Abstract
Objective
We studied the mechanistic links between fibrocalcific changes in the aortic valve and aortic valve function in mice homozygous for a hypomorphic epidermal growth factor receptor mutation (Wave mice). We also studied myocardial responses to aortic valve dysfunction in Wave mice.
Approach and Results
At 1.5 months of age, prior to development of valve fibrosis and calcification, aortic regurgitation, but not aortic stenosis, was common in Wave mice. Aortic valve fibrosis, pro-fibrotic signaling, calcification, osteogenic markers, lipid deposition, and apoptosis increased dramatically by 6 and 12 months of age in Wave mice. Aortic regurgitation remained prevalent, however, and aortic stenosis was rare, at all ages. Proteoglycan content was abnormally increased in aortic valves of Wave mice at all ages. Treatment with pioglitazone prevented abnormal valve calcification, but did not protect valve function. There was significant left ventricular volume overload, hypertrophy, and fetal gene expression, at all ages in Wave mice with aortic regurgitation. Left ventricular systolic function was normal until 6 months of age in Wave mice, but became impaired by 12 months of age. Myocardial transverse tubules were normal in the presence of left ventricular hypertrophy at 1.5 and 3 months of age, but became disrupted by 12 months of age.
Conclusions
We present the first comprehensive phenotypic and molecular characterization of spontaneous aortic regurgitation and volume-overload cardiomyopathy in an experimental model. In Wave mice, fibrocalcific changes are not linked to valve dysfunction, and are epiphenomena arising from structurally incompetent “myxomatous” valves.
Keywords: aortic regurgitation, aortic stenosis, valvular regurgitation, cardiomyopathy
Introduction
In patients with either aortic stenosis (AS) or aortic regurgitation (AR), morbidity and mortality accrue from exhaustion of compensatory mechanisms in the left ventricle (LV).1,2 Several mouse models of AS have been reported,3 and myocardial reponses to pressure-overload have been extensively investigated. Mechanisms of volume-overload heart failure are less completely understood. A recent Scientific Statement from the American Heart Association highlighted the absence of mouse models of valvular regurgitation, and suggested that discovery of such a model would be useful for understanding the mechanisms by which chronic LV volume-overload leads to heart failure.4
Epidermal growth factor receptor (EGFR) signaling regulates embryonic formation of semilunar heart valves.5 Inbred mice homozygous for a single-nucleotide substitution mutation in the gene encoding EGFR are known as EgfrWa2/Wa2, or waved-2 (Wave) mice, and have a global 90% reduction in EGFR-tyrosine kinase (tk) activity.6,7 Consequently, Wave mice develop histologic and functional abnormalities in the aortic valve which are strongly influenced by background strain, findings which have been interpreted as evidence for aortic valve stenosis.7
In the present study, we found that AR, not AS, is the predominant functional abnormality in Wave mice. We tested, and rejected, the hypothesis that valve fibrosis and calcification are temporally linked to progression of aortic valve dysfunction. We reported previously that treatment with pioglitazone, a PPAR-γ agonist, attenuated valve calcification and protected aortic valve function in hypercholesterolemic mice.8 In Wave mice, however, although treatment with pioglitazone prevented abnormal valve calcification, it did not protect valve function, findings which do not support a mechanistic link between valve calcification and valve dysfunction in this model. Finally, we report functional, molecular, and cardiomyocyte structural responses to volume-overload in the LV, with eventual progression to heart failure in Wave mice.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Morphometry and metabolism
Body mass and blood chemistries were normal in Wave mice (Supplemental Table I.).
Histological changes in aortic valve
Valve collagen levels, assessed using Masson’s Trichrome staining, were normal in Wave mice at 1.5 months of age, but were significantly increased at 6 and 12 months of age (Figure 1 A – C). After treatment with pioglitazone, collagen levels, assessed using Pircrosirius Red staining, remained elevated in Wave mice at 6 months of age.
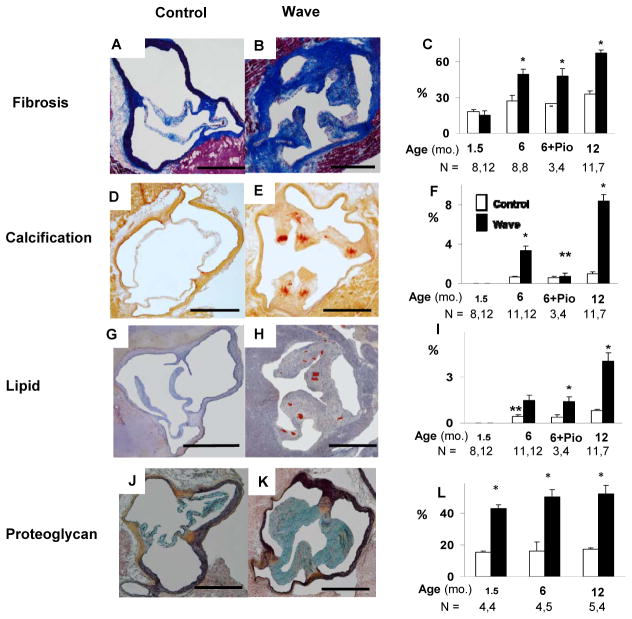
Figure 1. Histology of aortic valve.
A – C: Aortic valve collagen detected by Masson’s Trichrome or Picrosirius Red staining (for pioglitazone-treated [Pio] mice). D – F: Aortic valve calcification, assessed using Alizarin Red staining. G – I: Aortic valve lipid content, detected using Oil Red-O staining. J – L: Proteoglycan, assessed using Movat’s Pentachrome staining (blue-green). % refers to the proportion of valve tissue exhibiting positive staining. Scale bar = 500 μm. *p < 0.05 vs. age-matched Control; **p < 0.025 vs. 6 mo. Wave.
Valve calcification, assessed using Alizarin Red staining, was undetectable in Wave mice and Control mice at 1.5 months of age (Figure 1 D – F). In Wave mice, valve calcification was significantly increased, compared to age-matched Control mice, at 6 and 12 months of age. After treatment with pioglitazone, valve calcification was significantly reduced in Wave mice, compared to vehicle-treated Wave mice.
Lipid deposition, assessed using Oil Red-O staining, was undetectable in Wave mice and Control mice at 1.5 months of age. At 6 and 12 months of age, lipid levels were significantly increased in valves from Wave mice, compared to Control (Figure 1 G – I). After treatment with pioglitazone, lipid levels remained elevated in Wave valves.
Levels of proteoglycan in the aortic valve were significantly elevated in Wave mice at 1.5, 6, and 12 months of age (Figure 1 J – L).
Profibrotic signaling in the aortic valve
Levels of α-smooth muscle actin (SMA), which indicate transdifferentiation of valve interstitial cells from a quiescent state to a pro-fibrotic phenotype, were normal in Wave valves at 1.5 months of age. At 6 and 12 months of age, α-SMA levels were significantly elevated in Wave valves (Figure 2 A – C). After treatment with pioglitazone, α-SMA levels remained elevated in Wave valves.
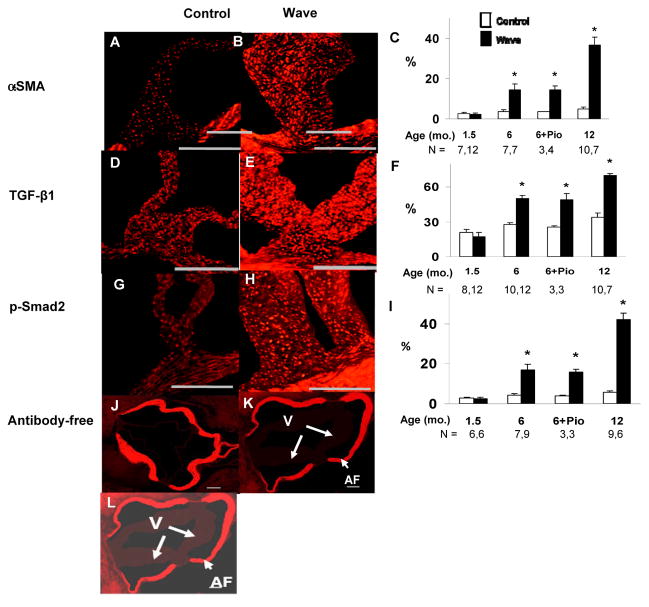
Figure 2. Immunostaining for pro-fibrotic signaling molecules in the aortic valve.
Examples are shown from mice at 12 months of age. A–C: transdifferentiation from quiescent valve interstitial cells to pro-fibrotic myofibroblasts, indicated by staining for α-smooth muscle actin (α-SMA). D–F: staining for transforming growth factor-β1 (TGF-β1), a pro-fibrotic signaling molecule. G–I: staining for p-Smad2, a mediator of pro-fibrotic TGF-β1 signaling. J–L: antibody-free negative controls for immunostaining. The image in Panel L is identical to the image in Panel K, except that Panel L was electronically brightened to show the location of valve tissue (V). % refers to the proportion of valve tissue exhibiting positive staining. Autofluorescence (AF) arises from coherently arranged fibers in valve annulus, but not in valve. Scale bar = 100 μm. *p < 0.05 vs. age-matched and treatment-matched Control.
Levels of the pro-fibrotic signaling molecule, transforming growth factor-β1 (TGF-β1) were normal in Wave valves at 1.5 months of age, but were significantly elevated at 6 and 12 months of age (Figure 2 D – F). After treatment with pioglitazone, TGF-β1 levels remained elevated in Wave valves.
Phosphorylated homologue of mothers against decapentaplegia-2 (p-Smad2) is a mediator of pro-fibrotic TGF-β1 signaling. Levels of p-Smad2 were normal in Wave valves at 1.5 months of age, but were significantly elevated at 6 and 12 months of age in Wave mice, compared to Control mice (Figure 2 G – I). After treatment with pioglitazone, p-Smad2 levels remained elevated in Wave valves.
Levels of pro-fibrotic signaling molecules in the aortic valve varied with age and genotype in a pattern that was similar to overall collagen levels in the aortic valve, as shown in Figure 1 A – C.
Osteogenic transdifferentiation in the aortic valve (Figure 3)
Figure 3. Immunostaining for osteogenic differentiation in the aortic valve.
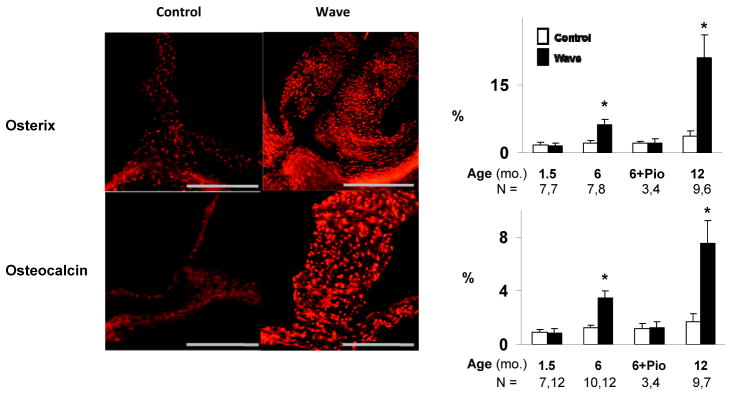
Examples were obtained at 12 months of age. % refers to the proportion of valve tissue exhibiting positive staining. Scale bar = 100 μm. *p < 0.05 vs. age-matched and treatment-matched Control.
Osterix and osteocalcin are pro-calcific signaling molecules produced by osteoblast-like cells. Osterix and osteocalcin levels were normal in Wave mice at 1.5 months of age. At 6 and 12 months of age, osterix and osteocalcin levels were significantly elevated in Wave mice, compared to Control mice. After treatment with pioglitazone, osterix and osteocalcin levels in the aortic valve were not significantly different from age-matched Control mice. Thus, changes in osterix and osteocalcin with age and genotype were similar to the pattern for overall valve calcification shown in Figure 1 D – F.
Proteoglycans in the aortic valve
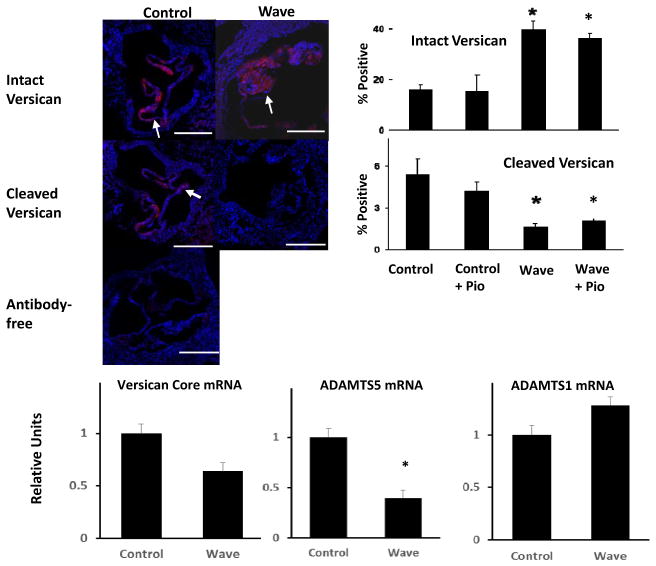
Intact versican, a proteoglycan that modulates properties of semilunar heart valves,9,10 was increased in aortic valves from Wave mice at 6 months of age (Figure 4). Cleaved versican, a product of enzymatic breakdown of intact versican, was significantly reduced in aortic valves from Wave mice at 6 months of age (Figure 4). Intact and cleaved versican levels remained elevated after treatment with pioglitazone in aortic valves from Wave mice. Expression of ADAMTS5, an enzyme which cleaves versican11, was decreased in Wave valves (Figure 4). Thus, increases in in tact versican and decreases in cleaved versican are consistent with increased levels of total proteoglycan in Wave valves, shown in Figure 1 J – L.
Figure 4. Proteoglycan homeostasis in aortic valve.
Histologic data were obtained at 6 months of age (N = 4). Red stain = intact versican or cleaved versican, respectively (arrows); to-Pro Blue stain = nuclei. PCR data were obtained at 12 months of age (N = 6). Scale bar = 100 μm. *p < 0.05 vs. untreated Control.
Preliminary studies in a few mice suggest that levels of biglycan, another proteoglycan, were normal in Wave mice (Supplemental Figure I). Expression of ADAMTS1, another proteoglycanolytic enzyme,12 was normal in Wave valves (Figure 4). Thus, changes in versican vs. biglycan suggest that changes in proteoglycans homeostasis in the aortic valve are selective for specific proteoglycan moieties.
Cell death in the aortic valve
Expression of activated caspase-3, a marker for programmed cell death, was not increased at 1.5 months in Wave valves (Supplemental Figure II). However, at 6 and 12 months of age, there was a very large increase in activated capase-3 levels in Wave mice, compared to Control mice. Increased apoptosis in Wave mice, at 6 months of age, was confirmed using TUNEL staining (Supplemental Figure III). Pioglitazone prevented increases in activated caspase-3 and TUNEL staining in Wave mice.
There was no difference between sexes for any histological parameter in the aortic valve (p = NS for male vs. female, data not shown).
Aortic valve function
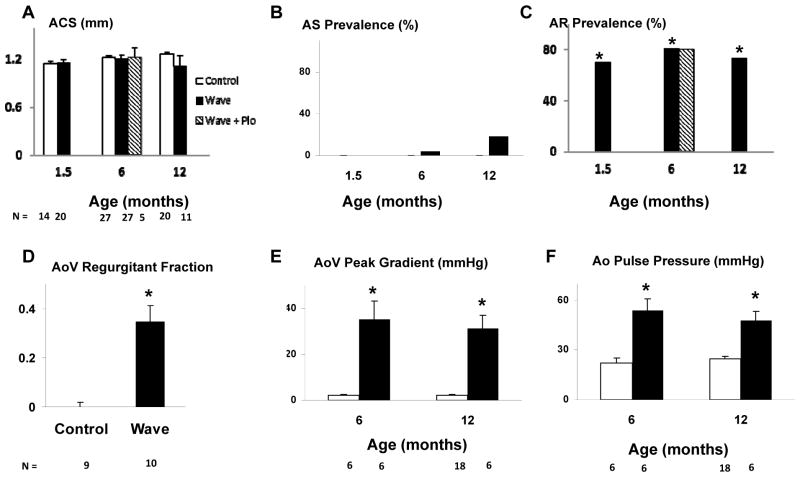
Representative valve function data are shown in Figure 5. A significant transvalvular gradient was observed, even when AS was not present in the Wave mouse with AR.
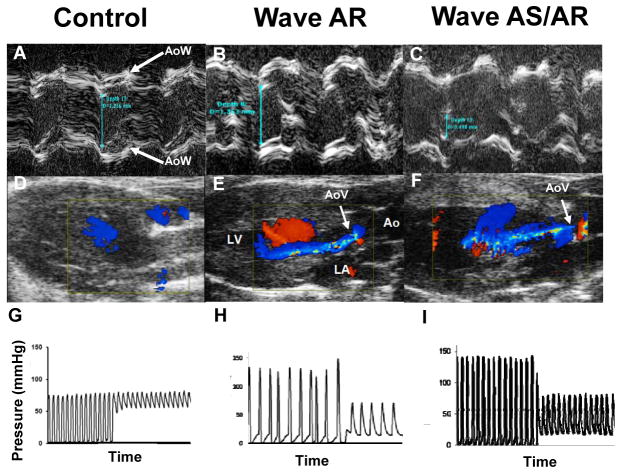
Figure 5. Aortic valve function.
A–C: M-mode echocardiograms demonstrating aortic cusp separation (blue-green line). D–F: Color Doppler frames acquired in mid-diastole. Blue jet indicates regurgitant flow across the aortic valve (AoV, white arrow). G–I: Pressure measurements during catheter pullback from left ventricle to aorta, which indicate a significant pressure gradient in the Wave mouse with normal aortic cusp separation (B, E, H). AoW aortic root wall.
Aortic cusp separation (ACS) was normal in Wave mice, as a group, at all ages (Figure 6A). In 3 of 55 Wave mice, however, ACS was ≤ 0.66 mm, which indicates that those 3 mice had hemodynamically important aortic stenosis13 (Figure 6B; p = NS vs. Control for AS prevalence). M-mode echo images only right and noncoronary aortic valve cusps. To address the hypothetical possiblilty that significant stenosis occurs in the left cusp, we performed Anatomical M-mode imaging on 7 additional Wave mice, and found that aortic cusp movement was symmetrical (Supplemental Figure IV), supporting the validity of standard M-mode measurements in the larger group of Wave mice. All 3 mice with severe AS also had severe AR with diastolic prolapse of one or more valve cusps into the left ventricular outflow tract (Supplemental Video SV1; legend for SV1 is included in the Data Supplement).
Figure 6. Aortic Valve Function.
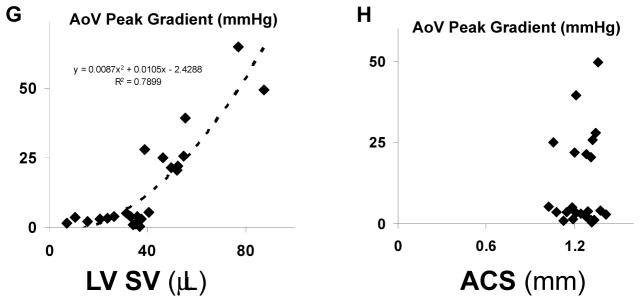
A–C: echocardiographic findings. D: MRI findings at 6 mo. E, F: invasive hemodynamic findings. G: Correlation between LV stroke volume and aortic valve peak gradient. H: Lack of correlation between aortic cusp separation and peak aortic valve gradient. ACS aortic cusp separation, AS aortic stenosis, AR aortic regurgitation, AoV aortic valve, Ao aorta, LV SV left ventricular stroke volume. *p < 0.05 vs. age-matched and treatment-matched Control.
The prevalence of moderate or severe AR, assessed using color Doppler echocardiography, was 70%, 81%, and 73% in Wave mice at 1.5, 6, and 12 months of age, respectively, and 0 in Control mice (p < 0.05 for all ages, Figure 6C). Aortic valve regurgitant fraction, quantitated at 6 months of age using MRI, was 0.34 ± 0.07 for all Wave mice vs. 0 ± 0.03 for Control mice (p < 0.05; Figure 6D). In instances where echocardiography demonstrated moderate or severe AR, MRI confirmed abundant diastolic retrograde flow across the aortic valve (Supplemental Video SV2;; legend for SV2 is included in the Data Supplement), and regurgitant fraction was 0.46 ± 0.06. For all mice without echocardiographic evidence of moderate or severe AR, regurgitant fraction was 0.06 ± 0.03 (p < 0.05 vs. mice with AR; Supplemental Figure V). Mitral regurgitation, assessed using color Doppler echocardiography, was absent or trivial in all mice. One Wave mouse, which had severe AR, also had mild pulmonic valve regurgitation observed on MRI.
Despite the presence of normal ACS, there were substantial systolic pressure gradients across the aortic valve in Wave mice, assessed using invasive hemodynamic measurements, (Figures 5H and 6E). The increased transvalvular gradients were associated with increased aortic pulse pressure (Figure 6F), a finding consistent with AR, and not AS.
In mice without AS, there was a remarkable quadratic relationship between left ventricular stroke volume and transvalvular systolic gradient (Figure 6G). Because the magnitude of increased LV stroke volume is determined by severity of AR, the correlation between stroke volume and systolic gradient implies that the gradient is produced by AR. Indeed, there was no correlation between aortic cusp separation and transvalvular gradient (Figure 6H).
Although treatment with pioglitazone prevented abnormal valve calcification, pioglitazone had no effect on aortic cusp separation or prevalence of moderate or severe aortic regurgitation (Figure 6A, C).
Proximal aorta
Aortic diameters measured using echocardiography at the level of the sinuses of Valsalva and at the level of the proximal ascending aorta, were increased in Wave mice vs. Control mice at all ages (Supplemental Figure VI A, B). Aortic enlargement was present at 1.5 months of age in Wave mice, even when aortic regurgitation was absent, which suggests genotype-related abnormalities in the aortic wall. Saccular or fusiform aneurysmal dilatation of the aortic root was not observed in any mouse. Increased expression of the pro-inflammatory injury response mediator Smad3, a TGFβ-1 response element, has been linked to increased incidence of aortic aneurysm in humans.14 We found that expression of Smad3, and also the anti-inflammatory mediator, Smad7, were not significantly increased in aorta of Wave mice (Supplemental Figure VI C, D).
Ventricular size and systolic function
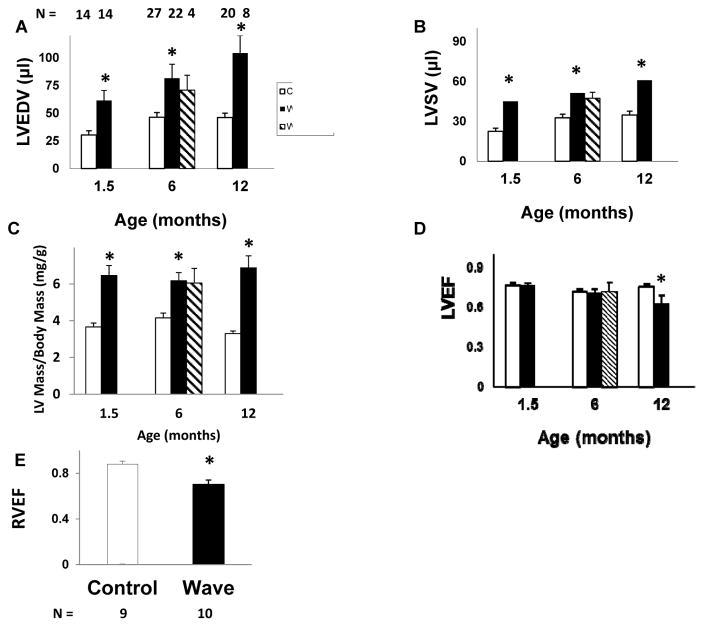
There was significant LV chamber enlargement, consistent with volume overload, in Wave mice with AR at 1.5, 6, and 12 months of age (Figure 7A). After treatment with pioglitazone, LV end-diastolic volume remained elevated in Wave mice.
Figure 7. Ventricular structure and function.
A–D: Echocardiography. For Wave mice, data are shown only for mice with moderate or severe aortic regurgitation. E: RVEF, assessed by MRI, at 6 months of age. LV left ventricular, EDV end-diastolic volume, SV stroke volume, EF ejection fraction, RV right ventricular. *p < 0.05 vs. age-matched and treatment-matched Control.
LV stroke volume was significantly increased in Wave mice at 1.5, 6, and 12 months of age (Figure 7B). At 1.5 months of age, end-diastolic volume and stroke volume were not increased in the 30% of Wave mice that did not have AR (p = NS vs. Control, data not shown). After treatment with pioglitazone, LV stroke volume remained elevated in Wave mice.
LV mass, indexed to body mass, was elevated in Wave mice at 1.5, 6, and 12 months of age (Figure 7C). After treatment with pioglitazone, LV mass remained elevated in Wave mice.
LV ejection fraction was normal in Wave mice at 1.5 and 6 months of age, but was significantly decreased by 12 months of age (Figure 7D). In Wave mice with AR, LV mass, LV end-diastolic volume, and LV ejection fraction were similar in males vs. females at 6 months of age. By 12 months of age, there was a trend toward greater LV dilation and lower LV ejection fraction in females. The trend did not achieve statistical significance, however, possibly because of greater variance at later stages of disease (p = NS for male vs. female, for each age, data not shown.) RV ejection fraction, quantitated at 6 months of age using MRI, was significantly decreased in Wave mice vs. Control (Figure 7E).
In summary, LV volumes and mass were increased in Wave mice at all ages, including the youngest age when valve collagen, calcification, and lipid levels were normal (Figure 1). LV systolic function was normal, despite high prevalence of moderate or severe aortic regurgitation, at 1.5 and 6 months of age, but was abnormal at 12 months of age.
Myocardial gene expression
At 1.5 months of age, expression of fetal genes15 was not increased in myocardium of Wave mice, with the exception of BNP (Supplemental Figure VII). When Wave mice with AR were analyzed separately, however, expression of skeletal muscle-type actin, and ANP were also increased (Supplemental Figure VIII).
Abnormal expression of several genes persisted or progressed during the course of disease (Supplemental Figure VII). By 6 months of age, myocardial expression of β-MyHC, myocyte-enriched calcineurin-interacting protein-1.4 (MCIP-1.4), collagen-1 and collagen-3 was significantly increased in Wave mice. At 12 months of age, fibrosis was histologically evident in myocardium from Wave mice. There was no difference between sexes for myocardial gene expression in Wave mice (p = NS for male vs. female, data not shown).
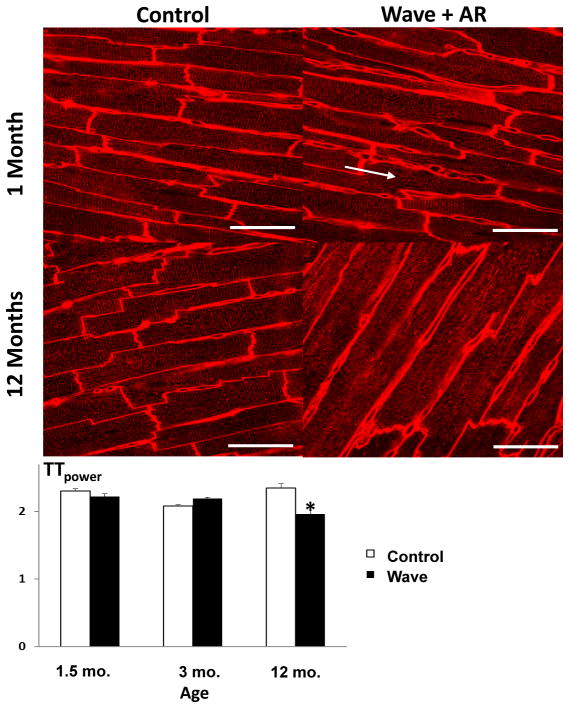
Organization of myocardial transverse tubules (TT)
In order to examine structural changes in individual cardiomyocytes in this model of valvular volume overload cardiomyopathy, we focused on TT. At 1.5 and 3 months of age, when Wave mice demonstrated LV hypertrophy and normal LV systolic function, TT organization was normal in Wave mice (Figure 8). At 12 months of age, when both LV hypertrophy and systolic dysfunction were present in Wave mice, TT organization was significantly disrupted.
Figure 8. T-tubule organization in myocardium.
At 1.5 months of age, and at 3 months of age, when left ventricular hypertrophy is already present in Wave mice, T-tubules are regularly spaced and oriented perpendicular to myocyte long-axes in Wave myocardium (arrow). By 12 months of age, when left ventricular systolic dysfunction occurs in Wave mice, T-tubules have become disorganized. TTpower is a statistical convention used to quantify the level of organization of T-tubules. (See Reference 12.) Sample sizes for WT and Wave mice at the 3 ages are 3,3; 2,3; and 4,8. Ten confocal micrographs were analyzed for each mouse. Scale bar = 50 μm. *p < 0.05 vs. Control.
Discussion
For the first time, we provide comprehensive functional, histological, and molecular characterization of spontaneous valvular volume overload cardiomyopathy in a mouse model. Aortic valve dysfunction occurs in the presence of excess proteoglycans, including versican, in valve cusps, but precedes fibrosis, calcification, apoptosis, and lipid deposition in the valve. Treatment with pioglitazone prevents abnormal calcification and apoptosis in the aortic valve in Wave mice, but does not protect aortic valve function. Over time, LV volume overload is associated with increased expression of fetal myocardial genes, progressive impairment of LV systolic function, myocyte TT disruption, and myocardial fibrosis.
Others have reported fibrosis, calcification, and elevated transvalvular gradients in aortic valves of Wave mice, and have interpreted the results as indicative of calcific AS.7 We provide multiple lines of evidence which indicate that AS is rare in Wave mice, and that AR predominates. Aortic cusp separation is normal in Wave mice, as a group, at all ages. Color Doppler and 2-D echocardiography clearly depict AR and increased LV stroke volume, respectively, in Wave mice. MRI demonstrates abundant retrograde diastolic flow across the aortic valve and regurgitant volumes which confirm the presence of moderate or severe aortic regurgitation in Wave mice. Systolic gradients across the aortic valve, assessed using invasive hemodynamic methods, follow a predictable quadratic relationship to LV stroke volume, which indicates that increased flow across the valve, not stenosis, accounts for elevated valve gradients in the vast majority of mice. Excluding 3 of 55 Wave mice in which echocardiography identified significant AS, there was no correlation between aortic valve pressure gradients and systolic aortic cusp separation. LV-to-aorta pressure gradients were higher in Wave mice than those typically observed in humans with pure AR. We speculate that this apparent disparity arises because normal LV systolic function is relatively hyperdynamic in mice (EF ~ 0.80) compared to humans (EF ~ 0.55).
Our findings are unique because aortic valve fibrosis, pro-fibrotic signaling, valve calcification, osteogenic transformation, and increased transvalvular pressure gradients are all features of acquired aortic valve stenosis in mice and humans, and together are often proffered as pathognomonic evidence for the disease. Indeed the levels of fibrocalcific changes in Wave mice actually exceed those previously reported in aortic stensosis-prone mice.16 Our findings in Wave mice, however, support the conclusion that abundant valve calcification and fibrosis alone are not sufficient to cause prevalent hemodynamically important aortic stenosis in mice.
Others have observed, anecdotally, spontaneous AR in mice, putatively in the presence of AS.5,17,18 However, the prevalence, quantitative severity, structural mechanism, and the impact of spontaneous chronic AR upon ventricular morphology, function, myocyte structure, and gene expression have not previously been reported.
Mechanisms of valve dysfunction
In the present study we identify myxomatous structural incompetence and consequent diastolic prolapse of valve cusps as major mechanisms of AR in Wave mice. Those findings are common in humans with isolated AR.19
Deficiency of proteoglycan breakdown occurs postnatally in Wave mice, a novel finding of the present study. Despite significant increases in polymeric intact versican, levels of cleaved versican are reduced in Wave mice at 6 months of age. Expression of ADAMTS5, an enzyme known to cleave versican,11 was reduced in Wave mice. Expression of the gene encoding versican core protein was not increased, further supporting a post-transcriptional mechanism for proteoglycan excess in Wave valves. Preliminary findings indicate that biglycan, another valve proteoglycan, was not increased in Wave valves. Together, the findings indicate that proteoglycan remodeling is selectively dysregulated in postnatal Wave mice. The findings may have translational implications, because the presence of postnatal proteoglycan dysregulation suggests a potential therapeutic target.
Lipid deposition
Prior studies have demonstrated electrostatic affinity of tissue proteoglycans for circulating lipoprotein particles, a putative early stage of atherosclerotic lesion formation.20 We speculate that the persistent excess of proteoglycans in the aortic valve in Wave mice may bind blood pool lipoprotein particles by similar interactions, resulting in lipid deposition in Wave valves. That speculation is supported by the observation that lipid deposition was almost exclusively observed in Wave aortic valve cusps and their attachment sites, where proteoglycans are abundant, but not in valve annulus or myocardium (Figure 1H). The finding may have clinical relevance, because lipid deposition is an early finding in humans with aortic valve disease.21
Valvular cardiomyopathy in Wave mice
Changes in LV morphology and function, paired with changes in myocardial gene expression and TT organization, reflect a longitudinal pattern of adaptation, then decompensation, in the presence of volume overload in Wave mice. In rats with traumatic acute AR, increased expression of some fetal genes and collagen isoforms occurs early, and persists essentially unchanged for months.22 Our findings in Wave mice with spontaneous chronic AR differ somewhat in that increased expression of fetal genes is progressive over 12 months, and myocardial expression of collagen-1,3 is normal during the early period of LV compensation in the presence of volume overload. Our findings thus dissociate upregulation of myocardial collagen isoform expression from volume overload per se, a novel finding.
Cardiomyocyte TT critically regulate excitation-contraction coupling by facilitating Ca2+ release from sarcoplasmic reticulum. TT disruption exacerbates disease progression from hypertrophy to heart failure.23 Strategies designed to inhibit or reverse the process of TT disruption can attenuate the transition to left ventricular systolic dysfunction.24 In experimental left ventricular pressure-overload hypertrophy, TT disruption occurs prior to the onset of LV systolic dysfunction,23 suggesting that TT disruption is an inherent component of myocyte remodeling in response to hemodynamic stress. In Wave mice, however, TT organization was preserved in the presence of volume-overload hypertrophy for a period of at least 3 months, and did not become disrupted until the development of contractile dysfunction. Wave mice at 1.5 and 3 months of age demonstrated similar severity of LV hypertrophy (~50%) to that reported previously in pressure-overload.23 Thus, we report a new finding with fundamental mechanistic importance: unlike pressure-overload LV hypertrophy, TT disruption is not an inherent feature of left ventricular hypertrophy induced by volume overload hemodynamic stress, but occurs during the transition to heart failure in Wave mice with aortic regurgitation. Future studies, using techniques which overcome the limited resolution of light microscopy25, may reveal disturbances within individual TT that occur in volume-overload left ventricular hypertrophy and heart failure. EGFR-tk signaling is trophic for LV myocardium, as overexpression produces cardiomyocyte enlargement and increased LV mass,26 whereas EGFR-tk deficiency results in LV wall thinning and decreased contractility.27 EGFR-tk activity is decreased about 90% in Wave mice. We found neither wall thinning nor impaired LV contractility in young Wave mice. The findings indicate that residual EGFR-tk activity in Wave mice is sufficient to prevent overt cardiomyopathy in the absence of volume overload. Our findings of LV hypertrophy in Wave mice at all ages, and preserved LV contractility until older age, argue strongly against a predominant effect of myocardial Wave genotype. Nevertheless, EGFR deficiency may modulate some aspects of LV responses to volume overload, possibly blunting the hypertrophic response.
Limitations
The most obvious histologic abnormality in young Wave mice is overabundance of proteoglycan in valve tissue, a finding which is common in patients with AR. We provide evidence for temporal association between proteoglycan excess and valve dysfunction, but cannot conclusively determine whether this association indicates causality, or is an epiphenomenon arising from other, as yet uncharacterized, abnormalities of valve matrix. A previous study indicated that valve abnormalities ascribed to EGFR-tk deficiency, including proteoglycan overabundance, are strongly influenced by background strain in mice7, suggesting the possibility of modifiers of valve homeostasis that could serve as therapeutic targets.
Conclusions
There are several novel findings in this study which challenge current paradigms of fibrocalcific aortic valve disease. Valve fibrosis, calcification, lipid accumulation, and apoptosis are common features of aortic stenosis in humans and mice, and are putative targets for therapeutic intervention. Here we report that, even when those features are present, aortic stenosis is uncommon in Wave mice, up to 12 months of age. Treatment with pioglitazone prevented valve calcification in Wave mice, but did not protect valve function, confirming further the dissociation between valve calcification and valve function. We speculate that fibrocalcific changes in the aortic valve reflect a response to injury arising from mechanical stresses upon structurally incompetent valve cusps.
Our findings reinforce the importance of comprehensive characterization of aortic valve function in vivo, when assessing the therapeutic efficacy of interventions designed to protect or improve valve function. Because phenotypic mechanisms of AR in Wave mice resemble those observed frequently in humans, a targeted search for abnormalities of EGFR-tk signaling in humans with isolated AR may be warranted.
Supplementary Material
Significance.
We provide the first comprehensive functional, histological, and molecular characterization of spontaneous valvular volume overload cardiomyopathy in a clinically relevant mouse model. Valve dysfunction is mechanistically dissociated from profound fibrocalcific changes in the aortic valve in Wave mice. Cardiomyocyte remodeling in this model of volume-overload left ventricular hypertrophy differs fundamentally from previously observed remodeling in pressure-overload hypertrophy. The findings reinforce the importance of comprehensive characterization of aortic valve function in vivo, when assessing the therapeutic efficacy of interventions designed to ameliorate fibrocalcific changes in the aortic valve.
Acknowledgments
We thank Stephanie Troyer, Wendy Meyers, and Teresa Ruggle for assistance with preparation of the manuscript, the University of Iowa Central Microscopy Research Facility for use of equipment, Katherine S. Walters for assistance with microscopy, Thomas Gerhold for assistance with the mouse colony. We also thank Loretha Myers and Jennifer Habashi, Johns Hopkins University School of Medicine, for helpful suggestions regarding p-Smad2 immunostaining.
Funding sources: These studies were supported by grants HL62984, HL090905, ODO019941, and RR 026293 from the National Institutes of Health.
Abbreviations
- ACS
aortic cusp separation
- α-SMA
alpha-smooth muscle actin
- ANP
atrial natriuretic peptide
- AR
aortic regurgitation
- AS
aortic stenosis
- β-MyHC
beta myosin heavy chain
- BNP
brain-type natriuretic peptide
- EGFR-tk
epithelial growth factor receptor-tyrosine kinase
- FCAVD
fibrocalcific aortic valve disease
- LV
left ventricle
- MCIP-1.4
myocyte-enriched calcineurin-interacting protein 1.4
- NFAT-1c
nuclear factor of activated T-cells-1c
- p-HH3
phospho-histone H3
- pSmad-2
phosphorylated homolog of mothers against decapentaplegic-2
- RV
right ventricle
- TGF-β1
transforming growth factor-beta1
- TT
cardiomyocyte transverse tubules
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- Wave mice
EgfrWa2/Wa2 mice
Footnotes
Disclosures: The authors have no potential conflicts of interest.
References
- 1.Carabello BA. Introduction to aortic stenosis. Circ Res. 2013;113:179–185. doi: 10.1161/CIRCRESAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 2.Lindman BR, Bonow RO, Otto CM. Current management of calcific aortic stenosis. Circ Res. 2013;113:223–237. doi: 10.1161/CIRCRESAHA.111.300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss RM, Miller JD, Heistad DD. Fibrocalcific aortic valve disease: opportunity to understand disease mechanisms using mouse models. Circ Res. 2013;113:209–22. doi: 10.1161/CIRCRESAHA.113.300153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, Magnuson T, Douglas PS, Morgan JP, Neel BG. Mice mutant for egfr and shp2 have defective cardiac semilunar valvulogenesis. Nat Genet. 2000;24:296–299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- 6.Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, Lee DC. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 1994;8:399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- 7.Barrick CJ, Roberts RB, Rojas M, Rajamannan NM, Suitt CB, O’Brien KD, Smyth SS, Threadgill DW. Reduced egfr causes abnormal valvular differentiation leading to calcific aortic stenosis and left ventricular hypertrophy in c57bl/6j but not 129s1/SvimJ mice. Am J Physiol: Heart Circulatory Physiol. 2009;297:H65–75. doi: 10.1152/ajpheart.00866.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu Y, Lund DD, Weiss RM, Brooks RM, Doshi H, Hajj GP, Sigmund CD, Heistad DD. Pioglitazone attenuates valvular calcification induced by hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2013;33:523–532. doi: 10.1161/ATVBAHA.112.300794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuis LE, Osinska H, Weinstein MB, Hinton RB, Kern CB. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J Mol Cell Cardiol. 2013;60:50–9. doi: 10.1016/j.yjmcc.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson CL, Gough PJ, Chang CA, Chan CK, Frey JM, Liu Y, Braun KR, Chin MT, Wight TN, Raines EW. Endothelial deletion of ADAM17 in mice results in defective remodeling of the semilunar valves and cardiac dysfunction in adults. Mech Dev. 2013;130:272–89. doi: 10.1016/j.mod.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupuis LE, Osinska H, Weinstein MB, Hinton RB, Kern CB. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J Mol Cell Cardiol. 2013;60:50–9. doi: 10.1016/j.yjmcc.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y1, Nagy JA, Brown LF, Shih SC, Johnson PY, Chan CK, Dvorak HF, Wight TN. Proteolytic cleavage of versican and involvement of ADAMTS-1 in VEGF-A/VPF-induced pathological angiogenesis. J Histochem Cytochem. 2011;59:463–73. doi: 10.1369/0022155411401748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114:2065–2069. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 14.van de Laar IM, Oldenburg RA, Pals G, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–6. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 15.Buttrick PM, Kaplan M, Leinwand LA, Scheuer J. Alterations in gene expression in the rat heart after chronic pathological and physiological loads. J Mol Cell Cardiol. 1994;26:61–67. doi: 10.1006/jmcc.1994.1008. [DOI] [PubMed] [Google Scholar]
- 16.Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 18.Kokubo H, Miyagawa-Tomita S, Nakashima Y, Kume T, Yoshizumi M, Nakanishi T, Saga Y. Hesr2 knockout mice develop aortic valve disease with advancing age. Arterioscler Thromb Vasc Biol. 2013;33:e84–92. doi: 10.1161/ATVBAHA.112.300573. [DOI] [PubMed] [Google Scholar]
- 19.Boodhwani M, de Kerchove L, Watremez C, Glineur D, Vanoverschelde JL, Noirhomme P, El Khoury G. Assessment and repair of aortic valve cusp prolapse: implications for valve-sparing procedures. J Thorac Cardiovasc Surg. 2011;141:917–25. doi: 10.1016/j.jtcvs.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Grande-Allen KJ, Osman N, Ballinger ML, Dadlani H, Marasco S, Little PJ. Glycosaminoglycan synthesis and structure as targets for the prevention of calcific aortic valve disease. Cardiovasc Res. 2007;76:19–28. doi: 10.1016/j.cardiores.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 22.Champetier S, Bojmehrani A, Beaudoin J, Lachance D, Plante E, Roussel E, Couet J, Arsenault M. Gene profiling of left ventricle eccentric hypertrophy in aortic regurgitation in rats: rationale for targeting the beta-adrenergic and renin-angiotensin systems. Am J Physiol Heart Circ Physiol. 2009;296:H669–77. doi: 10.1152/ajpheart.01046.2008. [DOI] [PubMed] [Google Scholar]
- 23.Guo A, Zhang C, Wei S, Chen B, Song LS. Emerging mechanisms of T-tubule remodelling in heart failure. Cardiovasc Res. 2013;98:204–15. doi: 10.1093/cvr/cvt020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo A, Zhang X, Iyer VR, Chen B, Zhang C, Kutschke WJ, Weiss RM, Franzini-Armstrong C, Song LS. Overexpression of junctophilin-2 does not enhance baseline function but attenuates heart failure development after cardiac stress. Proc Natl Acad Sci U S A. 2014;111:12240–5. doi: 10.1073/pnas.1412729111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim M1, Navaratnarajah M, Siedlecka U, Rao C, Dias P, Moshkov AV, Gorelik J, Yacoub MH, Terracciano CM. Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca(2+)-induced Ca(2+)release in a rodent model of heart failure. Eur J Heart Fail. 2012;14:571–80. doi: 10.1093/eurjhf/hfs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sysa-Shah P, Xu Y, Guo X, Belmonte F, Kang B, Bedja D, Pin S, Tsuchiya N, Gabrielson K. Cardiac-specific over-expression of epidermal growth factor receptor 2 (ErbB2) induces pro-survival pathways and hypertrophic cardiomyopathy in mice. PLoS One. 2012;7:e42805. doi: 10.1371/journal.pone.0042805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–65. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.