (B) shows total nicotine consumption across the entire study, where no significant effect of genotype was observed (t = 0.18, df = 16, p = 0.86). As seen in panel (C) there were no significant main effects of nicotine concentration (F4, 64 = 0.93, p = 0.45) or genotype (F1, 64 = 0.21, p = 0.65) on total fluid consumption. Finally, in panel (D) there was no main effect of genotype (F1, 64 = 0.15, p = 0.70) on preference for the nicotine bottle, however there was a significant main effect of nicotine concentration (F4, 64 = 111.4, p < 0.0001). Values are presented as mean (SEM) and n = 10 control and 8 MT2 null mutant.
Discussion
The experiments in this study demonstrate that both exogenous and endogenous melatonin are sufficient to reduce free-choice nicotine consumption. Importantly, this is found from two independent, but complimentary approaches; 1) melatonin supplementation in an inbred strain that does not produce endogenous melatonin (C57BL/6) reduces nicotine preference drinking, and 2) genetic deletion of both MT1 and MT2 in an inbred strain that does have endogenous melatonin production (C3H) increases nicotine consumption. Deletion of individual MT1 or MT2 receptors alone did not lead to increased nicotine consumption indicating that either receptor is sufficient to maintain reduced nicotine consumption in a melatonin producing animal. The effect of melatonin on nicotine intake is independent of alterations in total fluid intake. Importantly, melatonin supplementation at the doses sufficient to reduce nicotine intake does not alter gross daily patterns in activity. Previous literature has suggested a role for melatonin in drug-related behavior by comparing melatonin producing vs non-melatonin producing mouse strains (Akhisaroglu et al., 2004; Mexal et al., 2012; Uz et al., 2002), or by ablating the source of melatonin, the pineal gland (Kurtuncu et al., 2004; Uz et al., 2003). However, these methods have confounds and are only suggestive of a role of melatonin in drug-related behaviors. For example, making comparisons between different strains of mice suffers from the possibility that behavioral differences are due to genetic background effects rather than the ability or inability to synthesize melatonin. Also, pineal ablation does not account for other functions of the pineal. Therefore data presented here that directly examine the effect of melatonin on nicotine consumption in two independent models, extend previous findings and are the first demonstration that melatonin, and/or melatonin receptor activation, is able to reduce nicotine drug-seeking behavior.
The observation that 400 ng/mL melatonin supplementation in drinking water reduces nicotine consumption in the absence of gross alterations in locomotor activity is significant. This is consistent with, and extends results from a recent publication that showed that a 20 ng/mL melatonin supplementation in drinking water did not affect locomotor activity (Adamah-Biassi et al., 2013). Previous work in both humans and in rodents has shown that melatonin and melatonin agonists are able to shift the circadian clock and promote sleep when administered during the biological daytime (Benloucif and Dubocovich, 1996; Arendt and Skene, 2005; Markwald et al., 2010; Burke et al., 2013). One could speculate that this lack of effect could be because mice lacking endogenous melatonin (C57) do not have normal melatonin receptor expression. There is little direct evidence to support this possibility. On the contrary, there is a large body of literature showing that administration of physiologically relevant concentrations of melatonin to C57 mice produces the expected phase shifts, and that this effect is through the typical G-protein coupling (for a general review, see Dubocovich and Markowska, 2005). Given that the overall goal of work such as this is to not only understand how melatonin interacts with nicotine administration, but also to develop drugs to alleviate nicotine dependence, any novel therapeutics must be free from significant side effects. To date, there has only been a single paper on the potential for melatonin as a smoking cessation aid. Zhdanova and Piotrovskaya et al. (2000) found that when melatonin supplements are given to abstinent heavy smokers, self-reported measures of craving are reduced. Importantly, this is found in the absence of any alterations in self-reported measures of sleepiness (Zhdanova and Piotrovskaya, 2000). Given these data, combined with the findings presented here, further investigation of low dose melatonin or other melatonin agonists as novel therapeutics for smoking cessation should be considered.
It is also important to note that increases in nicotine consumption in the C3H background when both high affinity receptors are genetically deleted occur in the absence of changes to sweet or bitter taste preference. Previous work has shown that differences in nicotine preference drinking between inbred mouse strains are at least partially due to differences in taste preferences (Dahl et al., 1997). Furthermore, Gyekis et al. showed that mice that have been conditioned to avoid a bitter tasting quinine solution will also transfer this conditioning to nicotine solutions, and vice versa (Gyekis et al., 2012). This suggests that some portion of the sensory experience of nicotine is bitter tasting, and it could be possible that melatonin receptors play some role in bitter taste, but not in the rewarding properties of nicotine itself. This can be ruled out by the tastant drinking data, which shows that mice that have had both MT1 and MT2 melatonin receptors do not have alterations in tastant drinking as compared to wildtype littermates.
One potential limitation of the MT1/MT2 double mutant experiments is background strain. The double mutant mice were initially generated on a C3H/He background but have been maintained by double-homozygous breeding for minimally 30–40 generations following their initial development. As a result, a true control strain no longer exists for these animals. In the experiments in this study, C3H/Ibg mice were used as the control strain. This C3H substrain was chosen because it, like C3H/He, is a substrain of C3H with a functional Tlr4 gene. In contrast, the C3H/HeJ substrain has a known loss of function mutation in the toll-like receptor 4 (Tlr4). Having the same Tlr4 allele between controls and the MT1/MT2 double mutant mice is critical since it has been demonstrated that some actions of melatonin are dependent on Tlr4 (Xia et al., 2012). Nonetheless, even with a Tlr4-matched C3H strain some care must be taken with interpretation of these data. Given the potential control strain concern, it is especially important that the completely independent data from C57 mice with supplemental melatonin is consistent with the findings of the MT1/MT2 double mutant data.
One of the most interesting findings from this report is that expression of either MT1 or MT2 receptors alone is sufficient to maintain a reduced nicotine preference drinking in C3H mice. When both receptors are genetically deleted, there is a significant increase in nicotine consumption, however when MT1 alone or MT2 alone are removed, nicotine intake remains comparable to wildtype littermates. Melatonin receptors are G-protein coupled, and expressed throughout the body, though MT1 and MT2 have somewhat distinct patterns of expression (Reppert et al., 1994; Liu et al., 1997; Dubocovich et al., 1998; Reppert et al., 1995). Depending on tissue type, MT1 and MT2 can be coupled to different G-proteins (for review, see Hardeland, 2009), however if either melatonin receptor is sufficient to keep nicotine intake low, or if the high-affinity melatonin receptors are able to compensate for each other, this suggests a parallel signaling pathway. This also suggests a likely place to begin looking for the effect is in brain regions that express both receptors. There are a number of places of overlap, such as the hippocampus or hypothalamus (Liu et al., 1997; Reppert et al., 1995; Dubocovich et al., 1998), both of which have previously been implicated in reward and drug abuse pathways (Koob and Volkow, 2010).
Finally, because melatonin and/or melatonin signaling reduces nicotine preference drinking, and nicotine acts on nicotinic acetylcholine receptors (nAChRs), one of the next questions to answer is which nicotinic receptor subtypes are modulated by melatonin in a way that leads to decreased nicotine intake. Some preliminary evidence comes from a study by Lax who demonstrated that physiological levels of melatonin are able to alter function of nicotine induced currents in cultured cerebellar granular neurons, primarily through α4β2 nAChRs (Lax, 2008). Consistent with the possibility that melatonin modulates nicotine intake though modulating α4β2 nAChRs, previous studies have demonstrated that α4β2 receptors are involved in nicotine preference drinking (Butt et al., 2005; Wilking et al., 2010). Alternatively, there is also evidence that melatonin is able to alter function of α7 nAChRs, which have also been shown to affect rewarding properties of nicotine (Brunzell and McIntosh, 2012). Markus et al. used a combination of specific agonists and antagonists and found that melatonin is able to modulate nicotine-induced glutamate release in cerebellar slices through α7 nAChRs (Markus et al., 2003). Together these studies suggest a possible mechanistic explanation; that melatonin affects function of α4β2 and/or α7 nAChRs, which leads to reduction in nicotine preference drinking. Further research is needed to determine specifically which receptors are affected by melatonin, as well as the molecular mechanism by which these changes occur.
In summary, we found that melatonin signaling is able to reduce nicotine preference drinking in two independent mouse strains. Furthermore, the optimal dosage of melatonin used for this reduction in nicotine preference does not alter circadian patterns in locomotor activity. We also found that genetic deletion of MT1 and MT2 does not affect preference for sweet or bitter solutions, suggesting that alterations in nicotine preference are not due to changes in gustatory response. While there is still much work to be done to determine the exact mechanism by which melatonin effects nicotine preference, these studies may provide a foundation for novel smoking cessation therapies.
Acknowledgments
The authors wish to thank Vivian Nyguen, James Laughlin and David Sheneman for their expert technical assistance, and Dr. David Weaver for providing the melatonin receptor double null mutant mice. These studies were performed with support from NIH DA022462 and DA015663. WJH was supported by institutional training grant NIH DA017637 awarded to IBG.
Footnotes
Conflict of Interest Disclosure
The authors have no conflicts of interest to disclose
Reference List
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamah-Biassi EB, Stepien I, Hudson RL, Dubocovich ML. Automated video analysis system reveals distinct diurnal behaviors in C57BL/6 and C3H/HeN mice. Behav Brain Res. 2013;243:306–312. doi: 10.1016/j.bbr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacol Biochem Behav. 2004;79:37–42. doi: 10.1016/j.pbb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9:25–39. doi: 10.1016/j.smrv.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Dubocovich ML. Melatonin and light induce phase shifts of circadian activity rhythms in the C3H/HeN mouse. J Biol Rhythms. 1996;11:113–125. doi: 10.1177/074873049601100204. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P., III Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther. 1982;32:758–764. doi: 10.1038/clpt.1982.233. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–1143. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke TM, Markwald RR, Chinoy ED, Snider JA, Bessman SC, Jung CM, Wright KP., Jr Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep. 2013;36:1617–1624. doi: 10.5665/sleep.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, King NM, Hutton SR, Collins AC, Stitzel JA. Modulation of nicotine but not ethanol preference by the mouse Chrna4 A529T polymorphism. Behav Neurosci. 2005;119:26–37. doi: 10.1037/0735-7044.119.1.26. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756:22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–1220. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Grainge MJ, Shahab L, Hammond D, O’Connor RJ, McNeill A. First cigarette on waking and time of day as predictors of puffing behaviour in UK adult smokers. Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Gyekis JP, Dingman MA, Revitsky AR, Bryant BP, Vandenbergh DJ, Frank ME, Blizard DA. Gustatory, trigeminal, and olfactory aspects of nicotine intake in three mouse strains. Behav Genet. 2012;42:820–829. doi: 10.1007/s10519-012-9546-x. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors. 2009;35:183–192. doi: 10.1002/biof.23. [DOI] [PubMed] [Google Scholar]
- Hutchinson AJ, Ma J, Liu J, Hudson RL, Dubocovich ML. Role of MT1 melatonin receptors in methamphetamine-induced locomotor sensitization in C57BL/6 mice. Psychopharmacology (Berl) 2014;231:257–267. doi: 10.1007/s00213-013-3228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol. 2004;489:203–205. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Lax P. Melatonin inhibits nicotinic currents in cultured rat cerebellar granule neurons. J Pineal Res. 2008;44:70–77. doi: 10.1111/j.1600-079X.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- Li XC, Karadsheh MS, Jenkins PM, Brooks JC, Drapeau JA, Shah MS, Lautner MA, Stitzel JA. Chromosomal loci that influence oral nicotine consumption in C57BL/6J x C3H/HeJ F2 intercross mice. Genes Brain Behav. 2007;6:401–410. doi: 10.1111/j.1601-183X.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- Li XC, Karadsheh MS, Jenkins PM, Stitzel JA. Genetic correlation between the free-choice oral consumption of nicotine and alcohol in C57BL/6JxC3H/HeJ F2 intercross mice. Behav Brain Res. 2005;157:79–90. doi: 10.1016/j.bbr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- Markus RP, Santos JM, Zago W, Reno LA. Melatonin nocturnal surge modulates nicotinic receptors and nicotine-induced [3H]glutamate release in rat cerebellum slices. J Pharmacol Exp Ther. 2003;305:525–530. doi: 10.1124/jpet.102.045625. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Lee-Chiong TL, Burke TM, Snider JA, Wright KP., Jr Effects of the melatonin MT-1/MT-2 agonist ramelteon on daytime body temperature and sleep. Sleep. 2010;33:825–831. doi: 10.1093/sleep/33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mexal S, Horton WJ, Crouch EL, Maier SI, Wilkinson AL, Marsolek M, Stitzel JA. Diurnal variation in nicotine sensitivity in mice: role of genetic background and melatonin. Neuropharmacology. 2012;63:966–973. doi: 10.1016/j.neuropharm.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney M, Green C, Hatsukami D. Nicotine self-administration: cigarette versus nicotine gum diurnal topography. Hum Psychopharmacol. 2006;21:539–548. doi: 10.1002/hup.808. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Collins AC, Shiffman S, Pomerleau CS. Why some people smoke and others do not: new perspectives. J Consult Clin Psychol. 1993;61:723–731. doi: 10.1037//0022-006x.61.5.723. [DOI] [PubMed] [Google Scholar]
- Prat G, Adan A. Influence of circadian typology on drug consumption, hazardous alcohol use, and hangover symptoms. Chronobiol Int. 2011;28:248–257. doi: 10.3109/07420528.2011.553018. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Robinson SF, Marks MJ, Collins AC. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl) 1996;124:332–339. doi: 10.1007/BF02247438. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. A clinical model of genetic influences in alcohol dependence. J Stud Alcohol. 1994;55:5–17. doi: 10.15288/jsa.1994.55.5. [DOI] [PubMed] [Google Scholar]
- Stitzel JA, Lu Y, Jimenez M, Tritto T, Collins AC. Genetic and pharmacological strategies identify a behavioral function of neuronal nicotinic receptors. Behav Brain Res. 2000;113:57–64. doi: 10.1016/s0166-4328(00)00200-x. [DOI] [PubMed] [Google Scholar]
- Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci. 2002;70:3069–3075. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- Vivien-Roels B, Malan A, Rettori MC, Delagrange P, Jeanniot JP, Pevet P. Daily variations in pineal melatonin concentrations in inbred and outbred mice. J Biol Rhythms. 1998;13:403–409. doi: 10.1177/074873098129000228. [DOI] [PubMed] [Google Scholar]
- Wilking JA, Hesterberg KG, Crouch EL, Homanics GE, Stitzel JA. Chrna4 A529 knock-in mice exhibit altered nicotine sensitivity. Pharmacogenet Genomics. 2010;20:121–130. doi: 10.1097/FPC.0b013e3283369347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia MZ, Liang YL, Wang H, Chen X, Huang YY, Zhang ZH, Chen YH, Zhang C, Zhao M, Xu DX, Song LH. Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells. J Pineal Res. 2012;53:325–334. doi: 10.1111/j.1600-079X.2012.01002.x. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Piotrovskaya VR. Melatonin treatment attenuates symptoms of acute nicotine withdrawal in humans. Pharmacol Biochem Behav. 2000;67:131–135. doi: 10.1016/s0091-3057(00)00302-6. [DOI] [PubMed] [Google Scholar]