Abstract
Objective
Mice are typically housed at environmental temperatures below thermoneutrality, whereas humans live near thermoneutrality. This difference affects energy physiology and, potentially, anti-obesity drug efficacy. Here we compare β3-adrenergic agonist treatment at thermoneutrality (30°C) versus room temperature (22°C).
Methods
Male C57BL/6J mice were singly housed at 30°C or 22°C and treated with vehicle or CL316243, a β3-agonist, for four weeks. Food intake, energy expenditure, body and adipose weight, brown adipose activity, white adipose browning, and glucose tolerance were evaluated. CL316243 treatment was studied in both chow- and high fat diet- fed mice.
Results
Mice at 30°C, compared to 22°C, have reduced food intake, metabolic rate, and brown adipose activity, and increased adiposity. At both temperatures, CL316243 treatment increased brown adipose activation and energy expenditure, and improved glucose tolerance. At 30°C, CL316243 increased energy expenditure disproportionately to changes in food intake, thus reducing adiposity, while at 22°C these changes were matched, yielding unchanged adiposity.
Conclusions
CL316243 treatment can have beneficial metabolic effects in the absence of adiposity changes. In addition, the interaction between environmental temperature and CL316243 treatment is different from the interaction between environmental temperature and 2,4-dinitrophenol treatment reported previously, suggesting that each drug mechanism must be examined to understand the effect of environmental temperature on drug efficacy.
Keywords: obesity, drug development, thermoneutrality, energy expenditure, β3-adrenergic agonist, CL316243
Introduction
Mice are commonly used to study the physiology of energy homeostasis and obesity. However, their small body size means that there are profound differences in thermal biology as compared to larger mammals, such as adult humans. Typically, clothed humans at room temperature (e.g., 22°C) have cold-induced energy expenditure of ≤5% of basal metabolic rate (1, 2). In contrast, 22°C is well below thermoneutrality for mice (3, 4); cold-induced thermogenesis for singly-housed mice at this temperature is 120% of basal metabolic rate (5). Despite the modest level of adaptive thermogenesis in humans, the demonstration that adult humans do have functional brown adipose tissue (BAT) has increased interest in understanding BAT and thermal physiology and in stimulating BAT as a treatment for obesity (6, 7).
Mice studied at thermoneutrality might better model human physiology and predict obesity treatment efficacy (3, 8). Chronically housing mice at thermoneutrality minimizes heat generation, including inactivating BAT and reducing heat generation via uncoupling protein-1 (Ucp1) (9). Intriguingly, at thermoneutrality mice gain more body and adipose tissue weight than at 22°C (4, 10, 11, 12). Similarly, BAT-deficient mice develop obesity (13) and β3-receptor null mice have increased lipid stores (14). To date, the weight loss efficacy at room temperature versus thermoneutrality has been compared for only one anti-obesity drug mechanism, chemical uncoupling by 2,4-dinitrophenol (DNP) (15). At 22°C, DNP-mediated heat generation throughout the body substituted for BAT thermogenesis, inactivating BAT with no net effect on total energy expenditure or body weight/adiposity. In contrast, at thermoneutrality (30°C) the DNP-mediated increase in energy expenditure could not be compensated by a reduction in BAT activity. Thus, total energy expenditure was increased and body weight and adiposity were reduced compared to control mice.
We now explore the effect of environmental temperature on a second potential anti-diabetic weight-loss mechanism, activation of β3-adrenergic receptors (16, 17). Stimulation of β3-receptors causes lipolysis in white adipose tissue (WAT) and thermogenesis in BAT. Acutely, thermogenesis requires Ucp1 (18) and adipose tissue (19), but with chronic treatment, Ucp1-independent mechanisms contribute (20). This adipose activation elicited interest in using β3 agonists for the treatment of obesity and diabetes (21, 22). Here we investigate the effects of the rodent β3 agonist CL316243 at both 22°C and 30°C, in mice fed either a chow or high fat diet (HFD).
Methods
Animals and drug preparation
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were given ad libitum access to water and chow (7022 NIH-07 diet, 15% kcal fat, Harlan laboratories) or high fat diet (D12492, 60% kcal fat, 5.24 metabolizeable kcal/g; Research Diets, New Brunswick, NJ) with a 12:12-h dark-light cycle (lights on at 0600h) and cellulose bedding (7099W White TEK-Fresh Cellulose Bedding, Harlan laboratories). At 10 weeks of age, subcutaneous infusion of saline vehicle or CL316243 was begun in weight-matched mice (25 μg/day for 28 days using Alzet #1004 osmotic pumps infusing 0.11 μl/hr of 9.5 mg/ml CL316243, approximating 1000 μg/kg/day, see (19, 23, 24)). Pump infusion has been shown to be comparable to daily injections (24). Pumps were implanted subcutaneously using a dorsal incision with ketamine/xylazine anesthesia and banamine analgesia. After pump implantation, mice were singly housed to reduce variability (25) continuing at 22°C or immediately switched to 30°C for the duration of the experiment. In a separate experiment, mice were placed on the high-fat diet at 6 weeks of age and at 10 weeks of age they were started on a 28-day treatment with vehicle or CL316243 at 22°C or 30°C, as with the chow-fed cohort. Pharmacodynamically, the ED50 of CL316243 for an increase in metabolic rate at 60–180 min after an ip dose is 4 to 8 μg/kg (26)(Gavrilova, unpublished observations). Presumably the 25 μg/day (~1000 μg/kg/d) dose is saturating, but we are not aware of mouse CL316243 pharmacokinetic data. Body weight and food intake were measured twice weekly. Body composition was measured the day before pump implantation and again 3 weeks later by time domain Echo MRI 3-in-1 (Echo Medical Systems, Houston, TX). Energy expenditure was estimated by energy balance, which allows long-term measurement (weeks) in the native, undisturbed home cage environment. In brief, estimated energy expenditure is calculated from the metabolizeable caloric intake, corrected for the change in caloric content of the mouse (from the change in body composition over the measurement interval) (27). Protocols were approved by the NIDDK Animal Care and Use Committee.
Glucose tolerance test, hormone and metabolite profiles
Intraperitoneal glucose (2 g/kg for chow-fed mice, 1 g/kg for HFD-fed mice, with AUC calculated from 0 mg/dl) tolerance tests were performed at 1200, following a 4 hour fast. Glucose was measured with a Glucometer Contour (Bayer, Mishawaka, IN). Liver triglyceride was measured as glycerol after KOH hydrolysis (Cayman Chemical, #10010755). Serum was collected at 0900 by tail bleed 2 days before euthanasia for fed glucose, insulin, FFA, cholesterol, and triglycerides measurement. Serum for other measurements was taken from anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine, ip) mice by retro-orbital bleed at euthanasia and frozen until assayed using the following colorimetric assays: Free fatty acids (Roche Diagnostics Gmbh, Mannheim, Germany), triglycerides (Pointe Scientific Inc., Canton, MI), cholesterol (Thermo Scientific, Middletown, VA), and β-hydroxybutyrate (BioVision, Milpitas, CA). Insulin (Millipore, St. Charles, MO) was measured by RIA. Leptin (R&D Systems, Minneapolis, MN), adiponectin (ALPCO, Salem, NH), fibroblast growth factor 21 (Millipore), free T3 and free T4 (Eagle Biosciences, Nashua, NH) were measured by ELISA.
Quantitative RT-PCR
RNA was extracted (Qiagen RNeasy Plus Mini Kit, Germantown, MD), reverse transcribed (Roche Transcriptor High Fidelity cDNA Synthesis Kit, Indianapolis, IN), and quantified by real-time polymerase chain reaction (qRT-PCR, Applied Biosystems 7900HT, Foster City, CA) using SYBR green. Data are normalized to 18S RNA in the same sample and further normalized to 22°C vehicle-treated chow-fed mice.
Western blot analysis
Protein was prepared using RIPA buffer, quantified (Pierce BCA Protein Assay Kit, Thermo Scientific, Rockford, IL), separated in 4–20% SDS-PAGE gels (10 μg/lane), transferred to PVDF membrane, and probed with anti-UCP1 (1:5000, U6382, Sigma-Aldrich), or anti-α-Tubulin (1:5000, T6074, Sigma-Aldrich). Signals were detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific), using exposure times appropriate to signal intensity.
Histology
Tissues were fixed in 10% formalin, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined by light microscopy.
Data analysis
Data were analyzed using unpaired t-tests or two-way ANOVA with Holm-Sidak post-hoc testing using Sigmaplot 12.5 (Systat Software Inc), as appropriate. Statistical significance was defined as 2-tailed p<0.05.
Results
Effects of CL316243 in mice fed a chow diet
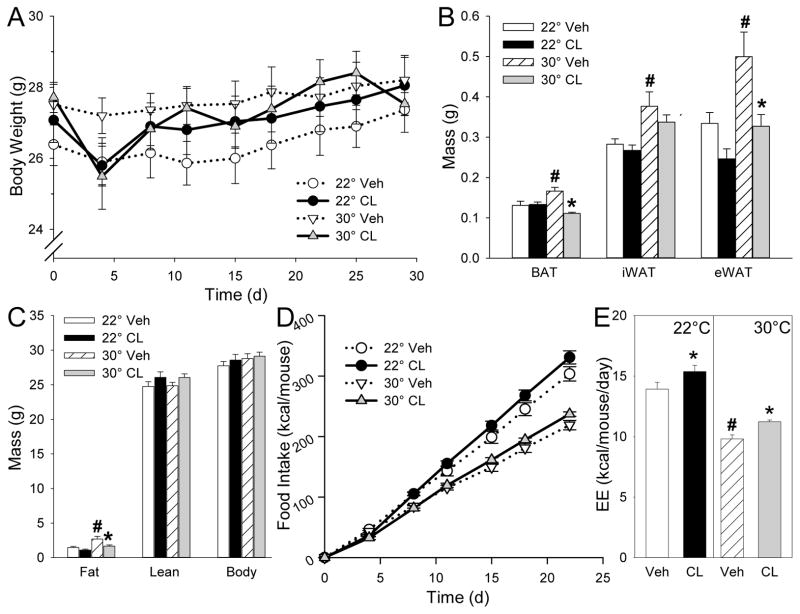
We treated 10-week old male C57BL/6J mice on a chow diet at either room temperature (22°C) or thermoneutrality (30°C) with CL316243 (25 μg/day) for 4 weeks. The effect of 30°C in vehicle-treated mice was to increase inguinal adipose tissue (iWAT), epididymal WAT (eWAT), BAT, and total fat mass, with no change in lean mass compared to mice at 22°C (Figure 1A–C). Mice at 30°C ate 28% less (p<0.001) and had a 30% (p<0.001) lower estimated free-living energy expenditure, calculated by energy balance from the caloric intake and change in body weight and composition (Figure 1D–E) (27).
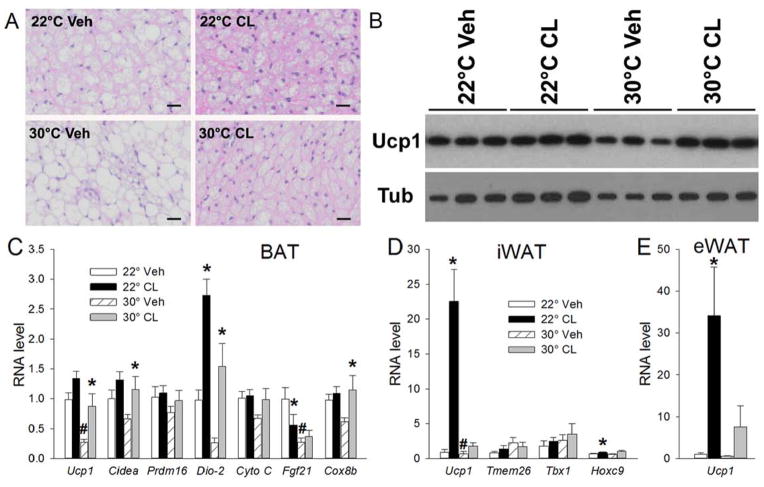
Figure 1.
Effect of CL316243 treatment on body composition and food intake in chow fed mice. A, body weight; B, fat mass; C, body composition; D, food intake ; E, estimated energy expenditure (EE). 10-week-old male mice were treated with CL316243 or vehicle for 4 weeks at the indicated temperatures. Fat pad mass was measured after 28 days of treatment. Body composition was measured after 3 weeks and thus estimated EE is averaged over first 3 weeks of treatment, avoiding the confounding effects of the testing done during the final week of drug treatment. Data are mean ± S.E.; n=8/group. #, p<0.05 for vehicle-vehicle comparison at different environmental temperatures; *, p<0.05 for vehicle-CL316243 comparison, within temperature. iWAT, inguinal white adipose tissue; eWAT, epididymal white adipose tissue; BAT, brown adipose tissue.
CL316243 treatment decreased BAT and eWAT weights at 30°C, with no significant effect at 22°C (Figure 1A–C). CL316243 increased food intake by 9% at 22°C (p=0.049) and 8% at 30°C (p=n.s.) and increased estimated energy expenditure by 10% at 22°C (p=0.025) and 14% (p=0.031) at 30°C (Figure 1D–E).
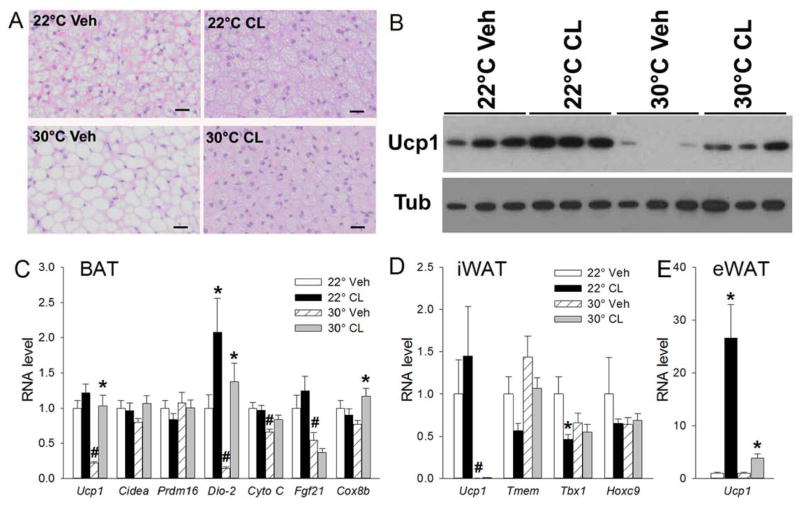
BAT lipid droplets were larger at 30°C than at 22°C in vehicle treated mice, consistent with the greater adiposity (Figure 2A). Ucp1 protein and mRNA levels were lower at 30°C than at 22°C, as were other BAT activation markers (Dio-2, CytoC, Fgf21) (Figure 2B–C). There was no clear effect of 30°C on iWAT and eWAT histology (not shown). In iWAT there was a large reduction in Ucp1 mRNA levels, while in eWAT the much lower 22°C levels were not reduced further by 30°C (Figure 2D–E, Table S1). CL316243 treatment decreased BAT lipid droplet size and increased Ucp1 protein levels at both temperatures (Figure 2A–B). CL316243 also increased Dio-2 and Cox8b mRNAs at 30°C, but only Dio-2 at 22°C (Figure 2C). Overall these data are consistent with modest BAT activation and slight WAT browning with chronic CL316243 treatment.
Figure 2.
CL316243 effect in BAT and WAT in chow fed mice after 28 days of CL316243 or vehicle treatment. A, BAT histology; B, BAT Ucp1 protein; C, BAT mRNA levels; D, iWAT mRNA levels; E, eWAT mRNA levels. Scale bar, 20 μm. mRNA levels are normalized to 22°C vehicle. Data are mean ± S.E.; n=8/group. #, p<0.05 for vehicle-vehicle comparison at different environmental temperatures; *, p<0.05 for vehicle-CL316243 comparison, within temperature.
In liver, there was no clear effect of either environmental temperature or CL316243 treatment on histology, weight, triglyceride content, metabolic mRNA levels (Pgc1a, Cpt1a, Fgf21, Pepck, Fas, Scd1, Mcad), or alanine aminotransferase levels (Figure S1A–E).
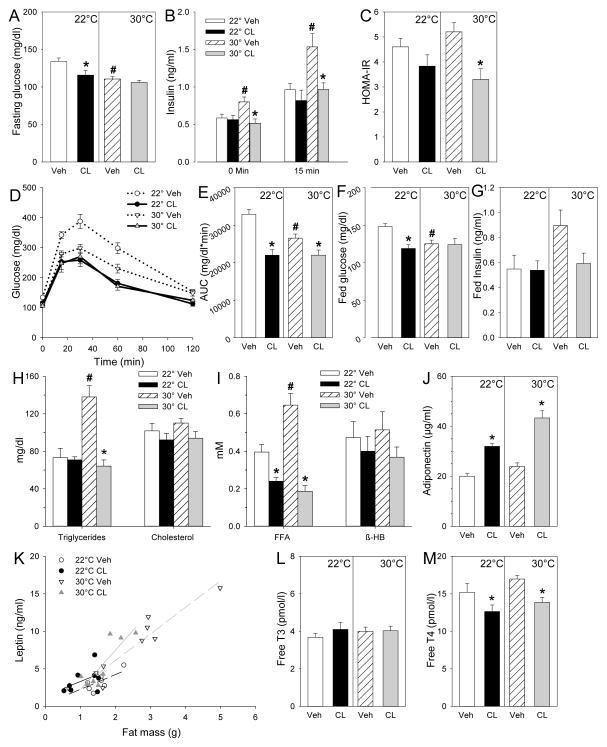
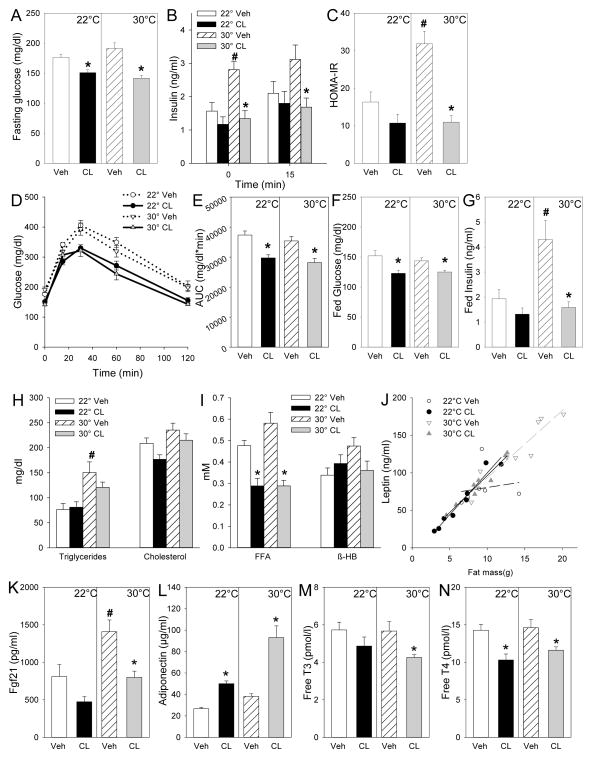
In this cohort, the vehicle group at 30°C unexpectedly had a lower fed and fasting glucose and improved glucose tolerance compared to 22°C (Fig 3A, D–F); these findings did not replicate on the high fat diet (see below). At 30°C, insulin, triglyceride, and free fatty acid levels were elevated (Figure 3B, H, I), with no significant change in cholesterol, β-hydroxybutyrate, adiponectin, leptin, free T3, or free T4 levels (Figure 3H–M).
Figure 3.
Effect of CL316243 on glucose homeostasis and circulating metabolites and hormones in chow fed mice. A, fasting blood glucose; B, plasma insulin before and 15 min after glucose administration; C, HOMA-IR; D, glucose tolerance test (GTT); E, area under the glucose excursion curve (AUC); F, fed blood glucose; G, fed serum insulin; H, total triglycerides and cholesterol; I, free fatty acids (FFA) and β-hydroxybutyrate (β-HB); J, adiponectin; K, leptin (the linear regression fit for each group is shown); L, free T3; M, free T4. GTT was conducted after 3 weeks of CL316243 or vehicle treatment with 4h fasting. After 26 days of treatment, serum was collected at 9am from fed animals to obtain fed glucose, insulin, and metabolite levels. Serum for the hormone levels was collected after 4h fasting at euthanasia, day 28 of CL316243 or vehicle treatment. Serum Fgf21 was below the limit of detection (<375pg/ml). Data are mean ± S.E.; n=8/group. #, p<0.05 for vehicle-vehicle comparison at different environmental temperatures; *, p<0.05 for vehicle-CL316243 comparison, within temperature.
CL316243 treatment at 22°C significantly reduced fasting and fed glucose levels and improved glucose tolerance. At 30°C, fasting insulin, HOMA-IR, and glucose tolerance were significantly improved by CL316243 treatment (Figure 3A–G). CL316243 also reduced triglyceride (30°C only), free fatty acid, and free T4 and increased adiponectin levels, with no significant effect on cholesterol, β-hydroxybutyrate, and free T3 (Figure 3H–M). Serum leptin levels were proportional to fat mass in each group and were decreased by CL316243 at 30°C (Figure 3K).
In summary, on a chow diet CL316243 treatment modestly activated BAT, causing increased energy expenditure and food intake, with no change in body weight and only a small decrease in fat mass. CL316243 treatment increased adiponectin, decreased free fatty acids, leptin (30°C only), and free T4 levels, and improved glucose homeostasis.
Effects of CL316243 in mice fed a high-fat diet
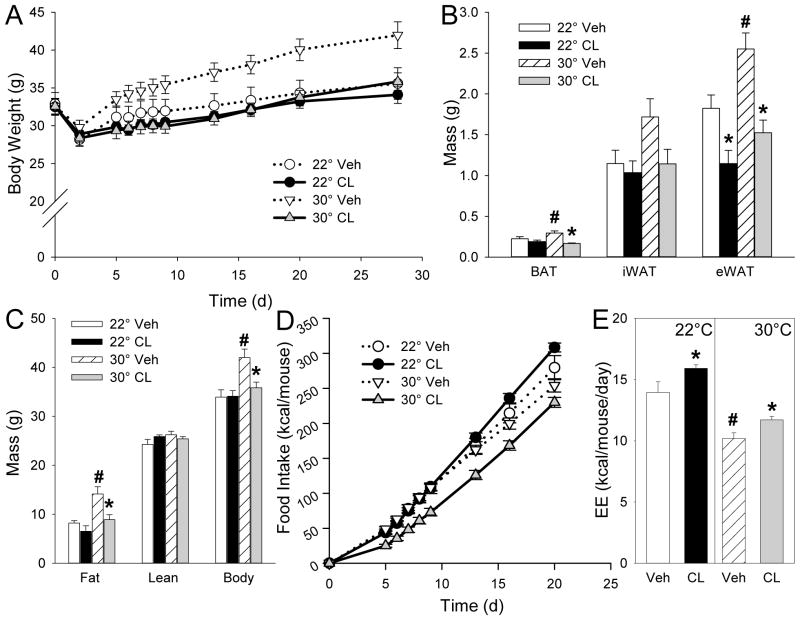
We applied the same experimental design to mice fed a HFD starting at 6 weeks of age, with CL316243 treatment starting at 10 weeks of age. HFD treatment is commonly used to produce insulin resistance and obesity. Vehicle-treated mice at 30°C gained considerably more weight (predominantly as fat) than at 22°C, while eating less and expending less energy (Figure 4A–E).
Figure 4.
Effect of CL316243 treatment on body composition and food intake in high-fat diet (HFD) fed mice. A, body weight; B, fat mass; C, body composition; D, food intake; E, estimated energy expenditure (EE). Ten-week-old male mice with HFD started at 6-weeks of age were treated with CL316243 or vehicle for 4 weeks at indicated temperatures. Body composition was measured after 3 weeks and estimated EE is averaged over first 3 weeks of treatment. Weight, fat mass, and composition are mean ± S.E.; n=8/group (n=7 for 22°C vehicle). #, p<0.05 for vehicle-vehicle comparison at different environmental temperatures; *, p<0.05 for vehicle-CL316243 comparison, within temperature. iWAT, inguinal white adipose tissue; eWAT, epididymal white adipose tissue.
CL316243 treatment at 30°C selectively prevented this increased adiposity, while decreasing food intake (not taking into account the lower body weight) by 10% (p=0.10) and increasing energy expenditure by 15% (p=0.038) (Figure 4A–E). At 22°C, the CL316243-treated mice showed no change in body weight or composition, with a 10% (p=0.064) increase in food intake and a 14% (p=0.011) increase in energy expenditure (Figure 4A–E).
In vehicle mice at 30°C, BAT had larger lipid droplets and lower Ucp1 protein and Ucp1 and Fgf21 mRNA levels than at 22°C (Figure 5A–C). At 30°C, CL316243 treatment reduced the BAT lipid droplet size, increased Ucp1 protein levels, and increased Ucp1 and other BAT activity mRNA markers including Cidea, Dio-2, and Cox8b (Figure 5A–C). At 22°C, only Dio-2 was increased by CL316243 treatment (Figure 5C). No obvious differences in iWAT and eWAT histology were observed (not shown). At 22°C, CL316243 increased iWAT and eWAT Ucp1 and iWAT Hoxc9 (Figure 5D–E, Table S1). The fat depot type is the predominant determinant of Ucp1 mRNA levels. Within each depot, multivariate regression (Table S1) demonstrated that Ucp1 expression is regulated differently in iWAT (temperature > drug ≫ diet) than in eWAT (drug > diet > temperature) or BAT (diet ≈ temperature ≈ drug).
Figure 5.
CL316243 effect in BAT and WAT in HFD fed mice. A, BAT histology; B, BAT Ucp1 protein; C, BAT mRNA levels; D, iWAT mRNA levels; E, eWAT mRNA levels. Scale bar, 20 μm. mRNA levels are normalized to 22°C vehicle from chow fed experiment. Mice were studied after 28 days of CL316243 or vehicle treatment. Data are mean ± S.E.; n=8/group (n=7 for 22°C vehicle). #, p<0.05 for vehicle-vehicle comparison at different environmental temperatures; *, p<0.05 for vehicle-CL316243 comparison, within temperature.
At 30°C (vs 22°C), liver showed no change in histology, weight, and most mRNAs, but an increase in liver Fgf21 mRNA and triglyceride levels, and in serum ALT levels (Figure S2A–E). CL316243 treatment had no significant effect on liver histology, weight, triglyceride, mRNA levels (except Fgf21, which was reduced by treatment at 30°C) or ALT levels (Figure S2A–E).
In vehicle mice at 30°C, insulin levels and HOMA-IR were higher, while glucose and glucose tolerance were similar, to measurements at 22°C (Figure 6A–G). CL316243 treatment reduced fasting and fed glucose levels and improved glucose tolerance at both temperatures. Insulin levels and HOMA-IR improved at 30°C only (Figure 6A–G).
Figure 6.
Effect of CL316243 on glucose homeostasis and circulating metabolites and hormones in HFD fed mice. A, fasting blood glucose; B, plasma insulin before and 15 min after glucose administration; C, HOMA-IR; D, glucose tolerance test (GTT); E, area under the glucose excursion curve (AUC); F, fed blood glucose; G, fed serum insulin; H, total triglycerides and cholesterol; I, free fatty acids (FFA) and β-hydroxybutyrate (β-HB); J, leptin (the linear regression fit for each group is shown); K, FGF21; L, adiponectin; M, free T3; N, free T4. GTT was conducted after 3 weeks of CL316243 or vehicle treatment at 12pm after 4h fast. After 26 days of treatment, serum was collected at 9am from fed animals to obtain fed glucose, insulin, and metabolite levels. Serum for the hormone levels was collected at euthanasia, day 28 of CL316243/vehicle treatment. Data are mean ± S.E.; n=8/group (n=7 for 22°C vehicle). #, p<0.05 for vehicle-vehicle comparison at different environmental temperatures; *, p<0.05 for vehicle-CL316243 comparison, within temperature.
At 30°C (vs 22°C), vehicle mice had increased serum triglyceride, Fgf21, and leptin levels, with no significant difference in cholesterol, free fatty acids, β-hydroxybutyrate, adiponectin, free T3, and free T4 (Figure 6H–N). At 30°C, CL316243 treatment reduced the serum free fatty acid, leptin, Fgf21, free T3, and free T4 levels and increased adiponectin levels; most of these changes were also observed at 22°C (Figure 6H–N). In summary, CL316243 treatment had similar effects on mice fed a HFD as those fed a chow diet, but with greater changes in some parameters.
Discussion
Effect of environmental temperature on energy homeostasis
This study examines the effect of environmental temperature on the treatment of obesity. The data confirm that housing mice at thermoneutrality causes inactivation of BAT and an increase in body weight due to increased adiposity, changes that are more pronounced on a high-fat diet (4, 9, 10, 11, 12). Insulin levels increase, possibly due to the increase in fat mass. Circulating triglyceride levels are also increased; flux studies will be required to characterize this effect at a mechanistic level.
It is unknown why, at thermoneutrality, the mouse reduces food intake less than the decrease in metabolic rate, causing the increase in adiposity. However, the metabolic response to reduced environmental temperature appears analogous to the response to increased physical activity (28). In both cases the animal in the unchallenged state (thermoneutrality or sedentary) shows increased adiposity. A small increase in energy expenditure (cooler environment or some physical activity) reduces adiposity, indicating an increase in energy expenditure in excess of the increase in food intake, while with further challenge (thermal or physical activity), the increase in food intake and energy expenditure are matched and the adiposity does not change further.
β3-agonist treatment and interaction with environmental temperature
β3-agonists activate WAT lipolysis and browning and BAT thermogenesis. We chose the C57BL/6J strain for these studies because it is commonly used in obesity and diabetes research. However, this genetic strain is a poor inducer of WAT Ucp1 (24), consistent with the moderate changes in Ucp1 mRNA induced by CL316243 in our study. Oxidation of fatty acids released from WAT in tissues besides BAT contributes to thermogenesis. However, in chronically CL316243-treated mice the magnitude of this non-BAT thermogenesis is not known (20).
We show that treatment with CL316243 at 22°C activated BAT and increased energy expenditure, but also increased food intake sufficiently to prevent a significant reduction in body weight/adiposity. However, despite the unchanged adiposity, the glucose tolerance improved. These results agree with prior rodent studies of chronic β3-agonist administration below thermoneutrality, which typically show modest or no weight loss, but often reduced fat mass and improved glucose tolerance (19, 23, 24, 29, 30, 31, 32, 33, 34). In a single study, body weight reduction by 24-day CL316243 treatment ranged from none to 22% over eight mouse lines (24). A contributing reason why our 22°C CL316243 treatment did not significantly reduce adiposity is that the mice, particularly the chow-fed group, were relatively lean.
CL316243 treatment at 30°C also activated BAT and increased energy expenditure, while food intake increased on the chow diet but not on the HFD. However at thermoneutrality, the food intake change was less than the increase in energy expenditure for both diets, causing a reduction in adiposity and body weight and improved glucose tolerance (Table 1).
Table 1.
Summary of intervention effects.
| Effect of: | TEE | Food intake | Adiposity | ΔFI vs ΔTEE | GTT | Reference |
|---|---|---|---|---|---|---|
| 30°C (vs 22°C) | ↓ | ↓ | ↑ | FI > TEE | no Δ | Current work, (15) |
| CL316243 (vs vehicle, at 22°C) | ↑ | ↑ | no Δ | FI = TEE | improved | Current work |
| CL316243 (vs vehicle, at 30°C) | ↑ | partial ↑ * | ↓ | FI < TEE | improved | Current work |
| DNP (vs vehicle, at 22°C) | no Δ | no Δ | no Δ | FI = TEE | no Δ | (15) |
| DNP (vs vehicle, at 30°C) | ↑ | no Δ | ↓ | FI < TEE | improved | (15) |
| Cold (vs 22°C) | ↑ | ↑ | no Δ | FI = TEE | trace improved | (39) |
Increase on chow, but not HFD.
TEE is total energy expenditure; FI is food intake; Δ FI vs Δ TEE indicates which change is greater, food intake or total energy expenditure; and GTT is glucose tolerance test. Data summarized are all from our laboratory using common methods.
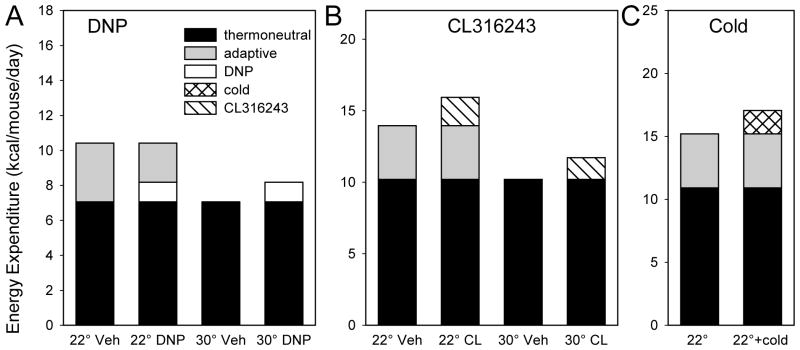
Chronic administration of CL314243 at 30°C caused a relatively small increase in energy expenditure (1.5 kcal/d in mice on HFD). For comparison, housing mice at 22°C vs 30°C increased energy expenditure by 3.8 kcal/day. Therefore, we were expecting to see little or no CL316243-induced increase in energy expenditure at 22°C, due to compensatory reduction of adaptive thermogenesis. To our surprise, CL316243 treatment at 22°C actually increased total energy expenditure by 2.0 kcal/d, slightly more than it did at 30°C (Figure 7).
Figure 7.
Effect of environmental temperature on mechanisms that increase metabolic rate. Interventions are: A, 2,4-dinitrophenol (DNP) treatment (15); B, CL316243 treatment (CL, current work); and C, exposure to 4°C (cold) for 8 hours three times per week (39). Both CL316243 and cold treatment increased energy expenditure compared to the 22°C control, while DNP did not. All data are from C57BL/6J mice on a high-fat diet. Thermonetural energy expenditure is defined as 30°C vehicle; for the cold experiment it was calculated using the mean fraction (22°C vehicle/30°C vehicle) from the DNP and CL316243 experiments.
It is possible that a compensatory reduction in energy expenditure did not occur because the CL316243 dose was high enough to completely substitute for any reduction in BAT sympathetic tone. But if this is true, why is the increase at 30°C with CL316243 less than the adaptive thermogenesis increment? A possible explanation could be that BAT stimulation by CL316243 produces hormones or neural signals that cause an increase in food intake. An example is that BAT stimulation increases production of neuregulin-4 (35), but it is not known if this hormone affects food intake.
The clinical prospects for β3 agonism as a treatment for human obesity are unclear (7). Treatment for 28 days with the β3 agonist L-796568 did not have an effect on energy expenditure, body composition, or glucose tolerance in obese men (36). More recently, β3 agonists have entered clinical use for overactive bladder and Cypess et al. showed that large single doses of mirabegron activate BAT and increase metabolic rate in men (37). There are no clinical reports of mirabegron effects on body weight, food intake, or insulin resistance/diabetes. Other considerations regarding clinical use of β3 agonists include possible cardiovascular effects (38) and the influence of a genetic variation in the receptor. Our thermoneutral data suggest that increased weight loss efficacy would result from combining reduction in food intake with a β3 agonist.
Thermoneutrality, BAT, and drug efficacy
CL316243 treatment is affected by thermoneutrality differently from DNP, the only other drug that has been studied in mice at thermoneutrality. When treated with DNP at a dose that increased energy expenditure at 30°C at an extent comparable to CL316243, there was no compensatory increase in food intake and adiposity was reduced (15) (Table 1, Figure 7). In contrast, with CL316243 treatment at 30°C on the chow diet, there was an increase in food intake, preventing some of the otherwise larger loss of adiposity. The CL316243 result shows homeostatic regulation—as energy stores were depleted, the body replaced them. We do not know why this did not occur with DNP.
In contrast at 22°C, the DNP-mediated increase in energy expenditure was quantitatively nullified by a reduction in BAT energy expenditure. Again, this differs from the energy expenditure increase seen with CL316243 at 22°C, discussed above (Table 1, Figure 7).
For comparison, chronic intermittent cold exposure looks physiologically similar to CL316243 treatment at 22°C, with increased energy expenditure and food intake with little or no effect on adiposity (39) (Table 1 and Figure 7).
Mouse experiments suggest a major role for BAT in glucose homeostasis. BAT transplantation increased energy expenditure, improved glucose tolerance at 8 weeks after transplantation, and reduced adiposity at 12 weeks (40). Both CL316243 treatment at 22°C and cold exposure also improved glucose tolerance, despite the increased food intake compensating for the increase in metabolic rate to the point that body weight/adiposity was not clearly affected (39) (Table 1, Figure 7). These observations suggest that an increase in energy expenditure, rather than a decrease in adiposity, better correlates with improved glucose tolerance, at least in mice (Table 1).
In summary, the interaction between environmental temperature and CL316243 treatment is starkly different from the interaction between environmental temperature and DNP treatment reported previously (15). Therefore, each anti-obesity mechanism must be examined individually to understand how environmental temperature affects its efficacy.
Supplementary Material
What is already known about this subject
Thermal physiology depends on body size
Mice are typically housed at environmental temperatures below thermoneutrality, while humans live close to thermoneutrality
β3-adrenergic agonists activate adipose tissue and are being considered for treatment of obesity
What this study adds
β3-adrenergic agonist treatment reduced adiposity more at thermoneutrality (30°C) than at 22°C
β3-agonist treatment increased metabolic rate similarly at thermoneutrality and 22°C
The effect of environmental temperature is different for different anti-obesity drug mechanisms, as evidenced by comparing β3-agonism to dinitrophenol uncoupling
Acknowledgments
We thank Tatyana Chanturiya and Ruifeng Teng for technical assistance. All authors conceived experiments and analyzed data. CX, MG, and OG carried out experiments. All authors were involved in writing the paper and had final approval of the submitted and published versions. This research was supported by the Intramural Research Program (DK075062, DK075064) of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH.
References
- 1.Celi FS, Brychta RJ, Linderman JD, Butler PW, Alberobello AT, Smith S, et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol. 2010;163:863–872. doi: 10.1530/EJE-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijers SL, Schrauwen P, van Baak MA, Saris WH, van Marken Lichtenbelt WD. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab. 2011;96:E598–605. doi: 10.1210/jc.2010-1957. [DOI] [PubMed] [Google Scholar]
- 3.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 4.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Thermal Biol. 2012;37:654–685. [Google Scholar]
- 5.Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab. 2015 doi: 10.1016/j.molmet.2015.03.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittle A, Relat-Pardo J, Vidal-Puig A. Pharmacological strategies for targeting BAT thermogenesis. Trends Pharmacol Sci. 2013;34:347–355. doi: 10.1016/j.tips.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speakman JR, Keijer J. Not so hot: Optimal housing temperatures for mice to mimic the environment of humans. Mol Metab. 2013;2:5–9. doi: 10.1016/j.molmet.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Rippe C, Berger K, Boiers C, Ricquier D, Erlanson-Albertsson C. Effect of high-fat diet, surrounding temperature, and enterostatin on uncoupling protein gene expression. Am J Physiol Endocrinol Metab. 2000;279:E293–300. doi: 10.1152/ajpendo.2000.279.2.E293. [DOI] [PubMed] [Google Scholar]
- 11.Nikonova L, Koza RA, Mendoza T, Chao PM, Curley JP, Kozak LP. Mesoderm-specific transcript is associated with fat mass expansion in response to a positive energy balance. FASEB J. 2008;22:3925–3937. doi: 10.1096/fj.08-108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Lowell BB, Susulic VS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 14.Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, et al. Targeted disruption of the beta 3-adrenergic receptor gene. J Biol Chem. 1995;270:29483–29492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 15.Goldgof M, Xiao C, Chanturiya T, Jou W, Gavrilova O, Reitman ML. The chemical uncoupler 2,4-dinitrophenol (DNP) protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. J Biol Chem. 2014;289:19341–19350. doi: 10.1074/jbc.M114.568204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arch JR. beta(3)-Adrenoceptor agonists: potential, pitfalls and progress. Eur J Pharmacol. 2002;440:99–107. doi: 10.1016/s0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- 17.Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Ann Rev Med. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- 18.Inokuma K, Okamatsu-Ogura Y, Omachi A, Matsushita Y, Kimura K, Yamashita H, et al. Indispensable role of mitochondrial UCP1 for antiobesity effect of beta3-adrenergic stimulation. Am J Physiol Endocrinol Metab. 2006;290:E1014–1021. doi: 10.1152/ajpendo.00105.2005. [DOI] [PubMed] [Google Scholar]
- 19.Gavrilova O, Marcus-Samuels B, Reitman ML. Lack of responses to a beta3-adrenergic agonist in lipoatrophic A-ZIP/F-1 mice. Diabetes. 2000;49:1910–1916. doi: 10.2337/diabetes.49.11.1910. [DOI] [PubMed] [Google Scholar]
- 20.Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285:E1230–1236. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- 21.de Souza CJ, Burkey BF. Beta 3-adrenoceptor agonists as anti-diabetic and anti-obesity drugs in humans. Curr Pharm Des. 2001;7:1433–1449. doi: 10.2174/1381612013397339. [DOI] [PubMed] [Google Scholar]
- 22.Weyer C, Gautier JF, Danforth E., Jr Development of beta 3-adrenoceptor agonists for the treatment of obesity and diabetes--an update. Diabetes Metab. 1999;25:11–21. [PubMed] [Google Scholar]
- 23.Bloom JD, Dutia MD, Johnson BD, Wissner A, Burns MG, Largis EE, et al. Disodium (R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino] propyl]-1,3-benzodioxole-2,2-dicarboxylate (CL 316,243). A potent beta-adrenergic agonist virtually specific for beta 3 receptors. A promising antidiabetic and antiobesity agent. J Med Chem. 1992;35:3081–3084. doi: 10.1021/jm00094a025. [DOI] [PubMed] [Google Scholar]
- 24.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagy TR, Krzywanski D, Li J, Meleth S, Desmond R. Effect of group vs. single housing on phenotypic variance in C57BL/6J mice. Obes Res. 2002;10:412–415. doi: 10.1038/oby.2002.57. [DOI] [PubMed] [Google Scholar]
- 26.Yu S, Gavrilova O, Chen H, Lee R, Liu J, Pacak K, et al. Paternal versus maternal transmission of a stimulatory G-protein alpha subunit knockout produces opposite effects on energy metabolism. J Clin Invest. 2000;105:615–623. doi: 10.1172/JCI8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravussin Y, Gutman R, LeDuc CA, Leibel RL. Estimating energy expenditure in mice using an energy balance technique. Int J Obes (Lond) 2013;37:399–403. doi: 10.1038/ijo.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer J, Marshall NB, Vitale JJ, Christensen JH, Mashayekhi MB, Stare FJ. Exercise, food intake and body weight in normal rats and genetically obese adult mice. Am J Physiol. 1954;177:544–548. doi: 10.1152/ajplegacy.1954.177.3.544. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Pennisi PA, Gavrilova O, Pack S, Jou W, Setser-Portas J, et al. Effect of adipocyte beta3-adrenergic receptor activation on the type 2 diabetic MKR mice. Am J Physiol Endocrinol Metab. 2006;290:E1227–1236. doi: 10.1152/ajpendo.00344.2005. [DOI] [PubMed] [Google Scholar]
- 30.Oana F, Takeda H, Matsuzawa A, Akahane S, Isaji M, Akahane M. Adiponectin receptor 2 expression in liver and insulin resistance in db/db mice given a beta3-adrenoceptor agonist. Eur J Pharmacol. 2005;518:71–76. doi: 10.1016/j.ejphar.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Perusse F, Bukowiecki LJ. Mechanisms of the antidiabetic effects of the beta 3-adrenergic agonist CL-316243 in obese Zucker-ZDF rats. Am J Physiol. 1998;274:R1212–R1219. doi: 10.1152/ajpregu.1998.274.5.R1212. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida T, Sakane N, Wakabayashi Y, Umekawa T, Kondo M. Anti-obesity effect of CL 316,243, a highly specific beta 3-adrenoceptor agonist, in mice with monosodium-L-glutamate-induced obesity. Eur J Endocrinol. 1994;131:97–102. doi: 10.1530/eje.0.1310097. [DOI] [PubMed] [Google Scholar]
- 33.Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, et al. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266:R1371–R1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 34.Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- 35.Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20:1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen TM, Toubro S, van Baak MA, Gottesdiener KM, Larson P, Saris WH, et al. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76:780–788. doi: 10.1093/ajcn/76.4.780. [DOI] [PubMed] [Google Scholar]
- 37.Cypess AM, Weiner LS, Roberts-Toler C, Franquet E, Kessler SH, Kahn PA, et al. Activation of Human Brown Adipose Tissue by a β3-Adrenergic Receptor Agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masutani S, Cheng HJ, Morimoto A, Hasegawa H, Han QH, Little WC, et al. beta3-Adrenergic receptor antagonist improves exercise performance in pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2013;305:H923–930. doi: 10.1152/ajpheart.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravussin Y, Xiao C, Gavrilova O, Reitman ML. Effect of intermittent cold exposure on brown fat activation, obesity, and energy homeostasis in mice. PLoS One. 2014;9:e85876. doi: 10.1371/journal.pone.0085876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.